Abstract
We have isolated a membrane fraction enriched in a class of transport carriers that form at the trans Golgi network (TGN) and are destined for the cell surface in HeLa cells. Protein kinase D (PKD) is required for the biogenesis of these carriers that contain myosin II, Rab6a, Rab8a, and synaptotagmin II, as well as a number of secretory and plasma membrane-specific cargoes. Our findings reveal a requirement for myosin II in the migration of these transport carriers but not in their biogenesis per se. Based on the cargo secreted by these carriers we have named them CARTS for CARriers of the TGN to the cell Surface. Surprisingly, CARTS are distinct from the carriers that transport vesicular stomatitis virus (VSV)-G protein and collagen I from the TGN to the cell surface. Altogether, the identification of CARTS provides a valuable means to understand TGN to cell surface traffic.
Keywords: CARTS, Golgi membranes, myosin II, protein kinase D, transport carriers
Introduction
Carrier-mediated transport along the secretory and the endocytic pathways is a key feature of eukaryotic cell compartmentation and protein secretion (Farquhar and Palade, 1981). Thus far, three different kinds of carriers have been isolated and characterized. These are (1) the clathrin-coated vesicles for endocytosis and Golgi to endosome transport (Pearse, 1976; Robinson, 1994); (2) COPI-coated vesicles for transport from the Golgi apparatus to the endoplasmic reticulum (ER) and perhaps also across the Golgi (Malhotra et al, 1989; Serafini et al, 1991; Emr et al, 2009); (3) COPII-coated vesicles for ER to early Golgi membranes (Barlowe et al, 1994). There are many different exit routes from the Golgi to the cell surface in mammalian cells and some cargoes traffic via endosomes en route to the cell surface (Bard and Malhotra, 2006; De Matteis and Luini, 2008). The molecular identity of the specific carriers for this step of protein secretion and their respective cargoes, however, remains largely elusive (de Curtis and Simons, 1989; Salamero et al, 1990; Wandinger-Ness et al, 1990; Lafont et al, 1998; Klemm et al, 2009).
In S. cerevisiae, transport of chitin synthase from the Golgi to the bud site is mediated by a class of carriers that contain specific proteins called the exomer (Wang et al, 2006). However, no exomer orthologues exist in higher eukaryotes. Simons and colleagues have reported the isolation of a class of vesicles that form at the Golgi and are destined for the cell surface in yeast (Klemm et al, 2009). These vesicles are suggested to be, in principle, similar to apically targeted vesicles of polarized mammalian cells. Although the polypeptide composition of these vesicles is not known, they are enriched in raft lipids. Clathrin was reported to be required for the export of vesicular stomatitis virus (VSV)-G protein from the trans Golgi network (TGN) and/or the endosomes in polarized MDCK cells (Deborde et al, 2008). However, the exact site of the clathrin-dependent trafficking of VSV-G protein or the composition of the respective carriers is not known. These findings reveal the possibility of numerous exit routes from the TGN and also suggest that the carriers are cargo specific. Advanced proteomics has revealed additional components of clathrin- and COPI-coated vesicles (Gilchrist et al, 2006; Borner et al, 2012); however, the identification, isolation, and characterization of the specific carriers from the Golgi to the cell surface have not been achieved to date.
Here, we report the identification of a novel class of TGN to cell surface carriers from HeLa cells. Based on their contents, we have dubbed them CARTS for CARriers of the TGN to the cell Surface pathway. Surprisingly, CARTS exclude collagen I and VSV-G protein. The description of CARTS follows.
Results
Generation of TGN46 containing transport carriers
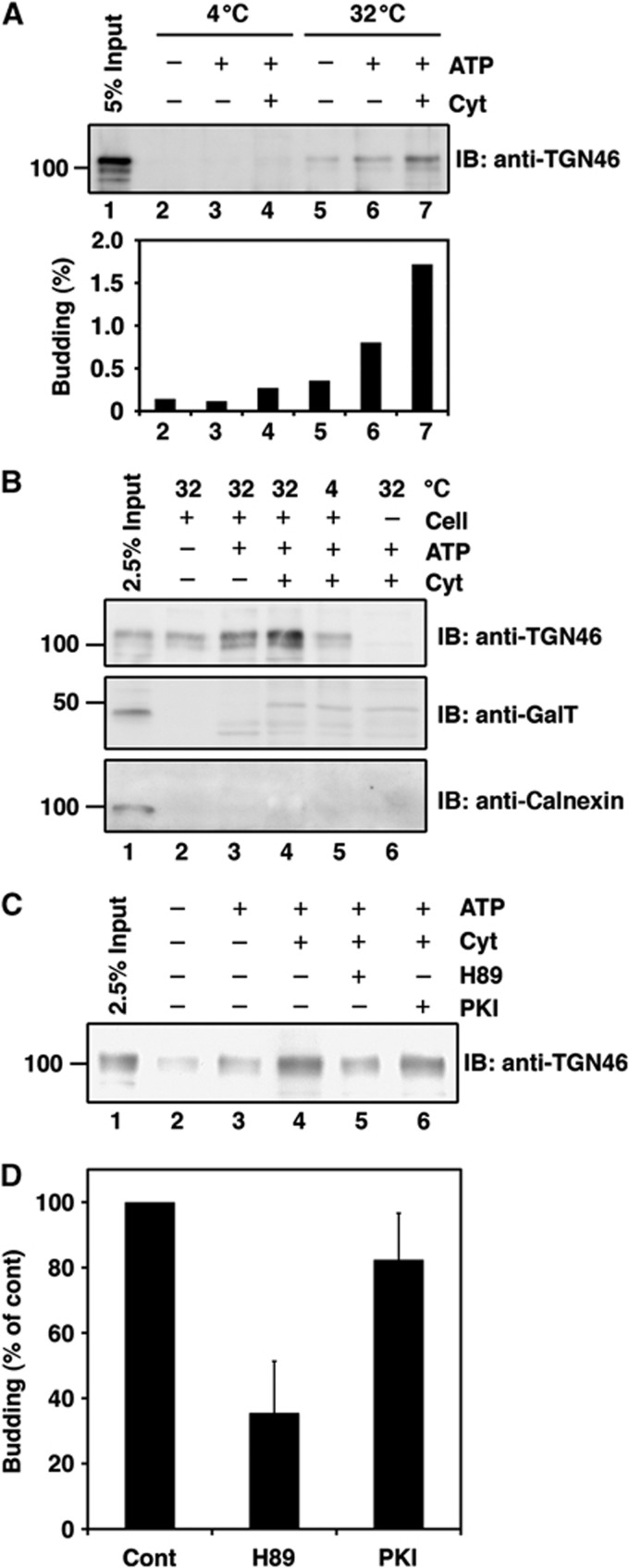
HeLa cells were permeabilized with digitonin and then incubated with an adenosine triphosphate (ATP) regenerating system and rat liver cytosol at 32°C for 45 min. The reaction mixture was centrifuged at low speed (10 000 g, 10 min) to remove cell debris and large membranes. The supernatant was centrifuged at high speed (100 000 g, 1 h) and the pellet was analysed by western blotting with an antibody to an integral membrane protein called TGN46 that is transported from the TGN to the cell surface (Rajasekaran et al, 1994; Ponnambalam et al, 1996; Banting and Ponnambalam, 1997). A reaction mixture that was incubated at 32°C was found to produce significantly a greater amount of TGN46 containing small membranes (high speed pellet) compared with that incubated at 4°C or at 32°C but without cytosol (Figure 1A). Throughout our study, ∼1–5% of the total TGN46 in the reaction mixture was contained in the small membranes. This apparent low level of budding efficiency can be explained by the fact that a very small portion of the total TGN46 is packed into transport carriers and our experimental procedures allow us to collect only a fraction of the total carriers.
Figure 1.
Biogenesis of the TGN46 containing transport carriers. (A, B) Permeabilized HeLa cells were incubated under conditions indicated. The reaction mixture was centrifuged at low speed (10 000 g for 10 min) and the supernatant was further centrifuged at high speed (100 000 g for 1 h). The high speed pellet was western blotted with antibodies against TGN46, GalT, and calnexin. The input in the figure refers to permeabilized HeLa cells used as starting material for these experiments. (C, D) The permeabilized HeLa cells were pretreated with H89 or PKI and then incubated with rat liver cytosol (Cyt) and an ATP regenerating system. The high speed pellet was western blotted with anti-TGN46. (D) Quantification of TGN46 in the high speed pellet. The average values of three independent experiments are shown (mean±s.d.). The control (Cont) refers to the permeabilized HeLa cells incubated with DMSO, ATP regenerating system and rat liver cytosol. Figure source data can be found with the Supplementary data.
The starting material (permeabilized cells: input) and the high speed pellet were western blotted with antibodies to the ER membrane chaperone, calnexin, and the resident integral membrane protein of the late Golgi cisternae, galactosyltransferase (GalT). Calnexin and GalT were detected in the starting material (Figure 1B, lane 1) but, unlike TGN46, not in the high speed pellet collected from reaction mixture containing ATP regenerating system with or without rat liver cytosol (Figure 1B, lanes 4 and 3, respectively). In the presence of rat liver cytosol, a protein with a slightly higher molecular weight was detected by the anti-GalT antibody in the high speed pellet even in reactions lacking the permeabilized cells; this protein is therefore a non-specific cytosolic protein that cross-reacts with the anti-GalT antibody (Figure 1B, lanes 4–6). These data suggest that the TGN46 containing small membranes are not produced as a result of Golgi membrane fragmentation in our experimental conditions.
Membrane fission mediated by a serine/threonine kinase, protein kinase D (PKD), is required for the biogenesis of TGN to cell surface carriers that transport cargoes to the basolateral surface (Liljedahl et al, 2001; Yeaman et al, 2004). We therefore tested the effect of the PKD inhibitor H89 (Johannes et al, 1995; Jamora et al, 1999) on the production of TGN46 containing small membranes. The reaction mixture was incubated with H89 or the protein kinase A-specific inhibitor PKI, processed to collect the high speed pellet, and western blotted with the anti-TGN46 antibody. H89, but not PKI, significantly inhibited the production of TGN46 containing small membranes (Figure 1C and D).
Altogether, these results indicate that permeabilized HeLa cells in the presence of an ATP regenerating system produce small membranes that contain TGN46 but are devoid of resident enzymes of the Golgi cisternae. The quantity of these membranes increases two- to three-fold upon addition of rat liver cytosol and the PKD inhibitor H89 inhibits the cytosol-dependent production of TGN46 containing small membranes.
Immunoisolation of TGN46 containing transport carriers
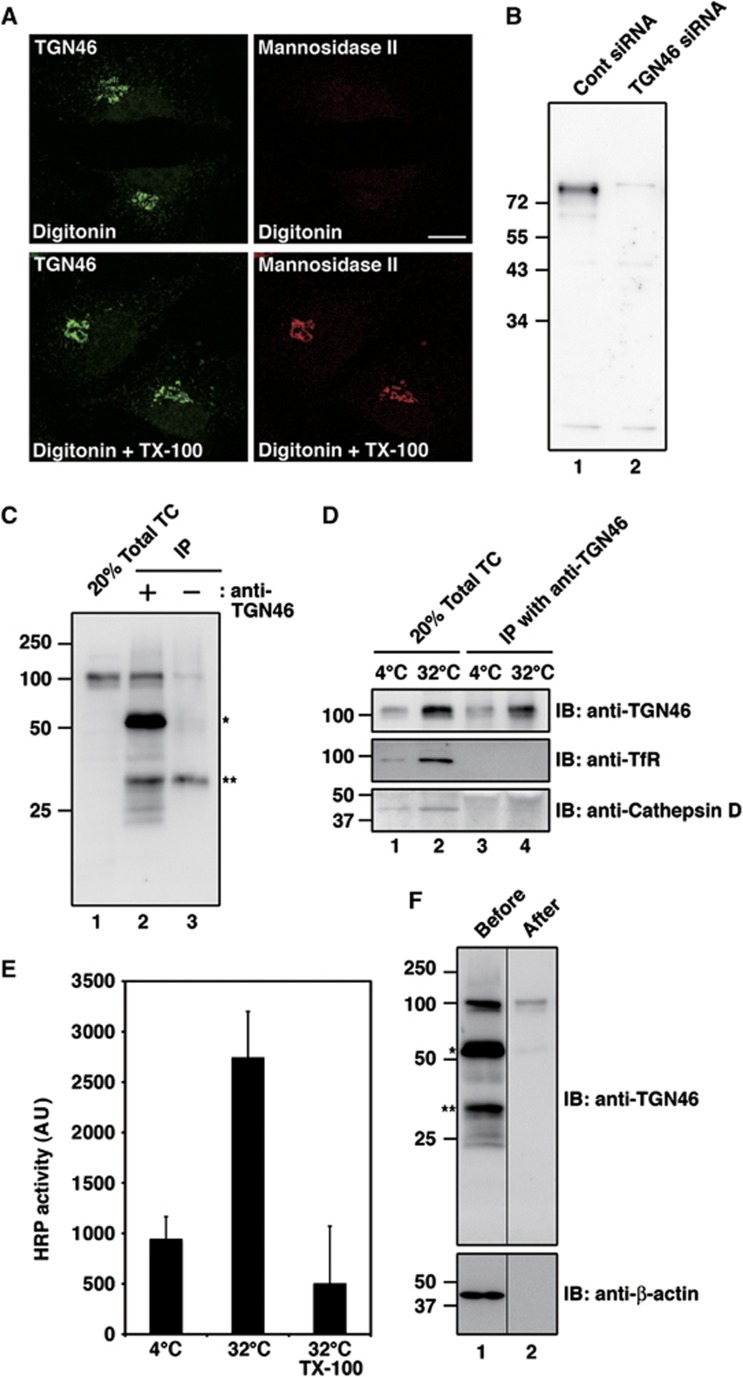
We reasoned that immunoisolation with an anti-TGN46 antibody would be the most effective approach to isolate the TGN46 containing Golgi to cell surface carriers. We therefore tested whether an affinity purified anti-TGN46 antibody recognized the cytoplasmic domain of TGN46. HeLa cells were permeabilized with digitonin and fixed with paraformaldehyde. The cells were then incubated with or without Triton X-100 and were visualized by immunofluorescence microscopy with the anti-TGN46 antibody. An antibody that recognizes the lumenal domain of the Golgi membrane-specific enzyme mannosidase II was used as a control. In digitonin-permeabilized cells, the anti-TGN46 antibody showed perinuclear staining of the Golgi membranes, unlike the mannosidase II antibody that required the incubation with Triton X-100 to bind its cognate polypeptide in the Golgi (Figure 2A). TGN46 is a heavily glycosylated protein and western blotting of HeLa cell lysates with the anti-TGN46 antibody reveals major polypeptides of 110 kDa apparent molecular weight. Lysates of HeLa cells transfected with control small interfering RNA (siRNA) or TGN46 siRNA were western blotted to test the specificity of the anti-TGN46 antibody and the result reveals depletion of almost the entire pool of the 110 kDa glycosylated TGN46 (Figure 2B). Although a few faint bands were detected even upon TGN46 knockdown, these non-specific bands were not present in the membrane fraction (total transport carries) collected from in-vitro system for the isolation of TGN46 containing potential transport carriers (Figure 2C, lane 1). These results show that the affinity purified anti-TGN46 antibody is highly specific for the cytoplasmic domain of TGN46 and therefore suitable for the immunoisolation of TGN46 containing transport carriers.
Figure 2.
Immunoisolation of the TGN46 containing transport carriers. (A) The anti-TGN46 antibody recognizes the cytoplasmic tail of TGN46. HeLa cells were permeabilized with digitonin, fixed with paraformaldehyde, incubated with or without Triton X-100 (TX-100), and then visualized with affinity purified anti-TGN46 and anti-mannosidase II antibodies. Bar, 10 μm. (B) HeLa cells were transfected with control (Cont) siRNA or siRNA oligos specific for TGN46. This procedure was repeated after 24 h. Seventy-two hours after the first transfection the cells were lysed and the lysates were western blotted with the anti-TGN46 antibody. (C) Permeabilized HeLa cells were incubated with an ATP regenerating system and rat liver cytosol at 32°C. The low speed supernatant of the reaction mixture was incubated with or without anti-TGN46 antibody and the immunoprecipitates were western blotted with anti-TGN46 antibody. An aliquot of the low speed supernatant was centrifuged at high speed (100 000 g for 1 h) and the pellet was analysed (Total transport carriers (TC)). The single and double stars denote immunoglobulin heavy chain and a non-specific polypeptide, respectively. (D) Permeabilized HeLa cells were incubated with an ATP regenerating system and rat liver cytosol at 4 or 32°C. Immunoprecipitates were western blotted with indicated antibodies. (E) Low speed supernatant of reaction mixture was prepared from HeLa-ssHRP cells and subjected to the immunoprecipitation in the presence or absence of TX-100. The HRP activity in immunoprecipitated materials was quantified by chemiluminescence. The average values of two experiments are shown (mean±s.d.). (F) Immunoisolated TGN46 containing membranes were released from the magnetic beads by incubation with 100 mM sodium carbonate (pH 11.5) and collected by high speed centrifugation (100 000 g for 1 h). The membranes were western blotted with indicated antibodies: single and double stars denote immunoglobulin heavy chain and a non-specific polypeptide, respectively. Figure source data can be found with the Supplementary data.
The low speed supernatant of the reaction mixture, described above, was incubated with the anti-TGN46 antibody and then with protein G conjugated magnetic beads. The beads were collected with a magnet and after extensive washing with an isotonic buffer the bound membranes were analysed by western blotting (Figure 2C and D). The immunoisolated membranes containing TGN46 were devoid of cathepsin D, which is transported from the TGN to the lysosomes (Zaidi et al, 2008), and transferrin receptor (TfR), which cycles between endosomes and the plasma membrane (Huebers and Finch, 1987; Figure 2D, lane 4). This result indicates that TGN46 containing transport carriers were not contaminated with transport carriers from the TGN that are destined for the endosomes/lysosomes.
HeLa cells stably expressing horseradish peroxidase (HRP) fused to a signal sequence (ss) (ssHRP) were used to test whether the secretory cargo HRP was contained in the TGN46 containing transport carriers. HeLa-ssHRP cells were incubated at 20°C to block exit of HRP from the Golgi. The immunoisolation procedure described above was then used to obtain TGN46 containing small membranes. The immunoisolated membrane fraction was solubilized with Triton X-100 to measure HRP activity by chemiluminescence as described previously (Bard et al, 2006). The membranes prepared from the reaction mixture at 32°C contained more functionally active HRP than the control (4°C) (Figure 2E). When the membranes were solubilized with Triton X-100 prior to the immunoisolation (32°C, TX-100) the HRP activity was comparable to that observed in the control prepared from the reaction mixture at 4°C. The results reveal that the immunoisolated TGN46 containing membranes also contained the secretory cargo HRP.
Polypeptide composition of TGN46 containing transport carriers
In order to release the TGN46 containing membranes from the magnetic beads, the beads were incubated with 100 mM sodium carbonate (pH 11.5) for 30 min. The beads were removed with the use of a magnet followed by low speed centrifugation (10 000 g, 5 min). The membranes released from the beads were collected by high speed centrifugation (100 000 g, 1 h), dissolved in SDS–PAGE sample buffer, and western blotted with anti-TGN46 and anti-β-actin antibodies to confirm the removal of non-specific cytosolic proteins (Figure 2F). This procedure was repeated with a larger reaction mixture and the high speed membrane fraction corresponding to the sample analysed by western blotting (Figure 2F, lane 2) was analysed by mass spectrometry. The complete list of peptides identified by this procedure is shown in Supplementary Table SI. It is important to note that many cytosolic proteins contained in this membrane fraction are a result of non-specific binding to the beads. We have therefore selected proteins that are likely to be secreted or inserted into the plasma membrane (and should therefore contain a signal sequence for targeting to the ER), and accessory proteins that likely aid in the trafficking of these carriers from the TGN to the cell surface (Table I). The final list of selected proteins includes three soluble (secreted) proteins, four integral membrane proteins, four peripheral membrane proteins, and six cytosolic proteins selected based on their localization and involvement in membrane trafficking. In principle, alkaline pH should release the peripheral and lumenal proteins from the membranes (Fujiki et al, 1982). Surprisingly, a number of proteins lacking transmembrane domains were not released from the membranes by our experimental procedures. The reasons remain unclear and therefore we used additional procedures to test their presence in the TGN46 containing potential transport carriers.
Table 1. Polypeptide composition of the TGN46 containing transport carriers.
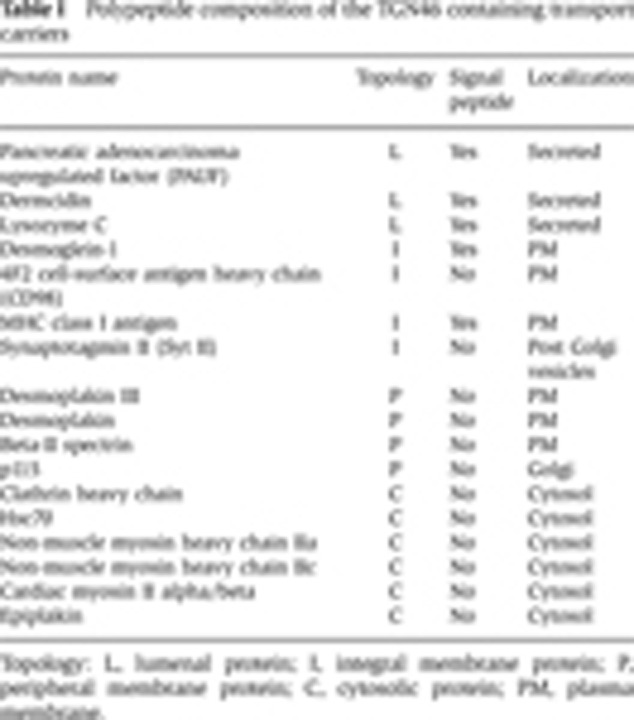
Topology: L, lumenal protein; I, integral membrane protein; P, peripheral membrane protein; C, cytosolic protein; PM, plasma membrane.
Pancreatic adenocarcinoma upregulated factor and lysozyme C
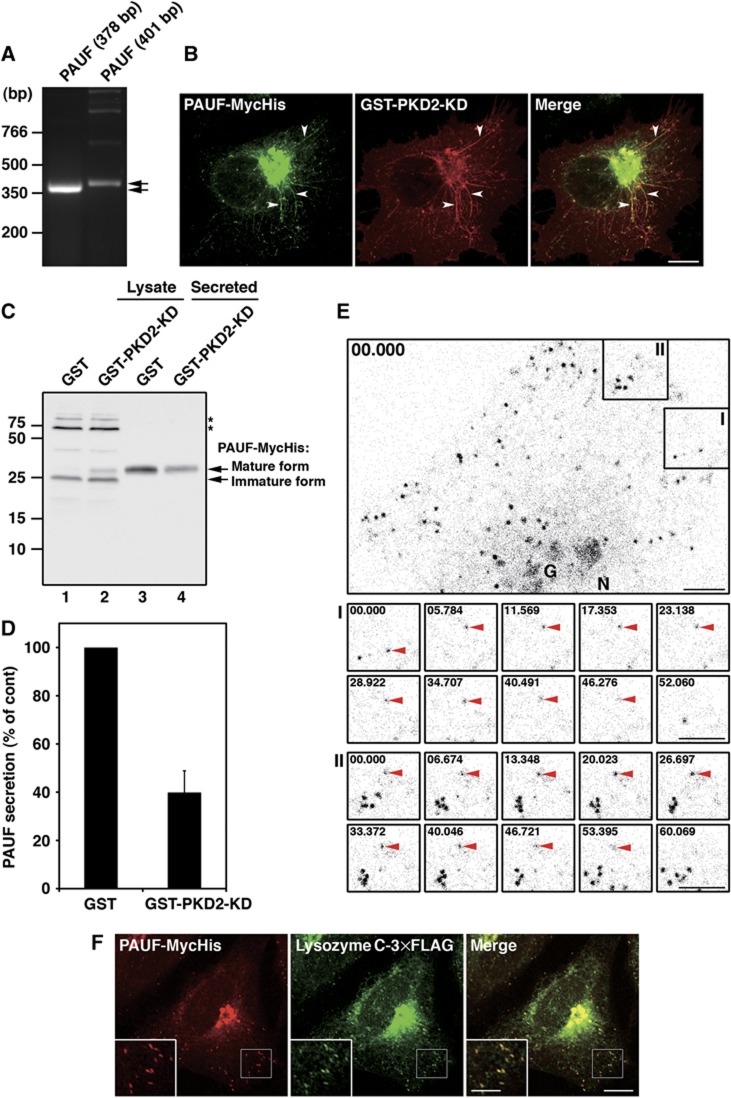
Expression of pancreatic adenocarcinoma upregulated factor (PAUF) in HeLa cells was confirmed by RT–PCR using two sets of primers designed to amplify cDNA fragments of 378 and 401 bp, respectively, in length from mRNA (Figure 3A).
Figure 3.
PKD is required for PAUF secretion. (A) PAUF cDNA was amplified from HeLa RNA by RT–PCR. Two sets of primers were designed to synthesize cDNA fragments of 378 and 401 bp, respectively, in length from mRNA. (B) PAUF-MycHis and GST-PKD2-KD were co-expressed in HeLa cells and visualized by fluorescence microscopy with anti-Myc and anti-GST antibodies, respectively. Arrowheads indicate tubules attached to the TGN. Bar, 10 μm. (C, D) PAUF-MycHis was co-expressed with GST (control) or GST-PKD2-KD in HeLa cells. The secretion of PAUF-MycHis was monitored by western blotting the cell lysate (lanes 1 and 2) and the medium (lanes 3 and 4) with anti-His antibody: stars denote a non-specific polypeptide. (D) Quantification of PAUF secretion. The average values of three independent experiments are shown (mean±s.d.). (E) Live-cell imaging of CARTS trafficking. HeLa cells expressing PAUF-mRFP were imaged at 440 ms intervals for ∼3 min. The kinetics of the disappearance of PAUF containing membranes in the areas marked I and II are shown in detail in the respective lower panels. The time (seconds) is indicated in each panel. Bars, 5 μm. G, Golgi. N, Nucleus. See also Supplementary Movie S1. (F) PAUF-MycHis and lysozyme C-3 × FLAG were co-expressed in HeLa cells and visualized by fluorescence microscopy with anti-Myc and anti-FLAG antibodies, respectively. High magnifications of small punctate elements are shown in the inset. Bars, 10 and 5 μm (inset). Figure source data can be found with the Supplementary data.
Is PAUF a secretory cargo? We expressed a MycHis-tagged PAUF in HeLa cells to monitor its secretion. As previously reported (Kim et al, 2009), western blotting of the cell lysate and the medium revealed that the intracellular pool of PAUF-MycHis (25 kDa) is modified and secreted as a higher molecular weight protein (Figure 3C). We have shown previously that expression of PKD-kinase dead (KD) mutant inhibits Golgi to cell surface transport and tubules containing the cargoes destined for the cell surface are found attached to the TGN (Liljedahl et al, 2001). These tubules contain TGN46, but not the late Golgi resident enzyme sialyltransferase (Supplementary Figure S1). We tested whether PAUF was contained in the tubules attached to the TGN in cells expressing PKD-KD. HeLa cells were co-transfected with plasmids for glutathione S transferase (GST)-PKD2-KD and PAUF-MycHis. The cells were visualized with anti-GST and anti-Myc antibodies to localize GST-PKD2-KD and PAUF-MycHis, respectively. They were found to co-localize at the Golgi apparatus (Figure 3B). Moreover, the GST-PKD2-KD induced tubules contained PAUF-MycHis. Thus, expression of PKD-KD arrested PAUF at the TGN and the tubules that fail to separate because of a defect in membrane fission. We further tested the effect of PKD-KD on the secretion of PAUF. HeLa cells were co-transfected with plasmids for GST-PKD2-KD and PAUF-MycHis. The medium and the cell lysate were western blotted with an anti-His antibody. The results revealed that GST-PKD2-KD expression decreased the secretion of PAUF-MycHis by 60% compared with the control cells expressing GST (Figure 3C and D). There was a concomitant increase in the amount of higher molecular weight PAUF-MycHis in the lysate of GST-PKD2-KD expressing cells (Figure 3C, lane 2). Together with the finding that H89 inhibited the formation of TGN46 containing membranes in the in-vitro system (Figure 1C and D), this result supports our proposal that the immunoisolated membranes require a PKD-mediated fission for their departure from the TGN.
HeLa cells expressing PAUF-monomeric red fluorescent protein (mRFP) were observed by live-cell imaging and PAUF-mRFP could be localized to the Golgi and small punctate elements (Figure 3E; Supplementary Movie S1). The arrowheads in Figure 3E show the disappearance of the PAUF-mRFP containing small punctate elements, presumably by fusion with the cell surface. HeLa cells expressing PAUF-MycHis were stained with antibodies specific for proteins of the COPI, COPII, and clathrin coats. PAUF containing carriers showed different distributions from the carriers containing these coat proteins (Supplementary Figure S2). PAUF containing carriers were also distinguished from the ER-Golgi intermediate compartment (ERGIC-53), endosomes (EEA1 and TfR) and lysosomes (Lamp-1) (Supplementary Figure S3). There was no co-localization of these carriers with mannose 6-phosphate receptor (M6PR), which is transported by clathrin-coated vesicles from the TGN to the endosomes. The carriers were also distinguished from the peripheral small elements stained with antibodies specific for proteins in TGN-derived clathrin-coated vesicles, γ-adaptin and GGA1 (Supplementary Figure S3). These results suggest that PAUF is likely transported directly from the TGN to the cell surface. PKD is required for the fission of TGN to cell surface-specific carriers (Liljedahl et al, 2001) and since PKD is required for the biogenesis of TGN46 containing membranes (Figure 1C and D) and secretion of PAUF, which was identified in the TGN46 containing isolated membranes (Figure 3C and D), we have named these membranes CARTS for CARriers from the TGN to the cell Surface.
We tested whether the secretory cargo, lysozyme C, was contained in the CARTS. Lysozyme C-3 × FLAG was co-expressed with PAUF-MycHis in HeLa cells. In all, 93% (n=613 punctate elements in 5 cells) of PAUF containing CARTS co-localized with lysozyme C-3 × FLAG (Figure 3F).
Ultrastructural analysis of CARTS
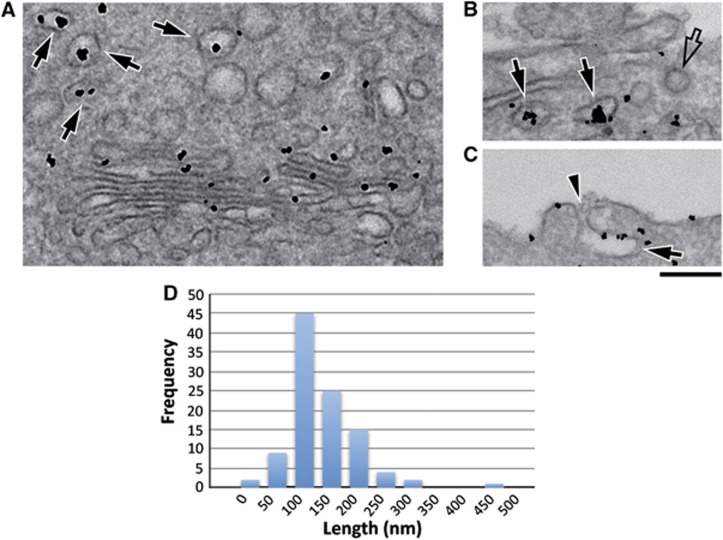
HeLa cells expressing PAUF-MycHis were incubated at 20°C for 5 h in the presence of cycloheximide. The cells were shifted to 37°C for 15 and 60 min, respectively, fixed and visualized by immuno-electron microscopy (EM) with anti-Myc antibody followed by a gold conjugated secondary antibody. Shortly after release from the 20°C block (15 min), gold particles associated with PAUF-MycHis were clearly detectable in the Golgi membranes and in a number of round and elongated profiles (Figure 4A, arrows). In cells visualized at 60 min after the shift from 20°C, we saw similar PAUF-MycHis containing membranes in the proximity of the plasma membrane (Figure 4B and C; Supplementary Figure S4, arrows). Gold particles were also observed at the cell surface at this time point, which might be due to the patches of the PAUF-MycHis release post fusion of CARTS with the cell surface (Figure 4C; Supplementary Figure S4, arrowheads). Based on the following criteria, we suggest the PAUF-MycHis-positive structures are not a result of endocytosis. First, PAUF-MycHis is not contained in clathrin-coated buds or vesicles emerging from the plasma membranes (Figure 4B; Supplementary Figure S4, empty arrows). Second, we employed tannic acid (Polishchuk et al, 2004) to block fusion of TGN-derived CARTS with the plasma membrane (and therefore PAUF release) and still observed PAUF containing CARTS-like structures proximal to the plasma membrane (Figure 4B, arrows). The CARTS vary in shape from round to elongated membranes of 100–250 nm diameter (158±62 nm, average±s.d.) (Figure 4D).
Figure 4.
Ultrastructure of CARTS. (A–D) HeLa cells were transfected with PAUF-MycHis and on the following day they were incubated at 20°C for 5 h in the presence of cycloheximide. The cells were shifted to 37°C to release PAUF-MycHis from the Golgi membranes and fixed at either 15 min (A) or 60 min (B, C). In a parallel incubation, cells were incubated with tannic acid post 20°C block to prevent fusion with the plasma membrane (B). Cells were then fixed and prepared for immuno-EM as described in Materials and methods. Arrows in all panels indicate CARTS labelled with the anti-Myc antibody; empty arrows show unlabelled clathrin-coated structures, and the sites of CARTS fusion with the plasma membrane are marked by arrowheads. (D) Quantitation of the number of CARTS and their corresponding size. Bar, 200 nm.
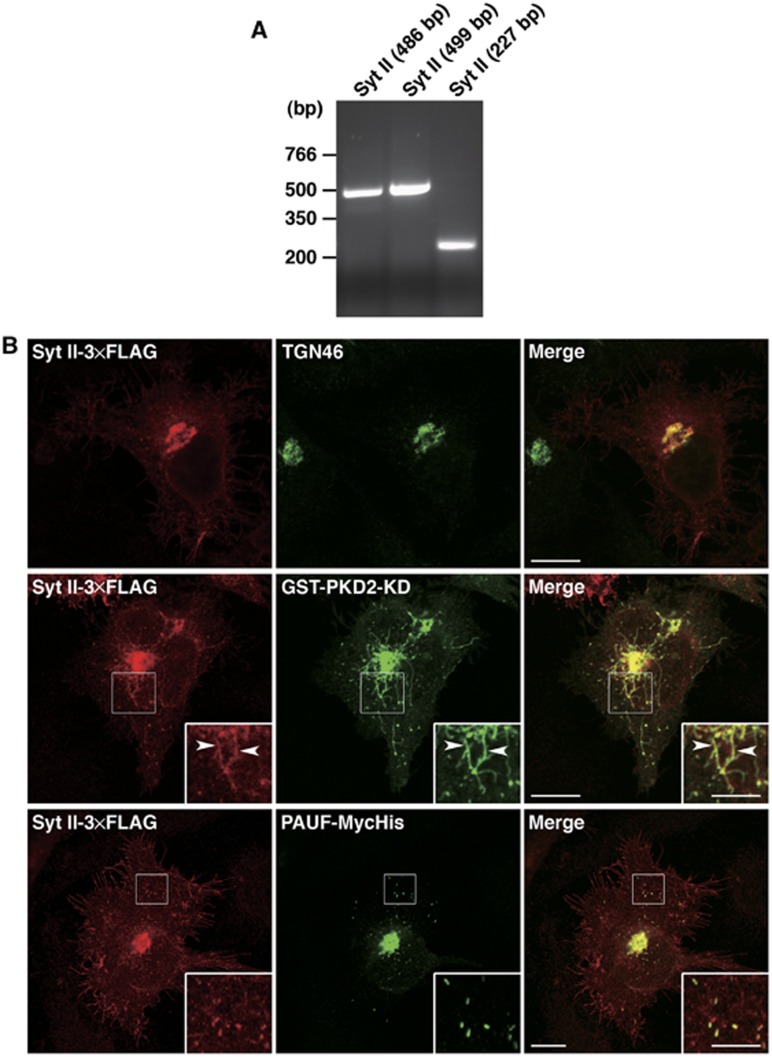
Synaptotagmin II
RT–PCR performed with three sets of primers confirmed expression of synaptotagmin (Syt) II in HeLa cells (Figure 5A). Syt II-3 × FLAG was expressed in HeLa cells and visualized by fluorescence microscopy. Syt II was found to co-localize with TGN46 (Figure 5B, top row). In cells co-expressing Syt II-3 × FLAG and GST-PKD2-KD, they were found to co-localize at the TGN and the attached tubules (Figure 5B, middle row). We next tested the localization of PAUF-MycHis and Syt II-3 × FLAG in cells co-expressing these proteins. Syt II-3 × FLAG was localized to the plasma membrane, the TGN, and a cluster of punctate elements (Figure 5B, bottom row). In all, 82% (n=484 punctate elements in 5 cells) of PAUF containing CARTS co-localized with these punctate elements of Syt II-3 × FLAG. In neurons, synaptotagmins are incorporated into synaptic vesicles at the Golgi complex and required for their fusion with the cell surface through interaction with phospholipids and SNAREs (Ullrich et al, 1994; Osborne et al, 1999; Tucker and Chapman, 2002). The incorporation of Syt II-3 × FLAG into the PKD-KD induced tubules and the punctate elements that contain PAUF and lysozyme C (Figures 3F and 5B) suggests that Syt II is carried in CARTS from the TGN to the cell surface.
Figure 5.
Syt II is contained in CARTS. (A) Syt II cDNA was amplified from HeLa RNA by RT–PCR. Three sets of primers were designed to synthesize cDNA fragments of 486, 499, and 227 bp, respectively, in length from mRNA. (B) Syt II-3 × FLAG was expressed alone (top row) or in combination with GST-PKD2-KD (middle row) or PAUF-MycHis (bottom row) in HeLa cells. The cells were double stained with anti-FLAG and anti-TGN46, anti-GST, or anti-Myc antibodies. The boxed areas are enlarged in the insets. Arrowheads indicate tubules attached to the TGN. Bars, 10 and 5 μm (inset).
CARTS contain Rab6a and Rab8a
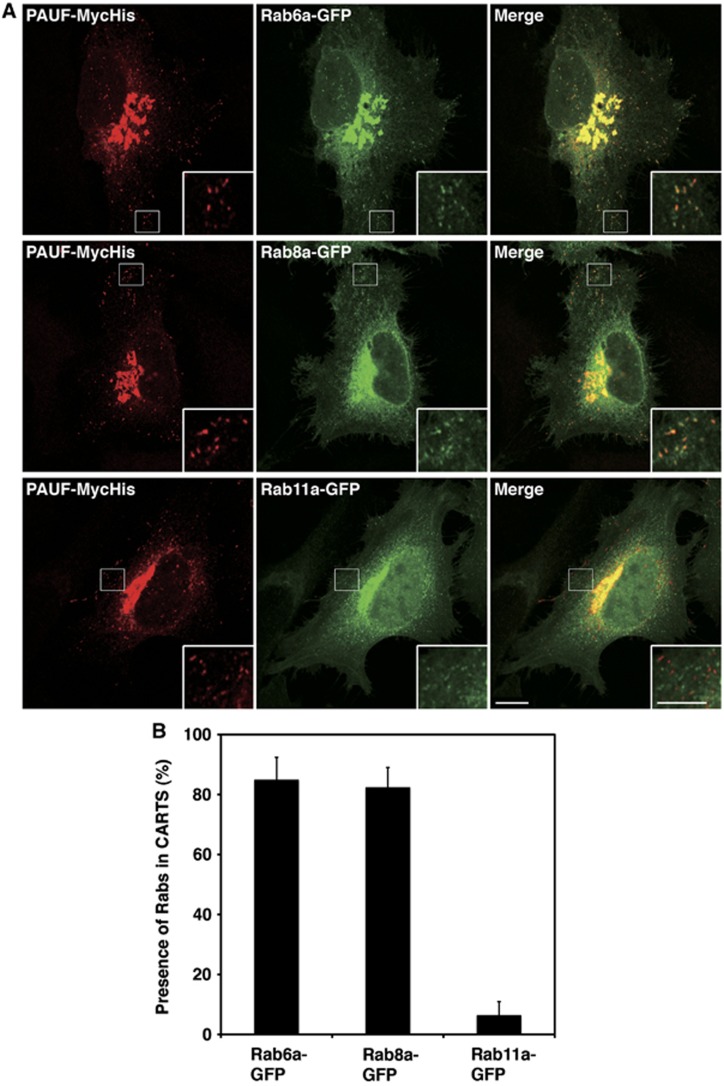
Rab proteins are small molecular weight GTPases required for both endocytosis and exocytosis (Pfeffer, 2001; Zerial and McBride, 2001; Stenmark, 2009; Hutagalung and Novick, 2011). We tested whether Rab proteins reported to be involved in post Golgi trafficking were contained in the CARTS. Rab6a-green fluorescent protein (GFP), Rab8a-GFP, and Rab11a-GFP were co-expressed with PAUF-MycHis in HeLa cells and the cells were visualized by fluorescence microscopy (Figure 6A). An anti-Myc antibody was used to identify PAUF containing CARTS and the results revealed the co-localization with both Rab6a and Rab8a, but not with Rab11a (Figure 6A and B). We also visualized HeLa cells expressing PAUF-MycHis with an anti-Rab6 antibody. The result reveals that PAUF containing CARTS contain endogenous Rab6 (Supplementary Figure S3, bottom row). We could not test endogenous Rab8a because of lack of anti-Rab8a antibodies available for immunofluorescence, but based on the fact that other GFP-tagged Rab proteins overexpressed in HeLa cells did not co-localize with PAUF-MycHis, it is reasonable to conclude that PAUF containing CARTS contain Rab6a and Rab8a.
Figure 6.
CARTS contain Rab6a and Rab8a. (A) HeLa cells were transfected with plasmids for PAUF-MycHis and Rab6a-GFP, Rab8a-GFP, or Rab11a-GFP and visualized by fluorescence microscopy. High magnifications of small punctate elements are shown in the inset. Bars, 10 and 5 μm (inset). (B) Quantification of co-localization of Rabs with CARTS. Rab6a-GFP: n=452 punctate elements in 6 cells, Rab8a-GFP: n=360 punctate elements in 5 cells, and Rab11a-GFP: n=364 punctate elements in 5 cells (mean±s.d.).
Myosin II
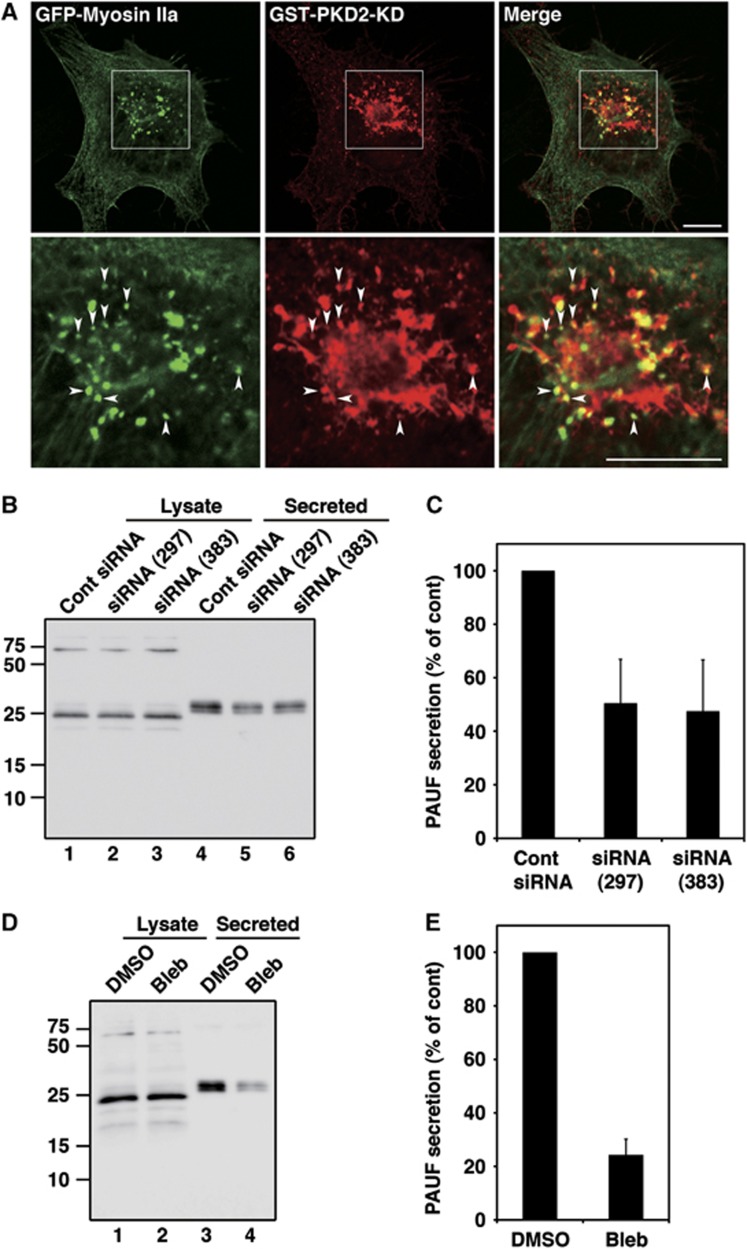
Since myosin II remains attached to the isolated membranes even after alkaline wash (sodium carbonate pH 11.5), we tested whether a pool of myosin II was attached to the Golgi membranes or the carriers that form at such membranes. Additionally, what is the role of myosin II in CARTS-dependent traffic to the cell surface? Myosin II is a cytosolic protein and by fluorescence microscopy found to be distributed throughout the cytoplasm. Permeabilization of the cells with digitonin followed by washing to remove the cytoplasmic pool improved the visualization of the membrane bound fraction of the cytoplasmic proteins. GFP-myosin IIa was found in the perinuclear region and many of the GFP-myosin IIa containing punctae co-localized with GST-PKD2-KD (Figure 7A). Because of the cell permeabilization clear tubules of PKD-KD were not observed.
Figure 7.
Myosin II is required for PAUF secretion. (A) Myosin IIa co-localization with PKD-KD at the TGN. HeLa cells co-expressing GFP-Myosin IIa and GST-PKD2-KD were visualized with fluorescence microscopy. Enlarged images of the perinuclear region (boxed) are shown in the lower panels. Arrowheads indicate the small punctate elements containing both GFP-Myosin IIa and GST-PKD2-KD. Bars, 10 μm. (B, C) HeLa cells were transfected with control (Cont) siRNA or siRNA oligos specific for myosin IIa. Forty-eight hours later, the cells were transfected with a plasmid for PAUF-MycHis. The secretion of PAUF-MycHis was monitored by western blotting the cell lysate and the medium with anti-His antibody. (D, E) The secretion of PAUF-MycHis from HeLa cells treated with DMSO (control) or 100 μM blebbistatin (Bleb) was monitored as described above. (C, E) Quantification of PAUF secretion. The amount of secreted PAUF was normalized with the expression level. The average values of three independent experiments are shown (mean±s.d.). Figure source data can be found with the Supplementary data.
HeLa cells were transfected with siRNA oligos targeting myosin IIa and the cell lysates were western blotted with an anti-myosin II antibody. Two siRNA oligos targeting a different sequence of myosin IIa, siRNA (297) and siRNA (383), decreased the expression of myosin IIa by 62 and 72%, respectively, compared with cells transfected with control siRNA (Supplementary Figure S5A and B). We tested the effect of myosin IIa knockdown on secretion of PAUF-MycHis. Forty-eight hours after siRNA transfection, cells were transfected with a plasmid encoding PAUF-MycHis. Twenty-four hours later, cells were washed with fresh medium and incubated at 20°C for 2 h to block cargo exit from the Golgi and then shifted to 32°C for 1 h to restore transport. Medium from myosin IIa knockdown and control cells was western blotted with anti-His antibody to quantitate PAUF-MycHis secretion. Myosin IIa knockdown inhibited PAUF-MycHis secretion by 50% compared with control levels (Figure 7B and C). Blebbistatin has been reported to specifically inhibit the activity of myosin II (Straight et al, 2003) and we therefore tested its effect on PAUF-MycHis secretion. PAUF-MycHis expressing cells were incubated at 20°C for 2 h, preincubated with blebbistatin containing medium for 15 min, and then shifted to 32°C for 45 min to restore transport. The medium from the dimethyl sulphoxide (DMSO)-treated cells (control) and the blebbistatin-treated cells was western blotted with anti-His antibody to quantitate PAUF-MycHis secretion. Blebbistatin treatment also efficiently inhibited PAUF-MycHis secretion (Figure 7D and E).
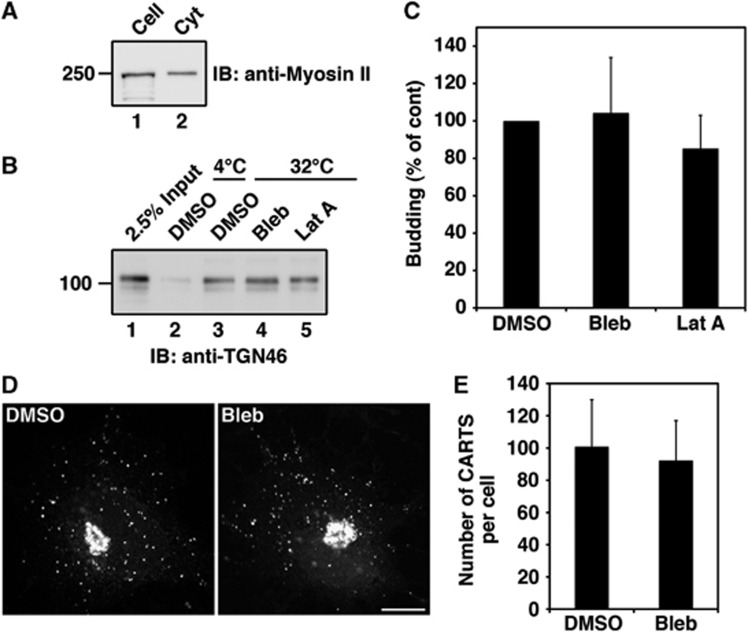
We then tested the role of myosin II in the biogenesis of TGN46 containing CARTS using the in-vitro transport carrier formation system. We found that about 70% of total myosin II was supplied from permeabilized cells in our in-vitro system (Figure 8A). Therefore, permeabilized HeLa cells were pretreated with blebbistatin for 10 min at 32°C followed by 10 min incubation with rat liver cytosol, and finally with ATP regenerating system for 45 min. A reaction mixture treated with DMSO was used as a control. Both the control and the blebbistatin-treated reaction mixtures were processed for the preparation of total transport carriers (high speed pellet) and then western blotted with the anti-TGN46 antibody to estimate the biogenesis of CARTS. Blebbistatin treatment did not affect the biogenesis of CARTS (Figure 8B, lane 4; Figure 8C). The same result was obtained with an actin-depolymerizing agent, latrunculin A (Figure 8B, lane 5 Figure 8C). We also incubated the permeabilized HeLa cells and the rat liver cytosol separately with blebbistatin for 10 min at 32°C and then mixed them in the presence of an ATP regenerating system for 45 min. This treatment also did not affect the biogenesis of CARTS (Supplementary Figure S5E and F).
Figure 8.
Myosin II is not required for the biogenesis of CARTS. (A) The starting materials for the biogenesis of CARTS, permeabilized HeLa cells and rat liver cytosol, were western blotted with anti-myosin II antibody. (B, C) The reaction mixture for the biogenesis of CARTS was incubated with DMSO (control), 100 μM blebbistatin (Bleb), or 500 nM latrunculin A (Lat A). The high speed pellet containing CARTS was western blotted with anti-TGN46 antibody. (C) Quantification of CARTS formation. The average values of three independent experiments are shown (mean±s.d.). (D, E) HeLa cells expressing PAUF-MycHis were incubated at 20°C for 2 h in the presence of 20 μg/ml cycloheximide. After pretreatment with DMSO or 100 μM Bleb for 15 min, the cells were shifted to 32°C. After 1 h incubation at 32°C, the cells were fixed and stained with anti-Myc antibody. Bars, 10 μm. (E) Quantification of CARTS. The number of CARTS in DMSO-treated cells (n=15) and blebbistatin-treated cells (n=15) was counted. The average number of CARTS per cell is shown (mean±s.d.). Figure source data can be found with the Supplementary data.
A block in the events leading to cutting of transport carriers is known to result in the accumulation of cargo containing tubules attached to the TGN and a concomitant decrease in the number of transport carriers. To further test the role of myosin II in the biogenesis of CARTS at the TGN, we asked whether blebbistatin treatment resulted in the accumulation of tubules containing PAUF-MycHis. HeLa cells expressing PAUF-MycHis were incubated at 20°C for 2 h in the presence of cycloheximide, preincubated with blebbistatin containing medium for 15 min, and then shifted to 32°C for 1 h to restore transport. We did not observe accumulation of tubules containing PAUF-MycHis and there was no difference in the number of peripheral punctae containing PAUF-MycHis under the experimental conditions (Figure 8D and E). Myosin IIa knockdown also did not alter the localization of PAUF-MycHis (Supplementary Figure S5C and D). These results suggest that myosin II is not required for the biogenesis of CARTS.
p115 is not required for secretion of PAUF
We examined the involvement of p115 in CARTS-mediated transport by monitoring the effect of p115 knockdown on the PAUF secretion. SiRNA targeting p115 caused 70% reduction in p115 expression levels compared with control siRNA (Supplementary Figure S6A and B). There was, however, no obvious defect in the secretion of PAUF-MycHis in p115 knockdown cells (Supplementary Figure S6C and D). Therefore, the significance of p115 in the isolated membranes remains unclear.
Since clathrin heavy chain was found in the mass spectrometric sequencing of the CARTS, we tested the effect of clathrin knockdown in HeLa cells on PAUF secretion. However, transfection with various siRNA oligos specific for clathrin heavy chain severely affected the synthesis of PAUF. We have therefore not been able to monitor the significance of clathrin in the secretion of PAUF or the biogenesis of CARTS.
CARTS exclude collagen I and VSV-G protein
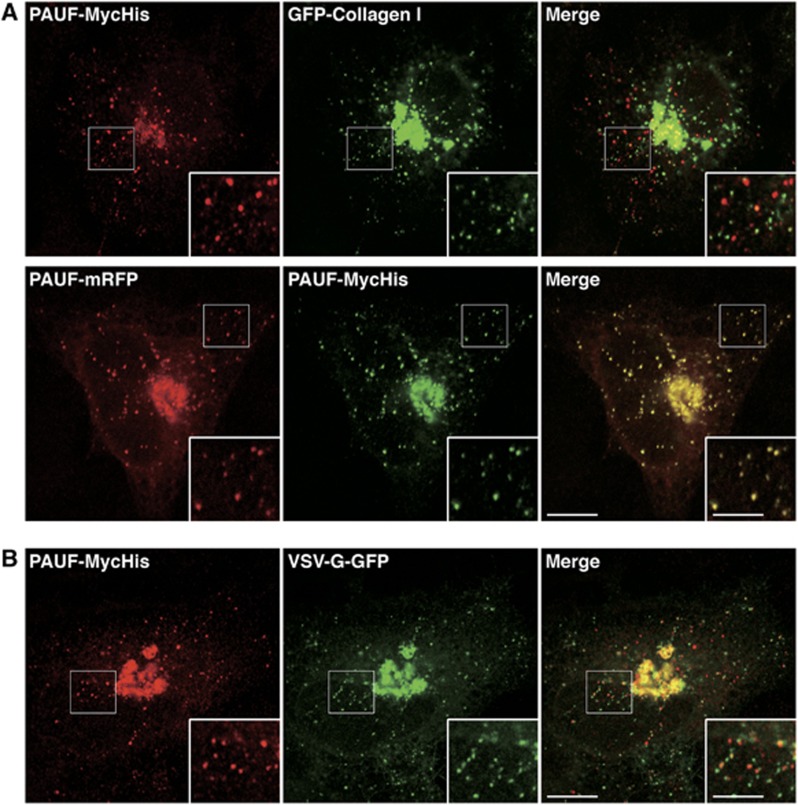
Collagen I is a bulky cargo and too big (300 nm) to be transported by small uniform size vesicles (50–100 nm) (Malhotra and Erlmann, 2011). We asked whether the CARTS contain collagen I. PAUF-MycHis and GFP-collagen I were co-expressed in HeLa cells and the cells were incubated at 20°C in the presence of cycloheximide for 2 h and then shifted to 32°C for 15 min. The cells were fixed and stained with anti-Myc antibody and visualized by fluorescence microscopy. GFP-collagen I was found in punctate elements that were distinct from the ER exit sites (Sec31) and, therefore, correspond to carriers derived from the TGN (Figure 9A; Supplementary Figure S7A). Quantification of 16 cells revealed that 87% (n=1655 punctate elements) of GFP-collagen I containing carriers did not co-localize with PAUF containing CARTS even though both of these cargoes were present in the TGN. These results reveal that bulky cargoes such as collagen I are excluded from the CARTS. As a control, PAUF-mRFP was co-expressed with PAUF-MycHis in HeLa cells and incubated as described above. PAUF-mRFP and PAUF-MycHis revealed >98% co-localization (n=827 punctate elements in 10 cells) (Figure 9A).
Figure 9.
CARTS exclude collagen I and VSV-G. (A) PAUF-MycHis and GFP-collagen I or PAUF-mRFP expressing HeLa cells were incubated at 20°C for 2 h in the presence of 20 μg/ml cycloheximide and then shifted to 32°C for 15 min. The cells were visualized with fluorescence microscopy. (B) PAUF-MycHis and VSV-G-GFP expressing HeLa cells were incubated at 40°C overnight, after which they were incubated at 20°C for 2 h in the presence of 20 μg/ml cycloheximide and then shifted to 32°C for 15 min. The cells were visualized with fluorescence microscopy. High magnifications of small punctate elements are shown in the inset. Bars, 10 and 5 μm (inset).
VSV-G protein is routinely used to study trafficking along the secretory pathway in mammalian cells. The 20°C block is often used to synchronize a wave of cargo export (by using VSV-G as a marker protein) from the Golgi to the cell surface. Incubations of cells at temperatures of 32–37°C restore the export of VSV-G from the Golgi. Is the transport of VSV-G from the TGN to the cell surface mediated by the same carriers that transport endogenous cargoes?
HeLa cells co-transfected with plasmids for PAUF-MycHis and VSV-G-GFP were incubated for 18 h at 40°C. The cells were shifted to 20°C for 2 h in the presence of cycloheximide and then after 0, 5, 15, or 60 min at 32°C fixed and stained with anti-Myc antibody for fluorescence microscopy (Figure 9B; Supplementary Figure S7B). At the earliest time point (0 min), PAUF-MycHis, VSV-G-GFP, and TGN46 clearly co-localized at the perinuclear region (Supplementary Figure S7B). A small number of PAUF containing carriers was evident even at the earliest time point (0 min). Probably, these carriers were generated during the 40°C incubation, after which their fusion with the plasma membrane was delayed by the incubation at 20°C. After 15 min at 32°C, there was a significant increase in the number of VSV-G-GFP and PAUF-MycHis containing carriers (Figure 9B; Supplementary Figure S7B). We found that <20% (n=1120 punctate elements in 9 cells) of the PAUF-MycHis containing CARTS contained VSV-G-GFP. Similarly, <20% (n=1073 punctate elements in 9 cells) of VSV-G-GFP containing carriers contained PAUF-MycHis. At 60 min, VSV-G-GFP was detected at the plasma membrane (Supplementary Figure S7). Interestingly, a number of these VSV-G-GFP and PAUF-MycHis containing carriers were located in close vicinity (Figure 9B; Supplementary Figure S7). They might be formed at the same exit site of the TGN and use the same track leading to the plasma membrane. We have not been able to detect TGN46 in the CARTS by immunofluorescence microscopy with the anti-TGN46 antibody (Supplementary Figure S7). This is probably due to a very small amount of TGN46 present in CARTS. This is also probably the reason for the failure to detect TGN46 by mass spectrometric analysis despite the fact that we could detect it by western blotting. Taken together, these results reveal that collagen I and VSV-G are predominantly excluded from the PAUF containing CARTS.
Discussion
Our reason for isolating the TGN to cell surface-specific carriers was mainly to reveal the endogenous cargoes secreted by the respective carriers and to identify other proteins that would be required for their biogenesis and trafficking. However, many different kinds of carriers travel this route and not all classes are conserved among species. We therefore chose to isolate the carriers containing the TGN to cell surface cargo TGN46. We have identified endogenous cargoes secreted by these carriers called CARTS and found that their formation requires the activity of the serine/threonine kinase PKD. The novel features of the CARTS follow.
Cargoes transported in the CARTS
We have identified both secretory and integral membrane proteins contained in the CARTS (Table I; Supplementary Table SI). Identification of secretory cargoes such as PAUF will help to address the mechanism of cargo sorting and packing, as well as the biogenesis of the CARTS. CARTS contain Syt II, which mediates Ca2+-dependent fusion of synaptic vesicles with the cell surface through interaction with phospholipids and SNAREs (Geppert et al, 1991; Ullrich et al, 1994; Osborne et al, 1999; Sudhof, 2002; Tucker and Chapman, 2002). CARTS contain Rab6a and Rab8a. It has recently been reported that Rab8a is recruited to exocytic (Golgi to cell surface) carriers in a Rab6a-dependent manner and together these proteins are required for docking and fusion of those transport carriers (Grigoriev et al, 2011). Although not tested, CARTS most likely use a similar mechanism for their docking/fusion with the plasma membrane. It is surprising that CARTS do not co-localize with collagen I containing transport carriers. We also found that VSV-G was predominantly excluded from the CARTS. The immuno-EM of the CARTS revealed their size in the range of 100–250 nm diameter, which is too small to transport mega cargoes such as the collagens. VSV-G and collagen I, in human fibroblasts, are exported from the TGN in the same carriers (Polishchuk et al, 2003). We have not tested this directly but together these findings raise the possibility that CARTS are predominantly involved in the trafficking of small secretory proteins.
CARTS contain the integral membrane protein desmoglein-1, which is a key component of desmosomes. The peripheral desmosomal components desmoplakins and the plakin family member epiplakin are also contained in the CARTS. Are desmosomal proteins assembled at the TGN and transported in a complex by the CARTS? This proposal merits further investigation.
The role of myosin II in Golgi to cell surface transport
In the 1990s, myosin II was localized to Golgi-derived carriers and suggested to be required for the biogenesis of Golgi-derived carriers containing VSV-G (Ikonen et al, 1997; Musch et al, 1997). Unfortunately, the anti-myosin II AD7 antibody used to block formation of VSV-G containing carriers reacts with native COPI coats thus casting a doubt on the validity of the proposal (Simon et al, 1998). It has recently been reported that myosin IIa knockdown or treatment with inhibitors of myosin II generates Rab6-positive tubules from the Golgi membranes in cells overexpressing GFP-Rab6 (Miserey-Lenkei et al, 2010). Removal of myosin II inhibitors was found to dissociate such tubules. In other words, myosin II inhibition blocked the fission of Rab6-containing tubules from the Golgi membranes. Some of these tubules contained both the retrograde cargo Shiga toxin and VSV-G. Why should Shiga toxin be in VSV-G containing Golgi to cell surface carriers? Moreover, knockdown of myosin IIa by siRNA caused only a slight delay in the arrival of VSV-G to the cell surface (about 25% decrease of the cell surface VSV-G) (Miserey-Lenkei et al, 2010). Regardless of the exact role of myosin II in the biogenesis/trafficking of VSV-G containing carriers, our data reveal that myosin II is required for PAUF secretion, but not for the biogenesis of CARTS.
Altogether, we believe that CARTS are bona fide transport carriers because they contain cargoes that are transported from the TGN to the cell surface. The biogenesis of CARTS requires PKD but not myosin II. The identification of CARTS will help reveal the mechanism of cargo export from the TGN during protein secretion.
Materials and methods
Buffers
Buffer A consisted of 20 mM Hepes, pH 7.4, 250 mM D-sorbitol, and 150 mM potassium acetate. Buffer B consisted of 1 M potassium chloride, 20 mM Hepes, pH 7.4, 250 mM D-sorbitol, and 150 mM potassium acetate. Buffer C consisted of 50 mM Hepes, pH 7.4, 250 mM D-sorbitol, and 70 mM potassium acetate, 15 mM EGTA, 0.5 mM magnesium acetate, 5 mM dithiothreitol, 1 μg/ml leupeptin, 2 μM pepstatin A, 2 μg/ml aprotinin, and 1 mM phenylmethylsulphonyl fluoride. Buffer D consisted of 100 mM Tris–HCl, pH 7.6, 4% sodium dodecyl sulphate (SDS), and 100 mM dithiothreitol. ATP regenerating system was composed of 1 mM ATP, 40 mM creatine phosphate, and 0.2 mg/ml creatine kinase.
Cell permeabilization
HeLa cells or HeLa-ssHRP cells were grown to 80–90% confluency in 10 cm dishes. The cells were incubated with trypsin and scraped into buffer A supplemented with 15 μg/ml soybean trypsin inhibitor. Cells were harvested by centrifugation at 1000 g for 3 min at 4°C. The cell pellet was resuspended in buffer A and then permeabilized with 40 μg/ml digitonin for 5 min on ice. The cell pellet was washed with buffer B and resuspended in buffer A (120 μl/dish). In all, 35 μl of cell suspension was used for each reaction mixture. For mass spectrometric analysis, HeLa cells from thirty 15 cm dishes were used to prepare TGN46 containing transport carriers.
Preparation of rat liver cytosol
A rat liver was excised, rinsed with phosphate-buffered saline (PBS), pH 7.4, and homogenized in 2 ml/g of buffer C with a Dounce homogenizer on ice. The homogenate was centrifuged at 1000 g for 10 min at 4°C. The resulting supernatant was cleared in the following centrifugation steps: 20 000 g for 20 min, 186 000 g for 1 h, and two times 186 000 g for 45 min. The final supernatant was frozen in liquid nitrogen and stored at −80°C.
Transport carrier formation in permeabilized cells
Permeabilized cells were incubated at 32°C for 45 min in buffer A supplemented with ATP regenerating system and 0.5 mg/ml rat liver cytosol. For H89 and PKI treatment, permeabilized cells were pretreated with 120 μM H89 or 130 nM PKI (Ki=36 nM) for 10 min at 32°C, incubated with rat liver cytosol for 10 min, and then further incubated with ATP regenerating system for 45 min. Transport carriers were separated from the permeabilized cells by centrifugation at 10 000 g (low speed) for 10 min at 4°C, and the supernatant was centrifuged at 100 000 g (high speed) for 1 h at 4°C. The pellet was solubilized with SDS sample buffer and western blotted with anti-TGN46 antibody.
Immunoisolation of transport carriers
After low speed centrifugation, the supernatant was mixed with anti-TGN46 antibody and incubated at 4°C overnight. TGN46 containing membranes were collected with the use of protein G conjugated magnetic beads (Dynabeads Protein G; Invitrogen), and washed extensively with buffer A. The membranes bound to the beads were analysed by western blotting or further subjected to extraction with 100 mM sodium carbonate, pH 11.5 for mass spectrometric analysis.
Detection of ssHRP activity
HeLa-ssHRP cells were incubated at 20°C for 2 h to accumulate HRP in the Golgi. The cells were permeabilized and then processed for the immunoisolation of TGN46 containing membranes. Magnetic beads were incubated in buffer A containing 1% Triton X-100 for 30 min at 4°C and removed by low speed centrifugation (10 000 g for 10 min). HRP activity in the supernatant was measured by using enhanced chemiluminescence (ECL) and a Victor 3 plate reader (Perkin-Elmer, Waltham, MA).
Extraction of membranes with sodium carbonate, pH 11.5
Magnetic beads associated with TGN46 containing membranes were incubated with 100 mM sodium carbonate, pH 11.5, for 30 min at room temperature. After removal of magnetic beads through the use of a magnet and by low speed centrifugation (10 000 g for 5 min at 4°C), the supernatant was further centrifuged at high speed (100 000 g for 1 h at 4°C). The precipitate was solubilized in buffer D and subjected to in-solution digestion for mass spectrometric analysis. See Supplementary data for protocol details.
PAUF secretion assay
HeLa cells were co-transfected with plasmids for PAUF-MycHis and GST or GST-PKD2-KD. Twenty hours after the transfection, the medium was replaced with Opti-MEM and incubated at 37°C for 6 h. The medium and cell lysates were analysed by western blotting with anti-His antibody. For experiments with blebbistatin, HeLa cells expressing PAUF-MycHis were incubated in Opti-MEM at 20°C for 2 h, pretreated with DMSO or 100 μM blebbistatin for 15 min, and then shifted to 32°C to allow transport from the Golgi. The medium was collected at 45 min after the temperature shift. Proteins in the medium were precipitated with trichloroacetic acid and the precipitates and cell lysates were analysed by western blotting.
Immuno-EM
HeLa cells transfected with PAUF-MycHis were incubated at 20°C for 5 h in the presence of cycloheximide and then shifted to 37°C to activate PAUF export from the Golgi. In one parallel experiment, tannic acid was added to cells to a final concentration of 0.5% to prevent fusion of CARTS with the plasma membranes (Polishchuk et al, 2004). Cells were fixed at 15 or 60 min after release from the 20°C block with a mixture of 4% paraformaldehyde and 0.05% glutaraldehyde, incubated with a monoclonal antibody against Myc according the gold-enhance protocol, embedded in Epon-812, and sectioned as described previously (Polishchuk et al, 2003). EM images were acquired from thin sections using a FEI Tecnai-12 electron microscope equipped with VELETTA CCD digital camera (FEI, Eindhoven, The Netherlands). Evaluation of CARTS size was done in EM images using the iTEM software (Soft Imaging Systems GmbH, Munster, Germany).
Supplementary Material
Acknowledgments
We thank Drs Sang Seok Koh (Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea), Robert S Adelstein (National Institute of Health, MD, USA), Ivan Stamenkovic (University of Lausanne, Lausanne, Switzerland), Francis A Barr (University of Oxford, Oxford, UK), Scottie Robinson (Cambridge University, Cambridge, UK), and Mitsuo Tagaya (Tokyo University of Pharmacy and Life Sciences, Tokyo, Japan) for providing materials. We thank all members of the Malhotra Laboratory for valuable discussions, Timo Zimmermann, Arrate Mallabiabarrena, and Raquel García for help with live-cell imaging and fluorescence microscopy. Elena Polishchuk and Simona Iacobacci are acknowledged for EM specimen preparation and morphometric analysis, and Telethon Electron Microscopy Core Facility (TeEMCoF, IBP, CNR, Naples; Telethon project #GTF08001) and Integrated Microscopy Facility (IGB, CNR, Naples) for EM support. V Malhotra is an Institució Catalana de Recerca i Estudis Avançats (ICREA) professor at the Center for Genomic Regulation, and the work in his laboratory is funded by grants from Plan Nacional (BFU2008-00414), Consolider (CSD2009-00016), Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) Grups de Recerca Emergents (SGR2009-1488; AGAUR-Catalan Government), and European Research Council (268692). The project has received research funding from the European Union. This paper reflects only the author’s views. The Union is not liable for any use that may be made of the information contained therein.
Author contributions: YW contributed to the design and execution of most of the experiments, data analysis, and writing of the manuscript. JG performed knockdown and immunofluorescence experiments. FM and MM performed mass spectrometric analysis. MS performed RT–PCR. RP performed immuno-EM analysis. VM designed experiments and wrote this manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Banting G, Ponnambalam S (1997) TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim Biophys Acta 1355: 209–217 [DOI] [PubMed] [Google Scholar]
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V (2006) Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439: 604–607 [DOI] [PubMed] [Google Scholar]
- Bard F, Malhotra V (2006) The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol 22: 439–455 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907 [DOI] [PubMed] [Google Scholar]
- Borner GH, Antrobus R, Hirst J, Bhumbra GS, Kozik P, Jackson LP, Sahlender DA, Robinson MS (2012) Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J Cell Biol 197: 141–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I, Simons K (1989) Isolation of exocytic carrier vesicles from BHK cells. Cell 58: 719–727 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Luini A (2008) Exiting the Golgi complex. Nat Rev Mol Cell Biol 9: 273–284 [DOI] [PubMed] [Google Scholar]
- Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E (2008) Clathrin is a key regulator of basolateral polarity. Nature 452: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S, Glick BS, Linstedt AD, Lippincott-Schwartz J, Luini A, Malhotra V, Marsh BJ, Nakano A, Pfeffer SR, Rabouille C, Rothman JE, Warren G, Wieland FT (2009) Journeys through the Golgi—taking stock in a new era. J Cell Biol 187: 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE (1981) The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol 91: 3 Part 277s–103s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB (1982) Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Archer BT 3rd, Sudhof TC (1991) Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem 266: 13548–13552 [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ (2006) Quantitative proteomics analysis of the secretory pathway. Cell 127: 1265–1281 [DOI] [PubMed] [Google Scholar]
- Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peranen J, Pasterkamp RJ, van der Sluijs P, Hoogenraad CC, Akhmanova A (2011) Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol 21: 967–974 [DOI] [PubMed] [Google Scholar]
- Huebers HA, Finch CA (1987) The physiology of transferrin and transferrin receptors. Physiol Rev 67: 520–582 [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91: 119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E, de Almeid JB, Fath KR, Burgess DR, Ashman K, Simons K, Stow JL (1997) Myosin II is associated with Golgi membranes: identification of p200 as nonmuscle myosin II on Golgi-derived vesicles. J Cell Sci 110: Part 182155–2164 [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V (1999) Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98: 59–68 [DOI] [PubMed] [Google Scholar]
- Johannes FJ, Prestle J, Dieterich S, Oberhagemann P, Link G, Pfizenmaier K (1995) Characterization of activators and inhibitors of protein kinase C mu. Eur J Biochem 227: 303–307 [DOI] [PubMed] [Google Scholar]
- Kim SA, Lee Y, Jung DE, Park KH, Park JY, Gang J, Jeon SB, Park EC, Kim YG, Lee B, Liu Q, Zeng W, Yeramilli S, Lee S, Koh SS, Song SY (2009) Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci 100: 828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol 185: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Lecat S, Verkade P, Simons K (1998) Annexin XIIIb associates with lipid microdomains to function in apical delivery. J Cell Biol 142: 1413–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V (2001) Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104: 409–420 [DOI] [PubMed] [Google Scholar]
- Malhotra V, Erlmann P (2011) Protein export at the ER: loading big collagens into COPII carriers. EMBO J 30: 3475–3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE (1989) Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58: 329–336 [DOI] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A (2010) Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol 12: 645–654 [DOI] [PubMed] [Google Scholar]
- Musch A, Cohen D, Rodriguez-Boulan E (1997) Myosin II is involved in the production of constitutive transport vesicles from the TGN. J Cell Biol 138: 291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne SL, Herreros J, Bastiaens PI, Schiavo G (1999) Calcium-dependent oligomerization of synaptotagmins I and II. Synaptotagmins I and II are localized on the same synaptic vesicle and heterodimerize in the presence of calcium. J Biol Chem 274: 59–66 [DOI] [PubMed] [Google Scholar]
- Pearse BM (1976) Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci USA 73: 1255–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR (2001) Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol 11: 487–491 [DOI] [PubMed] [Google Scholar]
- Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS (2003) Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains. Mol Biol Cell 14: 4470–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk R, Di Pentima A, Lippincott-Schwartz J (2004) Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol 6: 297–307 [DOI] [PubMed] [Google Scholar]
- Ponnambalam S, Girotti M, Yaspo ML, Owen CE, Perry AC, Suganuma T, Nilsson T, Fried M, Banting G, Warren G (1996) Primate homologues of rat TGN38: primary structure, expression and functional implications. J Cell Sci 109: Part 3675–685 [DOI] [PubMed] [Google Scholar]
- Rajasekaran AK, Humphrey JS, Wagner M, Miesenbock G, Le Bivic A, Bonifacino JS, Rodriguez-Boulan E (1994) TGN38 recycles basolaterally in polarized Madin-Darby canine kidney cells. Mol Biol Cell 5: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS (1994) The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol 6: 538–544 [DOI] [PubMed] [Google Scholar]
- Salamero J, Sztul ES, Howell KE (1990) Exocytic transport vesicles generated in vitro from the trans-Golgi network carry secretory and plasma membrane proteins. Proc Natl Acad Sci USA 87: 7717–7721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE (1991) ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell 67: 239–253 [DOI] [PubMed] [Google Scholar]
- Simon JP, Shen TH, Ivanov IE, Gravotta D, Morimoto T, Adesnik M, Sabatini DD (1998) Coatomer, but not P200/myosin II, is required for the in vitro formation of trans-Golgi network-derived vesicles containing the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci USA 95: 1073–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525 [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299: 1743–1747 [DOI] [PubMed] [Google Scholar]
- Sudhof TC (2002) Synaptotagmins: why so many? J Biol Chem 277: 7629–7632 [DOI] [PubMed] [Google Scholar]
- Tucker WC, Chapman ER (2002) Role of synaptotagmin in Ca2+-triggered exocytosis. Biochem J 366: Part 11–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich B, Li C, Zhang JZ, McMahon H, Anderson RG, Geppert M, Sudhof TC (1994) Functional properties of multiple synaptotagmins in brain. Neuron 13: 1281–1291 [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K (1990) Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol 111: 987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CW, Hamamoto S, Orci L, Schekman R (2006) Exomer: a coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol 174: 973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V (2004) Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol 6: 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi N, Maurer A, Nieke S, Kalbacher H (2008) Cathepsin D: a cellular roadmap. Biochem Biophys Res Commun 376: 5–9 [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.