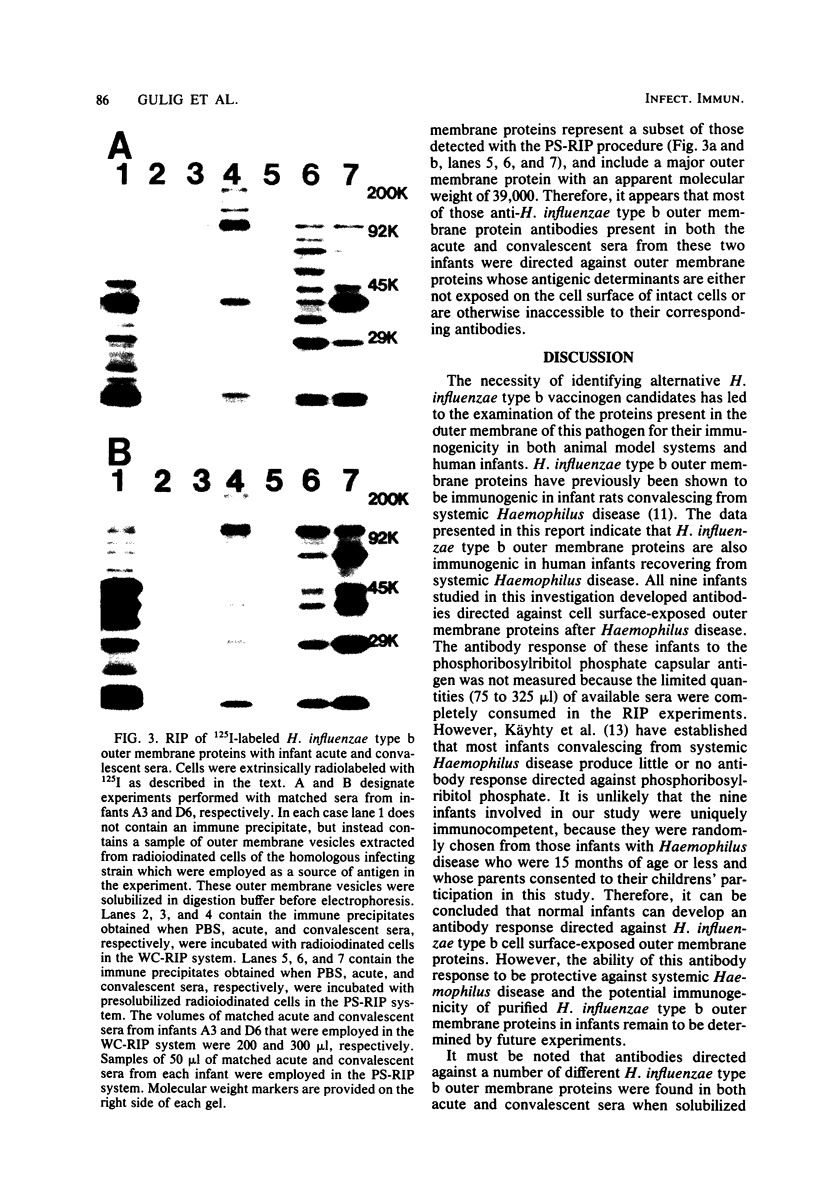
Abstract
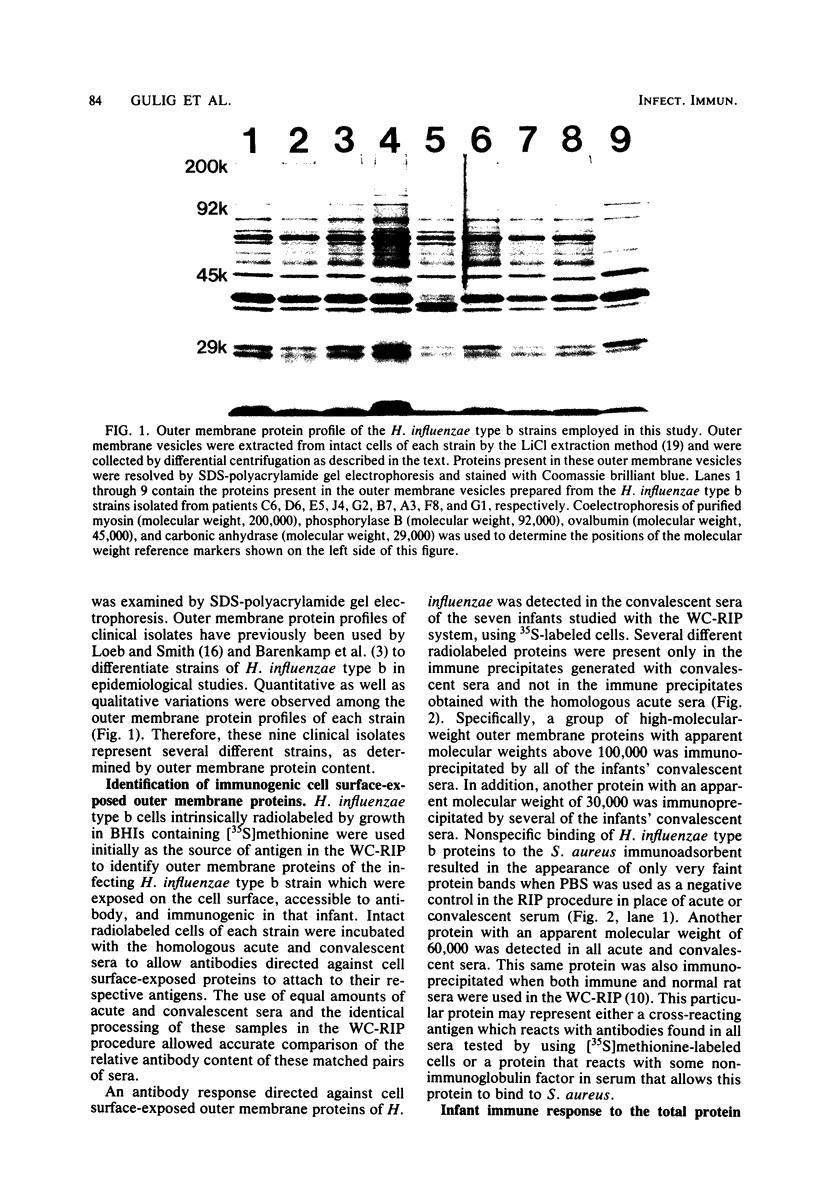
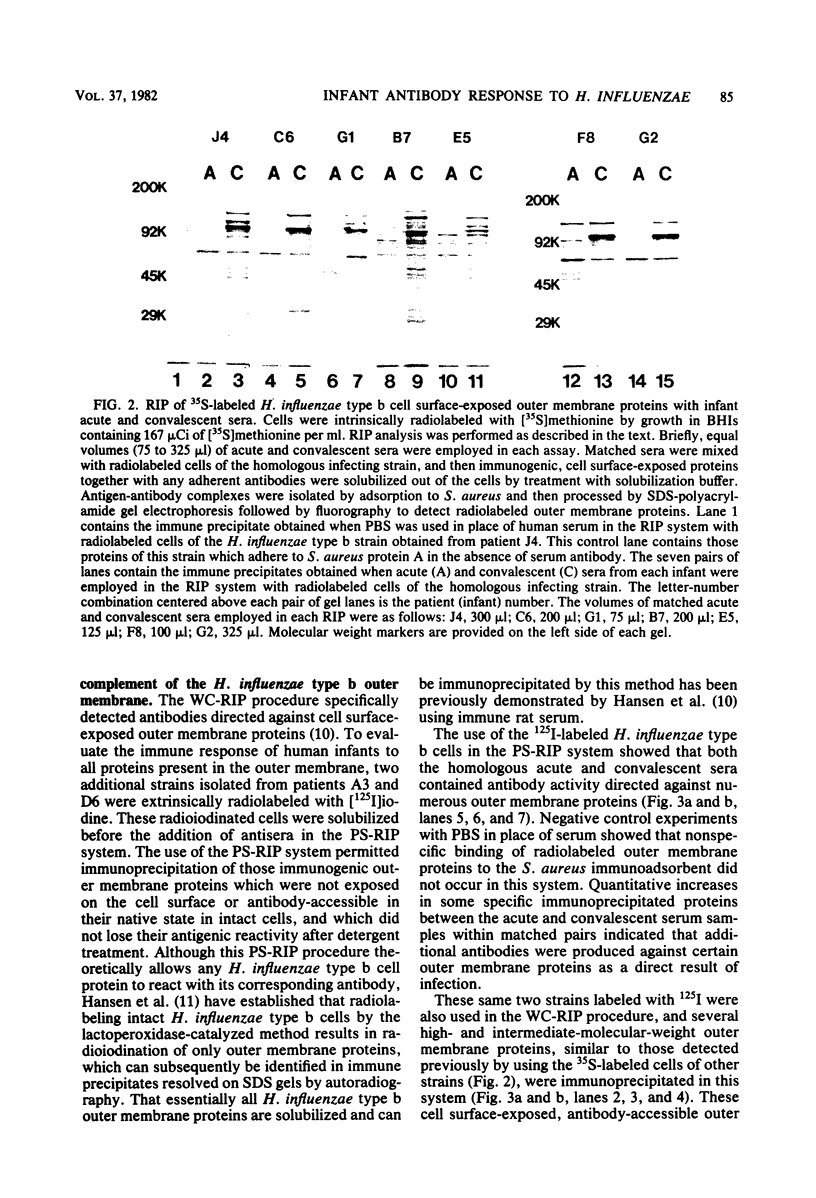
The immune response of nine infants with Haemophilus influenzae type b meningitis was examined by using a radioimmunoprecipitation procedure designed to detect antibodies directed against cell surface-exposed outer membrane proteins of this pathogen. Using intrinsically or extrinsically radiolabeled intact H. influenzae type b cells with acute- and convalescent-phase human sera in this radioimmunoprecipitation system, we found that all of the infants produced an antibody response directed against several different H. influenzae type b outer membrane proteins. Anti-H. influenzae type b outer membrane protein antibodies present in convalescent sera, but not found in acute sera, were directed against cell surface-exposed H. influenzae type b outer membrane proteins. In contrast, both acute and convalescent sera contained antibody activity directed against numerous H. influenzae type b outer membrane proteins whose antigenic determinants were apparently inaccessible to antibody on intact H. influenzae type b cells. The ability of infants to develop an antibody response to cell surface-exposed, antibody-accessible H. influenzae type b outer membrane proteins indicates that these proteins may have vaccinogenic potential.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoff S. P. On the surface of Haemophilus influenzae. J Infect Dis. 1981 May;143(5):747–748. doi: 10.1093/infdis/143.5.747. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Rockwell R. Experimental Haemophilus influenzae type b meningitis: immunological investigation of the infant rat model. Infect Immun. 1978 Jun;20(3):705–713. doi: 10.1128/iai.20.3.705-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Käyhty H., Jousimies-Somer H., Peltola H., Mäketä P. H. Antibody response to capsular polysaccharides of groups A and C neisseria meningitidis and Haemophilus influenzae type b during bacteremic disease. J Infect Dis. 1981 Jan;143(1):32–41. doi: 10.1093/infdis/143.1.32. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Norden C. W., Demchak T. A. Significance of noncapsular antigens in protection against experimental Haemophilus influenzae type b disease: cross-reactivity. Infect Immun. 1978 Aug;21(2):619–626. doi: 10.1128/iai.21.2.619-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Norden C. W. Variable susceptibility of Hemophilus influenzae, type B strains to serum bactericidal activity. Proc Soc Exp Biol Med. 1972 Jan;139(1):59–61. doi: 10.3181/00379727-139-36076. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins J., Williams F. F., Wehrle P. F., Portnoy B. Agglutinin response to pertussis vaccine. I. Effect of dosage and interval. J Pediatr. 1971 Aug;79(2):197–202. doi: 10.1016/s0022-3476(71)80101-4. [DOI] [PubMed] [Google Scholar]