Abstract
Background
Although MRI detected white matter disease has been correlated with cognitive decline in the elderly, it is unclear whether white matter disease is primarily responsible for the cognitive deterioration or whether another process is common to white matter disease and dementia.
Methods
We examined the relationship between Alzheimer type brain pathology at autopsy and MRI detected cerebral white matter disease in 50 participants from the Baltimore Longitudinal Study of Aging (BLSA) Autopsy Program, a prospective study of aging which includes detailed cognitive assessments.
Results
White matter disease was quantitated in pre- and postmortem MRI scans using the Cardiovascular Health Study (CHS) criteria in a blinded fashion. We found that several measures of Alzheimer disease (AD) pathology including CERAD score, Braak score and a composite AD pathology score, along with hypertension, were significantly associated with CHS white matter score using univariate and multivariate ordinal regression. In contrast, amyloid angiopathy was not independently related to with CHS score. While a clinical diagnosis of dementia was associated with CHS score in univariate analysis, the association disappeared after accounting for AD pathology.
Conclusion
Alzheimer’s pathology at autopsy is associated with MRI detected cerebral white matter disease. This relationship may explain, in part, the association between cerebral white matter disease and cognitive decline in the elderly.
Keywords: Alzheimer’s, Dementia, MRI, White Matter Disease, Hypertension, Atherosclerosis
1. Introduction
With the increasing use of MRI, cerebral white matter disease, characterized as hyperintensities on T2 or FLAIR sequences is commonly detected in the elderly [1]. White matter hyperintensities, spanning the spectrum from mild and focal to extensive and confluent are found in 96% of community volunteers over age 65 [2]. Of these, the vast majority (80%) of elderly subjects had low-grade abnormalities (Grades 1-3 out of a maximum possible of 9). In a large community based study (Mean Age 72 yrs), higher-grade white matter disease, stratified on the basis of MRI, was associated with greater age, asymptomatic brain infarction, higher systolic BP, female sex, history of hypertension and impaired cognitive function [3]. In the Framingham Offspring Study it was shown that white matter hyperintensities portended an increased risk of stroke, amnestic mild cognitive impairment, dementia, and death, independent of vascular risk factors and interim vascular events [4]. Importantly however, it is not known if white matter changes directly affect cognition or are merely a marker of another underlying process such as Alzheimer disease, which directly impacts cognition. The prevalence of both white matter abnormalities and Alzheimer’s disease increases with age. Autopsy studies of patients with Alzheimer’s disease have noted abnormalities in the subcortical or peri-ventricular white matter, raising the question of whether these white matter changes are coincidental or a result of the Alzheimer’s pathology. We undertook this analysis to determine the relative contribution of Alzheimer’s type brain pathology to the degree of white matter disease seen on MRI in our cohort of prospectively followed elderly patients. We report that Alzheimer’s type brain pathology, along with hypertension, is associated with white matter changes seen on MRI, suggesting that the cognitive effect of white matter changes in the elderly may be mediated by underlying Alzheimer’s disease.
2. Methods
2.1. Cohort
All 50 subjects in this study came from the Baltimore Longitudinal Study of Aging (BLSA) Autopsy Program, a prospective cohort of 579 participants from the larger Baltimore Longitudinal Study of Aging (BLSA) who have agreed to post-mortem brain exams [5]. As of December 2011, 241 subjects age 70 and above, who were cognitively and neurologically unimpaired at entry into the autopsy study, have died and undergone brain autopsy. In the past three years, all subjects who died within a 2-hour drive of Johns Hopkins Hospital (2/3 of all autopsy subjects) have had MRI scans of their brain prior to autopsy. This has resulted in 31 of the subjects for the current study. An additional group of 19 subjects had premortem MRI’s performed within 25 months of death; mean interval between MRI and death is 17 +/- 5 (SD) months (range 9-25 months). These subjects were part of the BLSA neuroimaging study [6,7], and the BLSA autopsy program. We limited the time from last MRI to death to 25 months, as MRI white matter changes in large cohorts are relatively modest over this time period [8]. Participants were predominantly white (96%). Five subjects had both premortem and postmortem MRI’s, which allowed comparison of the two modes of MRI acquisition; in subjects with pre and postmortem scans we used only the postmortem MRI for data analysis. Although the number of subjects in this study represents a small subset of the BLSA and the BLSA autopsy program, they have a similar dementia rate (26/50), brain infarction rate at autopsy (23/50), hypertension rate (34/50) and diabetes rate (13/50) as the autopsy cohort as a whole [5,9]. Given the high education level and socioeconomic status of this cohort we do not expect it to be representative of an epidemiologically rigorous community cohort.
All participants in the present study were cognitively and neurologically unimpaired at the time of entry into the BLSA and the autopsy cohort. They were assessed at baseline, within 18 months of death (mean 8.7±6.3 (SD) months prior to death), and annually in between. Evaluations included neuropsychological tests, neurological exam, interval medical history, medication review, and a structured informant and subject interview as described [8]. A history of diabetes or hypertension required both a documented history and the use of one medication for that condition. A history of coronary artery disease (CAD) required either a history of myocardial infarction, coronary bypass surgery or stenting, or a history of coronary artery disease plus medication prescribed to treat CAD. JNC-7 [10] blood pressure categories (which we categorized as 1-4) were derived from the mean of each individual’s annual blood pressure measurement between ages 65-85. The mean number of annual blood pressure measurements for the 50 participants was 11.5 +/- 2.3 (SD). Approximate ten-year Framingham risk scores [11] were calculated using the mean blood pressure recorded between ages 65-85, the fasting total cholesterol measured at study entry (between ages 60-75), sex, and a history of diabetes or smoking. We did not have HDL cholesterol values on most of our participants so we used a HDL cholesterol value of 40 mg/dl on all participants as a dummy variable. Subjects were grouped into 3 risk groups; 0-19% 10-year risk, 20-35% 10-year risk, and >35% 10-year risk.
Studies of this cohort are conducted under the auspices of the Johns Hopkins and MedStar Research Institute IRBs, and all participants provided written informed consent for the prospective cognitive evaluations and premortem MRIs, while next of kin provided written informed consent for the postmortem MRIs and autopsy.
2.2. Brain pathology
Postmortem examination of all brains was performed at Johns Hopkins by a neuropathologist. Neuritic plaques, neurofibrillary tangles, and infarcts were scored as described [5]. Twenty-three subjects had 33 macroscopic infarcts at autopsy. Thirteen of the infarcts were classified as macroscopic cortical infarcts and 20 as subcortical lacunar infarcts (some subjects had more than one type). AD pathology was examined on silver stains and graded according to CERAD and Braak criteria [12,13]. In addition, we generated a composite AD pathology score by summing the CERAD and Braak scores as described previously [5]. Intracranial atherosclerosis (graded on a 1-3 scale) and brain infarctions (graded as present or absent) were evaluated as previously described [14]. We did not do any quantitative pathological assessments of white matter disease in the post-mortem exam, as the slides used in microscopic pathological evaluations cover only a small amount of cerebral white matter, while gross visual assessment of cerebral white matter in fresh autopsy specimens is very insensitive in our hands. Amyloid angiopathy was assessed in a large (4 × 5 cm) block, taken from one temporal lobe, midway from the temporal tip to the parietal lobe. This block, which was the only one routinely immunostained with the 6E10 amyloid Aβ antibody, included all three temporal gyri as well as the hippocampus. More than 30 microscopic fields in the block were searched for Aβ immunostaining in leptomeningeal and parenchymal blood vessels. Amyloid angiopathy was said to be present if at least two blood vessels were observed to be immunopositive for Aβ in a circumferential manner.
2.3. Imaging
Both premortem and postmortem MRI scans were performed on 1.5T MR instruments. The imaging sequences used to evaluate the white matter were axial proton density and T2-weighted sequences (TR=3000, TE=34/100, FOV=24cm, matrix=256 × 192, NEX=0.5, 5mm slice thickness) acquired in sections oriented parallel to the anterior-posterior inter-commissural line. Proton density and T2-weighted sequences were chosen because fluid attenuated inversion recovery (FLAIR) data acquisition sequences were not widely available when the neuroimaging study protocol was initiated (1994). For longitudinal consistency of data acquisition, we continued with our original MRI imaging protocol throughout the duration of this study [15,18]. This also resulted in the absence of gradient echo sequences and an inability to quantify cerebral microbleeds. To evaluate the burden of white matter disease, we used the previously validated and published white matter disease scale from the Cardiovascular Health Study (CHS) [16,17]. This scale for measuring white matter disease is easy to apply and, in addition to having been validated extensively through the CHS study, has been used in previous studies of white matter in the BLSA participants [18]. Using this scale, a “0” represents virtually normal white matter and a “9” reflects a very thick ring of confluent periventricular white matter disease, along with marked subcortical disease. The signal abnormalities in the proton density and in the T2-weighted images were always concordant. All CHS white matter scores were assigned by a single radiologist (MK) blinded to all clinical and pathological variables. Subjects with both pre and postmortem scans had their premortem scans read at separate times from their postmortem scans, and the radiologist was not aware of the prior score. Running our analyses using a variable to indicate whether the MRI was done pre or postmortem did not alter the results. Blinded test-retest reliability rating of CHS scoring in 6 scans showed that 3 scans were graded within 1 point of the original rating and 3 were rated identically.
2.4. Statistics
To examine the relationship between increasing AD pathology scores and increasing CHS white matter scores we used standard error plots as well as univariate and multivariate ordinal logistic regression. Additional analyses, where appropriate, including linear regression, logistic regression and ANOVA (for variables which had only binary values) confirmed the ordinal regression analyses and are not shown in this manuscript. These statistical analyses were performed using Stata and NCSS. Comparison of the distribution of CHS white matter scores between pre and postmortem MRI’s was performed using a Komogorov-Smirnov analysis on SPSS.
3. Results
Fifty prospectively evaluated subjects in the BLSA, all cognitively and neurologically normal at the time of entry into the study had either an MRI within 25 months of death or an MRI immediately postmortem or both (Table 1A). All MRI’s were graded for white matter disease by a single blinded radiologist according to CHS criteria [17]. Premortem and postmortem MRI’s appeared to detect the same white matter abnormalities, as the distribution of CHS scores (Figure 1) and the mean CHS scores of premortem and postmortem MRI’s were similar (Table 1A). Five subjects had both a premortem and postmortem MRI scan available for comparison (Table 1B; Figure 2A); the CHS white matter scores did not differ by more than 1 point between the pre and postmortem evaluations in any of the 5 subjects.
Table 1. Subject and MRI Characteristics.
In A, the ages and CHS white matter scores of subjects with pre and postmortem MRI scans are shown. Age at death and age at last MRI were significantly different in the pre and postmortem MRI groups (ANOVA) while the CHS score was not. The total number of subjects with scans (50) is less than the sum of the pre and postmortem MRI’s (55) because 5 subjects had both (only the postmortem scans were used for subsequent analyses). In B, the CHS white matter scores of the 5 subjects with both pre and postmortem MRI scans are shown along with the interval from the last premortem scan until death. WM= White Matter.
| A | Subjects | Age at Last MRI | Age at Death | CHS WM Score |
| Total (n=50) | 87.7 +/- 6.0 | 88.6 +/- 5.8 | 4.0 +/- 1.9 | |
| Premortem MRI (n=24) | 84.4 +/- 5.4 | 86.1 +/- 5.6 | 4.1 +/- 1.9 | |
| Postmortem MRI (n=31) | 90.0 +/- 5.3 | 90.0 +/- 5.3 | 4.0 +/- 2.0 | |
| B | Subject | Premortem CHS WM Score | Postmortem CHS WM Score | Interval |
| #1 | 3 | 4 | 38 months | |
| #2 | 3 | 2 | 14 months | |
| #3 | 7 | 7 | 45 months | |
| #4 | 2 | 1 | 11 months | |
| #5 | 8 | 8 | 16 months |
Figure 1. Distribution of Pre and Postmortem CHS White Matter Scores.

The CHS white matter scores of 24 premortem MRI scans and 31 postmortem MRI scans on 50 subjects are shown. Using Komogorov-Smirnov analysis, these histograms do not differ (P=0.9).
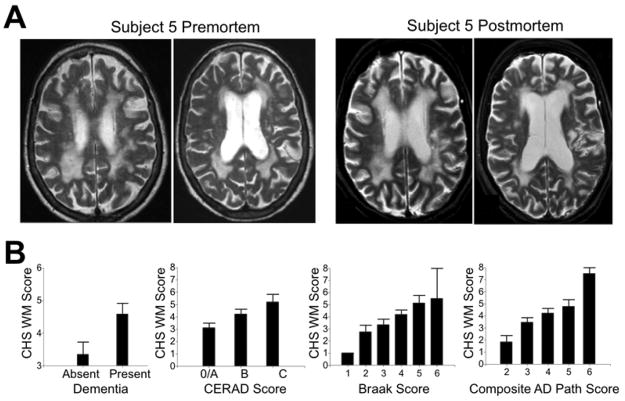
Figure 2. White Matter Disease and Alzheimer Pathology.
In A, premortem and postmortem MRI scans from Subject 5 of Table 1B were obtained 16 months apart. In B, the relationship between Dementia, AD Pathology and Cardiovascular Health Study (CHS) white matter ratings are shown for all 50 subjects in the study. Composite AD pathology refers to a weighted sum of the Braak and CERAD scores.
As shown in Figure 2B and Table 2, there is a relationship between measures of Alzheimer’s disease (AD) pathology and the CHS white matter score. A relationship was also seen between the presence and absence of dementia and the CHS white matter score. Using univariate ordinal regression (Table 2) all measures of AD pathology as well as JNC-7 hypertension grade and intracranial atherosclerosis were significantly associated with CHS score. Other variables such as any clinical history of hypertension, amyloid angiopathy, coronary artery disease, and the presence of a brain infarction also showed trends toward associations with CHS grade in this relatively small sample. Multivariate regression analyses (Table 3) confirmed that composite AD pathology score and JNC-7 hypertension grade were independently and significantly associated with white matter disease after adjusting for other variables while the role of intracranial atherosclerosis and brain infarction attenuated. Similar results were seen when CERAD and Braak scores were used in the multivariate analysis instead of composite AD pathology score. There was no statistical evidence of an interaction between AD pathology and JNC-7 hypertension grade using either ordinal regression or linear multivariate regression.
Table 2. Predictors of White matter Disease.
Various clinical and pathological risk factors for white matter disease were assessed in our cohort using univariate ordinal regression. The odds ratio refers to the increase in odds of the outcome due to a single step increase in the input variable. CAD = coronary artery disease. Composite AD pathology refers to a weighted sum of the Braak and CERAD scores. JNC-7 refers to the four categories of blood pressure detailed in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The “n” value for each risk factor refers to the number of individuals with that condition out of the 50 individuals in the cohort, while the other numbers in parentheses refer to the grades of the indicated risk factor.
| CHS White Matter Score as Outcome | ||
|---|---|---|
| Univariate Ordinal Regression | ||
| Input Variable | O.R. (95% CI) | P -Value |
| Age (75-101) | 1.0 (0.9-1.1) | 0.53 |
| Female Sex (n=19) | 1.7 (0.6-5.8) | 0.30 |
| Diabetes (n=13) | 0.9 (0.3-2.6) | 0.80 |
| Any Hypertension (n=34) | 2.5 (0.9-7.4) | 0.09 |
| JNC-7 Hypertension Grade (1-4) | 2.0 (1.3-3.4) | 0.03 |
| CAD (n=19) | 1.4 (0.5-4.3) | 0.40 |
| Intracranial Atherosclerosis (1-3) | 1.9 (1.0-3.6) | 0.04 |
| Framingham Risk Score (1-3) | 1.1 (0.6-2.1) | 0.71 |
| Brain Infarct (n=23) | 1.9 (0.7-5.2) | 0.21 |
| CERAD Score (0-3) | 2.9 (1.5-5.8) | 0.001 |
| Braak Score (1-6) | 2.1 (1.3-3.8) | 0.003 |
| Composite AD Score (2-6) | 3.2 (1.7-5.9) | 0.0001 |
| Composite AD Score ≥4 (n=29) | 4.7 (1.46-14) | 0.003 |
| Amyloid Angiopathy (n=23) | 2.1 (0.8-6.0) | 0.12 |
| Dementia Diagnosis (n=26) | 3.1 (1.2-8.9) | 0.02 |
Table 3. Multivariate Regression.
Two multivariate ordinal regression models were constructed, using CHS white matter score as the outcome, and the three indicated clinical or pathologic conditions plus age at death and sex as the independent variables for each model. The odds ratio refers to the increase in odds of the outcome due to a single step increase in the input variable. Composite AD pathology refers to a weighted sum of the Braak and CERAD scores. JNC-7 grade refers to the 4 categories of blood pressure detailed in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, while the intracranial atherosclerosis score was graded 1-3 as described in the Methods section.
| O.R. (95% CI) | P-Value | |
|---|---|---|
| Model 1 | ||
| Composite AD Pathology Score | 2.8 (1.5-5.3) | 0.001 |
| JNC-7 Hypertension Grade | 2.0 (1.1-3.7) | 0.03 |
| Intracranial Atherosclerosis Score | 1.1 (0.5-2.3) | 0.70 |
| Model 2 | ||
| Composite AD Pathology Score | 2.8 (1.5-5.1) | 0.001 |
| JNC-7 Hypertension Grade | 2.1 (1.2-3.8) | 0.01 |
| Presence of any Brain Infarct | 1.5 (0.5-4.3) | 0.41 |
Importantly, the relationship between CHS score and dementia, seen in a univariate ordinal regression analysis (Table 2), was no longer significant after adjusting for composite AD pathology score, a weighted sum of neuritic plaques and neurofibrillary tangles [5]. Specifically, the odds ratio (95% CI) for an increase in CHS score with a diagnosis of dementia drops from 3.1 (1.2-8.9) to 1.4 (0.5-4.4) when composite AD pathology score is included as a covariate. This result was confirmed using logistic regression with dementia as the outcome variable; the odds ratio (95% CI) for dementia associated with an increase in CHS white matter score dropped from 1.6 (1.2- 2.4) to 1.1 (0.7-1.8) after adjustment for composite AD pathology. Including JNC-7 hypertension grade as a covariate had no effect on the relationship between dementia and CHS white matter score, and there was no association between JNC-7 hypertension grade and dementia (O.R. 0.93; 95% CI 0.5-1.5). Similarly, the non-significant but potentially important relationship between amyloid angiopathy and CHS score (O.R. 2.1; 95% C.I. 0.8-6.0) seen in univariate analysis, was further attenuated (O.R. 1.1; 95% CI 0.4-3.1) when measures of AD pathology (in this case composite AD pathology score) were included in multivariate analysis. Conversely, the relationship between composite AD pathology and CHS score (O.R. 3.2; 95% CI 1.7 – 5.9) seen in univariate analysis, was not attenuated (O.R. 3.3; 95% CI 1.8-6.0) when the presence of amyloid angiopathy was included in the multivariate analysis.
4. Discussion
The interaction between white matter abnormalities detected on MRI, cerebrovascular risk factors and dementia is complex. Many cross-sectional and longitudinal studies have detected associations between white matter disease (also referred to as white matter hyperintensities) and impaired cognitive abilities [19-22], although this finding is not universal [23]. The CHS study found an association between extensive white matter disease and an increased risk of dementia [24]. In the CASCADE study, severe periventricular white matter disease was associated with impairment in several cognitive domains [25]. In the longitudinal Austrian stroke prevention study, progressive white matter lesion burden was associated with brain parenchymal loss and a decline in multiple cognitive tasks [21]. Most studies have attributed the relationship between white matter disease and cognition to coexisting atherosclerosis [16,26,27]. While our data includes too few subjects to be certain about the relationship between intracranial atherosclerosis and white matter disease, there is a strong relationship between white matter disease and Alzheimer’s disease (AD) type pathology that may explain in part the relationship between white matter disease and cognition. The surprisingly strong relationship we have seen between AD pathology and white matter disease may be a result of the older subjects enrolled in our study, whose mean age at death is much older than most of the other cohorts referenced. We have also found that hypertension is related to CHS white matter score but that it does not attenuate the effect of AD pathology. It should be noted that in a more epidemiologically rigorous study than ours, the role of vascular risk factors in white matter disease might be more prominent.
Like us, several previous studies have suggested a more complex relationship between white matter disease and dementia than simply a relationship to vascular risk factors. White matter abnormalities are frequently detected in the brain scans of patients with Alzheimer’s disease and white matter abnormalities are detected on pathology in the brains of patients suffering from Alzheimer’s disease with no vascular risk factors [28,29]. In the Rotterdam Scan Study, white matter lesions detected on MRI scans were shown to be associated with dementia despite adjusting for vascular risk factors like hypertension, diabetes mellitus, smoking and stroke [2]. Another recent large population based study also showed an association between MRI detected white matter hypertintensities and impaired cognition independent of vascular risk factors [4]. In a population based study of 1792 elderly subjects aged 65-80 yrs, Godin et al [30], observed that white matter lesion burden, assessed by MRI, was associated with gray matter and hippocampal volume atrophy, surrogate markers for Alzheimer’s pathology. Since these studies did not have a neuropathological component, the role of underlying AD pathology could not be assessed, although our findings would suggest the presence of such pathology.
Our results are similar to those of Polvikoski et al [31], who performed MRI scans on brains fixed from 3 months to 4 years in a cohort of elderly subjects and found that severe white matter changes on pathology were significantly more common in those with moderate to frequent neuritic plaques than in subjects with a lower density of cortical neuritic plaques. Our study extends that of Polvikoski and colleagues in that we have a prospective cohort with precise cognitive diagnoses and MRI scans that more closely approximate those performed clinically.
The mechanism behind the association of AD pathology and white matter disease was not the subject of this study, but several observations have been made in studies correlating histopathology to imaging abnormalities that suggest potential hypotheses. Scheltens et al [32], in a small study found that in persons with AD there was evidence of axonal loss, denudation of the ventricular lining and arteriosclerosis. In a similar study, Gouw et al [33], found that white matter abnormalities on MRI corresponded to axonal loss, myelin loss, astrogliosis and microglial activation. Cerebral amyloid angiopathy is frequently seen in patients with AD though it is not an invariant feature of the disease. By causing vascular injury it is hypothesized that amyloid angiopathy may contribute to white matter pathology. In our current study, we did not find that amyloid angiopathy was an independent contributor to white matter disease, but a larger study, or a more quantitative assessment of amyloid angiopathy may reveal a causal relationship. Gradient Echo sequences, which were not performed in this study, may help clarify the relationship between amyloid angiopathy and white matter disease, as would a more detailed pathologic correlation between amyloid angiopathy and white matter disease. Polvikoski et al [31] suggested that white matter disease associated with AD pathology might be a result of the vascular origin of AD pathology, a finding we have not observed in the BLSA autopsy study [14] or in the current study. Finally, it should be considered that AD pathology and white matter disease may be related because they both reflect, and are sensitive to, the presence of another, more basic process, still unknown.
Additional limitations of the current study includes its small size, non-representative cohort, lack of FLAIR sequences and the lack of hippocampal and total brain volumes determined on high resolution 3T scanners. Each of these would have improved our ability to correlate vascular risk factors, AD pathology and brain changes. However, the strong relationship we see between MRI detected white matter disease and AD pathology in the elderly is striking. Our findings will hopefully motivate future research on the pathophysiological mechanisms underlying this relationship and its potential impact on cognition.
Acknowledgments
Sources of Funding
Supported by National Institute on Aging grant P50 AG05146, the Burroughs Wellcome Fund for Translational Research, and the Intramural Research Program, National Institute on Aging, National Institutes of Health.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatr. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–4. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 4.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–6. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–13. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–71. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O’Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006 Oct;67:1363–9. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 12.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 14.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68:231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;5:464–72. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 16.Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–27. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 17.Yue NC, Arnold AM, Longstreth WT, Jr, Elster AD, Jungreis CA, O’Leary DH, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–9. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 18.Kraut MA, Beason-Held LL, Elkins WD, Resnick SM. The impact of magnetic resonance imaging-detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J Cereb Blood Flow Metab. 2008;28:190–7. doi: 10.1038/sj.jcbfm.9600512. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien JT, Wiseman R, Burton EJ, Barber B, Wesnes K, Saxby B, et al. Cognitive associations of subcortical white matter lesions in older people. Ann N Y Acad Sci. 2002;977:436–44. doi: 10.1111/j.1749-6632.2002.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 20.de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–51. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58:610–6. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 22.van der Flier WM, van Straaten ECW, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–20. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 23.Sinka L, Kövari E, Gold G, Hof PR, Herrmann FR, Bouras C, et al. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J Neuropathol Exp Neurol. 2010;69:1247–55. doi: 10.1097/NEN.0b013e3181ffc3b9. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 25.Söderlund H, Nilsson L-G, Berger K, Breteler MM, Dufouil C, Fuhrer R, et al. Cerebral changes on MRI and cognitive function: the CASCADE study. Neurobiol Aging. 2006;27:16–23. doi: 10.1016/j.neurobiolaging.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- 27.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–20. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 28.Scheltens P, Barkhof F, Valk J, Algra PR, van der Hoop RG, Nauta J, et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity Brain. 1992;115:735–48. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Gilley DW, Wilson RS, Huckman MS, Fox JH. Clinical correlates of high signal lesions on magnetic resonance imaging in Alzheimer’s disease. J Neurol. 1992;239:186–90. doi: 10.1007/BF00839137. [DOI] [PubMed] [Google Scholar]
- 30.Godin O, Maillard P, Crivello F, Alpérovitch A, Mazoyer B, Tzourio C, et al. Association of white-matter lesions with brain atrophy markers: the three-city Dijon MRI study. Cerebrovasc Dis. 2009;28:177–84. doi: 10.1159/000226117. [DOI] [PubMed] [Google Scholar]
- 31.Polvikoski TM, van Straaten ECW, Barkhof F, Sulkava R, Aronen HJ, Niinistö L, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–8. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology. 1995;45:883–8. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- 33.Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, et al. Heterogeneity of white matter hyperintensities in Alzheimer’s disease: post-mortem quantitative MRI and neuropathology. Brain. 2008;131:3286–98. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]