Abstract
Background
Peripheral arterial disease (PAD) increases cardiovascular risk in many patient populations. The risks associated with an abnormal ankle-brachial index (ABI) in patients with type 2 diabetes (T2D) and stable coronary artery disease (CAD) have not been well described with respect to thresholds and types of cardiovascular events.
Methods
We examined 2368 patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial that underwent ABIassessment at baseline. Death and major cardiovascular events (death, myocardial infarction (MI) and stroke) during follow-up (average 4.3 years) were assessed across the ABI spectrum and by categorizedABI: low (≤0.90), normal (0.91–1.3), high (>1.3), or non-compressible.
Results
A total of 12,568 person-years were available for mortality analysis. During follow-up, 316 patients died and 549 suffered major cardiovascular events. After adjustment for potential confounders, with normal ABI as the referent group, a low ABI conferred an increased risk of death (relative risk (RR) 1.6; C.I. 1.2, 2.2; p=.0005) and major cardiovascular events (RR 1.4; C.I. 1.1, 1.7; p=.004). Patients with a high ABI had similar outcomes as patients with a normal ABI, but risk again increased in patients with a non-compressible ABI with a risk of death (RR1.9; C.I. 1.3, 2.8; p=.001) and major cardiovascular event (RR 1.5, C.I. 1.1, 2.1; p=.01).
Conclusions
In patients with CAD and T2D ABI screening and identification of ABI abnormalities including a low ABI (<1.0) or non-compressible artery provide incremental prognostic information.
Keywords: coronary disease, diabetes mellitus, peripheral vascular disease
Peripheral arterial disease (PAD), defined as an ankle-brachial index (ABI) ≤ 0.90, is highly prevalent in patients with type 2 diabetes (T2D) and is associated with an increased risk of adverse cardiovascular and limb events.1, 2 On the other end of the ABI spectrum, an abnormally high value, or a non-compressible ankle artery related to arterial calcification, also carries a risk for mortality in studied populations.3–6 We have previously shown that patients with T2D and concomitant coronary artery disease (CAD) have a high prevalence of an abnormally low or supranormal ABI.7Whether there is an incremental prognostic value in determiningABI abnormalities in patients with established T2D and stable CAD is unknown. Additionally, the threshold for an abnormal ABI and the relationship between the severity of ABI abnormality and specific cardiovascular outcomes has not been described in this population. To address these questions, we analyzed death and cardiovascular outcomes according to baseline ABI in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Study (BARI 2D) population.
Methods
Study design
A detailed description of the BARI 2D study has been previously published.8, 9 In brief, the trial compared treatment strategies for angiographically documented stable CAD in patients with T2D: medical therapy with revascularization delayed until clinically required versus medial therapy with prompt revascularization for CAD; and insulin sensitizing strategy versus insulin providing strategy of glycemic control for diabetes. All patients received medical therapy according to current guidelines, with a target level for glycated hemoglobin of less than 7.0%, a low-density lipoprotein cholesterol level of less than 100 mg per deciliter (2.6 mmol per liter), and a blood pressure of 130/80 mm Hg or less. In addition, all patients received counseling regarding smoking cessation, weight loss, and regular exercise. The BARI 2D trial included 49 clinical sites throughout North America, South America, and Europe and was coordinated at the University of Pittsburgh (Pittsburgh, PA). Recruitment began in 2001 and continued until 2005. Treatment continued until the 6-year visit or until the last annual visit before December 1, 2008, resulting in an average follow-up of 4.3 years. Vital status was ascertained in the last quarter of 2008 for all patients, resulting in an additional year of follow-up (5.3 years), on average, for survival. About 90% of patients had vital status obtained at 4 years or longer following randomization. The local institutional review boards approved protocols, and all participants provided informed consent. The study population for BARI 2D consisted of 2368 patients, 2240 with ABI data and 128 with missing ABI data.
Funding
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) was funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804). BARI 2D received significant supplemental funding from GlaxoSmithKline, and additional funding from Lantheus Medical Imaging, Inc., AstellasPharma US, Inc., Merck & Co., Inc., Abbott Laboratories, Inc. and Pfizer, Inc. Medications and supplies were donated by Abbott Laboratories Ltd., MediSense Products, Bayer Diagnostics, Becton, Dickinson and Company, J. R. Carlson Labs, Centocor, Inc., Eli Lilly and Company, LipoScience, Inc., Merck Sante, Novartis Pharmaceuticals Corporation, and Novo Nordisk, Inc. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Ankle-Brachial Index Protocol
Sites received centralized training and followed a standard protocol for obtaining an ABI. Patients were placed in a supineposition for at least 5 minutes and the systolic blood pressure of the brachial artery of both arms and the posterior tibial artery of both ankles were measured using a doppler probe (Parks MedicalElectronics Inc., Aloha, OR). The highest blood pressure in the arms was used to calculate ABI. The ratio of ankle to arm systolic blood pressure was calculated for each leg and the lowest ratio was recorded as the ABI for the patient. ABIs were classified according to the BARI 2D protocol as: low ≤0.9, normal 0.91–1.3, high >1.3, or non-compressible artery when the operator was unable to occlude the ankle artery with maximum blood pressure cuff inflation. Patients that had an ankle to arm ratio of ≤0.9 in one leg who had the other limb non-compressible (n=19) were excluded.
Definitions
The primary endpoint was all cause mortality. The principal secondary endpoint of major adverse cardiac events included the composite of all cause mortality, MI and stroke. Nonfatal MI included spontaneous, silent and procedural events. Diagnosis of spontaneous MI required a doubling of either CK-MB or troponin and evidence of ischemia in the form of symptoms, electrocardiography (EKG), or imaging. Silent MI was defined as a Q-wave change of 2 grades on routine EKG according to the Minnesota code.10 Procedural MI required an increase in CK-MB of greater than 3 times normal for percutaneous coronary intervention and 10 times normal for cardiac surgical bypass. The core Electrocardiography Laboratory classified all MIs. Independent clinical events committees adjudicated cause of death and stroke.
Statistical Analysis
We compared clinical outcomes according to the ABI obtained at study enrollment using the normal ABI group (ABI 0.9–1.3) as referent. Baseline characteristics were compared with Student’s t-test for continuous variables and chi-square statistics for categorical variables. We compared rates of death and major cardiovascular events using Kaplan–Meier survival curves and log-rank tests with a two-sided alpha level of 0.05. Relative risks of death and major cardiovascular events for baseline ABI category were estimated with Cox regression models. The ABI analysis was adjusted for age, sex, black race, duration of diabetes, insulin therapy, glycated hemoglobin, body mass index, hypertension, smoking, hypercholesterolemia, chronic renal dysfunction, C reactive protein, albumin creatinine ratio, history of congestive heart failure, angina, peripheral neuropathy, left ventricular ejection fraction, myocardial jeopardy, and number of diseased regions. Death was censored at the time of the last contact with the patient, whereas data for MI and stroke were censored at the last study-clinic visit. We also examined the relative risk of the individual endpoints of all cause mortality, cardiovascular mortality, MI and stroke, excluding patients with missing ABI information and using a more stringent reference ABI range of 1.11–1.3 and ABI increments of 0.10 for values 0.61–1.1 and 1.31–1.5 to explore cut-off values at which the risk for these events increased significantly.
Results
Baseline characteristics according to ABI
An ABI was obtained and categorized in 94.6% (n=2240) of patients enrolled in the BARI 2D trial. The prevalence of a normal ABI, defined as a value of 0.91–1.3 was 66% (n=1489). A low ABI (≤ 0.9) was found in 430 patients (19%), and a high ABI (>1.3) or non-compressible artery was found in 182 (8%) and 139 (6%) patients respectively. The baseline characteristics of BARI 2D patients according to ABI have been previously described7 and are presented in Table 1.
Table 1.
Baseline Characteristics by Ankle-Brachial Index Group
| Normal N=1489 |
Low ABI N=430 |
High ABI N=182 |
NC artery N=139 |
|
|---|---|---|---|---|
| Age, mean (SD) | 61.9 (8.8) | 63.4 (8.9)† | 61.9 (8.5) | 64.2 (9.7)† |
| Female, % | 28.3 | 38.8§ | 17.6† | 30.2 |
| Black race, % | 15.2 | 27.4§ | 4.9‡ | 17.3 |
| Years with diabetes, mean (SD) | 9.7 (8.1) | 11.4 (9.0)‡ | 10.2 (9.1) | 15.1 (10)§ |
| Insulin therapy, % | 25.9 | 32.3† | 25.3 | 40.3‡ |
| Hemoglobin A1c, mean (SD) | 7.63 (1.62) | 7.79 (1.60) | 7.61 (1.70) | 7.76 (1.58) |
| Body mass index, mean (SD) | 31.5 (5.7) | 31.0 (5.7) | 33.7 (6.0)§ | 32.8 (6.7)* |
| Hypertension, % | 87.8 | 92.3† | 87.4 | 95.7† |
| Current smoking, % | 11.6 | 20.7§ | 3.8† | 7.3 |
| Hypercholesterolemia, % | 81.7 | 82.1 | 76.9 | 74.8* |
| Chronic renal dysfunction, % | 2.5 | 4.4* | 2.7 | 6.6† |
| Peripheral neuropathy, % | 24.8 | 28.1 | 28.0 | 48.9§ |
| History of CHF % | 5.1 | 11.0§ | 6.7 | 7.2 |
| CRP (log ug/ml), mean (SD) | 0.83 (1.24) | 1.04 (1.22)† | 0.82 (1.19) | 1.08 (1.41)* |
| ACR > 30 mg/g | 26.7 | 38.1§ | 26.4 | 47.5§ |
| LV ejection fraction, mean (SD) | 55.0 (16.2) | 53.5 (16.7) | 54.5 (16.5) | 49.8 (20.3)† |
| Angina | 82.1 | 81.2 | 83.0 | 88.5 |
| Myocardial jeopardy index, mean (SD) | 43.7 (24.4) | 46.8 (24.2)* | 41.4 (22.0) | 47.5 (25.1) |
| Number of diseased regions, mean (SD) | 1.9 (0.9) | 2.1 (0.8)‡ | 1.9 (0.8) | 2.0 (0.9) |
CHF indicated congestive heart failure; CRP, C reactive protein; ACR, Albumin creatinine ratio; LV, left ventricular
Clinical outcomes
A total of 12,568 person-years were available for mortality analysis, with a median and mean follow up duration of 5.2 and 5.3 years respectively (range 23 days to 7.8 years). During follow-up 316 patients died and 549 either died or had major cardiovascular events. For major cardiovascular events there were 61 strokes (5 fatal) and 272 MIs (25 fatal). The majority of MIs, 80% (n=218) were unrelated to a revascularization procedure. Among the 53 MI that were procedural, 40 were symptomatic. Therefore, only 4.8% of MIs overall were due to asymptomatic peri-procedural biomarker elevations.
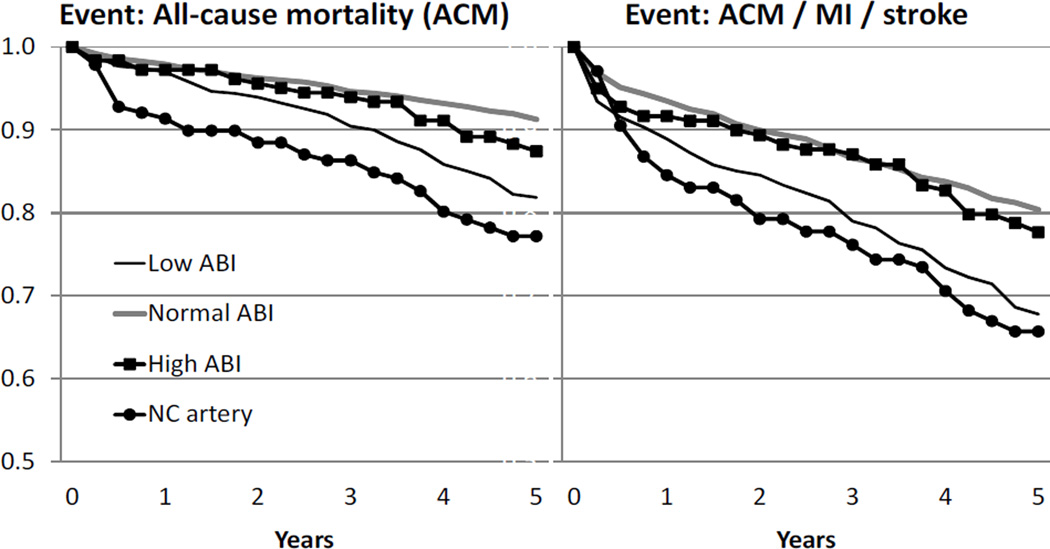
The 5 year rates of death from any cause and major cardiovascular events differed significantly according to ABI at study enrollment (Figure 1). Patients with a normal ABI had a 5 year survival of 91%. The 5 year rate of survival was significantly lower in patients with a low ABI (82%, p<0.0001 by log-rank test) or non-compressible artery (77%, p<0.0001). Similarly, the rate of freedom from major cardiovascular events was significantly lower in patients with a low ABI (68%) or non-compressible artery (66%), compared to patients with a normal ABI (80%). The rates of survival and of freedom from major cardiovascular events did not differ, however, between patients with a normal ABI and a high but compressible ABI (p=0.34 and p=0.85, respectively). We further evaluated the patients with an ABI between 1.31–1.4 (n=121) and >1.4 (n=61) compared those with an ABI 0.91–1.3 and found similar rates of adverse events over time (data not shown).
Figure 1.
Kaplan- Meier Event-Free Survival and Major Cardiovascular Event Curves According to Baseline ABI
ACM indicated all cause mortality; MI, myocardial infarction; ABI, ankle brachial index; NC, non-compressible. For ACM, compared to normal ABI, p<.0001 for low ABI and NC artery and p=0.34 for high ABI. For ACM/MI/stroke, compared to normal, p<.0001 for low ABI and NC artery and p=0.85 for high ABI.
After adjustment for baseline differences, compared to a normal ABI, a low ABI remained significantly associated with a higher risk of death (RR1.65, 95% C.I., 1.25–2.18, p=0.0005) and major cardiovascular events (RR1.37, 95% C.I., 1.10–1.70, p=0.004). Similarly, there was an increased risk of death (RR1.87, 95% C.I., 1.27–2.75, p=0.001) and major cardiovascular events (RR1.51, 95% C.I., 1.10–2.06, p=0.01) in patients with a non-compressible artery. In patients with a high ABI the risk of death and major cardiovascular events were similar to normal ABI patients (Table 2).
Table 2.
Relative Risks that compare all-cause mortality rates and major adverse cardiovascular event rates of various ABI groups to patients with normal ABI
| Mortality | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | |||||
| ABI group | RR | (95% CI) | p-value | RR | (95% CI) | p-value |
| Low ABI (<=0.9) High ABI (>1.3) Non-compressible artery |
2.16 1.24 2.93 |
1.66, 2.82 0.80, 1.92 2.03, 4.22 |
<.0001 0.34 <.0001 |
1.65 1.31 1.87 |
1.25, 2.18 0.84, 2.05 1.27, 2.75 |
.0005 0.24 .001 |
| Death, MI, and stroke | ||||||
| Unadjusted | Adjusted* | |||||
| ABI group | RR | (95% CI) | p-value | RR | (95% CI) | p-value |
| Low ABI (<=0.9) High ABI (>1.3) Non-compressible artery |
1.73 1.03 2.10 |
1.41, 2.12 0.73, 1.45 1.55, 2.83 |
<.0001 0.87 <.0001 |
1.37 1.11 1.51 |
1.10, 1.70 0.78, 1.58 1.10, 2.06 |
.004 0.55 .01 |
Adjusted for: Age, sex, black race, duration of diabetes, insulin therapy, glycated hemoglobin, body mass index, hypertension, smoking, hypercholesterolemia, chronic renal dysfunction, C reactive protein, albumin creatinine ratio, history of congestive heart failure, angina, peripheral neuropathy, left ventricular ejection fraction, myocardial jeopardy, and number of diseased regions.
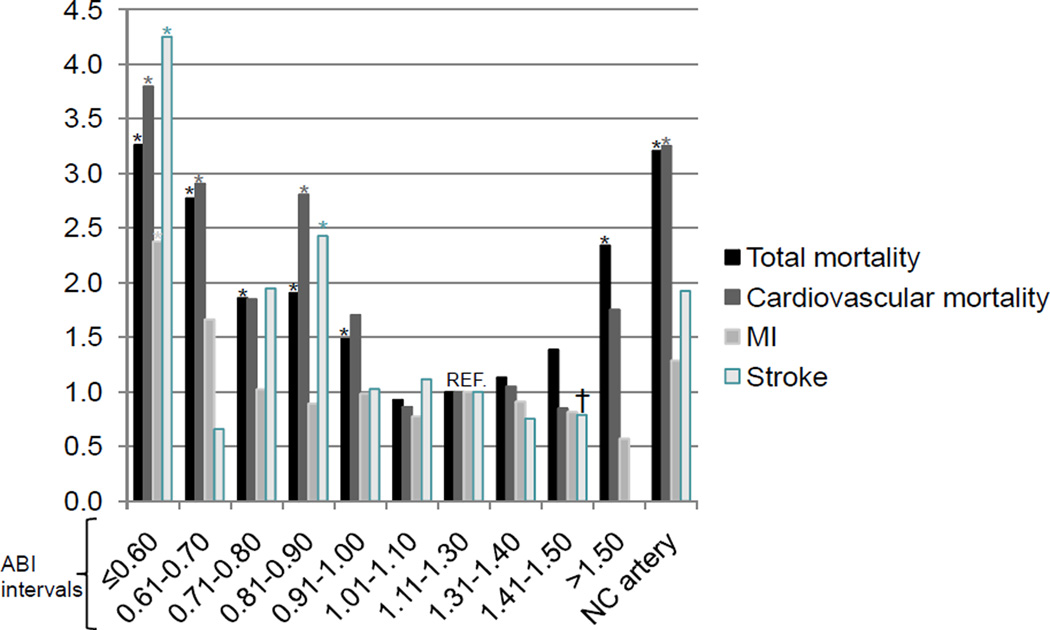
We further investigated the unadjusted relative risk of all cause mortality and individual outcomes of cardiovascular mortality, MI and stroke according to the severity of the ABI abnormality. Compared to a referent ABI of 1.11 to 1.3, the relative risk ratios for mortality formed a U-shaped curve (Figure 2). For levels of ABI below 1.0, the risk increased progressively with decreasing ABI. For levels of ABI above 1.3, the risk did not increase until the ABI was greater than 1.5 or the ankle artery was non-compressible. Similar, but less pronounced trends were observed for cardiovascular mortality. The risk of MI in ABI levels were nonsignificantly different from the referent group with the exception of the patients with ABI less than or equal to 0.6 (RR 2.4, p<.05). Trends in risk of stroke emerged with higher risk for levels of ABI below 0.91 and in non-compressible arteries; however the degree and consistency of the differences varied with decreasing ABI.
Figure 2.
Relative Risk (RR) of individual outcomes for ABI intervals compared to the interval 1.11 to 1.30
*p<0.05; † RR for stroke was calculated for the ABI interval ‘>1.40’ instead of ‘1.41–1.50’. NC indicates non-compressible; MI, myocardial infarction.
Discussion
We observed a U-shaped association between ABI and outcomes, both all cause mortality and major cardiovascular events, in a large cohort of patients with T2D and stable CAD. BARI 2D participants with ABI measurements ≤0.9 and non-compressible ankle arteries had higher mortality and cardiovascular risk than those with an ABI 0.91–1.3, with participants with non-compressible arteries at the highest risk. In comparison, those with a high but compressible ABI >1.3 had a similar 5 year risk as patients with a normal ABI. These findings are incremental to prior studies showing PAD has independent prognostic significance in patients with acute coronary syndromes, including patients with diabetes, by examining stable CAD and the entire ABI spectrum.11 In addition, our study highlights the importance of evaluating outcomes for patients with a high ABI separately from those with a non-compressible ankle artery.
The rates of all cause mortality and cardiovascular events we observed in the BARI 2D population are similar to those reported in the international Reduction of Atherothrombosis for Continued Health (REACH) registry.12, 13 Four year cardiovascular outcomes were reported in 45,227 outpatients with established coronary, cerebrovascular, or peripheral arterial disease, or multiple risk factors for atherothrombosis. All cause mortality was 10.7% in stable atherosclerotic disease patients. At 5 years, all cause mortality in BARI 2D patients with a normal ABI was 8.7%, with higher rates observed in patients with a low ABI (18.1%) or non-compressible ankle arteries (22.8%). In the REACH registry, the composite outcome cardiovascular death, MI, and stroke was observed in 15.5% of patients with stable atherosclerotic disease and diabetes, and 17.7% in patients with polyvascular disease.13 For major cardiovascular events, BARI 2D patients with a normal ABI had a rate of 19.6%, and with an abnormal ABI the rates were higher, 32.2% in low ABI and 34.3% in patients with non-compressible arteries. The higher risk of cardiovascular events observed in our study likely reflects the inclusion of all cause mortality as opposed to cardiovascular death in the composite endpoint and differences in baseline characteristics in the study populations with a larger diversity of risk due to varied atherosclerotic burden in REACH.
We found the presence of obstructive PAD or severe arterial stiffness was a stronger predictor of death and adverse outcomes than measures of diabetes duration or severity; and that the strength of ABI as a predictor of outcomes was similar to older age, chronic kidney disease and history of congestive heart failure (data not shown). The U-shape risk curve we observed for ABI has been previously described in patients without known CAD including older individuals, dialysis patients and Native Americans.4 In the Strong Heart Study of Native Americans, the adjusted risk estimates for all-cause mortality were 1.69 (1.34 to 2.14) for an ABI <0.9 and 1.77 (1.48 to 2.13) for ABI >1.4. In the high ABI group 44.3% of patients had a non-compressible ABI and these patients group accounted for the majority of risk.5 The high prevalence of diabetes and albuminuria in Native Americans likely resulted in the remarkable similar risk estimates compared with the BARI 2D population. In the Cardiovascular Health Study (CHS), which included Medicare eligible adults aged 65 years and older, a similar pattern of risk was observed.3 Using an ABI of 1.11 to 1.2 as the normal referent group, all cause and cardiovascular mortality were increased in patients with an ABI less than 1.0 and greater than 1.4. Cardiovascular events, including MI, stroke, coronary, and extremity revascularization were increased in patients with a low ABI, but not for high ABI. The CHS, however, was limited by a small number of patients with a high ABI and the lack of information on prevalence of non-compressible ankle arteries. Our findings are more congruent with the Health, Aging, and Body Composition Study of adults aged 70 to 79 years.6 In this study a normal ABI was considered 0.91 to 1.3 and a high ABI was found in 5% and non-compressible arteries in 2%. Both low and high ankle–arm index values were associated with increased risk of mortality and increased cardiovascular events. In particular, non-compressible arteries carried an independent risk for stroke and congestive heart failure.
In patients with known CAD the incremental risk of an abnormal ABI is less clear. In the setting of ACS, diabetes and PAD are highly prevalent, and patients with both conditions had a 4 fold risk of mortality in one study. Patients with non-compressible ankle arteries, however, were not examined.11 Conversely, in the setting of stable angina, the multicenter MERITO II registry in Spain found that in consecutive outpatients with a history of cardiovascular or cerebrovascular disease ABI was not an independent predictor of risk in patients with diabetes. 14 There are several potential reasons why our results differ. First, the patients in a randomized trial may not be representative of the general population of patients with DM and our patients were likely more symptomatic from CAD. Additionally; duration and treatment of diabetes were not considered in the prior study, and in our study outcomes were adjudicated and we had more patients and longer follow-up giving more strength to our findings.
The ability of the ankle brachial index to detect differing lower extremity large vessel characteristics, atherosclerotic PAD and arteriosclerosis resulting in arterial stiffness, explains the U-shaped association with ABI and outcomes. A high ABI is attributed to arterial calcification and the pathogenesis differs from atherosclerosis in that it is related to changes in the medial layer of the arterial wall.15 Patients with DM may have accelerated arterial stiffening due to the effects of hyperglycemia and insulin on connective tissue proteins and cellular proliferation respectively.16, 17 In a study of 16,493 individuals referred for outpatient, noninvasive lower extremity arterial testing, 17% had poorly compressible lower extremity arteries, defined as an ABI≥1.4 and/or an ankle systolic blood pressure >255 mm Hg. At a mean follow up of 5.8 years, the risk of death was 2 fold higher than in individuals with a normal ABI. This study and ours show that an abnormally high ABI is more prevalent than previously recognized and carries important prognostic implications.18
A limitation of our study is that we cannot investigate the mechanism of the association between ABI and outcomes. Potential causes include differences in functional status, extent and distribution of vascular disease, hemodynamic alterations with arterial stiffness, inflammation or other unmeasured confounders. Additionally, the ABI categories we used differ from the current recommendations. Based primarily on the AnkleBrachial Index Collaboration meta-analysis 19, the 2011 updated PAD guidelines 20 recommend the following ABI categories:Abnormal (ABI <0.90), Borderline (ABI 0.91–0.99), Normal (ABI 1.00 –1.40), and non-compressible arteries (ABI >1.40). We considered an ABI of 0.91–1.3 as normal; however when we analyzed outcomes for smaller ABI increments using 1.11 to 1.3 as the normal range our findings were consistent with the guidelines. We observed an increased risk of death in patients with an ABI of 0.91–1.0, but not in the 1.01 to 1.1 or 1.31–1.4 range. Our analysis of the individual endpoints of cardiovascular death, MI, and stroke by ABI increments is limited by sample size, therefore the results should be considered exploratory. Another limitation is that a non-compressible ankle artery can mask obstructive PAD and we cannot exclude concomitant atherosclerosis and arteriosclerosis and this may influence risk.
In summary, our results detailing the association between ABI and risk of mortality and cardiovascular events extend the findings of previous studies to include a population with high rates of non-compressible ankle arteries. The magnitude of mortality risk associated with a non-compressible ankle artery was similar to that of low ABI, highlighting the equal influence of these vascular pathologies on poor outcomes. Our study supports the utility of ABI as a tool for predicting mortality in individuals with established DM and CAD. Also, our data confirm an increased risk of mortality with a borderline low ABI. Whether the prognosis for these high risk patients can be improved with more aggressive medical therapy or exercise therapy should be investigated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
As an NIH funded trial, we are required to abide by the NIH PubMed Central Policy that we retain the right to provide a copy of the final manuscript to the NIH upon acceptance for publication by your journal, for public archiving in PubMed Central as soon as possible, but no later than 12 months after publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institute of Diabetes And Digestive And Kidney Diseases, or the National Institutes of Health.
Disclosures: none
References
- 1.Adler AI, Boyko EJ, Ahroni JH, et al. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22:1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 2.Norman PE, Davis WA, Bruce DG, et al. Peripheral arterial disease and risk of cardiac death in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2006;29:575–580. doi: 10.2337/diacare.29.03.06.dc05-1567. [DOI] [PubMed] [Google Scholar]
- 3.O'Hare AM, Katz R, Shlipak MG, et al. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 4.Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am SocNeph. 2003;14:1591–1598. doi: 10.1097/01.asn.0000065547.98258.3d. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 6.Sutton-Tyrrell K, Venkitachalam L, Kanaya AM, et al. Relationship of ankle blood pressures to cardiovascular events in older adults. Stroke. 2008;39:863–869. doi: 10.1161/STROKEAHA.107.487439. [DOI] [PubMed] [Google Scholar]
- 7.Singh P, Abbott J, Lombardero M, et al. The Prevalence and Predictors of an Abnormal Ankle-Brachial Index in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Diabetes Care. 2011;34:464–467. doi: 10.2337/dc10-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks MM, Frye RL, Genuth S, et al. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Bypass Angioplasty Revascularization Investigation 2 Diabetes Study G. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J. 2008;156:528–536. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prineas R, Crow R, Blackburn H, editors. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA: John Wright-PSG, Inc; 1982. [Google Scholar]
- 11.Quiles J, Morillas P, Bertomeu V, et al. Prevalence of Peripheral Arterial Disease in Patients with Acute Coronary Syndrome. Combination of ankle brachial index and diabetes mellitus to predict cardiovascular events and mortality after an acute coronary syndrome. Int J Cardiol. 2011;151:84–88. doi: 10.1016/j.ijcard.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 12.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 14.Mostaza JM, Manzano L, Suarez C, et al. Different prognostic value of silent peripheral artery disease in type 2 diabetic and non-diabetic subjects with stable cardiovascular disease. Atherosclerosis. 2011;214(1):191–195. doi: 10.1016/j.atherosclerosis.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Ledet T, Heickendorff L, Rasmussen L. Cellular mechanisms of diabetic large vessel disease. Chichester, England: John Wiley & Sons Ltd; 1992. [Google Scholar]
- 16.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. An Intern Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 17.Stout RW. Insulin as a mitogenic factor: role in the pathogenesis of cardiovascular disease. Am J Med. 1991;90:62S–65S. doi: 10.1016/0002-9343(91)90041-u. [DOI] [PubMed] [Google Scholar]
- 18.Arain FA, Ye Z, Bailey KR, et al. Survival in patients with poorly compressible leg arteries. J Am CollCardiol. 2012;59:400–407. doi: 10.1016/j.jacc.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowkes FGR, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(19):2020–2045. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]