Abstract
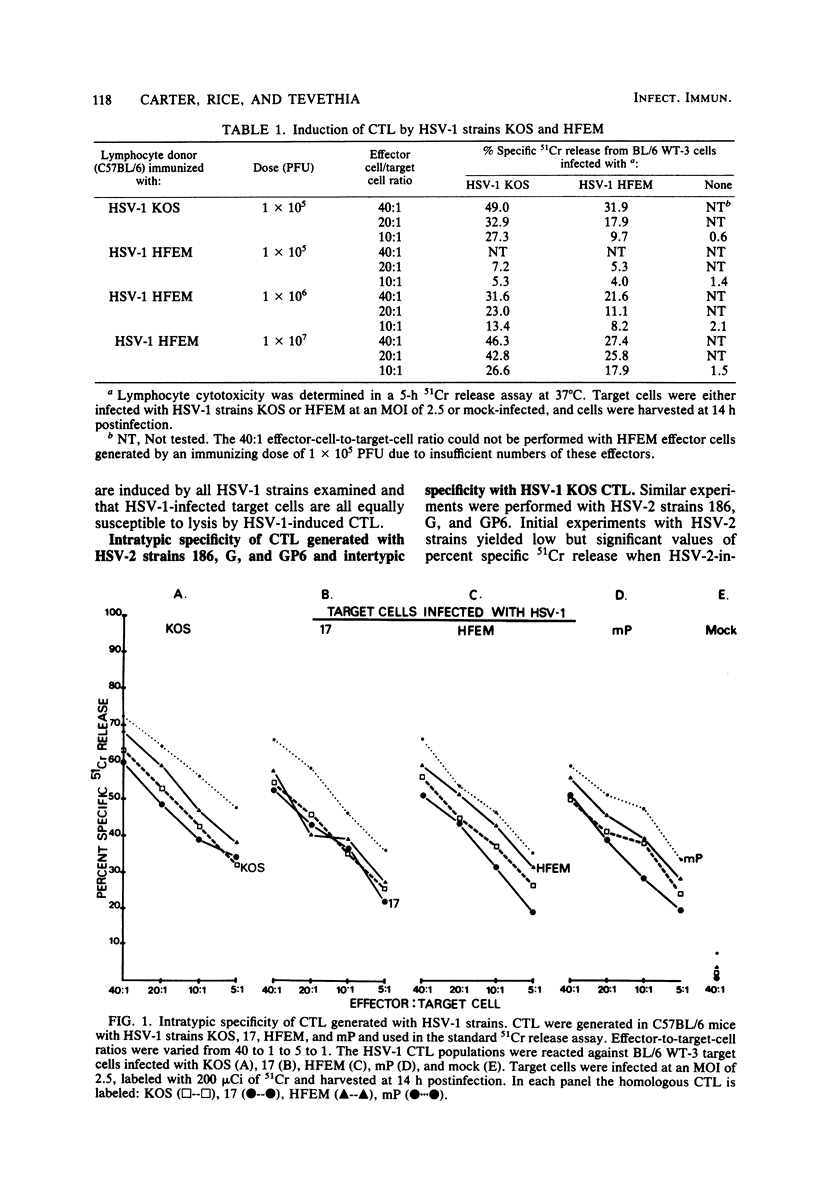
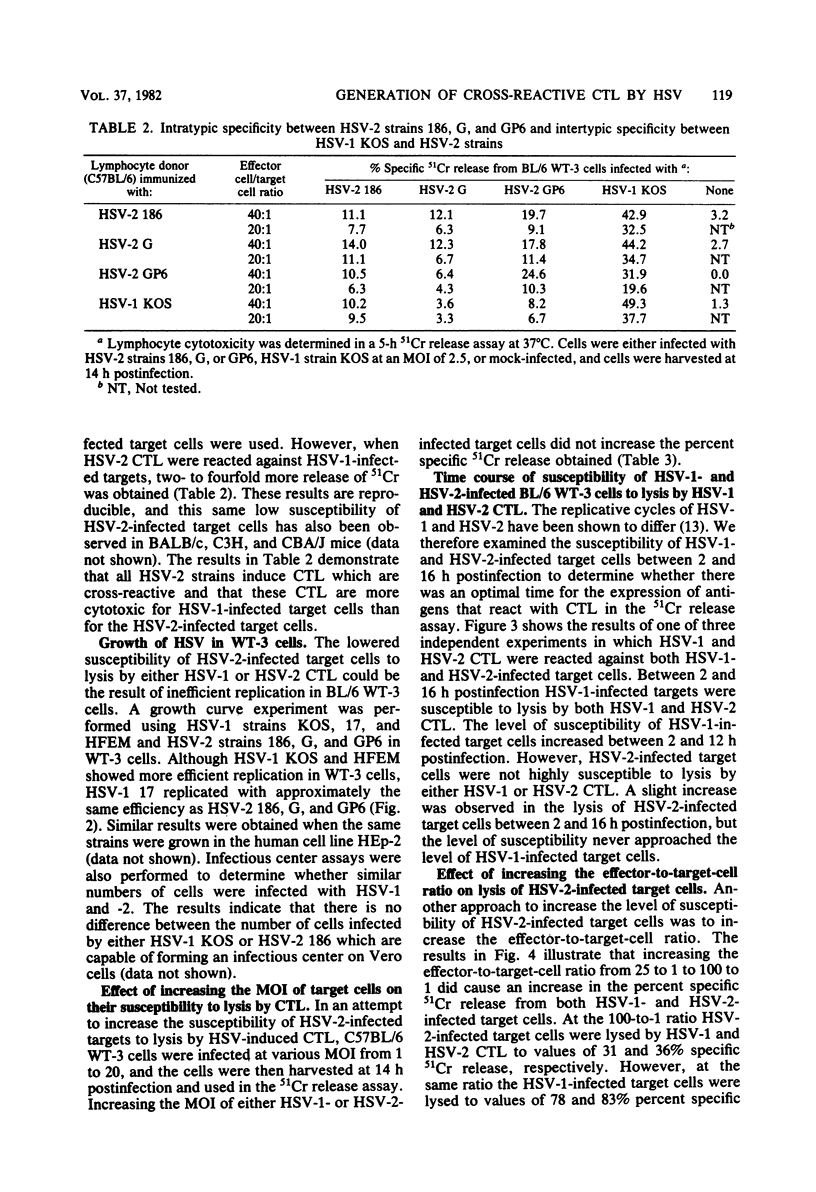
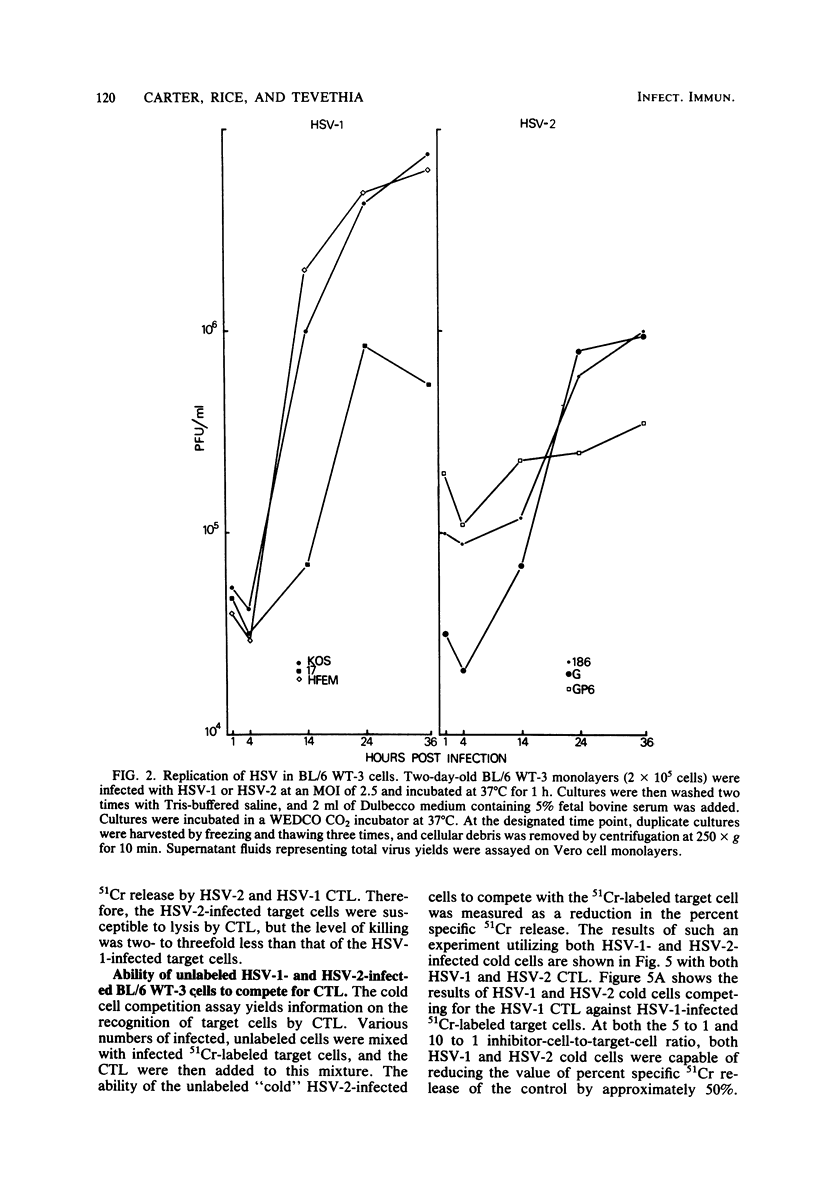
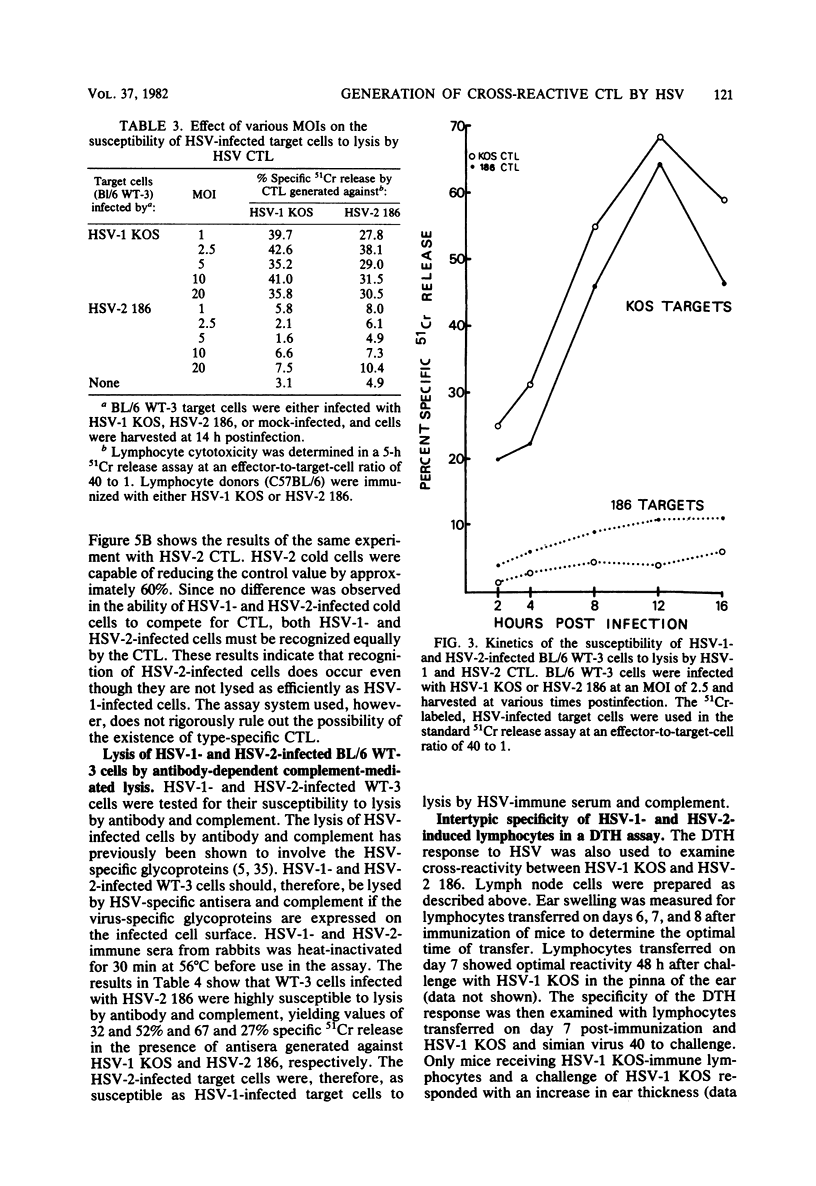
Cytotoxic T lymphocytes (CTL) were generated in C57BL/6 mice with herpes simplex virus type 1 (HSV-1) (strains KOS, 17, HFEM, and mP) and HSV-2 (strains 186, G, and GP6). Effector lymphocytes were tested for cytotoxicity against syngeneic HSV-1- and HSV-2-infected cells in a 5-h 51Cr release assay. HSV-1 strain HFEM was found to induce CTL efficiently only when 100-fold more virus was used as compared with HSV-1 strains KOS, 17, and mP. All HSV-1 and HSV-2 strains induced cross-reactive populations of CTL. CTL generated by HSV-1 KOS and HSV-2 186 also demonstrated cross-reactivity in an ear-swelling model for delayed-type hypersensitivity. Lymphocytes generated by all HSV-2 strains were highly efficient at lysing HSV-1-infected target cells. However, HSV-2-infected target cells were found to be less susceptible to lysis by either HSV-1 or HSV-2 CTL than were HSV-1-infected target cells. The lowered susceptibility of HSV-2-infected cells was not due to an inefficient infection of BL/6 WT-3 cells as measured by standard growth assays and infectious center assays. Varying the multiplicity of infection or the time of infection did not increase the susceptibility of HSV-2-infected target cells to lysis by CTL. Increasing the effector-to-target-cell ratio resulted in an increased lysis of both HSV-1- and HSV-2-infected target cells by CTL, but the level of HSV-2-infected target cell lysis still did not approach the level of HSV-1-infected target cell lysis. HSV-2-infected cells were as efficient as HSV-1-infected cells in the cold cell competition assay employed in reducing the lysis of 51Cr-labeled, HSV-1-infected target cells. In addition, HSV-2-infected cells were susceptible to lysis by HSV-immune serum and complement.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Webster R. G. Monoclonal antibodies to hemagglutinin and to H-2 inhibit the cross-reactive cytotoxic T cell populations induced by influenza. Eur J Immunol. 1980 Feb;10(2):151–156. doi: 10.1002/eji.1830100215. [DOI] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J. Specificity of cytotoxicity T cells directed to influenza virus hemagglutinin. J Exp Med. 1979 Apr 1;149(4):856–869. doi: 10.1084/jem.149.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Carter V. C., Schaffer P. A., Tevethia S. S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981 May;126(5):1655–1660. [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Cohen G. H. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1980 Aug;35(2):428–435. doi: 10.1128/jvi.35.2.428-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Esparza J., Purifoy D. J., Schaffer P. A., Benyesh-Melnick M. Isolation, complementation and preliminary phenotypic characterization of temperature-sensitive mutants of herpes simplex virus type 2. Virology. 1974 Feb;57(2):554–565. doi: 10.1016/0042-6822(74)90194-9. [DOI] [PubMed] [Google Scholar]
- Fenwick M., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XI. Apparent clustering of functions effecting rapid inhibition of host DNA and protein synthesis. J Virol. 1979 Feb;29(2):825–827. doi: 10.1128/jvi.29.2.825-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Wilson L. A., Fenger T. W., Smith J. W. Complement-mediated cytolysis of HSV-1 and HSV-2 infected cells: plasma membrane antigens reactive with type-specific and cross-reactive antibody. J Gen Virol. 1978 Aug;40(2):443–454. doi: 10.1099/0022-1317-40-2-443. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Randall R. E., Killington R. A., Watson D. H. Some properties of recombinants between type 1 and type 2 herpes simplex viruses. J Gen Virol. 1977 Sep;36(3):471–484. doi: 10.1099/0022-1317-36-3-471. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Howes E. L., Taylor W., Mitchison N. A., Simpson E. MHC matching shows that at least two T-cell subsets determine resistance to HSV. Nature. 1979 Jan 4;277(5691):66–68. doi: 10.1038/277067a0. [DOI] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausch R. N., Swyers J. S., Kaufman H. E. Delayed hypersensitivity to herpes simplex virus in the guinea pig. J Immunol. 1966 Jun;96(6):981–987. [PubMed] [Google Scholar]
- Lawman M. J., Naylor P. T., Huang L., Courtney R. J., Rouse B. T. Cell-mediated immunity to herpes simplex virus: induction of cytotoxic T lymphocyte responses by viral antigens incorporated into liposomes. J Immunol. 1981 Jan;126(1):304–308. [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L., McKenzie I. F. Specificity, Ly phenotype, and H-2 compatibility requirements of effector cells in delayed-type hypersensitivity responses to murine influenza virus infection. J Exp Med. 1980 Apr 1;151(4):815–826. doi: 10.1084/jem.151.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G. Cell-mediated immunity in herpes simplex virus-infected mice: suppression of delayed hypersensitivity by an antigen-specific B lymphocyte. J Gen Virol. 1980 Jun;48(Pt 2):359–364. doi: 10.1099/0022-1317-48-2-359. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: simultaneous induction of protective immunity and tolerance to delayed-type hypersensitivity. Immunology. 1981 May;43(1):153–159. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: studies on the mechanism of tolerance to delayed-type hypersensitivity. Immunology. 1981 Jun;43(2):363–369. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: H-2 mapping of the delayed-type hypersensitivity response and the antiviral T cell response. J Immunol. 1981 Apr;126(4):1260–1262. [PubMed] [Google Scholar]
- Nash A. A., Quartey-Papafio R., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980 Aug;49(2):309–317. doi: 10.1099/0022-1317-49-2-309. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Cromeans T. L., Nahmias A. J. Participation of three major glycoprotein antigens of herpes simplex virus type 1 early in the infectious cycle as determined by antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1980 Apr;28(1):38–44. doi: 10.1128/iai.28.1.38-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Norrild B., Roizman B. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5202–5206. doi: 10.1073/pnas.78.8.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Pfizenmaier K., Starzinski-Powitz A., Röllinghoff M., Falks D., Wagner H. T-cell-mediated cytotoxicity against herpes simplex virus-infected target cells. Nature. 1977 Feb 17;265(5595):630–632. doi: 10.1038/265630a0. [DOI] [PubMed] [Google Scholar]
- Plummer G. A review of the identification and titration of antibodies to herpes simplex viruses type 1 and type 2 in human sera. Cancer Res. 1973 Jun;33(6):1469–1476. [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Pretell J., Greenfield R. S., Tevethia S. S. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). V In vitro demonstration of SV40 TrAg in SV40 infected nonpermissive mouse cells by the lymphocyte mediated cytotoxicity assay. Virology. 1979 Aug;97(1):32–41. doi: 10.1016/0042-6822(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Rawls W. E., Laurel D., Melnick J. L., Glicksman J. M., Kaufman R. H. A search for viruses in smegma, premalignant and early malignant cervical tissues. The isolation of Herpesviruses with distinct antigenic properties. Am J Epidemiol. 1968 May;87(3):647–655. doi: 10.1093/oxfordjournals.aje.a120855. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Schulman J. L. Influenza type A virus M protein expression on infected cells is responsible for cross-reactive recognition by cytotoxic thymus-derived lymphocytes. Infect Immun. 1980 Aug;29(2):719–723. doi: 10.1128/iai.29.2.719-723.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. W., Scott L. V., Patnode R. A. Sensitization of guinea pigs to herpes simplex virus. J Immunol. 1972 Oct;109(4):801–807. [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. Specifically immune mouse T-cells can destroy H-2 compatible murine target cells infected with herpes simplex virus types 1 or 2. Z Immunitatsforsch Immunobiol. 1977 Jul;153(2):162–173. [PubMed] [Google Scholar]
- Sethi K. K., Wolff M. H. The nature of host-cell herpes-simplex virus interactions(s) that renders cells susceptible to virus-specific cytotoxic T cells. Immunobiology. 1980 Dec;157(4-5):365–378. doi: 10.1016/s0171-2985(80)80006-4. [DOI] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Adam E., Melnick J. L., Rawls W. E. Use of the 51 Cr release test to demonstrate patterns of antibody response in humans to herpesvirus types 1 and 2. J Immunol. 1972 Sep;109(3):554–564. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F., Norrild B. Crossed immunoelectrophoresis of a herpes simplex virus type 1-specific antigen: immunological and biochemical characterization. J Infect Dis. 1978 Nov;138(5):639–643. doi: 10.1093/infdis/138.5.639. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Askonas B. A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980 May;10(5):396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]



