Abstract
Background
The Dehalococcoides are strictly anaerobic bacteria that gain metabolic energy via the oxidation of H2 coupled to the reduction of halogenated organic compounds. Dehalococcoides spp. grow best in mixed microbial consortia, relying on non-dechlorinating members to provide essential nutrients and maintain anaerobic conditions.
A metagenome sequence was generated for the dechlorinating mixed microbial consortium KB-1. A comparative metagenomic study utilizing two additional metagenome sequences for Dehalococcoides-containing dechlorinating microbial consortia was undertaken to identify common features that are provided by the non-dechlorinating community and are potentially essential to Dehalococcoides growth.
Results
The KB-1 metagenome contained eighteen novel homologs to reductive dehalogenase genes. The metagenomes obtained from the three consortia were automatically annotated using the MG-RAST server, from which statistically significant differences in community composition and metabolic profiles were determined. Examination of specific metabolic pathways, including corrinoid synthesis, methionine synthesis, oxygen scavenging, and electron-donor metabolism identified the Firmicutes, methanogenic Archaea, and the ∂-Proteobacteria as key organisms encoding these pathways, and thus potentially producing metabolites required for Dehalococcoides growth.
Conclusions
Comparative metagenomics of the three Dehalococcoides-containing consortia identified that similarities across the three consortia are more apparent at the functional level than at the taxonomic level, indicating the non-dechlorinating organisms’ identities can vary provided they fill the same niche within a consortium. Functional redundancy was identified in each metabolic pathway of interest, with key processes encoded by multiple taxonomic groups. This redundancy likely contributes to the robust growth and dechlorination rates in dechlorinating enrichment cultures.
Background
The Dehalococcoides (Dhc) comprise a genus-level group of bacteria within the phylum Chloroflexi, notable for their ability to respire halogenated compounds including recalcitrant groundwater contaminants [1-4]. Their obligate use of halogenated organic compounds as an energy source has allowed successful development of Dhc-containing enrichment cultures for bioaugmentation of chlorinated ethene-contaminated sites [5,6]. Within the Dhc group, several strains have been isolated, and there are currently five published genome sequences available [2,7,8]. The Dhc genome sequences reveal small, approximately 1.4 Mb chromosomes which encode ~1500 genes and a relatively reduced metabolism [2,7,8]. Each Dhc strain contains a unique complement of genes that are homologs of known reductive dehalogenase genes (rdhs), the genes required for respiration of halogenated organic compounds [8]. The characterized reductive dehalogenases are iron-sulfur cluster- and cobalamin-containing membrane-bound components of the Dhc electron transport chain, catalyzing the H2-dependent dehalogenation of a chlorinated substrate with the resultant production of H+ and Cl- ions [9-11]. In a peculiar example of genome streamlining, the Dhc genomes encode only a partial corrinoid synthesis pathway [2,12], despite a cobalamin cofactor being required for reductive dehalogenase activity and hence Dhc energy production and growth. Isolate cultures of Dhc require exogenous cobalamin amended to the media to allow growth and dechlorination [2,13-15]. A constraint-based metabolic flux model examining the Dhc core metabolism highlighted the streamlined nature of the Dhc genomes; many typical bacterial pathways, including the TCA cycle and glycolysis are incompletely encoded by this group [12].
The majority of Dhc-containing cultures are maintained as mixed microbial consortia, primarily because Dhc are notoriously difficult to isolate, but also because mixed cultures exhibit higher Dhc growth rates, higher Dhc titer, and better stability [15-17]. Additionally, mixed cultures are more robust to oxygen exposure, which can kill a pure Dhc culture [18]. The non-dechlorinating members of these cultures are typically fermentative and acetogenic bacteria and methanogens [5,19-21]. Organic electron donors are fermented to hydrogen and acetate, which Dhc can subsequently utilize. Methanogenic Archaea in the cultures occupy the same niche as dechlorinators, relying on hydrogen and acetate from fermentative and acetogenic bacteria. Collectively, the non-dechlorinating populations help to maintain a reducing environment, and synthesize vitamins and other metabolites that Dhc requires [22-24]. It is hypothesized that the Dhc can scavenge the majority of its required metabolites from the organisms extant in the mixed cultures. This hypothesis was lent weight by experiments in which Dhc pure cultures amended with vitamin B12 (cobalamin) or a B12-producing acetogen showed increased growth and dechlorination rates compared to Dhc grown without an external source of cobalamin [16]. Still, the individual roles of community members in dechlorinating consortia are not well defined. The non-dechlorinating communities coexisting with Dhc show distinct taxonomic compositions in different enrichments; the structure of the community might depend on the electron donor and medium conditions that the cultures are maintained on [21,25].
The KB-1 consortium is a well-defined enrichment culture originally derived from a TCE-contaminated site in southern Ontario [26]. KB-1 robustly dechlorinates PCE through trichloroethene (TCE), cis-dichloroethene (cisDCE), and vinyl chloride (VC) to ethene, and has been commercialized for use as a bioremediation tool. Previous work with KB-1 has identified the major bacterial and archaeal community members [25], as well as gene sequences for 14 unique genes homologous to reductive dehalogenases [27]. It contains multiple strains of uncharacterized Dhc and a single Geobacter sp. as active dechlorinating organisms, as well as a variety of methanogens and acetogens [25].
A metagenome sequencing project for the KB-1 consortium was completed by the Joint Genome Institute (JGI), generating 103 Mb of sequence. Recently, the JGI has also sequenced metagenomes from two other Dehalococcoides-containing enrichment cultures: DonnaII and ANAS. The three microbial consortia, KB-1, DonnaII, and ANAS, are maintained in Toronto, Ontario, Canada, Ithaca, NY, and Berkeley, CA, respectively. They contain different strains of Dhc, with unique complements of rdh genes [7,27,28]. Prior to metagenome sequencing, a genome sequence for the Dhc strain in the DonnaII consortium was generated [7], and a draft genome sequence for the Dhc strain in the KB-1 culture has subsequently been generated from the metagenome sequence (in-house data). The consortia have each been maintained for over a decade, in different volumes, with differing growth conditions, and with different electron donors (Table 1). The KB-1 consortium is amended methanol as an electron donor, while DonnaII is amended butyrate and ANAS utilizes lactate as an electron donor. The consortia each contain methanogenic Archaea as well as acetogenic and/or fermentative organisms, but the dominant Archaea and acetogens differ taxonomically in each culture (see Figures 1, 2). In general, reference genomes for these organisms are not available from public databases. Two exceptions are the genome sequence of the δ-Proteobacterium Geobacter lovleyi strain SZ, which is a close relative to the Geobacter strain in the KB-1 consortium, and the euryarchaeote Methanospirillum hungatei in the DonnaII culture. Despite the physical differences in maintenance conditions and the taxonomic variation between the consortia, all three consortia are capable of the complete dechlorination of tetra- or trichloroethene to non-toxic ethene, indicating that the environmental differences present do not preclude sustained Dhc growth.
Table 1.
Physical maintenance conditions for the three Dehalococcoides-containing enrichment cultures
| KB-1 | DonnaII | ANAS | |
|---|---|---|---|
|
Cultures |
|
|
|
| Liquid volume |
1.6 L |
5.7 L |
0.4 L |
| Stirred |
No |
Yes |
Yes |
| Temperature |
20-22 °C |
30 °C |
25-28 °C |
| Electron acceptor |
TCE (858 μmol added/L)1 |
PCE (110 μmol added/L)2 |
TCE (278 μmol added/L)3 |
| Electron donor |
Methanol (4.3 mM)1 |
Butyrate (440 μM)2 |
Lactate (25 mM)3 |
| Feeding frequency |
14 days |
2 days |
4-7 days |
| Residence time |
2 years4 |
70-80 days5 |
16-47 days3 |
| Donor loading rate [(μmol/L/d)/(meeq/L/d)] |
0.31/1.84 |
0.22/4.44 |
4.55/54.5 |
| Acceptor loading rate [(mmol/L/d)/(meeq/L/d)] |
0.061/0.37 |
0.055/0.44 |
0.051/0.30 |
| Donor eeq/Acceptor eeq |
5 |
10 |
180 |
| Cobalamin amended |
B12 (0.005 μg/L) |
B12 (1 μg/L) |
B12 (0.1 μg/L) |
| Dhc strain(s) | KB-1/PCE & KB-1/VC1 | DET1955 | ANAS (2 strains) 3 |
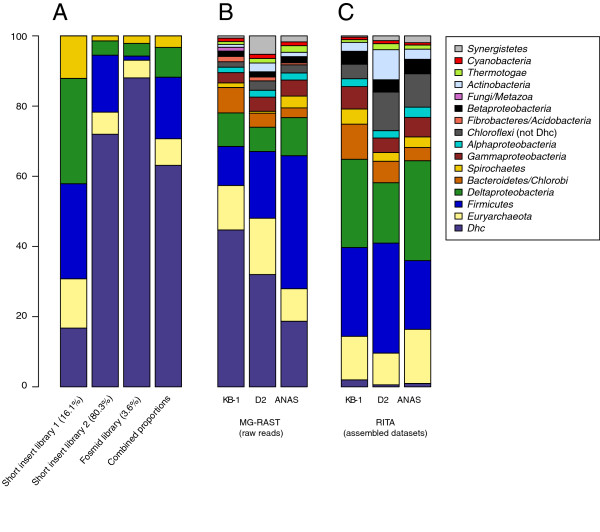
Figure 1.
Estimates of community composition for the three microbial consortia based on three different metrics. A: qPCR-based estimates of the KB-1 community from the gDNA used for generation of the three sequencing libraries, and the combined expected proportions given the amount of sequence generated from each library. qPCR estimates were based on tracking 10 dominant OTUs within the consortium [30]. B: MG-RAST-annotated prokaryotic proportions for the metagenomes’ raw reads. The MG-RAST automated annotation used was the SEED’s phylogenetic profile. All reads from the metagenomes were used to generate the initial profiles, with hits filtered by a maximum e-value of 10-5 and a minimum alignment length of 100. C: Proportion of taxonomic assignments for the assembled metagenome datasets based on RITA taxonomic assignments. All taxonomic groups present above 1% in any of the three metagenomes were included in the proportional representations in B and C.
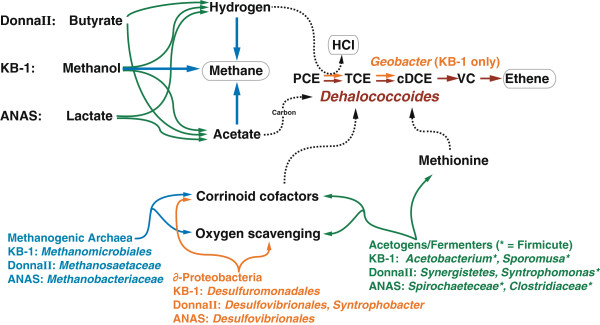
Figure 2.
Schematic overview of the examined metabolic processes taking place within the dechlorinating enrichment consortia, with the organisms implicated in each process highlighted. End products are boxed in light grey. Organisms are coloured by taxonomic affiliation and process: Methanogenesis and synthesis pathways encoded by methanogenic Archaea are in blue, acetogenesis and other processes largely attributed to Firmicutes are in green, and pathways encoded by ∂-Proteobacteria are in orange. NB: A hydrogen molecule is required for each dechlorination step from PCE to ethene: only the first dechlorination reaction is depicted here.
The availability of these metagenomes provides an opportunity to examine the microbial community composition and interactions present in these mixed microbial systems, bypassing the rigorous requirement of isolation of community members and complete genome sequencing. The rapid increase of available metagenome sequences has lead to the development of public servers and software for automated annotation and phylogenetic assignment of metagenome sequences [31-38], facilitating comparative metagenomic studies [39-45]. From metagenomic datasets, community compositions and metabolic functions can be examined. Further functional information in the form of culture-based experiments and/or transcriptomic or proteomic surveys is required to validate the predicted functions. Nevertheless, metagenomic data can suggest functional roles for organisms within a community, and inform follow-on experiments for examining these predicted roles in more detail.
Here we describe the KB-1 enrichment culture metagenome, and include a comparison with the ANAS and DonnaII enrichment consortia based on comparative metagenomics. We conduct an examination of the phylogenetic and metabolic differences and similarities among the three enrichment consortia, with a focus on the non-Dhc microbial population. In addition, we examine the presence and phylogenetic distribution of specific metabolic pathways of interest: cobalamin synthesis, methionine synthesis, oxygen utilization and scavenging, hydrogen production, and metabolism of the electron donors consumed by the enrichment cultures. This in-depth analysis of target pathways identifies taxonomic groups encoding synthesis pathways of required metabolites for Dhc, and strengthens the argument for maintenance of microbial diversity to safeguard functional redundancy within consortia.
Results and discussion
The KB-1 metagenome sequence
A metagenome sequence from the KB-1 Dhc-containing dechlorinating microbial consortium was generated by the Joint Genome Institute (JGI). The final KB-1 metagenome consists of 103 Mb of Sanger sequence data. Following assembly by the JGI’s in-house "pga.lucy" assembler, the unique sequence data is evenly split between assembled contigs (52.6% of sequence) and singletons (47.4% of sequence) (Table 2). The average contig size is quite short (2,356 bp), but 173 contigs have lengths greater than 10,000 bp, and 5 contigs have lengths greater than 100,000 bp. This uneven distribution of contig lengths is due to the varied levels of enrichment of the organisms within the KB-1 culture, whereby a dominant organism (e.g., Dehalococcoides) is over-represented in the community DNA and hence the metagenome sequence, yielding deeper coverage and assembly of longer contigs.
Table 2.
General features of the metagenome datasets
| Feature | KB-1 | DonnaII | ANAS |
|---|---|---|---|
| Type of sequencing |
Sanger |
454 |
454 & Sanger |
| Total number of bases pre-assembly |
106,515,530 |
930,446,714 |
330,964,688 |
| Number of contigs |
6,361 |
47,030 |
10,807 |
| Total length of contigs (bp) |
14,988,108 |
24,573,718 |
30,615,713 |
| Number of singletons |
18,629 |
105,608 |
15,486 |
| Total length of singletons (bp) |
13,487,233 |
57,708,799 |
10,450,264 |
| Largest contig (bp) |
155,970 |
121,460 |
921,258 |
| Average contig size (bp) |
2,356 |
522 |
2,832 |
| Average G + C content (%) |
52.33 |
52.28 |
51.91 |
| Protein coding genes |
40,766 |
194,527 |
60,992 |
| - with COGs |
21,857 |
116,001 |
39,920 |
| - connected to KEGG pathways |
8,077 |
36,685 |
11,878 |
| rRNA genes (5 S/16 S/23 S) |
18 (7/5/6) |
185 (11/62/112) |
40 (23/8/9) |
| tRNA genes |
330 |
818 |
525 |
| CRISPR count |
48 |
7 |
57 |
|
MG-RAST data |
|
|
|
| % Dhc in culture* |
43.7 |
31.3 |
18.2 |
| Metagenome size (bp)* |
106,508,248 |
916,191,214 |
330,396,345 |
| Average read length* |
958 |
477 |
547 |
| Number of sequences* |
111,162 |
1,920,396 |
603,841 |
| Number (%) identified for metabolic analysis† |
63,352 (57.0) |
363,424 (18.9) |
222,012 (36.8) |
| Number (%) identified for phylogenetic analysis† | 88,888 (80.0) | 540,785 (28.2) | 294,470 (48.8) |
* = post-MG-RAST preprocessing, which removed duplicate reads and nonsense reads from the datasets.
† = maximum e-value of 1x10-5, minimum alignment length ~100.
The KB-1 metagenome was sequenced from three separate clone libraries generated from DNA samples gathered at different dates. The original 3 kb-insert clone library was generated for creation of a shotgun microarray, while the subsequent two DNA samples were gathered specifically for metagenomic sequencing by the JGI, for 3 kb and 35 kb libraries. As a result, the expected community dynamic of the metagenome is a blend of the three DNA samples at the proportions to which they contributed to the final sequence dataset (Figure 1A).
A degenerate primer-based clone library of reductive dehalogenase genes generated previously [27] identified 14 unique reductive dehalogenase homologous genes in the KB-1 consortium, named KB1_rdhA1 through A14. Sequencing of the KB-1 metagenome resulted in the identification of a further 18 complete reductive dehalogenase homologous sequences, and 3 partial rdh sequences, bringing the total number of identified reductive dehalogenases in the KB-1 consortium to 35 (Additional file 1: Table S1).
In addition to acting as a general blueprint for the community, the KB-1 metagenome sequence has been instrumental in informing several targeted studies [46,47].
Comparative metagenomics of KB-1 with ANAS and DonnaII
The role of the non-dechlorinating community in Dhc-containing enrichment cultures is primarily to ferment the electron donor to H2. The additional processes contributing to the more robust dechlorination in mixed cultures compared to Dhc isolates are not well defined. In order to provide context to an examination of the roles of the KB-1 enrichment culture’s supporting community, we conducted a comparative metagenomic analysis of the three available dechlorinating enrichment culture metagenome sequences. The Joint Genome Institute conducted all three metagenome sequencing projects, under different modes of sequencing (see Table 2 for a comparison of metagenome properties).
The assembled datasets and the complete set of raw reads from the three metagenomes were uploaded as separate datasets into the MG-RAST server [33] for automated annotation and phylogenetic assignment. The distribution of different taxonomic groups among the three metagenomes as annotated by MG-RAST is depicted in Figure 1B. SEED data on the KB-1 consortium community composition was in general agreement with qPCR and clone library data (Figure 1A, [30]) with multiple rare organisms (>1% of the sequence) in the SEED classifications compared to the clone library. We used a hybrid taxonomic classification algorithm, RITA [48], to provide an independent assessment of the phylogenetic affiliations of the assembled datasets. RITA considers evidence from homology (via nucleotide vs. nucleotide and translated nucleotide vs. protein) and composition [49], and generated classifications that correlate well with the MG-RAST analysis on the raw read data (Figure 1B, Pearson correlation coefficient = 0.930). The main difference between these two sets of phylogenetic assessments is the proportion of Dhc within the datasets, where the annotated raw reads (MG-RAST) provide a more accurate depiction of the enrichment levels of Dhc within these consortia. Due to the small genome size and high enrichment levels, the Dhc contigs have higher read depths, causing the proportion of Dhc sequence in the assembled data to drop. Despite the differences in datasets and classifiers, the two methods largely agree on the taxonomic affiliations of the sequence data.
The MG-RAST analysis included generation of rarefaction curves for the unassembled metagenome sequences, which indicated that none of the three metagenomes have been sequenced to saturation (see MG-RAST metagenome projects for plots and data [MG-RAST IDs 4450840.3 (KB-1 raw reads), 4451142.3 (KB-1 assembled), 4451655.3 (ANAS raw reads), 4478350.3 (ANAS assembled), 4451259.3 (DonnaII raw reads) and 4447020.3 (DonnaII assembled). The distribution and diversity of the taxa identified from the three enrichment cultures indicates that certain organisms have been sequenced at much higher depths (Figure 1B). Combined with the absence of close reference genomes for these organisms, it is difficult to estimate the amount of missing information for each organism, meaning that all subsequent analyses are unable to distinguish between missing sequence data and true gene absences within these organisms.
For all subsequent pathway analyses, higher-level taxonomic assignments (phylum or class) were used to describe the presence/absence of pathways in order to reduce the effects of database bias in taxonomic assignments by MG-RAST. It must be noted that in all cases, assignment of a taxonomic identity and putative function to a sequence is dependent entirely on the presence of a known homolog within a sequenced relative in the MG-RAST database. In light of this, identification of novel features in Dhc was not anticipated. Indeed, the focus of this study is on non-Dhc populations, and on their functional gene complements. The requirement for a homolog within the database for gene identification undoubtedly caused a higher proportion of missing information in our analyses, but was an unavoidable constraint that we have attempted to work within.
Broad-scale phylogenetic and metabolic comparison of metagenomes
Phylogenetic and metabolic profiles of the three metagenomes, both including and excluding reads of Dhc origin, were imported into STAMP [31] for pair-wise statistical comparisons (see Additional file 1: supplemental information for STAMP parameters used). The annotated metagenomes varied in size as well as proportion of unknown proteins in each dataset (35%, 24.6%, and 18.5% for DonnaII, KB-1, and ANAS respectively). For all STAMP-based comparisons, the raw read datasets were utilized, to allow quantification of gene abundances. The strengths of the combined MG-RAST/STAMP analysis are its ability to identify the potentially interesting categories deserving of closer analysis and to bypass the differences in metagenomes’ sizes and sequence type by taking dataset size into account. As an example, KB-1 exhibits a comparatively high level of enrichment in the “Virus” category (Figure 3, #1). Although the effect size is large, the total number of reads assigned to the Virus category is small (1037 hits of a total of 1769825 assigned reads for the three metagenomes combined), and the difference among the three metagenomes cannot be confidently separated from a possible sampling effect. Hence, the apparent enrichment in KB-1 is not statistically significant. On a biological note for this example, the MG-RAST database contains limited sequence information for virus genomes, which may explain the low number of hits assigned to this category.
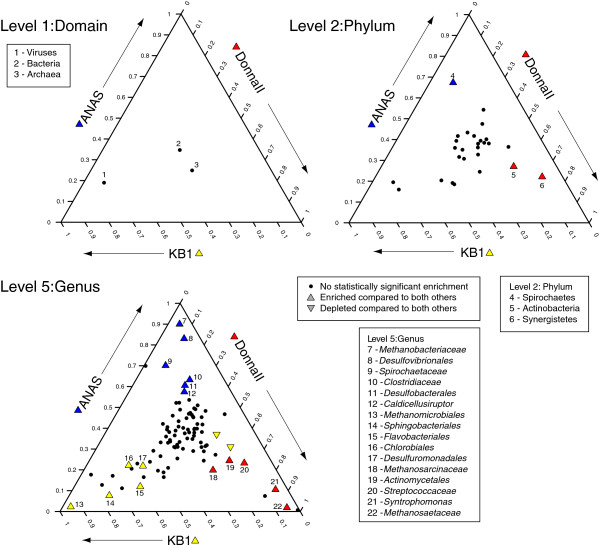
Figure 3.
Three-way statistical comparisons of phylogenetic differences within the enrichment culture metagenomes at different taxonomic levels. Points are displayed as the relative proportional enrichment among the metagenomes, where the closer to a corner of the plot a point falls, the more highly proportionally enriched that category is within the metagenome affiliated with that corner (arrows). Points located in the center of the plot are not enriched in one metagenome compared to the others. Only categories in which at least one metagenome’s proportion was above a threshold of 0.1% were plotted. Taxonomic levels were taken from MG-RAST phylogenetic profiles from the SEED database. Black circles indicate categories for which no pair-wise statistical significance between proportions was determined. Coloring of points indicates statistical significance for that category given a biological effect size filter in pair-wise comparisons conducted using the STAMP interface. Colors and shapes are as follows: Yellow = KB-1, Blue = ANAS, Red = DonnaII; up-pointing triangle = enriched in that metagenome above the other two, down-pointing triangle = depleted in the metagenome colored compared to the other two.
On a taxonomic level, the STAMP analysis identifies several meaningful differences among the consortia. At the level of phylum, DonnaII exhibits a biological enrichment in Actinobacteria and Synergistetes (Figure 3, #6,7), both groups that have been detected in DonnaII [20], but not in KB-1 or ANAS [1,19]. ANAS exhibits significant enrichment in Spirochaetes (Figure 3, #4). While all three consortia contain methanogenic Archaea at similar proportions (Figure 3, #3), the taxonomic affiliations of these methanogens are quite varied. In KB-1, the dominant Archaeal group is order Methanomicrobiales (Figure 3, #13), while DonnaII contains families Methanosarcinaceae and Methanosetaceae from order Methanosarcinales (Figure 3, #18, 22) and ANAS is enriched in family Methanobacteriaceae from order Metanobacteriales (Figure 3, #7). A similar scenario is seen in the Firmicute lineages present in the consortia, with DonnaII enriched in Streptococcaceae and Syntrophomonas (Figure 3, #20, 21), ANAS enriched in Clostridiaceae and Caldicellulosiruptor (Figure 3, #10, 12) and KB-1 not enriched in any Firmicute genus.
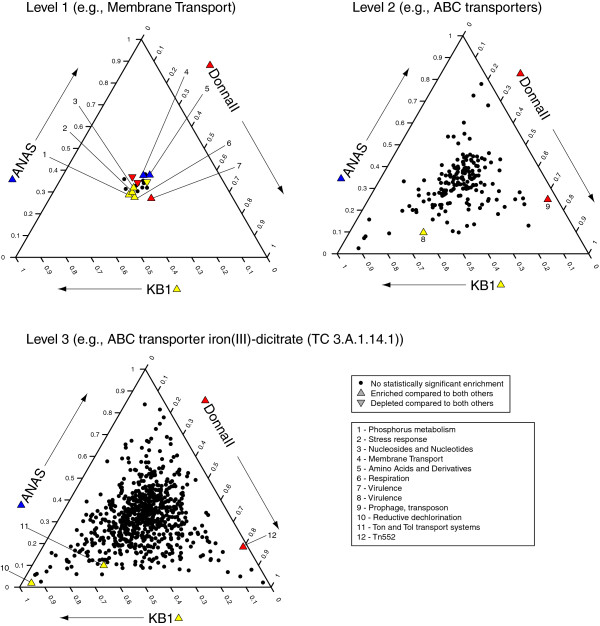
The MG-RAST metabolic assignments for the three metagenomes with all Dhc-assigned reads removed were used to examine the non-Dhc community metabolic differences. The SEED subsystems are available at three levels of increasing specificity, where level 1 provides broad categories similar to COG designations (e.g., Membrane Transport), and levels 2 and 3 provide more specific categories (e.g., Level 2 = ABC transporters, Level 3 = ABC transporter iron(III) dicitrate (TC_3.A.1.14.1)). Several categories from the SEED level 1 exhibit differences in enrichment among the three metagenomes. However, it is clear from the triangle plot that, while these are statistically significant differences, the enrichment seen is not strong (Figure 4, level 1). A more in-depth examination was required to distinguish the nature of this significance. When examining specific subsystems (SEED level 3) there are only three categories for which one metagenome shows significant enrichment above the other two (Figure 4, level 3). DonnaII exhibits significant enrichment in the transposon 552-associated subsystem (Figure 4, #12). This SEED subsystem contains the common elements of tn552 transposons, including mobility elements (transposase tnpA and recombinase binL) as well as β-lactamase (blaZ) and two regulatory genes (blaI and blaR) (SEED subsystem notes). Gene-specific examination reveals this enrichment is specifically due to the Firmicute-associated β-lactamase gene (E.C. 3.5.2.6) in DonnaII. β-lactamase confers antibiotic resistance against β-lactam antibiotics, including penicillin [50]. The DonnaII bioreactor has never been exposed to antibiotics, so this resistance likely stems from the DonnaII bioreactor’s origin, the Ithaca wastewater treatment plant. Examination of the DonnaII sequences assigned to this subsystem reveals that the reads assigned to transposon-associated genes are 700x less abundant than reads assigned to the β-lactamase gene. From the assembled metagenome data, the identified β-lactamase genes are not associated with annotated transposases, either on the same contig or within the same scaffold, indicating the β-lactamase may not be associated with a transposon, and hence may not be mobile.
Figure 4.
Three-way comparisons of metabolic differences within the enrichment culture metagenomes with all Dhc reads removed. Points are displayed as in Figure 3, with points here corresponding to MG-RAST assignments to categories of metabolic classification within The SEED database. The closer to a corner of the plot a point falls, the more highly proportionally enriched that category is within the metagenome affiliated with that corner (arrows). Points located in the center of the plot are not enriched in one metagenome compared to the others. Coloration and shapes are as in Figure 3. Labeled points correspond to all categories for which one metagenome was significantly enriched above the other two. For the metabolic level 1 class, the threshold for plotting was increased to 1% to reflect the lower number of possible categories.
The other two significantly enriched subsystems in metabolism were overrepresented in KB-1 (Figure 4, #10 & 11). The enrichment in the reductive dehalogenation subsystem in the KB-1 consortium stems from reads identified by MG-RAST as homologs to the Geobacter (class ∂-Proteobacteria) reductive dehalogenases. The Geobacter in KB-1 is known to be capable of reductively dechlorinating PCE to cDCE [5]. Examination of the non-Dhc rdh genes detected in the three metagenomes indicated that there are no non-Dhc rdh genes within DonnaII, while the ANAS metagenome had some reads most similar to Shewanella and Desulfitobacterium-type rdh genes, indicating there may be one or more non-Dhc dechlorinating organisms within that culture which have not previously been detected [19,21], or that the Dhc strains within the ANAS consortium encode rdh genes that were potentially acquired through lateral gene transfer. The second subsystem enriched in the KB-1 culture is the Ton and Tol transport subsystem (Figure 4, #11). The enrichment is largely within genes in the Ton pathway for iron transport (The SEED subsystem notes, [51]), specifically within the ∂-Proteobacteria, which for KB-1 corresponds to a Geobacter species. This enrichment may be linked to Geobacter’s requirement for corrinoids for active reductive dechlorination, as TonB has been shown to complex with the cobalamin transporter BtuB [52]. Examination of the cobalamin synthesis pathway (described below, Figure 5) suggests Geobacter, as the dominant ∂-Proteobacterium in KB-1, is potentially capable of de novo cobalamin synthesis, but a TonB/BtuB complex may be an additional avenue for cobalamin acquisition.
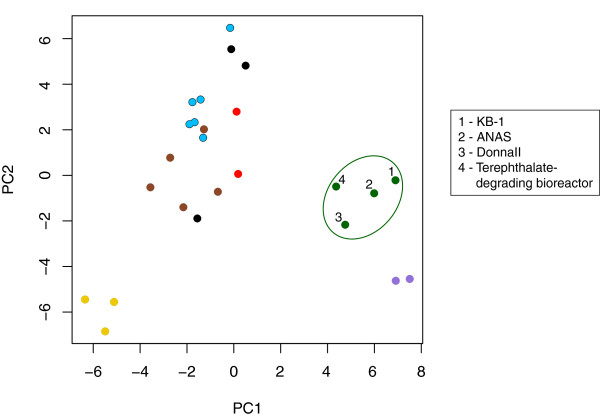
Figure 5.
Principal component analysis of 25 metagenomes based on frequencies of COG categories. COG frequencies were normalized to metagenome size. Points are colored by sample type: green = contaminant-degrading microbial consortia, black = waste water/sludge samples, light blue = pristine groundwater and sediment sites, brown = soil samples, yellow = Hawaii Ocean Time Series samples, red = ammonia-oxidizing communities, purple = non-contaminant degrading microbial consortia. See Additional file 1: Table S9 for a full list of metagenomes used. All samples are publically available from the JGI IMG-M site (merced.jgi-psf.org/cgi-bin/mer/main.cgi).
In summary, the broad-scale comparison of the three metagenome sequences identified that the three enrichment consortia metagenomes differ more based on taxonomy (Figure 3, Level 5) than they do based on the proportions of the metabolic pathways encoded (Figure 4, Level 1). The identified variance in metabolic pathways was confined to either a highly specific function, as in reductive dechlorination by a non-Dhc organism in KB-1, or to specific resistance or mobile element markers, as in the tn552 transposon in DonnaII. Thus at a broad functional level, the communities are remarkably similar. In order to examine whether this similarity was specific to dechlorinating enrichment cultures, or merely the result of a comparison of mixed anaerobic samples, we compared the proportional distribution of genes within COG categories among 25 anaerobic mixed microbial community metagenomes using principal components analysis (Figure 5). This analysis also indicated that the three dechlorinating enrichment cultures share a higher level of similarity to each other, on a functional level, than to other, non-bioremediation-related enrichment cultures or anaerobic environmental samples.
Examination of pathways mediating ecological interactions with Dehalococcoides
From information available from published Dehalococcoides genome sequences [2,7,8], a metabolic flux model of the group’s metabolism [12], and culture-based observations [18], several limitations in Dhc growth and metabolism have been identified. Specifically, the Dhc are incapable of de novo corrinoid synthesis, are obligately anaerobic and highly susceptible to oxygen, and are not able to transform electron donor substrates amended to mixed cultures into the H2 required for energy. In addition, the methionine synthesis pathway in Dhc has not been identified, though Dhc are not methionine auxotrophs [53]. We conducted in-depth examinations of selected metabolic pathways to determine how the mixed consortia may address these deficits and promote Dhc growth. Each pathway of interest was defined through literature searches and the SEED database, and genes pertinent to the processes mined from the MG-RAST annotated metagenomes. Annotations were kept at the phylum or class level to minimize database bias, and gene frequencies were recorded. The numeric data presented in the pathway-specific discussions is primarily from the unassembled datasets, as the assembled datasets corroborated the results from the unassembled data. Complete lists of the specific genes examined for each pathway of interest, as well as the exact gene counts for each phylum within each metagenome for both the unassembled and assembled datasets are available in the Additional file 2.
Fermentation of electron donors to hydrogen
A significant difference among the three enrichment cultures examined is the substrate amended to the culture to serve as an electron donor (i.e., source of hydrogen) for the dechlorination reaction. In the above broad-level analysis of the metagenomes’ metabolic capacity, none of the pathways for degradation of the electron donors were proportionally enriched in a given metagenome. In a more detailed examination of the genes annotated by MG-RAST, we found that, rather than an increased proportion of the specific degradation pathway for a donor within a metagenome, there is instead a trend for increased diversity of organisms present in the culture capable of utilizing the specific donor (Table 3). We examined the presence and taxonomic attribution of the enzymes responsible for the initial steps of electron donor metabolism to identify the organisms putatively capable of the direct utilization of the three electron donors. We typically did not examine the entire pathway: once the product was a common compound in many metabolic pathways (e.g., pyruvate, acetoacetate), the organisms identified as utilizing this metabolite were not considered informative to our question.
Table 3.
Presence of metabolic pathways for the utilization of the electron donor substrates amended to the three enrichment cultures as detected using MG-RAST annotations
| |
# Pathways complete/partial* |
Taxonomic Classification of Pathways |
||||
|---|---|---|---|---|---|---|
| DonnaII | ANAS | KB-1 | DonnaII | ANAS | KB-1 | |
|
LACTATE (ANAS) |
|
|
|
|
|
|
| A - Lactate to Acetate via Pyruvate (3 genes) |
2/2 |
1/6 |
0/1 |
α-P, Firm/Act, BC |
Firm/α-P, BC, β-P, Cya, γ-P, Planc |
/γ-P |
| B – Lactate to Ethanol (4 genes) |
2/2 |
1/5 |
0/1 |
α-P, Firm/Act, BC |
Firm/α-P, BC, β-P, Fib, γ-P |
/Fib |
| C - L-lactate to Acetate directly (EC 1.13.12.4) |
0 |
0 |
0 |
|
|
|
|
BUTYRATE (DonnaII) |
|
|
|
|
|
|
| To Acetyl-CoA and Acetoacetate (5–7 genes) |
4/8 |
2/6 |
1/2 |
δ-P, Firm, γ-P, β-P/Act, α-P, BC, Cflx, CrenA, EurA, Fib, Fus |
δ-P, Firm/Act, α-P, β-P, EurA, Fus, γ-P |
Firm/BC, δ-P |
|
METHANOL (KB-1) |
|
|
|
|
|
|
| A - to Formaldehyde (ECs 1.1.99.8/1.11.1.6/1.11.1.7) |
9 |
6 |
6 |
Act, BC, β-P, DT, δ-P, ϵ-P, EurA, Firm, γ-P |
BC, β-P, δ-P, EurA, Firm, γ-P |
BC, β-P, δ-P, EurA, Firm, γ-P |
| B - to Methyl-CoM, eventually to coenzyme M (4 genes) | 1 | 1 | 1 | EurA | EurA | EurA |
Abbreviations are as follows: Act = Actinobacteria, α/β/δ/ϵ/γ-P = Proteobacteria, BC = Bacteroidetes/Chlorobi group, Cya = Cyanobacteria, Cflx = Chloroflexi, CrenA = Crenarchaeota, Dhc = Dehalococcoides, DT = Deinococcus/Thermus group, EurA = Euryarchaeota, Fib = Fibrobacteres/Acidobacteria, Firm = Firmicutes, Fus = Fusobacteria, Planc = Planctomycetes, Therm = Thermotogae.
*partial – 40% or greater of pathway must be detected, for lactate to acetate conversions, initial step was required to be present.
ANAS is amended lactate as an electron donor, which can be metabolized via pyruvate to acetyl-CoA. From acetyl-CoA, either acetate and hydrogen or ethanol can be formed (KEGG pathway: pyruvate metabolism, Additional file 2: Table S3). Another alternative is for lactate to be metabolized through lactaldehyde to propionate. The SEED database does not contain the NADH 1,2-propanediol oxidoreductase required for propionate production. BLAST-based searches of the assembled datasets for this gene indicate it is not present in any of the three metagenomes. This is reasonable, as under low hydrogen partial pressures, and in the presence of methanogens, the energetically favored pathway is via lactate dehydrogenase to pyruvate with acetate as an end product [54], which is what has been predicted for the ANAS culture [55]. ANAS contains a higher number of taxa encoding this pathway compared to the other two metagenomes (Table 3). DonnaII, by comparison, utilizes butyrate as an electron donor, which is ultimately converted to acetyl-CoA and acetoacetate and hydrogen (KEGG pathway: butanoate metabolism, Additional file 2: Table S4). The observed trend of organismal diversity exists for butyrate degradation as well, with DonnaII having more hits to distinct phyla associated with this process. KB-1 is provided methanol as an electron donor, which can be transformed to formaldehyde by acetogens or to methyl-coenzyme M en-route to methane formation by methanogens (KEGG pathway: methane metabolism, Additional file 2: Table S5). Here, the number of taxonomic groups with complete methanol degradation pathways is highest for DonnaII, with ANAS and KB-1 exhibiting the same number of groups. The electron donor for each enrichment culture was originally chosen based on comparisons of several different donors, which resulted in three different substrates being independently chosen [21,26,56]. Interestingly, this analysis predicts that each metagenome still encodes the capacity to utilize any of the electron donors examined here, despite years of exposure to only one of the three substrates. This is valuable information, as a culture’s ability to metabolize multiple electron donors increases its flexibility as a bioremediation tool, though this inferred function would need to be verified prior to implementing a change in electron donor during bioremediation.
The required electron donor for Dhc reductive dechlorination is H2, making it the central intermediate for dechlorination activity in the three consortia. Synthesis of H2 is primarily catalyzed by hydrogenases, a family of enzymes which can reversibly convert hydrogen cations to H2[57,58]. The three enrichment consortium metagenomes encode a wide diversity of hydrogenases, including nickel-only, nickel-iron, and iron-only hydrogenases, primarily identified in Dhc, the Firmicutes, Euryarchaeota, and the ∂-Proteobacteria (see Additional file 1: Table S2 for a more detailed analysis of the hydrogenase family). The identified hydrogenases did not show specific taxonomic trends or enrichment in any of the three metagenomes.
Corrinoid cofactor synthesis
Dhc’s inability to synthesize corrinoid cofactors de novo for reductive dehalogenases required for energy generation means that these cofactors must be scavenged from the environment for Dhc growth [2,12]. In laboratory cultures, cobalamin is typically amended to the media in the form of vitamin B12 (cyanocobalamin) (see Table 1), but in the natural environment, organisms coexisting with Dhc must synthesize it.
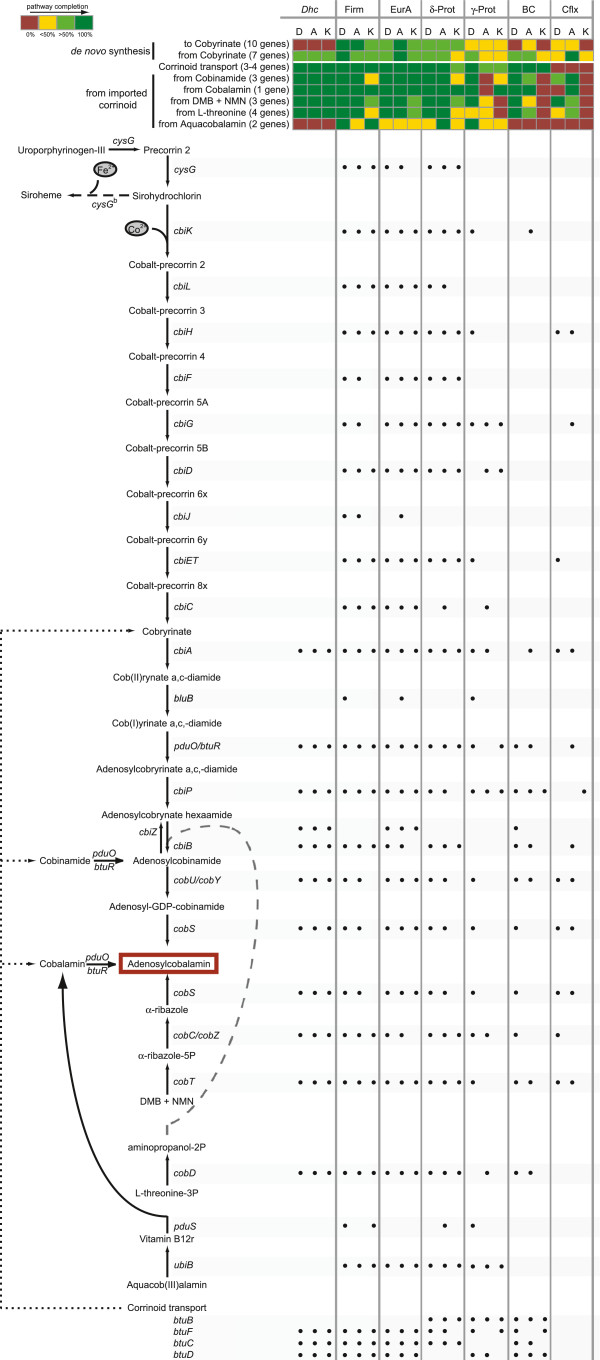
An examination of the prokaryotic corrinoid synthesis pathway [59] within the three metagenomes demonstrated the absence of a complete de novo synthesis pathway in the strains of Dhc contained in these cultures. The beginning of the anaerobic corrinoid synthesis pathway, from uroporphyrinogen-III or siroheme through to cobyrinate (10 genes of a total of 17) is categorically absent from all sequenced Dhc genomes, including strain 195 from the DonnaII consortium [8,12]. An in-house draft genome of the Dehalococcoides in KB-1 also lacks homologs to this portion of the corrinoid synthesis pathway. In addition, all isolated Dehalococcoides strains require the addition of cobalamin for growth, including strain 195, two strains from ANAS, and an in-house isolate from KB-1 [14][13]. In contrast, the genes required for synthesis of adenosylcobalamin from partially synthesized corrin-containing products including cobyrinate, cobinamide, 5,6-dimethylbenzimidazole, and cobalamin are all present within Dhc in these metagenomes and sequenced genomes, as are the genes for import of these corrin-containing precursors into the cell (Figure 6, Dhc column). An exception to this is aquacobalamin, which does not appear to be utilized as a precursor by Dhc. This indicates that while Dhc are typically incapable of de novo synthesis of a corrinoid [12], they encode multiple options for import and subsequent adaptation of varied cobalamin molecules, suggesting Dhc are highly versatile in their ability to scavenge corrinoids from an environment and repurpose them for insertion in a reductive dehalogenase enzyme.
Figure 6.
The anaerobic cobalamin synthesis pathway with gene distributions within the three metagenomes by taxonomic classification. The heat map depicts presence/absence of cobalamin synthesis pathways where red = genes absent; yellow = partial pathway, less than 50% of genes detected; light green = partial pathway, greater than 50% of genes detected; and dark green = complete pathway detected. Black circles indicate the specific genes detected for each microbial group. Abbreviations: DMB = 5,6-dimethylbenzimidazole, NMN = nicotinamide mononucleotide, Dhc = Dehalococcoides, Firm = Firmicutes, EurA = Euryarchaeota, δ/γ-Prot = Proteobacteria, BC = Bacteroidetes/Chlorobi group, Cflx = Chloroflexi.
An examination of cobalamin synthesis in the non-dechlorinating members of the enrichment cultures yielded no evidence of the aerobic corrinoid synthesis pathway, which involves a later addition of the cobalt cation compared to the anaerobic synthesis pathway [59]. The complete de novo cobalamin synthesis pathway (all 17 genes) was detected in the Euryarchaeota in DonnaII and ANAS, and the Firmicutes in ANAS (Figure 6, bottom portion, see Additional file 2: Table S6 for more detail). KB-1 showed near-complete representation of the pathway for the Euryarchaeota (13 of 17 genes detected) and both DonnaII and KB-1 exhibited near-completion of the pathway in the Firmicutes (>50% of genes detected, Figure 6). The only other phylogenetic group with a significant proportion of the pathway detected was the ∂-Proteobacteria, (14, 15, and 10 of 17 genes detected for DonnaII, ANAS, and KB-1 respectively, Figure 6). The incomplete detection of this pathway in the ∂-Proteobacteria is likely the result of sampling effects, as the ∂-Proteobacteria represent a smaller proportion of each of the metagenomes compared to the Firmicutes and Euryarchaeota. Further arguments in favour of a complete corrinoid synthesis pathway within the ∂-Proteobacteria in these enrichment consortia include the uneven distribution of the “absent” genes, which argues against a possible hand-off of a partial product for completion in a different organism; there is no specific break point in the detected pathway in the ∂-Proteobacteria as there is for Dehalococcoides. In addition, in contrast to sequenced Dehalococcoides genomes, which do not encode the upper portion of the corrinoid synthesis pathway, typically complete genomes of ∂-Proteobacteria (e.g., the Geobacteraceae) do encode a complete cobalamin synthesis pathway [60,61]. It is possible the pathway is indeed incomplete within the ∂-Proteobacteria present in these enrichment cultures, but the available evidence does not favour this hypothesis. The heatmap in Figure 6 depicts the extent of the corrinoid synthesis pathway in non-dechlorinating members, either de novo or from various precursor products. Aside from synthesis, import and conversion of corrin-containing molecules functional genes are also primarily found in the Euryarchaeota, the Firmicutes, and the ∂-Proteobacteria.
Methionine synthesis
Despite published experimental evidence for methionine synthesis in Dhc[53], no known methionine synthesis or uptake genes have been identified in Dhc genomes [2,12]. Dhc are capable of methionine uptake from the environment [62], and hence may take advantage of exogenous methionine produced by non-dechlorinating organisms. The organisms present in the enrichment cultures predicted to contain the various methionine synthesis pathways are summarized in Table 4 (see Additional file 2: Table S7 for more detail). In general, methionine synthesis is predominantly encoded by the Firmicutes in all three metagenomes, despite the enrichment cultures containing different Firmicute genera.
Table 4.
Presence of methionine biosynthesis pathways in the three enrichment cultures as detected using MG-RAST annotations
| |
# Pathways complete/partial* |
Taxonomic Classification of Pathways |
||||
|---|---|---|---|---|---|---|
| Pathway | DonnaII | ANAS | KB-1 | DonnaII | ANAS | KB-1 |
|
Methionine synthesis | ||||||
| Methylation pathway (from methylene-tetrahydrofolate and L-homocysteine) |
2 |
3 |
3 |
Firm, γ-P |
δ-P, Firm, γ-P |
BC, δ-P, Firm |
| betaine-homocysteine S-methyltransferase (BhmT) |
0 |
0 |
0 |
|
|
|
|
Uptake of Methionine | ||||||
| Methionine transporter MetT |
5 |
3 |
2 |
δ-P, Firm, Fuso, γ-P, Spiro |
δ-P, Firm, Fuso |
BC, γ-P |
| ABC Met transporter (3 components) | 5/2 | 4/2 | 0/1 | α-P, β-P, DT, ϵ-P, Firm/Act, γ-P | α-P, ϵ-P, Firm, γ-P/Act, β-P | None/Firm |
A full diagram of this system is available through the SEED viewer at ( http://seed-viewer.theseed.org/seedviewer.cgi). Taxonomic abbreviations are as in Table 3.
*partial – 40% or greater of pathway must be detected.
Based on genome and metagenome sequence data, the enzymes Dhc are utilizing to generate or acquire methionine are divergent from any currently known methionine synthesis enzymes. Identification of the Dhc genes active in methionine synthesis and transport will require targeted experiments examining transcriptomics or protein expression profiles under growth conditions lacking methionine and/or in co-cultures with the Firmicute genera highlighted here as putative methionine producers.
Oxygen tolerance and scavenging
The maintenance of an anaerobic environment is critical for continued growth of Dhc, of which all known strains are obligate anaerobes. The presence of oxygen leads to a complete loss of reductive dechlorination activity, which is typically irreversible in pure cultures [14,18]. Based on the SEED annotations for the three metagenomes, the Dhc strains present contain two mechanisms for scavenging oxygen free-radicals: an [Mn]-superoxide dismutase (SOD) and the ruberythrin/rubredoxin putative scavenging system (Table 5, Additional file 2: Table S8). The Dhc sequenced genomes do not encode any identified mechanism for the direct utilization of oxygen.
Table 5.
Presence of oxygen-scavenging mechanisms within the three enrichment cultures
| |
Presence |
Taxonomic Classification |
||||
|---|---|---|---|---|---|---|
| D | A | K | DonnaII | ANAS | KB-1 | |
|
Direct O2removal |
|
|
|
|
|
|
| Glycolate oxidase |
- |
+ |
- |
|
Cya |
|
| Cytochrome c oxidase |
+ |
+ |
+ |
Act, β-P*, Cya*, δ-P*, ϵ-P*, Firm* |
γ-P* |
δ-P, ϵ-P* |
| Cytochrome d ubiquinol oxidase |
+ |
+ |
+ |
Act*, BC, δ-P*, ϵ-P*, Firm |
BC, δ-P |
δ-P, Firm |
| Ferroxidase |
+ |
- |
- |
Firm |
|
|
|
Radical O2species |
|
|
|
|
|
|
| Catalase |
+ |
+ |
+ |
Act, BC, β-P, DT, δ -P, ϵ-P, EurA, Firm, γ-P |
BC, β-P, δ-P, EurA, Firm, γ-P |
BC, β-P, δ-P, Eury, Firm, γ-P |
| Peroxidase |
+ |
+ |
+ |
β-P, δ-P |
δ-P, Firm, γ-P |
γ-P |
| Cytochrome c551 peroxidase |
+ |
+ |
- |
ϵ-P, γ-P |
ϵ-P, γ-P |
|
| Glutathione peroxidase |
+ |
+ |
+ |
α-P, Firm |
Firm |
Cya, Firm, Spiro |
| [Cu-Zn]-SOD |
+ |
+ |
- |
BC, Firm, |
α-P |
|
| [Mn]-SOD |
+ |
+ |
+ |
α-P, β-P, Cya, DT, Dhc, Firm, γ-P, Therm |
Dhc, Firm, γ-P, Therm |
Dhc, EurA, γ-P |
| [Fe]-SOD |
+ |
+ |
+ |
α-P, BC, β-P, Cya, δ-P, γ-P |
β-P |
β-P, δ-P |
| [Mn/Fe]-SOD |
+ |
+ |
- |
EurA |
EurA |
|
| Superoxide reductase |
+ |
+ |
+ |
δ-P, EurA, Firm, Therm |
δ-P, EurA, Firm |
δ-P, Firm |
|
Putative scavenging systems |
|
|
|
|
|
|
| Ruberythrin |
+ |
+ |
+ |
BC, Dhc, δ-P, ϵ-P, EurA, Firm |
BC, Dhc, δ-P, EurA, Firm |
BC, Dhc, δ-P, EurA, Firm |
| Rubredoxin |
+ |
+ |
+ |
Dhc, ϵ-P, EurA, Firm, γ-P |
Dhc, δ-P. EurA, Firm |
Dhc, EurA |
| Rubredoxin-oxygen oxidoreductase | + | + | + | δ-P, EurA, Firm | δ-P, EurA, Firm | δ-P, EurA, Firm |
Taxonomic abbreviations are as in Table 3.
* = partial representation of a multi-subunit enzyme complex from MG-RAST annotation.
The ability of the mixed cultures to mitigate oxygen free radical damage is significantly more robust than that of isolate Dhc strains in vivo. In support of this, each metagenome had evidence for at least 2 oxidases, a catalase, peroxidase, multiple kinds of SODs, superoxide reductase, and multiple ruberythrin/rubredoxin scavenging systems in multiple phylogenetic groups (Table 5, Additional file 2: Table S8). The main organisms encoding these oxygen scavenging systems are the ∂-Proteobacteria, the Firmicutes, and the Euryarchaeota, with Actinobacteria contributing in DonnaII as well. From this, it seems reasonable to conclude that mixed enrichment cultures are more robust to exposure to oxygen because they encode many more enzymes for the complete removal of oxygen and damaging free radical species. Even if only a fraction of these genes are expressed as active proteins, it would still represent a substantial increase in oxygen scavenging mechanisms at work compared to pure Dhc isolate cultures.
Conclusions
Examination of the metabolic and phylogenetic differences among three dechlorinating enrichment cultures revealed that, despite substantial statistically significant differences in phylogenetic groups present, the enrichment cultures show a highly conserved relative abundance of different metabolic pathways. The statistically significant metabolic differences among the three cultures’ non-Dhc populations were restricted to small differences in genes encoding reductive dechlorination and, as seen in many comparative metagenomic studies, the mobile element-associated genes [42,44,63]. In addition, a comparison of the metabolic signatures in the metagenomes of a wide variety of anaerobic microbial consortia confirmed that this conserved metabolic profile in the contaminant-degrading consortia is distinct from those found in non-contaminant-degrading anaerobic microbial communities (Figure 5).
In particular, the metabolic functions important in supporting Dhc growth were primarily encoded by the Firmicutes, the Euryarchaeota, and the ∂-Proteobacteria, with different genera of each enriched in the three consortia (see Figure 2 for an overview of all of the systems examined here). Our results corroborate earlier enrichment culture comparison hypotheses that a diversity of genera can inhabit overlapping functional niches [25,64].
We postulate that these taxonomic groups are highlighted in the examined pathways because cultures containing them have been best able to support Dhc growth; laboratory enrichment for Dhc activity over time has required the parallel enrichment of these organisms. This analysis identifies a new importance for the Euryarchaeota in these enrichment cultures: while the Euryarchaeota are never the sole predicted source of a metabolite, it is clear from these examinations that they encode pathways predicted to provide essential nutrients, including corrinoids, to the Dhc. The euryarchaeotal contributions to Dhc growth may mitigate or even outweigh their role as H2 competitors with the Dhc, particularly when excess electron donor is present, allowing them to remain abundant in mixed cultures without adverse effects on Dhc growth and dechlorination. The pathway-specific examinations demonstrated that the primary communities maintained in these enrichment consortia metabolize methanol, lactate, or butyrate such that a niche for Dhc growth is created. Notably, each enrichment consortium is maintained on an electron donor chosen for culture performance [21,26,56], which has seemingly maintained increased organismal diversity related to donor substrate metabolism in at least two of the three cultures.
In all of the above examinations, it must be reiterated that the identified functions and phylogenetic assignments are predicated on the presence of homologs within the database utilized, and represent predicted functions only. The absence of specific genes on organisms’ genomes cannot be separated from missing data given the unsaturated status of the metagenome sequences. Lateral gene transfers will occlude correct taxonomic identifications. Given the lack of sequence saturation, it is also possible further sequencing of these communities could alter the enrichments identified here. As this analysis relies solely on genomic material, the active function of the pathways examined cannot be confirmed without further proteomic or expression data. Instead, what we have presented here represents a preliminary identification of potential organisms, genes, and pathways of interest within dechlorinating microbial consortia that may serve as guidance for subsequent targeted studies.
Each of these enrichment consortia represents a robust ecosystem allowing an examination of the global halogen cycle, where dechlorination is maintained by the collective activities of the microbial community. The metabolic differences between the enrichment consortia are subtle, which was unexpected given the consortia originated from three disparate environments and have been maintained under different conditions for many years. Taken together, the observations described here illustrate the importance of functional redundancy within dechlorinating enrichment cultures.
Methods
Culture and metagenome information
The KB-1 consortium was maintained in batch culture in defined mineral medium [65] with trichloroethene (TCE) as electron acceptor and methanol as electron donor. The culture was routinely allowed to dechlorinate TCE completely to ethene prior to a new amendment of acceptor/donor approximately every two weeks. The DonnaII reactor and ANAS semi-batch reactor were maintained as described previously [19,20]. See Table 1 for a summary comparison of maintenance conditions, and Table 2 for metagenome sizes and modes of sequencing used.
For the KB-1 metagenome, DNA was extracted from the T3 MP1 KB-1 culture just after completion of a dechlorination cycle using a Cetyl trimethylammonium bromide (CTAB) protocol [66] with volumes scaled up for higher yield as described in the alternate protocol, omitting subsequent cesium chloride gradient centrifugation steps. Clone libraries with 35-kb and 3-kb inserts were created by the JGI using their in-house protocols ( http://www.jgi.doe.gov/sequencing/protocols), and an additional 3 kb short insert library generated for construction of a shotgun metagenome microarray was constructed by Genome Atlantic. A total of 103 MB of metagenome sequence was generated on AB13730xl Sanger sequencers from the three clone libraries. The metagenome was assembled using the JGI’s in-house bacterial assembly pipeline, utilizing lucy [67] for vector and quality screening. The KB-1 metagenome sequence and assembly were made publically available by the JGI on May 2nd, 2009 ( http://genome.jgi-psf.org/aqukb/aqukb.download.ftp.html). Community composition of the three KB-1 DNA samples utilized for sequencing the KB-1 metagenome was determined by Dr. Alison S. Waller using qPCR [30].
The DonnaII metagenome was generated from 454 Titanium libraries according to the JGI’s in-house protocols ( http://www.jgi.doe.gov/sequencing/protocols). Metagenome assembly was conducted using the Newbler program from Roche [68]. The sequence data, including an in-house assembly draft for the DonnaII metagenome was made publically available by the JGI on January 31st, 2012 (IMG-M taxon ID: 2032320001 ( http://img.jgi.doe.gov/cgi-bin/m/main.cgi)).
The ANAS metagenome was composed of a combination of Sanger sequencing and Titanium 454 sequencing as described above. The sequence data, including an in-house assembly draft for the ANAS metagenome was made publically available by the JGI on August 20th, 2009 (IMG-M taxon ID: 2014730001 ( http://img.jgi.doe.gov/cgi-bin/m/main.cgi)).
Comparative metagenome analysis
The raw reads from all three metagenomes as well as the assembled datasets were submitted to the MG-RAST server [33] for automated annotation utilizing the SEED database [34]. All phylogenetic and metabolic profiles discussed herein were generated utilizing a maximum e-value of 1x10-5 and a minimum alignment length match of 100 required, criteria designed to reduce noise and poorly supported assignments, but to still allow imperfect matches between sequences and novel homologs. A global criterion is not ideal for preventing any erroneous annotations, but is required when working with datasets of this size. Vector sequences were identified and removed from the analysis (see Additional file 1: materials and methods). In order to facilitate certain comparisons of the non-Dhc communities, all reads assigned to Dhc under MG-RAST’s phylogenetic profiles for the SEED, Silva LSU, Silva SSU, RDP, and Greengenes databases were removed from the datasets, and a second automated annotation conducted for the “Dhc-subtracted” metagenomes.
The assembled datasets were input to the RITA pipeline [48] to generate a second assessment of community composition. RITA combines homology-based predictions with the Naïve Bayes approach to compositional classification used in [49] to generate predictions with increased precision relative to a one-step BLASTN search performed by MG-RAST. The RITA pipeline was used to assign taxonomy to fragments using a reference database of 1479 genomes using the following matching protocols: (i) agreement between the best match with UBLASTX (maximum e-value = 10-15) [69] and the Naïve Bayes classification of a fragment; (ii) a difference of at least ten orders of magnitude between the e-value of the best match and that of the second-best matching group; (iii)-(iv) same as (i)-(ii), but using BLASTN instead of UBLASTX; and (v) matches based on the Naïve Bayes prediction alone. Fragments assigned to set (v) have a much lower confidence than sets (i)-(iv) which are based on homology.
Three-way comparisons were conducted among the metagenomes, adapting the method described by Tringe et al.[45] for MG-RAST output files. In brief, subsystem counts (for metabolic analyses) or taxonomic counts (for a chosen phylogenetic class analysis) were converted to a proportional count for each metagenome. Pseudocounts proportional to the size of each dataset were added to prevent selection for rare categories [45]. For each category, the relative proportions for the three metagenomes were normalized to one. The data were displayed as a barycentric plot, allowing 3-dimensional data to be displayed in 2 dimensions utilizing scripts developed by Tringe et al.[45]. Trends seen in this three-way comparison test were confirmed by the pair-wise statistical testing in STAMP with a p-value of 0.05 (full statistical methods available in the Additional file 1: methods), allowing the biological effect size filtering to be indirectly applied to observed three-way differences.
Specific pathway comparisons
For each of the identified pathways or functions of interest, a literature search was conducted to determine all enzymes associated with the system. The SEED database was searched for the subsystem location(s) of the identified genes using enzyme commission (EC) numbers where available, and enzyme names or synonyms as listed in BRENDA ( http://www.brenda-enzymes.org) where EC numbers were not available or omitted in the SEED. The complete tabular data for each of subsystems on the resultant list were exported from MG-RAST for each of the three metagenomes.
The SEED phylogenetic identifiers associated with each sequence read were converted from the SEED-specific nomenclature to the NCBI taxonomic identifier, and a phylum-level taxonomic assignment was added. Dhc was designated as a phylum-level classification to distinguish between reads assigned to Dhc and reads assigned to other Chloroflexi. The presence and proportion of the genes of interest across phylogenetic groups were examined (summary tables available in Additional file 2).
Abbreviations
PCE: Tetrachloroethene; TCE: Trichloroethene; DCE: Dichloroethene; VC: Vinyl chloride; SOD: Superoxide dismutase.
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
LAH conceived of the experiments, carried out the analyses, and drafted the manuscript. RGB and ARR assisted with the analyses and helped to draft the manuscript. RER and EAE participated in the design of the experiments, and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Contains supplemental methods as referenced in the main text, and a discussion of the hydrogenase distribution within the enrichment consortia, including supplemental tables S1, S2, and S9 [58,70].
Contains supplemental tables S3-S8; detailed tables of the pathways discussed in the manuscript, including all genes implicated and the number of assigned reads to each gene from each phylum examined. Presented as a single MS Excel workbook, with each sheet corresponding to a different pathway of interest. Each enzyme examined within a pathway is listed in the left-most column, with number of assigned reads from MG-RAST for each phylum within each metagenome listed. Metagenomes are indicated as follows; D = DonnaII, A = ANAS, K = KB-1. Enzymes found within the metagenomes are highlighted in green, while enzymes absent from specific phyla are highlighted in red. Enzymes of interest may be absent either because they are not present in the SEED database (denoted with (NOT FOUND) in the enzyme name), or were not detected in the metagenome datasets.
Contributor Information
Laura A Hug, Email: laura.hug@utoronto.ca.
Robert G Beiko, Email: beiko@cs.dal.ca.
Annette R Rowe, Email: arr36@cornell.edu.
Ruth E Richardson, Email: rer26@cornell.edu.
Elizabeth A Edwards, Email: elizabeth.edwards@utoronto.ca.
Acknowledgments
We would like to thank Mr. Donovan Parks (Dalhousie University) for an introduction to the STAMP program, and Dr. Radhakrishnan Mahadevan (University of Toronto) for critical discussions. Metagenome sequencing for KB-1, DonnaII, and ANAS was conducted by the United States Department of Energy Joint Genome Institute (JGI) community sequencing program. RGB acknowledges the support of Genome Atlantic and the Canada Research Chairs program. LAH was supported by a University of Toronto Fellowship. The authors acknowledge support from the Government of Canada through the Genome Canada and the Ontario Genomics Institute (2009-OGI-ABC-1405) and the United States Department of Defense Strategic Environmental Research and Development Program (SERDP) project ER-1586.
Author details
1Department of Cell and Systems Biology, University of Toronto, Toronto, Canada. 2Faculty of Computer Science, Dalhousie University, Halifax, NS, Canada. 3Civil & Environmental Engineering, Cornell University, Ithaca, NY, USA. 4Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, ON, Canada.
References
- Duhamel M, Mo K, Edwards EA. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl Environ Microbiol. 2004;70:5538–5545. doi: 10.1128/AEM.70.9.5538-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol. 2005;23:1269–1273. doi: 10.1038/nbt1131. [DOI] [PubMed] [Google Scholar]
- Grostern A, Edwards EA. Growth of Dehalobacter and Dehalococcoides spp. during degradation of chlorinated ethanes. Appl Environ Microbiol. 2006;72:428–436. doi: 10.1128/AEM.72.1.428-436.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian L, Dudkova V, Demnerova K, Bedard DL. "Dehalococcoides" sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture aroclor 1260. Appl Environ Microbiol. 2009;75:4516–4524. doi: 10.1128/AEM.00102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel M, Edwards EA. Growth and yields of dechlorinators, acetogens, and methanogens during reductive dechlorination of chlorinated ethenes and dihaloelimination of 1,2-dichloroethane. Environ Sci Technol. 2007;41:2303–2310. doi: 10.1021/es062010r. [DOI] [PubMed] [Google Scholar]
- Major DW, McMaster ML, Cox EE, Edwards EA, Dworatzek SM, Hendrickson ER, Starr MG, Payne JA, Buonamici LW. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ Sci Technol. 2002;36:5106–5116. doi: 10.1021/es0255711. [DOI] [PubMed] [Google Scholar]
- Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe BA, Ward NL, Nelson WC, Deboy RT, Khouri HM. et al. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science. 2005;307:105–108. doi: 10.1126/science.1102226. [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, Behrens SF, Muller JA, Goke J, Ritalahti KM, Wagner R, Goltsman E, Lapidus A, Holmes S, Löffler FE, Spormann AM. Localized plasticity in the streamlined genomes of vinyl chloride respiring Dehalococcoides. PLoS Genet. 2009;5:e1000714. doi: 10.1371/journal.pgen.1000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JA, Rösner BM, Von Abendroth G, Meshulam-Simon G, McCarty PL, Spormann AM. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Appl Environ Microbiol. 2004;70:4880–4888. doi: 10.1128/AEM.70.8.4880-4888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard J, Schumacher W, Vazquez F, Regeard C, Hagen WR, Holliger C. Characterization of the corrinoid iron-sulfur protein tetrachloroethene reductive dehalogenase of Dehalobacter restrictus. Appl Environ Microbiol. 2003;69:4628–4638. doi: 10.1128/AEM.69.8.4628-4638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian L, Rahnenfuhrer J, Gobom J, Hölscher T. Identification of a chlorobenzene reductive dehalogenase in Dehalococcoides sp. strain CBDB1. Appl Environ Microbiol. 2007;73:7717–7724. doi: 10.1128/AEM.01649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsanul Islam M, Edwards EA, Mahadevan R. Characterizing the metabolism of Dehalococcoides with a constraint-based model. PLoS Comput Biol. 2010;6(8):e1000887. doi: 10.1371/journal.pcbi.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PK, Cheng D, Hu P, West KA, Dick GJ, Brodie EL, Andersen GL, Zinder SH, He J, Alvarez-Cohen L. Comparative genomics of two newly isolated Dehalococcoides strains and an enrichment using a genus microarray. ISME J. 2011;5:1014–1024. doi: 10.1038/ismej.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maymó-Gatell X, Chien Y, Gossett JM, Zinder SH. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- He J, Sung Y, Krajmalnik-Brown R, Ritalahti KM, Löffler FE. Isolation and characterization of Dehalococcoides sp strain FL2, a trichloroethene (TCE)- and 1,2-dichloroethene-respiring anaerobe. Environ Microbiol. 2005;7:1442–1450. doi: 10.1111/j.1462-2920.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- He J, Holmes VF, Lee PK, Alvarez-Cohen L. Influence of vitamin B12 and cocultures on the growth of Dehalococcoides isolates in defined medium. Appl Environ Microbiol. 2007;73:2847–2853. doi: 10.1128/AEM.02574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, He J. Isolation and characterization of "Dehalococcoides" sp. strain MB, which dechlorinates tetrachloroethene to trans-1,2-dichloroethene. Appl Environ Microbiol. 2009;75:5910–5918. doi: 10.1128/AEM.00767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos BK, Ritalahti KM, Cruz-Garcia C, Padilla-Crespo E, Löffler FE. Oxygen effect on Dehalococcoides viability and biomarker quantification. Environ Sci Technol. 2008;42:5718–5726. doi: 10.1021/es703227g. [DOI] [PubMed] [Google Scholar]
- Richardson RE, Bhupathiraju VK, Song DL, Goulet TA, Alvarez-Cohen L. Phylogenetic characterization of microbial communities that reductively dechlorinate TCE based upon a combination of molecular techniques. Environ Sci Technol. 2002;36:2652–2662. doi: 10.1021/es0157797. [DOI] [PubMed] [Google Scholar]
- Rowe AR, Lazar BJ, Morris RM, Richardson RE. Characterization of the community structure of a dechlorinating mixed culture and comparisons of gene expression in planktonic and biofloc-associated "Dehalococcoides" and Methanospirillum species. Appl Environ Microbiol. 2008;74:6709–6719. doi: 10.1128/AEM.00445-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeborn RA, West KA, Bhupathiraju VK, Chauhan S, Rahm BG, Richardson RE, Alvarez-Cohen L. Phylogenetic analysis of TCE-dechlorinating consortia enriched on a variety of electron donors. Environ Sci Technol. 2005;39:8358–8368. doi: 10.1021/es048003p. [DOI] [PubMed] [Google Scholar]
- Magnuson JK, Stern RV, Gossett JM, Zinder SH, Burris DR. Reductive dechlorination of tetrachloroethene to ethene by a two-component enzyme pathway. Appl Environ Microbiol. 1998;64:1270–1275. doi: 10.1128/aem.64.4.1270-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher T, Görisch H, Görisch H, Adrian L. Reductive dehalogenation of chlorobenzene congeners in cell extracts of Dehalococcoides sp. strain CBDB1. Appl Environ Microbiol. 2003;69:2999–3001. doi: 10.1128/AEM.69.5.2999-3001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis I, Zinder SH. Characterization of hydrogenase and reductive dehalogenase activities of Dehalococcoides ethenogenes strain 195. Appl Environ Microbiol. 2005;71:1664–1667. doi: 10.1128/AEM.71.3.1664-1667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel M, Edwards EA. Microbial composition of chlorinated ethene-degrading cultures dominated by Dehalococcoides. FEMS Microbiol Ecol. 2006;58:538–549. doi: 10.1111/j.1574-6941.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Duhamel M, Wehr SD, Yu L, Rizvi H, Seepersad D, Dworatzek S, Cox EE, Edwards EA. Comparison of anaerobic dechlorinating enrichment cultures maintained on tetrachloroethene, trichloroethene, cis-dichloroethene and vinyl chloride. Water Res. 2002;36:4193–4202. doi: 10.1016/S0043-1354(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Waller AS, Krajmalnik-Brown R, Löffler FE, Edwards EA. Multiple reductive-dehalogenase-homologous genes are simultaneously transcribed during dechlorination by Dehalococcoides-containing cultures. Appl Environ Microbiol. 2005;71:8257–8264. doi: 10.1128/AEM.71.12.8257-8264.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes VF, He J, Lee PK, Alvarez-Cohen L. Discrimination of multiple Dehalococcoides strains in a trichloroethene enrichment by quantification of their reductive dehalogenase genes. Appl Environ Microbiol. 2006;72:5877–5883. doi: 10.1128/AEM.00516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahm BG, Morris RM, Richardson RE. Temporal expression of respiratory genes in an enrichment culture containing Dehalococcoides ethenogenes. Appl Environ Microbiol. 2006;72:5486–5491. doi: 10.1128/AEM.00855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller AS. Molecular investigation of chloroethene reductive dehalogenation by the mixed microbial community KB1. Chemical Engineering and Applied Chemistry: University of Toronto; 2010. [Google Scholar]
- Parks DH, Beiko RG. Identifying biologically relevant differences between metagenomic communities. Bioinformatics. 2010;26:715–721. doi: 10.1093/bioinformatics/btq041. [DOI] [PubMed] [Google Scholar]
- Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A. et al. The metagenomics RAST server - a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinforma. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, de Crecy-Lagard V, Diaz N, Disz T, Edwards R. et al. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005;33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Klar B, Huson DH. Visual and statistical comparison of metagenomes. Bioinformatics. 2009;25:1849–1855. doi: 10.1093/bioinformatics/btp341. [DOI] [PubMed] [Google Scholar]
- Seshadri R, Kravitz SA, Smarr L, Gilna P, Frazier M. CAMERA: a community resource for metagenomics. PLoS Biol. 2007;5:e75. doi: 10.1371/journal.pbio.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Brito B, Rohwer F, Edwards RA. An application of statistics to comparative metagenomics. BMC Bioinforma. 2006;7:162. doi: 10.1186/1471-2105-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yooseph S, Sutton G, Rusch DB, Halpern AL, Williamson SJ, Remington K, Eisen JA, Heidelberg KB, Manning G, Li W. et al. The Sorcerer II Global Ocean Sampling expedition: expanding the universe of protein families. PLoS Biol. 2007;5:e16. doi: 10.1371/journal.pbio.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon I, Battchikova N, Aro EM, Giglione C, Meinnel T, Glaser F, Pinter RY, Breitbart M, Rohwer F, Beja O. Comparative metagenomics of microbial traits within oceanic viral communities. ISME J. 2011;5:1178–1190. doi: 10.1038/ismej.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis KT, Braff J, Karl DM, DeLong EF. Comparative metagenomic analysis of a microbial community residing at a depth of 4,000 meters at station ALOHA in the North Pacific subtropical gyre. Appl Environ Microbiol. 2009;75:5345–5355. doi: 10.1128/AEM.00473-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Wang F, Guo L, Chen Z, Sievert SM, Meng J, Huang G, Li Y, Yan Q, Wu S. et al. Comparative metagenomics of microbial communities inhabiting deep-sea hydrothermal vent chimneys with contrasting chemistries. ISME J. 2011;5:414–426. doi: 10.1038/ismej.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, Takami H, Morita H, Sharma VK, Srivastava TP. et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tringe SG, von Mering C, Kobayashi A, Salamov AA, Chen K, Chang HW, Podar M, Short JM, Mathur EJ, Detter JC. et al. Comparative metagenomics of microbial communities. Science. 2005;308:554–557. doi: 10.1126/science.1107851. [DOI] [PubMed] [Google Scholar]
- Waller AS, Hug LA, Mo K, Radford DR, Maxwell KL, Edwards EA. Transcriptional analysis of a Dehalococcoides-containing microbial consortium reveals prophage activation. Appl Environ Microbiol. 2012;78:1178–1186. doi: 10.1128/AEM.06416-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ, Hug LA, Edwards EA, Holmes S, Spormann AM. Site-specific mobilization of vinyl chloride respiration islands by a mechanism common in Dehalococcoides. BMC Genomics. 2011;12:287. doi: 10.1186/1471-2164-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald NJ, Parks DH, Beiko RG. Rapid identification of high-confidence taxonomic assignments for metagenomic data. Nucleic Acids Res. 2012. 10.1093/nar/gks335. [DOI] [PMC free article] [PubMed]
- Parks DH, MacDonald NJ, Beiko RG. Classifying short genomic fragments from novel lineages using composition and homology. BMC Bioinforma. 2011;12:328. doi: 10.1186/1471-2105-12-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese I, Haine V, Delrue RM, Tibor A, Lestrate P, Stevaux O, Mertens P, Paquet JY, Godfroid J, De Bolle X, Letesson JJ. The Ton system, an ABC transporter, and a universally conserved GTPase are involved in iron utilization by Brucella melitensis 16 M. Infect Immun. 2004;72:5783–5790. doi: 10.1128/IAI.72.10.5783-5790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultis DD, Purdy MD, Banchs CN, Wiener MC. Outer membrane active transport: structure of the BtuB:TonB complex. Science. 2006;312:1396–1399. doi: 10.1126/science.1127694. [DOI] [PubMed] [Google Scholar]
- Tang YJ, Yi S, Zhuang WQ, Zinder SH, Keasling JD, Alvarez-Cohen L. Investigation of carbon metabolism in "Dehalococcoides ethenogenes" strain 195 by use of isotopomer and transcriptomic analyses. J Bacteriol. 2009;191:5224–5231. doi: 10.1128/JB.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner G, Neudorfer F, Diekmann H. Degradation of lactate by an anaerobic mixed culture in a fluidized-bed reactor. Water Res. 1994;28:1337–1340. doi: 10.1016/0043-1354(94)90299-2. [DOI] [Google Scholar]
- Men Y, Feil H, VerBerkmoes NC, Shah MB, Johnson DR, Lee PKH, West KA, Zinder SH, Andersen GL, Alvarez-Cohen L. Sustainable syntrophic growth of Dehalococcoides ethenogenes strain 195 with Desulfovibrio vulgaris Hildenborough and Methanobacterium congolense: global transcriptomic and proteomic analyses. International Society for Microbial Ecology. 2012;2:410–421. doi: 10.1038/ismej.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell DE, Gossett JM. Modeling the production of and competition for hydrogen in a dechlorinating culture. Environ Sci Technol. 1998;32:2450–2460. doi: 10.1021/es980136l. [DOI] [Google Scholar]
- Adams MW, Stiefel EI. Biological hydrogen production: not so elementary. Science. 1998;282:1842–1843. doi: 10.1126/science.282.5395.1842. [DOI] [PubMed] [Google Scholar]
- Vignais PM, Billoud B, Meyer J. Classification and phylogeny of hydrogenases. FEMS Microbiol Rev. 2001;25:455–501. doi: 10.1111/j.1574-6976.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem. 2003;278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- Aklujkar M, Krushkal J, DiBartolo G, Lapidus A, Land ML, Lovley DR. The genome sequence of Geobacter metallireducens: features of metabolism, physiology and regulation common and dissimilar to Geobacter sulfurreducens. BMC Microbiol. 2009;9:109. doi: 10.1186/1471-2180-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methe BA, Nelson KE, Eisen JA, Paulsen IT, Nelson W, Heidelberg JF, Wu D, Wu M, Ward N, Beanan MJ. et al. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science. 2003;302:1967–1969. doi: 10.1126/science.1088727. [DOI] [PubMed] [Google Scholar]
- Zhuang WQ, Yi S, Feng X, Zinder SH, Tang YJ, Alvarez-Cohen L. Selective utilization of exogenous amino acids by Dehalococcoides ethenogenes strain 195 and the effects on growth and dechlorination activity. Appl Environ Microbiol. 2011;77(21):7797–7803. doi: 10.1128/AEM.05676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A, Brulc JM, Wilson MK, Law BF, Theoret JR, Joens LA, Konkel ME, Angly F, Dinsdale EA, Edwards RA. et al. Comparative metagenomics reveals host specific metavirulomes and horizontal gene transfer elements in the chicken cecum microbiome. PLoS One. 2008;3:e2945. doi: 10.1371/journal.pone.0002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daprato RC, Löffler FE, Hughes JB. Comparative analysis of three tetrachloroethene to ethene halorespiring consortia suggests functional redundancy. Environ Sci Technol. 2007;41:2261–2269. doi: 10.1021/es061544p. [DOI] [PubMed] [Google Scholar]
- Edwards EA, Grbic-Galic D. Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl Environ Microbiol. 1994;60:313–322. doi: 10.1128/aem.60.1.313-322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. 2001;Chapter 2:Unit 2–4. doi: 10.1002/0471142727.mb0204s56. [DOI] [PubMed] [Google Scholar]
- Chou HH, Holmes MH. DNA sequence quality trimming and vector removal. Bioinformatics. 2001;17:1093–1104. doi: 10.1093/bioinformatics/17.12.1093. [DOI] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z. et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains supplemental methods as referenced in the main text, and a discussion of the hydrogenase distribution within the enrichment consortia, including supplemental tables S1, S2, and S9 [58,70].
Contains supplemental tables S3-S8; detailed tables of the pathways discussed in the manuscript, including all genes implicated and the number of assigned reads to each gene from each phylum examined. Presented as a single MS Excel workbook, with each sheet corresponding to a different pathway of interest. Each enzyme examined within a pathway is listed in the left-most column, with number of assigned reads from MG-RAST for each phylum within each metagenome listed. Metagenomes are indicated as follows; D = DonnaII, A = ANAS, K = KB-1. Enzymes found within the metagenomes are highlighted in green, while enzymes absent from specific phyla are highlighted in red. Enzymes of interest may be absent either because they are not present in the SEED database (denoted with (NOT FOUND) in the enzyme name), or were not detected in the metagenome datasets.