Abstract
Background
The impact of hepatitis B virus (HBV) vaccination campaigns on HBV epidemiology needs to be evaluated, in order to assess the long-term immunity offered by vaccines against HBV.
Objectives
To evaluate the current status of anti-HBV vaccine coverage among healthcare workers (HCWs) in Southern Italy, and to determine the long-term persistence of antibodies to hepatitis B surface antigens (anti-HBs) in such a cohort of subjects.
Patients and Methods
A longitudinal, retrospective seroepidemiological survey was conducted among 451 HCWs, who were working at or visiting, the Occupational Health Department of a city hospital, in Catania, Italy, between January 1976 and December 2010.
Results
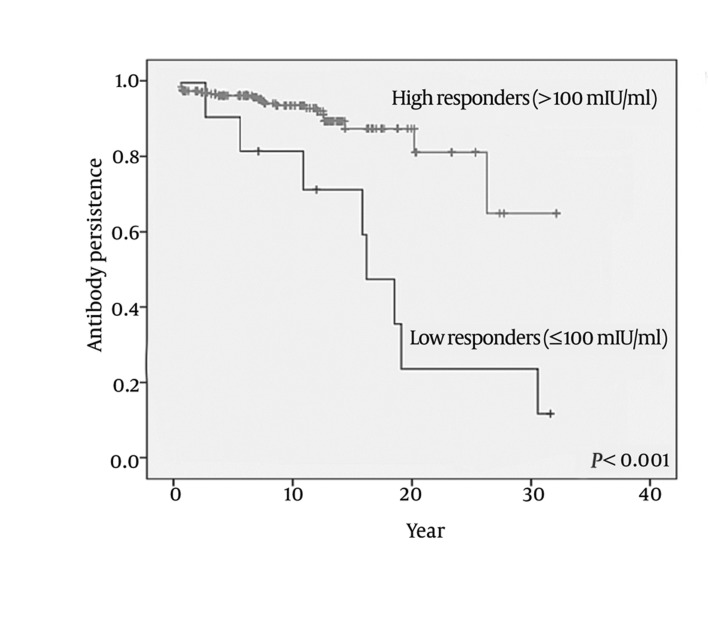
At the 30-year follow-up (mean follow-up 10.15 ± 5.96 years, range 0.74-30), 261 HCWs had detectable anti-HBs titers indicating a persistence of seroprotection of 89.4% (out of 292 anti-HBs positive results, three months after vaccination). An inadequate vaccination schedule was the strongest predictor of antibody loss during follow-up (OR = 8.37 95% CI: 5.41-12.95, P < 0.001). A Kaplan-Maier survival curve revealed that the persistence of anti-HBs 30 years after vaccination, was 92.2% for high responders, while it was only 27.3% for low responders (P = 0.001).
Conclusions
A good level of seroprotection persisted in 57.9% of the subjects after 30 years. Factors related to this immunization status confirmed the importance of vaccinating HCWs early in their careers and ensuring an adequate vaccination schedule. However, with particular reference to the low rate of hepatitis B vaccine coverage among HCWs in Southern Italy, the implementation of a new educational intervention as part of an active vaccination program is needed.
Keywords: Hepatitis B Virus, Vaccines, Health Personnel, Vaccination
1. Background
In Italy, during the 1980s, the hepatitis B virus (HBV) infection was one of the major causes of mortality, leading to about 9 000 deaths per year from HBV-related diseases, such as; chronic active hepatitis, hepatocellular carcinoma, and cirrhosis (1). Hepatitis B infection was a common infectious occupational disease among healthcare workers (HCWs), thus a vaccination campaign was conducted in 1983 to sensitize high-risk groups, such as HCWs, for whom vaccination was strongly suggested (2). However, despite the long-standing existence of recommendations for such high-risk groups (3), hepatitis B vaccinations only reached a small percentage of these populations, who remained susceptible to the virus (4). Indeed, the incidence of hepatitis B in HCWs continued to be higher than in the general population, reflecting poor vaccine coverage (5). This may be attributed to the lack of perceived risk of hepatitis B infection in this job category, or the absence of appropriate healthcare programs targeting vaccination against HBV infection (6). However, a survey conducted in Italy during the 1990s showed an insufficient overall coverage against HBV, with lower rates in the south of the country (7), that was mostly endemic for HBV infection in association with the hepatitis delta virus (8).
In recent years, no difference has been reported in the incidence rate of acute HBV between HCWs and the general population (9), however, this may not necessarily be due to vaccination programs, but to non-specific prophylactic measures such as; more accuracy in hospital procedures, training programs for HCWs, and proper sterilization. Thus, the impact of HBV vaccination campaigns on HBV epidemiology should be evaluated at the present time, with the aim of establishing the long-term immunity offered by vaccines against HBV. Indeed, the antigenic stimulation of the vaccine leads to the production of antibodies to hepatitis B surface antigens (anti-HBs).
2. Objectives
The aim of the present study was to evaluate the current status of anti-HBV vaccine acceptance among HCWs in the city of Catania, Southern Italy. Moreover, we aimed to determine the long-term persistence of anti-HBs in such a cohort of subjects.
3. Patients and Methods
A longitudinal retrospective seroepidemiological survey was conducted among HCWs of the Vittorio Emanuele Hospital, Catania, Southern Italy, who visited the Occupational Health Department between January 1976 and December 2010, and this process is still ongoing. The study was approved by the ethic committee of the hospital and subjects information were assured of complete anonymity. Data from 956 HCWs were collected. Out of the overall HCW population, only 451 HCWs (mean age 32.8 years, range 25-70 years) had received vaccination and were included in the survey.
3.1. Study Population
Each newly employed HCW must undergo a medical examination in the Occupational Health Department prior to signing his or her contract and joining the hospital in their area of their expertise. During such visits, subjects were screened for HBV infection and those having anti-HBs levels less than 10 mIU/mL were referred to be vaccinated.
3.2.Vaccination
The vaccine was provided free of charge by the hospital, to all HCWs, but the vaccination itself was not mandatory. The vaccine schedule was delivered by the laboratory staff in the Outpatient Department of the hospital. In most cases, a yeast-derived recombinant HBV vaccine was administered and subjects were advised to take three doses at zero, one, and six months of 1 mL (20 μg/mL) intramuscularly (in the deltoid muscle). We considered ‘vaccinated‘ subjects as having received at least the first vaccination dose, while considering ‘compliant‘ as those who had received the complete schedule. Subjects younger than 12 years old were mandatorily vaccinated during the vaccination campaign introduced by law since 1981, and they did not need to prove that they had been vaccinated from their health records.
3.3. Serologic Tests
Blood samples were checked for anti-HBs levels by an enzyme immunoassay (DiaSorin, s.r.l, Saluggia, Italy) during the initial screening of HCWs, and this process was repeated three months after the third dose of anti-HBV vaccine and routinely every two years. Moreover, anti-HBs levels of antibodies were often checked after accidental exposure to blood or needle stick injuries. The serologic status of subjects, who were vaccinated during the vaccination campaign in 1981, was assessed at the first visit after the recruitment, but no data about post-primary immunization titers were available.
3.4. Data Collection
Demographic data; age, gender, occupation and working hours, as well as clinical data; BMI, medical history, smoking status, age at primary vaccination, date and concentration (mIU/ml) of the initial anti-HBs antibody measurement, were collected retrospectively by three investigators using a structured form to collect data from the chart of each subject included in the survey. Occupational categories included; physicians (non-surgeon and surgeon), nurses, technicians and laboratory workers. Working hours were divided into day (approximately 4 to 8 consecutive hours, depending if they were full-time or part-time, from 7 am to 9 pm), night (from 9 pm to 7 am), and night and day shift (with varying amounts of night-shift depending on the type of ward) categories. BMI was calculated according to international standards by weight (kg)/height (m2) and subjects were considered normal if it was less than or equal to 25, overweight between 25 and 28, and obese if higher than 28. Subjects’ smoking status was classified as; (i) non-smokers, (ii) smoking one to 20 cigarettes per day, and (iii) more than 20 cigarettes per day. Medical history comprised of diseases that may interfere with the immune system (e.g., allergies, asthma, splenectomy, sarcoidosis, autoimmune diseases, chronic viral hepatitis and cancer), and diseases not-known to interfere with the immune system. We considered subjects as seropositive against hepatitis B if their anti-HBs antibody concentration was greater than or equal to 10 mIU/ml, and seronegative if it was less than 10 mIU/ml as recommended by the United States Advisory Committee on Immunization Practices and the World Health Organization (WHO) (10). Antibody titers following primary vaccination were classified into low responders (10–99 mIU/ml) and high responders (≥ 100 mIU/ml). Antibody loss and antibody persistence were defined as testing negative or positive, respectively, for anti-HBs according to the results of the commercial kits.
3.5. Statistical Analysis
Categorical variables were presented as frequency of occurrence and percentage and differences between groups were assessed by a chi-square test. Continuous variables were presented as mean and standard deviations, and differences between groups were assessed by a Student’s t-test. The cumulative incidence was calculated using the Kaplan–Meier method. The crude odds ratios (ORs) were used for the association of immunization loss, with the characteristics of subjects evaluated by univariate analysis. Adjusted ORs and their 95% confidence intervals were calculated by stepwise logistic regression analysis to identify independent predictors of becoming seronegative. Only variable results associated at univariate analysis were entered into the logistic model. P ≤ 0.05 was considered significant. Analyses were carried out using the Statistical Package for the Social Sciences version 17.0 (SPSS, Chicago, IL, USA).
4. Results
Sociodemographic and medical characteristics of the 451 HCWs included in the study are listed in Table 1. Fully compliant subjects with a completed vaccination schedule consisted of 157 (34.8%) HCWs. Three months following vaccination, the overall anti-HBs prevalence in vaccinated HCWs was 64.7% (292 subjects). Vaccination at a young age, either at birth or before the age of 12, as well as having received an inadequate schedule of vaccination, were significantly associated with being seronegative when tested (P < 0.0001). Among the 181 subjects vaccinated at job commencement or during their working life, the response after primary immunization was 52.1% (158 subjects). At the 30-year follow-up (mean follow-up 10.15 ± 5.96 years, range 0.74-30), 261 HCWs (57.9% out of the overall 451 vaccinated HCWs) had detectable anti-HBs titers, indicating a persistence of seroprotection of 89.4% (out of the 292 anti-HBs positive HCWs three-months after vaccination). Characteristics significantly associated with becoming seronegative are listed in Table 2. Younger and overweight HCWs with 10-20 years in their occupation were less likely to lose seroprotection against HBV (OR = 0.47, 95% CI: 0.3-0.72, P = 0.01; OR = 0.7, 95% CI: 0.51-0.95, P = 0.022 and OR = 0.65, 95% CI: 0.43-0.98, P = 0.41, respectively), compared with older, normal weight HCWs employed for less than 10 years. By contrast, an inadequate vaccination schedule was found to be most strongly associated with antibody loss during follow-up, even after adjusting for other significant covariates (OR = 8.37, 95% CI: 5.41-12.95, P < 0.001). Results of anti-HBs titer levels detected in subjects vaccinated at job commencement or during their working life were similar to those for all vaccinated HCWs (Table 2). At the 30-year follow-up, the odds for antibody loss were higher in subjects that had received an inadequate schedule (OR = 16.97, 95% CI: 7.12-40.41, P < 0.001). Kaplan-Maier survival curve of time from primary vaccination to an anti-HBs antibody measurement outcome less than 10 mIU/ml, revealed that low post-immunization titers (10–99 mIU/ml) was a predictor of antibody loss during the follow-up period. The persistence of anti-HBs 30 years after vaccination was 92.2% for high responders, while it was only 27.3% for low responders (P < 0.001) (Figure 1). When analyzing risk factors associated with becoming seronegative against hepatitis B, being a low-responder after primary immunization was a predictor of antibody loss after the follow-up period (OR = 6.18, 95% CI: 2.48-17.01, P < 0.001), but after adjusting for the vaccination schedule, only the last factor remained significant (OR = 11.76, 95% CI: 2.99-46.2, P < 0.001).
Table 1. Demographic, Occupational and Clinical Characteristics of the Study Population by Serologic Status.
| Seropositive, No. (%) (n = 292) | Seronegative, No. (%) (n = 159) | Total, No. (%) (n = 451) | P value | |
| Gender | 0.549 | |||
| Male | 153 (52.4) | 88 (55.3) | 241 (53.4) | |
| Female | 139 (47.6) | 71 (44.7) | 210 (46.6) | |
| Age group, y | < 0.0001 | |||
| 25-35 | 204 (69.9) | 139 (87.4) | 343 (76.1) | |
| 36-45 | 73 (25) | 14 (8.8) | 87 (19.3) | |
| > 45 | 15 (5.1) | 6 (3.8) | 21 (4.7) | |
| BMI, kg/m2 | 0.277 | |||
| > 25 | 120 (41.1) | 54 (34) | 174 (38.6) | |
| > 30 | 13 (4.5) | 6 (3.8) | 19 (4.2) | |
| Medical history | 0.131 | |||
| Group A a | 187 (64) | 113 (71.1) | 300 (66.5) | |
| Group B b | 105 (36) | 46 (28.9) | 151 (33.5) | |
| Smoking status | 0.745 | |||
| Non smoker | 163 (55.8) | 89 (56) | 252 (55.9) | |
| 1-20 cigarettes per day | 101 (34.6) | 58 (36.5) | 159 (35.3) | |
| > 20 cigarettes per day | 28 (9.6) | 12 (7.5) | 40 (8.9) | |
| Occupational category | 0.794 | |||
| Physician | 46 (18.6) | 21 (17.5) | 67 (18.3) | |
| Nurse | 201 (81.4) | 99 (82.5) | 300 (81.7) | |
| Hospital department | 0.782 | |||
| Medical department | 117 (45.5) | 53 (42.1) | 170 (44.4) | |
| Surgical department | 130 (50.6) | 67 (53.2) | 197 (51.4) | |
| Laboratory department | 10 (3.9) | 6 (4.8) | 16 4.2) | |
| Years in occupation | 0.1 | |||
| > 10 | 233 (79.8) | 144 (90.6) | 377 (83.6) | |
| 20-Oct | 55 (18.8) | 13 (8.2) | 68 (15.1) | |
| > 20 | 4 (1.4) | 2 (1.3) | 6 (1.3) | |
| Working schedule | 0.679 | |||
| Day shift | 193 (66.1) | 103 (64.8) | 296 (65.6) | |
| Night shift | 24 (8.2) | 17 (10.7) | 41 (9.1) | |
| Rotating day/night shift | 75 (25.7) | 39 (24.5) | 114 (25.3) | |
| Vaccine status | < 0.0001 | |||
| Vaccinated = 12 years | 140 (47.9) | 130 (81.8) | 270 (59.9) | |
| Vaccinated > 12 years | 152 (52.1) | 29 (18.2) | 181 (40.1) | |
| Vaccination schedule | < 0.0001 | |||
| Adequate | 267 (91.4) | 27 (17) | 294 (65.2) | |
| Inadequate | 25 (8.6) | 132 (83) | 157 (34.8) |
aDiseases not known to interfere with the immune system
bDiseases that could interfere with the immune system
Table 2. Factors Associated With Becoming Seronegative Against Hepatitis B by Vaccination Status.
| Overall Vaccinated (n = 451) | Vaccinated > 12 years (n = 181) | |||||||
| Unadjusted OR (95 % CI), No. (%) | P value | Adjusted OR (95 % CI), No. (%) | P value | Unadjusted OR (95 % CI) , No. (%) | P value | Adjusted OR (95 % CI), No. (%) | P value | |
| Gender | - | |||||||
| Male | 1 | - | - | - | 1 | - | - | - |
| Female | 1.01 (0.76-1.35) | 0.918 | - | - | 1.02 (0.56-1.84) | 0.952 | - | - |
| Age group, y | ||||||||
| 25-35 | 1 | - | 1 | - | 1 | - | - | - |
| 36-45 | 0.47 (0.3-0.72) | 0.001 | 0.68 (0.37-1.26) | 0.228 | 1.29 (0.6-2.75) | 0.507 | - | - |
| > 45 | 0.54 (0.3-0.97) | 0.043 | 0.49 (0.18-1.32) | 0.16 | 1.46 (0.6-3.58) | 0.401 | - | - |
| BMI, kg/m2 | ||||||||
| < 25 | 1 | - | 1 | - | 1 | - | - | - |
| 25-28 | 0.7 (0.51-0.95) | 0.022 | 0.71 (0.5-1) | 0.054 | 0.67 (0.37-1.22) | 0.196 | - | - |
| > 28 | 0.6 (0.28-1.3) | 0.191 | 0.61 (0.22-1.67) | 0.335 | 0 (0) | 0.978 | - | - |
| Medical history | ||||||||
| Group A a | 1 | - | - | - | 1 | - | - | - |
| Group B b | 0.86 (0.63-1.17) | 0.328 | - | - | 1.12 (0.61-1.2) | 0.729 | - | - |
| Smoking status | ||||||||
| Non smoker | 1 | - | - | 1 | - | - | - | |
| 1-20 cigarettes/day | 0.92 (0.67-1.25) | 0.601 | - | - | 0.58 (0.3-1.12) | 0.109 | - | - |
| >20 cigarettes/day | 1.06 (0.63-1.77) | 0.819 | - | - | 0.69 (0.16-2.93) | 0.62 | - | - |
| Occupational category | ||||||||
| Physician | 1 | - | - | - | 1 | - | - | - |
| Nurse | 1.06 (0.7-1.6) | 0.78 | - | - | 1.38 (0.61-3.12) | 0.431 | - | - |
| Hospital department | ||||||||
| Medical | 1 | - | 1 | - | 1 | - | - | - |
| Surgical | 1.53 (1.09-2.12) | 0.012 | 1.36 (0.97-1.91) | 0.072 | 1.61 (0.88-2.95) | 0.117 | - | - |
| Laboratory | 1 (0.46-2.54) | 0.846 | 0.45 (0.2-1.08) | 0.076 | 0 (0) | 1 | - | - |
| Years in occupation | ||||||||
| < 10 | 1 | - | 1 | - | 1 | - | - | - |
| 10-20 | 0.65 (0.43-0.98) | 0.041 | 0.96 (0.47-1.99) | 0.93 | 1.62 (0.88-3) | 0.119 | - | - |
| > 20 | 0.34 (0.1-1.08) | 0.068 | 0.51 (0.12-2.21) | 0.373 | 0.7 (0.19-2.57) | 0.597 | - | - |
| Vaccination schedule | ||||||||
| Adequate | 1 | - | 1 | - | 1 | - | 1 | - |
| Inadequate | 7.5 (5.14-10.96) | < 0.001 | 8.37 (5.41-12.95) | < 0.001 | 16.97 (7.12-40.41) | < 0.001 | 11.76 (2.99-46.2) | < 0.001 |
| Immunization | ||||||||
| High responder | - | - | - | - | 1 | - | 1 | - |
| Low responder | - | - | - | - | 6.18 (2.48-17.01) | < 0.001 | 1.14 (0.31-4.1) | 0.841 |
aDiseases not known to interfere with the immune system
bDiseases that could interfere with the immune system
Figure 1. Anti-HBsAg Persistence According to Post-Vaccination Anti-HBsAg Titers.
5. Discussion
This longitudinal, retrospective seroepidemiological survey aimed to collect and analyze important information on vaccination against HBV in a hospital environment, including compliance rates and serological status of those vaccinated. Based on our knowledge, this is the first study to assess compliance rates of vaccination and markers kinetics over a follow-up period of 30 years. Moreover, this is the first report that underlines the situation of immunization status against HBV, due to poor compliance rates to the vaccine, among HCWs in Sicily. Indeed, according to our study, the current overall vaccine coverage among HCWs in Catania was only 61.2% (451 of 956). Several other studies have assessed lower vaccination rates among HCWs in Southern Italy than in the northern regions, but in our opinion these works underestimated this trend. In fact, in Southern Italy overall vaccine coverage has been documented to have increased from 44.3% in a survey conducted in 1996 (7), to 77.7% in another survey conducted in 2006 (11), however, our findings are much lower than these results. Moreover, taking into account the introduction of the compulsory-by-law HBV vaccination in Italy for all newborns in 1981 and 12-year-old children in 1991, as well as HCWs vaccinated only after a percutaneous exposure incident, we can conclude that compliance rates to the vaccination against HBV among HCWs in Southern Italy at the start of their employment, considered as a positive attitude towards the vaccination program, were even lower. Accordingly, compared with other countries, compliance rates in our study are similar to those reported in the USA (54%) (12), Spain (47.1%) (13), Australia (55.8%) (14), and Nigeria (53.8%) (15), but lower than those reported in the UK (80%) (16), and Brazil (80.7%) (17). According to the job category, physicians were shown to be compliant, as well as nurses. Compliance rates among physicians assessed in our study were similar to those reported in; Australia (14), the UK (16), and Brazil (17), ranging between 69% and 98.6%, much higher than those reported elsewhere, which range from 30% to 40.3% (12, 15). However, an indifferent attitude in regard to hepatitis B vaccine among physicians is also well recognized (18-20), resulting in doctors being relatively overlooked, compared with other professional groups, with regard to both education and vaccination (21, 22).
Among the 181 HCWs vaccinated at job commencement or after, we analyzed antibody kinetics to assess whether subjects were still immunized during a follow-up period of 30 years, finding that the cumulative percentage of seroprotection was 52.1%. The persistence of anti-HBs antibodies is well documented and our study seems to confirm existing findings in the literature (23, 24). However, how long immunity might be expected to last after vaccination and its role in immunization is still a controversial issue. Indeed, no scientific evidence supports the assumption that immunity depends entirely on anti-HBs antibodies. Thus, according to the WHO, individuals with anti-HBs levels which dropped below 10 mIU/ml are supposed to remain immunized against HBV, due to the stronger role in the anamnestic response of cellular immunity. On the other hand, it has been documented that subjects with post-vaccination anti-HBs levels lower than 50 mIU/ml may contract a HBV infection, experience hepatitis B surface antigen reactivity (25), or develop the disease. Thus, in European countries the threshold which considers that a subject is immunized, is recommended to be at least 100 mIU/ml (26). However, we recorded no cases of hepatitis B in non-responder subjects or in those with anti-HBs levels lower than 10 mIU/ml or 50 mIU/ml after vaccination.
The issues concerning anti-HBs levels and the persistence of seroprotection are important in determining the potential need for booster doses. Although current data on children leads to the assumption that no booster dose is needed before 10 years of age (27), the need for booster doses to maintain HBV seroprotection is still debated in adults, given that results from previous surveys conducted in adult HCWs are in contrast to international guidelines (24, 28, 29). However, subjects vaccinated at 12 years or earlier, and those who did not receive the booster doses at job recruitment, were seronegative at the first visit. Seropositivity status was influenced by the age of subjects at vaccination. Indeed, a younger age at primary vaccination was predictive of seropositivity, confirming the recommendations of the WHO (30). This finding is supported by other studies conducted on HCWs (28, 31, 32). We checked if demographic and clinical variables had an effect on serological status. Being overweight (33, 34, 35, 36), male (37), having a chronic disease (38, 39), and smoking (33), have previously been documented as predictive of seronegative status after vaccination. In our study, univariate analysis only confirmed that being overweight was a predictor of failed response to primary vaccination, while being a low-responder had a higher risk of becoming seronegative during the follow-up period. However, none of these variables remained significant in the multivariate analysis. Among the occupational data, we decided to include the type of occupation and working hours, thinking that such variables could influence immunization status over time, due to the increased risk of HBV infection among different groups, but no significant predictive variable was found. Findings of retrospective seroepidemiological surveys such as ours must be read in light of several limitations. Missing data due to the retrospective nature of the work should be taken in to account. We have also included only those subjects who are still working at the hospital, and whose medical data was still available (ie, excluding fired, deceased, and retired subjects), which may lead to a potential bias. However, detailed information about the time of vaccination as well as serologic data performed soon after vaccination, has been documented to reliably determine whether a seronegative subject was primary (anti-HBs < 10 mIU/ml since vaccination), or secondary (anti-HBs < 10 mIU/ml after a certain time following the vaccination), non-responders to anti-HBV vaccine, and if variables supposed to be associated to seronegativity were related to becoming or staying seronegative. Conversely, a limitation of this study in regard to the role of such variables occurred, because they were collected at the time of recording the HCW’s chart, thus we cannot assure the status of the subject at the time of vaccination and we can only hypothesize that characteristics collected at the start of their work and joining the hospital staff, remained stable as far as possible over time.
In conclusion, the implementation of universal precautions such as; safety procedures in hospitals pre-dating the availability of anti-HBV vaccine, were associated with decreased high-risk exposures and the decreased incidence of HBV (40, 41), leading to a certain degree of controversy when attributing the lower rates solely to HBV vaccination programs (42). On the other hand, several studies have shown that a vaccination program in healthcare workers against HBV was cost-effective, decreased the anxiety of an employee after needle stick and sharp injuries, and prevented the transmission of HBV after exposure in the majority of cases (3, 43, 44). Thus, according to the results of this study, a good level of seroprotection persisted in 57.9% of subjects after 30 years, and factors related to immunization status confirmed the importance of vaccinating HCWs early in their career and assuring an adequate vaccination schedule. However, with particular reference to the low rate of hepatitis B vaccine coverage among HCWs in Southern Italy, implementation of new educational interventions as part of an active vaccination program is still required.
Acknowledgments
Giuseppe Grosso was supported by the International Ph.D. Program in Neuropharmacology, University of Catania Medical School, and Catania, Italy. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors are grateful to Susanne Lukowicz for English language editing.
Footnotes
Implication for health policy/practice/research/medical education: Occult hepatitis B infection is important entity in healthcare workers and study of this article is recommended to the epidemiologists and other researchers who are interested in public health issues and in methods to assess, contain and monitoring the risk of hepatitis B virus infection after vaccination.
Please cite this paper as: Grosso G, Mistretta A, Marventano S, Ferranti R, Mauro L, Cunsolo R, et al. Long-Term Persistence of Seroprotection by Hepatitis B Vaccination in Healthcare Workers of South Italy. Hepat Mon. 2012;12(9):e6025. DOI: 10.5812/hepatmon.6025
Authors’ Contribution: None declared.
Financial Disclosure: None declared.
Funding/Support: Authors did not receive any sources of support in the form of grants, equipment, or drugs.
References
- 1.Zanetti AR. Update on hepatitis B vaccination in Italy 10 years after its implementation. Vaccine. 2001;19(17-19):2380–3. doi: 10.1016/S0264-410X(00)00458-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonanni P, Bonaccorsi G. Vaccination against hepatitis B in health care workers. Vaccine. 2001;19(17-19):2389–94. doi: 10.1016/s0264-410x(00)00460-6. [DOI] [PubMed] [Google Scholar]
- 3.Immunization of health-care workers: recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 1997;46(RR-18):1–42. [PubMed] [Google Scholar]
- 4.Ippolito G, De Carli G, Puro V, Petrosillo N, Arici C, Bertucci R, et al. Device-specific risk of needlestick injury in Italian health care workers. JAMA. 1994;272(8):607–10. [PubMed] [Google Scholar]
- 5.Stroffolini T, Palumbo F, Galanti C, Moiraghi A, Novaco F, Corona R, et al. Hepatitis B in health workers in Italy. Public Health. 1994;108(6):433–7. doi: 10.1016/S0033-3506(94)80101-0. [DOI] [PubMed] [Google Scholar]
- 6.Francois G, Hallauer J, Van Damme P. Hepatitis B vaccination: how to reach risk groups. Vaccine. 2002;21(1-2):1–4. doi: 10.1016/S0264-410X(02)00440-1. [DOI] [PubMed] [Google Scholar]
- 7.Stroffolini T, Petrosillo N, Ippolito G, Lopalco A, Sagliocca L, Adamo B, et al. Hepatitis B vaccination coverage among healthcare workers in Italy. Infect Control Hosp Epidemiol. 1998;19(10):789–91. doi: 10.1086/647727. [DOI] [PubMed] [Google Scholar]
- 8.Gaeta GB, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, Lobello S, et al. Chronic hepatitis D: a vanishing Disease? An Italian multicenter study. Hepatology. 2000;32(4 Pt 1):824–7. doi: 10.1053/jhep.2000.17711. [DOI] [PubMed] [Google Scholar]
- 9.Tosti ME, Mariano A, Spada E, Pizzuti R, Gallo G, Ragni P, et al. Incidence of parenterally transmitted acute viral hepatitis among healthcare workers in Italy. Infect Control Hosp Epidemiol. 2007;28(5):629–32. doi: 10.1086/513728. [DOI] [PubMed] [Google Scholar]
- 10.West DJ, Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine. 1996;14(11):1019–27. doi: 10.1016/0264-410X(96)00062-X. [DOI] [PubMed] [Google Scholar]
- 11.Stroffolini T, Coppola R, Carvelli C, D’Angelo T, De Masi S, Maffei C, et al. Increasing hepatitis B vaccination coverage among healthcare workers in Italy 10 years apart. Dig Liver Dis. 2008;40(4):275–7. doi: 10.1016/j.dld.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Murata PJ, Young LC. Physicians’ attitudes and behaviors regarding hepatitis B immunization. J Fam Pract. 1993;36(2):163–8. [PubMed] [Google Scholar]
- 13.Mayo Ferreiro F, Smyth Chamosa E, Figueiras A. [Vaccination for the hepatitis B virus in primary care health staff: prevalence, affecting factors and need]. Aten Primaria. 1998;22(1):13–20. [PubMed] [Google Scholar]
- 14.Stanford MP, Black TR, March LM, Holt DA, Campbell DH. Hepatitis B vaccination rates among staff at a district general hospital. Med J Aust. 1995;162(6):304–6. doi: 10.5694/j.1326-5377.1995.tb139905.x. [DOI] [PubMed] [Google Scholar]
- 15.Fatusi AO, Fatusi OA, Esimai AO, Onayade AA, Ojo OS. Acceptance of hepatitis B vaccine by workers in a Nigerian teaching hospital. East Afr Med J. 2000;77(11):608–12. doi: 10.4314/eamj.v77i11.46734. [DOI] [PubMed] [Google Scholar]
- 16.Gyawali P, Rice PS, Tilzey AJ. Exposure to blood borne viruses and the hepatitis B vaccination status among healthcare workers in inner London. Occup Environ Med. 1998;55(8):570–2. doi: 10.1136/oem.55.8.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manso VF, Castro KF, Matos SM, Junqueira AL, Souza SB, Sousa MM, et al. Compliance with hepatitis B virus vaccination and risk of occupational exposure to blood and other body fluids in intensive care department personnel in Brazil. Am J Infect Control. 2003;31(7):431–4. doi: 10.1067/mic.2003.77. [DOI] [PubMed] [Google Scholar]
- 18.Burden AD, Whorwell PJ. Poor uptake of hepatitis B immunization amongst hospital-based health care staff. Postgrad Med J. 1991;67(785):256–8. doi: 10.1136/pgmj.67.785.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panhotra B, Saxena A, Al-Arabi AGAM. The effect of a continuous educational program on handwashing compliance among healthcare workers in an intensive care unit. Brit J Infect Control. 2004;5(3):15–8. doi: 10.1177/14690446040050030401. [DOI] [Google Scholar]
- 20.Smith ER, Banatvala JE, Tilzey AJ. Hepatitis B vaccine uptake among surgeons at a London teaching hospital: how well are we doing? Ann R Coll Surg Engl. 1996;78(5):447–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Brotherton JM, Bartlett MJ, Muscatello DJ, Campbell-Lloyd S, Stewart K, McAnulty JM. Do we practice what we preach? Health care worker screening and vaccination. Am J Infect Control. 2003;31(3):144–50. doi: 10.1067/mic.2003.24. [DOI] [PubMed] [Google Scholar]
- 22.Rosen E, Rudensky B, Paz E, Isacsohn M, Jerassi Z, Gottehrer NP, et al. Ten-year follow-up study of hepatitis B virus infection and vaccination status in hospital employees. J Hosp Infect. 1999;41(3):245–50. doi: 10.1016/s0195-6701(99)90023-3. [DOI] [PubMed] [Google Scholar]
- 23.Durlach R, Laugas S, Freuler CB, Rodriguez VE, Costa M. Ten-year persistence of antibody to hepatitis B surface antigen in healthcare workers vaccinated against hepatitis B virus, and response to booster vaccination. Infect Control Hosp Epidemiol. 2003;24(10):773–6. doi: 10.1086/502132. [DOI] [PubMed] [Google Scholar]
- 24.Floreani A, Baldo V, Cristofoletti M, Renzulli G, Valeri A, Zanetti C, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine. 2004;22(5-6):607–10. doi: 10.1016/j.vaccine.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann F, Kralj N. Criteria for successful hepatitis B vaccination in adults: results of a case study. Infection. 2009;37(3):266–9. doi: 10.1007/s15010-008-7410-y. [DOI] [PubMed] [Google Scholar]
- 26.Are booster immunisations needed for lifelong hepatitis B immunity?European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355(9203):561–5. [PubMed] [Google Scholar]
- 27.Yuen MF, Lim WL, Cheng CC, Lam SK, Lai CL. Twelve-year follow-up of a prospective randomized trial of hepatitis B recombinant DNA yeast vaccine versus plasma-derived vaccine without booster doses in children. Hepatology. 1999;29(3):924–7. doi: 10.1002/hep.510290327. [DOI] [PubMed] [Google Scholar]
- 28.Locquet C, Marande JL, Choudat D, Vidal-Trecan G. Hepatitis B vaccination in women healthcare workers: a seroepidemiological survey. Eur J Epidemiol. 2007;22(2):113–9. doi: 10.1007/s10654-006-9094-x. [DOI] [PubMed] [Google Scholar]
- 29.Williams JL, Christensen CJ, McMahon BJ, Bulkow LR, Cagle HH, Mayers JS, et al. Evaluation of the response to a booster dose of hepatitis B vaccine in previously immunized healthcare workers. Vaccine. 2001;19(28-29):4081–5. doi: 10.1016/S0264-410X(01)00112-8. [DOI] [PubMed] [Google Scholar]
- 30.Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis. 2002;35(11):1368–75. doi: 10.1086/344271. [DOI] [PubMed] [Google Scholar]
- 31.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15(1):1–8. doi: 10.1016/S0749-3797(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 32.Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA. 1993;270(24):2931–4. doi: 10.1001/jama.1993.03510240043029. [DOI] [PubMed] [Google Scholar]
- 33.Alimonos K, Nafziger AN, Murray J, Bertino JS, Jr. Prediction of response to hepatitis B vaccine in health care workers: whose titers of antibody to hepatitis B surface antigen should be determined after a three-dose series, and what are the implications in terms of cost-effectiveness? Clin Infect Dis. 1998;26(3):566–71. doi: 10.1086/514575. [DOI] [PubMed] [Google Scholar]
- 34.Cardell K, Fryden A, Normann B. Intradermal hepatitis B vaccination in health care workers. Response rate and experiences from vaccination in clinical practise. Scand J Infect Dis. 1999;31(2):197–200. doi: 10.1080/003655499750006272. [DOI] [PubMed] [Google Scholar]
- 35.Goldwater PN. Randomized, comparative trial of 20 micrograms vs 40 micrograms Engerix B vaccine in hepatitis B vaccine non-responders. Vaccine. 1997;15(4):353–6. doi: 10.1016/s0264-410x(96)00202-2. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman JN, Zuckerman AJ. Recombinant hepatitis B triple antigen vaccine: Hepacare. Expert Rev Vaccines. 2002;1(2):141–4. doi: 10.1586/14760584.1.2.141. [DOI] [PubMed] [Google Scholar]
- 37.Yu AS, Cheung RC, Keeffe EB. Hepatitis B vaccines. Infect Dis Clin North Am. 2006;20(1):27–45. doi: 10.1016/j.idc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Bronowicki JP, Weber-Larivaille F, Gut JP, Doffoel M, Vetter D. [Comparison of immunogenicity of vaccination and serovaccination against hepatitis B virus in patients with alcoholic cirrhosis]. Gastroenterol Clin Biol. 1997;21(11):848–53. [PubMed] [Google Scholar]
- 39.Fleming SJ, Moran DM, Cooksley WG, Faoagali JL. Poor response to a recombinant hepatitis B vaccine in dialysis patients. J Infect. 1991;22(3):251–7. doi: 10.1016/S0163-4453(05)80007-6. [DOI] [PubMed] [Google Scholar]
- 40.Dale JC, Pruett SK, Maker MD. Accidental needlesticks in the phlebotomy service of the Department of Laboratory Medicine and Pathology at Mayo Clinic Rochester. Mayo Clin Proc. 1998;73(7):611–5. doi: 10.1016/S0025-6196(11)64883-0. [DOI] [PubMed] [Google Scholar]
- 41.Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database syst Rev. 2002;(3):CD003087. doi: 10.1002/14651858.CD003087. [DOI] [PubMed] [Google Scholar]
- 42.Osterholm MT, Garayalde SM. Clinical viral hepatitis B among Minnesota hospital personnel. Results of a ten-year statewide survey. JAMA. 1985;254(22):3207–12. doi: 10.1001/jama.1985.03360220073032. [DOI] [PubMed] [Google Scholar]
- 43.Agerton TB, Mahoney FJ, Polish LB, Shapiro CN. Impact of the bloodborne pathogens standard on vaccination of healthcare workers with hepatitis B vaccine. Infect Control Hosp Epidemiol. 1995;16(5):287–91. doi: 10.1086/647109. [DOI] [PubMed] [Google Scholar]
- 44.Murphy E. Hepatitis B, vaccination and healthcare workers. Occup Med (Lond) 2000;50(6):383–6. doi: 10.1093/occmed/50.6.383. [DOI] [PubMed] [Google Scholar]