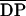Abstract
Chitin, a linear polysaccharide composed of (1→4)-linked 2-acetamido-2-deoxy-β-d-glucopyranose (GlcNAc) residues, and chitosan, the fully or partially N-acetylated, water-soluble derivative of chitin composed of (1→4)-linked GlcNAc and 2-amino-2-deoxy-β-d-glucopyranose (GlcN), have been proposed as elicitors of defense reactions in higher plants. We tested and compared the ability of purified (1→4)-linked oligomers of GlcNAc (tetramer to decamer) and of GlcN (pentamer and heptamer) and partially N-acetylated chitosans with degrees of acetylation (DA) of 1%, 15%, 35%, 49%, and 60% and average degrees of polymerization between 540 and 1100 to elicit phenylalanine ammonia-lyase (PAL) and peroxidase (POD) activities, lignin deposition, and microscopically and macroscopically visible necroses when injected into the intercellular spaces of healthy, nonwounded wheat (Triticum aestivum L.) leaves. Purified oligomers of (1→4)-linked GlcN were not active as elicitors, whereas purified oligomers of (1→4)-linked GlcNAc with a degree of polymerization ≥ 7 strongly elicited POD activities but not PAL activities. Partially N-acetylated, polymeric chitosans elicited both PAL and POD activities, and maximum elicitation was observed with chitosans of intermediate DAs. All chitosans but not the chitin oligomers induced the deposition of lignin, the appearance of necrotic cells exhibiting yellow autofluorescence under ultraviolet light, and macroscopically visible necroses; those with intermediate DAs were most active. These results suggest that different mechanisms are involved in the elicitation of POD activities by GlcNAc oligomers, and of PAL and POD activities by partially N-acetylated chitosan polymers and that both enzymes have to be activated for lignin biosynthesis and ensuing necrosis to occur.
Plant cells can respond to fungal infection by the activation of induced resistance reactions upon recognition of fungal cell wall components (Albersheim and Anderson-Prouty, 1975; Ride, 1992; Boller, 1995). In plant tissues extracellular chitinases (Collinge et al., 1993) and possibly chitosanases (Grenier and Asselin, 1990), in concert with β-(1→3)-glucanases (Mauch et al., 1988), are likely to partially degrade fungal cell wall polysaccharides. This not only leads to a weakening of the fungal cell wall (Arlorio et al., 1992) but also produces soluble and diffusable fragments that may indicate the presence of a potential pathogen to the plant tissues (Ham et al., 1991; Ryan and Farmer, 1991).
Chitin, a linear polysaccharide composed of GlcNAc residues, forms the fibrillar component of typical fungal cell walls. The fungal cell wall may in addition to chitin also contain chitosan (Peberdy, 1990), the deacetylated counterpart of chitin. Fungal chitosan is most likely biosynthesized from chitin by the action of chitin deacetylase, converting in chain GlcNAc residues to GlcN residues (Araki and Ito, 1975; Davis and Bartnicki-Garcia, 1984; Kafetzopoulos et al., 1993). Thus, fungal cell walls can in principle contain chitin and chitosans with DAs from 0% to 100%.
It has been shown previously that chitin (Pearce and Ride, 1982), chitosan (Pearce and Ride, 1982; Moerschbacher et al., 1986; Gotthardt and Grambow, 1992), and oligomers of β-(1→4)-linked GlcNAc (Barber et al., 1989; Gotthardt and Grambow, 1992), but not GlcN (Barber et al., 1989), are active elicitors of defense-related lignification in wounded (Pearce and Ride, 1982; Barber et al., 1989) and intact (Moerschbacher et al., 1986) wheat (Triticum aestivum L.) leaves and in suspension-cultured wheat cells (Gotthardt and Grambow, 1992). The activity of chitosan was thought to reside in the acetylated regions of the molecule, since fully deacetylated chitosan was inactive (Barber and Ride, 1988). Recently, enzymatic hydrolysates of highly acetylated chitosan (DA of 80%) were shown to be active elicitors in wounded wheat leaves (Mitchell et al., 1994). Problems in the interpretation of these studies arose because of the use of relatively poorly characterized chitins and chitosans as elicitors, as well as because of the relatively unspecific and qualitative rather than quantitative assay of elicitor action. Assays involving wounding of the wheat leaves further complicate the interpretation, since wounding alters the reactivity of the tissues (Barber et al., 1989).
In the current study we used, in addition to purified
β-(1→4)-linked oligomers of GlcN and GlcNAc, chemically
well-characterized and fully water-soluble chitosans. The chitosans had
known (average) Mrs
(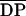 n = 540–1100) and a wide range of
known (average) DAs (1%, 15%, 35%, 49%, and 60%). Furthermore, the
distribution of GlcNAc residues along the polymer chain has been
characterized and found to be random (Vårum et al., 1991a, 1991b).
Samples were applied to the leaves by injection into the intercellular
spaces using a hypodermic syringe, minimizing wounding of the leaves
(Moerschbacher et al., 1989). The elicitation of symptoms known to be
involved in the defense mechanism of wheat to fungal pathogens, such as
an increase in enzyme activities involved in lignin biosynthesis, the
deposition of lignin or a lignin-like polymer, and the formation of
macroscopically and microscopically visible foliar necroses (Beardmore
et al., 1983; Moerschbacher et al., 1988; Moerschbacher, 1989; Ride et
al., 1989; Tiburzy et al., 1990; Moerschbacher and Reisener,
1997), could thus be quantified under near-natural conditions.
n = 540–1100) and a wide range of
known (average) DAs (1%, 15%, 35%, 49%, and 60%). Furthermore, the
distribution of GlcNAc residues along the polymer chain has been
characterized and found to be random (Vårum et al., 1991a, 1991b).
Samples were applied to the leaves by injection into the intercellular
spaces using a hypodermic syringe, minimizing wounding of the leaves
(Moerschbacher et al., 1989). The elicitation of symptoms known to be
involved in the defense mechanism of wheat to fungal pathogens, such as
an increase in enzyme activities involved in lignin biosynthesis, the
deposition of lignin or a lignin-like polymer, and the formation of
macroscopically and microscopically visible foliar necroses (Beardmore
et al., 1983; Moerschbacher et al., 1988; Moerschbacher, 1989; Ride et
al., 1989; Tiburzy et al., 1990; Moerschbacher and Reisener,
1997), could thus be quantified under near-natural conditions.
MATERIALS AND METHODS
Preparation and Characterization of GlcNAc and GlcN Oligomers
Purified GlcNAc oligomers with DPs from 4 to 10 were prepared as described by Bosso et al. (1986), and oligomers of glucosamine with DPs of 5 and 7 were prepared according to the method of Domard and Cartier (1989).
Characterization of the purified GlcNAc oligomers and glucosamine were obtained by 13C-NMR and steric-exclusion chromatography (Bosso et al., 1986; Domard and Cartier, 1989), as well as by MS (Bosso and Domard, 1992).
Preparation and Characterization of Partially N-Acetylated Chitosan Polymers
Water-soluble polymeric chitosans
(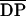 n 540–1100) with DAs from 1% to
60% (Table I), in which the GlcNAc and
GlcN residues are randomly distributed along the polymer chain, were
prepared and the chemical composition (DA) and the intrinsic
viscosities ([η]), which are converted to the number of
average Mr or DPn
(Anthonsen et al., 1993) were determined. It should be emphasized that
the Mr is an average and that the samples
are polydisperse both with respect to Mr
and chemical composition (Anthonsen et al., 1993; Vårum et al., 1994).
n 540–1100) with DAs from 1% to
60% (Table I), in which the GlcNAc and
GlcN residues are randomly distributed along the polymer chain, were
prepared and the chemical composition (DA) and the intrinsic
viscosities ([η]), which are converted to the number of
average Mr or DPn
(Anthonsen et al., 1993) were determined. It should be emphasized that
the Mr is an average and that the samples
are polydisperse both with respect to Mr
and chemical composition (Anthonsen et al., 1993; Vårum et al., 1994).
Table I.
Characterization of chitosans used in this study (Anthonsen et al. [1993]; Vårum et al. [1994])
| DAa | Intrinsic Viscosity | No. of Average Mrb |
|
|
|---|---|---|---|---|
| % | mL g−1 | kD | ||
| 1 | 610 | 170 | 1100 | |
| 15 | 740 | 190 | 1100 | |
| 35 | 410 | 96 | 550 | |
| 49 | 450 | 98 | 540 | |
| 60 | 820 | 180 | 970 |
Determined by NMR.
Determined from intrinsic viscosity measurements.
Calculated from the average Mr.
Bioassays for Elicitor Activity
Primary leaves of 7-d-old wheat (Triticum aestivum L. cv Prelude) plants grown in automatically regulated growth chambers were injected with the test solutions using a hypodermic syringe (Moerschbacher et al., 1989). Leaf segments were harvested into liquid nitrogen 24 h after injection.
A crude enzyme extract (0.1 m borate buffer, pH 8.8, 1 mm EDTA, 1 mm DTT, 25 mg mL−1 insoluble PVP, and 25 mg mL−1 Dowex 1 × 2 [Cl−] quartz sand) was prepared, and the activities of PAL (EC 4.3.1.5.) and POD (EC 1.11.1.7.) were determined (PAL: 0.1 m borate buffer, pH 8.8, 6 mm Phe, 40°C, 290 nm; POD: 0.1 m phosphate buffer, pH 5.8, 18 mm guaiacol, 8 mm H2O2, 30°C, 470 nm) as described previously by Moerschbacher et al. (1988). The protein concentration of the extracts was estimated with Biuret reagent (Gornall et al., 1949).
Determination of Lignin Deposition
Lignin content of elicitor-treated leaves was estimated using the acetyl bromide procedure (Iiyama and Wallis, 1988). Cell walls were prepared from frozen wheat leaves by grinding in liquid nitrogen and then in methanol:water (1:1), followed by sequential extraction with methanol:water (1:1), methanol:chloroform (2:1), acetone, ethanol, and distilled water. Lyophilized and vacuum oven-dried cell walls (5–15 mg) were incubated for 30 min in 2.5 mL of acetyl bromide reagent (25% [v/v] acetyl bromide in acetic acid) and 0.1 mL of 70% perchloric acid at 70°C. Ice-cooled samples were then transferred to a mixture of 10 mL of 2 m NaOH and 12 mL of acetic acid, and the volume was adjusted with acetic acid to 50 mL. After 60 to 120 min, the extinction of the solution was read at 280 nm, and the lignin content was calculated using the molar extinction coefficient for graminaceous lignin of 20 g−1 L cm−1 (Iiyama and Wallis, 1988).
RESULTS
PAL and POD Elicitation by Purified GlcN and GlcNAc Oligomers
Fully acetylated GlcNAc oligomers with DPs from 4 to 10 were injected into the intercellular spaces of wheat leaves at a concentration of 1 mg mL−1, and PAL and POD activities were determined 24 h after injection (Fig. 1). Only oligomers with a DP ≥ 7 induced POD activities, whereas PAL activity was not activated by any of the oligomers. Fully deacetylated GlcN oligomers of DPs 5 and 7 induced neither POD nor PAL activities in the leaves (data not shown).
Figure 1.
Influence of the DP on the activity of purified GlcNAc oligomers (1 mg mL−1; 24 h after injection) as elicitors of PAL and POD in wheat leaves. Data are means ± sd of triplicate samples from one representative of two independent experiments. The dotted lines indicate enzyme activities of water-treated control plants. prot., Protein.
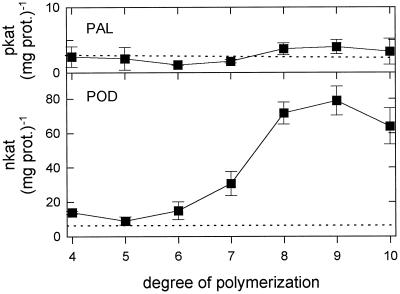
Dose-response curves for PAL and POD activation by the GlcNAc oligomer with a DP of 8 and by the GlcN oligomer with a DP of 7 were established, with concentrations ranging from 0.01 to 1000 μg mL−1 (Fig. 2). Only the GlcNAc oligomer induced POD activities but not PAL activities at concentrations ≥ 500 μg mL−1, whereas neither PAL nor POD activities were activated by the GlcN oligomer.
Figure 2.
Dose-response curves of PAL and POD elicitation by the GlcNAc octamer (▪) and the GlcN heptamer (•) 24 h after injection into the intercellular spaces of primary wheat leaves. Data are means ± sd of triplicate samples from one representative of two independent experiments. The dotted lines indicate enzyme activities of water-treated control plants. prot., Protein.
PAL and POD Elicitation by Polymeric Chitosans with Different DAs
Polymeric chitosans with 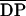 ns
from 540 to 1100 and DAs ranging from 1% to 60% were injected
into intact wheat leaves at a concentration of 100 μg
mL−1, and PAL and POD activities were determined
24 h after injection (Fig. 3).
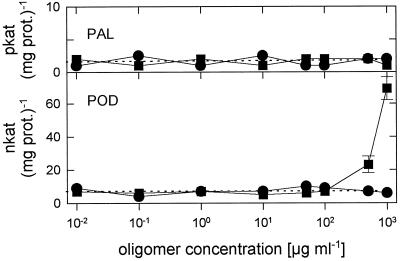
Chitosans with a DA of less than 20% strongly induced PAL activity,
but POD activities were only weakly activated. With increasing DA up to
35%, both PAL and POD induction increased. No further increase in PAL
induction was seen with DAs of 50% to 60%, whereas POD induction
increased dramatically.
ns
from 540 to 1100 and DAs ranging from 1% to 60% were injected
into intact wheat leaves at a concentration of 100 μg
mL−1, and PAL and POD activities were determined
24 h after injection (Fig. 3).
Chitosans with a DA of less than 20% strongly induced PAL activity,
but POD activities were only weakly activated. With increasing DA up to
35%, both PAL and POD induction increased. No further increase in PAL
induction was seen with DAs of 50% to 60%, whereas POD induction
increased dramatically.
Figure 3.
Influence of the DA on the activity of chitosan
polymers (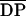 n = 540–1100; 100 μg
mL−1; 24 h after injection) as elicitors of PAL and
POD activities in wheat leaves. Data are means ± sd
of triplicate samples from one representative of two independent
experiments. The dotted lines indicate enzyme activities of
water-treated control plants. prot., Protein.
n = 540–1100; 100 μg
mL−1; 24 h after injection) as elicitors of PAL and
POD activities in wheat leaves. Data are means ± sd
of triplicate samples from one representative of two independent
experiments. The dotted lines indicate enzyme activities of
water-treated control plants. prot., Protein.
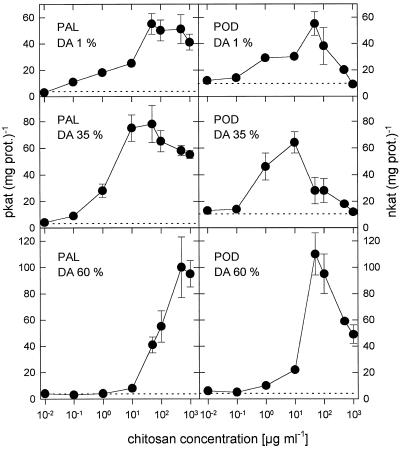
Dose-response curves for PAL and POD activation by the chitosans with DAs of 1%, 35%, and 60% were established with concentrations ranging from 0.01 to 1000 μg mL−1 (Fig. 4). Almost fully deacetylated chitosan polymers (DA of 1%) elicited both enzymes, with half-maximum enzyme elicitation at a concentration of about 10 μg mL−1. With increasing DA the chitosans became more potent elicitors of both enzymes, with about 1 μg mL−1 of the DA 35% chitosan achieving half-maximum enzyme elicitation. When the DA of the chitosans was increased even further, their elicitor activity decreased; about 100 μg mL−1 DA 60% chitosan was required for half-maximum PAL elicitation and about 20 μg mL−1 achieved half-maximum POD elicitation. PAL activation remained about constant at higher chitosan concentrations, whereas POD activation drastically declined: at 1 mg mL−1 DA 1% and DA 35% chitosans, POD activities were not higher than in water-treated control leaves.
Figure 4.
Dose-response curves of PAL and POD elicitation by polymeric chitosans with DAs of 1%, 35%, and 60% 24 h after injection into the intercellular spaces of primary wheat leaves. Data are means ± sd of triplicate samples from one representative of two independent experiments. The dotted lines indicate enzyme activities of water-treated control plants. prot., Protein.
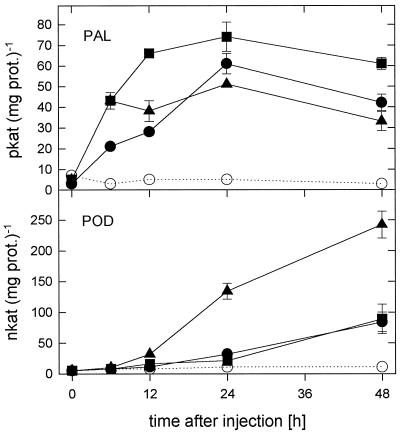
Time-response curves for PAL and POD activation by the chitosans with DAs of 1%, 35%, and 60% at 100 μg mL−1 were established (Fig. 5). PAL activity was already increased 6 h after injection of either of the chitosans and reached maximum activities at 24 h after injection. In contrast, POD activities started to increase only from 12 to 24 h after chitosan injection but were still increasing 48 h past injection. A strong increase in POD activities was especially striking with the chitosan DA of 60%.
Figure 5.
Time-response curves of PAL and POD elicitation by polymeric chitosans with DAs of 1% (circles), 35% (squares), and 60% (triangles) at 100 μg mL−1 upon injection into the intercellular spaces of primary wheat leaves. Data are means ± sd of triplicate samples from one representative of two independent experiments. The dotted lines indicate enzyme activities of water-treated control plants. prot., Protein.
Macroscopically Visible Necrosis Elicited by GlcNAc Oligomers, GlcN Oligomers, and Partially Deacetylated Chitosan Polymers
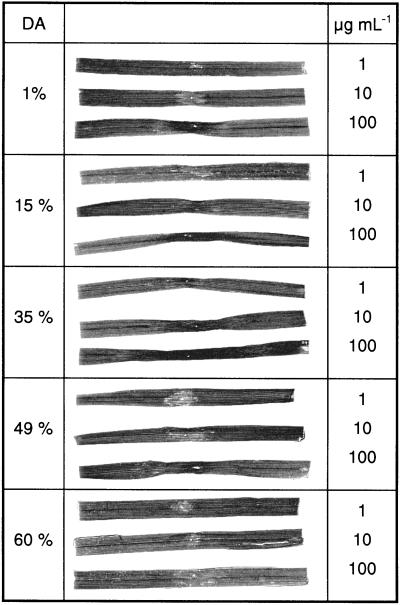
When fully acetylated GlcNAc oligomers with a DP varying from 4 to 10 or fully deacetylated GlcN oligomers with a DP of 5 or 7 were injected into the intercellular spaces of wheat leaves at a concentration of 1 mg mL−1, no symptoms were visible macroscopically up to several days after injection. In contrast, necroses were visible by 24 h after injection of 1 to 100 μg mL−1 partially deacetylated polymeric chitosans (Fig. 6). The chitosan with a DA of 35% clearly was the most potent elicitor, because the whole injected area exhibited severe necrosis. The almost fully deacetylated chitosan with a DA of 1% elicited strong necrosis in the zone surrounding the injection site, but the more distal portions of the injected area appeared more or less healthy. Symptoms were least marked with the most highly acetylated chitosan used (DA of 60%), which induced necrotic spots in a slightly chlorotic area around the injection site smaller than the chitosan-injected area of the leaves.
Figure 6.
Macroscopically visible symptoms induced in wheat leaves by polymeric chitosans with DAs of 1%, 15%, 35%, 49%, and 60%, at 1, 10, and 100 μg mL−1, 24 h after injection into the intercellular spaces of primary wheat leaves.
Fluorescence-Microscopically Visible Symptoms Elicited by GlcNAc Oligomers, GlcN Oligomers, and Partially Deacetylated Chitosan Polymers
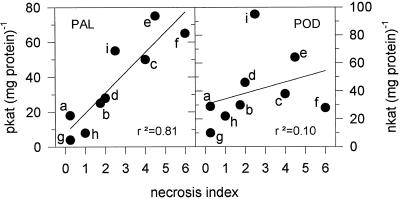
No symptoms other than a narrow ring of necrotic cells surrounding the injection site were visible after injection of water, fully acetylated GlcNAc oligomers (DPs from 4–10), or fully deacetylated GlcN oligomers (DP of 5 or 7). In contrast, necrotic cells exhibiting alkali-stable (0.5 n NaOH, 48 h, 20°C), yellow autofluorescence indicative of lignin deposition appeared in the injected area after injection of partially deacetylated polymeric chitosans. In all cases, the extent of microscopically visible autofluorescence closely correlated with the severity of macroscopically visible necroses. The extent of necroses correlated more closely with the induced PAL activities than with POD activities (Fig. 7).
Figure 7.
Correlation between the extent of foliar necroses and the induction of PAL and POD activities by chitosans with DAs of 1% (a–c), 35% (d–f), and 60% (g–i), at 1 (a, d, and g), 10 (b, e, and h), and 100 (c, f, and i) μg mL−1, 24 h after injection into the intercellular spaces of primary wheat leaves. Necroses were arbitrarily quantified by macroscopic and UV-fluorescence microscopic examination on a necrosis index scale from 0 to 6. Macroscopic effects: 0, no symptoms visible; 2, central part of the injected area chlorotic; 4, central part of injected area necrotic, single chlorotic spots toward the periphery; 6, all of the injected area necrotic. Microscopic effects: 0, only cells directly at the injection site showed yellow autofluorescence under UV light; 2, many collapsed and yellow autofluorescing cells in the central part of the injected area, sparse clusters of such cells toward the periphery; 4, all of the cells in the central part of the injected area and many cells in the periphery collapsed and exhibited yellow autofluorescence; 6, all of the cells in the injected area collapsed and exhibited yellow autofluorescence.
Lignin Deposition Elicited by Partially Deacetylated Polymeric Chitosans
Polymeric chitosans with 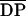 ns
from 540 to 1100 and DAs of 1%, 35%, and 60% were injected into
intact wheat leaves at a concentration of 100 μg
mL−1, and the lignin content of the treated leaf
areas was determined 24 h after injection (Table
II). All chitosans induced a significant
accumulation of lignin, with the most active being the chitosan with a
DA of 35%.
ns
from 540 to 1100 and DAs of 1%, 35%, and 60% were injected into
intact wheat leaves at a concentration of 100 μg
mL−1, and the lignin content of the treated leaf
areas was determined 24 h after injection (Table
II). All chitosans induced a significant
accumulation of lignin, with the most active being the chitosan with a
DA of 35%.
Table II.
Lignin content of cell walls isolated from wheat leaves 24 h after injection of polymeric chitosans with different DAs
| Sample | Lignin Content | Control |
|---|---|---|
| % (w/w) of cell wall | % | |
| Water | 10.92 ± 0.79 | 100 |
| DA-1% | 12.84 ± 0.82 | 118 |
| DA-35% | 14.51 ± 0.33 | 133 |
| DA-60% | 12.82 ± 0.28 | 117 |
Data are means ± sd of triplicate samples. The lignin contents determined in chitosan-treated leaves were significantly (P < 0.05) different from those of water-treated control plants.
DISCUSSION
Our studies corroborated some of the earlier findings, namely, elicitor activity of partially N-acetylated chitosan polymers and β-(1→4)-linked GlcNAc oligomers, but not GlcN oligomers. Surprisingly, however, differential effects of the elicitors toward different enzymes used as markers for active resistance responses were observed, and the macroscopic symptoms exhibited by the elicitor-treated leaves differed significantly. Fully deacetylated GlcN oligomers elicited neither PAL nor POD activities, fully acetylated GlcNAc oligomers strongly elicited POD activities at rather high concentrations, but PAL activity was not induced at all. Neither of the oligomers induced any macroscopically or microscopically visible symptoms. In contrast, partially acetylated chitosan polymers elicited both enzymes and led to lignin deposition and development of foliar necroses. These differences between the effects of GlcNAc oligomers and chitosan polymers in wheat leaves suggest different modes of action of these elicitors.
GlcNAc oligomers are most likely recognized by specific lectin-like receptors. Lectins specific for GlcN or GlcNAc have been implicated in the recognition of chitosan (Liénart et al., 1991) and chitin (Etzler, 1985) oligomers, respectively, and high-affinity binding sites for GlcNAc oligomers that are not inhibited by GlcN oligomers have been described in suspension-cultured rice and tomato cells that can be elicited by chitin but not by chitosan oligomers (Shibuya et al., 1993; Baureithel et al., 1994). In wheat leaves the GlcNAc-specific lectin wheat germ agglutinin might be involved in the recognition of chitin oligomers (Ride et al., 1989), triggering a specific induction of POD, which alone does not lead to lignin deposition. GlcNAc oligomers did induce lignification in prewounded wheat leaves (Barber et al., 1989), possibly because of the activation of PAL and the general phenylpropanoid pathway by mechanical wounding (Menden, 1995). The rather high concentrations of GlcNAc oligomers needed for elicitation in intact wheat leaves (1 mg mL−1 = 30 μg per leaf) are in the same range as reported for wounded wheat leaves (40 μg per wound, Barber et al. [1989]) and melon plants (50 μg per plant, Roby et al. [1987]). Suspension-cultured wheat cells, on the other hand, were much more sensitive, reacting in the nanomolar range (Vander, 1995), as reported for suspension-cultured cells of tomato and rice (Felix et al., 1993; Yamada et al., 1993).
In contrast, the observed elicitor activity of highly deacetylated chitosan polymers and the inactivity of GlcN oligomers may be explained by a mechanism independent of specific receptors (Kauss et al., 1989). The polycationic polymers may interact with negatively charged phospholipids, thus disturbing the integrity of the plant plasma membrane. Consequently, their elicitor activity should decrease with increasing DAs (Kauss et al., 1989). However, this was not the case in our study. The maximum PAL and POD activities induced increased with increasing DA, whereas lignin deposition and symptom development were strongest with chitosans of intermediate DA. The extent of foliar necrotization correlated more closely with the induced PAL activities than with POD activities, possibly because POD became trapped in the developing lignin polymer during heavy necrotization (McDougall, 1993). The chitosan with the highest DA (60%) induced only weak necroses despite substantial elicitation of both PAL and POD activities. It is interesting that this resembles the recently reported results of the toxicity of partially acetylated chitosans toward human epithelial cells, which decreased with increasing DA (Schipper et al., 1996). The discrepancy seen with the chitosan with a DA of 60% may hint at a different step, such as the reduction of the phenylpropanoic acids to the corresponding alcohols or the production of hydrogen peroxide, being the limiting step in the biosynthesis of lignin in these elicited leaves.
We suggest that the interpretation of the observed elicitor activities of partially acetylated chitosan polymers is complex because of their expected degradation by apoplastic wheat leaf endochitinases (Ride and Barber, 1990). Whereas the fully water-soluble chitosans used in this study were well characterized with respect to DA and the random distribution of GlcNAc residues along the polymer chain (Vårum et al., 1991a, 1991b, 1994), the substrate specificity of the wheat leaf chitinases and/or chitosanases toward partially acetylated chitosans is unknown. Consequently, the detailed chemical composition and DP of the oligomers resulting from a possible degradation of an injected chitosan by apoplastic wheat leaf chitinases are unknown. Our results suggest that some of the fragments resulting from enzymic degradation of partially acetylated chitosans may be highly elicitor active and that studies of the biological activity of purified partially acetylated oligomers and small polymers of β-(1→4)-linked GlcNAc and GlcN are needed to obtain a more detailed understanding of the resistance reactions in wheat leaves elicited by partially acetylated chitosans and, hence, fungal cell walls.
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of U. Noll and U. Beike and the committed help of S. Horn with preparation of the manuscript. We also thank Dr. K. Hicks for initial provision of chitin oligomers of defined DP.
Abbreviations:
- DA
degree of acetylation
- DP
degree of polymerization
-
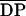 n
n
number of average degree of polymerization
- GlcN
2-amino-2-deoxy-β-d-glucopyranose
- GlcNAc
2-acetamido-2-deoxy-β-d-glucopyranose
- PAL
Phe ammonia-lyase
- POD
peroxidase
Footnotes
This project was supported in part by the Deutsche Forschungsgemeinschaft and the Norwegian Research Council.
LITERATURE CITED
- Albersheim P, Anderson-Prouty AJ. Carbohydrates, proteins, cell surfaces, and biochemistry of pathogenesis. Annu Rev Plant Physiol. 1975;26:31–52. [Google Scholar]
- Anthonsen MW, Vårum KM, Smidsrød O. Solution properties of chitosans: conformation and chain stiffness of chitosans with different degrees of N-acetylation. Carbohydr Polym. 1993;22:193–201. [Google Scholar]
- Araki Y, Ito E. A pathway of chitosan formation in M. rouxii. Eur J Biochem. 1975;55:71–78. doi: 10.1111/j.1432-1033.1975.tb02139.x. [DOI] [PubMed] [Google Scholar]
- Arlorio M, Ludwig A, Boller T, Bonfante P. Inhibition of fungal growth by plant chitinases and β-1,3-glucanases. A morphological study. Protoplasma. 1992;171:34–43. [Google Scholar]
- Barber MS, Bertram RE, Ride JP. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol Mol Plant Pathol. 1989;34:3–12. [Google Scholar]
- Barber MS, Ride JP. A quantitative assay for induced lignification in wounded wheat and its use to survey potential elicitors of the response. Physiol Mol Plant Pathol. 1988;32:185–197. [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high-affinity binding of chitin fragments to tomato cells and membranes. Competitive inhibition of binding by derivatives of chitooligosaccharides and a nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Beardmore J, Ride JP, Granger JW. Cellular lignification as a factor in the hypersensitive resistance of wheat to stem rust. Physiol Plant Pathol. 1983;22:209–220. [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Bosso C, Defaye J, Domard A, Gadelle A. The behavior of chitin towards anhydrous hydrogen fluoride. Preparation of β-(1→4)-linked 2-acetamido-2-deoxy-d-glucopyranosyl oligosaccharides. Carbohydr Res. 1986;156:57–68. [Google Scholar]
- Bosso C, Domard A. Characterization of glucosamine and N-acetylglucosamine oligomers by fast atom bombardment mass spectrometry. Org Mass Spectrom. 1992;27:799–806. [Google Scholar]
- Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. Plant J. 1993;3:31–40. doi: 10.1046/j.1365-313x.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- Davis LL, Bartnicki-Garcia S (1984) A model for the mechanism and regulation of chitosan synthesis in Mucor rouxii. In WM Dugger, S Bartnicki-Garcia, eds, Structure, Function, and Biosynthesis of Plant Cell Walls. American Society of Plant Physiologists, Rockville, MD, pp 400–408
- Domard A, Cartier N. Glucosamine oligomers. 1. Preparation and characterization. Int J Biol Macromol. 1989;11:297–302. doi: 10.1016/0141-8130(89)90023-8. [DOI] [PubMed] [Google Scholar]
- Etzler ME. Plant lectins: molecular and biological aspects. Annu Rev Plant Physiol. 1985;36:209–234. [Google Scholar]
- Felix G, Regenass M, Boler T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Gotthardt U, Grambow HJ. Near-isogenic wheat suspension cultures: establishment, elicitor induced peroxidase activity and potential use in the study of host/pathogen-interactions. J Plant Physiol. 1992;139:659–665. [Google Scholar]
- Grenier J, Asselin A. Some pathogenesis-related proteins are chitosanases with lytic activity against fungal spores. Mol Plant-Microbe Interact. 1990;3:401–407. [Google Scholar]
- Ham KS, Kauffmann S, Albersheim P, Darvill AG. Host-pathogen interactions. XXXIX. A soybean pathogenesis-related protein with β-1,3-glucanase activity releases phytoalexin elicitor-active heat-stable fragments from fungal walls. Mol Plant-Microbe Interact. 1991;4:545–552. [Google Scholar]
- Iayama K, Wallis AFA. Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agric. 1988;51:145–161. [Google Scholar]
- Kafetzopoulos D, Martiniou A, Bouriotis V. Proc Natl Acad Sci USA. 1993;90:2564–2568. doi: 10.1073/pnas.90.7.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W, Domard A. The degrees of polymerization and N-acetylation of chitosan determine its ability to elicit callose formation in suspension cells and protoplasts of Catharanthus roseus. Planta. 1989;178:385–392. doi: 10.1007/BF00391866. [DOI] [PubMed] [Google Scholar]
- Liénart Y, Gautier C, Domard A. Isolation from Rubus cell-suspension cultures of a lectin specific for glucosamine oligomers. Planta. 1991;184:8–13. doi: 10.1007/BF00208229. [DOI] [PubMed] [Google Scholar]
- Mauch F, Mauch-Mani B, Boller T. Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. Solubilization of wall-bound peroxidases by limited proteolysis. Phytochemistry. 1993;33:765–767. [Google Scholar]
- Menden B (1995) Biochemische Analysen zur Bedeutung von Phenolsäuren und Lignin für Resistenz und Anfälligkeit von Weizenpflanzen gegen Schwarzrost (Puccinia graminis f. sp. tritici). PhD thesis. Rheinisch-Westfälische Technische Hochschule, Aachen, Germany
- Mitchell HJ, Hall JL, Barber MS. Elicitor induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol. 1994;104:551–556. doi: 10.1104/pp.104.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerschbacher B, Kogel KH, Noll U, Reisener HJ. An elicitor of the hypersensitive lignification response in wheat leaves isolated from the rust fungus Puccinia graminis f. sp. tritici. I. Partial purification and characterization. Z Naturforsch. 1986;41c:830–838. [Google Scholar]
- Moerschbacher BM. Lignin biosynthesis in stem rust infected wheat. ACS Symp Ser. 1989;399:370–382. [Google Scholar]
- Moerschbacher BM, Flott BE, Noll U, Reisener HJ. On the specificity of an elicitor preparation from stem rust which induces lignification in wheat leaves. Plant Physiol Biochem. 1989;27:305–314. [Google Scholar]
- Moerschbacher BM, Noll UM, Flott BE, Reisener HJ. Lignin biosynthetic enzymes in stem rust infected, resistant and susceptible near-isogenic wheat lines. Physiol Mol Plant Pathol. 1988;33:33–46. [Google Scholar]
- Moerschbacher BM, Reisener HJ (1997) The hypersensitive resistance reaction. In H Hartleb, R Heitefuss, HH Hoppe, eds, Resistance of Crop Plants against Fungi. Gustav Fischer Verlag, Jena, Germany, pp 126–158
- Pearce RB, Ride JP. Chitin and related compounds as elicitors of the lignification response in wounded wheat leaves. Physiol Plant Pathol. 1982;20:119–123. [Google Scholar]
- Peberdy JF. Fungal cell walls—a review. In: Kuhn PJ, Trinci APJ, Jung MJ, Goosey MW, Copping LG, editors. Biochemistry of Cell Walls and Membranes in Fungi. Berlin: Springer Verlag; 1990. pp. 5–30. [Google Scholar]
- Ride JP. Recognition signals and initiation of host responses controlling basic incompatibility between fungi and plants. Soc Exp Biol Semin Ser. 1992;48:213–237. [Google Scholar]
- Ride JP, Barber MS. Purification and characterization of multiple forms of endochitinase from wheat leaves. Plant Sci. 1990;71:185–197. [Google Scholar]
- Ride JP, Barber MS, Bertram RE. Infection-induced lignification in wheat. ACS Symp Ser. 1989;399:361–369. [Google Scholar]
- Roby D, Gadelle A, Toppan A. Chitin oligosaccharides as elicitors of chitinase activity in melon plants. Biochem Biophys Res Commun. 1987;143:885–892. doi: 10.1016/0006-291x(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Ryan CA, Farmer EE. Oligosaccharide signals in plants: a current assessment. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:651–674. [Google Scholar]
- Schipper NG, Vårum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1. Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res. 1996;13:1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Kaku H, Kuchitsu K, Maliarik MJ. Identification of a novel high-affinity binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction of suspension-cultured rice cells. FEBS Lett. 1993;329:75–78. doi: 10.1016/0014-5793(93)80197-3. [DOI] [PubMed] [Google Scholar]
- Tiburzy R, Noll U, Reisener HJ. Resistance of wheat to Puccinia graminis f. sp. tritici: histological investigation of resistance caused by the Sr5 gene. Physiol Mol Plant Pathol. 1990;36:95–108. [Google Scholar]
- Vander P (1995) Solvolysen in wasserfreiem Fluorwasserstoff als Methode zur Fraktionierung pilzlicher Zellwände und Untersuchungen zur biologischen Aktivität von Chitin und Chitosanen in Weizen. PhD thesis. Rheinisch-Westfälische Technische Hochschule, Aachen, Germany
- Vårum KM, Anthonsen MW, Grasdalen H, Smidsrød O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field n.m.r spectroscopy. Carbohydr Res. 1991a;211:17–23. doi: 10.1016/0008-6215(91)84142-2. [DOI] [PubMed] [Google Scholar]
- Vårum KM, Anthonsen MW, Grasdalen H, Smidsrød O. 13C-N.m.r studies of the acetylation sequences in partially N-deacetylated chitins (chitosans) Carbohydr Res. 1991b;217:19–27. doi: 10.1016/0008-6215(91)84113-s. [DOI] [PubMed] [Google Scholar]
- Vårum KM, Ottøy MH, Smidsrød O. Water-solubility of partially N-acetylated chitin (chitosans) at neutral pH: effect of chemical composition and depolymerization. Carbohydr Polym. 1994;25:65–70. [Google Scholar]
- Yamada T, Shibuya N, Kodama O, Akatsuka T. Induction of phytoalexin formation in suspension-cultured rice cells by N-acetyl-chitooligosaccharides. Biosci Biotech Biochem. 1993;57:405–409. [Google Scholar]