Abstract
In this study we present data to support the role for Cdk2ap2 in regulating self-renewal of mouse embryonic stem cells (mESCs) under permissive conditions, and cell survival during differentiation of the mESCs into terminally differentiated cell types. To understand the function of Cdk2ap2 during early development, we generated mESCs with homozygous disruption of the endogenous Cdk2ap2 locus (Cdk2ap2tr/tr). The Cdk2ap2tr/tr mESCs, when grown in a complete growth medium containing leukemia inhibitory factor (LIF), showed an early differentiation phenotype characterized by flattened colonies and a distinct intercellular boundary. We also observed downregulation of Nanog and upregulation in markers of mesoderm and endoderm differentiation, including Brachyury (T), Afp, and S100a, when compared to Wt mESCs. Cdk2ap2tr/tr mESCs were able to form embryoid bodies (EBs); however, those EBs were unhealthy and had an increased level of apoptosis. Furthermore, Cdk2ap2tr/tr mESCs were unable to form teratomas in severe combined immunodeficiency (SCID) mice. Cdk2ap2 under normal conditions has a biphasic expression, suggesting regulatory roles in early-versus-late stem cell differentiation. These data begin to add to our understanding of how Cdk2ap2 may be involved in the regulation of self-renewal of stem cells during early embryogenesis.
Introduction
The Cdk2-associated protein 2 gene, CDK2AP2, was identified in an in silico analysis of the expressed sequence-tag database using the CDK2AP1 sequence as the bait [1]. CDK2AP2, which maps to chromosome 11q13.1 in humans and 19qA in mouse, is expressed ubiquitously in adult tissues [1,2]. The CDK2AP2 protein shows a very high degree of similarity with orthologs from lower order vertebrates, suggesting an evolutionarily conserved function for this gene.
Terret et al. identified Cdk2ap2 as an interacting partner of mitogen-activated protein kinase (MAPK), and a target for phosphorylation by MAPK and Cyclin B/Cdc2 during meiosis in mouse oocytes [3]. In a subsequent study, Buajeeb et al. showed an interaction between CDK2AP2 and CDK2AP1 in a yeast-two hybrid [4]. Although cellular and in vivo functions of CDK2AP2 remain largely unknown, the study of Cdk2ap2 function in mouse oocytes revealed a potential role for Cdk2ap2 in the meiosis process. Analyses of intracellular localization of Cdk2ap2 in oocytes showed a shift from diffused nucleocytoplasmic expression to distinct localization to meiotic spindles in maturing oocytes [3]. Depletion of Cdk2ap2 expression in oocytes resulted in multiple and aberrant aster formation and a severe arrest of oocytes in metaphase II stage, thus supporting a role for Cdk2ap2 in meiosis and terminal maturation of oocytes. In a recent study to identify genes involved in leukemia inhibitory factor (LIF)-mediated maintenance of pluripotency and self-renewal in mouse embryonic stem cells (mESCs), Zhu et al. identified that both Cdk2ap1 and Cdk2ap2 preferentially clustered with the cell cycle-related genes that are important in self-renewal of mESCs [5]. These data, taken together, place Cdk2ap2 in a role central to early mammalian development.
To understand the function of Cdk2ap2 during early development, we generated mESCs with homozygous disruption of the endogenous Cdk2ap2 locus (Cdk2ap2tr/tr). Analyses of these cells showed a spontaneous early differentiation phenotype under self-renewing conditions. Cdk2ap2tr/tr mESCs were incapable of forming terminally differentiated cells/tissues in a teratoma assay and fail to form a proper embryoid body (EB) after induction of differentiation. Forced terminal differentiation resulted in increased apoptotic cell death in the Cdk2ap2tr/tr EB, suggesting that Cdk2ap2 may be required for cell survival during terminal differentiation. Together, our data support a novel role for Cdk2ap2 in maintenance of mESC self-renewal under permissive conditions and for cell survival during differentiation.
Materials and Methods
mES cell culture and generation of homozygous clones
The gene-trap clone PST11316 and the corresponding Wt D3 ESC lines were obtained from the Mammalian Functional Genomics Centre at the University of Manitoba. ESCs were cultured on gelatinized tissue culture plates in an ES complete growth medium (ESCGM; high-glucose Dulbecco's modified Eagle's medium supplemented with 15% knockout serum replacement [Invitrogen], nonessential amino acids, 150 μM β-mercaptoethanol, and ∼1000 U/mL of LIF), on a 0.2%-gelatin-coated plate. The medium was changed every 3 days, and cells were passaged at ∼70%–80% confluence. All cell culture reagents were purchased from HyClone™ unless stated otherwise.
ESCs were grown in 1.5 mg/mL of G418 to force homologous recombination of the Cdk2ap2 locus. Individual colonies were picked, expanded, and genotyped by polymerase chain reaction (PCR).
EB formation assay
EBs were formed according to the hang-drop protocol [6]. EBs were harvested at days 0, 2, 5, and 10 of use in the expression analysis. For further differentiation, EBs were plated onto 48-well gelatinized plates and treated with 5 μM retinoic acid (RA) or dimethyl sulfoxide (DMSO).
Lentiviral cDNA construct
The CDK2AP2 open-reading frame was PCR-amplified and cloned into the pHAGE lentivirus vector. Viral transduction of mESCs was carried out in the ESCGM supplemented with 5 μg/mL Polybrene (Sigma). The medium was replaced after 6 h with a fresh ESCGM, and cells were grown for 48 h before use in further experiments.
Teratoma formation assay
The teratoma assay was performed as described [7]. Animals were euthanized at 4 weeks after the ES cell injection. Tumors and testes were extracted, fixed in 4% paraformaldehyde overnight, and embedded in paraffin for histology. Various structures in the tissues were visualized using hematoxylin and eosin (H&E) staining.
Imaging
Cultured cells and EBs were imaged on a Leica DM IL inverted microscope. Histology sections were imaged on a Leica LMD microscope. Images were captured with an AxioCam MR CCD monochrome camera and AxioVision software (Carl Zeiss). Immunofluorescence was performed using an Olympus IX80 light fluorescent microscope. EBs were stained with a primary antibody against Annexin V (abcam) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (vectashield).
Reverse transcriptase–quantitative PCR
EBs were maintained for up to 10 days in culture and harvested for total RNA using the TRI Reagent (Molecular Research Center), according to the manufacturer's protocol. An equal amount of total RNA (2 μg) was used to quantitatively compare the expression level of various genes. An ABI 7700 real-time PCR machine with SYBR Green PCR master mix (Applied Biosystems) was used for quantitative analysis. The experiment was normalized against glyceraldehyde-3-phosphate dehydrogenase. The fold change was determined by the ΔΔCt method. Primer sequences will be available upon request.
Western blot analysis
To detect Oct4 and Nanog protein expression, cells were lyzed in an EDTA lysis buffer (ELB) (50 mM HEPES [pH 7.2], 250 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 0.1% NP-40, and 1 mM dithiothreitol) containing phosphatase and protease inhibitors. Twenty-five micrograms of total protein lysate was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane by standard procedures. α-Nanog (Bethyl Laboratories; No. A300-397A) and α-Oct-4 (Cell Signaling; No. 2750) antibodies were used, and proteins were detected using horseradish peroxidase-conjugated donkey anti-mouse or anti-rabbit antibodies (Jackson Immunosciences).
DNA-laddering assay
EBs and floating cells in the differentiating cultures were collected by centrifugation. Genomic DNA from cell pellets was isolated according to a previously published protocol [8]. DNA laddering was analyzed by electrophoresis on a 0.8% agarose gel [9].
Statistical analysis
Statistical significance was determined using the 2-tailed Student's t-test.
Results
Cdk2ap2tr/tr mESCs display spontaneous differentiation in vitro
We generated homozygous gene-trap mESCs from the International Gene Trap Consortium (IGTC) clone PST11316 (Cdk2ap2tr/+). These cells have a retroviral neomycin trap cassette with a strong acceptor site inserted into the third intron that disrupts endogenous Cdk2ap2 gene expression. Multiple mESC clones with a loss of heterozygosity (LoH) of the Cdk2ap2 locus were generated by growing the cells in the presence of high concentrations of G418 (1.5–2 mg/mL) for 1 week. Individual colonies were identified, expanded, and analyzed for the presence of homozygous Cdk2ap2tr alleles by PCR genotyping (Fig. 1A), northern blot (Fig. 1B), and reverse transcriptase–quantitative PCR (RT-qPCR) analyses (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). No Cdk2ap2 expression was seen in the northern blot analyses, suggesting that the gene-trap vector abolished almost all expression of the endogenous Cdk2ap2 gene, rendering this virtually a Cdk2ap2-null allele. Low levels of Cdk2ap2 expression seen in the RT-qPCR analysis could possibly be from the contaminating murine embryonic fibroblast (MEF) cells that were used to culture the mESCs.
FIG. 1.
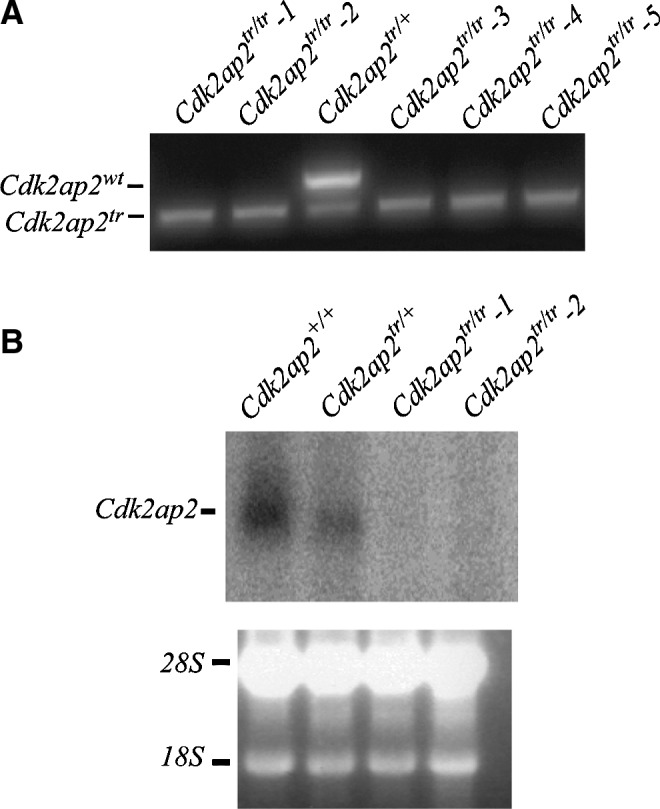
Genotyping and expression analyses of Cdk2ap2tr/tr mESCs. (A) PCR genotyping of various mESC clones with LoH of the Cdk2ap2 locus after high G418 selection. The lower band shows presence of the neoR allele, and the upper band indicates endogenous Wt Cdk2ap2 allele. (B) Northern blot analyses for Cdk2ap2 expression. Lower panel, ethidium bromide-stained gel showing equal loading. mESCs, mouse embryonic stem cells; PCR, polymerase chain reaction; LoH, loss of heterozygosity.
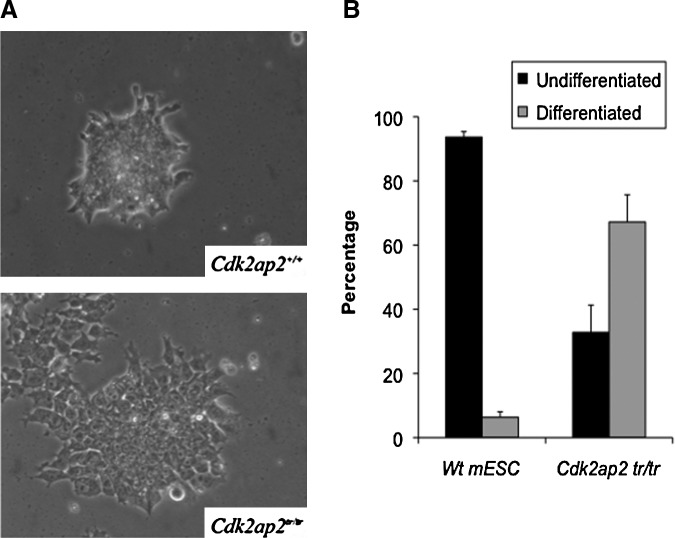
The Cdk2ap2tr/tr mESCs, when grown in a complete growth medium containing LIF, showed an early differentiation phenotype characterized by flattened colonies and a distinct intercellular boundary (Fig. 2A). This is in contrast to Wt and Cdk2ap2tr/+ mESCs, which grow as tight colonies with smooth borders. These phenotypes have been correlated with loss of stem cell self-renewal and spontaneous in vitro differentiation [10]. Furthermore, a colony formation assay was performed to assess what percentage of mESC colonies differentiated in the Wt compared to the Cdk2ap2tr/tr mESCs. In the Cdk2ap2tr/tr mESCs, there were significantly more differentiated colonies at 67.2% compared to the minor 6.3% differentiation seen in the Wt mESCs (Fig. 2B). These data suggest that the loss of Cdk2ap2 expression resulted in an inability of the mESCs to self-renew and possibly maintained there a pluripotent state.
FIG. 2.
Spontaneous differentiation phenotype in Cdk2ap2tr/tr mESCs. (A) Deletion of Cdk2ap2 resulted in an inability of the mESCs to grow in defined colonies with smooth borders that are characteristic of mESCs (upper panel). Instead, the Cdk2ap2tr/tr mESCs grew in a monolayer with a distinctly visible cytoplasm compared to the Wt cells where the nucleus and cytoplasm are indistinct. (B) Colony formation assay was performed by comparing Wt and Cdk2ap2tr/tr mESCs. Cdk2ap2tr/tr mESCs had significantly greater differentiation compared to the Wt colonies.
Cdk2ap2tr/tr mESCs differentially downregulated Nanog expression
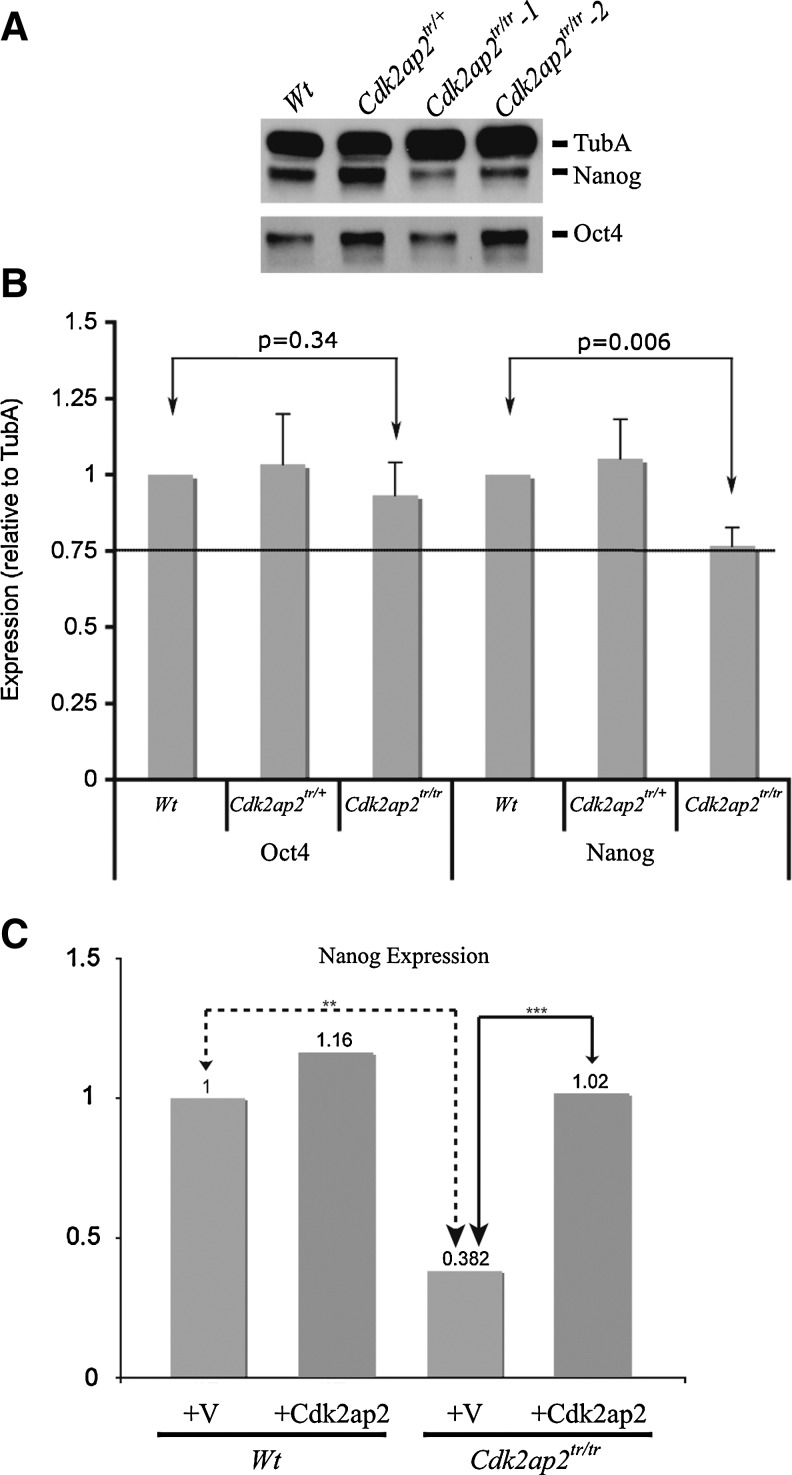
ESCs are characterized by the expression of pluripotency factors, Oct4 and Nanog [11]. After induction of differentiation, expression of Oct4 and Nanog is downregulated, either equally or differentially, in the resulting germ lineages [12,13]. Because the Cdk2ap2tr/tr mESCs fail to maintain the normal growth phenotype, we analyzed the expression of Nanog and Oct4 by Western blot and RT-qPCR analyses in the Wt, Cdk2ap2tr/+, and Cdk2ap2tr/tr mESCs (Fig. 3). Densitometric analysis showed that the levels of the Nanog protein were downregulated in the Cdk2ap2tr/tr mESCs by about 25% (P=0.006; Fig. 3B). No significant change in Oct4 expression was observed in these cells.
FIG. 3.
Loss of Cdk2ap2 expression results in downregulation of Nanog expression. Western blot analyses (A) and quantitative densitometry (B) for Oct4 and Nanog expression in Cdk2ap2tr/tr mESCs showed reduced expression of Nanog (P<0.05), but not of the Oct4 protein. RT-qPCR analyses showed that this reduction of expression was transcriptional, and re-expression of wt-Cdk2ap2 rescued Nanog expression to almost Wt levels (C). RT-qPCR, reverse transcriptase–quantitative PCR.
To test if Nanog expression was a direct result of loss of Cdk2ap2 expression, Wt and Cdk2ap2tr/tr mESCs were transduced with a lentivirus expressing the wt-CDK2AP2 cDNA. RT-qPCR analysis showed that Nanog expression was rescued to Wt levels (Fig. 3C). This supports that downregulation of Nanog expression is a direct consequence from the loss of Cdk2ap2 expression.
Differential upregulation of mesoderm and endoderm lineage markers in Cdk2ap2tr/tr mESCs
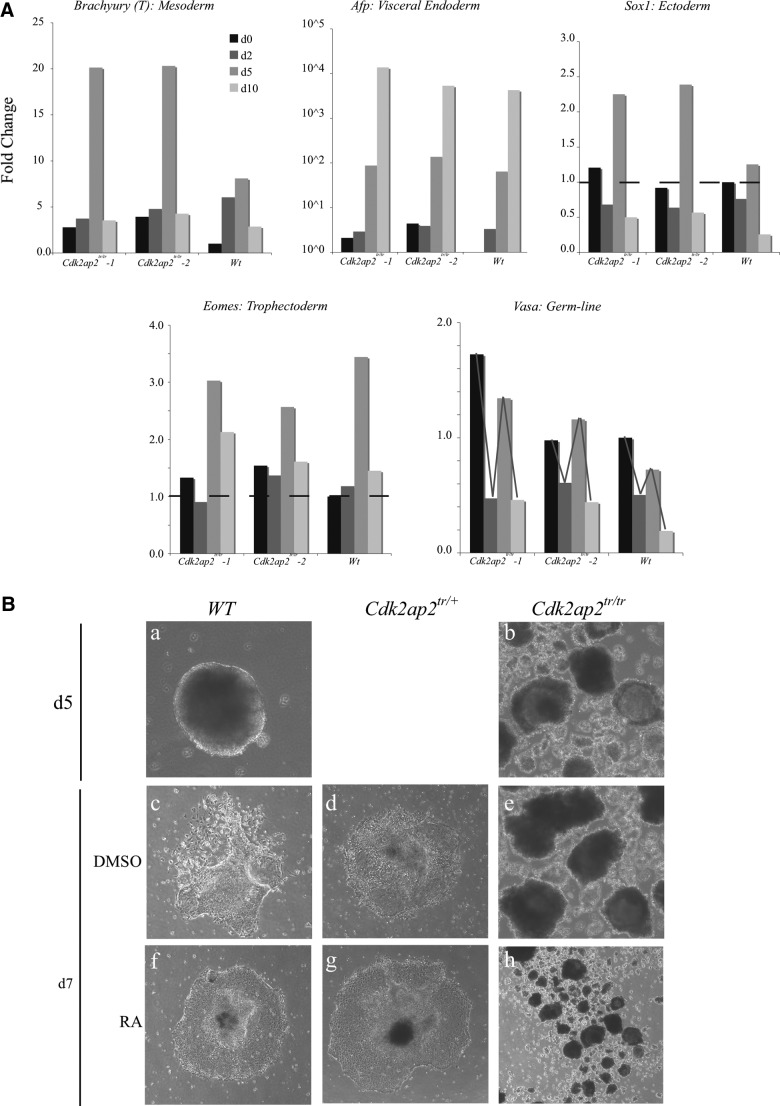
We wanted to determine which lineage markers would be preferentially upregulated during differentiation of the Cdk2ap2tr/tr mESCs. To assess this, an RT-qPCR analysis was performed to identify lineage markers during EB formation. Wt and Cdk2ap2tr/tr mESCs were differentiated into EBs by hanging-drop [14]. Total RNA was extracted from EBs collected on days 0, 2, 5, and 10, and used in the RT-qPCR analyses. As anticipated, markers of mesoderm and endoderm differentiation, including Brachyury (T), Afp, and S100a, showed a 5–10-fold increase in the mRNA levels in 2 different knockout mESC lines (Fig. 4A and Supplementary Fig. S2). In contrast, a little or no difference was observed in the levels or timing of expression of Eomes, Keratin18, Sox1, and Vasa, suggesting that Cdk2ap2 does not play a significant role in commitment of mESCs to the ectoderm or the trophectoderm lineages. Additional markers for mesoderm and endoderm lineages were tested (Supplementary Fig. S3). Some of these markers were highly upregulated in the Cdk2ap2tr/tr mESCs, early on such as Acta2 and CA3 compared to the Wt, whereas other markers had a similar or repressed profile compared to the Wt such as Hnf1a and Hnf4 (Supplementary Figs. S2 and S3). Some of these data further suggest an endodermal or mesodermal lineage early on, and an early differentiation potential. However, all the endodermal and mesodermal markers in the Cdk2ap2tr/tr mESCs are not highly upregulated, suggesting that there may be some stress on the EBs that can be seen by the altered morphology in Fig. 4B. Differences were observed in the temporal expression of lineage markers in the Cdk2ap2tr/tr-derived EB, and the relative patterns of gene expression for some of the markers during the differentiation time course were well conserved in both Wt and Cdk2ap2tr/tr EB (tracking line, Fig. 4A and Supplementary Fig. S3). These results suggest that Cdk2ap2 expression is required to maintain balanced commitment to the embryonic lineages during development, through regulation of Nanog expression, and/or by some other, as yet undefined, mechanism.
FIG. 4.
Cdk2ap2tr/tr EBs show an increase in mesoderm/endoderm commitment, and reduced cell survival after terminal differentiation. Markers of mesoderm and endoderm lineage markers (Brachyury (T) and Afp) were upregulated in the differentiating EBs derived from Cdk2ap2tr/tr mESCs (A). These levels are approximately equivalent to those at day 2 of differentiation of the Wt EBs (black bars; dashed lines indicate the day-1 levels of gene expression in the Wt EBs). In contrast, no significant difference was observed in the expression of markers of ectoderm (Sox1), trophectoderm (Eomes), or the germ-cell lineage (Vasa). In addition, there was no difference in the pattern of gene expression (red-tracking line) during differentiation. Each of the 4 bars represents days 0, 2, 5 and 10 of mESC differentiation into EBs. A representative plot of the various markers is depicted in the figure. Phenotype analyses of differentiating EB showed abnormal structures in the EBs derived from the Cdk2ap2tr/tr mESCs [B (b)] compared to the Wt EBs [B (a)]. In addition to the rough uneven edges at day 5, Cdk2ap2tr/tr EBs showed extensive disintegration of the EB structures in the presence of strong inducers of differentiation, DMSO and RA [B (e, h)]. Treatment of Wt and Cdk2ap2tr/+ cells with RA and DMSO resulted in adherent differentiating colonies [B(c,d,f,g)]. Images were obtained at 100× magnification. EB, embryoid body; DMSO, dimethyl sulfoxide; RA, retinoic acid.
Cdk2ap2tr/tr mESCs fail to survive during in vitro differentiation
We further analyzed the ability of the Wt and Cdk2ap2tr/tr mESCs to terminally differentiate in the presence of strong inducers of differentiation, DMSO and RA (Fig. 4B). Wt, Cdk2ap2tr/+, and Cdk2ap2tr/tr mESCs were suspended in a hanging-drop for 5 days [Fig. 4B (a, b)], followed by plating on gelatin-coated plates, with DMSO [Fig. 4B (c–e)] or with RA [Fig. 4B (f–h)]. Although Cdk2ap2tr/tr mESCs formed EBs, these were unhealthy, with rough borders, and the culture contained an excess amount of cell debris [Fig. 4B (b, e, h)]. In contrast to the Wt and the Cdk2ap2tr/+ cells, EBs obtained from Cdk2ap2tr/tr also failed to adhere to the gelatin-coated plate and differentiate [Fig. 4b (b, e, h)]. Additionally, Cdk2ap2tr/tr EB showed further deterioration of the EB structure, especially in the presence of strong differentiation enhancers, suggesting enhanced cell death. This suggests that Cdk2ap2 expression is potentially also required for survival of cells as they progress through differentiation.
Cdk2ap2tr/tr mESCs fail to form teratoma in vivo
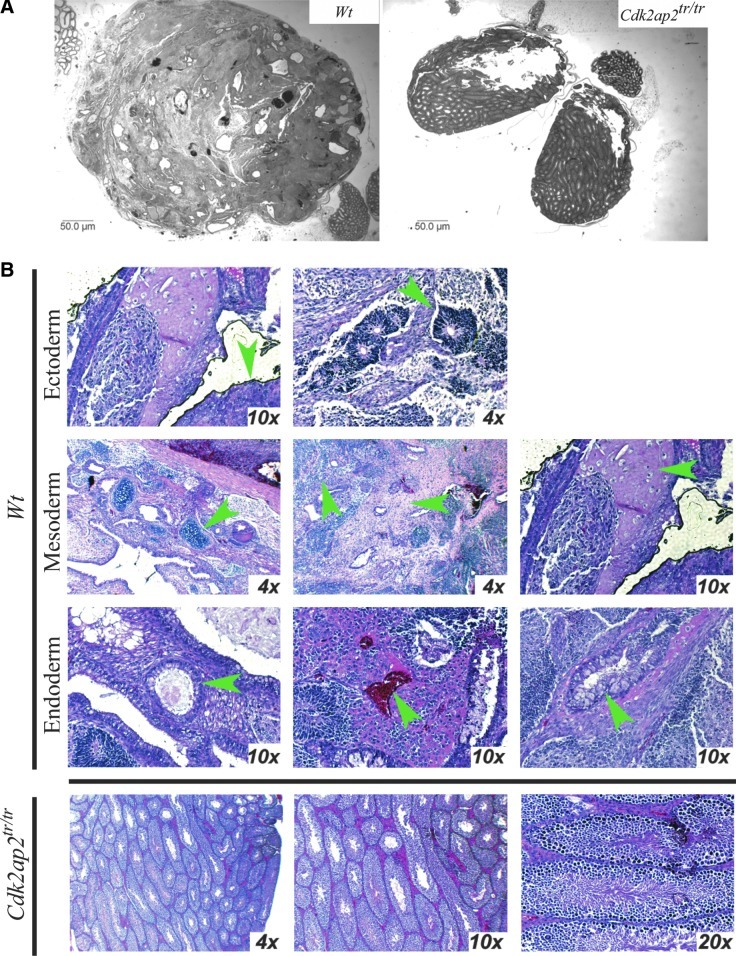
Since the Cdk2ap2tr/tr mESCs showed a preferential commitment to the mesoderm and endoderm lineages, the ability of these cells to give rise to mesoderm and endoderm tissues in vivo was tested by the teratoma assay [15–17]. For this, 500,000 mESCs were injected into the testes of SCID mice in duplicate [7]. Animals were monitored for tumor formation and sacrificed at 4 weeks. Tumors and any surrounding testicular tissue were extracted, fixed in formalin, and analyzed by H&E staining. While the Wt mESCs formed large, well-defined, and contained teratomas (Fig. 5A, left panel), the Cdk2ap2tr/tr cells failed to form any tumors (Fig. 5A, right panel). H&E analyses of the tissue sections showed the presence of all 3 germ lineages (endoderm, mesoderm, and ectoderm) in the tumors derived from the Wt mESCs. In contrast, the Cdk2ap2tr/tr cells failed to form tumor, and only the testis tissue was observed (Fig. 5B). Lack of tumor formation by the Cdk2ap2tr/tr mESCs could be a result of (1) rapid and robust differentiation and a limited cell proliferation capacity of the Cdk2ap2tr/tr cells in the absence of differentiation-inhibiting signals (LIF), or (2) loss of the survival capacity of the Cdk2ap2tr/tr cells in the absence of LIF and potential activation of apoptotic signals.
FIG. 5.
Cdk2ap2tr/tr mESCs fail to form teratoma in vivo. Wt and Cdk2ap2tr/tr mESCs were injected into the testes of SCID mice and allowed to form teratomas. Tumors were harvested, fixed, and analyzed for differentiation by H&E analyses. (A) Shows a 1× magnification of the tissues. As seen, while the Wt mESCs formed well-defined and differentiated teratomas (left panel), no tumors were observed in the testes from the Cdk2ap2tr/tr mESC injections. (B) Analysis of the H&E sections showed that the Wt mESCs formed tissues derived from all 3 germ lineages (green arrowheads). These include ectoderm-derived tissues—melanising epithelium (left) and neuroepithelium (middle); mesoderm-derived tissues—cartilage (left), dense connective tissue (middle), and bone (left); endoderm-derived tissues—glandular epithelium (left), hematopoietic tissue (middle), and ciliated epithelium (right). In contrast, only the testicular tissue was observed in the Cdk2ap2tr/tr injections. Appropriate magnifications were used to show each tissue and are indicated in each panel. H&E, hematoxylin and eosin.
Cdk2ap2tr/tr mESCs undergo enhanced apoptosis after LIF withdrawal
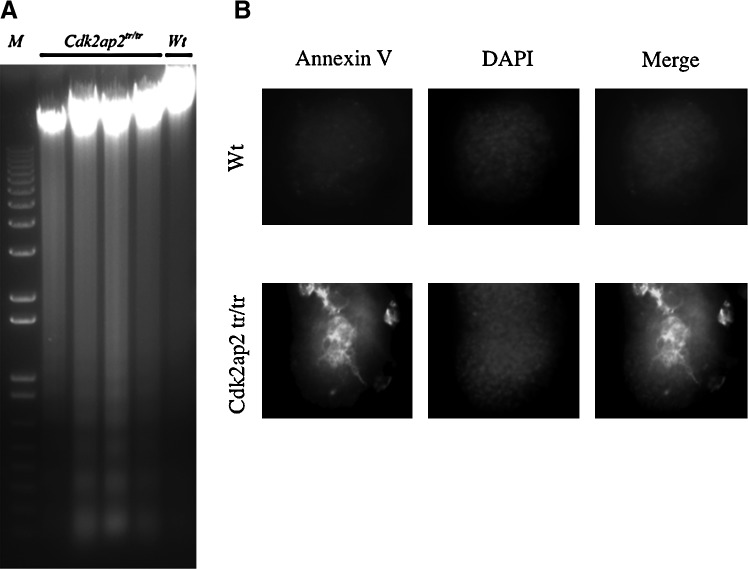
As seen in Fig. 4B, in vitro differentiation of mESCs resulted in unhealthy Cdk2ap2tr/tr ES clones. These structures fail to form differentiating colonies when plated onto gelatin-coated plates and progressively deteriorated when cultured in the presence of differentiation inducers. In addition, the Cdk2ap2tr/tr mESCs fail to form teratoma in vivo (Fig. 5). To analyze if the Cdk2ap2tr/tr ESC were undergoing apoptosis during differentiation, we compared day 7 EBs derived from Wt and Cdk2ap2tr/tr ESCs. Genomic DNA was isolated from EBs and floating cells and was subjected to electrophoresis on a 0.8% agarose gel. As seen in Fig. 6A, Cdk2ap2tr/tr EBs showed an increase in the fragmented DNA. This pattern is similar to that seen during cell death. Furthermore, an Annexin V immunostain was performed (Fig. 6B), showing greater Annexin V levels in the Cdk2ap2tr/tr EBs when compared to the Wt. These data taken together suggest that loss of Cdk2ap2 expression in mESCs leads to an increase in apoptosis, and hence an inability of the Cdk2ap2tr/tr ESCs to survive during terminal differentiation. Although apoptosis with low levels is a normal occurrence during early embryonic differentiation and has also been shown to occur during EB formation, and excessive levels of apoptosis could result in an inability of ESCs to survive and to give rise to differentiated tissue [18–20]. Together, these data suggest that, in addition to promoting self-renewal, Cdk2ap2 also promotes cell survival during terminal differentiation of the mESCs.
FIG. 6.
Increased cell death (apoptosis) in Cdk2ap2tr/tr mESCs. (A) Extensive cell death was observed in the EBs derived from Cdk2ap2tr/tr mESCs. The DNA-laddering assay for apoptosis showed an increase in fragmented DNA in the Cdk2ap2tr/tr EBs compared to the Wt EBs. (B) Immunofluorescent analysis was performed for Annexin V in Wt and Cdk2ap2tr/tr mESC EBs. The Cdk2ap2tr/tr EBs had greater Annexin V levels and an altered EB morphology, suggesting an increase in apoptosis.
Cdk2ap2 expression is downregulated during normal mESC differentiation
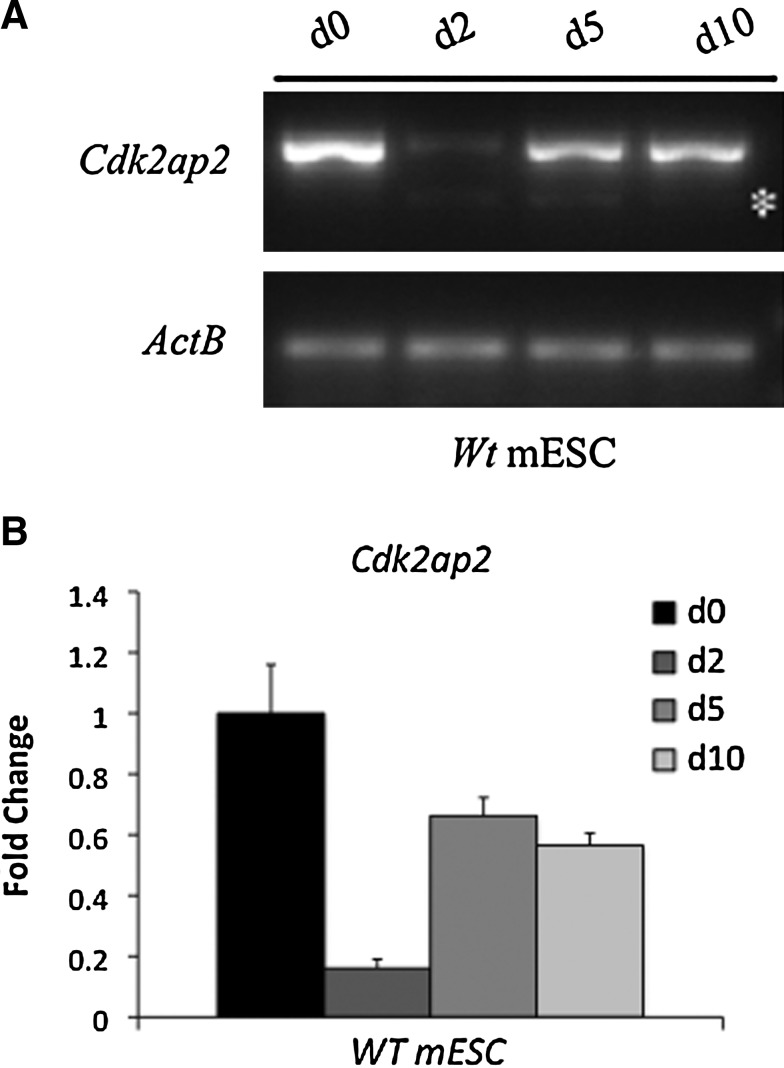
As seen from the data above, mESCs lacking Cdk2ap2 exit self-renewal and exhibit an early differentiation phenotype. In addition, sustained expression of Cdk2ap2 is required for cell survival during terminal differentiation of EB in vitro and in vivo. Next, we wanted to determine how Cdk2ap2 expression was throughout mESC differentiation. To assess this, we analyzed the expression of Cdk2ap2 in Wt mESCs during differentiation. Wt mESCs were differentiated into EBs and were used to isolate total RNA at days 0, 2, 5, and 10 to analyze expression of the Cdk2ap2 during differentiation. As seen in Fig. 7A, B, expression of Cdk2ap2 is significantly downregulated by day 2 of differentiation, followed by reappearance of expression by day 5. These data suggest that expression of Cdk2ap2 follows a biphasic pattern during differentiation of mESCs and potentially during embryogenesis. Taking all our data together, this biphasic pattern may occur to allow mESCs to exit self-renewal, thereby creating the proper cellular environment for differentiation into the germ lineages, followed by re-expression of Cdk2ap2 to ensure survival during terminal differentiation.
FIG. 7.
Cdk2ap2 expression is downregulated during differentiation of mESCs. (A) During normal differentiation of mESCs into EBs, expression of Cdk2ap2 was observed to be significantly downregulated at day 2 of differentiation followed by recovery of expression by day 5. (B) RT-qPCR was performed to confirm the return in the expression level of Cdk2ap2 by day 5 in Wt mESCs.
Discussion
In this study, we have started to explore the function of Cdk2ap2 during mammalian development. mESCs with a significant reduction of Cdk2ap2 expression (Cdk2ap2tr/tr) show a spontaneous exit from self-renewal. Analyses of expression of pluripotent markers, Nanog and Oct4, showed reduced expression of Nanog, but not of Oct4. Next, we observed that Cdk2ap2tr/tr mESCs showed an increase in mesoderm and endoderm lineage-specific genes. These data suggest that Cdk2ap2 is required to sustain the self-renewal capacity of mESCs, and loss of Cdk2ap2 expression results in an exit of mESCs into lineage commitment. This was further supported by an expression analysis during differentiation of Wt mESCs, wherein expression of Cdk2ap2 was seen to significantly downregulate at day 2 of differentiation when mESCs exit self-renewal and commit to the various germ lineages.
Experiments to identify the differentiation potential of the knockout mESCs to give rise to the 3 germ lineages showed that Cdk2ap2tr/tr mESCs failed to form differentiated teratoma tumors in vivo. In vitro analysis showed that these cells fail to form differentiating colonies after RA treatment and show an increase in the apoptotic fraction when forced to terminally differentiate. These data support a role for the Cdk2ap2 gene in maintaining cell survival during terminal differentiation of mESCs. This is again supported by the reappearance of Cdk2ap2 expression at day 5 and 10 in an EB formation assay.
Although the detailed mechanism explaining the function of Cdk2ap2 remains to be understood, the data presented here support a novel role for the Cdk2ap2 gene in regulating self-renewal of mESCs and survival of differentiating cells. Our group has previously shown that Cdk2ap1 is important in stem cell pluripotency, but the effects are opposite to those seen with Cdk2ap2, ie, loss of Cdk2ap1 expression maintains a pluripotent state. We can speculate that Cdk2ap2 may be interacting with protein complexes similar to Cdk2ap1, but are having the opposite effect. Further studies need to be done to elucidate the exact mechanism of Cdk2ap2 function, especially in the context of how this mechanism works in relation to other Cdk2-associated proteins.
Supplementary Material
Acknowledgments
This work was supported by grants from NIH NIDCR (R01 DE014857 to D.T.W.W.; T32 DE07269 to O.K.) and CIRM Basic Biology Award II (RB2-01562 to Y.K.).
Author Disclosure Statement
A.D., O.K., Y.K., A.L., and A.C. have no conflicts of interest and nothing to disclose. D.T.W.W. is associated with RNAmeTRIX, Inc.
References
- 1.Zhang X. Tsao H. Tsuji T. Minoshima S. McBride J. Majewski P. Todd R. Shimizu N. Wong DT. Housman DE. Haluska FG. Identification and mutation analysis of DOC-1R, a DOC-1 growth suppressor-related gene. Biochem Biophys Res Commun. 1999;255:59–63. doi: 10.1006/bbrc.1999.0148. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Biotechnology Information, Homo sapiens CDK2AP2. 2008. www.ncbi.nlm.nih.gov/nuccore?.Db=gene&DbFrom=nuccore&Cmd=Link&LinkName=nuccore_gene&ldsFromResult=39725675 www.ncbi.nlm.nih.gov/nuccore?.Db=gene&DbFrom=nuccore&Cmd=Link&LinkName=nuccore_gene&ldsFromResult=39725675
- 3.Terret ME. Lefebvre C. Djiane A. Rassinier P. Moreau J. Maro B. Verlhac MH. DOC1R: a MAP kinase substrate that control microtubule organization of metaphase II mouse oocytes. Development. 2003;130:5169–5177. doi: 10.1242/dev.00731. [DOI] [PubMed] [Google Scholar]
- 4.Buajeeb W. Zhang X. Ohyama H. Han D. Surarit R. Kim Y. Wong DT. Interaction of the CDK2-associated protein-1, p12(DOC-1/CDK2AP1), with its homolog, p14(DOC-1R) Biochem Biophys Res Commun. 2004;315:998–1003. doi: 10.1016/j.bbrc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H. Yang H. Owen MR. Combined microarray analysis uncovers self-renewal related signaling in mouse embryonic stem cells. Syst Synth Biol. 2007;1:171–181. doi: 10.1007/s11693-008-9015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X. Yang P. In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. J Vis Exp. 2008;(17):e825. doi: 10.3791/825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway AE. Lindgren A. Galic Z. Pyle AD. Wu H. Zack JA. Pelligrini M. Teitell MA. Clark AT. A self-renewal program controls the expansion of genetically unstable cancer stem cells in pluripotent stem cell-derived tumors. Stem Cells. 2009;27:18–28. doi: 10.1634/stemcells.2008-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nellissery MJ. Padalecki SS. Brkanac Z. Singer FR. Roodman GD. Unni KK. Leach RJ. Hansen MF. Evidence for a novel osteosarcoma tumor-suppressor gene in the chromosome 18 region genetically linked with Paget disease of bone. Am J Hum Genet. 1998;63:817–824. doi: 10.1086/302019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung MC. Accelerated apoptotic DNA laddering protocol. Biotechniques. 2002;33:734–736. doi: 10.2144/02334bm03. [DOI] [PubMed] [Google Scholar]
- 10.Wells D. In vitro isolation of murine embryonic stem cells. Methods Mol Biol. 2002;180:93–126. doi: 10.1385/1-59259-178-7:093. [DOI] [PubMed] [Google Scholar]
- 11.Chen L. Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–R27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 12.Chambers I. The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells. 2004;6:386–391. doi: 10.1089/clo.2004.6.386. [DOI] [PubMed] [Google Scholar]
- 13.Niwa H. Miyazaki J. Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 14.Desbaillets I. Ziegler U. Groscurth P. Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85:645–651. [PubMed] [Google Scholar]
- 15.Martin GR. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209:768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa S. Jakt LM. Era T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat Rev Mol Cell Biol. 2007;8:502–507. doi: 10.1038/nrm2189. [DOI] [PubMed] [Google Scholar]
- 17.Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–1250. doi: 10.1634/stemcells.2005-0014. [DOI] [PubMed] [Google Scholar]
- 18.Brooks DG. James RM. Patek CE. Williamson J. Arends MJ. Mutant K-ras enhances apoptosis in embryonic stem cells in combination with DNA damage and is associated with increased levels of p19(ARF) Oncogene. 2001;20:2144–2152. doi: 10.1038/sj.onc.1204309. [DOI] [PubMed] [Google Scholar]
- 19.Duval D. Reinhardt B. Kedinger C. Boeuf H. Role of suppressors of cytokine signaling (Socs) in leukemia inhibitory factor (LIF) -dependent embryonic stem cell survival. FASEB J. 2000;14:1577–1584. doi: 10.1096/fj.14.11.1577. [DOI] [PubMed] [Google Scholar]
- 20.Okazawa H. Shimizu J. Kamei M. Imafuku I. Hamada H. Kanazawa I. Bcl-2 inhibits retinoic acid-induced apoptosis during the neural differentiation of embryonal stem cells. J Cell Biol. 1996;132:955–968. doi: 10.1083/jcb.132.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.