Abstract
Bone morphogenetic protein (BMP) signaling is known to support differentiation of human embryonic stem cells (hESCs) into mesoderm and extraembryonic lineages, whereas other signaling pathways can largely influence this lineage specification. Here, we set out to reinvestigate the influence of ACTIVIN/NODAL and fibroblast growth factor (FGF) pathways on the lineage choices made by hESCs during BMP4-driven differentiation. We show that BMP activation, coupled with inhibition of both ACTIVIN/NODAL and FGF signaling, induces differentiation of hESCs, specifically to βhCG hormone-secreting multinucleated syncytiotrophoblast and does not support induction of embryonic and extraembryonic lineages, extravillous trophoblast, and primitive endoderm. It has been previously reported that FGF2 can switch BMP4-induced hESC differentiation outcome to mesendoderm. Here, we show that FGF inhibition alone, or in combination with either ACTIVIN/NODAL inhibition or BMP activation, supports hESC differentiation to hCG-secreting syncytiotrophoblast. We show that the inhibition of the FGF pathway acts as a key in directing BMP4-mediated hESC differentiation to syncytiotrophoblast.
Introduction
Human embryonic stem cells (hESCs) originate from the inner cell mass of the blastocyst are self-renewing and pluripotent with an innate ability to give rise to embryonic and extraembryonic lineages [1,2]. ACTIVIN/NODAL and fibroblast growth factor (FGF) signaling supports hESC self-renewal [3–8]. They also form a subset of the key developmental pathways, also including bone morphogenetic protein (BMP) and WNT, instrumental in differentiation, which play important roles in embryonic development [9,10].
In mouse, ACTIVIN/NODAL AND FGF signaling pathways are crucial for primitive streak (PS) formation, leading to mesoderm and endoderm induction [11–15]. The lineage specification into the initial cell types, epiblast, trophectoderm (TE), and primitive endoderm (PE) is dependent on the FGF pathway [16–19]. Excessive FGF signaling is crucial for PE formation [19] and is functional in switching the BMP4-induced hESC differentiation to the mesendoderm [20]. FGF signaling can also promote or inhibit neuroectodermal differentiation of ES cells in a context-dependent manner [21–25]. Neuroectodermal differentiation can be induced by inhibition of ACTIVIN/NODAL or BMP signaling or a combination of both [5,7,26–28]. On the other hand, BMP signaling can support differentiation of hESCs into multiple lineages, including both embryonic [29–31] and extraembryonic lineages [2,32], being essential for trophoblast induction [33].
These independent findings emphasize the importance of BMP, ACTIVIN/NODAL, and FGF signaling in a broad range of cellular events, like self-renewal and differentiation, which imply their influence on a contextual basis. Both ACTIVIN/NODAL and FGF signaling can influence BMP-mediated differentiation of hESCs [5,34,35]. BMP4- and FGF2-induced signaling are known to cooperate in inducing mesoderm and inhibiting endoderm differentiation of hESCs, where the latter is functional in switching the BMP4-induced hESC differentiation to mesendoderm [20,36]. Therefore, the importance of FGF signaling in mesendoderm induction has been well deciphered. In this article, we show that upon FGF inhibition, BMP4 directs hESC differentiation to syncytiotrophoblast, preventing the other BMP4-driven lineages such as mesendoderm and PE. In this study, we reinvestigated the effect of these pathways on the outcome of BMP4-driven differentiation of hESCs. Here, we show that BMP activation, in combination with inhibition of both ACTIVIN/NODAL and FGF pathways, specifically drives the differentiation of hESCs to syncytiotrophoblast, circumventing mesendoderm and PE induction. Furthermore, we elucidate the crucial role of FGF inhibition in specifying this differentiation process of hESCs to syncytiotrophoblast.
Materials and Methods
Cell culture
The experiments were carried out using the Human ES lines H1 and H9 obtained from WiCell and cultured on Matrigel-coated plates under defined conditions (N2B27) [37]. The cells were passaged and maintained in an MEF-conditioned medium [38] till they reach a confluency of ∼40%–50%. They were rinsed with PBS, followed by the various treatments (Fig. 1A, Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). The recombinant proteins and reagents used are the following: BMP4 (R&D 314-BP-010): 10 ng/mL; SB-431542 (Sigma S4317): 20 μM; SU5402 (Calbiochem 572630): 20 μM; FGF2 (Peprotech): 4–20 ng/mL.
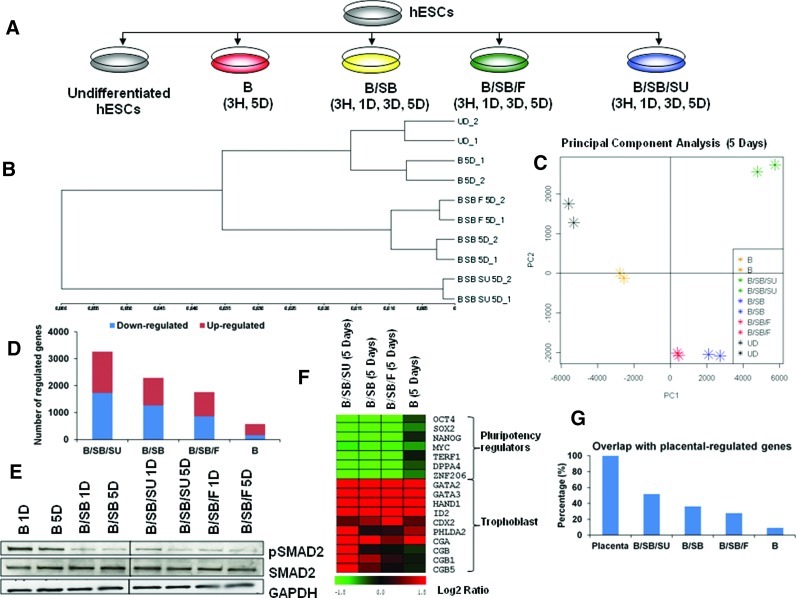
FIG. 1.
Experimental design and BMP4-induced differentiation are accelerated and accentuated with additional inhibition of ACTIVIN/NODAL and FGF signaling. (A) Details of treatments [BMP4 (B): 10 ng/mL; SB431542 (SB): 20 μM; SU5402 (SU): 20 μM; FGF2 (F): 20 ng/mL] [Hour (H); Day (D)]. (B) Dendrogram based on Pearson's correlation coefficient between samples (5 days). (C) Principal Component Analysis on the regulated genes (2-fold), with respect to their expression in hESCs in at least one of the indicated (5 days). (D) Number of significantly regulated genes after 5 days of the indicated treatments, with respect to hESCs. Blue, downregulated; red, upregulated. (E) Western blot analysis for the indicated proteins and samples. (F) Heat map depicting the regulation of genes with respect to hESCs in the indicated samples. (G) Percentage overlaps of regulated genes (2-fold) in each sample (5 days) with respect to hESCs, with placental-regulated genes. BMP, bone morphogenetic protein; hESCs, human embryonic stem cells. Color images available online at www.liebertpub.com/scd
Real-time PCR and microarray-based gene expression analyses
RNA was isolated using the RNeasy Mini Kit (Qiagen). The cells were directly lyzed with a lysis buffer in the cell culture wells, after rinsing with PBS. Total RNA from human placenta (normal human placentas pooled from 15 Caucasians, ages: 19–33) was purchased from Clontech (636527).
Reverse transcription was carried out using M-MLV reverse transcriptase (Promega) and Oligo-dT primer (Invitek). Real-time PCR was carried out using SYBR Green mix (ABI) with validated gene-specific primers (Supplementary Table S2). The ΔΔCt method was employed for calculations, employing the housekeeping genes (ACTB and GAPDH) for normalization.
For microarray-based gene expression profiling, 500 ng DNAse-free total RNA was biotin-labeled to generate cRNA, employing a linear amplification kit (Ambion). The labeled cRNA samples were hybridized onto HumanRef-8 v3 chips following the manufacturer's instructions (Illumina). Data were analyzed using the software, Bead Studio, TIGR-MEV, R (Bioconductor), MS Excel, MS Access, DAVID, and VENNY interactive tool (Refer Supplementary Data).
Immunoblotting, immunostaining, and ELISA
Standard procedures were followed for immunoblotting and immunostaining. Cells were rinsed with PBS before performing these assays. For immunoblotting, cells were scraped-off after adding the lysis buffer, and further lyses were performed on ice to preserve the phosphorylation status of the proteins. ELISA was carried out using the hCG ELISA Kit (Bio-Quant, BQ 047F).
Transmission electron microscopy
Undifferentiated hESCs were cultured on Matrigel-coated Thermanox plastic coverslips (Nunc; www.nuncbrand.com) till they reached ∼50% confluency, and B/SB/SU treatment was carried out for 5 days. Cells were then rinsed with PBS and fixed with 2.5% glutaraldehyde in 50 mM sodium cacodylate buffer (pH 7.4), supplemented with 50 mM sodium chloride for at least 30 min at room temperature (refer Supplementary Data).
Mitosis index
Cells were immunostained following standard procedures to detect phosphorylated Histone H3 (H3p). The mitotic index was computed as the ratio of H3p-positive nuclei to the total number of nuclei [diamidino-2-phenylindoldihydrochloride-positive (DAPI)]. The number of nuclei was determined using the image processing software, ImageJ. At least 2,000 cells were counted for each sample.
Fusion index
Cells were immunostained following standard procedures to detect expression of E-cadherin (CDH1). For the estimation of the fusion index, edges of the B/SB/SU-treated (5 days) colonies were taken into consideration, as the βhCG-positive cells were concentrated toward the edges (Figs. 3B and 4H, Supplementary Fig. S3F). The fusion index was computed as the number of nuclei (DAPI) in fused regions to the total number of nuclei analyzed. The number of nuclei was determined using the image processing software, ImageJ. At least 600 nuclei were counted from 3 independent experiments.
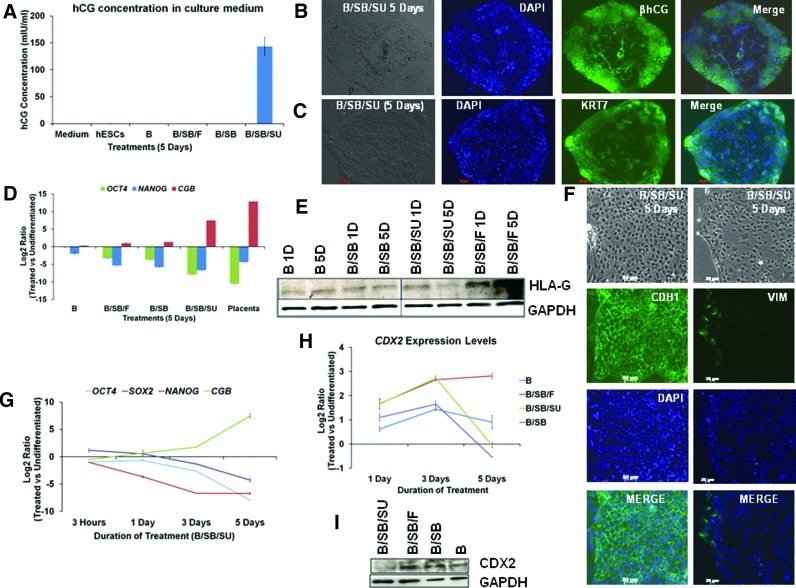
FIG. 3.
B/SB/SU treatment transiently upregulates CDX2 and induces differentiation to βhCG-secreting syncytiotrophoblast and does not support extravillous trophoblast formation. (A) ELISA-mediated estimation of hCG concentration in growth media in the indicated samples. (B) Representative immunostain for βhCG expression in B/SB/SU-treated cells. (C) Representative immunostain for KRT7 expression in B/SB/SU-treated cells. (D) Analysis of OCT4, NANOG, and CGB expression in the indicated samples. (E) Western blot analysis of HLA-G expression in the indicated samples. (F) Representative immunostains for E-Cadherin (CDH1) and Vimentin (VIM) expression in B/SB/SU-treated cells. (G) Analysis of OCT4, SOX2, NANOG, and CGB, during B/SB/SU treatment. (H) Analysis of CDX2 expression in the indicated samples. (I) Western blot analysis of CDX2 expression in the indicated samples (5 days). Color images available online at www.liebertpub.com/scd
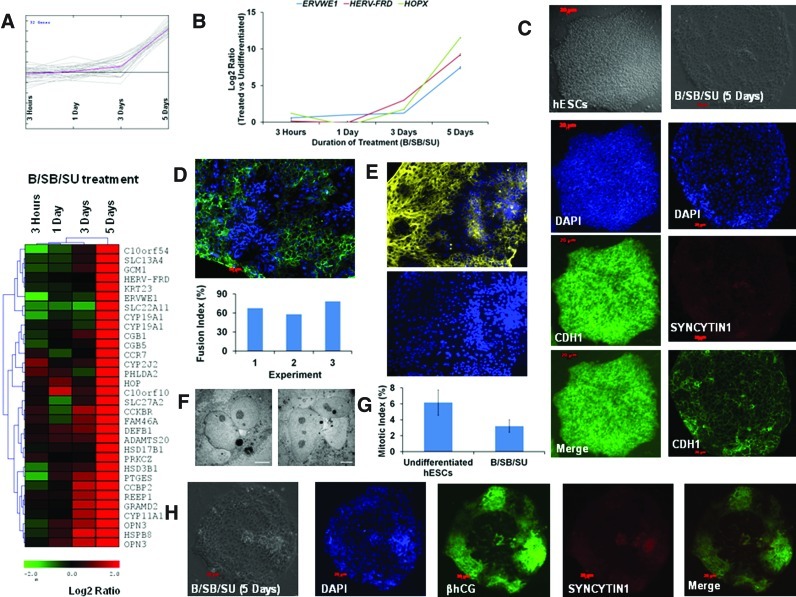
FIG. 4.
B/SB/SU treatment induces the expression of fusogens and differentiation to multinucleated syncytiotrophoblast. (A) Heat map and expression profile of coregulated and induced genes in comparison to hESCs during the B/SB/SU treatment. (B) Real-time PCR-based expression analysis of the indicated genes during B/SB/SU treatment. (C) Representative immunostain for E- Cadherin (CDH1) expression in the indicated samples. (D) Representative immunostain for CDH1 in the B/SB/SU-treated samples (5 days), exhibiting multinucleated regions in the colony edges and fusion indices of 3 different experiments. CDH1, green; DAPI, blue. (E) Representative immunostain for ß-Actin (ACTB) in the B/SB/SU-treated samples (5 days), exhibiting multinucleated regions in the colony edges. ACTB: yellow; DAPI: blue. (F) Representative electron microscopy images depicting multinucleated cells in B/SB/SU-treated samples (5 days). Scale bar: 5 μm. (G) Mitotic index for the indicated samples. (H) Representative immunostains for βhCG (green) and SYNCYTIN1 (red) expression in B/SB/SU-treated cells. DAPI, diamidino-2-phenylindoldihydrochloride. Color images available online at www.liebertpub.com/scd
Results
FGF inhibition, in addition to ACTIVIN/NODAL inhibition, accelerates and accentuates BMP4-mediated hESC differentiation, leading to increased overlap with placental-regulated genes
BMP signaling promotes differentiation of hESCs into multiple lineages [2,29,31,32], and ACTIVIN/NODAL and FGF signaling can influence this lineage specification [5,34,35]. Based on this evidence, we adopted a time-course whole-genome expression profiling analysis to decipher the influence of ACTIVIN/NODAL and FGF signaling on BMP4-mediated differentiation of hESCs (Fig. 1A and Supplementary Table S1). Pearson's correlation coefficient (Fig. 1B, Supplementary Fig. S1A, Table S3), principal component analysis (Fig. 1C, Supplementary Fig. S2), and the number of differentially regulated genes (Fig. 1D) together revealed the degree of segregation of each sample from the other, and speed and extent of differentiation. BMP4-treated cells (B) were the least differentiated (Fig. 1B–D, Supplementary Figs. S1A and S2) and also shared the least overlap with placental-regulated genes (genes regulated in placenta, in comparison to hESCs) (Fig. 1G, Supplementary Table S4). The reason for this could be the persistent pSMAD2 activity in these cells (B) (Fig. 1E), which might have led to the higher expression of NANOG and other pluripotency-supporting genes than all the other treatments that involved ACTIVIN/NODAL inhibition (B/SB, B/SB/F, and B/SB/SU) (Fig. 1F, Supplementary Fig. S1C). The overlap with placental-regulated genes increased upon additional ACTIVIN/NODAL inhibition (B/SB) (Fig. 1G, Supplementary Table S4), also showing increased kinetics (Fig. 1C, Supplementary Fig. S2) and extent of differentiation (Fig. 1D). When BMP activation and ACTIVIN/NODAL inhibition were combined with FGF activation (B/SB/F), there was a reduction in the extent of differentiation (Fig. 1C, D) and also, overlap with that of placental-regulated genes (Fig. 1G, Supplementary Table S4). ACTIVIN/NODAL and/or FGF signaling blocks TE differentiation of hESCs induced by BMP4 [8,34,39], and this is in corroboration with our data. In contrast, upon BMP activation, coupled with the inhibition of both ACTIVIN/NODAL and FGF signaling (B/SB/SU), the transcriptome of these cells segregated from the transcriptomes of cells treated with other treatments (B, B/SB, and B/SB/F) in terms of maximum acceleration and extent of differentiation (Fig. 1B, D, Supplementary Figs. S1A and S2). Furthermore, B/SB/SU-treated cells acquired maximum overlap with the placental-regulated genes (Fig. 1G, Supplementary Table S4). These results suggest that additional inhibition of FGF signaling, along with ACTIVIN/NODAL, leads to an increase in the kinetics and extent of BMP4-mediated differentiation and enhanced overlap with placental-regulated genes.
Additional inhibition of FGF signaling impairs BMP4-driven differentiation into mesendoderm and PE, but supports trophoblast induction
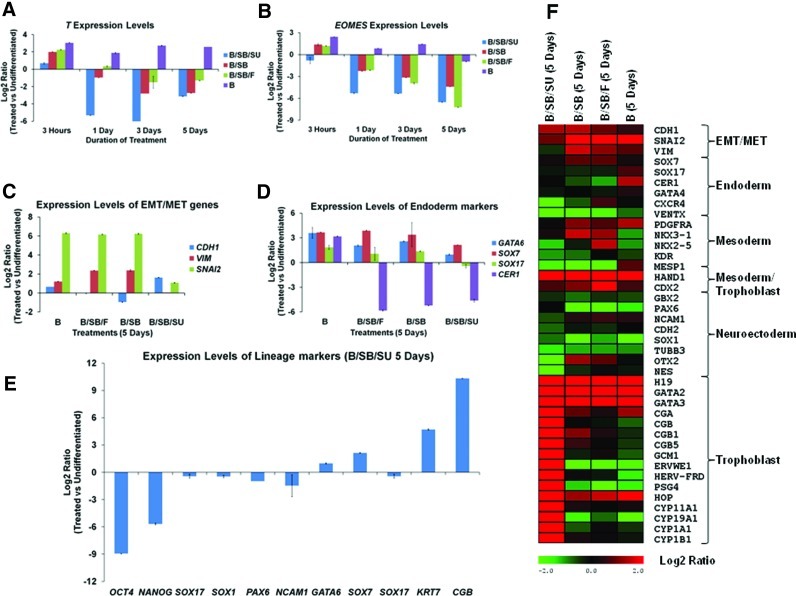
Transient upregulation of PS markers, T (Fig. 2A) and EOMES (Fig. 2B), induction of epithelial-mesenchymal transition (EMT) markers, VIM and SNAI2 (Fig. 2C), mesoderm markers, PDGFRA and MESP1 (Fig. 2F), and endoderm markers, SOX17, CER1, GATA6, and SOX7 (Fig. 2D) indicates that BMP4 treatment can support mesendoderm and PE differentiation when the FGF pathway is not inhibited (B, BSB, and B/SB/F). ACTIVIN/NODAL plays an important role in endodermal differentiation of hESCs [15], and this explains the decreased expression of the endodermal markers, SOX17 and CER1, upon its inhibition (B/SB, B/SB/F, and B/SB/SU) (Fig. 2D). However, the expression of PE markers, GATA6 and SOX7, still remained high upon B/SB and B/SB/F treatments, but diminished (GATA6: ∼3–10-fold lower and SOX7: ∼8–12-fold lower) with FGF inhibition (B/SB/SU) (Fig. 2D, F). Although FGF signaling is required for PE induction in mice [19], it has been shown not to be required for human PE induction [40]. However, the reduction in expression of PE markers upon B/SB/SU treatment could be a combinatorial effect. On the other hand, B/SB/SU treatment led to the induction of the epithelial marker, E-Cadherin (CDH1), and did not support induction of the mesenchymal marker, Vimentin (VIM), and the mesodermal, endodermal, or neuroectodermal markers (Fig. 2C–F). The data also revealed that none of the treatments (B, BSB, B/SB/F, and B/SB/SU) supported neuroectodermal differentiation (Fig. 2E, F). BMP activation is known to block neuroectodermal differentiation [5,26,27,35,41]. Although a number of trophoblast-related genes were induced upon all treatments (H19, GATA2, and GATA3), the degree of induction of trophoblast-related genes, such as CGB, CGB1, CGB5, KRT7, GCM1, and CYP19A1, was much higher in B/SB/SU-treated cells, compared to the others (B, B/SB, and B/SB/F) (Fig. 2E, F, Supplementary Table S5). However, B/SB/SU treatment led to the induction of only trophoblast markers, but not any other lineage markers analyzed (Fig. 2E, F).
FIG. 2.
FGF and ACTIVIN/NODAL inhibition prevents BMP4-driven differentiation into mesendoderm and PE and specifically supports trophoblast induction. (A) Real-time PCR based analysis of T expression in the indicated samples. (B) Analysis of of EOMES expression in the indicated samples. (C) Analysis of CDH1, SNAI2 and VIM expression in the indicated samples (5 days). (D) Analysis of GATA6, SOX7, SOX17, and CER1 expression in the indicated samples. (E) Analysis of the indicated genes in B/SB/SU-treated samples (5 days). (F) Heat map depicting the regulation of genes with respect to hESCs in the indicated samples. PE, primitive endoderm. Color images available online at www.liebertpub.com/scd
Taken together, our results illustrate that BMP signaling, irrespective of the presence or absence of ACTIVIN/NODAL signaling and in the presence of FGF signaling (B, B/SB, and B/SB/F), can support both mesendoderm and PE differentiation. However, inhibition of both ACTIVIN/NODAL and FGF signaling in conjunction with BMP activation (B/SB/SU) affects PE induction and supports neither mesendoderm nor neuroectoderm induction, but however supports trophoblast induction of hESCs.
BMP activation, in conjunction with the inhibition of ACTIVIN/NODAL and FGF signaling (B/SB/SU), leads to differentiation of hESCs to βhCG-secreting syncytiotrophoblast, with relatively compromised CDX2 expression, and does not support the formation of extravillous trophoblast
Trophoblast stem (TS) cells can adopt 2 mutually exclusive differentiation paths, either the noninvasive villous syncytiotrophoblast that remains epithelial or invasive extravillous cytotrophoblasts that undergo EMT [42]. The 2 trophoblast cell types are distinguishable from each other by the expression of specific markers [43–47]. ELISA-based results strongly indicated hCG secretion by cells in which both the self-renewal supporting pathways were blocked, along with BMP activation (B/SB/SU), whereas no secretion was detected as a consequence of the other treatments in a span of 5 days (B, B/SB, and B/SB/F) (Fig. 3A). Both mRNA (CGB) and protein levels revealed a high proportion of syncytiotrophoblast-specific βhCG-expressing cells among the B/SB/SU-treated cells (Figs. 3B, D and 4H, Supplementary Fig. S3A). The induction of CGB at such high levels was reproducible (Fig. S3B). The pattern of βhCG expression reflects that the differentiation proceeded from edges of the colonies toward inside (Figs. 3B and 4H, Supplementary Fig. S3A). Even phenotypically, the colony edges of the B/SB/SU-treated colonies looked prominently different from the inner region, unlike the other treatments (Supplementary Fig. S5). The B/SB/SU-treated cells also expressed the general marker of the trophoblast lineage KRT7 [47] with more intense expression toward edges of the colonies (Fig. 3C). In addition, the expression of the extravillous trophoblast marker HLA-G (Fig. 3E, Supplementary Fig. S3C) was highly compromised upon B/SB/SU treatment. However, the difference in HLA-G levels was only seen at the protein level and not at the mRNA level that could be due to probable post-transcriptional regulation and requires further investigation. In addition, the epithelial marker E-CADHERIN (CDH1) was induced both at the mRNA and protein levels in the B/SB/SU-treated cells, while the mesenchymal markers, SNAI2 and VIMENTIN, were downregulated (Figs. 2C and 3F). The other treatments (B, B/SB, and B/SB/F) resulted in high induction of both SNAI2 and VIM and downregulation of CDH1 expression (Fig. 2C).
Silencing of OCT4, SOX2, or NANOG in hESCs supports trophoblast differentiation [48–50], and the ability of OCT4 to silence hCGα and hCGβ gene expression has been described [51]. Systematic loss of expression of the pluripotency markers, such as OCT4, SOX2, and NANOG, coincided with the induction of the syncytiotrophoblast marker CGB (Fig. 3G). The reduction in the pluripotency-associated markers was observed as a result of all the treatments (B, B/SB, B/SB/F, and B/SB/SU) (Figs. 1F, 3D, 3G). The TE marker CDX2 was induced by all the treatments (B, B/SB, B/SB/F, and B/SB/SU) (Fig. 3H). The treatments involving ACTIVIN/NODAL inhibition (B/SB and B/SB/SU) led to a transient induction of CDX2, whereas the treatment involving FGF stimulation (B/SB/F) led to its sustained induction. At the protein level, CDX2 expression was relatively the lowest in B/SB/SU-treated cells (Fig. 3I). CDX2 expression depends on an FGF4-induced MEK-ERK pathway in mouse TS cells [52], and upon FGF4 withdrawal [53], TS cells differentiate, preferential to spongiotrophoblast and syncytiotrophoblast [54]. Taken together, our results present strong evidence that BMP activation, coupled with ACTIVIN/NODAL and FGF inhibition, results in a transient induction of CDX2, leading to differentiation of hESCs to epithelial, βhCG-secreting syncytiotrophoblast, thus evading extravillous trophoblast induction.
BMP activation, in conjunction with inhibition of ACTIVIN/NODAL and FGF signaling (B/SB/SU), induces cell fusion
Our microarray data revealed coregulation of several syncytiotrophoblast-expressed genes with those coding for βhCG (CGB, CGB1, CGB5, and CGB7) (Fig. 4A, Supplementary Fig. S3D). They include the genes involved in estrogen/progesterone biosynthesis and metabolism (HSD17B1, HSD3B1, CYP19A1, and CYP11A1) [55], the tumor suppressor HOP (NECC1) [56], CYP19A1 (aromatase), and fusogens [GCM1 and Syncytins (ERVWE1 and HERV-FRD)] [57–59]. Although fusogens and HOP were induced by the other treatments (B, B/SB, and B/SB/F), the induction was much higher (∼200–300-fold) with the B/SB/SU treatment (Fig. 4A, B, Supplementary Fig. S3E). In addition, SYNCYTIN 1 (ERVWE1) was seen to be expressed (Fig. 4C, H). The cellular regions positive for β-hCG exhibited the presence of clustered nuclei that were mostly concentrated toward the edges of the colony (Figs. 3B and 4H, Supplementary Fig. S3A, F). A closer examination of the E-CADHERIN- or ß-Actin-stained colony edges led us to discover that several multinucleated regions were present in these regions (Fig. 4C–E, Supplementary Fig. S4A). The fusion index ranged ∼60%–80% (Fig. 4D). Furthermore, electron microscopy analysis also confirmed the presence of multinucleated cells among the B/SB/SU-treated cell population (Fig. 4F). In addition, several genes and pathways functional in human placenta, such as PPAR signaling, xenobiotic, and fatty acid metabolisms, were also active after B/SB/SU treatment (Supplementary Fig. S4B). Taken together, our data reemphasize that BMP activation, in conjunction with inhibition of ACTIVIN/NODAL and FGF signaling, leads hESC differentiation to cell fusion mediated-βhCG-secreting syncytiotrophoblast formation.
Pathway analyses also revealed the regulation of the cell cycle and p53 pathways in the B/SB/SU-treated cells (Supplementary Fig. S4B). Cell cycle disruption [60] as well as cell fusion are involved in the formation of syncytiotrophoblast, which is nonproliferative and considered to represent a mitotically end-stage cell [61,62]. Induction of the CDK/CYCLIN inhibitors (GADD45G, p21, and p57), downregulation of mitotic genes (CDK1, CCNA1, CCNA2, CCNB1, and CCNB2) (Supplementary Fig. S4C, Table S6), and a reduction in mitotic index, based on histone H3 phosphorylation (H3p) (Fig. 4G, Supplementary Fig. S4D), suggest that cell cycle was affected in the B/SB/SU-treated cells. H3p is a downstream marker for chromosome condensation and entry into mitosis [63], and its expression is regulated by the ERK/MAPK pathway (Chambard). Cell division is affected in dominant negative FGF receptor mouse embryos, though there is no increase in cell death [13]. The DNA damage-driven apoptosis-inducing factors CHEK1, CHEK2, and TP53 [64] were seen to be downregulated in B/SB/SU-treated cells (Supplementary Fig. S4D, Table S6), during which the TP53 inhibitor MDM2 [65] was induced. Both the apoptotic (BAD) and antiapoptotic (BCL-2 and BCL2L1) BCL-2 family members were upregulated after B/SB/SU treatment (Supplementary Fig. S4D, Table S6). BCL-2 protein is expressed in syncytiotrophoblast and syncytial knots, thus suggesting their involvement in protection from apoptosis [66]. Thus, our results indicate that the differentiation of hESCs to syncytiotrophoblast, mediated by B/SB/SU treatment, is brought about via the induction of fusogens and involves cell cycle exit.
Autocrine signaling leads to abrogation of BMP signaling and WNT activation when hESCs differentiate to syncytiotrophoblast
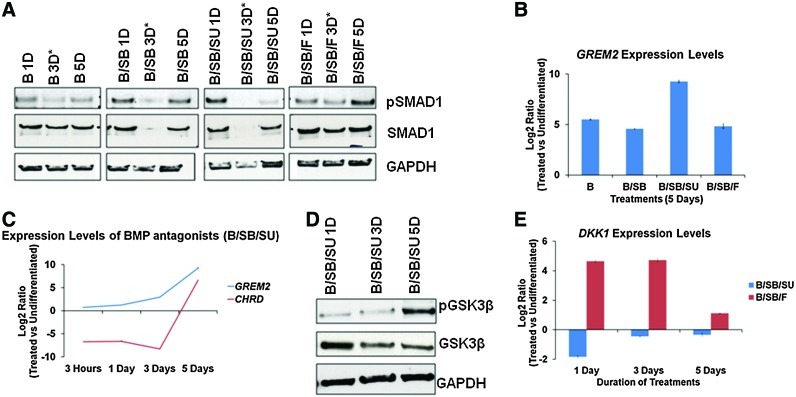
Although all treatments involved the activation of BMP signaling, only the B/SB/SU-treated cells had a reduction in pSMAD1 (phosphorylated SMAD1) after 5 days (Fig. 5A). This was unexpected as the cells were treated with a fresh medium, along with the respective treatments every day. The reduction in pSMAD1 levels in the B/SB/SU-treated samples coincided with the induction of the BMP-signaling antagonists, Gremlin2 (GREM2) and Chordin (CHRD) (Fig. 5B, C), and increased phosphorylation of GSK3β (Fig. 5D). The phosphorylation of the SMAD1 protein at the linker region by GSK3β causes degradation of SMAD1 (reviewed in [67]). The increased phosphorylation of GSK3β also indicates the activation of WNT signaling. The WNT antagonist Dickkopf homolog 1 (DKK1) was not induced upon B/SB/SU treatment, whereas when FGF signaling was kept active (B/SB/F), it was induced (Fig. 5E). The reduction in pSMAD1, along with the induction of GREM2 and CHRD, indicates a decrease in BMP signaling, and the increased phosphorylation of GSK3β, along with diminished DKK1 levels, indicates activation of WNT signaling. These events coincided with the activation of syncytiotrophoblast genes and βhCG-secretion (5 days of B/SB/SU treatment) (Figs. 2F, 3A–C, and 4A, Supplementary Fig. S4A). A recent finding has shown the importance of WNT signaling for the fusion inducing-GCM1/syncytin pathway in human choriocarcinoma cells [68]. Taken together, our data indicate that although trophoblast induction requires BMP activation, it is affected via autocrine signaling during subsequent differentiation to syncytiotrophoblast, when WNT signaling is active.
FIG. 5.
Autocrine signaling leads to the abrogation of BMP signaling and WNT activation in B/SB/SU-treated cells (5 days). (A) Western blot analysis for the indicated proteins and samples. The 3-day (3D) samples were not considered for analysis due to unequal loading. (B) Expression analysis of GREM2 in the indicated samples. (C) Expression analysis of CHRD, FST, and GREM2 in the indicated samples. (D) Western blot analysis for the indicated proteins in B/SB/SU-treated samples. (E) Expression analysis of DKK1 in the indicated samples. GREM2, Gremlin2; CHRD, Chordin; DKK1, dickkopf homolog 1. Color images available online at www.liebertpub.com/scd
Inhibition of the FGF pathway supports differentiation of hESCs to hCG-secreting syncytiotrophoblast, and additional BMP activation and ACTIVIN/NODAL inhibition accelerate as well as specify the differentiation toward syncytiotrophoblast
Our results revealed that all the treatments (B, B/SB, and B/SB/F), except the one involving FGF inhibition (B/SB/SU), support mesendoderm induction (Fig. 2). Although all the treatments led to the induction of CGB, βhCG secretion was detected only upon B/SB/SU treatment, indicative of syncytiotrophoblast formation (Figs. 3A, B, D and 4H) that involved additional FGF inhibition. From this, we hypothesized that the additional blocking of FGF signaling might be responsible for the diversion of hESCs to a syncytrophoblast lineage and hCG secretion, preventing mesendoderm and neuroectoderm induction. To confirm this, we investigated the effect of FGF inhibition on syncytiotrophoblast induction of hESCs, using the FGF inhibitor SU5402 (SU) (Supplementary Table S1).
ELISA results revealed hCG secretion upon FGF inhibition alone (SU) or when coupled with BMP activation (B/SU) or ACTIVIN/NODAL inhibition (SB/SU) (Fig. 6A). CGB was induced upon FGF inhibition (Fig. 6B, D, J, Supplementary Fig. S6B, D). The pluripotency-associated genes, such as OCT4, SOX2, and NANOG, were downregulated as a result of FGF inhibition (SU) (Fig. 6B, J, Supplementary Fig. S6E).
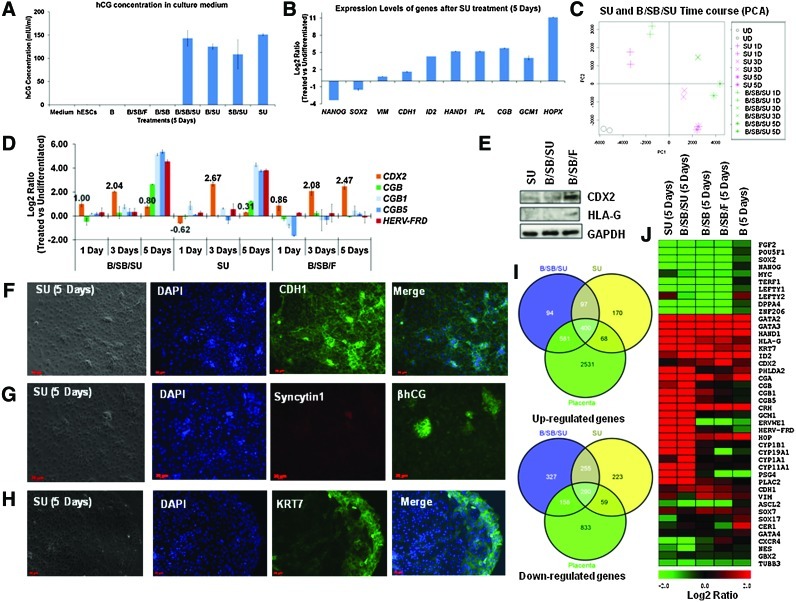
FIG. 6.
FGF inhibition in hESC supports differentiation to hCG-secreting syncytiotrophoblast. (A) ELISA-based estimation of hCG concentration in the growth medium after the indicated treatments. (B) Expression analysis of indicated genes in SU-treated samples (5 days). (C) Principal Component Analysis of regulated genes (2-fold) in the indicated samples. (D) Expression of CDX2, βhCG-encoding genes (CGB, CGB1, and CGB5), and HERV-FRD in the indicated samples, in comparison to hESCs (microarray data). (E) Western blot analysis for the expression of the indicated proteins and samples. (F) Representative immunostain for E-Cadherin (CDH1) expression in SU-treated cells. (G) Representative immunostain for SYNCYTIN1 (ERVWE1) and βhCG expression in SU-treated cells. (H) Representative immunostain for KRT7 expression in SU-treated cells. (I) Venn diagrams representing the overlap of the up- or downregulated genes between the indicated samples (5 days), in comparison to hESCs. (J) Heat map depicting the regulation of genes with respect to hESCs in the indicated samples. Color images available online at www.liebertpub.com/scd
Similar to the B/SB/SU-treatment, after 5 days, FGF inhibition (SU) led to the induction of the epithelial marker, CDH1, the trophoblast markers, such as ID2 and IPL, and also the syncytiotrophoblast genes, such as the βhCG-encoding genes (CGB, CGB1, and CGB5), the fusogens (GCM1 and Syncytins), aromatase (CYP19A1), and HOPX (Supplementary Fig. S1B, Figs. 3D, 4A, and 6B, D, J, Supplementary Fig. S6B). At the protein level, the SU-treated cells expressed E-CADHERIN (CDH1), SYNCYTIN1 (ERVWE1), βhCG, and KRT7 (Fig. 6F–H). The expression of SYNCYTIN1 was rather weak (Fig. 6G). Unlike the B/SB/SU-treated cells that exhibited very high proportion of βhCG-positive cells toward the colony edges, also having marked morphological demarcation from the inner areas, βhCG-positive cells were haphazardly distributed among the SU-treated colonies with no demarcating morphology of the edges (Figs. 3B, 4H, and 6G, Supplementary Figs. S4 and S6J). The expression profile of the extraembryonic endoderm marker GATA6 was quite similar to that of the B/SB/SU-treated samples (Fig. S6F). In addition, SOX7 was induced upon FGF inhibition (Fig. S6G), but both these genes were expressed at much lower levels (GATA6: ∼3–10-fold lower and SOX7: ∼8–12-fold lower) than that of all the other treatments (B, B/SB, and B/SB/F) (Supplementary Fig. S6F, G, Fig. 2D). CDX2 was transiently induced upon FGF inhibition (SU), similar to the consequence of B/SB/SU treatment, reflecting further differentiation of the cells, leaving the TS cell status (Figs. 3H, I and 6D, E, Supplementary Fig. S6H). Also, similar to the B/SB/SU-treated cells, the expression of the extravillous trophoblast marker HLA-G was relatively lower than that of the B/SB/F-treated cells that had FGF signalling activated, indicating its possible role in extravillous trophoblast induction (Fig. 6E). Principal Component Analysis of the global time-course transcription profiles revealed that both the B/SB/SU- and SU-treated cells followed a similar pattern of differentiation, but seemed to be less differentiated than the B/SB/SU-treated cells (Fig. 6C, Supplementary Fig. S6I). The expression pattern of some of the analyzed cell cycle-related genes, upon FGF inhibition (SU), was similar to that seen in B/SB/SU-treated cells, and also a reduction in the mitotic index showed compromise of cell cycle as a result of FGF inhibition (Fig. S6K, L). These results suggest that inhibition of FGF could be the key to differentiating hESCs to the syncytiotrophoblast lineage.
In spite of many similarities, morphological analysis during the course of treatments and the transcriptome changes revealed that the B/SB/SU treatment induced a more robust and faster differentiation than the SU treatment (Supplementary Fig. S6J, Fig. 6C, Supplementary Fig. S6I). Global transcriptional profiles revealed that the B/SB/SU- and SU-treated cells correlated more with each other than with the other cells after 5 days of treatment (B, B/SB, and B/SB/F) (Fig. 6C, Supplementary Fig. S6A, I). The B/SB/SU-treated cells had a higher overlap of the regulated genes, with that of the placental-regulated genes, than the SU-treated cells (Fig. 6I). The expression levels of the βhCG-encoding genes (CGB, CGB1, CGB, and CGB7) were higher than that of the SU-treated cells (Fig. 6D, Supplementary Fig. S6C).
Taken together, our results show that the inhibition of the FGF pathway (SU) supports differentiation of hESCs to hCG-secreting syncytiotrophoblast, acting as a key to syncytiotrophoblast induction of hESCs. When FGF inhibition is coupled with BMP activation and ACTIVIN/NODAL inhibition (B/SB/SU), it leads to a more robust differentiation of hESCs, specifically into syncytiotrophoblast.
Discussion
Activation of BMP signaling has the potential to direct hESCs to both the embryonic mesoderm lineage [29–31] and extraembryonic lineages, such as PE and trophoblast [2,20,32]. The BMP-driven differentiation potential of these cells can be restricted to specific lineages from the broad range of choices, based on the activity and influence of other pathways, such as ACTIVIN/NODAL and FGF signaling [5,34,35]. With this prior knowledge, we set out to decipher the influence of these pathways on BMP4-mediated lineage decisions imposed on hESCs.
All our treatments involved BMP activation, which is known to have an inhibitory capability on neuroectodermal differentiation, and therefore this lineage induction was averted (Fig. 2E, F) in spite of ACTIVIN/NODAL inhibition that supports neuroectodermal induction [5,7,26–28]. When the ACTIVIN/NODAL pathway is blocked, while both BMP and FGF pathways are active, mesendoderm induction is inhibited [20]. Our results revealed induction of mesoderm markers, such as PDGFRA, NKX3-1, and NKX2-5, upon BMP activation and ACTIVIN/NODAL inhibition (B/SB) and upon additional FGF activation (B/SB/F), respectively (Fig. 2F). It could be possible that even if ACTIVIN/NODAL inhibition can grossly affect mesendoderm differentiation, it might have a positive effect on certain mesoderm sublineages at this particular BMP4 concentration (10 ng/ml). This however requires further investigation.
Our results revealed that when BMP activation and ACTIVIN/NODAL inhibition were coupled with FGF inhibition (B/SB/SU), both the PS markers, T and EOMES, were highly downregulated, and none of the mesendoderm markers investigated were upregulated (Fig. 2). FGF inhibition is known to oppose neural and mesendodermal induction [12,13,20,69]. FGF signaling has a role in switching the outcome of BMP4-driven differentiation of hESCs to the mesendoderm via the maintenance of NANOG [20]. Our results also show continuous downregulation of NANOG upon B/SB/SU treatment (Fig. 3G, Supplementary Fig. S1C). Along with this, considering the downregulation of PS markers and noninduction of mesoderm markers, it can be concluded that B/SB/SU treatment circumvents mesoderm induction (Fig. 2). Excessive FGF activation promotes PE formation, and FGF inhibition causes failure of PE formation in mice [19], but it has been shown not to be required for human PE induction [40]. Our results show that the expression of both the PE markers, GATA6 and SOX7, was affected upon FGF inhibition (Fig. 2D), and also, the treatments involving FGF inhibition (SU and B/SB/SU) led to a similar expression pattern of GATA6 (Supplementary Fig. S6F). As our data show a significant reduction in the induction of PE markers upon FGF inhibition (SU and B/SB/SU), it could be possible that although FGF inhibition cannot solely prevent PE induction from hESCs, its inhibition might contribute to this, in combination with other factors, such as ACTIVIN/NODAL inhibition. This requires further investigation. Taken together, FGF signaling has an important role in switching the outcome of BMP4-driven differentiation not only to mesendoderm but also to PE.
Other than mesoderm and PE, BMP signaling can lead to trophoblast induction (Xu et al.; Das et al.). All our experiments were conducted under defined culture conditions, in contrast to other studies on BMP-induced differentiation of hESCs to trophoblast that were conducted in an MEF-conditioned medium that could be a source of many growth factors, such as FGF (Xu et al.; Das et al.). Our results show that upon inhibition of both ACTIVIN/NODAL and FGF pathways, along with BMP activation (B/SB/SU), hESCs differentiate toward syncytiotrophoblast and lacked extravillous trophoblast characteristics (Figs. 3 and 4, Supplementary Figs. S3 and S4).
FGF and ACTIVIN/NODAL signaling has a conserved role in TS cell maintenance [53,70], and the FGF-responsive transcription factors, Eomes and CDX2, are crucial for TE development [71]. FGF4 withdrawal or MEKK4 inactivation in TS cells induces differentiation, accompanied by the downregulation of these factors [53]. Our results reveal transient induction of CDX2, when FGF signaling was either exogenously blocked (B/SB/SU and SU) or not blocked (B/SB), but persistent induction with continuous FGF activation (B/SB/F) (Figs. 3H, I and 6D, E, Supplementary Fig. S6H). However, EOMES was persistently downregulated upon B/SB/SU treatment (Fig. 2B). Similar observations in the expression patterns of both CDX2 and EOMES upon ACTIVIN/NODAL inhibition have been reported [39]. In spite of the dependence of CDX2 expression on an FGF4-induced MEK-ERK pathway in mouse TS cells [52], its inhibition in mouse embryos caused neither an alteration in the TE cell fate nor a loss of CDX2 expression [19]. Hence, our results suggest that upon BMP induction, CDX2 is induced even in the absence of FGF signaling and is downregulated upon further differentiation to syncytiotrophoblast (Figs. 3H, I and 6D, E, Supplementary Fig. S6H). Although the possibility of the requirement of EOMES in the extravillous trophoblast differentiation cannot be ruled out, our results suggest the possibility of it not being required for the differentiation of hESCs into syncytiotrophoblast.
Syncytiotrophoblast is the nonproliferative layer of the placenta, which is formed by continuous fusion of cytotrophoblasts, and is thought to represent a mitotically end stage [61,62]. Although FGF signaling is required for the proliferation and growth of extraembryonic ectoderm, its absence neither affects its formation nor increases apoptosis [13], and this is in corroboration with our findings. Our further study of the B/SB/SU-induced differentiation to syncytiotrophoblast revealed a reduction in the mitotic index, and also, transcriptome analysis revealed regulation of cell cycle-related genes, favoring exit of the cell cycle (Fig. 4G, Supplementary Fig. S4B–D). However, this was accompanied by the induction of both apoptotic and antiapoptotic factors, such as the BCL2 family and the p53-MDM2 pathway. BCL2 is expressed in syncytiotrophoblast and syncytial knots, suggesting protection from apoptosis [66], and the importance of the p53-Mdm2 pathway in the development of trophoblast and its lineages has been reported [72]. Hence, our results demonstrate that syncytiotrophoblast formation involves cell cycle exit.
Another feature in the B/SB/SU-treated cells was that in spite of BMP signaling, a reduction in pSMAD1 was observed after 5 days when the syncytiotrophoblast characteristics were evident. This coincided with the enhanced expression of pGSK3β and the WNT antagonist, DKK1 (Fig. 5), reflecting WNT activation, which has been shown to be important for the GCM1/syncytin pathway in human choriocarcinoma cells, leading to syncytiotrophoblast formation [68]. Phosphorylation of GSK3β can induce degradation of SMAD1 protein through phosphorylation of its linker region [67]. Our results clearly indicate that though BMP signaling initially supports the differentiation of hESCs to trophoblast, BMP signaling is negatively modulated during further differentiation to syncytiotrophoblast.
As B/SB/SU treatment specifically supported differentiation of hESCs to syncytiotrophoblast and not mesendoderm, extravillous trophoblast and PE, unlike all the other treatments (B, B/SB, and B/SB/F), we hypothesized that FGF inhibition might be causing this specific lineage commitment. Our findings reveal that inhibition of FGF signaling alone in hESCs supports their differentiation to syncytiotrophoblast, also exhibiting a reduction in the mitotic index (Fig. 6, Supplementary Fig. S6). FGF signaling is essential for cell cycle progression [73], and Mekk4 inactivation in TS cells lead to differentiation, preferential to spongiotrophoblast and syncytiotrophoblast [54]. The coupling of FGF inhibition with BMP activation and ACTIVIN/NODAL inhibition led to syncytiotrophoblast induction, with a greater overlap with placental-regulated genes and prevention of mesendoderm, neuroectoderm, extravillous trophoblast, and PE induction (Figs. 2–4 and 6I). In fact, FGF inhibition alone (SU), or in combination with either BMP activation or ACTIVIN/NODAL inhibition (B/SU and SB/SU), also led to CGB induction and hCG hormone secretion, a characteristic feature of syncytiotrophoblasts (Figs. 3A, B, D, J and 6A, B, Supplementary Fig. S6B, D). Further, the SU-treated cells expressed E-CADHERIN, SYNCYTIN1, βhCG, and KRT7 (Fig. 6F–H). Taken together, the findings from our study strongly emphasize that FGF inhibition acts as a key in supporting syncytiotrophoblast differentiation of hESCs, thus preventing BMP-mediated differentiation to mesoderm, extravillous trophoblast, and PE (Fig. 7). Also, BMP signaling in conjunction with inhibition of both ACTIVIN/NODAL and FGF signaling specifically drives hESCs toward syncytiotrophoblast, preventing differentiation toward mesendoderm, neuroectoderm, extravillous trophoblast, and PE (Fig. 7).
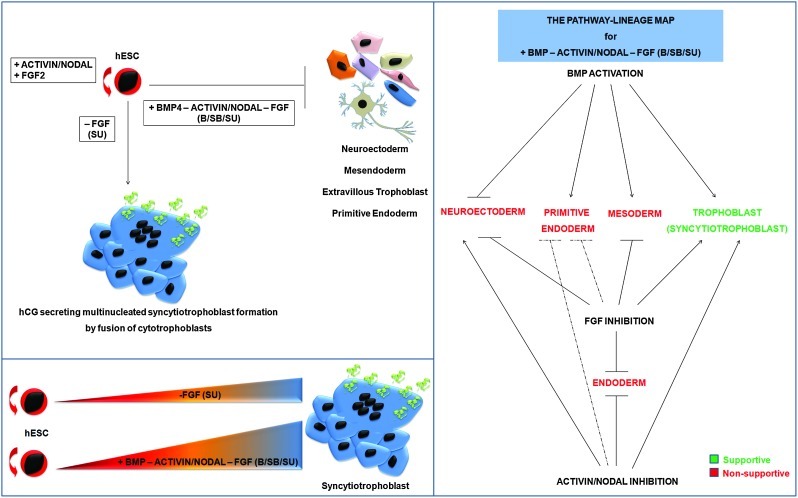
FIG. 7.
A model summarizing the outcome of B/SB/SU treatment of hESCs that leads to syncytiotrophoblast induction (“+” and “−” represent activation and inhibition, respectively). FGF inhibition (SU) supports syncytiotrophoblast induction, but to a lesser extent compared to B/SB/SU treatment. The pathway–lineage map, prepared on the basis of our findings and that of other laboratories, represents the consolidated outcome of BMP4 activation and inhibition of both ACTIVIN/NODAL and FGF pathways (B/SB/SU). Dotted lines: the reason for the inhibition of PE induction from hESCs upon B/SB/SU treatment could be a combinatorial effect of inhibition of both ACTIVIN/NODAL and FGF, but this requires further investigation. Color images available online at www.liebertpub.com/scd
We believe that this study provides valuable information for directed in vitro differentiation of hESCs. Furthermore, the data from this study can also be used in understanding the dynamics of gene regulation during syncytiotrophoblast formation that could be useful in understanding placental disorders, such as pre-eclampsia.
Supplementary Material
Acknowledgments
We thank Ms. Aydah Sabah and Dr. Rudi Lurz for providing support for Illumina microarray hybridizations and transmission electron microscopy, respectively. We are extremely grateful to Prof. Petra Knaus, Freie University, and Prof. Dr. Berthold Huppertz, University of Graz, for subject-oriented discussions. We thank Dr. Justyna Jozefczuk for reading the article and helpful comments. This work was part funded by the Max Planck Society. We acknowledge support from the German Federal Ministry of Education and Research (BMBF) (grants 01GN1005 and 0315717A), which is a partner of the ERASysBio+ initiative supported under the EU ERA-NET Plus scheme in FP7.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Xu RH. Chen X. Li DS. Li R. Addicks GC. Glennon C. Zwaka TP. Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 3.Amit M. Shariki C. Margulets V. Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 4.Beattie GM. Lopez AD. Bucay N. Hinton A. Firpo MT. King CC. Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 5.Greber B. Lehrach H. Adjaye J. Control of early fate decisions in human ES cells by distinct states of TGFbeta pathway activity. Stem Cells Dev. 2008;17:1065–1077. doi: 10.1089/scd.2008.0035. [DOI] [PubMed] [Google Scholar]
- 6.James D. Levine AJ. Besser D. Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 7.Vallier L. Reynolds D. Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Xu RH. Peck RM. Li DS. Feng X. Ludwig T. Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 9.Tam PP. Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SJ. Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 11.Ciruna B. Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 12.Kunath T. Saba-El-Leil MK. Almousailleakh M. Wray J. Meloche S. Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 13.Chai N. Patel Y. Jacobson K. McMahon J. McMahon A. Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol. 1998;198:105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- 14.Conlon FL. Lyons KM. Takaesu N. Barth KS. Kispert A. Herrmann B. Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 15.D'Amour KA. Agulnick AD. Eliazer S. Kelly OG. Kroon E. Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 16.Arman E. Haffner-Krausz R. Chen Y. Heath JK. Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman B. Poueymirou W. Papaioannou VE. DeChiara TM. Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- 18.Wilder PJ. Kelly D. Brigman K. Peterson CL. Nowling T. Gao QS. McComb RD. Capecchi MR. Rizzino A. Inactivation of the FGF-4 gene in embryonic stem cells alters the growth and/or the survival of their early differentiated progeny. Dev Biol. 1997;192:614–629. doi: 10.1006/dbio.1997.8777. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka Y. Lanner F. Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- 20.Yu P. Pan G. Yu J. Thomson JA. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 2011;8:326–334. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greber B. Wu G. Bernemann C. Joo JY. Han DW. Ko K. Tapia N. Sabour D. Sterneckert J. Tesar P. Scholer HR. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6:215–226. doi: 10.1016/j.stem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Schulz TC. Palmarini GM. Noggle SA. Weiler DA. Mitalipova MM. Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter MK. Inokuma MS. Denham J. Mujtaba T. Chiu CP. Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 24.Reubinoff BE. Itsykson P. Turetsky T. Pera MF. Reinhartz E. Itzik A. Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 25.Greber B. Coulon P. Zhang M. Moritz S. Frank S. Muller-Molina AJ. Arauzo-Bravo MJ. Han DW. Pape HC. Scholer HR. FGF signalling inhibits neural induction in human embryonic stem cells. EMBO J. 30:4874–4884. doi: 10.1038/emboj.2011.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaulden J. Reiter JF. Neur-ons and neur-offs: regulators of neural induction in vertebrate embryos and embryonic stem cells. Hum Mol Genet. 2008;17:R60–66. doi: 10.1093/hmg/ddn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Sanjuan I. Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- 28.Smith JR. Vallier L. Lupo G. Alexander M. Harris WA. Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P. Li J. Tan Z. Wang C. Liu T. Chen L. Yong J. Jiang W. Sun X, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 30.Boyd NL. Dhara SK. Rekaya R. Godbey EA. Hasneen K. Rao RR. West FD., 3rd Gerwe BA. Stice SL. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 2007;232:833–843. [PubMed] [Google Scholar]
- 31.Chadwick K. Wang L. Li L. Menendez P. Murdoch B. Rouleau A. Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 32.Pera MF. Andrade J. Houssami S. Reubinoff B. Trounson A. Stanley EG. Ward-van Oostwaard D. Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 33.Chen G. Ye Z. Yu X. Zou J. Mali P. Brodsky RA. Cheng L. Trophoblast differentiation defect in human embryonic stem cells lacking PIG-A and GPI-anchored cell-surface proteins. Cell Stem Cell. 2008;2:345–355. doi: 10.1016/j.stem.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das P. Ezashi T. Schulz LC. Westfall SD. Livingston KA. Roberts RM. Effects of fgf2 and oxygen in the bmp4-driven differentiation of trophoblast from human embryonic stem cells. Stem Cell Res. 2007;1:61–74. doi: 10.1016/j.scr.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallier L. Touboul T. Chng Z. Brimpari M. Hannan N. Millan E. Smithers LE. Trotter M. Rugg-Gunn P. Weber A. Pedersen RA. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One. 2009;4:e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardo AS. Faial T. Gardner L. Niakan KK. Ortmann D. Senner CE. Callery EM. Trotter MW. Hemberger M, et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao S. Chen S. Clark J. Hao E. Beattie GM. Hayek A. Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu C. Inokuma MS. Denham J. Golds K. Kundu P. Gold JD. Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z. Zhang W. Chen G. Cheng L. Liao J. Jia N. Gao Y. Dai H. Yuan J. Cheng L. Xiao L. Combinatorial signals of activin/nodal and bone morphogenic protein regulate the early lineage segregation of human embryonic stem cells. J Biol Chem. 2008;283:24991–25002. doi: 10.1074/jbc.M803893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roode M. Blair K. Snell P. Elder K. Marchant S. Smith A. Nichols J. Human hypoblast formation is not dependent on FGF signalling. Dev Biol. 2012;361:358–363. doi: 10.1016/j.ydbio.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greber B. Lehrach H. Adjaye J. Fibroblast growth factor 2 modulates transforming growth factor beta signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells. 2007;25:455–464. doi: 10.1634/stemcells.2006-0476. [DOI] [PubMed] [Google Scholar]
- 42.Vicovac L. Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 43.Tarrade A. Lai Kuen R. Malassine A. Tricottet V. Blain P. Vidaud M. Evain-Brion D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001;81:1199–1211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 44.Handschuh K. Guibourdenche J. Cocquebert M. Tsatsaris V. Vidaud M. Evain-Brion D. Fournier T. Expression and regulation by PPARgamma of hCG alpha- and beta-subunits: comparison between villous and invasive extravillous trophoblastic cells. Placenta. 2009;30:1016–1022. doi: 10.1016/j.placenta.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Loke YW. King A. Burrows T. Gardner L. Bowen M. Hiby S. Howlett S. Holmes N. Jacobs D. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50:135–146. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 46.Jameson JL. Hollenberg AN. Regulation of chorionic gonadotropin gene expression. Endocr Rev. 1993;14:203–221. doi: 10.1210/edrv-14-2-203. [DOI] [PubMed] [Google Scholar]
- 47.Muhlhauser J. Crescimanno C. Kasper M. Zaccheo D. Castellucci M. Differentiation of human trophoblast populations involves alterations in cytokeratin patterns. J Histochem Cytochem. 1995;43:579–589. doi: 10.1177/43.6.7539466. [DOI] [PubMed] [Google Scholar]
- 48.Babaie Y. Herwig R. Greber B. Brink TC. Wruck W. Groth D. Lehrach H. Burdon T. Adjaye J. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2007;25:500–510. doi: 10.1634/stemcells.2006-0426. [DOI] [PubMed] [Google Scholar]
- 49.Hay DC. Sutherland L. Clark J. Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- 50.Matin MM. Walsh JR. Gokhale PJ. Draper JS. Bahrami AR. Morton I. Moore HD. Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- 51.Liu L. Roberts RM. Silencing of the gene for the beta subunit of human chorionic gonadotropin by the embryonic transcription factor Oct-3/4. J Biol Chem. 1996;271:16683–16689. doi: 10.1074/jbc.271.28.16683. [DOI] [PubMed] [Google Scholar]
- 52.Murohashi M. Nakamura T. Tanaka S. Ichise T. Yoshida N. Yamamoto T. Shibuya M. Schlessinger J. Gotoh N. An FGF4-FRS2alpha-Cdx2 axis in trophoblast stem cells induces Bmp4 to regulate proper growth of early mouse embryos. Stem Cells. 2010;28:113–121. doi: 10.1002/stem.247. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka S. Kunath T. Hadjantonakis AK. Nagy A. Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 54.Abell AN. Granger DA. Johnson NL. Vincent-Jordan N. Dibble CF. Johnson GL. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol Cell Biol. 2009;29:2748–2761. doi: 10.1128/MCB.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mesiano S. Roles of estrogen and progesterone in human parturition. Front Horm Res. 2001;27:86–104. doi: 10.1159/000061038. [DOI] [PubMed] [Google Scholar]
- 56.Asanoma K. Matsuda T. Kondo H. Kato K. Kishino T. Niikawa N. Wake N. Kato H. NECC1, a candidate choriocarcinoma suppressor gene that encodes a homeodomain consensus motif. Genomics. 2003;81:15–25. doi: 10.1016/s0888-7543(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 57.Yu C. Shen K. Lin M. Chen P. Lin C. Chang GD. Chen H. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277:50062–50068. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 58.Yamada K. Ogawa H. Honda S. Harada N. Okazaki T. A GCM motif protein is involved in placenta-specific expression of human aromatase gene. J Biol Chem. 1999;274:32279–32286. doi: 10.1074/jbc.274.45.32279. [DOI] [PubMed] [Google Scholar]
- 59.Huppertz B. Borges M. Placenta trophoblast fusion. Methods Mol Biol. 2008;475:135–147. doi: 10.1007/978-1-59745-250-2_8. [DOI] [PubMed] [Google Scholar]
- 60.Crocker IP. Arthur P. Heazell AE. Baker PN. The mitotic manipulation of cytotrophoblast differentiation in vitro. Placenta. 2007;28:408–411. doi: 10.1016/j.placenta.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Ichikawa N. Zhai YL. Shiozawa T. Toki T. Noguchi H. Nikaido T. Fujii S. Immunohistochemical analysis of cell cycle regulatory gene products in normal trophoblast and placental site trophoblastic tumor. Int J Gynecol Pathol. 1998;17:235–240. doi: 10.1097/00004347-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Wakuda K. Yoshida Y. DNA ploidy and proliferative characteristics of human trophoblasts. Acta Obstet Gynecol Scand. 1992;71:12–16. doi: 10.3109/00016349209007940. [DOI] [PubMed] [Google Scholar]
- 63.Juan G. Traganos F. James WM. Ray JM. Roberge M. Sauve DM. Anderson H. Darzynkiewicz Z. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry. 1998;32:71–77. doi: 10.1002/(sici)1097-0320(19980601)32:2<71::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 64.Ullah Z. Kohn MJ. Yagi R. Vassilev LT. DePamphilis ML. Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev. 2008;22:3024–3036. doi: 10.1101/gad.1718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Momand J. Zambetti GP. Olson DC. George D. Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 66.Ratts VS. Tao XJ. Webster CB. Swanson PE. Smith SD. Brownbill P. Krajewski S. Reed JC. Tilly JL. Nelson DM. Expression of BCL-2, BAX and BAK in the trophoblast layer of the term human placenta: a unique model of apoptosis within a syncytium. Placenta. 2000;21:361–366. doi: 10.1053/plac.1999.0486. [DOI] [PubMed] [Google Scholar]
- 67.Sieber C. Kopf J. Hiepen C. Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Matsuura K. Jigami T. Taniue K. Morishita Y. Adachi S. Senda T. Nonaka A. Aburatani H. Nakamura T. Akiyama T. Identification of a link between Wnt/beta-catenin signalling and the cell fusion pathway. Nat Commun. 2:548. doi: 10.1038/ncomms1551. [DOI] [PubMed] [Google Scholar]
- 69.Na J. Furue MK. Andrews PW. Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 2010;5:157–169. doi: 10.1016/j.scr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Erlebacher A. Price KA. Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol. 2004;275:158–169. doi: 10.1016/j.ydbio.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 71.Rossant J. Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 72.Chiu SY. Asai N. Costantini F. Hsu W. SUMO-specific protease 2 is essential for modulating p53-Mdm2 in development of trophoblast stem cell niches and lineages. PLoS Biol. 2008;6:e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chambard JC. Lefloch R. Pouyssegur J. Lenormand P. ERK implication in cell cycle regulation. Biochimica et biophysica acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.