Abstract
The mouse mammary gland is an outstanding developmental model that exemplifies the activities of many of the effector pathways known to organize mammalian morphogenesis; furthermore, there are well-characterized methods for the specific genetic manipulation of various mammary epithelial cell components. Among these signaling pathways, Wnt signaling has been shown to generate plasticity of fate determination, expanding the genetic programs available to cells in the mammary lineage. It is responsible first for the appearance of the mammary fate in embryonic ectoderm and then for maintaining bi-potential basal stem cells in adult mammary ductal trees. Recent technical developments have led to the separate analysis of various mammary epithelial cell subpopulations, spurring the investigation of Wnt-dependent interactions. Although Wnt signaling was shown to be oncogenic for mouse mammary epithelium even before being identified as the principle oncogenic driver for gut epithelium, conclusive data implicating this pathway as a tumor driver for breast cancer lag behind, and we examine potential reasons.
The Wnt pathway is responsible for the appearance of the mammary fate in embryonic ectoderm, and then for maintaining bi-potential basal stem cells in adult mammary ductal trees.
There are several excellent recent reviews that cover aspects of Wnt signaling and mammary gland development and transformation (Boras-Granic and Wysolmerski 2008; van Amerongen and Nusse 2009; Incassati et al. 2010; Roarty and Rosen 2010; Wend et al. 2010; Jarde and Dale 2011). The aim of this article is to focus attention on the open questions in this area. There are a remarkable number of tools available to assist with this, given the focus on breast cancer research in the past 10 years. They include large collections of human breast cancer cell lines (Neve et al. 2006; Hoeflich et al. 2009; Hollestelle et al. 2010) and dozens of strains of mice that are useful for analysis of different aspects of Wnt signaling and biology (van Amerongen and Berns 2006). This battery of genetic tools includes transgenic mice that express (or induce conditional ablation of) genes in either of the two main mammary epithelial cell types that comprise the mammary gland (the organization of the mammary gland is shown in Fig. 1). These are the luminal cells—typically targeted by one of three drivers, namely, MMTV LTR (mouse mammary tumor virus long terminal repeat), WAP (whey acidic protein promoter, expressed during milk production/terminal differentiation), or BLG (β-lactoglobulin, another milk whey protein) promoters)—and the basal cells (typically targeted using keratin-5 or -14 promoters, also expressed in other stratified epithelia, notably skin). The definitions of luminal and basal cells vary between studies because they are based on the analytical method used. These may be (1) location in tissue sections (facing the lumen or adherent to the basement membrane); (2) expression of molecular markers usually associated with basal cells (e.g., expression of basal-specific cytokeratin 5 [CK 5] or p63, or luminal-specific CK8 or Muc1); or (3) copurification with subgroups of cells isolated by flow cytometry (e.g., using Lin/CD29/CD24 or Lin/EpCAM/CD49f). These latter cell populations are often subsequently typed using their expression of histological markers or mRNA profile to generate a “luminal” or “basal” descriptor. The cell groups described by these means are not always the same, and this becomes important to experimental interpretation.
Figure 1.
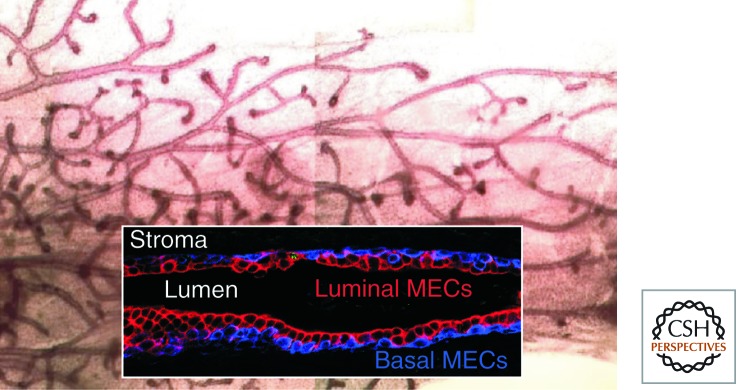
The mouse mammary gland, organization, and cell types. The (10) mouse mammary gland(s) comprise fat pads attached to the ventral mouse skin, colonized by a branched tree of hollow, epithelial mammary ducts (stained with carmine red) that are connected to the nipple. During pregnancy, there is massive proliferation of lobuloalveolar side branches to colonize the interstitial spaces between ducts, becoming filled with milk, expressed by oxytocin-induced contraction of myoepithelial cells. (Inset) An immunofluorescent stain of a longitudinal cross section of a non-pregnant duct, stained with a basal cell anti-cytokeratin (CK5) together with a luminal cell anti-cytokeratin (CK8), to show the bilayered structure of the epithelium.
Not all cells in a given lineage are identical. For luminal cells, perhaps one of their most obvious differences is their expression of ERα (estrogen receptor-α). Thus, for both mouse and human, ∼15% of luminal cells express ERα (at any one time), irrespective of their stage of development (Clarke 2003; Mastroianni et al. 2009). ERα-positive and -negative cells can be purified by flow cytometry and analyzed separately (Kendrick et al. 2008; Lim et al. 2010). The ERα-negative luminal cell subpopulation contains a proliferative activity, leading it to be labeled “luminal progenitor cells” (a heterogeneous group), whereas the ERα-positive group is non-clonogenic in vitro and therefore labeled “mature” (Table 1).
Table 1.
Characterized expression and function of cell surface Wnt signaling components in mammary cells and tissues
| Gene | Array | mRNA | Protein | Cell/tissue type | Function | Citation |
|---|---|---|---|---|---|---|
| Wnt1 | X | All sources | ||||
| X (N) | Normal mouse MG | Tumorigenic in transgenic mice (MMTV-Wnt1) Induces branching and lobuloalveolar hyperplasia |
Tsukamoto et al. 1988 | |||
| ✓ (R) | T-47D cells | Suzuki et al. 2008 | ||||
| Highly transforming in C57MG cells | Wong et al. 1994 | |||||
| Transfection into HC11 cells and transplantation into cleared fat pads leads to fibrotic outgrowths | Humphreys and Rosen 1997 | |||||
| Wnt2 (Wnt2a) | ✓ | ✓ (I) | TEB stroma | Kouros-Mehr and Werb 2006 | ||
| ✓ (R, I, N) | Basal cells and TEBs | mRNA decreases 10×–40× during pregnancy and lactation | Buhler et al. 1993 | |||
| Transforms C57MG cells | Wong et al. 1994 | |||||
| ✓ (RP, I) | Normal human breast fibroblasts and human breast tumor epithelium | Low expression levels in normal tissue and overexpression (5×–8×) in some carcinomas | Dale et al. 1996 | |||
| Transfection into HC11 cells and transplantation into cleared fat pads leads to fibrotic outgrowths | Humphreys and Rosen 1997 | |||||
| ✓ | Mouse breast cancer cell line bone metastasis model | Significantly down-regulated at tumor–bone interface | Sadanandam et al. 2011 | |||
| Wnt2b (Wnt13) | X | All sources | ||||
| ✓ (R) | Normal human mammoplasty tissue and infiltrating ductal carcinoma biopsies | Low expression levels in normal tissue and overexpression (5×–8×) in some carcinomas | Bergstein et al. 1995 | |||
| Wnt3 | X | All sources | ||||
| ✓ (N) | Normal mouse mammary gland | MMTV CIS | Roelink et al. 1990; Theodorou et al. 2007; Callahan and Smith 2008 | |||
| ✓ (RP) | Normal human breast tissue, MTSV1-7, BT20, MCF7adr (ER−, EGFR+) | Huguet et al. 1994 | ||||
| Wnt3a | X | All sources | ||||
| Highly transforming in C57MG cells | Wong et al. 1994 | |||||
| MMTV CIS | Roelink et al. 1990; Theodorou et al. 2007; Callahan and Smith 2008 | |||||
| Wnt4 | ✓ | Mature luminal | Lim et al. 2010 | |||
| ✓ | Differentiated luminal | Grigoriadis et al. 2006 | ||||
| ✓ | Luminal ER+ cells | Kendrick et al. 2008 | ||||
| ✓ | NDE | Kendrick et al. 2008 | ||||
| ✓ | ✓ (I) | TEBs/mature ducts | Kouros-Mehr and Werb 2006 | |||
| ✓ (I) | Luminal | Brisken et al. 2000 | ||||
| X (W) | C3H 10T1/2 cells | Plays a role in branching during pregnancy | Bradbury et al. 1995; Brisken et al. 2000 | |||
| ✓ (R) | Luminal cells from mammary glands treated with estrogen | Mediates progesterone-induced stem cell expansion | Joshi et al. 2010 | |||
| ✓ (N) | Highest expression in virgin also in cell lines C57MG, C127I, and NMuMG cells | Gavin and McMahon 1992 | ||||
| ✓ (RP) | MDA415 | Huguet et al. 1994 | ||||
| Wnt5a | ✓ | ✓ | Luminal ER+ cells | Kendrick et al. 2008 | ||
| ✓ | Mature luminal cells | Lim et al. 2010 | ||||
| ✓ | Luminal cells | Grigoriadis et al. 2006 | ||||
| ✓ | ✓ (I) | TEB epithelium | Kouros-Mehr and Werb 2006 | |||
| ✓ (N) | Weakly expressed in 12-wk-old virgin and induced during pregnancy | Weber-Hall et al. 1994 | ||||
| ✓ (N) | Whole mammary gland | Expression detectable during pregnancy (peak at 10 days), no expression during lactation | Buhler et al. 1993 | |||
| Required for MG development and TGFβ-mediated inhibition of ductal growth. Wnt5a KO mice show accelerated ductal morphogenesis |
Roarty and Serra 2007 | |||||
| ✓ (N) | Virgin, increase during pregnancy; C57MG and C127I cells | Gavin and McMahon 1992 | ||||
| ✓ (N) | Human breast carcinoma | Does not transform C57MG cells | Iozzo et al. 1995 | |||
| ✓ (RP, I) | Little to no expression in normal breast tissue Increased expression in benign proliferations and invasive breast cancer ISH signal localized to epithelial compartment |
Lejeune et al. 1995 | ||||
| Overexpression results in lactation defect in MMTV-Wnt5a mice | Baxley et al. 2011 | |||||
| Wnt5b | ✓ | Bipotent CFCs | Raouf et al. 2008 | |||
| ✓ | Luminal | Grigoriadis et al. 2006 | ||||
| ✓ | ✓ (I) | TEBs/mature ducts | Kouros-Mehr and Werb 2006 | |||
| ✓ (N) | Whole mammary gland | Expression detectable during pregnancy (d10-18), no expression during lactation | Buhler et al. 1993 | |||
| Transforms C57MG cells | Wong et al. 1994 | |||||
| ✓ (N) | Virgin mammary gland, increases during pregnancy | Gavin and McMahon 1992 | ||||
| Wnt6 | ✓ | Basal cells | Kendrick et al. 2008 | |||
| ✓ | ✓ (I) | TEBs/mature ducts | Kouros-Mehr and Werb 2006 | |||
| ✓ (N) | Expressed in virgin, increase during pregnancy | Gavin and McMahon 1992 | ||||
| Wnt7a | X | All sources | ||||
| Highly transforming in C57MG cells | Wong et al. 1994 | |||||
| ✓ (N) | NMuMG cell line | Gavin and McMahon 1992 | ||||
| Wnt7b | ✓ | ✓ | Luminal ER+ cells | Kendrick et al. 2008 | ||
| ✓ | Luminal ER- cells | Kendrick et al. 2008 | ||||
| ✓ | Mature luminal cells | Lim et al. 2010 | ||||
| ✓ | ✓ (I) | TEB epithelium | Kouros-Mehr and Werb 2006 | |||
| Does not alter MG development Transforms C57MG cells |
Wong et al. 1994; Naylor et al. 2000 | |||||
| Transfection into HC11 cells and transplantation into cleared fat pads leads to fibrotic outgrowths and palpable adenocarcinomas | Humphreys and Rosen 1997 | |||||
| ✓ (N) | Highest expression in virgin glands | Gavin and McMahon 1992 | ||||
| ✓ (RP) | Normal human breast tissue, MTSV1-7, MCF7, ZR75, T47D, MCF7adr, MDA231, MDA361, BT20, MDA415, MDA457, MDAMB157, ZR9B11 | Huguet et al. 1994 | ||||
| Wnt9a (Wnt14) | ✓ | Luminal cells | Grigoriadis et al. 2006 | |||
| ✓ | Primary human breast cancer | Overexpressed in one of nine cases of breast cancer | Kirikoshi et al. 2001 | |||
| Wnt9b (Wnt14b Wnt15) | ✓ | Whole mammary gland | Transforms C57MG cells (weaker than Wnt1) | Qian et al. 2003 | ||
| ✓ | Primary human breast cancer | Kirikoshi et al. 2001 | ||||
| Wnt10a | ✓ | ✓ | Basal/myoepithelial | Kendrick et al. 2008 | ||
| Wnt10b (Wnt12) | Tumorigenic in transgenic mice (MMTV-Wnt10b) MG hyperplasia, increased proliferation and branching |
Lane and Leder 1997 | ||||
| ✓ (RP,R) | MCF-7 Adrr, MDA-MB-435, and MDA-MB-157 cells Human breast samples, and some primary human breast carcinomas |
Bui et al. 1997 | ||||
| ✓ (N) | Virgin mouse mammary glands (not pregnant) | Lee et al. 1995 | ||||
| ✓ | Mammary ridge (E11.5) Dual abdominal MG anlagen (12.5 dpc) |
Christiansen et al. 1995 | ||||
| Wnt11 | X | All sources | ||||
| Transforms C57MG cells | Christiansen et al. 1996 | |||||
| ✓ (W) | MDA-MB-231 | ERRα- and β-catenin-regulated gene Involved in cancer cell migration |
Dwyer et al. 2010 | |||
| Wnt16 | ✓ | Induced by Wnt4 | Kim et al. 2009 | |||
| RSpo1 | ✓ | Luminal ER- | Kendrick et al. 2008 | |||
| ✓ (I) | Mammary mesenchyme (E15.5) | Nam et al. 2006, 2007 | ||||
| KD might result in absence of ductal side-branching and alveolar formation | Chadi et al. 2009 | |||||
| RSpo2 | X | Not in adult tissues | ||||
| ✓ (R) | Detected at very low levels early in MG development but not detectable in MG of day 15 pregnant mice | Lowther et al. 2005 | ||||
| X (R) | X (W, ICC) | HC11, C57MG mouse cell lines | Klauzinska et al. 2011 | |||
| X (R) | HC11 cells | SA Fakhraldeen and CM Alexander, unpubl. | ||||
| RSpo3 | X | Not in adult tissues | ||||
| CIS for MMTV | Theodorou et al. 2007; Callahan and Smith 2008 | |||||
| Fzd1 | ✓ | ✓ | Basal/myoepithelial | Kendrick et al. 2008 | ||
| ✓ | Down-regulated in mature luminal cells | Lim et al. 2010 | ||||
| ✓ | Mature myoepithelial cells | Raouf et al. 2008 | ||||
| ✓ | ✓ (I) | TEB stroma | Kouros-Mehr and Werb 2006 | |||
| ✓ (R) | HMEC, MDA-MB-468, MDA-MB-453, MCF-7, T-47D, BT-20, and BT-474 cells | Benhaj et al. 2006 | ||||
| Fzd2 | ✓ | ✓ | Basal/myoepithelium | Kendrick et al. 2008 | ||
| ✓ | ✓ (I) | TEB | Kouros-Mehr and Werb 2006 | |||
| ✓ (R) | MDA-MB-468, MDA-MB-453, MCF-7, T47D, BT-20, and BT-474 cells | Benhaj et al. 2006 | ||||
| Fzd3 | ✓ | Basal/myo | Kendrick et al. 2008 | |||
| ✓ | Myo | Grigoriadis et al. 2006 | ||||
| ✓ (R) | MDA-MB-231 and SUM-159 cells | Functions in MDA-MB-231 cell motility | Valastyan et al. 2009 | |||
| ✓ (W) | MDA-MB-231 cells | |||||
| ✓ (R) | HMEC, MDA-MB-468, MCF-7, T-47D, BT-20, and BT-474 cells | Benhaj et al. 2006 | ||||
| Fzd4 | X | All sources | ||||
| Fzd5 | ✓ | Luminal | Grigoriadis et al. 2006 | |||
| ✓ (R) | MDA-MB-468 cells | Benhaj et al. 2006 | ||||
| Fzd6 | ✓ | Basal/myoepithelium | Jones et al. 2004 | |||
| ✓ | Basal/myoepithelium | Grigoriadis et al. 2006 | ||||
| ✓ (R) | HMEC, MDA-MB-468, MDA-MB-453, MCF-7, T-47D, BT-20, and BT-474 cells | Benhaj et al. 2006 | ||||
| Fzd7 | ✓ | ✓ | Basal/myoepithelium | Kendrick et al. 2008 | ||
| ✓ | Luminal-restricted CFCs | Raouf et al. 2008 | ||||
| ✓ (R) | HMEC, MDA-MB-468, MDA-MB-453, T-47D, and BT-20 cells | Benhaj et al. 2006 | ||||
| Plays a role in proliferation and invasiveness of TNBC cell lines | Yang et al. 2011 | |||||
| Fzd8 | ✓ | ✓ | Basal/myoepithelium | Kendrick et al. 2008 | ||
| ✓ | MaSC-enriched fraction | Lim et al. 2010 | ||||
| ✓ (R) | HMEC and MDA-MB-453 cells | Benhaj et al. 2006 | ||||
| X (R) | MDA-MB-468 | |||||
| Fzd9 | ✓ | Differentiated luminal cells | Raouf et al. 2008 | |||
| Fzd10 | X | |||||
| Lrp5 | ✓ | Bipotent CFCs | ||||
| ✓ (F, IHC) | Basal cells | Badders et al. 2009 | ||||
| ✓ | Translation induced in Wnt1-induced luminal cells | Kim et al. 2011 | ||||
| ✓ (R) | HMEC, MDA-MB-468, MDA-MB-453, MCF-7, T-47D, BT-20, and BT-474 cells | Benhaj et al. 2006 | ||||
| ✓ (R) | Breast cancer specimens and normal breast tissue specimens | Bjorklund et al. 2009 | ||||
| Mammary stem cell maintenance | Lindvall et al. 2006 | |||||
| Maintenance of basal cell population | Badders et al. 2009 | |||||
| Lrp6 | ✓ | Luminal | Grigoriadis et al. 2006 | |||
| ✓ (W) | SUM1315 cells | DiMeo et al. 2009 | ||||
| ✓ (R) | HMEC, MDA-MB-468, MDA-MB-453, MCF-7, T-47D, BT-20, and BT-474 cells | Benhaj et al. 2006 | ||||
| ✓ (R) | Breast tumor, MDA-MB-231 and MDA-MB-468 cells | Li et al. 2004 | ||||
| OE in mice (MMTV-Lrp6) induces hyper-lobular development and increased TEB number | Zhang et al. 2010a | |||||
| ✓ (F, IHC) | Basal in adult Basal and luminal in newborns |
Loss interferes with mammary placode, fat pad, and branching development during embryogenesis Heterozygosity for inactivating mutation leads to reduced TEBs and branches |
Badders et al. 2009; Lindvall et al. 2009 | |||
| Ror1 | ✓ | Luminal CFCs | Raouf et al. 2008 | |||
| ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 | ||||
| Ror2 | ✓ (R) | ✓ (IHC) | Expressed in basal/myoepithelial cells and luminal cells of mouse mammary gland | K Roarty and JM Rosen, pers. comm. | ||
| ✓ (R) | Expressed in breast cancer cell lines (MCF7 and MDA-MB231) and brain metastases of brCA in vivo | Klemm et al. 2011 | ||||
| Lgr4 | ✓ | Basal cells | Kendrick et al. 2008 | |||
| X | Normal luminal epithelium | Grigoriadis et al. 2006 | ||||
| Lgr5 | ✓ | Basal cells | Kendrick et al. 2008 | |||
| Dkk1 | ✓ | Basal cells | Kendrick et al. 2008 | |||
| ✓ | NDE | Kendrick et al. 2008 | ||||
| ✓ | Luminal cells | Grigoriadis et al. 2006 | ||||
| ✓ (W) | SUM1315 cells | DiMeo et al. 2009 | ||||
| OE prevents mammary placode development | Chu et al. 2004 | |||||
| X (R) | HC11 mouse cells | SA Fakhraldeen and CM Alexander, unpubl. | ||||
| Dkk2 | X | All sources | ||||
| X (R) | HC11 mouse cells | SA Fakhraldeen and CM Alexander, unpubl. | ||||
| Dkk3 | ✓ | ✓ | Bipotent CFCs | Raouf et al. 2008 | ||
| ✓ | Differentiated basal cells | Raouf et al. 2008 | ||||
| ✓ | MaSC-enriched | Lim et al. 2010 | ||||
| ✓ | Myoepithelium/basal cells | Jones et al. 2004 | ||||
| ✓ | Myoepithelium/basal cells | Grigoriadis et al. 2006 | ||||
| ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 | ||||
| X (R) | HC11 cells | SA Fakhraldeen and CM Alexander, unpubl. | ||||
| Dkk4 | X | All sources | ||||
| X (R) | SA Fakhraldeen and CM Alexander, unpubl. | |||||
| WIF1 | ✓ | ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 | ||
| ✓ | MaSC-enriched | Lim et al. 2010 | ||||
| ✓ (R) | T47D, normal human breast tissue | Targeted for epigenetic silencing in human breast cancer | Ai et al. 2006 | |||
| ✓ (IHC) | Normal breast tissue | Down-regulated in invasive ductal breast carcinoma | Wissmann et al. 2003 | |||
| ✓ | Mouse breast cancer cell line bone metastasis model | Increased expression at tumor-bone interface | Sadanandam et al. 2011 | |||
| SFRP1 | ✓ | ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 | ||
| ✓ | Down-regulated in mature luminal | Lim et al. 2010 | ||||
| Maintains/reduces canonical signaling levels | Cowling et al. 2007 | |||||
| mRNA expression lost in >80% of invasive breast carcinomas (medullary) | Ugolini et al. 2001 | |||||
| ✓ (IHC) | Mammary epithelial cells | Tumor suppressive function Lost in tumors Loss associated with poor prognosis |
Klopocki et al. 2004 | |||
| X (W) | SUM1315 cells | DiMeo et al. 2009 | ||||
| ✓ | Mature luminal | Grigoriadis et al. 2006 | ||||
| ✓ (R) | Normal breast tissue MDA-MB-435S, MDA-MB-436, MDA-MB-468, MDA-MB-157, MDA-MB-361, and ZR-75-1 cells |
Suzuki et al. 2008 | ||||
| X (R) | MCF-7, MDA-MB-231, T47D, and SK-BR-3 cells | |||||
| SFRP2 | ✓ | Myoepithelium/basal cells | Grigoriadis et al. 2006 | |||
| ✓ (R) | Normal breast tissue and MDA-MB-157 cells | Suzuki et al. 2008 | ||||
| X (R) | MCF-7, MDA-MB-231, MDA-MB-435S, MDA-MB-468, T-47D, SK-BR-3, MDA-MB-453, and ZR-75-1 cells | |||||
| SFRP4 | ✓ | Luminal | Grigoriadis et al. 2006 | |||
| ✓ | Mouse breast cancer cell line bone metastasis model | Increased expression at tumor-bone interface | Sadanandam et al. 2011 | |||
| SFRP5 | X | All sources | ||||
| WISP1 | ✓ | Myoepithelium/basal cells | Grigoriadis et al. 2006 | |||
| ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 | ||||
| WISP2 | ✓ | Luminal | Grigoriadis et al. 2006 | |||
| ✓ (R) | ✓ (W) | MCF7, T47D, ZR-75.1, SKBr3 | Knockdown promotes proliferation (including E-independent) of MCF7 cells Inducing expression inhibits proliferation of MCF7 and MDA-MB-231 cells |
Fritah et al. 2008 | ||
| X (R) | X (W) | HMEC, MDA-MB-231 | ||||
| WISP3 | ✓ | Bipotent CFCs | Grigoriadis et al. 2006 | |||
| Naked1 | ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 | |||
| Naked2 | ✓ | Bipotent CFCs | Raouf et al. 2008 | |||
| ✓ | Myoepithelium/basal cells | Kendrick et al. 2008 |
The expression patterns of Wnt-receptor signaling components are summarized, deduced from significant expression by mRNA species by microarray analysis (Array), or more specific analysis by qPCR/RT-PCR (R), in situ hybridization (I), RNase protection (RP), or Northern blotting (N), and of cognate proteins, shown by flow cytometry (F), immunohistochemical/cytochemical localization (IHC/ICC), or Western blotting (W). Functional information for each component is summarized.
Specificity of expression in mammary gland cell types: The array results reported are from several separate studies, and the cell fractions and gene chips are as follows:
1. (Raouf et al. 2008) Normal human mammary epithelial cells were sorted into four fractions. Both two stem/progenitor-enriched fractions (a bipotent basal stem cell and a luminal-restricted luminal progenitor; both scored as colony-forming cells in vitro, CFCs) showed high EpCAM expression compared with their mature myoepithelial and luminal counterparts. Two mature cell types extracted from the low-EpCAM fraction were luminal cells (high Muc1/CD133, low Thy1/CD10) and myoepithelial cells (high Thy1 and low Muc1). mRNA was analyzed using Affymetrix human X3P GeneChip arrays or PCR-LongSAGE libraries.
2. (Lim et al. 2010) Normal mouse mammary glands were sorted into three epithelial cell fractions, and one stromal. A basal stem cell–enriched fraction (MaSC) was separated as β1 integrin (CD29)-high, CD24-low, and CD61-positive. Luminal cells were isolated as CD29-low (CD24-positive), and the progenitors were CD61-negative, whereas the mature cells were CD61-positive. For normal human mammary gland cells, the sorting parameters were different, but the cell populations were broadly similar. Three fractions described were a basal, mammary stem cell–enriched (MaSC) fraction—α6 integrin (CD49f)-high, EpCAM-negative—and two luminal fractions—EpCAM-positive, the progenitor fraction CD49f-positive, and the mature fraction CD49f-negative. mRNA was analyzed using Illumina MouseWG-6 v 2.0 BeadChips.
3. (Kendrick et al. 2008) Normal mouse mammary glands were sorted into three epithelial cell fractions, broadly similar to those described by Lim et al. (2010), although the cell surface markers used are different, as is the preparation and exclusion of other non-epithelial cell types. A basal cell fraction (stem/myoepithelial) was collected as CD24-low and Sca1-negative. Two luminal cell fractions both expressed CD24; the ERα-negative, luminal progenitor cell fraction was isolated as Sca1-low; and the mature, ERα-positive cells were Sc11-positive. mRNA analysis was performed used Mouse Affymetrix Mouse Expression MOE430 2.0 arrays.
4. (Grigoriadis et al. 2006) This is the only study to use cultured primary cells. Cells from normal human breast and from primary tumors were separated by double immunomagnetic sorting, either as EMA+/integrin β4-negative luminal cells, or CD10+/BerEP4-negative myoepithelial cells. The mRNA was analyzed using MPSS and Affymetrix Human Genome U133 Plus 2.0 GeneChip, CodeLink Human Whole Genome Bioarray, Agilent Whole Human Genome Oligo Microarray 44K cDNA array, and 20K cDNA microarray (constructed at the Breakthrough Breast Cancer Research Center, UK).
(CFC) Colony-forming cell; (NDE) not differentially expressed; (TNBC) triple negative breast cancer; (OE) overexpression; (KO) knockout; (ISH) in situ hybridization; (IHC) immunohistochemistry; (CIS) common integration site (applied to MMTV retroviral tagging studies). Wnt ligand and Fzd receptor expression in embryonic mammary tissues has been previously described (Chu et al. 2004).
(✓) Detectable expression.
(X) No detectable expression.
Using lacZ reporter strains, the heterogeneity of expression of MMTV- and WAP-driven transgenes is obvious, but the basis for this is unknown (e.g., Wagner et al. 2001). Indeed, the MMTV LTR is a remarkable 1200-bp gene expression motif that condenses all of the key elements of mammary regulation, to include the specification of mammary fate (expression starting early in the ectoderm of embryogenesis), together with the hormone inducibility that appears during puberty in females (Rouault et al. 2007), with further up-regulation during pregnancy and lactation (Mink et al. 1990). Cre expression has effects on mammary morphogenesis and lactation; therefore, experiments that use this tool need to be interpreted with caution (Chan et al. 2007; Robinson and Hennighausen 2011).
There are several Wnt reporter lines that show highly regulated (but slightly different) expression patterns in mammary gland (including a number of strains based on Wnt response elements [WRE] driving lacZ [for review, see Barolo 2006], and two newer strains based on WRE-GFP, perhaps more useful for purifying Wnt-responsive cell types [Currier et al. 2010; Ferrer-Vaquer et al. 2010]). Because of the redundant expression of many Fzd (8/10), both Ror receptors (2/2) and Wnt ligand types (11/19) in mammary gland (Table 1), initial experiments have focused on producing gain or loss of function for canonical Wnt signaling. Thus, overexpression of the forerunner Wnt ligand species, Wnt1- (using an MMTV-Wnt1 construct) (Table 1) (Tsukamoto et al. 1988) induced ductal hyperplasia (not correctly described as mid-pregnant equivalent) and basaloid tumors (Li et al. 2003a; Liu et al. 2004; Vaillant et al. 2008), whereas the cell-autonomous Wnt signaling effector ΔNβ-catenin (using MMTV-LTR) induced later onset hyperplasia (luminal in origin) and luminal-type tumors (Imbert et al. 2001) or ductal hyperplasia and basaloid tumors when expressed by a CK5 construct (Teuliere et al. 2005). Loss of function for β-catenin, induced by overexpression of Axin or a dominant-negative β-catenin effector in luminal cells, has implicated β-catenin in lobulo-alveolar development during pregnancy (Hsu et al. 2001; Tepera et al. 2002). This is despite a lack of clear evidence for activation of canonical Wnt reporter activity during pregnancy (Chu et al. 2004; Boras-Granic et al. 2006a). If a canonical βcat/TCF signaling is indeed activated in luminal cells, this presents a paradox. Cell surface presentation of Lrp is considered to be the limiting factor for canonical Wnt signaling (Brennan and Brown 2004), and Wnt1 responses are confined to Lrp5-positive cells (Badders et al. 2009; Teissedre et al. 2009; Baker et al. 2010; Kim et al. 2011). Only basal cells appear to be competent to generate a canonical response in response to Wnt ligands (they express the obligate canonical Lrp5 and Lrp6 receptors). Therefore, the identity of the cell surface receptor able to mediate β-catenin/TCF-induced luminal cell division is not yet known.
Lrp6 knockout (KO) mice die at birth, and the functionality of Lrp6 KO mammary rudiments has not yet been evaluated, although Lrp6 hypomorphs (Lrp6+/−) are underbranched (Lindvall et al. 2009). Although dominant-negative TCF molecules have been used effectively in other cell types (van de Wetering et al. 2002), their use has not yet been reported for mammary gland. Table 1 summarizes the expression data and functional information that are available to describe Wnt signaling effectors from studies of normal and transgenic mice and human tissues (including some observations from cell lines).
The vast majority of data that describe the molecular basis of signaling via Lrp5/6 derives from ectopic expression of Wnt components in HEK293T cells (Brennan et al. 2004; Cong et al. 2004; Zeng et al. 2005, 2008). Thus, the specific molecular complexes that form in mammary epithelial cells have yet to be described (Fig. 2). For example, there are convincing data for the activation of LRP (at the PPSP motif) by a range of different kinases, including GSK, PKA, Pftk1, Grk5/6, and CK1 (Niehrs and Shen 2010), and for the control of kinase recruitment by local phospho-lipid effectors such as P(4,5)IP2 (Tanneberger et al. 2011); however, the relative significance of each of these mechanisms for mammary epithelial cells is not yet known.
Figure 2.
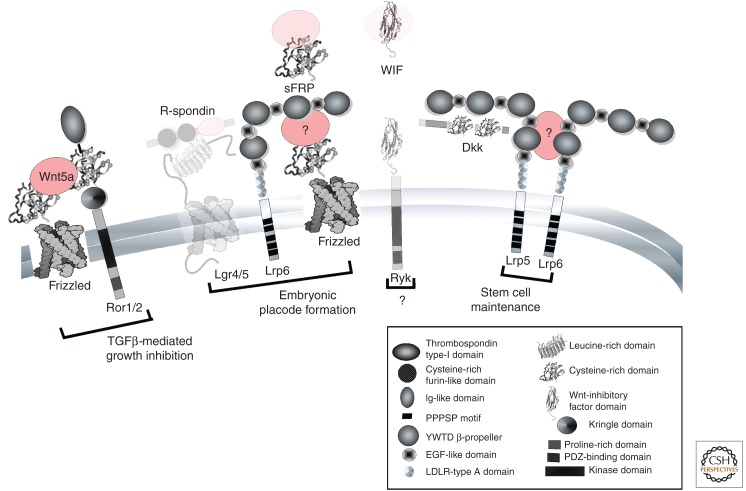
Cell surface Wnt signaling components shown to function during various phases of mammary gland growth and development. Structural and function information is drawn from data from He et al. (2004), Liepinsh et al. (2006), Kikuchi et al. (2007), Green et al. (2008), Bourhis et al. (2010), and Weis (2011). The information presented here can be cross-referenced to Table 1 for expression data. Physiological activities are detailed in the text. The Lgr4/5/RSpo component of embryonic placode formation is likely but has not yet been shown (hence it is grayed out). Although both Lrp5 and Lrp6 are required to maintain mammary stem cells, the Lrp5-6 heterodimer shown here is implied from our data (Goel et al. 2012a). It is not known whether the inhibitors shown (dkks, WIF, and sFRPs) are functional, but several are expressed. When components are illustrated with ribbon diagrams, their structure is known in some detail. (Wnt ligands are shown in pink.)
PLASTICITY OF MAMMARY EPITHELIAL CELL FATE REQUIRES A WNT SIGNAL
When mammary precursor cells are set aside from the embryonic ectoderm, there is a pulse of Wnt signaling that can be visualized by the expression of Wnt reporter genes (the lacZ Wnt reporter TOPgal) (Chu et al. 2004). If dkk1 is overexpressed in the basal keratinocyte layer (using a CK14 promoter) or when Lef1 function is missing (Boras-Granic et al. 2006b; Boras-Granic and Wysolmerski 2008), mammary specification is inhibited. Wnt10b is expressed at the earliest time during differentiation of the mammary placode (Veltmaat et al. 2004; Boras-Granic and Wysolmerski 2008), suggesting that Wnt10b could be the placode specifier. Thus Wnt signaling is essential for increasing the plasticity of embryonic skin cells so that they initiate mammary-specific programs. The induction of plasticity is a typical function associated with pulses of Wnt signaling during mammalian developmental processes (Wend et al. 2010; Domyan and Sun 2011).
Somatic stem cells are different from the majority of cells in mammalian organs, owing to their plasticity and pluripotency. For mammary gland, ductal mammary stem cells are defined by their capacity to regenerate a new mammary gland comprising a bilayered epithelial ductal network following transplantation to cleared fat pads. These stem cells have been shown to comprise a specific subpopulation of basal epithelial cells (with high expression of integrins α6 and β1, and other cell surface-associated antigens such as epithelial cell adhesion molecule [EpCAM] and CD24, a GPI-linked sialoglycoprotein). When ectopic Wnt signals are present, mammary stem cells comprise a larger fraction of the total population (Li et al. 2003b; Liu et al. 2004; Shackleton et al. 2006), and undifferentiated cells (lacking expression of marker proteins) are evident in mammary glands (Liu et al. 2004). There are data to support a role for Wnt proteins as inhibitors of differentiation. Analysis of a mouse mammary cell line (HC11 cells) showed that Wnt3a inhibited differentiation (assayed as β-casein expression) in response to endocrine effectors (including prolactin/dexamethasone/insulin), and that was reversible by sFRP4 expression (Constantinou et al. 2008). Furthermore, cells from Wnt1-induced glands show higher expression of the ΔN-isoform of the p53 family member, p63 (Badders et al. 2009), and less TA-p63; a profile typically associated with dividing progenitors of basal epithelial cell types and functionally implicated in tissue senescence and aging (Wu et al. 2003).
Ductal mammary stem cells require an Lrp5-dependent, Wnt-associated signal for their induction or maintenance, and their activity is much reduced when the Lrp5 receptor species is missing (Lindvall et al. 2006; Badders et al. 2009). After the embryonic phase of development, there is little evidence for the expression of TCF-consensus concatamer-based Wnt reporters, including BAT-gal (Chu et al. 2004; Boras-Granic and Wysolmerski 2008; Lindvall et al. 2009). This is somewhat of a conundrum, given the importance of canonical Wnt signaling to adult stem cells. However, more recently, Zeng and Nusse (2010) and R van Amerongen and R Nusse (pers. comm.) identified rare lacZ+ cells in postnatal glands of Axin2LacZ mice. These cells were quantified using flow cytometric analysis, separated using a substrate loading approach, and found to be enriched in basal stem cells (Zeng and Nusse 2010). Furthermore, the addition of high concentrations of Wnt3a ligand sustained a long-lived mammary stem cell activity (measured by clonogenicity in vitro and in vivo) when added to cultures of primary mammary epithelial cells.
Note that the absence of Lrp5 does not significantly affect embryonic mammary placode development (even though that too is Wnt dependent), nor does it affect lobulo-alveolar development during pregnancy. The Wnt factors that regulate these processes are therefore likely to be different and Lrp5 independent. The role of Lgr4/5 and RSpo proteins in the maintenance of other stem cell types is described later. This together with the expression pattern of RSpo proteins during embryogenesis (Table 1) suggests that Lgr/RSpo may be the embryonic counterpart of the Lrp5-Lrp6 complex that is essential to the adult gland (Fig. 2).
Furthermore, in the absence of stem cells (due either to the absence of Lrp5 or β1 integrin), ductal outgrowth is almost normal, as indeed are all other functions associated with mammary gland growth and differentiation (Lindvall et al. 2006; Taddei et al. 2008; Badders et al. 2009). This may appear to be counterintuitive. However, recently, it has emerged that bi-potential stem cell activity is not activated during normal mammary development. Thus, Van Keymeulen et al. (2011) genetically tagged CK14-expressing or CK5-expressing mammary epithelial basal cells and showed that this cell population contained bipotent stem cell activity when transferred to cleared fat pads. When tagged during puberty, the bi-potential activity (stem cell activity) of these basal cells was not expressed throughout ductal extension or pregnancy, deduced from the lack of tagged luminal cell daughters (Van Keymeulen et al. 2011). Vice versa, CK8-expressing or CK18-expressing tagged luminal cells did not appear in the basal cell compartment. In fact, the bipotency of basal cell stem cells could be suppressed during fat pad transfer if luminal cells were included at a cell ratio of one per five basal cells. These investigators propose that there are long-lived mono-potent basal and luminal stem/progenitor cell types that organize the morphogenesis of mammary gland after birth. It is implied that the activity of each stem/progenitor activity cell activity would primarily affect the directly related lineage. Indeed, the loss of basal stem cell activity (by targeted gene mutations) typically results in a decrease in the proportion of basal cells (compared with luminal cells), without affecting overall mammary gland proliferation (Taddei et al. 2008; Badders et al. 2009). Using other constructs and reporters, some of these results have been reproduced by other investigators (R Van Amerongen, R Nusse, and J Jonkers, pers. comm.). This represents a profound change in our view of stem cell function during adult organogenesis.
What, then, is “stem cell” activity scored by fat pad transfer, and is it a relevant assay? Stem cell activity is scored by assaying the outgrowth of mammary ductal trees after inoculating limiting doses of dissociated, singlized cells from mammary glands. The regenerative activity must therefore be able to survive cell isolation, to subsequently differentiate in vivo, and to create both basal and luminal mammary epithelial cells. Without ectopic endocrine support (e.g., pituitary extract), outgrowths of purified luminal cells are limited in extent (Kamiya et al. 1998; Visvader and Smith 2011). Given the evidence for luminal stem/progenitor cells provided by these studies, it is important to be able to assay this activity somehow, preferably in vitro. Perhaps it is this activity that is measured by the mammosphere assay (spheres contain no dividing basal cells) (S Kim and C Alexander, unpubl.).
IS THERE A WNT SIGNAL THAT CREATES THE MAMMARY STEM CELL NICHE?
Given that mammary stem cells are dependent on Wnt signaling, the question then turns to the identity of the ligand that interacts with Lrp5/Fzd to maintain these cells. Lrp5 is expressed and presented, alongside Lrp6, on all basal mammary epithelial cells (within the limits of detection) (Badders et al. 2009), meaning that the stem cells are not alone in being able to respond to Wnt ligands. Indeed, it is interesting that Lrp5 is specifically required as a maintenance factor for stem cells, given that Lrp6 is often described as the “better,” more potent receptor (MacDonald et al. 2008). However, we have shown that a subgroup of Wnt ligands (including Wnt1 and Wnt9b) requires both Lrp5 and Lrp6 to signal in some cell contexts (Goel et al. 2012b). One of this subgroup is presumably responsible for adult MaSC function (Fig. 2).
It is likely that there is a localized source of a Wnt ligand that is responsible for the stem cell niche. The phenotype of Lrp5−/− mammary glands maps to the mammary epithelial cells, and not the Lrp5−/− fat pads (NM Badders and CM Alexander, unpubl.). Most of the Wnt ligands expressed in mammary gland (Table 1) are known to have a short range of effect in vivo (due to binding to heparan sulfates or their lipidation). In fact, the diffusion range of a Wnt ligand has been visualized in mouse limb bud, where Wnt is produced by the surface ectoderm. In this case, the range appears to be approximately five cells, through the relatively loosely packed cells of the limb bud (and assuming no influence of cross gradients of other molecules) (ten Berge et al. 2008). The ligand responsible for stem cell maintenance may be expressed by other epithelial cells, or by a stromal host cell (for candidates, see Table 1).
WNT RESPONSES: A SUM TOTAL
Given the variety of Wnt signaling receptors (illustrated in Fig. 2) together with the fact that these receptors are commonly coexpressed, any one Wnt ligand is likely to generate several different signals in any one cell. The signaling outcome will depend on the absolute concentration of each ligand and receptor species, along with their relative affinity. Whether these pathways synergize with one another, or antagonize each other, depends on the context and molecules involved (Semenov et al. 2007; van Amerongen and Nusse 2009). Thus, for melanoma cells, non-canonical Wnt signaling, mediated by Wnt5a/Ror2, promotes malignant changes in the presence of high levels of canonical signaling, and these pathways are considered to be synergistic during tumor development (O’Connell and Weeraratna 2009).
For mammary gland, the “old-fashioned” distinction of canonical and non-canonical Wnt ligands appears to hold up, presumably because of the specific repertoire of cell surface receptors expressed (van Amerongen et al. 2008). Thus, Wnt5a, although it can acquire canonical signaling activity when Lrp5 and Fzd4 are present (Mikels and Nusse 2006), has no βcat/TCF trans-activation activity in mammary gland (not surprising because Fzd4 is not expressed) (Table 1). Indeed, as shown for other cell types, Wnt5a not only does not induce Wnt reporter expression, it inhibits Wnt3A/Wnt1 canonical responses. In fact, TGF-β (a well-known mammary gland growth inhibitor) induces Wnt5a expression (directly via Smad binding sites on the promoter), and Wnt5a may be necessary to limit and control mammary gland growth. Thus, when Wnt5a is absent (Wnt5a KO), mammary gland development is hyperproliferative (Roarty and Serra 2007), whereas in the presence of ectopic Wnt5a (produced by slow release beads), ductal extension is inhibited. These phenotypes are the inverse of gain of function of canonical Wnt activity. It is puzzling then that this inhibition is not reproduced in transgenic MMTV-Wnt5a strains (Baxley et al. 2011).
How does a non-canonical ligand inhibit the canonical response? Various ideas have support; the first relies on competition; thus, if Wnt5a competes effectively for Fzd binding (in association with Ror1/2) (Mikels et al. 2009; Grumolato et al. 2011), the amount of the rate-limiting LRP receptor available to other ligands could be reduced. The second is that Wnt5a may generate an intracellular signal that is inhibitory for canonical responses; thus, when Ror1/2 binds Wnt ligands, Rac is activated (leading to JNK activation) and Fzd2 is internalized and inactivated by a clathrin-dependent mechanism (Sato et al. 2010). Witte et al. (2010) suggest that Ror2 generates an inhibitory signal via CK1-mediated dvl phosphorylation and that CK1 constitutes a switch that directs the signaling output (studies of HEK293 and Cos cells) (Foldynova-Trantirkova et al. 2010; Witte et al. 2010). Indeed, the effects of the morphogenetic planar cell polarity pathway, typically mediated by ligands such as Wnt11, are largely unexplored for mammary gland. During development, it is often true that multiple Wnt signals are perceived by cells simultaneously (see the examples drawn from genetic analysis of Caenorhabditis elegans, summarized by van Amerongen and Nusse 2009). These signals are key to tube building and the convergent extension processes associated with polarization of epithelial layers (Kouros-Mehr and Werb 2006; Miller and McCrea 2010). In addition, it is likely that there are polarized cell divisions that occur either in the plane of the basal and luminal layers, or that cross between them. Evidence suggests that Notch signaling and p53 are important to determining these division planes, and both pathways are highly cross-regulated by, or interact with, Wnt signaling (Callahan and Egan 2004; Collu and Brennan 2007; Bouras et al. 2008; Cicalese et al. 2009; Yan et al. 2010).
Wnt signaling in other tissue contexts also serves to organize the growth and development of more differentiated cells that emerge from the stem cell compartment. Thus, for intestinal cells, a battery of genes are induced by βcat/TCF signaling (van de Wetering et al. 2002), and these can be divided functionally into at least three programs (maintenance of progenitor phenotype, promotion of differentiation of Paneth cells in the crypt, and compartmentalization of differentiated intestinal cells from progenitors). The latter is regulated by ephrins (EphB2 and EphB3). Thus, in Apcmin mice, when ephrins are missing and cell sorting is defective, tumor development proceeds even more rapidly (Batlle et al. 2002). For the retinotectum, opposing gradients of ephrin and Wnt signals (via Ryk or Fzd) organize and map the cell function (Schmitt et al. 2006). For mammary gland, the ephrin Robo1 is a suppressor of mammary branching and basal cell division (Macias et al. 2011) and promotes the accumulation of cytoplasmic E-cadherin and β-catenin. Whether ephrin expression is downstream and/or upstream of Wnt signals in this cell type is not fully understood. Other Wnt target genes identified in intestine by van de Wetering et al. (2002) include the orphan GPCR receptors Lgr4 and Lgr5 (indeed, for intestine, an Lgr5-dependent tag serves to identify intestinal stem cells in studies of growth and pathogenesis) (Barker et al. 2007). Lgr4/5 functions to feed forward to amplify the stem cell function in this tissue, by binding RSpo proteins, forming a complex with Lrp/Fzd molecules that potentiates a canonical Wnt (Wnt3a) signal (Carmon et al. 2011; de Lau et al. 2011). Because all of the same components are also expressed in mammary gland, the same principle may apply (Fig. 2).
It is possible that Wnt signaling could act indirectly on mammary morphogenesis and function via its effects on non-epithelial components of the mammary gland, including blood vessels, macrophages, fibroblasts, and adipocytes. For example, endothelial cell maturation is highly Wnt dependent (Parmalee and Kitajewski 2008), including a relatively little characterized pathway that depends on the Norrin ligand and Fzd4/Lrp5 (Ye et al. 2010). Macrophages and other immunomodulatory cells are well known to affect mammary development, involution, and tumor progression (Schwertfeger et al. 2006; Schaale et al. 2011; O'Brien et al. 2012). Fibroblasts are activated by local Wnt signals to initiate a desmoplastic response (Xu et al. 2000) that elicits a mobilization and proliferation response from circulating cells and bone marrow precursors (Kim et al. 2008). These cells are recruited to the tumor, where they participate in the angiogenic and inflammatory response, creating leaky vessels and macrophage activation, neither of which is associated with the tumors that develop in response to the intracellular Wnt effector β-catenin (Kim et al. 2008). Adipocyte differentiation is controlled by Wnt proteins (specifically Wnt10b), and adipocyte differentiation/fat content varies substantially throughout pregnancy and lactation (Prestwich and Macdougald 2007). Indeed, it is likely that the controlled spacing and branching of mammary ducts in the fat pad represents an interaction between the host tissue and the expanding ductal epithelial cell population (Sakakura 1987).
Furthermore, responses to Wnt signals can be modulated by cross talk from other effectors and pathways, including metabolic checkpoints such as AMPK, mitochondrial function, autophagy, and lipid effectors (Inoki et al. 2006; Gao et al. 2010; Yoon et al. 2010; Kang et al. 2011). Prostaglandins (Goessling et al. 2009) have been shown to cross-talk to Wnt signaling to maintain hematopoietic stem cells, and there are other pathways that regulate the turnover and metabolism of β-catenin (Incassati et al. 2010). Although the significance of these is largely unknown for normal mammary development and tumor initiation, the interaction of Wnt signaling with EGFR or with nuclear hormone receptors suggests that these could be particularly important for dissecting mammary behaviors (Schlange et al. 2007; Mastroianni et al. 2009; Roarty and Rosen 2010).
WNT4: A WNT PROBLEM CHILD?
Wnt4 knockout glands were reported to show a delay of lobulo-alveolar development (Brisken et al. 2000) during pregnancy, implying that this ligand could be a mammary epithelial cell growth factor with similar properties to Wnt1 (and Wnt10b). Indeed, when mammary epithelial cells were transduced with a Wnt4 expression vector, the outgrowths were hyperplastic (Bradbury et al. 1995). However, unlike Wnt1, Wnt4 does not induce mammary gland hyperplasia when expressed as an MMTV-based transgene. This is despite effective expression levels (equivalent to mid-pregnancy) and evidence of Wnt4-induced gene expression (e.g., Wnt16) (Kim et al. 2009), both in vitro (in HC11 cells) and in vivo in virgin glands. In contrast to Wnt1 (and the other canonical ligands), Wnt4 does not induce axin2 expression or TOP-FLASH activation in mammary epithelial cells or fibroblasts, and does not induce the typical developmental phenotypes associated with canonical Wnt ligand expression in zebrafish embryos.
It may be that Wnt4 is just one arm of a progesterone-induced program, making Wnt4 necessary but not sufficient. Other progesterone-induced components, such as RANKL (Beleut et al. 2010) and/or the differentiation/specification factor bHLH protein Id4 (Fernandez-Valdivia et al. 2005), could also be required for an effective response. Wnt4 may be a prime candidate to explain the regulation of mammary stem cell function by endocrine factors. Thus, recent reports describe a dynamic twofold expansion and retraction of the stem cell activity during the 4-d estrus cycle (Joshi et al. 2010), and an 11-fold difference in activity in estrogen/progesterone positive/negative mammary environments (Asselin-Labat et al. 2010). These hormonal dynamics closely resemble the expression of Wnt4 mRNA and protein expression in luminal mammary epithelial cells (Table 1) (Silberstein et al. 2006).
Information derived from other Wnt4-dependent processes, such as kidney development, could provide a useful precedent for mammary gland. For kidney, several Wnt proteins are expressed by the epithelial ureteric bud, including Wnt6, 7b, 9b, and 11, and two Wnt proteins by the morphogenic mesenchyme, Wnt 2b and Wnt4. Of these, Wnt4, 9b, and 11 are essential, and none show redundant functions, suggesting that the nature of the signal generated by each is unique (Carroll et al. 2005; Pulkkinen et al. 2008). In MDCK and other kidney cell types, Wnt4 can induce a canonical signal, but the receptor mediating this signaling event is still mysterious (although Wnt4 binds Fzd6, this is not the canonical signaling reaction) (Lyons et al. 2004). Interestingly, Bernard et al. (2008) suggest that Wnt4 elicits a distinct response from that induced by Wnt5a, namely, accumulation of β-catenin at the cell membranes (of HEK293T cells). These, or other factors, may be important to understanding the molecular mechanism of Wnt4 activity.
IS WNT SIGNALING A DRIVER FOR BREAST TUMOR GROWTH?
Wnt signaling has been known to be highly oncogenic for mouse mammary glands since 1982, when Nusse and Varmus showed that mouse mammary tumors induced by MMTV were caused by activation of Wnt ligand gene expression. In fact, not only were Wnt1, Wnt3, Wnt3A, and Wnt10b activated to induce mammary tumors, so indeed were Wnt-pathway coactivators RSpo2 and RSpo3 (Nusse and Varmus 1982; Nusse 1988; Theodorou et al. 2007; Callahan and Smith 2008). Of these, only Wnt10b and the RSpo ligands are usually expressed in significant amounts in mammary gland (Table 1). It may be significant that other Wnt components are not on that list—including Lrp6, even though overexpressed Lrp6 can induce hyperplasia in mouse models (Lindvall et al. 2009; Liu et al. 2010; Zhang et al. 2010a).
However, although it is true that the vast majority of colorectal tumors have a Wnt signaling driver (initiated by mutations in one of three genes in the canonical Wnt signaling pathway, namely, inactivating mutations of the tumor suppressors Apc or axin or activating mutations of the oncogene β-catenin), there is little evidence to suggest that breast tumors share this etiology. Mice with an Apc mutation (Apcmin), although they develop intestinal polyps, do not spontaneously develop breast tumors. (They are, however, sensitized to tumor development, revealed by the enhanced rate of tumorigenesis after the administration of carcinogens [Moser et al. 1993].) Indeed, unlike mammary glands expressing Wnt ligands, Apcmin mammary glands are not hyperplastic, so this loss of function is insufficient to generate a growth signal. The mechanisms governing inactivation of Wnt signaling components may be different for mammary cells; for example, data derived from combination microarray–CGH suggest that the expression of genes in 5q22.2 is reduced in basaloid breast tumors (Geyer et al. 2011). This interval includes Apc. Perhaps total inactivation of the typical Wnt tumor suppressors by mutation is lethal for mammary epithelial cells.
Preneoplastic lesions with ectopic Wnt signaling often require cooperating mutation(s) to progress. Perhaps the lack of Wnt mutations in breast tumors could be explained if these cooperating mutations did not arise readily during breast cancer development, or were not tolerated. For example, gut tissues in Apcmin mice (after loss of heterozygosity) accumulate β-catenin, and this is sufficient to prevent differentiation and induce morphological changes in tissue architecture. Note that this tissue is remarkable for its high rate of continuous growth, which has no parallel in adult mammary gland. However, nuclear β-catenin does not arise until later in tumor development, after Ras is mutated (Phelps et al. 2009). Ras is rarely mutated in any type of breast tumor, although Ras mutations are relatively common in breast tumor cell lines (Hollestelle et al. 2007). Ectopic Ras activity is often detected by surveillance checkpoints (Zhu et al. 2005), and cells are eliminated in response (typically a p53-dependent process). Thus, ras activation has to be carefully titrated to contribute effectively to tumor development. Perhaps it is no coincidence that the Fgf signaling pathway is frequently activated during Wnt-induced tumor development (where Ras is activated as a downstream effector in a regulated manner) (Callahan and Smith 2008). In developmental models, when Wnt and Fgf are coordinately expressed, the signal generated is distinct from either alone (ten Berge et al. 2008). This may also explain the observation that human basaloid breast tumor aggression is associated with (and dependent on) hyperactivation of Fgf signaling (Turner et al. 2010a,b; Sharpe et al. 2011). Similarly, activation of the tyrosine kinase Ron has been associated with β-catenin phosphorylation (Y654 and Y670), together with enhanced nuclear translocation and transactivation activity (Wagh et al. 2011). Furthermore, when a Wnt signal is present, two reports suggest that Akt activation is sufficient to activate the nuclear translocation of β-catenin, in human and mouse breast tumor epithelial cells (Korkaya et al. 2009; Zhang et al. 2010b). Akt has been shown to phosphorylate β-catenin (S-552) to enhance nuclear translocation (He et al. 2007), and an inhibitor of Akt signaling (perifosine) inhibits the accumulation and translocation of β-catenin, Wnt signaling reporters, and stem cell activity, in normal cells and tumor cells deficient in PTEN and/or p53. Up-regulation of Akt signaling in the absence of Wnt signals is unlikely to cross-talk to Wnt end points, despite the fact that they share a signaling component (GSK3β) (Korkaya et al. 2009; Ng et al. 2009); indeed, the data to date suggest that the pools of GSK3β allocated to these pathways are different and separate (Ding et al. 2000).
It is useful to outline the limitations of assays designed to test whether Wnt signaling is a tumor driver, in the following sections.
Wnt-Dependent Subpopulations Are Obscured by General Wnt Signatures
The tumorigenic effects of ectopic Wnt signaling may be associated with only a subpopulation of (stem/progenitor) cells (especially early in tumor development), whereas the tumor majority shows a different Wnt signaling response. This has been illustrated in a Wnt1–Lrp5-dependent transgenic tumor model system (Gunther et al. 2003; Lindvall et al. 2006). These tumors contain a minority “responder” cell population that depends on a paracrine canonical Wnt ligand (Lrp5-positive cells) from the cell majority (Kim et al. 2011). This cell majority is therefore unable to generate a canonical Wnt signal (Lrp5-negative; visualized by axin2 expression), but is nonetheless recruited to grow in a Wnt1-dependent manner. Thus, the Wnt signature is not activated in the cell majority, and the tumor cell population grows in response to a field effect, with so far unknown mediators.
A “field effect” for growth is common for other tumor drivers, such as the estrogen-ERα axis. Thus, for ERα-positive tumors, the tumorigenic ERα receptor is typically expressed by a minority population (ERα-positive cells comprise 1%–100% of tumor cells, depending on the exact pathological criteria), but the Ki67/mitotic index is raised throughout (Clarke 2003; Schlange et al. 2007; Mastroianni et al. 2009). Extrapolating from this idea, it is clear that transcriptional signatures of whole populations of mixed cell types could be difficult to interpret (Huang et al. 2005). The markers c-myc and cyclinD1 that characterize Wnt-dependent transcription for intestinal cells are not prominent (or required for tumorigenesis) for mammary gland (Huang et al. 2005), and indeed are not specific to Wnt signaling. Data also suggest that activation of Akt signaling may be a component of Wnt ligand-mediated oncogenesis, and this outcome may make ectopic Wnt signaling difficult to recognize by a genetic signature (Korkaya et al. 2009; Zhang et al. 2010b).
Nuclear β-Catenin
As discussed above, a combination of signaling activities may be required to generate nuclear β-catenin. Nuclear β-catenin has been convincingly observed in some breast tumors (Geyer et al. 2011). However, even when Wnt signaling is known to be the tumor driver, it may not be associated with significant immunostaining of nuclear β-catenin, thus the breast tumors identified with ectopic Wnt signaling by this assay may underrepresent the prevalence of this tumor driver. For example, although the growth of Wnt1-induced breast tumors is known to require the continuous presence of Wnt1/Lrp5, there is little support for the presence of nuclear β-catenin (data not shown), despite high expression of Wnt reporters in responder cells (Kim et al. 2011). Furthermore, basaloid breast tumors (in particular) are demonstrably heterogeneous with respect to basal and luminal cell subpopulations (Rakha et al. 2009; Kim et al. 2012), and the Wnt-dependent subpopulation may be rare.
Loss of E-Cadherin
Loss of function for the tumor suppressor E-cadherin (characteristic of many breast tumors) can lead to redistribution or elimination of β-catenin, an E-cadherin binding partner (van de Wetering et al. 2001; Incassati et al. 2010). However, this is not necessarily accompanied by the functional recruitment of β-catenin to the Wnt signaling pathway (Gumbiner 1998; Geyer et al. 2011).
These issues do not eliminate Wnt signaling as a significant tumor driver. Indeed, there is a large body of literature that describes a disproportionate expression of mRNA for positive effectors of Wnt signaling in tumors, either with respect to normal tissue, sometimes with respect to a larger group of breast tumors (for review, see Incassati et al. 2010). All of these studies have the caveat that the cell composition of tumors is different from subtype to subtype (e.g., the luminal vs. basaloid subtypes, where the latter are mixtures of both basal and luminal cells), and the samples are seldom microdissected, perhaps including contributions from cells that may express high amounts of Wnt signaling components (such as macrophages). However, the accumulated data suggest that there are significant expression of Wnt ligands (summarized by Benhaj et al. 2006), loss of non-canonical signaling (Jonsson et al. 2002; Serra et al. 2011), and shutdown of expression of extracellular inhibitors. Numerous extracellular inhibitors of Wnt signaling are expressed by mammary gland (including 2/4 dkk species, WIF1, and 3/4 sFRP species) (Fig. 2; Table 1). Thus, Ugolini et al. (2001) originally described the epigenetic inhibition of sFRP1 expression in ERα-positive tumors, and Schlange et al. (2007) showed that autocrine Wnt signaling (measured by dvl phosphorylation) was inhibited by pan siRNA to Dvl species, and by the ectopic administration of sFRP1, for a group of breast tumor cells lines that represent all the major subtypes of breast tumor. This relied partly on transactivation of EGFR (and ERK phosphorylation), mediated by Wnt-induced MMP activation and the release of erbB2 ligands from extracellular matrix. Note that this study (and others) may not implicate canonical Wnt signaling, but Wnt ligand/Fzd-dependent growth and invasion. Alterations in mRNA species for Wnt signaling components require cautious interpretation, because the effects of so-called inhibitors (in particular) are often difficult to predict a priori. Thus, loss of dkk3 (reported in breast tumors) (Suzuki et al. 2008; Veeck et al. 2008) induces apoptosis of lung cancer cells (Jung et al. 2010), and ectopic dkk3 can potentiate Wnt signaling (probably by binding and disabling Kremen) (Nakamura and Hackam 2010).
However, despite all these caveats, Geyer et al. (2011) have recently published clear pictures of the localization and amount of β-catenin (using two different monoclonal antibodies) visualized for a cohort of 245 breast tumors, and showed that nuclear β-catenin is prevalent in a subpopulation of tumor epithelial cells in approximately one-third of basaloid breast tumors. Basaloid breast tumors comprise about one-sixth of total breast tumors and show high levels of expression of basal cell markers (both protein and mRNA) alongside luminal cell markers. The Wnt receptor, Lrp6, is overexpressed in some of these tumors (by approximately twofold to fivefold) (Lindvall et al. 2009; Liu et al. 2010), and inhibition of Lrp6 in MDA-MB231 basaloid cells in culture inhibits TOP-FLASH (10×–100×), growth (2×), Axin2 expression (2×), colony formation (5×) in vitro, and tumor growth in vivo (Matsuda et al. 2009).
The focus is now on the inhibitors of Wnt signaling for their possible use in tumor treatment, and given the data described above, the most likely target cohort may be patients with basaloid tumors. Drug development of inhibitors of Wnt signaling tend to assay HEK293 cells, and they have been highly successful in producing molecules that inhibit signaling outside and inside of the cell (Barker and Clevers 2006; Dasgupta 2009). It may be possible to exploit unique aspects of Wnt signaling in breast tumor cells to enhance these more generic approaches.
CONCLUDING REMARKS
This is not a comprehensive review, in part because it focuses on the cell surface components of Wnt signaling, and does not detail how that signal is modified by (1) cytoplasmic modifiers; (2) nuclear uptake mechanisms for β-catenin; (3) the nature of the TCF species expressed, together with the associated transcription factors (Archbold et al. 2012); or (4) the epigenetic chromatin status (Hatzis et al. 2008; Gu et al. 2009; Wend et al. 2010). This article mentions these mechanisms only in passing because there is little specific information published to describe the importance of these aspects with respect to mammary gland or mammary cell lines. We have focused instead on some of the outstanding questions likely to be our preoccupation in the near future, such as the evident plasticity of fate induced by Wnt signaling (indicated by transitions between basal and luminal mammary epithelial cell fates), the molecules that regulate the mammary stem cell niche, the combinatorial outputs that are offered by the multiplicity of Wnt signals perceived by mammary epithelial cells, the difficulties of conclusively implicating Wnt signaling in tumor growth or tumor cell survival, and the potential Wnt responses of the Lrp-deficient luminal cell population. Key Wnt signaling components that discriminate luminal and basal cell function, such as Lrp5, are regulated at the posttranscriptional level (Joshi et al. 2010; Kim et al. 2011), and this requires more investigation. Much of the information available to describe the regulation of Wnt signaling involves ectopic expression of various components. At many levels, this does not reflect the obvious competition for binding partners that regulates the transcriptional response and the response to Wnt ligands at the cell surface. The next generation of data will focus more heavily on studying the function of Wnt signaling components when expressed at endogenous levels. Given the new generation of Wnt signaling inhibitors, there are new horizons for investigations of mechanism and therapeutic potential.
ACKNOWLEDGMENTS
This work is supported by the BCRP/DOD Era of Hope Scholars Award W81XWH (to C.M.A., S.G., S.K., and S.F.).
Footnotes
Editors: Roel Nusse, Xi He, and Renee van Amerongen
Additional Perspectives on Wnt Signaling available at www.cshperspectives.org
REFERENCES
- Ai L, Tao Q, Zhong S, Fields CR, Kim WJ, Lee MW, Cui Y, Brown KD, Robertson KD 2006. Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis 27: 1341–1348 [DOI] [PubMed] [Google Scholar]
- Archbold HC, Yang YX, Chen L, Cadigan KM 2012. How do they do Wnt they do?: Regulation of transcription by the Wnt/β-catenin pathway. Acta Physiol (Oxf) 204: 74–109 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802 [DOI] [PubMed] [Google Scholar]
- Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM 2009. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS ONE 4: e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Kent CV, Silbermann RA, Hassell JA, Young LJ, Howe LR 2010. Pea3 transcription factors and Wnt1-induced mouse mammary neoplasia. PloS ONE 5: e8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H 2006. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 5: 997–1014 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Barolo S 2006. Transgenic Wnt/TCF pathway reporters: All you need is Lef? Oncogene 25: 7505–7511 [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263 [DOI] [PubMed] [Google Scholar]
- Baxley SE, Jiang W, Serra R 2011. Misexpression of Wingless-related MMTV integration site 5A in mouse mammary gland inhibits the milk ejection response and regulates connexin43 phosphorylation. Biol Reprod 85: 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C 2010. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci 107: 2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhaj K, Akcali KC, Ozturk M 2006. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep 15: 701–707 [PubMed] [Google Scholar]
- Bergstein I, Schultz R, Osborne MP, Welcsh PL, Bowcock AM, Brown AM 1995. Investigation of the possible role of WNT genes in human breast cancer. Ann NY Acad Sci 768: 257. [DOI] [PubMed] [Google Scholar]
- Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E 2008. Wnt4 inhibits β-catenin/TCF signalling by redirecting β-catenin to the cell membrane. Biol Cell 100: 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund P, Svedlund J, Olsson AK, Akerstrom G, Westin G 2009. The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS ONE 4: e4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boras-Granic K, Wysolmerski JJ 2008. Wnt signaling in breast organogenesis. Organogenesis 4: 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boras-Granic K, Chang H, Grosschedl R, Hamel PA 2006a. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol 295: 219–231 [DOI] [PubMed] [Google Scholar]
- Boras-Granic K, Grosschedl R, Hamel PA 2006b. Genetic interaction between Lef1 and Alx4 is required for early embryonic development. Int J Dev Biol 50: 601–610 [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE 2008. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3: 429–441 [DOI] [PubMed] [Google Scholar]
- Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, Costa M, Cochran AG, Hannoush RN 2010. Reconstitution of a frizzled8·Wnt3a·LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem 285: 9172–9179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JM, Edwards PA, Niemeyer CC, Dale TC 1995. Wnt-4 expression induces a pregnancy-like growth pattern in reconstituted mammary glands in virgin mice. Dev Biol 170: 553–563 [DOI] [PubMed] [Google Scholar]
- Brennan KR, Brown AM 2004. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia 9: 119–131 [DOI] [PubMed] [Google Scholar]
- Brennan K, Gonzalez-Sancho JM, Castelo-Soccio LA, Howe LR, Brown AM 2004. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize β-catenin independently of Frizzled proteins. Oncogene 23: 4873–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarra T, Elenbaas B, Tan J, Dey SK, McMahon AP, Weinberg R 2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14: 650–654 [PMC free article] [PubMed] [Google Scholar]
- Buhler TA, Dale TC, Kieback C, Humphreys RC, Rosen JM 1993. Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev Biol 155: 87–96 [DOI] [PubMed] [Google Scholar]
- Bui TD, Rankin J, Smith K, Huguet EL, Ruben S, Strachan T, Harris AL, Lindsay S 1997. A novel human Wnt gene, WNT10B, maps to 12q13 and is expressed in human breast carcinomas. Oncogene 14: 1249–1253 [DOI] [PubMed] [Google Scholar]
- Callahan R, Egan SE 2004. Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia 9: 145–163 [DOI] [PubMed] [Google Scholar]
- Callahan R, Smith GH 2008. Common integration sites for MMTV in viral induced mouse mammary tumors. J Mammary Gland Biol Neoplasia 13: 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q 2011. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci 108: 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP 2005. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292 [DOI] [PubMed] [Google Scholar]
- Chadi S, Buscara L, Pechoux C, Costa J, Laubier J, Chaboissier MC, Pailhoux E, Vilotte JL, Chanat E, Le Provost F 2009. R-spondin1 is required for normal epithelial morphogenesis during mammary gland development. Biochem Biophys Res Commun 390: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Chan EL, Peace BE, Toney K, Kader SA, Pathrose P, Collins MH, Waltz SE 2007. Homozygous K5Cre transgenic mice have wavy hair and accelerated malignant progression in a murine model of skin carcinogenesis. Mol Carcinog 46: 49–59 [DOI] [PubMed] [Google Scholar]
- Christiansen JH, Dennis CL, Wicking CA, Monkley SJ, Wilkinson DG, Wainwright BJ 1995. Murine Wnt-11 and Wnt-12 have temporally and spatially restricted expression patterns during embryonic development. Mech Dev 51: 341–350 [DOI] [PubMed] [Google Scholar]
- Christiansen JH, Monkley SJ, Wainwright BJ 1996. Murine WNT11 is a secreted glycoprotein that morphologically transforms mammary epithelial cells. Oncogene 12: 2705–2711 [PubMed] [Google Scholar]
- Chu E, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE 2004. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 131: 4819–4829 [DOI] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG 2009. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138: 1083–1095 [DOI] [PubMed] [Google Scholar]
- Clarke RB 2003. Steroid receptors and proliferation in the human breast. Steroids 68: 789–794 [DOI] [PubMed] [Google Scholar]
- Collu GM, Brennan K 2007. Cooperation between Wnt and Notch signalling in human breast cancer. Breast Cancer Res 9: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H 2004. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115 [DOI] [PubMed] [Google Scholar]
- Constantinou T, Baumann F, Lacher MD, Saurer S, Friis R, Dharmarajan A 2008. SFRP-4 abrogates Wnt-3a-induced β-catenin and Akt/PKB signalling and reverses a Wnt-3a-imposed inhibition of in vitro mammary differentiation. J Mol Signal 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, D’Cruz CM, Chodosh LA, Cole MD 2007. c-Myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol Cell Biol 27: 5135–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier N, Chea K, Hlavacova M, Sussman DJ, Seldin DC, Dominguez I 2010. Dynamic expression of a LEF-EGFP Wnt reporter in mouse development and cancer. Genesis 48: 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale TC, Weber-Hall SJ, Smith K, Huguet EL, Jayatilake H, Gusterson BA, Shuttleworth G, O’Hare M, Harris AL 1996. Compartment switching of WNT-2 expression in human breast tumors. Cancer Res 56: 4320–4323 [PubMed] [Google Scholar]
- Dasgupta R 2009. Functional genomic approaches targeting the wnt signaling network. Curr Drug Targets 10: 620–631 [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. 2011. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297 [DOI] [PubMed] [Google Scholar]
- DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C 2009. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial–mesenchymal transition in basal-like breast cancer. Cancer Res 69: 5364–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem 275: 32475–32481 [DOI] [PubMed] [Google Scholar]
- Domyan ET, Sun X 2011. Patterning and plasticity in development of the respiratory lineage. Dev Dyn 240: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer MA, Joseph JD, Wade HE, Eaton ML, Kunder RS, Kazmin D, Chang CY, McDonnell DP 2010. WNT11 expression is induced by estrogen-related receptor α and β-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res 70: 9298–9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Mulac-Jericevic B, Conneely OM, DeMayo FJ, Amato P, Lydon JP 2005. Revealing progesterone’s role in uterine and mammary gland biology: Insights from the mouse. Semin Reprod Med 23: 22–37 [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK 2010. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol 10: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldynova-Trantirkova S, Sekyrova P, Tmejova K, Brumovska E, Bernatik O, Blankenfeldt W, Krejci P, Kozubik A, Dolezal T, Trantirek L, et al. 2010. Breast cancer-specific mutations in CK1ε inhibit Wnt/β-catenin and activate the Wnt/Rac1/JNK and NFAT pathways to decrease cell adhesion and promote cell migration. Breast Cancer Res 12: R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritah A, Saucier C, De Wever O, Bracke M, Bieche I, Lidereau R, Gespach C, Drouot S, Redeuilh G, Sabbah M 2008. Role of WISP-2/CCN5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol Cell Biol 28: 1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T, Fu W, Zhang J, Wu W, Zhang X, et al. 2010. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol 12: 781–790 [DOI] [PubMed] [Google Scholar]
- Gavin BJ, McMahon AP 1992. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol Cell Biol 12: 2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, Mackay A, Natrajan R, Reis-Filho JS 2011. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol 24: 209–231 [DOI] [PubMed] [Google Scholar]
- Goel S, Fakhraldeen SA, Chin EN, Alexander CM 2012a. Some Wnt ligands require both Lrp receptors (Lrp5 and Lrp6) for normal canonical signaling. J Biol Chem (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Chin EN, Fakhraldeen SA, Berry SM, Beebe DJ, Alexander CM 2012b. Both Lrp5 and Lrp6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells (and fibroblasts). J Biol Chem 10.1074/jbc.M112.362137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, et al. 2009. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136: 1136–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Kuntz SG, Sternberg PW 2008. Ror receptor tyrosine kinases: Orphans no more. Trends Cell Biol 18: 536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis A, Mackay A, Reis-Filho JS, Steele D, Iseli C, Stevenson BJ, Jongeneel CV, Valgeirsson H, Fenwick K, Iravani M, et al. 2006. Establishment of the epithelial-specific transcriptome of normal and malignant human breast cells based on MPSS and array expression data. Breast Cancer Res 8: R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbary R, Biswas R, Arroyave R, Vijayakumar S, Economides KD, Aaronson SA 2011. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 24: 2517–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, Agrawal A, Rheaume C, Bilanchone V, Veltmaat JM, et al. 2009. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol 185: 811–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM 1998. Propagation and localization of Wnt signaling. Curr Opin Genet Dev 8: 430–435 [DOI] [PubMed] [Google Scholar]
- Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, Cardiff RD, Chodosh LA 2003. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev 17: 488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, et al. 2008. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol 28: 2732–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X 2004. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: Arrows point the way. Development 131: 1663–1677 [DOI] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. 2007. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, Januario T, Savage H, Punnoose E, Truong T, et al. 2009. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 15: 4649–4664 [DOI] [PubMed] [Google Scholar]
- Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M 2007. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res 5: 195–201 [DOI] [PubMed] [Google Scholar]
- Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ, et al. 2010. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat 121: 53–64 [DOI] [PubMed] [Google Scholar]
- Hsu W, Shakya R, Costantini F 2001. Impaired mammary gland lymphoid development caused by inducible expression of Axin in transgenic mice. J Cell Biol 155: 1055–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Li Y, Chen Y, Podsypanina K, Chamorro M, Olshen AB, Desai KV, Tann A, Petersen D, Green JE, et al. 2005. Changes in gene expression during the development of mammary tumors in MMTV–Wnt-1 transgenic mice. Genome Biol 6: R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet EL, McMahon JA, McMahon AP, Bicknell R, Harris AL 1994. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res 54: 2615–2621 [PubMed] [Google Scholar]
- Humphreys RC, Rosen JM 1997. Stably transfected HC11 cells provide an in vitro and in vivo model system for studying Wnt gene function. Cell Growth Differ 8: 839–849 [PubMed] [Google Scholar]
- Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P 2001. DN89b-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol 153: 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incassati A, Chandramouli A, Eelkema R, Cowin P 2010. Key signaling nodes in mammary gland development and cancer: β-catenin. Breast Cancer Res 12: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, et al. 2006. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968 [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Eichstetter I, Danielson KG 1995. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res 55: 3495–3499 [PubMed] [Google Scholar]
- Jarde T, Dale T 2011. Wnt signalling in murine postnatal mammary gland development. Acta Physiol (Oxf) 204: 118–127 [DOI] [PubMed] [Google Scholar]
- Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, Dexter T, Davies S, Bulmer K, Ford E, et al. 2004. Expression profiling of purified normal human luminal and myoepithelial breast cells: Identification of novel prognostic markers for breast cancer. Cancer Res 64: 3037–3045 [DOI] [PubMed] [Google Scholar]
- Jonsson M, Dejmek J, Bendahl PO, Andersson T 2002. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res 62: 409–416 [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R 2010. Progesterone induces adult mammary stem cell expansion. Nature 465: 803–807 [DOI] [PubMed] [Google Scholar]
- Jung IL, Kang HJ, Kim KC, Kim IG 2010. Knockdown of the Dickkopf 3 gene induces apoptosis in a lung adenocarcinoma. Int J Mol Med 26: 33–38 [DOI] [PubMed] [Google Scholar]
- Kamiya K, Gould MN, Clifton KH 1998. Quantitative studies of ductal versus alveolar differentiation from rat mammary clonogens. Proc Soc Exp Biol Med 219: 217–225 [DOI] [PubMed] [Google Scholar]
- Kang DW, Choi K-Y, Min DS 2011. Phospholipase D meets Wnt signaling: A new target for cancer therapy. Cancer Res 71: 293–297 [DOI] [PubMed] [Google Scholar]
- Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ 2008. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Kishida S 2007. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal 19: 659–671 [DOI] [PubMed] [Google Scholar]
- Kim YC, Clark RJ, Ranheim EA, Alexander CM 2008. Wnt1 expression induces short-range and long-range cell recruitments that modify mammary tumor development and are not induced by a cell-autonomous β-catenin effector. Cancer Res 68: 10145–10153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Clark RJ, Pelegri F, Alexander CM 2009. Wnt4 is not sufficient to induce lobuloalveolar mammary development. BMC Dev Biol 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Goel S, Alexander CM 2011. Differentiation generates paracrine cell pairs that maintain basaloid mouse mammary tumors: Proof of concept. PLoS ONE 6: e19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Roopra AS, Alexander CM 2012. A phenotypic mouse model of basaloid breast tumors. PLoS ONE 7: e30979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirikoshi H, Sekihara H, Katoh M 2001. Expression of WNT14 and WNT14B mRNAs in human cancer, up-regulation of WNT14 by IFNγ and up-regulation of WNT14B by β-estradiol. Int J Oncol 19: 1221–1225 [DOI] [PubMed] [Google Scholar]
- Klauzinska M, Baljinnyam B, Raafat A, Rodriguez-Canales J, Strizzi L, Greer YE, Rubin JS, Callahan R 2011. Rspo2/Int7 regulates invasiveness and tumorigenic properties of mammary epithelial cells. J Cell Physiol 227: 1960–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm F, Bleckmann A, Siam L, Chuang HN, Rietkotter E, Behme D, Schulz M, Schaffrinski M, Schindler S, Trumper L, et al. 2011. β-Catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis 32: 434–442 [DOI] [PubMed] [Google Scholar]
- Klopocki E, Kristiansen G, Wild PJ, Klaman I, Castanos-Velez E, Singer G, Stohr R, Simon R, Sauter G, Leibiger H, et al. 2004. Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol 25: 641–649 [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS 2009. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol 7: e1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Werb Z 2006. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn 235: 3404–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF, Leder P 1997. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene 15: 2133–2144 [DOI] [PubMed] [Google Scholar]
- Lee FS, Lane TF, Kuo A, Shackleford GM, Leder P 1995. Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. Proc Natl Acad Sci 92: 2268–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune S, Huguet EL, Hamby A, Poulsom R, Harris AL 1995. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res 1: 215–222 [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. 2003a. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci 100: 15853–15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. 2003b. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induced mammary cancers from progenitor cells. Proc Natl Acad Sci 100: 15853–15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu W, He X, Schwartz AL, Bu G 2004. LRP6 expression promotes cancer cell proliferation and tumorigenesis by altering β-catenin subcellular distribution. Oncogene 23: 9129–9135 [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Banyai L, Patthy L, Otting G 2006. NMR structure of the WIF domain of the human Wnt-inhibitory factor-1. J Mol Biol 357: 942–950 [DOI] [PubMed] [Google Scholar]
- Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE 2010. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res 12: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO 2006. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem 281: 35081–35087 [DOI] [PubMed] [Google Scholar]
- Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO 2009. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PloS ONE 4: e5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, McDermott SP, Khwaja SS, Alexander CM 2004. The transforming ability of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci 101: 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Prior J, Piwnica-Worms D, Bu G 2010. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci 107: 5136–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther W, Wiley K, Smith GH, Callahan R 2005. A new common integration site, Int7, for the mouse mammary tumor virus in mouse mammary tumors identifies a gene whose product has furin-like and thrombospondin-like sequences. J Virol 79: 10093–10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, Barth AM, McCrea PD 2004. Wnt-4 activates the canonical β-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: Functional implications of Wnt/β-catenin activity in kidney epithelial cells. Exp Cell Res 298: 369–387 [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Yokota C, Tamai K, Zeng X, He X 2008. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J Biol Chem 283: 16115–16123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias H, Moran A, Samara Y, Moreno M, Compton JE, Harburg G, Strickland P, Hinck L 2011. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev Cell 20: 827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni M, Kim S, Kim YC, Esch A, Wagner C, Alexander CM 2009. Wnt signaling can substitute for estrogen to induce division of ERα-positive cells in a mouse mammary tumor model. Cancer Lett 289: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE 2009. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res 11: R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R 2006. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R 2009. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem 284: 30167–30176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, McCrea PD 2010. Wnt to build a tube: Contributions of Wnt signaling to epithelial tubulogenesis. Dev Dyn 239: 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink S, Ponta H, Cato AC 1990. The long terminal repeat region of the mouse mammary tumour virus contains multiple regulatory elements. Nucleic Acids Res 18: 2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN 1993. ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci 90: 8977–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RE, Hackam AS 2010. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 28: 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK 2006. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate β-catenin-dependent gene expression. J Biol Chem 281: 13247–13257 [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK 2007. Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns 7: 306–312 [DOI] [PubMed] [Google Scholar]
- Naylor S, Smalley MJ, Robertson D, Gusterson BA, Edwards PA, Dale TC 2000. Retroviral expression of Wnt-1 and Wnt-7b produces different effects in mouse mammary epithelium. J Cell Sci 113: 2129–2138 [DOI] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. 2006. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10: 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, Schutte M, Clevers H 2009. Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J Biol Chem 284: 35308–35313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Shen J 2010. Regulation of Lrp6 phosphorylation. Cell Mol Life Sci 67: 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R 1988. The int genes in mammary tumorigenesis and in normal development. Trends Genet 4: 291–295 [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109 [DOI] [PubMed] [Google Scholar]
- O'Brien J, Martinson H, Durand-Rougely C, Schedin P 2012. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 139: 269–275 [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Weeraratna AT 2009. Hear the Wnt Ror: How melanoma cells adjust to changes in Wnt. Pigment Cell Melanoma Res 22: 724–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmalee NL, Kitajewski J 2008. Wnt signaling in angiogenesis. Curr Drug Targets 9: 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW, Jones DA 2009. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 137: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich TC, Macdougald OA 2007. Wnt/β-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19: 612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen K, Murugan S, Vainio S 2008. Wnt signaling in kidney development and disease. Organogenesis 4: 55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Jiang Z, Li M, Heaphy P, Liu YH, Shackleford GM 2003. Mouse Wnt9b transforming activity, tissue-specific expression, and evolution. Genomics 81: 34–46 [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Reis-Filho J, Ellis IO 2009. Patho-biological aspects of basal-like breast cancer. Breast Cancer Res Treat 113: 411–422 [DOI] [PubMed] [Google Scholar]
- Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, Iscove N, Jones S, McKinney S, Emerman J, et al. 2008. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3: 109–118 [DOI] [PubMed] [Google Scholar]
- Roarty K, Rosen JM 2010. Wnt and mammary stem cells: Hormones cannot fly wingless. Curr Opin Pharmacol 10: 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roarty K, Serra R 2007. Wnt5a is required for proper mammary gland development and TGF–β-mediated inhibition of ductal growth. Development 134: 3929–3939 [DOI] [PubMed] [Google Scholar]
- Robinson GW, Hennighausen L 2011. MMTV-Cre transgenes can adversely affect lactation: Considerations for conditional gene deletion in mammary tissue. Anal Biochem 412: 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H, Wagenaar E, Lopes da Silva S, Nusse R 1990. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci 87: 4519–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault F, Nejad Asl SB, Rungaldier S, Fuchs E, Salmons B, Gunzburg WH 2007. Promoter complex in the central part of the mouse mammary tumor virus long terminal repeat. J Virol 81: 12572–12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandam A, Futakuchi M, Lyssiotis CA, Gibb WJ, Singh RK 2011. A cross-species analysis of a mouse model of breast cancer-specific osteolysis and human bone metastases using gene expression profiling. BMC Cancer 11: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura T 1987. Mammary embryogenesis. In The mammary gland (ed. Neville MC, Daniel CW), pp. 37–66 Plenum, New York [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A 2010. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 29: 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N 2011. Wnt signaling in macrophages: Augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol 90: 553–559 [DOI] [PubMed] [Google Scholar]
- Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE 2007. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res 9: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y 2006. Wnt–Ryk signalling mediates medial–lateral retinotectal topographic mapping. Nature 439: 31–37 [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL, Rosen JM, Cohen DA 2006. Mammary gland macrophages: Pleiotropic functions in mammary development. J Mammary Gland Biol Neoplasia 11: 229–238 [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X 2007. SnapShot: Noncanonical Wnt signaling pathways. Cell 131: 1378. [DOI] [PubMed] [Google Scholar]
- Serra R, Easter SL, Jiang W, Baxley SE 2011. Wnt5a as an effector of TGFβ in mammary development and cancer. J Mammary Gland Biol Neoplasia 16: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE 2006. Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Sharpe R, Pearson A, Herrera-Abreu MT, Johnson DA, Mackay A, Welti JC, Natrajan R, Reynolds AR, Reis-Filho JS, Ashworth A, et al. 2011. FGFR signalling promotes the growth of triple-negative and basal-like breast cancer cell lines both in vitro and in vivo. Clin Cancer Res 17: 5275–5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB, Van Horn K, Hrabeta-Robinson E, Compton J 2006. Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: Evidence for a novel timing system. J Endocrinol 190: 225–239 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, et al. 2008. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer 98: 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA 2008. β1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol 10: 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V, Schulte G, Bryja V, Behrens J 2011. Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J 30: 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissedre B, Pinderhughes A, Incassati A, Hatsell SJ, Hiremath M, Cowin P 2009. MMTV-Wnt1 and -ΔN89β-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PloS ONE 4: e4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Brugmann SA, Helms JA, Nusse R 2008. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135: 3247–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepera SB, McCrea PD, Rosen JM 2002. A β-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci 116: 1137–1149 [DOI] [PubMed] [Google Scholar]
- Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze’ev A, Thiery JP, Glukhova MA 2005. Targeted activation of β-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development 132: 267–277 [DOI] [PubMed] [Google Scholar]
- Theodorou V, Kimm MA, Boer M, Wessels L, Theelen W, Jonkers J, Hilkens J 2007. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nat Genet 39: 759–769 [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE 1988. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55: 619–625 [DOI] [PubMed] [Google Scholar]
- Turner N, Lambros MB, Horlings HM, Pearson A, Sharpe R, Natrajan R, Geyer FC, van Kouwenhove M, Kreike B, Mackay A, et al. 2010a. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene 29: 2013–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, Natrajan R, Marchio C, Iorns E, Mackay A, et al. 2010b. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 70: 2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini F, Charafe-Jauffret E, Bardou VJ, Geneix J, Adelaide J, Labat-Moleur F, Penault-Llorca F, Longy M, Jacquemier J, Birnbaum D, et al. 2001. WNT pathway and mammary carcinogenesis: Loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene 20: 5810–5817 [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE 2008. The mammary progenitor marker CD61/β3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 68: 7711–7717 [DOI] [PubMed] [Google Scholar]
- Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA 2009. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137: 1032–1046 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- van Amerongen R, Berns A 2006. Knockout mouse models to study Wnt signal transduction. Trends Genet 22: 678–689 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R 2009. Towards an integrated view of Wnt signaling in development. Development 136: 3205–3214 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R 2008. Alternative wnt signaling is initiated by distinct receptors. Sci Signal 1: pre9 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Barker N, Harkes IC, van der Heyden M, Dijk NJ, Hollestelle A, Klijn JG, Clevers H, Schutte M 2001. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res 61: 278–284 [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. 2002. The β-catenin/TCF4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C 2011. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479: 189–193 [DOI] [PubMed] [Google Scholar]
- Veeck J, Bektas N, Hartmann A, Kristiansen G, Heindrichs U, Knuchel R, Dahl E 2008. Wnt signalling in human breast cancer: Expression of the putative Wnt inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter hypermethylation in mammary tumours. Breast Cancer Res 10: R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S 2004. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn 229: 349–356 [DOI] [PubMed] [Google Scholar]
- Visvader JE, Smith GH 2011. Murine mammary epithelial stem cells: Discovery, function, and current status. Cold Spring Harb Perspect Biol 3: a004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, Waltz SE 2011. β-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene 30: 3694–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Cavis B, Wiseman R, Hennighausen L 2001. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res 10: 545–553 [DOI] [PubMed] [Google Scholar]
- Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC 1994. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 57: 205–214 [DOI] [PubMed] [Google Scholar]
- Weis W 2011. Proceedings of WNT2011, June 29-July 3, Los Angeles, CA [Google Scholar]
- Wend P, Holland JD, Ziebold U, Birchmeier W 2010. Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol 21: 855–863 [DOI] [PubMed] [Google Scholar]
- Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, et al. 2003. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol 201: 204–212 [DOI] [PubMed] [Google Scholar]
- Witte F, Bernatik O, Kirchner K, Masek J, Mahl A, Krejci P, Mundlos S, Schambony A, Bryja V, Stricker S 2010. Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J 24: 2417–2426 [DOI] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP 1994. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol 14: 6278–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, Dong SM, Guo Z, Benoit N, Cohen Y, et al. 2003. ΔNp63α and TAp63α regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res 63: 2351–2357 [PubMed] [Google Scholar]
- Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ 2000. WISP-1 is a Wnt-1- and β-catenin-responsive oncogene. Genes Dev 14: 585–595 [PMC free article] [PubMed] [Google Scholar]
- Yan H, Blackburn AC, McLary SC, Tao L, Roberts AL, Xavier EA, Dickinson ES, Seo JH, Arenas RB, Otis CN, et al. 2010. Pathways contributing to development of spontaneous mammary tumors in BALB/c-Trp53+/− mice. Am J Pathol 176: 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, et al. 2011. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 30: 4437–4446 [DOI] [PubMed] [Google Scholar]
- Ye X, Wang Y, Nathans J 2010. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med 16: 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ 2010. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev 24: 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng YA, Nusse R 2010. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6: 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X 2005. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al. 2008. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via Frizzled, Dishevelled and Axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Liu Q, Lu W, Bu G 2010a. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: Implication for breast cancer tumorigenesis. Oncogene 29: 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Atkinson RL, Rosen JM 2010b. Selective targeting of radiation-resistant tumor-initiating cells. Proc Natl Acad Sci 107: 3522–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF 2005. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]