Summary
Drosophila Hibris (Hbs), a member of the Nephrin IgG-superfamily, has been implicated in myogenesis and eye patterning. Here, we uncover a role of Hbs in Notch (N)-signaling and γ-secretase processing. Loss of hbs results in classical N-signaling associated phenotypes in Drosophila, including eye patterning, wing margin, and sensory organ specification defects. In particular, hbs mutant larvae display altered γ-secretase-dependent Notch proteolytic processing. Hbs also interacts molecularly and genetically with Presenilin (Psn) and other components of the γ-secretase complex. This Hbs function appears conserved, as mammalian Nephrin also promotes N-signaling in mammalian cells. Our data suggest that Hbs is required for Psn maturation. Consistent with its role in Psn processing, Hbs genetically interacts with the Drosophila β-amyloid protein precursor-like (Appl) protein, the mammalian homologue of APP, the cleavage of which is associated with Alzheimer's disease. Thus, Hbs/Nephrin appear to share a general requirement in Psn/γ-secretase regulation and associated processes.
Keywords: Drosophila, Hbs, Psn, Notch, Cell polarity
Introduction
Notch (N)-signaling is a conserved pathway and required in many processes during animal development and organ patterning (Artavanis-Tsakonas and Muskavitch, 2010; Fortini, 2009; Kopan and Ilagan, 2009) and has been extensively studied in Drosophila (Fortini, 2009). Signaling is initiated by the binding of the ligands Delta (Dl) or Serrate (Ser) to the transmembrane N receptor (Fehon et al., 1990). The ligand-receptor interaction stimulates extracellular cleavage of N by ADAM/TACE metalloproteases (Kuzbanian in Drosophila) (Hartmann et al., 2002; Pan and Rubin, 1997; Sotillos et al., 1997; Wen et al., 1997) and a subsequent intracellular cleavage close to the transmembrane domain by the gamma-secretase complex (Struhl and Greenwald, 1999), releasing the N intracellular domain (NICD or Nintra) (Schroeter et al., 1998; Struhl and Adachi, 1998). NICD can then transit to the nucleus to form a complex with the transcription factor Suppressor of Hairless Su(H)/CSL and activate gene transcription (Fortini and Artavanis-Tsakonas, 1994; Mumm and Kopan, 2000; Selkoe and Kopan, 2003).
Gamma-secretase is a multi-subunit protease complex that has been shown to cleave Type I single-pass transmembrane proteins including Amyloid Precursor protein (APP) and the N receptor. The complex itself consists of four proteins Presenilin, Nicastrin (Nct), APH-1 (anterior pharynx-defective 1), and PEN-2 (presenilin enhancer 2) with Psn acting as the catalytic subunit of the complex (De Strooper, 2003). Elimination of Presenilin1 in mammals leads to reduced γ-secretase mediated cleavage of APP (De Strooper et al., 1998; Naruse et al., 1998). In mammals, biochemical studies have shown that Presenilin is synthesized as an immature holoprotein that undergoes endoproteolytic cleavage in one of the cytoplasmic loops that produces a larger N-terminal and a smaller C-terminal fragment, together forming the functional protein (Nowotny et al., 2000; Thinakaran et al., 1996). This endoproteolytic processing also requires Nct, APH-1 and PEN-2, which are required for proper Psn maturation (De Strooper, 2003; Hu and Fortini, 2003).
In Drosophila, N-signaling is involved in almost every step of eye development, ranging from a role in growth control to lateral inhibition and cell fate specification of photoreceptors. In addition, it is required for correct planar cell polarity (PCP) specification in the eye (Mlodzik, 1999; Strutt and Strutt, 1999). PCP in the Drosophila eye is established by an interplay between the Frizzled (Fz)/PCP and N-signaling pathways in 3rd instar larval eye discs, leading to correct specification of the photoreceptor R3 and R4 precursors (Cooper and Bray, 1999; Fanto and Mlodzik, 1999; Tomlinson and Struhl, 1999). At the five-cell precluster stage, the R3 precursor, which is closer to the D/V-midline, sees higher levels of Fz/PCP signaling (Tomlinson and Struhl, 1999; Zheng et al., 1995) and adopts the R3 fate. Fz activity then activates Dl transcription in R3, which in turn signals to N in the adjacent cell, specifying it as R4 (Cooper and Bray, 1999; Fanto and Mlodzik, 1999). Thus, N-signaling acts downstream of Fz/PCP signaling to control PCP-mediated cell fate specification in the eye.
In a search for molecules involved in PCP regulation in the Drosophila eye we have identified the Immunoglobulin Super Family (IgSF) member hibris (hbs, J.S. and M.M. unpublished). Hbs is a transmembrane protein with multiple extracellular Ig-repeats and is homologous to vertebrate Nephrins (Dworak et al., 2001; Artero et al., 2001). It is expressed dynamically during all developmental stages (Dworak et al., 2001) and can interact across cell membranes with the related Roughest (Rst) and Kirre proteins, members of the Neph family of adhesion factors (Bao and Cagan, 2005). This cell adhesion function of Hbs has been implicated in embryonic myogenesis and late pupal eye patterning (Artero et al., 2001; Bao and Cagan, 2005; Dworak et al., 2001). Recently it has also been suggested that hbs acts in PCP establishment through its genetic interaction with Mtl, a member of the Rho GTPase subfamily (Munoz-Soriano et al., 2011), though the mechanism(s) by which Hbs might influence PCP have not been addressed.
In this study we have uncovered a role of the IgG-super family member Hbs in N and Appl signaling via Psn processing. Hbs loss gives classical N loss-of-function phenotypes including PCP associated and other cell fate specification defects in the eye, wing margin defects, and sensory organ specification defects. Hbs acts as a positive regulator of N-signaling. Loss of hbs suppresses phenotypes caused by expression of membrane-tethered active Notch, but not a constitutively active intracellular form of Notch (Nintra), suggesting defects in Notch activation downstream of ligand binding. Consistently, hbs mutant larvae display altered Notch protein cleavage/processing. Importantly, hbs genetically and molecularly also interacts with Presenilin (Psn) and Nicastrin (Nct), components of the γ-secretase complex. Strikingly, Hbs is required for Psn maturation. Consistent with these findings hbs genetically also interacts with Drosophila APPL, the homologue of mammalian APP, a well known Psn substrate in the context of Alzheimer's disease. The mammalian Hbs orthologue, Nephrin, also promotes Notch-signaling in mammalian cells, and thus this function of Hbs/Nephrin likely represents a conserved requirement in Psn associated processes.
Results
Hbs Loss and gain-of-function cause PCP phenotypes in the eye
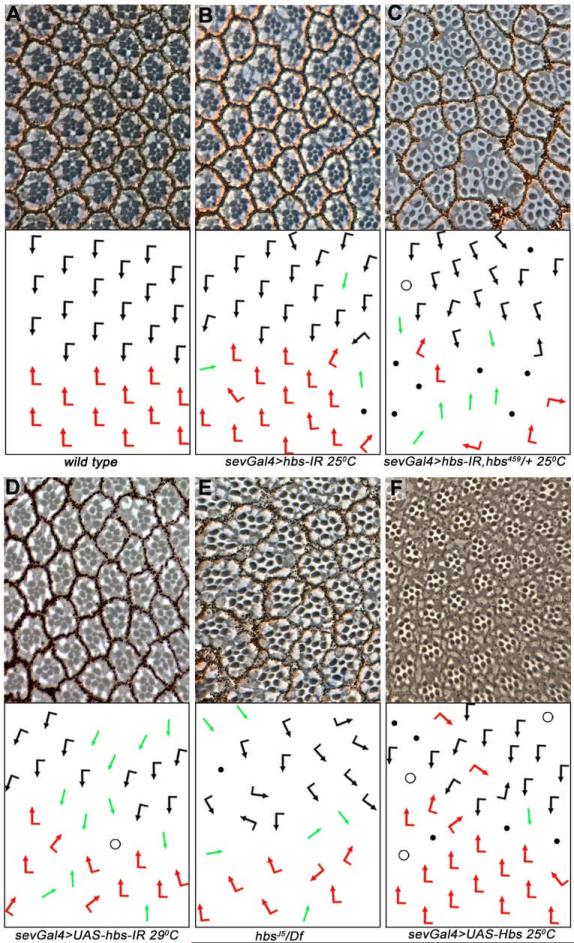
To determine a specific role of Hbs in eye PCP specification, we expressed UAS-hbsRNAi (hbs-IR) using sevenless-Gal4 (sevGal4), which drives expression in the R3/R4 precursors during PCP establishment (and later also in R1/R6 and R7). Fz/PCP-signaling activity in R3 precursors leads to the upregulation of Dl and neuralized expression, which in turn activates N-signaling in the adjacent cell, specifying it as R4 (Cooper and Bray, 1999; del Alamo and Mlodzik, 2006; Fanto and Mlodzik, 1999; Tomlinson and Struhl, 1999). If this interplay is disrupted by mutations in components of either the Fz/PCP or N pathways, the R3-R4 cell fate decision is either randomized, or both precursors adopt the R3 fate (when Fz-signaling is increased or N-signaling is reduced) or the R4 fate (when N-signaling is activated in both precursors; Cooper and Bray, 1999; del Alamo and Mlodzik, 2006; Fanto and Mlodzik, 1999; Tomlinson and Struhl, 1999). Eyes of sevGal4, UAS-hbs-IR flies displayed dosage-dependent PCP defects manifest as symmetrical clusters of the R3-R3 type, ommatidial rotation defects, and occasional loss of photoreceptors (Figure 1A-C and Table 1). Removal of a genomic copy of hbs (hbs459/+) enhanced the hbs-IR phenotypes, confirming its specificity (Figure 1B-C). As Gal4 activity is temperature dependent, expression of hbs-IR at higher temperature (29°C) resulted in stronger PCP defects (Figure 1D). Although several hbs alleles have been reported, none of them are protein null (Suppl. Figure S1D-D”). We thus generated a new strong hbs allele (hereafter hbsJ5) by excising the genomic region between two piggyBac/FRT insertions (see Exp. Procedures for details). All hbs alleles (hbsJ5, hbsJ5/Df(2R)ED2423, and the previously reported hbs459) produced similar eye phenotypes as hbs-IR (Figure 1E, Table 1, and Suppl. Figure S1A; and not shown). We confirmed with an antibody to Hbs that hbs-IR eliminates Hbs protein expression (engrailedGal4>hbs-IR; Suppl. Figure S2A-A’), indicating that hbs-IR mimics a strong LOF allele. Clones of another hbs allele (hbsEP) also displayed similar eye defects (Suppl. Figure S1B). We next analyzed whether Hbs overexpression could affect R3/R4 cell fate specification, as PCP factors generally affect the process in both loss and gain-of-function scenarios (Boutros et al., 1998; Tomlinson and Struhl, 1999). Expression of Hbs (UAS-Hbs, under sevGal4 control) resulted in classical eye PCP defects (including chirality and ommatidial rotation defects) and also some R-cell loss (Figure 1F and Table 1). The gain-of-function phenotypes were stronger at 29° with many symmetrical clusters and an increase in photoreceptor loss (Table 1 and Suppl. Figure S1C). Taken together, these data suggest that Hbs is involved in establishing PCP in the eye and also in specification or differentiation of other R-cells.
Figure 1. hbs loss and gain-of-function displays PCP eye phenotypes.
(A–F) Tangential eye sections of adult eyes of indicated genotypes, centered around the equator; bottom panels: schematic representations of ommatidial polarity. Anterior is left and dorsal up in all panels. Black and red arrows represent dorsal and ventral chiral forms, respectively, green arrows represent R3-R3 symmetrical clusters, open circles represent ommatidia with loss of R7, black dots represent ommatidia with a reduced number of R-cells.
(A) Wild-type: regular ommatidial arrangement of opposing chiralities forming a mirror image across the equator
(B) sevGal4, UAS-hbs-IR at 25°C: note symmetrical clusters (green arrows), rotation defects, and some loss of R-cells.
(C) sevGal4, UAS-hbs-IR is enhanced by hbs heterozygosity (sev>hbs-IR; hbs459/+), confirming specificity of RNAi.
(D) sevGal4, UAS-hbs-IR at 29°C: note stronger defects as compared to 25°C.
(E) hbs459/Df(2R)ED2423: note PCP defects and R-cell loss comparable to the hbs-RNAi experiments, compare to also (D) for similarity to UAS-hbs-IR phenotypes. Quantified in Table 1.
(F) Overexpression of Hbs using sevGal4 (sevGal4, UAS-Hbs) causes similar eye phenotypes (compare to C-E).
See also Suppl. Figure S1.
Table 1.
| Genotype | Wild Type | Chirality Defects | Rotation Defects | Not Scorable |
|---|---|---|---|---|
| sevGal4°hbs-IR 25°C | 77.7±2.2 | 3.0±1.3 | 16.4±1.1 | 2.9±0.1 |
| sevGal4°hbs-IR, hbs459/+ 25°C | 60.5±5.7 | 11.7±2.2 | 16.6±1.1 | 12.5±1.4 |
| sevGal4°hbs-IR 29°C | 56.9±5.9 | 19.4±2.3 | 14.0±2.4 | 9.7±6.9 |
| sevGal4°UAS-Hbs 25°C | 60.4±3.0 | 2.9±1.8 | 5.6±2.7 | 31.1±3.8 |
| sevGal4°UAS-Hbs 29°C | 24.3±6.9 | 7.4±1.5 | 9.6±1.9 | 60.5±9.1 |
| hbsJ5/Df(2R)ED2423 | 51.0±11.7 | 11.0±3.6 | 29.9±10.2 | 8.2±1.5 |
| N55e11/+; sevGal4°hbs-IR 25°C | 80.2±7.9 | 12.9±3.1 | 6.6±5.6 | 0.9±1.6 |
| sevNΔECD 29°C | 35.9±0.5 | 32.5±5.5 | - | 43.7±10.3 |
| sevNΔECD, sevGal4°hbs-IR 29°C | 23.7±6.1 | 9.4±4.1 | - | 54.6±4.3 |
| sevGal4°hbs-IR, kuze29-4/+ 25°C | 81.5±8.2 | 4.3±1.3 | 12.4±6.7 | 1.7±1.9 |
| sevGal4°hbs-IR, psn143/+ 25°C | 53.9±3.4 | 19.6±1.0 | 19.1±3.8 | 7.4±3.4 |
| sevGal4°hbs-IR, psn9/+ 25°C | 63.7±2.5 | 11.8±1.1 | 18.8±2.4 | 5.7±1.7 |
| sevGal4°hbs-IR, psn-IR/+ 25°C | 64.4±4.2 | 14.3±1.3 | 13.5±3.2 | 7.8±4.0 |
| sevGal4°hbs-IR, nctA7/+ 25°C | 83.2±2.7 | 12.1±2.8 | - | 4.8±1.2 |
| sevGal4°appl-IR 25°C | 33.5±8.7 | 7.9±4.2 | - | 59.0±5.2 |
| sevGal4°hbs-IR, appl-IR 25°C | 22.1±5.7 | 34.6±5.0 | - | 43.3±8.2 |
For each genotype at least 3 independent eyes were scored and more than 300 ommatidia were counted. Numbers represent percentages of ommatidia ± standard deviation. Unscorable clusters consist of fewer or more than the normal R-cell complement and thus their orientation cannot be scored.
Hbs is expressed in developing R-cell preclusters with membrane and vesicular localization
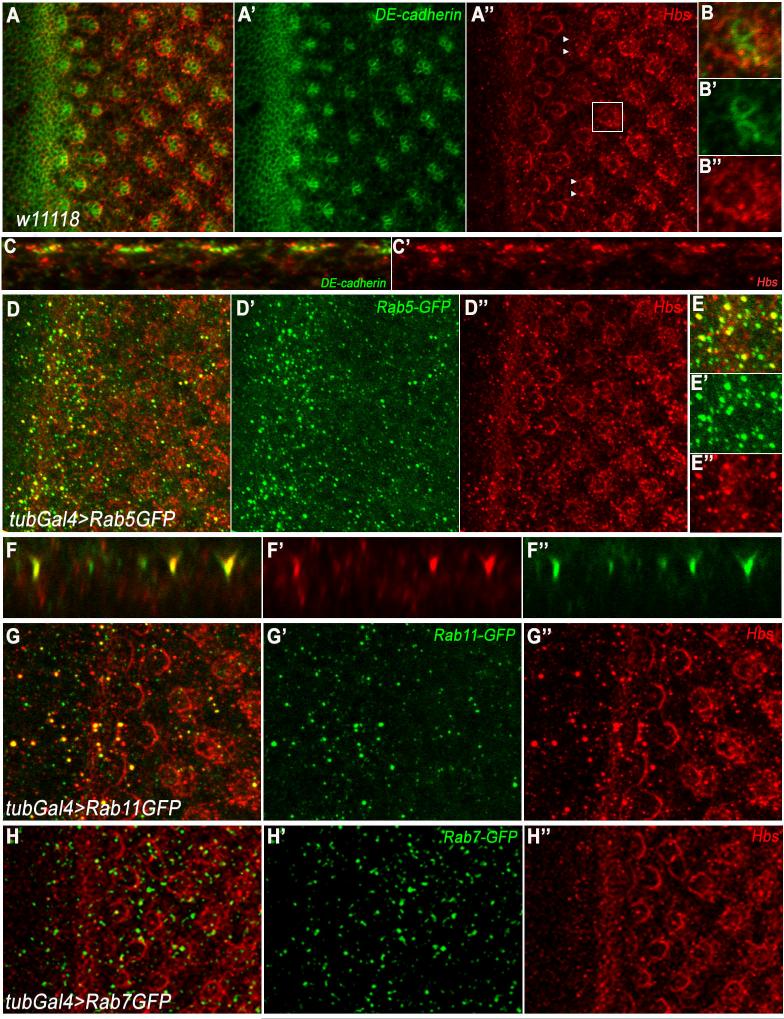
Consistent with a role in eye PCP establishment and R-cell specification, hbs expression is upregulated in the morphogenetic furrow (MF; arrow in Figure 2A-A”) and in developing clusters as they emerge as arcs from the MF (in addition to a lower level ubiquitous expression in all cells). Subsequently, by row 2-4 Hbs expression was enriched around ommatidial preclusters and was also detected in all developing photoreceptors (including R3/R4) at the time of PCP establishment (arrowheads in Figure 2A”). This expression pattern is consistent with the notion that Hbs is required in the R3/R4 specification context and/or other photoreceptor cell fate decisions. Hbs expression is maintained at later stages of eye development, consistent with its reported role in pupal eye patterning (Bao and Cagan, 2005). Intriguingly, besides its membrane association, we also consistently detected a significant portion of Hbs in intracellular puncta in imaginal discs (Figure 2B-B” and D,D”, when imaged at a more basal plane, see also x/z-sections in 2C-C’). To determine the nature of the Hbs positive intracellular puncta, we co-stained eye discs with vesicular markers. Most Hbs puncta co-localized with Rab5, a marker for early endosomes (Figure 2D-E, also x/z-section in 2F-F”). Some co-localization was also seen with Rab11 positive vesicles (recycling endosomes), while in contrast no co-localization was seen with Rab7 (Figure 2G-G” and 2H-H”). These data suggest that Hbs not only gets enriched apically at plasma membranes near junctions, but is also present in early and recycling endosomes.
Figure 2. Hbs is localized at membranes/junctions and in intracellular puncta.
(A-B) Confocal projections depicting localization of Hbs (red) and DE-cadherin (green, marking cellular outlines at junctional level and highlighting developing photoreceptor clusters) in third instar eye discs. Hbs is upregulated in the furrow (MF, white arrow in A and A”) and R-cell preclusters, as they emerge from MF. Hbs is enriched at membranes surrounding ommatidial preclusters (A”, magnified in B-B”), reflecting expression in all R-cells.
(C-C’) x/z-section of third instar eye disc in A. Note that Hbs is not only enriched in junctional membranes but also present in intracellular puncta throughout R cells.
(D-E) x/y-section of third instar eye disc of tubGal4>Rab5GFP genotype, stained for Hbs (red) and Rab5GFP (green). Note Hbs positive puncta co-localize (magnified in E-E’) with Rab5GFP puncta, a marker for early endosomes.
(F-F’) x/z-section of third instar eye disc in D, showing co-localization of Hbs and Rab5 positive puncta.
(G-G’) tubGal4>Rab11GFP eye discs showing co-localization of Hbs (red) with recycling endosomal marker Rab11 (green).
(H-H’) tubGal4>Rab7GFP eye discs showing expression of Hbs (red) and Rab7GFP (green). Hbs does not co-localize with late endosomal marker Rab7 (green).
See also Suppl. Figure S2.
Loss of hbs mimics Notch loss-of-function phenotypes in several tissues
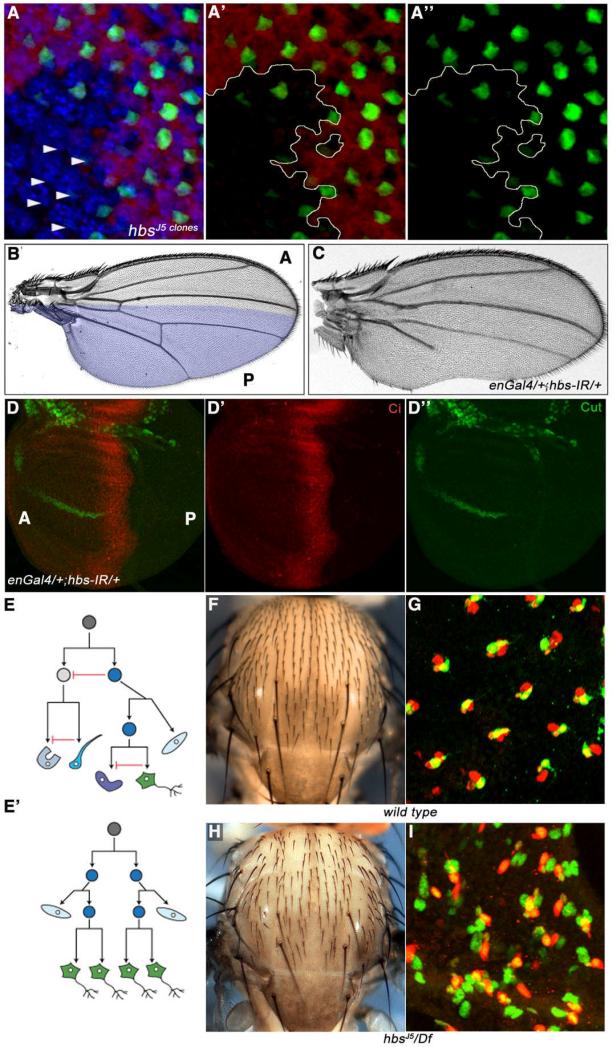
To define the role of Hbs in eye PCP specification and to establish whether it acts in Fz/PCP or N-signaling, we tested the N-signaling reporter mδ0.5, a 500-base-pair fragment of the E(spl)mδ promoter (Cooper and Bray, 1999), which serves as a marker for R4 specification and a molecular read-out of N activity (mδ0.5 is first expressed at low levels in both R3/R4 cells and then up-regulated specifically in R4 in response to N-activation). Strikingly, expression of mδ0.5Gal4, UAS-GFP (mδ0.5>GFP) was reduced or lost in hbs mutant clones in third instar discs (Figure 3A, arrowheads), suggesting defects in N-signaling and R4 cell fate specification. To determine whether hbs is generally required for N-signaling, we tested its functions in other tissues patterned by N-signaling. In the wing, N-signaling is required, among others, for the formation of the dorsal/ventral (D/V) compartment boundary and wing margin specification, and loss-of-function N alleles cause wing notching phenotypes (de Celis et al., 1996; Shellenbarger and Mohler, 1978). We tested whether hbs has a role in modulating N-signaling in this context. Expression of enGal4>hbs-IR (expressed in the posterior compartment [P], highlighted in Figure 3B) resulted in wing notching phenotypes reminiscent of N signaling defects (Figure 3C; wing notching phenotypes were also seen in clones of two hbs alleles, Suppl. Figure S3A-B). Consistently, en>hbs-IR resulted in reduced expression of the N-signaling target gene cut at the prospective posterior wing margin (Figure 3D-D”, anterior compartment [marked by Ci in red] acts as internal control as hbs-IR is not expressed there). Of note, overexpression of Hbs (enGal4>UAS-Hbs) also resulted in wing notching phenotypes and reduction of Cut expression in the posterior compartment, suggesting that too much Hbs acts as dominant negative (Suppl. Figure S3C-E, see Discussion for details).
Figure 3. hbs loss-of-function resembles N-signaling loss-of-function defects.
(A-A”) Confocal projections of third instar eye discs stained for mδ0.5Gal4>GFP (green; N-signaling reporter in R4), neuronal marker Elav (blue, labeling all R-cells), and lacZ (red, labeling wild-type tissue), hbs mutant tissue (marked by absence of red) is outlined by yellow lines in A’-A”. mδ0.5Gal4>GFP expression is reduced or often lost in preclusters mutant for hbs, suggesting a failure of N-signaling target activation in R4 cells.
(B-C) Adult wings. (B) wild-type wing with highlighted engrailed (en) expression domain in posterior compartment (P); (C) enGal4, UAS-hbs-IR (enGal4>hbs-IR, at 29°C) results in wing notching and vein formation defects.
(D-D”) Confocal projections of enGal4, UAS-hbs-IR third instar wing disc stained for the N-signaling target Cut (green) and Ci (red, marking anterior compartment [A]). Cut expression is lost/reduced in P compartment. en-expression expands into the A compartment at this stage and hence Cut expression is also affected near the border in the A compartment.
(E-E’) Schematic of divisions within a sensory organ, consisting of two external cells: shaft and socket, and two internal cells: neuron (green) and sheath (glia-like), which arise from a series of asymmetric divisions from a single sensory organ precursor (SOP) cell. Directional Delta/N signaling specifies the two daughter cells to adopt different fates. Red inhibitory arrow represents N-signaling in (E). In N loss-of-function (E’) all four cells are specified as neurons (green), leading to loss of external bristles.
(F-G) Wild-type adult thorax showing mechanosensory bristles, patterned uniformly across the notum (F), and (G) confocal projections of anterior notum at 24–28h APF, stained for Elav (green; neuronal marker) and Cut (red; labeling all SOP cluster cells). Note: each cluster has only one Elav positive cell.
(H-I) hbs mutant thorax (hbsJ5/Df(2R)ED2423) showing bald patches as a result of missing bristle cells in adults (H), and confocal projections of equivalent notum at 24-28h APF (I) with multiple Elav positive (green) cells per SOP cluster, suggesting failure of N-signaling based cell fate specification in hbs mutants.
See also Suppl. Figure S3
Asymmetric activation of N signaling in sensory organ precursor cells is required for proper development of thoracic sensory bristles, consisting of shaft, socket, sheath and neuronal cells (Figure 3E). Failure of N-signaling during sensory bristle group development results in all cells adopting a neuronal fate at the expense of the accessory fates (Figure 3E’; Lai and Orgogozo, 2004). Since higher levels of Hbs are expressed in the developing sensory bristle clusters (as compared to surrounding epithelial cells; Suppl. Figure S2B-B”), we tested whether hbs mutants display bristle group phenotypes. In wild type, a regular pattern of bristles was observed (Figure 3F), while hbs mutants (hbsJ5/Df(2R)ED2423 and hbs459/Df(2R)ED2423) displayed a reduction in bristle numbers (Figure 3H; Suppl. Figure S3H). To test whether this is due to aberrant N-signaling mediated cell fate specification within the SOP group, we analyzed their development at 24h APF with the neuronal marker Elav and Cut (marking all cells of the SOP cluster). In wild type, each Cut-expressing cluster includes a single Elav-expressing neuron (Figure 3G). In contrast, in hbs mutants most SOP clusters displayed several Elav-positive cells (Figure 3I), implying defects in N-signaling. Similar to hbs mutants, loss of external bristle structures was also observed when hbs-IR was expressed under pannier-gal4 control (expressed centrally in the developing thorax; Suppl. Figure S3F-G). These phenotypes suggest a general requirement in N-signaling.
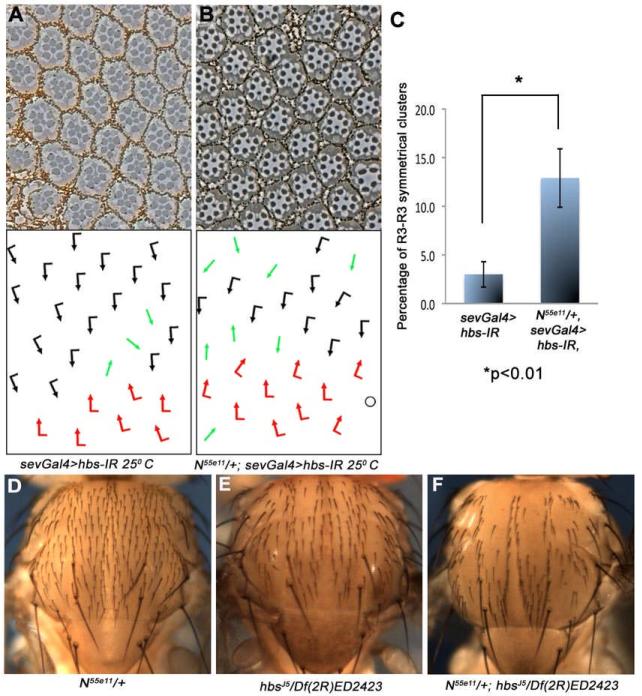
To further address the role of hbs in N-signaling, we tested whether reduction in N levels can modify hbs LOF phenotypes. In the eye, removing one copy of N (N55e11/+, which by itself has no eye phenotype, not shown) strongly enhanced the sevGal4>hbs-IR phenotypes (Figure 4A-C and Table 1). Specifically, N55e11/+, sevGal4>hbs-IR displayed a marked increase in R3-R3 symmetrical clusters (compared to sevGal4>hbs-IR alone), consistent with a reduction in N-dependent R4 specification. In the thorax, reducing N dosage resulted in an enhancement of the hbsJ5/Df(2R)ED2423-associated bristle loss phenotype (Figure 4D-F; N-/+ has no thorax phenotype alone). Similar interactions were also observed between several N and other hbs alleles (Suppl. Figure S3H-I). Taken together, these data confirmed a role of hbs in N-signaling.
Figure 4. hbs genetically interacts with N.
(A-C) Tangential eye sections of adult eyes of indicated genotypes (at 25°C; centered around the equator); bottom panels show schematic representations of ommatidial polarity. Anterior is left and dorsal is up. (A) sevGal4, UAS-hbs-IR and (B) N-/+; sevGal4, UAS-hbs-IR: The N55e11/+ background enhances the hbs-IR phenotype, most evident in the increase in R3-R3 symmetrical clusters, quantified in (C; graph shows mean standard deviation [s.d.] of three eyes, n>300 ommatidia scored, P-value: *p<0.01 as determined by student t-test).
(D-F) Dorsal thorax view of indicated genotypes, anterior is up. (D) N55e11/+ thorax (control) showing normal sensory bristles arrangement, (E) hbsJ5/Df(2R)ED2423 thorax with some missing bristles, (F) N55e11/+; hbsJ5/Df(2R)ED2423 thorax, note strong enhancement of the hbsJ5/Df(2R)ED2423 phenotype by removing a copy of N.
Strikingly, in addition to these N-signaling phenotypes, hbs expression is upregulated in most contexts where high levels N-signaling are required. Besides the MF and R-cell preclusters in the eye (Fig. 2), Hbs is also expressed at high levels at the wing margin (Supp. Fig. S2A and S2-F”) and within the SOP groups (Suppl. Fig. S2B-B”). Interestingly, these expression patterns are under the control of several signaling pathways, including Wg-signaling in the wing or Egfr/Ras-signaling in the eye (Suppl. Fig. S2 and not shown), which could thus affect N-signaling levels (see Discussion). In summary, the phenotypic data, genetic interactions, and expression in imaginal discs are all consistent with the notion that hbs is generally required for (and possibly a component of) N-signaling
hbs is required for the activity of membrane-tethered Notch but not for Notchintra in vivo
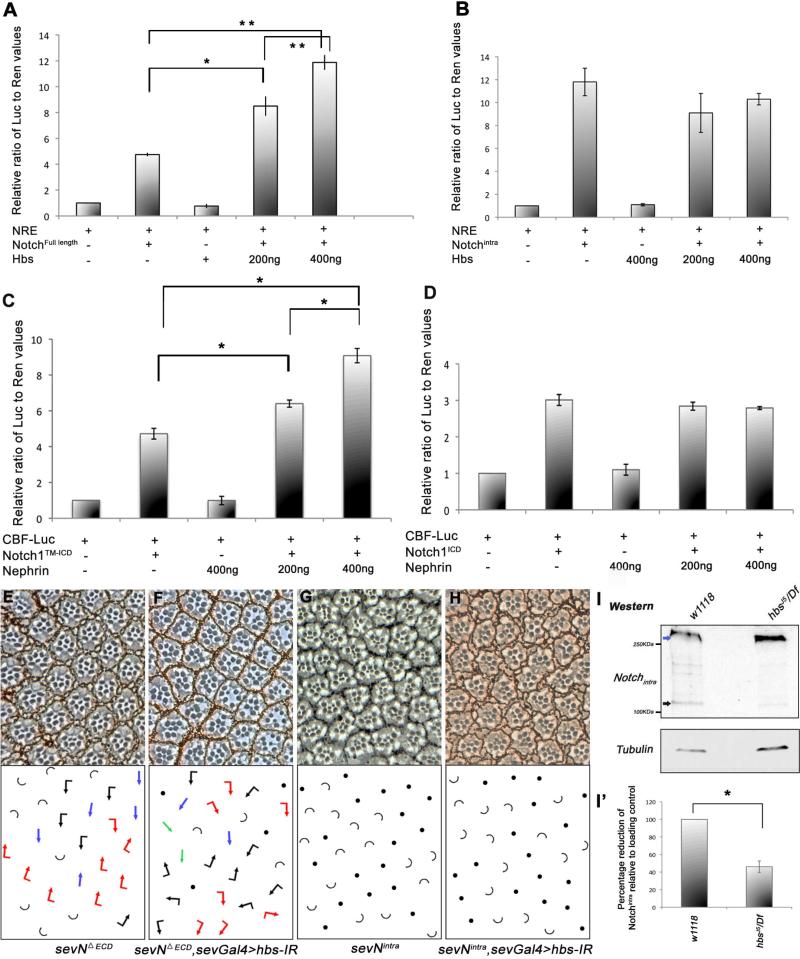
To address molecular mechanism(s) of how Hbs could affect N-signaling, we first tested the effect of Hbs on a N-responsive reporter (NRE) (Bray et al., 2005). Full-length N-mediated induction of the NRE in S2 cells was potentiated in a dosage-dependent manner by Hbs co-expression (Figure 5A). In contrast, co-transfection of Hbs did not affect the level of NRE activation by the constitutively active cytoplasmic fragment of N (Nintra; Figure 5B). As Nintra acts independently of upstream regulation this result suggested that Hbs is required for activation of membrane tethered N, or related membrane-associated processes, rather than downstream signaling events.
Figure 5. hbs is required for the activity of membrane-tethered Notch.
(A-B) Activation of N-signaling induced luciferase reporter containing Su(H) binding sites (NREs) in S2 cells co-transfected with Hbs along with either full length N (A) or NICD (B). Hbs expression causes an increase in activity of full length N in a dose dependent manner, while reporter activity induced by intracellular NICD remains unaffected by Hbs. Values represent mean ratio of luciferase/renilla control (internal control). Representative experiment from three independent experiments is shown (error bars represent standard deviations within each experiment). P-values were *P<0.05 and **P<0.005 (student t-test).
(C-D) Activation of mammalian CBF Notch luciferase reporter in 293T cells co-transfected with Nephrin along with either mN1TM-ICD (C) or mN1ICD (D); co-expression of Nephrin enhanced mN1TM-ICD based CBF reporter activation in a dose dependent manner (*P<0.005), while mN1ICD mediated reporter induction remained unchanged. Error bars denote standard deviations within each experiment.
(E-H) Tangential sections of adult eyes of indicated genotypes, anterior is left and dorsal up. Arrows are as in Figure 1, with blue arrows representing R4-R4 type symmetrical clusters and half circles ommatidia with >1 R7 (at the expense of R1/R6, as frequently observed in N overactivation caused by transformation of R1 and/or R6 to R7; Fortini et al., 1993; Tomlinson and Struhl, 1999).
(E-F) Knock-down of hbs (sevGal4>hbs-IR) markedly suppresses the phenotype of membrane tethered sevNΔECD (compare E and F; note even some R3-R3-type clusters; quantified in Suppl. Figure S4),
(G-H) The effect of “active” cytoplasmic sev-Nintra (G) is not affected by hbs-IR (H), suggesting a requirement of Hbs at the membrane.
(I-I’) Western blot of third instar larval eye-brain complexes showing cleavage pattern of endogenous N protein. In wild-type larvae higher molecular weight full-length N (blue arrow) and smaller cleavage products, N-intra fragments (~120K, black arrow) are detected. In samples from hbs mutants, there is a significant reduction in the levels of N cleavage fragments, whereas levels of full-length N protein are unchanged (γ-Tubulin, lower panel, serves as loading control). (I’) Quantification showing percentage reduction of Notchintra cleavage products (black arrows), which are reduced in hbs mutant larval eye-brain complexes (error bars represent standard deviations with*p<0.01).
See also Suppl. Figure S4.
Hbs encodes a transmembrane IgSF protein and shares extensive homology to mammalian Nephrins. Nephrins are expressed in diverse organisms ranging from worms to mammals (Dworak et al., 2001). In vertebrates, Nephrin has been studied mostly in the context of the slit diaphragm, a specialized junction in the kidney glomerulus, which is involved in filtering blood (Holzman et al., 1999; Ruotsalainen et al., 1999). We next tested whether the N-signaling-related function of Hbs/Nephrin is conserved in mammals and asked whether Nephrin can potentiate N-signaling in mammalian cells. We co-expressed mTM-Notch1 (a mouse Notch1 isoform, inserted in the membrane and requires the final [S3] cleavage for activation) along with Nephrin in the presence of a Notch reporter. mTM-Notch1 expression alone resulted in induction of the reporter as expected (Figure 5C). Strikingly, co-expression of Nephrin along with mTM-Notch1 caused a dosage dependent increase in N-reporter activity (Figure 5C). Consistent with the Drosophila data, expression of Nephrin did not modify the level of N reporter activity induced by mNotch1intra (Figure 5D). Taken together, the role of Hbs and Nephrin in regulating membrane-associated events in N-signaling appears conserved.
To corroborate this hypothesis in vivo, we tested the requirement of Hbs for N-signaling activity in the Drosophila eye. Expression of a truncated membrane tethered form of N (NΔECD) that lacks most of the extracellular domain and functions in a ligand-independent manner (Fortini et al., 1993), resulted in frequent R4-R4 symmetrical clusters (sev-NΔECD; with 32.6±5.5% R4-R4 clusters, Figure 5E, Table 1, and Suppl. Figure S4A) and frequent transformations of outer R1/R6 to inner R7s as reported (Fortini et al., 1993; Tomlinson and Struhl, 1999). The number of R4-R4 clusters was markedly reduced by co-expression of hbs-IR in sev-NΔECD eyes (Figure 5E-F, quantified in Table 1 and Suppl. Figure S4A). Notably, even R3-R3 symmetrical clusters, never seen in a sev-NΔECD background, were observed in sevGal4>hbs-IR, sev-NΔECD, suggesting repression of N activity. Similar suppression of sev-NΔECD was seen when we removed a genomic copy of hbs (not shown). In contrast to the membrane-tethered NΔECD, the equivalent eye phenotypes of the soluble Nintra isoform (Tomlinson and Struhl, 1999) were not affected by hbs-IR (Figure 5G-H; sev-Nintra and sevGal4>hbs-IR, sev-Nintra displayed indistinguishable phenotypes; also Table 1). Taken together, these data are consistent with a positive requirement of Hbs in N-signaling. Importantly, our data indicated that Hbs was required for activation of trans-membrane N proteins, but dispensable for shorter soluble isoforms, not requiring membrane associated cleavage. Hence we tested whether Hbs affected cleavage of Notch. Western analysis of hbs mutant eye/brain complexes revealed an altered banding pattern of N, with the levels of the fully cleaved low molecular bands (equivalent to Nintra, ~120KDa fragment) markedly reduced (Figure 5I, black arrow; quantified in 5I’). Similar reduction in Notch-intra levels were observed in hbsIR wing discs under nubGal4 driven expression (expressed in all wing blade cells, Suppl. Figure S4B).
Loss of hbs affects the Psn/γ-secretase mediated Notch cleavage pattern
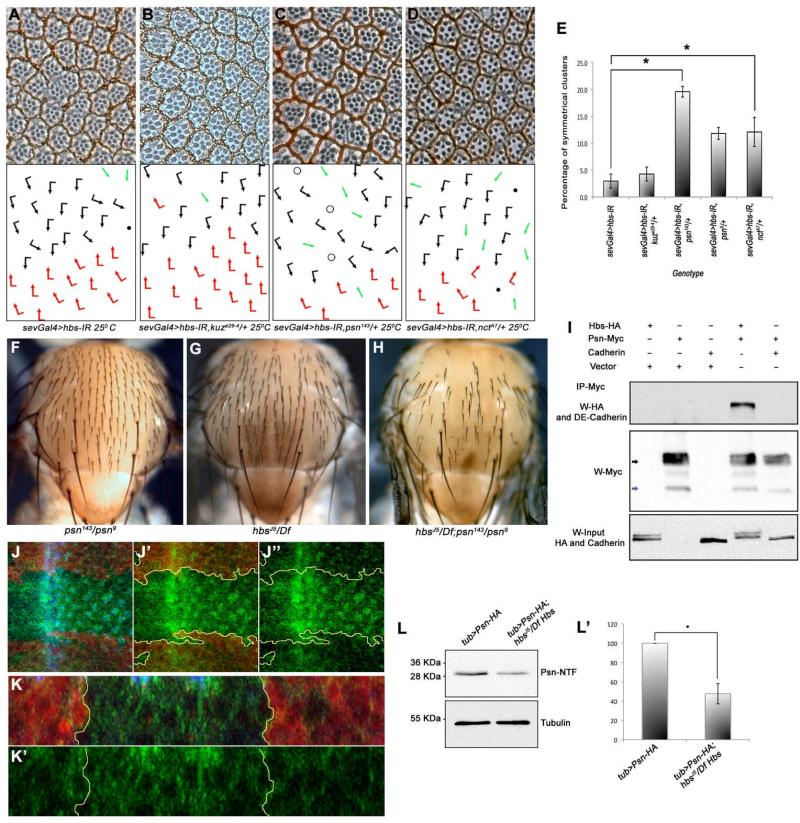
The Notch receptor undergoes a ligand-dependent cleavage mediated by the Kuzbanian/TACE metalloproteases at an extracellular site close to the membrane (Pan and Rubin, 1997; Sotillos et al., 1997; Wen et al., 1997). Subsequently, Notch undergoes an intra-membranous cleavage mediated by the γ-secretase complex consisting of Psn, Nct, Aph-1 and Pen-2 (De Strooper et al., 1999; Ray et al., 1999; Struhl and Greenwald, 1999; Ye and Fortini, 1998). The NΔECD construct represents an isoform of N that does not require the extracellular TACE/Kuzbanian mediated (S2) cleavage for activation. As the activity of NΔECD (which does not require Kuz mediated S2 cleavage) was suppressed by reducing hbs levels (hbs-IR, Figure 5F), this would suggest that Hbs functions downstream of TACE/Kuz. Accordingly, reduction of Kuz levels (kuz-/+) in hbs-IR backgrounds had no detectable effect on hbs-IR eye phenotypes. (Figure 6A-B, quantified in 6E and Table 1). We next tested whether Hbs has a role in Psn-mediated cleavage of N (Struhl and Greenwald, 1999). Strikingly, removing a genomic copy of psn (via either the psn143 or psn9 alleles) resulted in a dominant enhancement of the frequency of R3-R3 symmetrical clusters induced by sev>hbs-IR (Figure 6C-D and Table 1). Similarly, expression of psn-IR in the eye enhanced the sev>hbs-IR PCP phenotype (Suppl. Figure S5A-B). Removing a genomic copy of psn (psn143/+) enhanced the sevGal4>UAS-Hbs PCP phenotype, consistent with the notion that overexpression of Hbs in vivo acts as a dominant negative (Table 1 and Suppl. Figure S5C-D, also Discussion). Importantly, psn also enhanced the effects of hbs mutants in other tissues. For example, the hypomorphic psn143/psn9 trans-heterozygous background, which displayed no thoracic abnormalities by itself (Figure 6F), strongly enhanced the bristle loss of hbsJ5/ Df(2R)ED2423 and hbs459/ Df(2R)ED2423 mutants (Figure 6F-H and not shown). Of note, removing a genomic copy of nct (nctA7/+), another component of γ-secretase, also enhanced hbs-IR PCP phenotypes (Figure 6D-E and Table 1), suggesting a role of Hbs in γ-secretase complex function.
Figure 6. Hbs is required for Psn function and processing.
(A-D) Tangential adult eye sections of indicated genotypes, anterior is left and dorsal up (arrows and dots as in Figure 1). sevGal4>hbs-IR (A) is not affected by kuze29-4/+ heterozygosity (B), whereas dosage reduction of psn (sevGal4>hbs-IR, psn143/+) (C), and nct (sevGal4>hbs-IR, nctA7/+) (D) strongly enhances the hbs-IR phenotypes, suggesting a positive relationship between Psn and Hbs. (E) Quantification of R3-R3 symmetrical clusters in genotypes shown in B-D. Error bars represent standard deviations (**p<0.001).
(F-H) Adult nota of genotypes indicated, anterior is up. (F) heteroallelic hypomorphic psn143/psn9 combination with normal mechanosensory bristle arrangement; (G) hbsJ5/Df(2R)ED2423 mutant thorax displaying occasional “bald” patches as a result of missing bristles; and (H) hbsJ5/Df(2R)ED2423; psn143/psn9 double mutant thorax with many bristles missing, suggesting synergistic interaction and hbs and psn acting in the same molecular context.
(I) Hbs is co-immunoprecipated (co-IP) by Psn: immunoblot from S2 cell whole cell lysates expressing Hbs-HA either alone or in combination with Psn–Myc or DE-Cadherin (acting as negative control). Cell lysates were immunoprecipitated with anti-Myc (IP-Myc) and blots were probed with anti-HA (Hbs) and anti-DE-Cadherin antibodies, revealing specific Co-IP of Hbs with Psn while DE-Cadherin (a control protein similar to Hbs) does not bind to Psn-Myc (bottom panel: Hbs input). See also Suppl. Fig. S5I for additional controls.
(J-J”) Confocal projections of third instar larval eye discs stained for Psn-HA (green), DE Cadherin (blue, labeling apical surfaces of all cells), and lacZ (red, marking wild-type tissue: hbs mutant tissue marked by absence of red; outlined by yellow lines in J’-J”). hbs mutant tissue shows marked accumulation of PsnHA. (K-K”) Transverse (x/z)-section of eye disc shown in (J); note accumulation of Psn in the hbs mutant tissue (marked by absence of lacZ).
(L-L’) Western blot of third instar larval eye-brain complexes showing Psn-NTF fragment in wild-type and hbs mutant larvae. In hbs mutant larvae there is a marked reduction of Psn-NTF fragment (quantified in L’) as compared to control flies (γ-Tubulin, lower panel, serves as loading control). Error bars represent standard deviations, with *p<0.001.
See also Suppl. Figure S5.
Next we investigated whether the genetic hbs-psn interactions were supported by a molecular association. First, we observed Hbs and Psn co-localization in S2 cells (Suppl. Figure S5E-E”; both proteins localizing to the same subcellular compartments). Co-localization was confirmed in third instar imaginal discs at endogenous levels: PsnHA [a biologically active, HA-epitope-tagged, form of Psn (Chung and Struhl, 2001)] and endogenous Hbs co-localized to the same subcellular compartments (Suppl. Figure S5F-G”). Similar co-localization of PsnHA and Hbs was also detected in developing wing discs (Suppl. Figure S5H-H”). Second, we tested whether Hbs can physically associate with Psn. Hbs-HA (haemagglutinin-tag) was transfected alone or together with Psn-Myc, Fz-Myc and/or DE-Cadherin (Fz-Myc and DE-Cadherin act as negative controls to rule out non-specific binding of Hbs-HA and Psn-Myc to other trans-membrane proteins). Immunoprecipitation of Psn-Myc specifically co-immonoprecipitated Hbs-HA (Fig. 6I), while DE-Cadherin (Fig. 6I) and Fz-Myc did not pull-down Hbs-HA (Suppl. Fig. S5I), indicating a specific molecular interaction between Hbs and Psn. Of note, very similar physical interactions were also observed between Hbs and Nct in S2 cells (data not shown). To confirm a potential physiological significance of Hbs co-localization and binding to Psn, we examined the subcellular distribution of Psn in hbs mutant clones. Relative to wild-type cells (marked in red, Figure 6J-K), Psn accumulated in hbs mutant cells (6J-K), suggesting that Psn processing or maturation might be affected. To test this hypothesis we analyzed the levels of processed Psn fragments [Psn is synthesized as an immature holoprotein that exists as two closely linked endoproteolytic fragments (Thinakaran et al. 1996)]. In the PsnHA construct the HA-tag is associated with the N-terminal NTF. Strikingly, in hbs mutant larvae (hbsJ5/Df), there is a marked reduction in Psn-NTF as compared to wild-type control (Figure 6L-L’). These data suggest that Hbs affects Psn processing, which results in a reduction of active, cleaved Psn in hbs mutant cells.
Hbs genetically interacts with Drosophila APPL
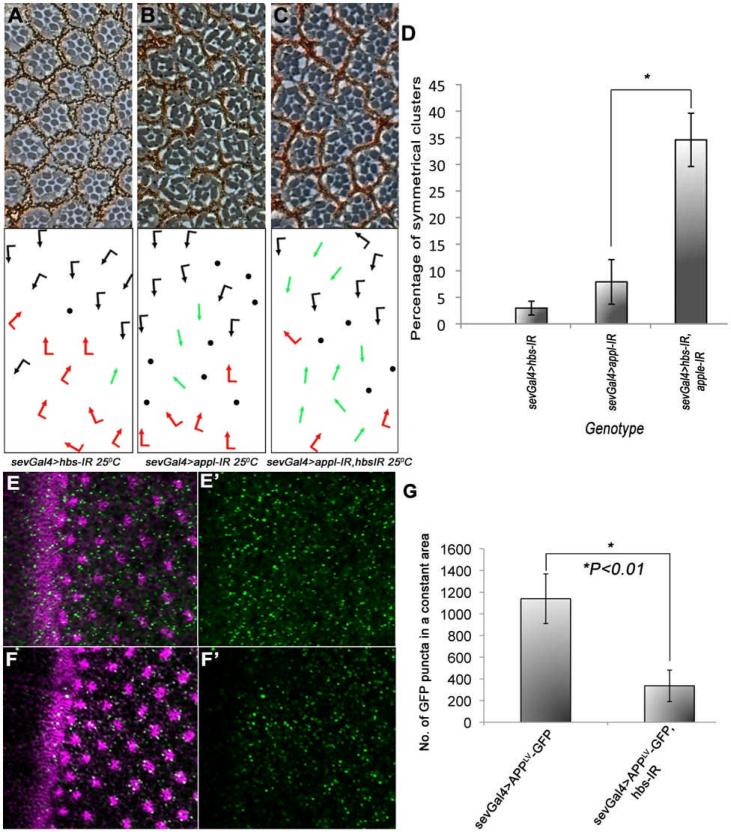
Another well studied cleavage substrate of Psn is APP (De Strooper et al., 1998; Thinakaran and Koo, 2008). Similar to Notch, APP also undergoes several cleavages, one of which (mediated by Psn) leads to the generation of amyloid-β peptide, a major component of the neurotoxic plaques found in Alzheimer's disease patients (Goate et al., 1991; Thinakaran et al., 1996). Amyloid-β peptide is generated by sequential cleavage of APP by β-secretase followed by γ-secratase. In Drosophila, an APP-related protein (Appl) has been identified (Rosen et al., 1989). Intriguingly, one report suggests that Appl behaves in a similar manner to mammalian APP in that it can aggregate, forming toxic amyloid deposits in neurons (Carmine-Simmen et al., 2009). As our data suggest that Hbs is required for the activity of Psn and Drosophila Appl is processed by Psn in the same way as its mammalian orthologue, we tested whether Hbs can affect the function of Drosophila Appl. Strikingly, knock-down of appl in the eye (sevGal4>UAS-appl-IR) resulted in PCP-like phenotypes (besides loss of photoreceptors), characterized by the presence of R3-R3 symmetrical clusters (Figure 7B). Co-expression of hbs-IR with appl-IR (sevgal4>UAS-appl-IR, UAS-hbsIR) significantly enhanced the appl-IR PCP phenotype (Figure 7C-D), arguing for a role of Hbs in Appl function. To test whether Hbs affected processing of Appl via Psn, we used an established Appl-reporter (Loewer et al., 2004; Suppl. Figure S6), which provides a direct read-out of Psn mediated cleavage of Appl-ICD (AICD) at the membrane, by activating GFP transcription and thus this effect can be quantified. The fusion reporter construct is under Gal4/UAS control and thus can be expressed in a tissue specific manner. Expression of the reporter alone in developing eyes (sevGal4>UAS-AppLV-GFP) yields a GFP signal due to endogenous Psn-mediated processing in photoreceptor cells (Figure 7E). Strikingly, expression of hbs-IR (under sevGal4) markedly suppresses the cleavage of this fusion protein, resulting in a significant reduction of GFP (Figure 7F-G). These data establish that Hbs is generally required for Psn-mediated cleavages, as it affects both N and Appl functions, and corroborate our conclusion that Hbs is required/important for the stability or cleavage of Psn.
Figure 7. hbs genetically interacts with dAppl.
(A-C) Tangential sections of adult eyes of indicated genotypes, anterior is left and dorsal up.
(A) sevGal4, UAS-hbs-IR eye at 25°C with phenotypes as seen previously (e.g. Figure 1B); (B) sevGal4, UAS-appl-IR eye at 25°C, note several ommatidia that adopt R3-R3 type symmetrical arrangement; (C) sevGal4, UAS-appl-IR, UAS-hbs-IR at 25°C. Note enhancement of either inidividual RNAi phenotype, indicating a positive (synergistic) relationship between Appl and Hbs. (D) Quantification of R3-R3 symmetrical clusters in the genotypes shown in A-C. Error bars represent standard deviations (*p<0.01).
(E-G) Confocal projections of third instar larval eye discs expressing the APPLV-GFP fusion protein under the control of sevGal4 (sevGal4>AppLV-GFP) showing expression of GFP behind the MF and its quantification (G). Expression of the GFP reporter is significantly reduced in eye discs co-expressing hbs-IR (sevGal4>AppLV-GFP, UAS-hbsIR), indicating that there is reduction of APPL-ICD membrane cleavage when Hbs is compromised (F). Eerror bars show standard deviations, with *p<0.01 in (G). See also Suppl. Fig. S6F for schematic of reporter assay. See also Suppl. Figure S6.
Discussion
In this study we have identified the IgG-superfamily member Hbs/Nephrin as a positive regulator of Psn processing, thus affecting N-signaling and Appl function. Previous work has primarily defined a role for Hbs/Neprhins in heterophyllic cell-cell adhesion contexts, including podocyte function in mammalian kidneys (Kawachi et al., 2006), muscle cell fusion and morphogenesis in Drosophila, and the organization of interommatidial precursor cells (IPCs) in late pupal eye patterning in Drosophila (Artero et al., 2001; Bao and Cagan, 2005; Araujo and Logothetis, 2010; Shelton et al., 2009). Here, we show that Hbs physically associates with Psn and is required for its processing and/or stability. In hbs mutant tissue, Psn-mediated cleavage and subsequent N-signaling is compromised. In addition to N, Psn cleaves Amyloid precursor protein (APP) to generate beta amyloid(Aβ) peptides, the primary components of amyloid plaques implicated in Alzheimer's disease (De Strooper, 2003; Thinakaran et al., 1996). Accordingly, Hbs also affects the cleavage of the Drosophila Amyloid precursor protein-like (Appl) protein. It is likely that the intracellular region of Hbs is acting in the Psn associated function as a hbs mutant allele causing a truncation of the intracellular region, hbs459, shows similar N-signaling associated phenotypes as the general Hbs knock-down, Mpreover, we did not detect a requirement for the cell-adhesion binding partners of Hbs, Roughest and Kirre (the Drosophila Neph orthologues), in N-signaling (not shown), again suggesting that Hbs/Nephrin regulation of N-signaling is independent of its role in cell adhesion.
Hbs and γ–secretase complex function
Regulated membrane proteolysis is important for many signaling pathways. γ–secretase is a large multi-protein complex, whose function is to catalyze cleavage of type I membrane proteins, including the well-studied N receptor and APP. The γ–secretase complex is 220 kDa protein complex, consisting of Psn, Nct, APH-1 (anterior pharynx defective) and PEN-2 (presenilin enhancer 2). Psn constitutes the catalytic subunit of the complex, while Nct has been suggested to function in substrate recognition and Psn stability (Chung and Struhl, 2001; De Strooper and Annaert, 2010; Hu et al., 2002; Lopez-Schier and St Johnston, 2002; Struhl and Greenwald, 1999). Our study highlights the role of Hbs in N and Appl by affecting Psn maturation, whether mammalian Nephrin can also affect APP or other type I membrane protein cleavage remains unknown.
What is the function of Hbs in the γ–secretase complex? Nct, Aph-1 and Pen-2 have been shown to affect the stability of Psn (Lopez-Schier and St Johnston, 2002). Hbs also appears to be required for the proper maturation and/or stability of Psn (Figure 6J-L). We did not see a change in Notch expression levels in hbs loss-of-function scenarios (e.g. when hbs-IR was expressed in the posterior compartment), suggesting that Hbs acts on Psn rather than N and/or Appl. Furthermore, co-localization of Psn and Hbs in imaginal discs suggests that Hbs and Psn are present in the same subcellular vesicular compartment(s). This is consistent with previous studies, showing that most of Psn inside cells accumulates in ER and vesicular structures (Hu et al., 2002). Significant amounts of Hbs are present in intracellular puncta and this pool of Hbs might affect Psn processing or stability, possibly in conjunction with other γ-secretase components. Thus Hbs is likely to affect N and Appl cleavage via its effect on Psn.
Experiments in Drosophila suggest that overexpression of Hbs gives similar phenotypes to hbs loss-of-function. One possibility to explain this conundrum could be that overexpression of Hbs acts as a dominant negative. Indeed removing a genomic copy of hbs in the Hbs overexpression background enhances its phenotype (data not shown), consistent with this model. This is similarly consistent with existing data for Psn: overexpression of Psn causes similar phenotypes as psn loss-of-function (Ye and Fortini, 1999). It has been hypothesized that overexpression of Psn forms insoluble complexes that down regulate signaling. Indeed, several studies have highlighted that all four components of the γ-secretase complex have to be expressed for it to be active (Edbauer et al., 2003; Stempfle et al., 2010). Thus, it is likely that an excess of one component of the complex renders it less active. These results are consistent with and supporting our hypothesis that Hbs behaves in a similar manner to components of the γ-secratase complex that are important for Psn processing.
Transcriptional regulation of hbs expression
In contrast to Nct and Psn, which appear to be uniformly expressed in Drosophila (Nowotny et al., 2000; Ye and Fortini, 1998), hbs expression is highly regulated and under the control of several signaling pathways in different tissues (although hbs is also expressed constitutively at low levels in all cells). As such, different endogenous levels of Hbs could affect the stability/processing of Psn and the γ–secretase complex, boosting its activity where needed. This is notion consistent with the expression pattern of hbs, which is upregulated where N-signaling is highest, for example at the wing margin (via Wg-signaling, Figure S3) or during eye development in ommatidial preclusters (likely via Egfr/Ras signaling, not shown). In developing embryos hbs expression is regulated via N and Ras signaling (Artero et al., 2001). As increased hbs expression correlates with cells that require high N-signaling levels (wing margin, SOP clusters [Suppl. Fig. S1], or ommatidial preclusters), hbs might integrate input from several signaling pathways to modulate Psn activity and hence N-signaling levels.
Nephrins and Notch signaling
In mammalian cells, co-expression of Nephrin resulted in increased Notch reporter activity (Figure 5C-D) when membrane tethered form of Notch was expressed, while soluble Notchintra was not affected. This behavior was very similar to the equivalent experiments in Drosophila S2 cells and in vivo. Thus, there is a likely mechanistic conservation of Hbs and Nephrin function to regulate N-signaling activity via the γ–secretase complex. In mammals, like in Drosophila, most studies related to Nephrin family members have focused on their role in cell adhesion with focus on the kidney. In kidney podocytes, Nephrin has been studied extensively in the context of the slit diaphragm, involved in filtering blood (Holzman et al., 1999; Ruotsalainen et al., 1999). Mutations in Nephrin are associated with congenital nephritic syndrome of the Finnish-type (Kallinen et al., 2001; Koziell et al., 2002). Our study supports the idea that Nephrin family members play important roles in other tissues. This is consistent with a recent report where Nephrin has been shown to be involved in cardiac function (Wagner et al., 2011), corroborating the importance of Nephrin in biological contexts where Notch signaling plays an important role during development.
Experimental Procedures
Drosophila Stocks
Fly crosses were grown on standard food at 25°C unless otherwise stated. The following stocks were used and sources are as indicated:
sevGal4; tubGal4; mδ0.5Gal4, UAS-GFP; enGal4; pnrGal4, hs-FLP; actin>y>Gal4, UAS-GFP;
Rab5GFP; Rab11GFP; and Rab7GFP are our lab stocks;
UAS-Hbs, hbsLacZ, and hbs459 (R. Cagan);
tub>PsnHA (G. Struhl);
nctA7, sevNintra and sevNΔECD (Mark Fortini);
UAS-hbs-IR and UAS-wg-IR were from the Vienna Drosophila RNAi Center;
Df(2R)ED2423, hbsEP, N55e11, psn143, psn9, UAS-appl-IR (stock no. 28043), UAS-dicer2, kuze29-4 and w1118; P{w[+mc]-UAS-mycAPP.LV} (II) were from the Bloomington stock center.
hbsj5 was generated using a FLP-recombinase mediated excision of two piggyBac/FRT insertions (PBac{RB}e04215 and PBac{WH}f04302) as described (Parks et al., 2004).
Immunofluorescence and histology
Third instar larvae or 24hr old white pupae were dissected in ice cold PBS and fixed in 4% paraformaldehyde for 20 mins. After washing in PBT (PBS+0.1% Triton), discs were incubated in primary antibody over night at 4°C followed by washes in PBT, secondary antibody incubation for 2hrs, and again washed in PBT and subsequently mounted in Mowiol. The following primary antibodies were used: mouse anti-Cut, rat anti-DE Cadherin and rat anti-Elav (DSHB), rabbit anti-Hbs (gift from Karl Fischbach), and anti-GFP (Molecular Probes).
S2 cells were grown on coverslips placed in 6 well plates containing Schneider's Drosophila medium (Gibco, Invitrogen) supplemented with 10% FBS and antibiotics. For immunofluorescence cells were washed in PBS, fixed in 4% paraformaldehyde for 20 mins at RT, permeabilized in PBT (0.1% Triton-X 100 in PBS) for 10 mins and “blocked” with 0.1% BSA in PBS. Cells were then incubated with mouse anti-Myc and rat anti-HA antibodies (Santa Cruz and Roche) overnight at 4°C, followed by washes with PBS. Cells were then incubated with secondary antibodies and Hoechst dye (to stain nuclei), for 2 hrs at room temperature, followed washes in PBS and mounted in Vectashield (Vector Labs).
Western blotting and co-immunoprecipitation
For co-immunoprecipitation experiments, S2 cells were transfected with Hbs-HA and/or Psn-Myc (gift from Mark Fortini) or Fz-Myc using Effectene (Qiagen) according to manufacturers instructions. Cells were harvested after 48 hrs, washed and lysed in ice-cold lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 5 mM β-glycerophosphate, protease inhibitor cocktail I and II (Sigma) and 1% Triton-X100). Lysed samples were immunoprecipitated using mouse anti-Myc (9E10, Santa Cruz Biotechnology) overnight at 4°C and later protein-antibody complexes were pulled down using Protein G-Agarose. Beads were washed in lysis buffers and Western blots were carried out with immunoprecipitated samples using rat anti-HA, mouse anti-γ-Tubulin or mouse anti-Myc antibodies.
For in-vivo Notch cleavage analyses, 5 larval eye/brain complexes were lysed in ice cold hypotonic lysis buffer (10 mM KCl, 20 mM Tris (pH 7.5), 0.1% mercaptoethanol, 1 mM EDTA along with protease and phosphatase inhibitors[Sigma]). Supernatant from these extracts wereresolved and subjected to standard Western blotting procedures using mouse anti Notchintra (DSHB, 1:500) and mouse anti γ-tubulin (Sigma, 1:1000) antibodies.
Notch reporter assays
S2 cells were transfected with N-responsive Su(H) sites NRE plasmid (gift from Sarah Bray) together with pMTNotchFull length or pMTNotchintra (from DGRC) either alone or along with varying conc. of Hbs (200ng and 400ng). Renilla-expressing plasmid was co-transfected as an internal control. 24 hrs after transfection, N-constructs were induced with 600μM CuSO4 for 18 hrs. Cells were then lysed and luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega). Reporter activity was calculated via the luciferase/renilla values. In mammalian 293T cells, CBF reporter (gift from Reshma Taneja) was co-transfected with Nephrin (gift from Christan Faul) along with either mNTM-ICD or mNICD. 48 hrs after transfection cells were lysed and luciferase activity monitored as described above.
Supplementary Material
Highlights.
- Loss of hbs results in classical Notch-signaling associated phenotypes
- Hbs is required for Psn mediated Notch cleavage
- Hbs regulates Psn processing and possibly stability of γ-secretase
- Hbs affects the processing/activity of Drosophila APP-like protein
Acknowledgements
We thank the Bloomington Stock Center, DSHB, Spyros Artavanis-Tsakonas, Sujin Bao, Sarah Bray, Ross Cagan, Mark Fortini, Christian Faul, Edward Giniger, Gary Struhl, and Reshma Taneja for various fly stocks, plasmid DNAs, and antibodies, and all Mlodzik lab members for helpful suggestions and discussion. We are grateful to Karl Fischbach for a gift of the limited Hbs antibody. We thank Mark Fortini, William Gault, Edward Giniger, Ruth Johnson, Lindsay Kelly, and Robert Krauss for helpful comments on drafts of the manuscript, and Aurore Dussert, Susanna Franks, Joyce Lau, and Sophy Okello for technical help. Confocal laser microscopy was performed at the MSSM Microscopy SRF, supported by a NIH/National Cancer Institute shared instrumentation grant. This research was supported by NIH grants from the NEI and NIGMS to MM.
References
- Artavanis-Tsakonas S, Muskavitch MA. Notch: the past, the present, and the future. Curr Top Dev Biol. 2010;92:1–29. doi: 10.1016/S0070-2153(10)92001-2. [DOI] [PubMed] [Google Scholar]
- Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development. 2001;128:4251–4264. doi: 10.1242/dev.128.21.4251. [DOI] [PubMed] [Google Scholar]
- Artero RD, Monferrer L, Garcia-Lopez A, Baylies MK. Serpent and a hibris reporter are co-expressed in migrating cells during Drosophila hematopoiesis and Malpighian tubule formation. Hereditas. 2006;143:117–122. doi: 10.1111/j.2006.0018-0661.01928.x. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Bray S, Musisi H, Bienz M. Bre1 is required for Notch signaling and histone modification. Dev Cell. 2005;8:279–286. doi: 10.1016/j.devcel.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Carmine-Simmen K, Proctor T, Tschape J, Poeck B, Triphan T, Strauss R, Kretzschmar D. Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol Dis. 2009;33:274–281. doi: 10.1016/j.nbd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HM, Struhl G. Nicastrin is required for Presenilin-mediated transmembrane cleavage in Drosophila. Nat Cell Biol. 2001;3:1129–1132. doi: 10.1038/ncb1201-1129. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Garcia-Bellido A, Bray SJ. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development. 1996;122:359–369. doi: 10.1242/dev.122.1.359. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Novel research horizons for presenilins and gamma-secretases in cell biology and disease. Annu Rev Cell Dev Biol. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- del Alamo D, Mlodzik M. Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Dev Cell. 2006;11:887–894. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Dworak HA, Charles MA, Pellerano LB, Sink H. Characterization of Drosophila hibris, a gene related to human nephrin. Development. 2001;128:4265–4276. doi: 10.1242/dev.128.21.4265. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Fanto M, Mlodzik M. Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature. 1999;397:523–526. doi: 10.1038/17389. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Fortini ME. Different cofactor activities in gamma-secretase assembly: evidence for a nicastrin-Aph-1 subcomplex. J Cell Biol. 2003;161:685–690. doi: 10.1083/jcb.200304014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ye Y, Fortini ME. Nicastrin is required for gamma-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2:69–78. doi: 10.1016/s1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- Kallinen J, Heinonen S, Ryynanen M, Pulkkinen L, Mannermaa A. Antenatal genetic screening for congenital nephrosis. Prenat Diagn. 2001;21:81–84. doi: 10.1002/1097-0223(200102)21:2<81::aid-pd1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Miyauchi N, Suzuki K, Han GD, Orikasa M, Shimizu F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology (Carlton) 2006;11:274–281. doi: 10.1111/j.1440-1797.2006.00583.x. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P. Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet. 2002;11:379–388. doi: 10.1093/hmg/11.4.379. [DOI] [PubMed] [Google Scholar]
- Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol. 2004;269:1–17. doi: 10.1016/j.ydbio.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Loewer A, Soba P, Beyreuther K, Paro R, Merdes G. Cell-type-specific processing of the amyloid precursor protein by Presenilin during Drosophila development. EMBO Rep. 2004;5:405–411. doi: 10.1038/sj.embor.7400122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Drosophila nicastrin is essential for the intramembranous cleavage of notch. Dev Cell. 2002;2:79–89. doi: 10.1016/s1534-5807(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Munoz-Soriano V, Belacortu Y, Durupt FC, Munoz-Descalzo S, Paricio N. Mtl interacts with members of Egfr signaling and cell adhesion genes in the Drosophila eye. Fly (Austin) 2011;5:88–101. doi: 10.4161/fly.5.2.15457. [DOI] [PubMed] [Google Scholar]
- Naruse S, Thinakaran G, Luo JJ, Kusiak JW, Tomita T, Iwatsubo T, Qian X, Ginty DD, Price DL, Borchelt DR, et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron. 1998;21:1213–1221. doi: 10.1016/s0896-6273(00)80637-6. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Nowotny P, Gorski SM, Han SW, Philips K, Ray WJ, Nowotny V, Jones CJ, Clark RF, Cagan RL, Goate AM. Posttranslational modification and plasma membrane localization of the Drosophila melanogaster presenilin. Mol Cell Neurosci. 2000;15:88–98. doi: 10.1006/mcne.1999.0805. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Yao M, Nowotny P, Mumm J, Zhang W, Wu JY, Kopan R, Goate AM. Evidence for a physical interaction between presenilin and Notch. Proc Natl Acad Sci U S A. 1999;96:3263–3268. doi: 10.1073/pnas.96.6.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Martin-Morris L, Luo LQ, White K. A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci U S A. 1989;86:2478–2482. doi: 10.1073/pnas.86.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Shellenbarger DL, Mohler JD. Temperature-sensitive periods and autonomy of pleiotropic effects of l(1)Nts1, a conditional notch lethal in Drosophila. Dev Biol. 1978;62:432–446. doi: 10.1016/0012-1606(78)90226-9. [DOI] [PubMed] [Google Scholar]
- Shelton C, Kocherlakota KS, Zhuang S, Abmayr SM. The immunoglobulin superfamily member Hbs functions redundantly with Sns in interactions between founder and fusion-competent myoblasts. Development. 2009;136:1159–1168. doi: 10.1242/dev.026302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S, Roch F, Campuzano S. The metalloprotease-disintegrin Kuzbanian participates in Notch activation during growth and patterning of Drosophila imaginal discs. Development. 1997;124:4769–4779. doi: 10.1242/dev.124.23.4769. [DOI] [PubMed] [Google Scholar]
- Stempfle D, Kanwar R, Loewer A, Fortini ME, Merdes G. In vivo reconstitution of gamma-secretase in Drosophila results in substrate specificity. Mol Cell Biol. 2010;30:3165–3175. doi: 10.1128/MCB.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Polarity determination in the Drosophila eye. Curr Opin Genet Dev. 1999;9:442–446. doi: 10.1016/S0959-437X(99)80067-7. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, Struhl G. Decoding vectorial information from a gradient: sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development. 1999;126:5725–5738. doi: 10.1242/dev.126.24.5725. [DOI] [PubMed] [Google Scholar]
- Wagner N, Morrison H, Pagnotta S, Michiels JF, Schwab Y, Tryggvason K, Schedl A, Wagner KD. The podocyte protein nephrin is required for cardiac vessel formation. Hum Mol Genet. 2011;20:2182–2194. doi: 10.1093/hmg/ddr106. [DOI] [PubMed] [Google Scholar]
- Wen C, Metzstein MM, Greenwald I. SUP-17, a Caenorhabditis elegans ADAM protein related to Drosophila KUZBANIAN, and its role in LIN-12/NOTCH signalling. Development. 1997;124:4759–4767. doi: 10.1242/dev.124.23.4759. [DOI] [PubMed] [Google Scholar]
- Ye Y, Fortini ME. Characterization of Drosophila Presenilin and its colocalization with Notch during development. Mech Dev. 1998;79:199–211. doi: 10.1016/s0925-4773(98)00169-5. [DOI] [PubMed] [Google Scholar]
- Ye Y, Fortini ME. Apoptotic activities of wild-type and Alzheimer's disease-related mutant presenilins in Drosophila melanogaster. J Cell Biol. 1999;146:1351–1364. doi: 10.1083/jcb.146.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.