Summary
Mammalian Two-Pore Channels (TPC1, 2; TPCN1, TPCN2) encode ion channels in intracellular endosomes and lysosomes and were proposed to mediate endolysosomal calcium release triggered by the second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP). By directly recording TPCs in endolysosomes from wild-type and TPC double knockout mice, here we show that, in contrast to previous conclusions, TPCs are in fact sodium-selective channels activated by PI(3,5)P2, and are not activated by NAADP. Moreover, the primary endolysosomal ion is Na+, not K+, as had been previously assumed. These findings suggest that the organellar membrane potential may undergo large regulatory changes, and may explain the specificity of PI(3,5)P2 in regulating the fusogenic potential of intracellular organelles.
Keywords: Whole-endolysosome patch-clamp; Two-pore channel (TPC); PIKfyve; phosphoinositide; PI(3,5)P2; Na+ channel; lysosome; membrane trafficking
Introduction
Two-pore channel proteins (TPC1, 2; TPCN1, TPCN2) (Calcraft et al., 2009; Morgan et al., 2011) are localized in the intracellular endosomes and lysosomes (collectively endolysosomes) previously inaccessible to conventional patch clamp assays. Consistent with this localization, human genetic studies have identified TPC2 as a regulator of pigmentation (Sulem et al., 2008), and a number of recent studies suggest that TPCs mediate Ca2+ release from endolysosomes in response to an elevation of the potent Ca2+-mobilizing second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP) (Brailoiu et al., 2009; Calcraft et al., 2009; Ruas et al., 2010; Zong et al., 2009) (but also see ref. (Guse, 2009)). Unlike plasma membrane-localized NaV and CaV channels, the primary structures of TPCs contain two, instead of four, 6 transmembrane (6TM) domains (Yu and Catterall, 2004). Like NaV and CaV channels, they contain multiple positively charged amino acid residues in their voltage sensor domains, and negatively-charged amino acid residues in their pore domains, but their intracellular localization has prevented characterization of basic channel properties such as selectivity and gating.
PI(3,5)P2 is an endolysosome-specific phosphoinositide (PIP) of low abundance (Dove et al., 2009; Shen et al., 2011). Upon cellular stimulation PIKfyve/Fab1, PI 5-kinase phosphorylates PI(3)P to increase PI(3,5)P2 from low nM to μM concentrations (Dove et al., 2009; Shen et al., 2011). Human mutations in PI(3,5)P2-metabolizing enzymes and their regulators result in muscle and neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Charcot-Marie-Tooth (CMT-4B, CMT-4J) disease (Chow et al., 2007). PI(3,5)P2-deficient cells have enlarged endolysosomes/vacuoles, suggestive of impaired ion homeostasis and/or defective membrane trafficking (Chow et al., 2007; Dove et al., 2009; Kerr et al., 2010; Shen et al., 2011). We recently found that TRPML1 mediates PI(3,5)P2-dependent Ca2+ release from endolysosomes (Dong et al., 2010a). However, PI(3,5)P2 deficiency results in a much more severe phenotype than TRPML1 mutations, suggesting that there are additional PI(3,5)P2 effectors (Shen et al., 2011). Here, we find by direct patch-clamp of endolysosomal membranes, that PI(3,5)P2 specifically activates TPCs, and unexpectedly, that NAADP does not. TPC-mediated currents are selective for Na+, which we demonstrate is the predominant cation in the lysosome. TPCs represent the first intracellular Na+-selective channels, and suggest a new model for ion channel control of endolysosomal fusion.
Results
PI(3,5)P2 activation of a large endogenous current in the endolysosome
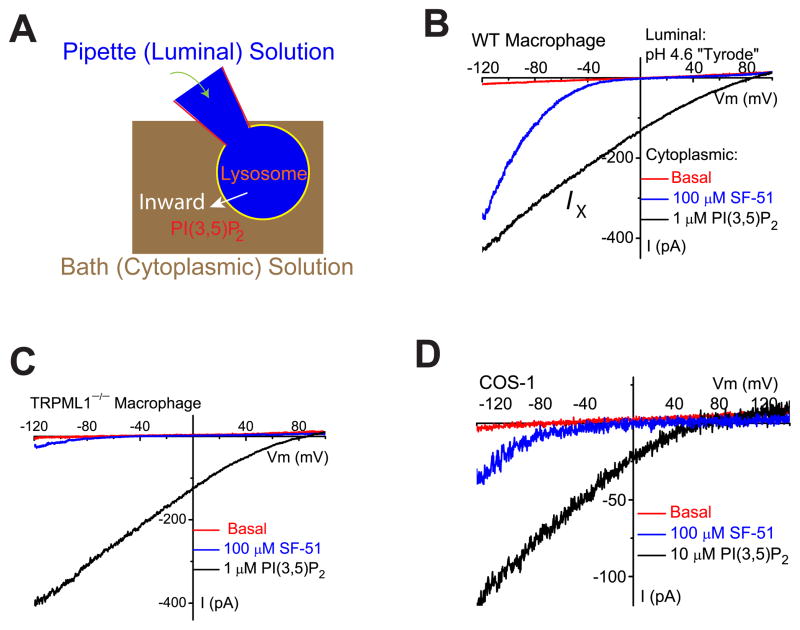
Cells were pretreated with vacuolin-1, a lipid-soluble polycyclic triazine (Huynh and Andrews, 2005) that can selectively increase the size of endosomes and lysosomes from < 0.5 μm to up to 5 μm (Dong et al., 2010a). The enlarged endolysosomes were manually isolated and then patch clamped in the whole-endolysosome configuration (Fig. 1A; see Suppl. fig. S1). We previously reported that TRPML1 was the primary PI(3,5)P2-activated conductance (reversal potential, Erev ~ 0 mV) in the endolysosomes of human fibroblasts (Dong et al., 2010a). However, in several other cell types including skeletal muscles (data not shown) and macrophages, we observed that bath (cytoplasmic) application of diC8 PI(3,5)P2 (abbreviated as PI(3,5)P2), a water-soluble analog of PI(3,5)P2 (Dong et al., 2010a), activated a distinct whole-endolysosome conductance with an Erev > + 60 mV (defined as IX; Fig. 1B). Strongly inwardly-rectifying TRPML-like currents (ITRPML-L; (Dong et al., 2010a)) were also present in macrophages, but PI(3,5)P2-activated ITRPML-L was often masked by IX due to its positive Erev. ITRPML-L could be activated by SF-51 (100 μM (Grimm et al., 2010); Fig. 1B). ITRPML-L, but not IX, was dramatically reduced in TRPML1−/− macrophages (Fig. 1C). In 9 out of 23 enlarged endolysosomes isolated from non-transfected COS-1 cells, high concentrations of PI(3,5)P2 (10 μM) activated IX, which was distinct from ITRPML-L activated by 1 μM PI(3,5)P2 (Dong et al., 2010a) or SF-51 (Fig. 1D).
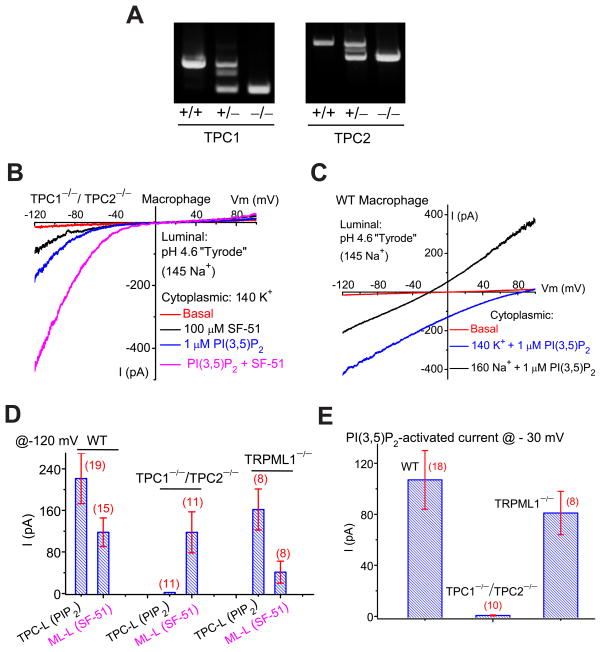
Figure 1. PI(3,5)P2 activates endogenous TRPML1-independent inward currents in endolysosomes.
(A). Illustration of the whole-endolysosome recording configuration. Pipette (luminal) solution was a standard external (modified Tyrode’s) solution adjusted to pH 4.6 to mimic the acidic environment of the lysosomal lumen. Bath (internal/cytoplasmic) solution was a K+-based internal solution (140 mM K+-gluconate). Note that the inward current indicates cations flowing out of the endolysosome (arrow). (B). Bath application of PI(3,5)P2 (diC8, 1 μM) to the cytoplasmic side of enlarged endolysosome/vacuoles isolated from vacuolin-treated WT primary macrophage cells activated whole-endolysosome currents (296 ± 28pA/pF at −120mV, n = 24 vacuoles/endolysosomes) with positive Erev (79 ± 2.4mV, n = 20). K+-based cytoplasmic/bath solution contained (in mM) 140K+/4Na+/2Mg2+ (pH 7.2, free Ca2+ ~ 100 nM); luminal/pipette solution was a pH 4.6 modified Tyrode solution, which contained (in mM) 145Na+/5K+/1Mg2+/2 Ca2+ (pH 4.6); the equilibrium potential of Na+ (ENa) was estimated to be ~ +90mV. Inwardly rectifying TRPML-like currents (ITRPML-L) with Erev = 3.7 ± 1.7 mV (n = 20) were induced by a TRPML-specific small molecule agonist (SF-51) in the same vacuoles (blue trace). (C). PI(3,5)P2 activated a current with a positive Erev in an enlarged endolysosome/vacuole isolated from a TRPML1−/− primary macrophage cell. (D). PI(3,5)P2 (10 μM) activated whole-endolysosome current (from 30 to 420 pA measured at −120 mV) with positive Erev in enlarged endolysosome/vacuoles isolated from non-transfected COS-1 cells. Note the small ITRPML-L (Erev ~ 0mV) activated by SF-51 in the same vacuoles. Data are presented as the mean ± standard error of the mean (SEM). See also Figure S1.
PI(3,5)P2 activates recombinant TPC1 and TPC2 channels in the endolysosome
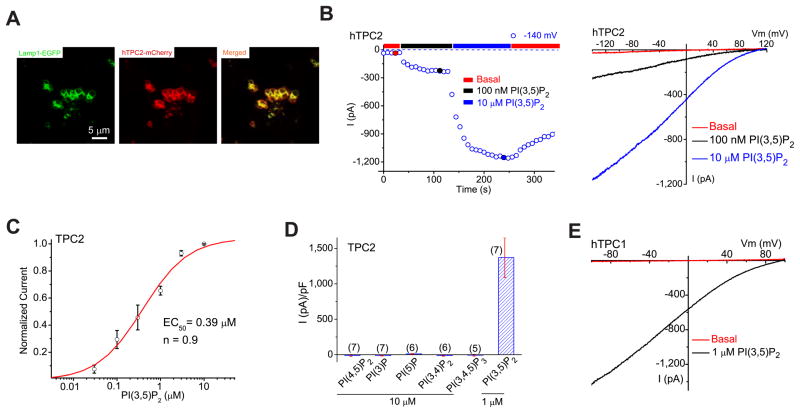
To search for the identity of the protein mediating IX, a number of fluorescently-tagged putative intracellular channels or transporter-like lysosomal membrane proteins were transfected into COS-1 cells. As described below, endolysosomes from TPC1- and TPC2-transfected cells exhibit large Ix. The majority (> 80%) of vacuolin-1-treated TPC2-positive vacuoles were Lamp-1+ (Fig. 2A), confirming that TPC2-positive vacuoles were enlarged late endosomes and lysosomes (LELs). In TPC2 (hTPC2)-positive enlarged LELs isolated from transfected COS1 cells, little or no basal currents were detected in the whole-endolysosome configuration (Fig. 2B). Bath application of PI(3,5)P2 rapidly activated hTPC2-mediated currents (ITPC2; Erev = + 83 ± 3 mV; Fig. 2B), but not those that expressed a mutant hTPC2 carrying a charge-reversal mutation in the putative pore domains (D276K; see Suppl. fig. S2A, B); ITPC2 gradually declined upon the washout of PI(3,5)P2 (Fig. 2B) with variable time courses (depending on treatment time). The I-V and Erev of ITPC2 are similar to the endogenous PI(3,5)P2-activated IX.
Figure 2. PI(3,5)P2 activates recombinant TPCs in endolysosomes.
(A). TPC2 proteins are localized in Lamp1-positive late endosomes and lysosomes in COS-1 cells that were transfected with TPC2 and Lamp-1 fusion proteins and treated with vacuolin-1. (B). PI(3,5)P2 activated a large whole-endolysosome current with Erev > + 80mV in an EGFP (hTPC2)-positive endolysosome isolated from an hTPC2-EGFP-transfected COS-1 cell. Whole-endolysosome currents were elicited by repeated voltage ramps (−140 to +140 mV; 400 ms) with a 4s interval between ramps; current amplitudes measured at −140 mV were used to plot the time course of activation. The right panel shows representative I-V traces of hTPC2-mediated whole-endolysosome currents (IhTPC2) before (red; −20 ± 4pA/pF at −120 mV, n = 9) and after (black and blue) PI(3,5)P2 bath application at three different time points, as indicated in the left panel (red, blue and black circles). Only a portion of the voltage protocol is shown; holding potential (HP) = 0 mV. (C). Dose-dependence of PI(3,5)P2-dependent activation (EC50 = 390 ± 94 nM, Hill slope (n) = 0.9, n= 13 vacuoles). (D). Specific activation of TPC2 by PI(3,5)P2 (in 1 μM), but not other PIPs (all in 10 μM). On average, ITPC2 in the presence of 1μM PI(3,5)P2 was 1410 ± 360pA/pF at −120mV (n = 7). (E). Activation of IhTPC1 by 1 μM PI(3,5)P2. Data are presented as the mean ± SEM. See also Figure S2.
PI(3,5)P2-dependent activation of ITPC2 was dose-dependent (EC50= 390 ± 94 nM; Fig. 2C). IhTPC2 was inhibited > 80% by the PI(3,5)P2 chelators (Nilius et al., 2008; Suh and Hille, 2008) poly-L-lysine and anti-PI(3,5)P2 antibody (Suppl. fig. S2C, D). Other phosphoinositides, PI(3)P, PI(5)P, PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3, did not activate ITPC2 (10 μM; Fig. 2D Suppl. fig. S2E). Thus PI(3,5)P2 activated ITPC2 with striking specificity. In contrast, 1 μM PI(3,5)P2 failed to activate the lysosome-localized (Lange et al., 2009) recombinant TRPM2 channel (Suppl. fig. S2F) or modulate the endogenous outward currents that were present in a subset of endolysosomes isolated from INS1 pancreatic β-cells (Suppl. fig. S2G). TPC1, also localized in the endolysosome (Calcraft et al., 2009) (but primarily in Lamp-1-negative compartments; Suppl. fig. S2A), was also activated by PI(3,5)P2 (Fig. 2E).
TPC-mediated currents are Na+-selective
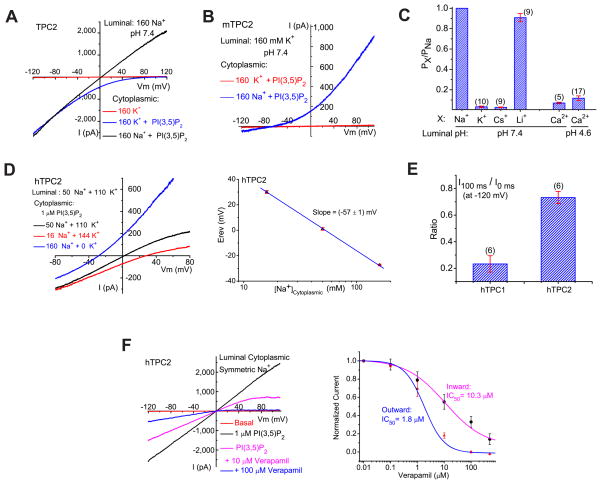
The measured Erev of ITPC under standard recording conditions (with a low pH modified Tyrode’s solution in the pipette/lumen and a K+-based solution in the bath/cytosol) dictates that the channels are selective for Na+, Ca2+, or H+, but not K+. Increasing the luminal pH from 4.6 to 7.4 had minimal effects on ITPC2 (Fig. 3A; Suppl. fig. S3A) and ITPC1. Conversely, replacement of luminal cations (Na+, K+, Mg2+, and Ca2+) with NMDG+ at pH 4.6 completely abolished inward ITPC2 (Suppl. fig. S3B), suggesting that ITPC2 is impermeable to H+ or NMDG+. Under bi-ionic conditions (luminal Na+, pH 7.4; cytoplasmic K+), the Erev of ITPC2 was + 89 ± 5 mV (n=8; see Fig. 3A). In contrast, under reversed bi-ionic conditions (luminal K+, pH 7.4; cytoplasmic Na+), the Erev of ITPC2 was −68 ± 3 mV (n = 5; see Fig. 3B). These results indicated that ITPC2 was selective for Na+ over K+. Consistent with this conclusion, switching cytoplasmic K+ to Na+ in the presence of luminal Na+ resulted in a leftward shift of the Erev and the appearance of large outward currents (Fig. 3A). Addition of 2 mM Ca2+ to the luminal side of the symmetric Na+ solutions did not result in any significant change of the Erev or the amplitude of the inward currents (see Suppl. fig. S3C), suggesting that luminal Ca2+ contributed insignificantly to inward ITPC2. Consistently, under bi-ionic conditions (luminal isotonic Ca2+, pH 4.6 or 7.4; cytoplasmic Na+), the Erev of ITPC2 was −68 ± 2 mV (n = 12; see Suppl. fig. S3D), in dramatic contrast to the Erev of ITRPML1 (+ 47 ± 2 mV, n = 3; see Suppl. fig. S3E). With cytoplasmic K+, however, a small inward ITPC2 could be resolved with luminal isotonic Ca2+ (105 mM), but not NMDG+ (Suppl. fig. S3B, D), suggesting a very limited Ca2+ permeability for TPC2. By estimating the permeability ratios based on Erev measurements, we determined the sequence of ion permeability or selectivity of ITPC2 as Na+>Li+≫Ca2+≫K+ ~ Cs+ (Fig. 3C & Suppl. fig. S3F). PCa/PNa and PK/PNa were about 0.10 and 0.03, respectively, which are similar to the values for canonical NaV channels (0.08–0.11) (Favre et al., 1996; Hille, 1972). Consistent with the low PK/PNa, with a mixture of K+ and Na+ at both luminal and cytoplasmic sides, the Na+-dependence of Erev was fit with a Nernstian slope of 57 mV per 10-fold change of [Na+]cyto (Fig. 3D). Taken together, these ion substitution analyses demonstrate that TPC2 is a highly Na+-selective channel in the endolysosome.
Figure 3. PI(3,5)P2-activated TPC currents are Na+-selective.
(A). PI(3,5)P2-activated ITPC2 (Erev= + 89 mV ± 5mV, n = 8) under bi-ionic conditions with luminal/pipette Na+ and cytoplasmic/bath K+. Large outward IhTPC2 was observed in cytoplasmic Na+. (B). ImTPC2 Erev (−68 ± 3mV, n = 5) under bi-ionic conditions with luminal K+ and cytoplasmic Na+. (C). Relative cationic permeability ratios of IhTPC2 based on Erev measurement under bi-ionic conditions. (D). Na+-dependence of IhTPC2 Erev. The left panel shows I-V relations of IhTPC2 with cytoplasmic solutions containing various concentrations of Na+ and K+. (E). Distinct inactivation kinetics of IhTPC2 and IhTPC1 at −120 mV. (F). Verapamil inhibited outward and inward IhTPC2 with different dose-dependencies under luminal and cytoplasmic symmetric Na+ (luminal pH 7.4). Data are presented as the mean ± SEM. See also Figure S3.
Because the S4 segments of TPC1 and TPC2 contain several positively-charged amino acid residues, we investigated the voltage-dependence of ITPC. Unlike canonical NaV and CaV channels, ITPC was not directly activated by membrane depolarization. Instead, in response to a step voltage protocol, PI(3,5)P2-activated ITPC inactivated at negative voltages (Suppl. fig. S3G), with ITPC1 exhibiting faster inactivation than ITPC2 (Fig. 3E). Inactivation recovered rapidly after a brief pulse to positive voltages (Suppl. fig. S3H). Despite being Na+ selective, ITPC2 was insensitive to the NaV blocker, TTX (Suppl. fig. S3I), but was sensitive to low concentrations of the nonselective CaV blocker, verapamil, in a voltage-dependent manner (Fig. 3F).
Na+ is the major cation in the lysosome
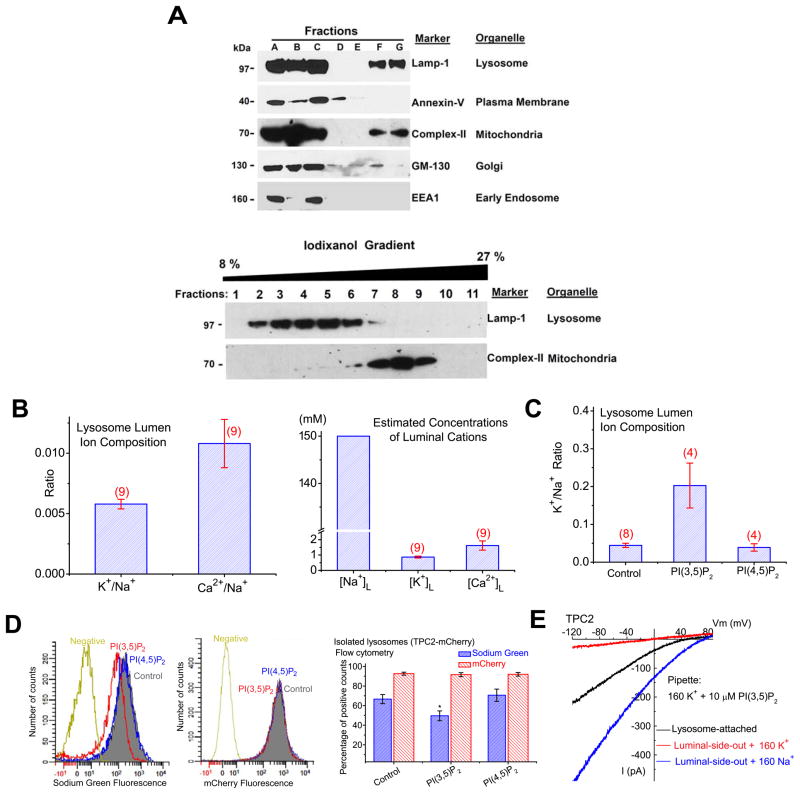
The existence of Na+-selective channels in the lysosome was unexpected because the lysosomal lumen, like the cytosol and the ER lumen (Morgan et al., 2011), has been presumed to contain high K+ and low Na+ (Morgan et al., 2011; Steinberg et al., 2010), suggesting the lack of a significant Na+ or K+ concentration gradient across the lysosomal membrane. To directly measure the ionic composition of the lysosome lumen, we enriched the lysosome fraction of HEK293T cells using density gradient centrifugation (Dong et al., 2010a; Graves et al., 2008b) (Suppl. fig. S4A; Fig. 4A), and then determined the ratios of major cations (Na+, K+, Ca2+, and Mg2+) using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) analysis. All centrifugation steps were performed at 4 °C (1 h in the Percoll density gradient + 2.5 h in the iodixanol gradient; see Suppl. fig. S4A). At this temperature, the rate of ion transport across the lysosomal membrane is expected to be extremely low. In addition, the sucrose -based homogenization buffer contains few ions. Thus, lysosomal ion transporters/exchangers are not likely to be operative. Hence, we presume that the lysosomal ion composition is largely maintained during the isolation procedure. Similar approaches have been used to determine ionic compositions in a number of intracellular organelles, including mitochondria and synaptic vesicles (Cohn et al., 1968; Schmidt et al., 1980). Although the absolute concentrations of ions could not be accurately measured due to the lack of information about lysosome volume, this approach allowed us to determine the relative abundance/ratios of the total, but not free ions in the lumen. Interestingly, the K+/Na+ and Ca2+/Na+ ratios were only about 0.01 (Fig. 4B), which were not significantly affected by the trace amount of ions in the buffer (Suppl. fig. S4B). Similar results were obtained from human fibroblasts and mouse macrophages. Thus Na+ is the predominant cation in the lumen of the lysosome (estimated to be ~ 140–150 mM, assuming that its lumen is iso-osmotic relative to the cytosol, and all the cations are osmotically-active) (Fig. 4B), indicating that in contrast to previous indirect measurements (Morgan et al., 2011; Steinberg et al., 2010), a large Na+ concentration gradient is present across the lysosomal membrane.
Figure 4. Na+ is the major cation in the lysosome.
(A). Western blotting was performed for each fraction (A–G) using various organelle markers: Lamp-1 for the lysosome; annexin-V for the plasma membrane; complex II for the mitochondria; GM-130 for the Golgi; EEA1 for the early endosome. Cellular fractionation protocol is described in Suppl. Fig. S4. Centrifugation of cell homogenate (fraction A) of HEK293T cells resulted in a pellet (fraction B) and a supernatant (fraction C). The supernatant was then layered over a discontinued gradient containing a cushion of 2.5 M sucrose and 18% Percoll in the HM buffer. Further centrifugation of the gradient resulted in the light membrane fraction (fraction D), the medium membrane fraction (fraction E), and the heavy membrane fraction (fraction G). Lower panel: fraction G was then layered over a discontinuous iodixonal (8–27%) gradient (fractions 1–11); Western blotting was performed for each fraction using Anti-Lamp-1 and Anti- complex II. (B). Ionic composition (Na+, K+, and Ca2+) in the lysosomal lumen of HEK293T cells determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Right panel: estimated concentrations of Na+, K+, and Ca2+ in the lysosome lumen; estimates were based on the assumptions that the lysosome lumen is iso-osmotic relative to the cytosol, and all the cations are osmotically-active. (C). Luminal K+/Na+ ratios were significantly increased for isolated lysosomes that were treated with PI(3,5)P2 (20 μM), but not PI(4,5)P2 (20 μM). (D). Left panel: application of PI(3,5)P2 (red), but not PI(4,5)P2 (blue) to isolated lysosomes decreased Sodium Green, but not mCherry fluorescence. Lysosomes were isolated from TPC2-mCherry-expressing HEK293 cells and loaded with Sodium Green dyes. “Control” and “Negative” indicate fluorescence levels in isolated lysosomes with and without Sodium Green dye loading, respectively. Right panel: decreased Sodium Green fluorescence intensity (reflecting luminal Na+ concentration) from PI(3,5)P2-treated lysosomes; data are presented as the percentage of lysosomes that were Sodium Green-positive. mCherry fluorescence remained constant upon PI(3,5)P2/PI(4,5)P2 application. (E). ITPC2 measured in lysosome-attached configuration. ITPC2 was activated in the lysosome-attached configuration with PI(3,5)P2 (10 μM) in the K+ pipette solution. ITPC2 was detected with luminal Na+, but not K+ upon excision into the luminal-side-out configuration. Note that the indicated voltages in the lysosome-attached configuration contained a contribution from the lysosomal membrane potential, for which no accurate measurement is available. The smaller current amplitude seen in the lysosome-attached configuration might be due to luminal [Na+] lower than 160 mM, relief from luminal inhibition, or both. Data are presented as the mean ± SEM. See also Figure S4.
To directly test whether the lysosomal lumen is a high Na+-compartment, isolated lysosomes were treated with TPC agonists. Application of PI(3,5)P2, but not PI(4,5)P2, significantly increased the K+/Na+ ratios (Fig. 4C). Similarly, in isolated TPC2-mCherry lysosomes loaded with Sodium Green (Suppl. fig. S4C), a Na+-sensitive dye (Carrithers et al., 2007), PI(3,5)P2, but not PI(4,5)P2 application significantly decreased Sodium Green fluorescence (Fig. 4D). These results suggest that sustained activation of TPCs may reduce luminal Na+ content. Consistent with the lysosome being a high Na+ compartment rather than high K+, when PI(3,5)P2 was included in the pipette solution, a large ITPC2 was observed under the lysosome-attached configuration (Fig. 4E) in which the lysosomal content and hence the Na+ gradient were maintained. Collectively, these results suggest that TPC-mediated Na+ flux in response to a localized increase in PI(3,5)P2 may rapidly depolarize endolysosomal membranes (luminal-side positive ~ + 30–110 mV at rest (Dong et al., 2010b; Morgan et al., 2011)), and facilitate membrane fusion (Suppl. fig. S4D). Consistently, TPC2-positive compartments were significantly enlarged in COS1 cells transfected with WT, but not D276K mutant hTPC2 (Suppl. fig. S4E, E′), suggesting that TPC2-expressing endolysosomes might have increased fusogenic potentials.
TPC1 and TPC2 underlie endogenous TPC-like currents in the endolysosome
Mice lacking TPC1 or TPC2 were generated and crossed to make double knockout (TPC1−/−/TPC2−/−) mice (Fig. 5A). In our targeting strategy, the first exons of the TPC1 and TPC2 genes were deleted, and the resulting recombinant transcripts failed to generate ITPC (Suppl. fig. S5A). In vacuoles isolated from TPC1−/−/TPC2−/− primary macrophages, PI(3,5)P2 activated ITRPML-L, but Na+-selective (ITPC-like; ITPC-L) currents were absent (Fig. 5B). In contrast, PI(3,5)P2 activated ITPC-L in the majority (> 90%) of vacuoles in WT (Fig. 5C) and TRPML1−/− (Fig. 1C) macrophages. The current amplitudes of ITRPML-L were not significantly different in TPC1−/−/TPC2−/− compared to WT macrophages (Figs. 1B, 5B, 5D), but were dramatically reduced in TRPML1−/− macrophages (Figs. 1C, 5D). Although ITRPML-L and ITPC-L are both activated by PI(3,5)P2, their I-V and Erev differed significantly from each other. When we analyzed PI(3,5)P2-activated currents at − 30 mV, large differences were noted between WT, TRPML1−/−, and TPC1−/−/TPC2−/− macrophages (Fig. 5E). Consistently, the PI(3,5)P2-activated current was selective for Na+ over Ca2+ (Suppl. fig. S5B). Collectively, these results suggest that ITPC-L is mediated by TPC2 and/or TPC1.
Figure 5. Genetic inactivation of TPC1 and TPC2 abolishes TPC currents in the endolysosome.
(A). PCR genotyping of TPC1 KO (TPC1−/−) and TPC2 KO (TPC2−/−) mice. (B). Lack of significant PI(3,5)P2-activated TPC-like current (ITPC-L) in vacuoles isolated from a TPC1−/−/TPC2−/− mouse macrophage. Instead, in 15/15 vacuoles, PI(3,5)P2 activated ITRPML-L that was further potentiated by SF-51. (C). An endogenous ITPC-L activated by PI(3,5)P2 (1 μM) in a vacuole isolated from a WT mouse macrophage cell. Switching the cytoplasmic solution from K+ to Na+ resulted in a leftward shift of Erev, and an increase of the current in the outward direction. (D). Summary of ITPC-L and ITRPML-L in WT, TPC1−/−/TPC2−/−, and TRPML1−/− macrophages. (E). Summary of PI(3,5)P2-activated whole-endolysosome inward currents in WT, TPC1−/−/TPC2−/−, and TRPML1−/− macrophages at −30mV. IPIP2 was 107 ± 23pA/pF (n= 18) and 0.7 ± 0.4pA/pF (n=10) for WT and TPC1−/−/TPC2−/− macrophages, respectively. Data are presented as the mean ± SEM. See also Figure S5.
TPCs are not activated by NAADP
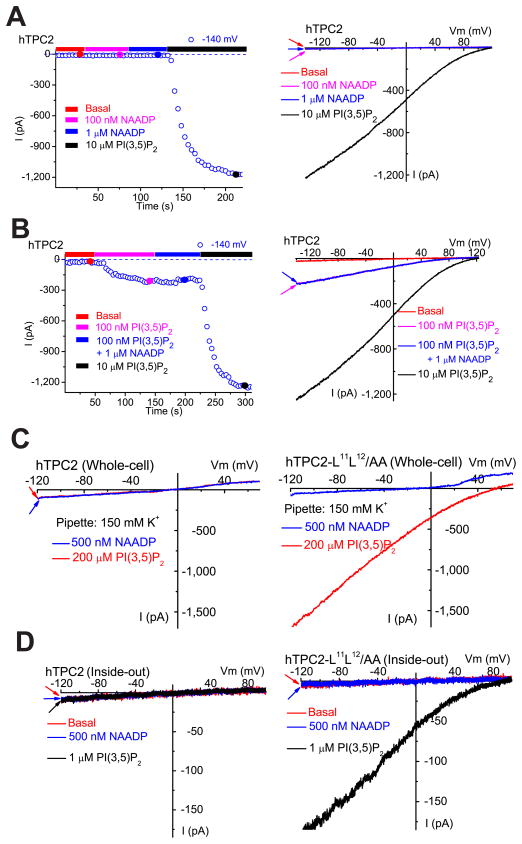
Since TPC1 and TPC2 were reportedly activated by NAADP in endolysosomes (Brailoiu et al., 2009; Calcraft et al., 2009; Morgan et al., 2011; Ruas et al., 2010; Zong et al., 2009), we measured endolysosomal currents after direct application of NAADP. Surprisingly, in TPC2-positive enlarged vacuoles, no significant current activation was seen with varying concentrations of NAADP (Fig. 6A; Suppl. fig. S6A). In contrast, PI(3,5)P2 (10 μM) reliably (> 90%) and robustly activated ITPC2 in the same vacuoles. NAADP (1–10 μM) also failed to modulate or desensitize ITPC2 that was activated by a low concentration of PI(3,5)P2 (100 nM; Fig. 6B). Similar results were seen with two NAADP analogs (4-methyl NAADP and 5-methyl NAADP) that induce Ca2+ release from sea urchin egg homogenates (Jain et al., 2010). ITPC1 was also insensitive to NAADP (Suppl. fig. S6A). To exclude the possibility that NAADP responsiveness was impaired in vacuolin-enlarged endolysosomes, we also tested NAADP on surface-expressed mutant hTPC2 channels (i.e. hTPC2-L11L12/AA; see ref. (Brailoiu et al., 2010)). However, plasma membrane ITPC2, which exhibited no notable difference in channel properties to lysosomal ITPC2, was also insensitive to NAADP in inside-out patches and in the whole-cell configuration (Fig. 6C, D). In contrast, NAADP (100 μM) activated NAADP-sensitive plasma membrane ITRPM2 (see ref. (Toth and Csanady, 2010)) (Suppl. fig. S6B, C), demonstrating that NAADP was active.
Figure 6. NAADP does not activate TPCs.
(A). NAADP (100 nM or 1 μM) failed to activate whole-endolysosome IhTPC2. In contrast, PI(3,5)P2 robustly activated IhTPC2 in the same vacuole. The right panel shows representative I-V traces of whole-endolysosome currents at 4 different time points (indicated by color-coded circles) shown in the left panel. (B). NAADP (1 μM) failed to modulate PI(3,5)P2–activated IhTPC2. (C). Pipette dialysis of PI(3,5)P2 (200 μM) or NAADP (500 nM) failed to elicit whole-cell current in HEK293T cells transfected with WT hTPC2. In contrast, pipette dialysis of PI(3,5)P2 (200 μM), but not NAADP (500 nM), activated whole-cell ITPC2 in HEK293T cells transfected with a surface-expressed hTPC2 mutant (hTPC2-L11L12/AA). (D). PI(3,5)P2, but not NAADP, activated ITPC2-LL-AA in inside-out macro-patches isolated from hTPC2-L11L12/AA -transfected HEK293T cells. Data are presented as the mean ± SEM. See also Figure S6.
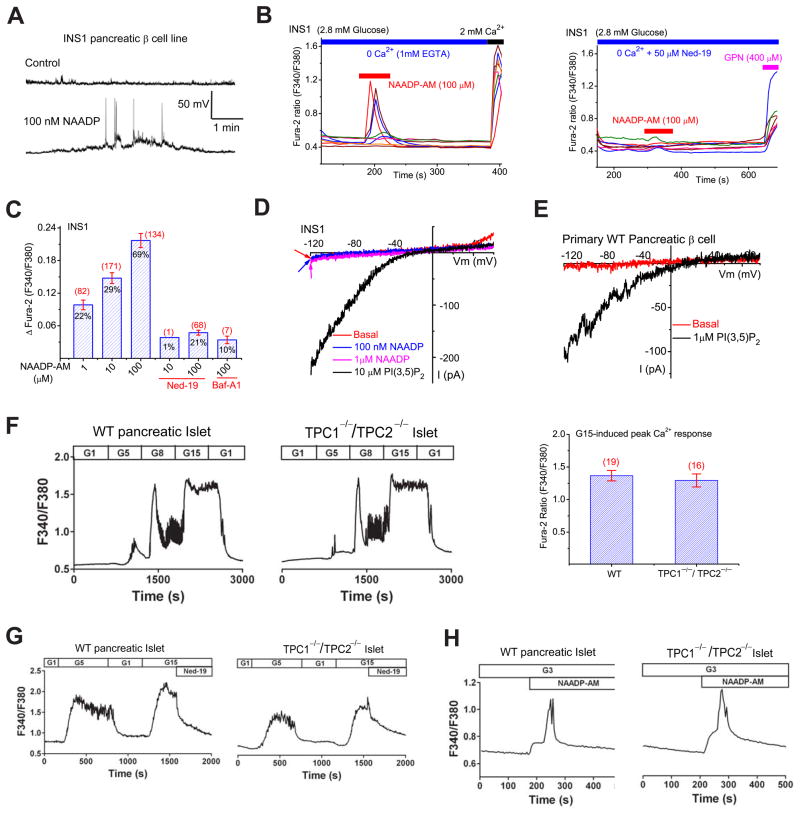
Pancreatic β–cells exhibit robust NAADP-mediated Ca2+ responses and have been commonly used as a cellular model to study endogenous NAADP signaling (Morgan et al., 2011). In INS1 pancreatic β-cell lines, intracellular perfusion with 100 nM NAADP in the whole-cell current-clamp configuration induced membrane depolarization and spike generation (Fig. 7A). It has been reported that Ned-19, a membrane-permeable inhibitor of the NAADP receptor, completely inhibits NAADP- or glucose-induced Ca2+ responses at high μM concentrations (Naylor et al., 2009). However, Ned-19 had only a weak inhibitory effect on IhTPC2, even at very high concentrations (1 mM; Suppl. fig. S6D). Together with the fact that TPCs have limited Ca2+ permeability, these results suggest that TPCs do not contribute directly to NAADP-induced endolysosomal Ca2+ release.
Figure 7. NAADP induces lysosomal Ca2+ release in cells that lack TPC currents or proteins.
(A). Pipette dialysis of NAADP (100 nM) induced membrane depolarization and spike generation in an INS1 pancreatic β-cell line under the whole-cell current-clamp configuration. (B). In the absence of external Ca2+ (free [Ca2+] < 10 nM), NAADP-AM (100 μM) induced Ca2+ release measured with Fura-2 (F340/F380) ratios from intracellular stores in INS1 cells; Ned-19 (50 μM) abolished the majority of the NAADP-induced response. (C). Average peak Ca2+ responses induced by NAADP-AM (1, 10, 100 μM) with and without Ned-19 and Baf-A1 (500 nM). Percentage (from a total of 100–400 cells) of responding (ΔFura-2> 0.02) cells; the number of the responding cells are indicated. (D). NAADP (100 nM or 1 μM) failed to induce measurable whole-endolysosomal current in a vacuole isolated from an INS1 cell; PI(3,5)P2 activated large ITRPML-L (Erev =3.4 ± 3 mV, n=14; the ratio of current amplitudes at − 30 mV versus −120 mV was ~ 1%) in the same vacuole. (E). PI(3,5)P2 activated ITRPML-L in a vacuole from a WT primary pancreatic β-cell. (F). TPC1−/−/TPC2−/− primary pancreatic islets exhibit normal concentration-dependent glucose-induced Ca2+ responses. Cytosolic [Ca2+] increased significantly in both WT and TPC1−/−/TPC2−/− pancreatic islets in response to elevations of the glucose concentration (in mM; 1, 5, 8, 15; G1, G5, G8, and G15) in the perfusion solution (2.5 mM Ca2+). The traces shown are representative of 19 WT and 16 TPC1−/−/TPC2−/− islets, respectively. The right panel shows the average peak Ca2+ responses induced by 15 mM glucose (G15) in WT and TPC1−/−/TPC2−/− pancreatic islets. (G). Glucose-induced Ca2+ responses in WT and TPC1−/−/TPC2−/− pancreatic islets were inhibited by Ned-19 (100 μM). The traces shown are representative of the results obtained in 7 WT and 7 TPC1−/−/TPC2−/− islets, respectively. (H). NAADP-AM (200 μM) induced bi-phasic Ca2+ increases in the presence of 3 mM glucose (Yamasaki et al., 2004) in both WT and TPC1−/−/TPC2−/− islets. Traces shown are representative of the results obtained in 2 WT and 3 TPC1−/−/TPC2−/− islets, respectively. Data are presented as the mean ± SEM. See also Figure S7.
TPC currents are absent in pancreatic β-cell lines that exhibit NAADP-induced lysosomal Ca2+ release
Consistent with the results obtained from the pipette dialysis experiments (Fig. 7A), cell-permeant NAADP-AM (1–100 μM) (Parkesh et al., 2007) induced Ca2+ transients in INS1 (Fig. 7B, C; also see Suppl. fig. S7A, B) and MIN6 (see Suppl. fig. S7D) pancreatic β-cell lines, in the absence or presence of external Ca2+. NAADP-AM-induced Ca2+ responses in INS-1 cells were abolished by the NAADP receptor blocker, Ned-19 (Fig. 7B, C & Suppl. fig. S7A), or by pretreatment with Bafilomycin A1, which inhibits V-ATPase to deplete acidic Ca2+ stores (Morgan et al., 2011) (Fig. 7C; also see Suppl. fig. S7C). These results suggest that, consistent with previous studies, NAADP induces Ca2+ release from lysosomal stores. Surprisingly, no measurable NAADP-activated whole-endolysosomal current was seen in INS1 (Fig. 7D; Suppl. fig. S7E) or MIN6 (Suppl. fig. S7F) cells. Furthermore, PI(3,5)P2 (10 μM) activated ITRPML-L in 14/14 vacuoles, but IX or ITPC-L were not detected (Fig. 7D & Suppl. fig. S7E, F). Collectively, NAADP-mediated responses appeared to be distinct from ITPC-L in pancreatic β-cell lines, suggesting that TPCs do not contribute to the NAADP-mediated response.
TPC1 and TPC2 are not required for NAADP- or glucose- induced Ca2+ responses in pancreatic islets
Glucose induces robust Ca2+ responses in pancreatic β-cells mediated via NAADP and its receptor localized in the endolysosome (Morgan et al., 2011; Naylor et al., 2009). However, in WT primary pancreatic β-cells, we did not observe significant whole-endolysosome ITPC-L (Fig. 7E). Glucose (5, 8, and 15 mM) induced significant increases of intracellular [Ca2+] (measured with Fura-2 Ca2+-sensitive dyes) in pancreatic islets (Fig. 7F), which were dramatically inhibited by Ned-19 (100 μM; Fig. 7G), but the glucose-induced Ca2+ response was still largely intact in TPC1−/−/TPC2−/− islets (Fig. 7F, G). Finally, the NAADP-AM-induced Ca2+ response was not significantly reduced in TPC1−/−/TPC2−/− pancreatic islets (Fig. 7H). These results demonstrate that TPCs are not essential for NAADP- and glucose-induced Ca2+ responses in pancreatic β cells.
Discussion
TPCs have been reported to serve as the receptors (Brailoiu et al., 2009; Calcraft et al., 2009; Ruas et al., 2010; Zong et al., 2009) or co-receptors (Lin-Moshier et al., 2012) for NAADP, and NAADP-activated TPC currents were shown to be K+-permeable (Pitt et al., 2010), Cs+-permeable (Brailoiu et al., 2010), or Ca2+-selective (Schieder et al., 2010). Those studies contrast drastically with our direct measurements of TPCs as NAADP-insensitive PI(3,5)P2-activated Na+-selective channels using whole-endolysosome patch-clamp recordings. Because NAADP-induced Ca2+ responses are robust in cells that lack ITPC-L and are largely intact in TPC1−/−/TPC2−/− cells, and because our direct measurements of TPCs show that they are insensitive to NAADP, it should be clear that TPCs are not the NAADP receptor. Supporting this argument, recent studies using photoaffinity-labeled NAADP suggested that TPCs are unlikely to be the genuine NAADP binding sites (Lin-Moshier et al., 2012). Thus, the reported effects of TPCs on NAADP-induced Ca2+ release from endolysosomes (Morgan et al., 2011) could arise from indirect mechanisms, for example, secondary to endolysosomal enlargement associated with TPC overexpression, or the change of the local Na+ gradient across the lysosomal membrane. Given that we have not been able to detect any NAADP-activated whole-endolysosome current, it is also possible that vacuolin treatment could impair the detection of NAADP-sensitive conductance. On the other hand, one cannot rule out that NAADP, like Bafilomycin-A1 and Glycyl-L-phenylalanine 2-naphthylamide (GPN) (Morgan et al., 2011), might act via non-channel-mediated Ca2+ release mechanisms. NAADP might also target an unidentified anion conductance (low Cl− recording solutions are used in the current study) to induce lysosomal Ca2+ release. Finally, NAADP might also induce Ca2+ release from non-lysosomal Ca2+ stores, but in a lysosome-dependent manner. Molecular identification of the NAADP-bound 22–23-kDa lysosomal proteins (Lin-Moshier et al., 2012) may help distinguish these possibilities.
The lysosomal lumen has been presumed to contain high K+ and low Na+ (Morgan et al., 2011; Steinberg et al., 2010), which would suggest the lack of a significant Na+ or K+ concentration gradient across the lysosomal membrane. These conclusions contrast directly with the high Na+/low K+ (like that of the extracellular media) we have found here using subcellular fractionation of organelles, which has been successfully applied to measure ionic compositions in a number of intracellular organelles, including mitochondria and synaptic vesicles (Cohn et al., 1968; Schmidt et al., 1980). Because the isolation procedures were performed at 4 °C using a homogenization buffer that limits ion exchange, the lysosomal ion composition is presumed to be largely maintained. Indeed, lysosome fractions prepared using this protocol are of relatively normal size (lysosome swelling could be caused by the loss of luminal ions) and are functional (Graves et al., 2008a; Radhakrishnan et al., 2008). Finally, the significant Na+-selective current observed in the lysosome-attached configuration provides an independent verification that the lysosome lumen contains high concentrations of Na+. It is worth mentioning that although a putative lysosomal K+ release channel was proposed to provide counter ion flux for lysosomal acidification (Steinberg et al., 2010), such a scenario is unlikely to occur due to the high cytosolic K+, hence the low or opposite electrochemical gradient of K+ across the lysosome membrane. Instead, the existence of a large Na+ gradient and lysosomal Na+ channels are more likely to fulfill this function (counter-ion flux).
What is the purpose of Na+ selective, PI(3,5)P2-activated TPC channels? Increases in PI(3,5)P2 will allow Na+ to move down its concentration gradient, rapidly reducing and reversing (Suppl. Fig. S4D) the endolysosomal potential, which is presumed to be luminal-side positive at rest (estimated to be + 30 to + 110 mV) (Dong et al., 2010b). In model membranes, it has been demonstrated that Na+ and K+ exhibit differential effects on membrane curvature (Kraayenhof et al., 1996). While oppositely-charged lipid bilayers tend to fuse (Anzai et al., 1993), Na+ influx into the cytoplasm reportedly affects membrane fusion during exocytosis (Parnas et al., 2000). Thus, TPC-mediated Na+ flux in response to a localized increase in PI(3,5)P2 may rapidly depolarize endolysosomal membranes and promote fusion (Suppl. Fig. S4D). Consistent with a previous study (Ruas et al., 2010), we found that TPC overexpression results in enlarged endolysosomes; this might be caused by enhanced endolysosomal fusion, decreased fission, or both. However, unlike TRPML1 (Shen et al., 2011), TPCs are not expressed in every cell type, suggesting that their role in membrane trafficking is more specific. Furthermore, because membrane fusion could occur even in in vitro reconstitution systems, neither TPCs nor TRPMLs are necessarily required as direct participants in the basic membrane fusion machinery (Shen et al., 2011). However, they may regulate the direction and specificity of lysosomal trafficking in vivo. Indeed, lysosomal trafficking is significantly delayed, although not blocked in cells lacking TRPML1 (Shen et al., 2011). Future research may reveal the relative importance of Na+ versus Ca2+, and TRPMLs versus TPCs in spatial and temporal regulation of lysosomal trafficking. Finally, in addition to defining organelle specificity and determining the fusogenic potential of endolysosomes, the proposed cellular functions of PI(3,5)P2 also include regulating endolysosomal ion homeostasis, especially H+ homeostasis (Kerr et al., 2010; Shen et al., 2011). The proposed role of a putative monovalent cation (K+ or Na+) conductance in lysosomal acidification (Steinberg et al., 2010), together with our demonstration of a large Na+ gradient across the endolysosomal membrane, suggest that PI(3,5)P2-sensitive Na+-permeable TPCs, but not K+ release channels (see above), may participate in endolysosomal pH regulation in a transient and localized manner. In addition, rapid changes in Na+ content will drive Na+/H+ exchangers in the organelle membrane, thus changing organellar pH. The exact sequence of events will depend on expanding our knowledge of transporters in endolysosomal membranes, and finding more accurate methods to measure endolysosomal potentials in intact cells.
Experimental Procedures
Targeted deletion of TPC1 and TPC2 in mice
TPC1 and TPC2 double knockout mice were generated as described in Extended Experimental Procedures in the Supplementary Information.
Endolysosomal electrophysiology
Endolysosomal electrophysiology was performed in isolated enlarged endolysosomes using a modified patch-clamp method (Dong et al., 2010a). Cells were treated with 1 μM vacuolin-1, a lipid-soluble polycyclic triazine that can selectively increase the size of endosomes and lysosomes (Huynh and Andrews, 2005), for at least 1h, or up to 12h. Large vacuoles (up to 5 μm; capacitance = 1.1 ± 0.1 pF, n= 29 vacuoles) were observed in most vacuolin-treated cells. Occasionally, enlarged vacuoles were also seen in non-treated cells; no significant difference in TPC channel properties were seen for enlarged vacuoles obtained with or without vacuolin-1 treatment. Whole-endolysosome recordings were performed on manually isolated enlarged endolysosomes (Dong et al., 2010a). In brief, a patch pipette was pressed against a cell and quickly pulled away to slice the cell membrane. Enlarged endolysosomes were released into a dish and identified by monitoring EGFP-TPC1/2, mCherry-TPC1/2, or EGFP-Lamp1/mCherry-Lamp1 fluorescence. After formation of a gigaseal between the patch pipette and the enlarged endolysosome, capacitance transients were compensated. Voltage steps of several hundred mVs with ms duration were then applied to break into the vacuolar membrane (see Suppl. fig. S1). The whole-endolysosome configuration was verified by the re-appearance of capacitance transients after break-in (see Suppl. fig. S1).
Unless otherwise stated, bath (internal/cytoplasmic) solution contained (in mM) 140 K-gluconate, 4 NaCl, 1 EGTA, 2 MgCl2, 0.39 CaCl2, 20 HEPES (pH adjusted with KOH to 7.2; free [Ca2+]i ~ 100 nM). In a subset of experiments, 2 mM Na2-ATP and 0.1 mM GTP were added to the bath solution, and pH was re-adjusted. Pipette (luminal) solution was standard extracellular solution (modified Tyrode’s; in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 MES, 10 glucose (pH adjusted with NaOH to pH 4.6). In a subset of experiments, a low Cl− pipette solution containing Na-gluconate replaced NaCl. All bath solutions were applied via a perfusion system that allowed us to achieve complete solution exchange within a few seconds. Data were collected using an Axopatch 2A patch clamp amplifier, Digidata 1440, and pClamp 10.2 software (Axon Instruments). Whole-endolysosome currents were digitized at 10 kHz and filtered at 2 kHz. All experiments were conducted at room temperature (21–23°C), and all recordings were analyzed with pClamp 10.2 and Origin 8.0 (OriginLab, Northampton, MA). All PIPs were from A.G. Scientific; water-soluble diC8-PIPs, prepared in high-concentration stock solutions, were dissolved in the bath solutions, and delivered via the perfusion system at low concentrations (0.1–1 μM), and direct bath application at higher concentrations (10 μM). NAADP, 4-methyl NAADP, and 5-methyl NAADP were from Tocris Bioscience, Sigma, or kindly provided by Dr. Slama (Jain et al., 2010). The permeability to cations (relative to PNa) was estimated based on Erev measurement under bi-ionic conditions as described in Extended Experimental Procedures in the Supplementary Information.
Ca2+ imaging
Ca2+ imaging was performed using an EasyRatioPro system (PTI) as described in Extended Experimental Procedures in the Supplementary Information.
Lysosome isolation by subcellular fractionation
Lysosomes were isolated as described previously (Dong et al., 2010a; Graves et al., 2008b). Briefly, cell lysates were obtained by Dounce homogenization in a homogenizing buffer (HM buffer; 0.25 M sucrose, 1 mM EDTA, 10 mM HEPES, and pH 7.0), and then centrifuged at 1900 g (4, 200 rpm) at 4°C for 10 min to remove the nuclei and intact cells. Post-nuclear supernatants then underwent ultracentrifugation through a Percoll density gradient using a Beckman L8–70 ultracentrifuge. An ultracentrifuge tube was layered with 2.5 M sucrose, 18% Percoll in HM buffer. Centrifugation speed was 67,200 g (14,000 rpm) at 4°C for 1 h using a Beckman Coulter 70.1 Ti Rotor. Samples were fractionated into light, medium, and heavy membrane fractions. Heavy membrane fractions contained concentrated bands of cellular organelles and were further layered over a discontinuous iodixonal gradient. The iodixonal gradient was generated by mixing iodixonal in the HM buffer with 2.5 M glucose (in v/v; 27%, 22.5%, 19%, 16%, 12%, and 8%); the osmolarity of all solutions was ~ 300 mOsm. After centrifugation at 4°C for 2.5 h, the sample was divided into 10 fractions (0.5 ml each) for biochemical and atomic absorption analyses. Note that the ionic composition of the lysosome was largely maintained due to the low rate of ion transport across the lysosomal membrane at 4°C. Antibodies used for Western blots: anti-Lamp1 (Iowa Hybridoma bank), 1:5000 dilution; anti-Annexin V (Abcam), 1:2000 dilution; anti-GM130 (Abcam), 1:2000 dilution; anti-EEA1 (Santa Cruz Biotechnology), 1:500 dilution; anti-Complex II (Abcam), 1:5000 dilution; anti-GFP (Covance), 1:5000 dilution.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Lysosomal fractions were prepared for atomic absorption by diluting the samples in a 1:1 ratio with concentrated nitric acid. After digestion (10min, 60°C), the ionic composition was measured using a Thermo Scientific Finnigan Element inductively coupled with a plasma-high resolution mass spectrometer (ICP-HRMS)(Seby et al., 2003).
Data analysis
Data are presented as the mean ± standard error of the mean (SEM). Statistical comparisons were made using analysis of variance (ANOVA). A P value < 0.05 was considered statistically significant.
Supplementary Material
TPC proteins are sodium-selective channels in the lysosome.
TPC channels are activated specifically by PI(3,5)P2.
TPC channels are not NAADP receptors.
Lysosomes are high sodium compartments.
Acknowledgments
This work was supported by NIH RO1 grants (NS062792 to H.X., NS055293 to D.R., GM081658 to M.X.Z., and HHMI to D.C.) and a Sloan Research Fellowship (to H.X). We thank the Gene Manipulation Facility of the Children’s Hospital Boston (TPC1) and the Transgenic & Chimera Mouse Facility at the University of Pennsylvania (TPC2) for ES cell injection.
Footnotes
Author contribution: the authors were listed in the order of their institutions; X.W. initiated the project; X.W., X.Z, and X.D. performed the electrophysiology experiments; X.W also performed the Ca2+ imaging experiments; M.S. and A. G. performed the ionic composition experiments; X. L., X. C., D.S., and M.X.Z contributed the reagents; D.R. and J.H. developed the TPC1, TPC2 knockout mouse lines, the hTPC1, hTPC2, hTPC1ΔN and hTPC2ΔN constructs; Y.Z. measured the glucose- and NAADP -induced Ca2+ responses in the TPC mutant islets compared with wild-type; development of TPC1 knockout was carried out by D.R. in the laboratory of D.E.C; H.X. and D.E.C. wrote the paper with input from all authors. We are grateful to Drs. Susan Slaugenhaupt and James Slama for sharing the reagents, Drs. Ted Huston, Hollis Showalter, Yafei Jin, Leslie Satin, Jianhua Ren, Peter Arvan, and Dr. Gautam Rajpal for assistance, and Dr. Richard Hume for comments on an earlier version of the manuscript. We appreciate the encouragement and helpful comments from other members of the Xu, Ren, and Clapham laboratories.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anzai K, Masumi M, Kawasaki K, Kirino Y. Frequent fusion of liposomes to a positively charged planar bilayer without calcium ions. J Biochem. 1993;114:487–491. doi: 10.1093/oxfordjournals.jbchem.a124204. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated Two-pore Channel Targeted to the Plasma Membrane Uncouples Triggering from Amplifying Ca2+ Signals. J Biol Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers MD, Dib-Hajj S, Carrithers LM, Tokmoulina G, Pypaert M, Jonas EA, Waxman SG. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol. 2007;178:7822–7832. doi: 10.4049/jimmunol.178.12.7822. [DOI] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn DV, Bawdon R, Newman RR, Hamilton JW. Effect of calcium chelation on the ion content of liver mitochondria in carbon tetrachloride-poisoned rats. J Biol Chem. 1968;243:1089–1095. [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, et al. PI(3,5)P(2) Controls Membrane Traffic by Direct Activation of Mucolipin Ca Release Channels in the Endolysosome. Nat Commun. 2010a:1. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010b;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J. 2009;419:1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- Favre I, Moczydlowski E, Schild L. On the structural basis for ionic selectivity among Na+, K+, and Ca2+ in the voltage-gated sodium channel. Biophys J. 1996;71:3110–3125. doi: 10.1016/S0006-3495(96)79505-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Curran PK, Smith CL, Mindell JA. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008a;453:788–792. doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- Graves AR, Curran PK, Smith CL, Mindell JA. The Cl(−)/H(+) antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008b doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- Grimm C, Jors S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Small molecule activators of TRPML3. Chem Biol. 2010;17:135–148. doi: 10.1016/j.chembiol.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse AH. Second messenger signaling: multiple receptors for NAADP. Curr Biol. 2009;19:R521–523. doi: 10.1016/j.cub.2009.05.045. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972;59:637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Andrews NW. The small chemical vacuolin-1 alters the morphology of lysosomes without inhibiting Ca2+-regulated exocytosis. EMBO Rep. 2005;6:843–847. doi: 10.1038/sj.embor.7400495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Slama JT, Perez-Haddock LA, Walseth TF. Nicotinic acid adenine dinucleotide phosphate analogues containing substituted nicotinic acid: effect of modification on Ca(2+) release. J Med Chem. 2010;53:7599–7612. doi: 10.1021/jm1007209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Wang JT, Castro NA, Hamilton NA, Town L, Brown DL, Meunier FA, Brown NF, Stow JL, Teasdale RD. Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J. 2010;29:1331–1347. doi: 10.1038/emboj.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraayenhof R, Sterk GJ, Wong Fong Sang HW, Krab K, Epand RM. Monovalent cations differentially affect membrane surface properties and membrane curvature, as revealed by fluorescent probes and dynamic light scattering. Biochim Biophys Acta. 1996;1282:293–302. doi: 10.1016/0005-2736(96)00069-7. [DOI] [PubMed] [Google Scholar]
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity Labeling of Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Targets in Mammalian Cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J. 2008;27:2809–2816. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkesh R, Lewis AM, Aley PK, Arredouani A, Rossi S, Tavares R, Vasudevan SR, Rosen D, Galione A, Dowden J, et al. Cell-permeant NAADP: A novel chemical tool enabling the study of Ca(2+) signalling in intact cells. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Parnas H, Segel L, Dudel J, Parnas I. Autoreceptors, membrane potential and the regulation of transmitter release. Trends Neurosci. 2000;23:60–68. doi: 10.1016/s0166-2236(99)01498-8. [DOI] [PubMed] [Google Scholar]
- Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J Biol Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, et al. Purified TPC Isoforms Form NAADP Receptors with Distinct Roles for Ca(2+) Signaling and Endolysosomal Trafficking. Curr Biol. 2010 doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J Biol Chem. 2010;285:21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Zimmermann H, Whittaker VP. Metal ion content of cholinergic synaptic vesicles isolated from the electric organ of Torpedo: effect of stimulation-induced transmitter release. Neuroscience. 1980;5:625–638. doi: 10.1016/0306-4522(80)90060-3. [DOI] [PubMed] [Google Scholar]
- Seby F, Gagean M, Garraud H, Castetbon A, Donard OF. Development of analytical procedures for determination of total chromium by quadrupole ICP-MS and high-resolution ICP-MS, and hexavalent chromium by HPLC-ICP-MS, in different materials used in the automotive industry. Anal Bioanal Chem. 2003;377:685–694. doi: 10.1007/s00216-003-2133-5. [DOI] [PubMed] [Google Scholar]
- Shen D, Wang X, Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics: Phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays. 2011;33:448–457. doi: 10.1002/bies.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BE, Huynh KK, Brodovitch A, Jabs S, Stauber T, Jentsch TJ, Grinstein S. A cation counterflux supports lysosomal acidification. J Cell Biol. 2010;189:1171–1186. doi: 10.1083/jcb.200911083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Jakobsdottir M, Steinberg S, Gudjonsson SA, Palsson A, Thorleifsson G, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- Toth B, Csanady L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem. 2010;285:30091–30102. doi: 10.1074/jbc.M109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJ, Galione A. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.