Abstract
Aims
Anecdotally, methamphetamine is considered to have a greater abuse potential compared to d-amphetamine, but there are no studies directly comparing self-administration of these drugs. This study characterized and compared self-administration as well as the mood, performance, and physiological effects of intranasal methamphetamine- and d-amphetamine.
Design
A randomized, double-blind, placebo-controlled, cross-over study.
Setting
An outpatient research unit at the New York State Psychiatric Institute.
Participants
Male recreational methamphetamine users (n = 13).
Measurements
Five 2-day blocks of sessions were conducted. On the first day of each block, participants “sampled” a single methamphetamine or d-amphetamine dose (0, 12, 50 mg/70 kg) and a monetary reinforcer ($5 or $20). Amphetamines plasma levels, cardiovascular, mood, and psychomotor performance effects were assessed before drug administration and repeatedly thereafter. On the second day of each block, participants chose between the sampled reinforcers (drug or money).
Findings
There were no significant differences between the drugs on the majority of measures. Under the $5 condition, both amphetamines dose-dependently increased self-administration, whereas under the $20 condition, few drug options were selected. Overall, participants selected more drug choices under the $5 condition compared with the $20 condition (41% versus 17%). Both drugs increased cardiovascular activity and “positive” mood, although methamphetamine produced more prominent effects on some measures (e.g., heart rate and ratings of ‘high’).
Conclusions
These data are consistent with previous findings suggesting that the two amphetamines produce a similar dose-related profile of acute effects in humans, with methamphetamine producing greater effects on some mood and cardiovascular measures. The amphetamines were self-administered equally indicating their equivalence for abuse potential.
Keywords: methamphetamine, d-amphetamine, amphetamines, self-administration, performance, subjective effects, humans
Introduction
Methamphetamine and d-amphetamine have nearly identical chemical structures. Methamphetamine is the N-methylated analog of d-amphetamine and both are FDA-approved for similar medical conditions. Despite their structural similarities, d-amphetamine is commonly regarded as a safe and effective therapeutic, whereas methamphetamine is rarely prescribed [1]. It is possible that methamphetamine is infrequently used therapeutically because of the perception that it produces greater deleterious effects. For example, there is an extensive human literature suggesting that illicit methamphetamine produces cognitive impairments [2] and psychological disturbances [3]. A comparable literature for d-amphetamine does not exist. Furthermore, epidemiological evidence indicates that methamphetamine abuse rates are greater than those of d-amphetamine. According to the U.S. Treatment Episode Data Set [4], in 2007 methamphetamine users comprised approximately 96% of all amphetamine treatment admissions. One possible explanation for the greater incidence of methamphetamine abuse is that illicit methamphetamine is more readily available due to its purported ease of synthesis.
Another explanation is that the addition of the N-methyl group to the basic amphetamine structure makes methamphetamine more lipophilic compared to d-amphetamine [5]. Consequently, methamphetamine may more readily cross the blood brain barrier, making it more potent than d-amphetamine [6]. It is important to note, however, that data from studies directly comparing the amphetamines in laboratory animals do not support the notion that methamphetamine is more potent. For example, Melega and colleagues [7] observed that the drugs had equivalent pharmacokinetic profiles and similarly increased striatal dopamine in rats. In contrast, others found that, although the amphetamines similarly increased dopamine in the rat nucleus accumbens, methamphetamine released dopamine in the prefrontal cortex less effectively than d-amphetamine [8]. Furthermore, although some researchers observed that larger methamphetamine doses (e.g., 2.5–10 mg/kg) increased locomotor activity to a greater extent than d-amphetamine in mice [9], others reported that, at smaller doses (e.g., 0.5–1 mg/kg), the drugs were equipotent at activating locomotion [10]. Finally, data from studies comparing the two amphetamines on measures believed to be predictive of abuse potential (i.e., drug discrimination and self-administration) indicate that equivalent doses of the drugs produced similar responses, further indicating that the drugs are equipotent [11,12,13].
Concordant with the literature obtained with laboratory animals, direct comparisons of the effects of oral methamphetamine and d-amphetamine in humans indicate the drugs produce overlapping effects on measures of cardiovascular activity, mood, and drug discrimination [14,15,16]. An important consideration of these studies, however, is that they compared relatively low oral doses (i.e., 2.5–30 mg). It is unclear to what degree these findings generalize to illicit methamphetamine use. Recreational methamphetamine use is purportedly used in larger doses via routes of administration that produce a more rapid onset of effects (e.g., intranasal, intravenous, and smoked: [17]). The onset speed of drug-related effects is a critical determinant of the intensity of mood and behavioral effects of a drug [18,19]. Thus, it is possible that potential differences between methamphetamine and d-amphetamine may only be detected following a route of administration associated with a faster onset of effects. There have been no direct comparisons of these amphetamines using a route commonly associated with abuse.
It is also important to note that previous comparisons of oral methamphetamine and d-amphetamine primarily examined drug-related effects on mood and/or drug discrimination. Although these measures provide potentially useful information about the abuse potential of a given drug, they are indirectly related to actual drug-taking behavior and may not correspond with self-administration data. Results from studies indicating that drug-related subjective effects and self-administration can be dissociable highlight this point [20]. For example, using a choice procedure during which participants had several opportunities to self-administer oral d-amphetamine (5 mg) or placebo, Johanson and Uhlenhuth [21] reported that d-amphetamine-related subjective effects were comparable in all subjects but did not predict choice to self-administer the drug. These results underscore the importance of assessing drug-taking behavior in the human laboratory.
In an effort to further understand the impact of modifications of the basic amphetamine structure on human behavior, the present investigation directly compared self-administration as well as the subjective, cardiovascular, and psychomotor performance effects of intranasal methamphetamine and d-amphetamine (0, 12, and 50 mg/70 kg). During a “sample” session, participants were administered a single drug dose and given a monetary reinforcer (US$5 or $20). On the following day, participants had the opportunity to choose between the sampled reinforcers (drug or money). Data from several self-administration studies indicate that increasing the value of an alternative non-drug reinforcer decreases drug choice in laboratory animals [22,23] and humans [24,25]. Thus, we hypothesized that methamphetamine and d-amphetamine would similarly increase drug self-administration when $5 was the alternative reinforcer but amphetamine-related self-administration would be attenuated when $20 was the alternative reinforcer. Furthermore, we predicted that both drugs would dose-dependently increase “positive” subjective-effects ratings and cardiovascular values, and improve psychomotor performance.
Methods and Materials
Participants
Male research volunteers (N=13: one Asian, six Black, two Hispanic, four White) completed this study. They were 37.4 ± 7.3 (mean ± SD) years of age and had completed 14.8 ± 2.0 years of formal education. All passed comprehensive medical examinations and psychiatric interviews and were within normal weight ranges according to the 1983 Metropolitan Life Insurance Company height/weight table (body mass index: 24.9 ± 2.7). All participants reported current methamphetamine use (9.4 ± 4.7 days/month). Seven participants reported current alcohol use (4–10 drinks/week), seven reported current cocaine use (1–8 days/month), three participants reported current marijuana use (4–12 days/month), and four smoked 3–20 tobacco cigarettes/day. Three met criteria for current methamphetamine dependence but none were seeking treatment for drug use and none met criteria for any other Axis I disorder.
All participants were solicited via word-of-mouth referral and newspaper and online advertisement in New York City. Before enrollment, each signed a consent form that was approved by the Institutional Review Board of The New York State Psychiatric Institute (NYSPI). Upon discharge, each participant was informed about experimental and drug conditions and paid for participation at a rate of $60 per day.
Pre-study Training
Prior to starting the study, participants completed two training sessions (3–4 hours each) on the computerized psychomotor tasks that would be used during the study. Additionally, on a separate day, they received the largest active methamphetamine dose (50 mg/70 kg) to be administered during the study in order to monitor any adverse reactions and provide them with experience with a study drug. No untoward effects were noted.
Design
This 10-session outpatient study consisted of five 2-day blocks of sessions, during which physiological measures were assessed and participants completed visual analog mood scales and computerized psychomotor task batteries. Table 1 shows the study design. Briefly, the first day of each block was a sample session, during which participants received an intranasal amphetamine dose (0, 12, 50 mg/70 kg) and a monetary reinforcer. The monetary reinforcer was US$5 for seven participants and US$20 for six participants. The second day of each block was a choice session, during which participants could work for all or part of the drug and/or money they received on the previous day. Each block of sessions was separated by at least 48 hours and each participant experienced all dosing conditions, which were counterbalanced.
Table 1.
Study design
| Week | Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|---|
| 1 | MA (mg/70 kg) | S (50) | C (50) | Off | S (12) | C (12) |
| 2 | AMPH (mg/70 kg) | S (12) | C (12) | Off | S (50) | C (50) |
| 3 | S (placebo) | C (placebo) |
Note: Sample administration and Choice procedure occurred at 1000 h.
MA=methamphetamine; AMPH=d-amphetamine. S=sample session; C=choice session. All participants completed five 2-day blocks of sessions, one for each dosing condition. Dosing order was varied across participants.
Procedure
Sample sessions
Each session began at approximately 0900 h and lasted for nearly 6 hours. Upon reporting to the laboratory, participants passed a field sobriety test and gave a urine sample that was negative for several drug metabolites, excluding amphetamines and THC. Following a light breakfast, they completed a visual analog sleep questionnaire and the baseline subjective-effects questionnaire and psychomotor task battery (described below). After baseline assessments, participants were given the monetary reinforcer and drug, which was insufflated immediately. Then, they completed four task batteries, took a 45-min lunch break period, and completed two additional task batteries. Subjective effects and cardiovascular measures were assessed at baseline and 5, 15, 30, 60, 90, 120, 180, and 240 mins post drug administration. Blood samples were collected at baseline and 15, 60, 90, 120, 180, and 240 mins post drug administration via an i.v. line, which was kept patent by a physiological saline solution drip.
Upon completion of a session, participants were evaluated for signs of intoxication, passed a field sobriety test, provided fare for public transportation and excused.
Choice (self-administration) sessions
The second day of each block was identical to the first with two exceptions: 1) blood samples were not collected; and 2) after baseline assessment, participants completed a 50-min computerized self-administration task. On this task, participants were given 10 opportunities to choose between 10% of the drug dose or 10% of the monetary reinforcer that they received on the previous day. Responses consisted of finger presses on a mouse manipulandum. The response requirements to choose drug or money increased independently as follows: 50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, and 2800 responses. In order to receive 100% of either reinforcer, a participant had to select that reinforcer on all 10 trials and make a total of 11,550 responses. Following completion of the task, participants received the chosen amount of drug and/or money.
Subjective-Effects and Psychomotor Battery
The computerized visual analog questionnaire (VAS) consisted of a series of 100-mm lines labeled ‘not at all’ at one end and ‘extremely’ at the other end [26]. The lines were labeled with adjectives describing a mood (e.g., ‘I feel…’ ‘irritable,’ ‘talkative’), a drug effect (e.g., ‘I feel…’ ‘stimulated,’ ‘a good drug effect’), or a physical symptom (‘I feel nauseous,’ ‘I have a headache’). Additionally, at 45 min post drug administration participants completed a drug-effect questionnaire (DEQ), during which they were required to rate ‘good effects’ and ‘bad effects’ on a five-point scale: 0 = ‘not at all’ and 4 = ‘very much.’ They were also asked to rate the drug strength as well as their ‘desire to take the drug again.’ Participants were also asked to rate how much they liked the drug effect on a 9-point scale: −4 = ‘disliked very much,’ 0 = ‘feel neutral, or feel no drug effect,’ and 4 = ‘liked very much.’
The computerized psychomotor task battery consisted of two tasks: 1) the digit-symbol substitution task (DSST), designed to assess changes in visuospatial processing [27]; and 2) the divided attention task (DAT), designed to assess changes in vigilance and inhibitory control [28].
Drug
Methamphetamine HCl (provided by the NIDA) and d-amphetamine sulfate (provided by Cambrex, Charles City, IA) were prepared by the NYSPI Pharmacy. Lactose powder was used as a placebo and added to each active amphetamine dose (12 and 50 mg/70 kg) to achieve a final weight of 60 mg/70 kg. A research nurse placed each dose in a small medicine cup, along with a plastic straw (~7 cm). Participants were instructed to insufflate the entire dose within a 30-s period in either one or two nostrils. This procedure has been shown to produce dose-dependent changes in subjective-effects measures and cardiovascular activity [26]. All drugs were administered in a double-blind manner.
Data Analysis
For each choice session, choice data were analyzed using a single-factor repeated-measures analyses of variance (ANOVA); the factor was drug condition (0, 12, 50 mg methamphetamine and d-amphetamine). Separate analyses were conducted for each group (i.e., those who received the $20 monetary reinforcer [N = 6] and those that received the $5 reinforcer [N = 7]). For each sample session, cardiovascular effects, plasma levels, and psychomotor performance data were analyzed using two-factor ANOVAs: the first factor was drug condition and the second factor was time (time and number of assessments varied depending on the measure). Subjective-effect ratings were summed across the session and analyzed using single-factor ANOVAs. The two groups did not differ on any physiological, subjective, or performance measure; therefore, we combined these data for these analyses (N = 13). In order to assess the residual effects of the amphetamines, single-factor ANOVAs were conducted for subjective-effect ratings, cardiovascular measures, and psychomotor performance data obtained 24 hours after drug administration (i.e., baseline measures on choice days). For all analyses, ANOVAs provided the error terms needed to calculate within-drug planned comparisons (0 mg vs. all other doses, 12 mg vs. 50 mg) and between-drug planned comparisons (methamphetamine vs. d-amphetamine). Values were considered statistically significant at p<0.05, using Huynh-Feldt corrections when appropriate.
Results
Plasma Methamphetamine and d-Amphetamine Levels
Acute Effects
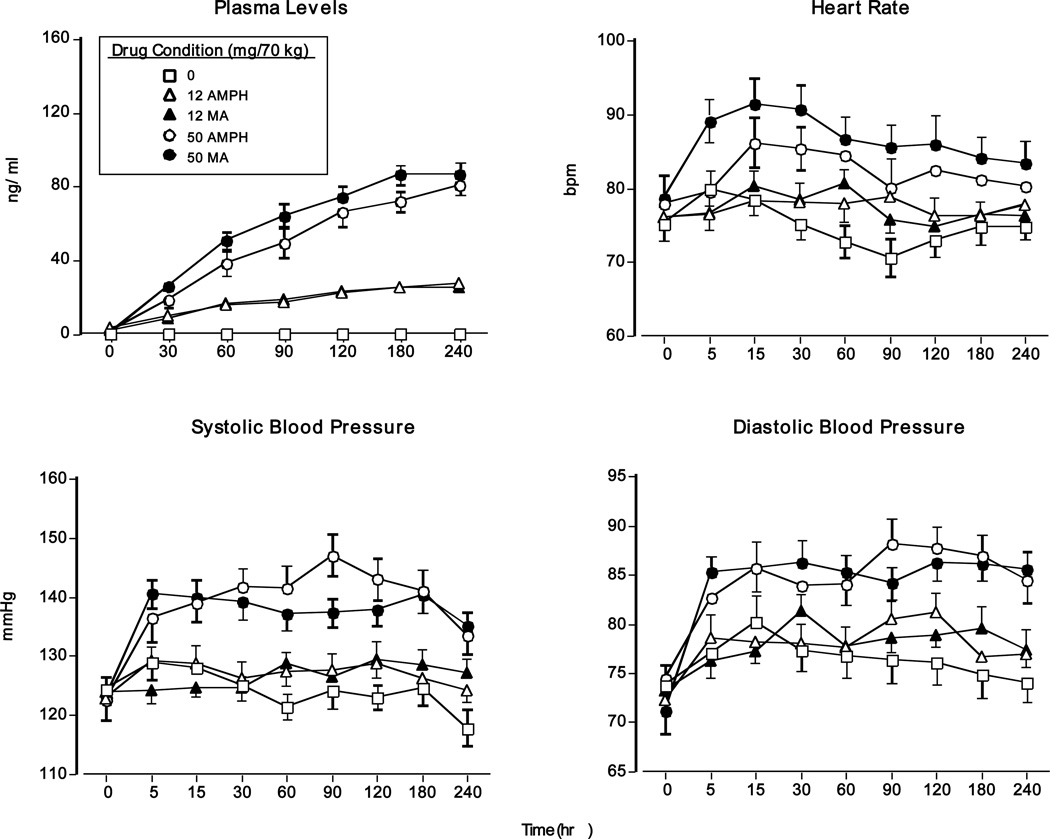
Figure 1 (top left panel) demonstrates that methamphetamine and d-amphetamine dose-dependently increased plasma concentrations. Peak concentrations for both drugs were observed 3–4 h after drug administration. All amphetamine doses significantly increased plasma concentrations compared to placebo and the 50-mg doses produced larger increases than the 12-mg doses (p<0.0001 for all comparisons).
Figure 1.
Upper panel (left): drug plasma levels as a function of drug dose and time (N = 13). Upper panel (right): heart rate as a function of drug dose and time. Lower panels: systolic and diastolic pressure as a function of drug dose and time. Error bars represent one SEM. Overlapping error bars were omitted for clarity.
Methamphetamine and d-Amphetamine Choice (self-administration)
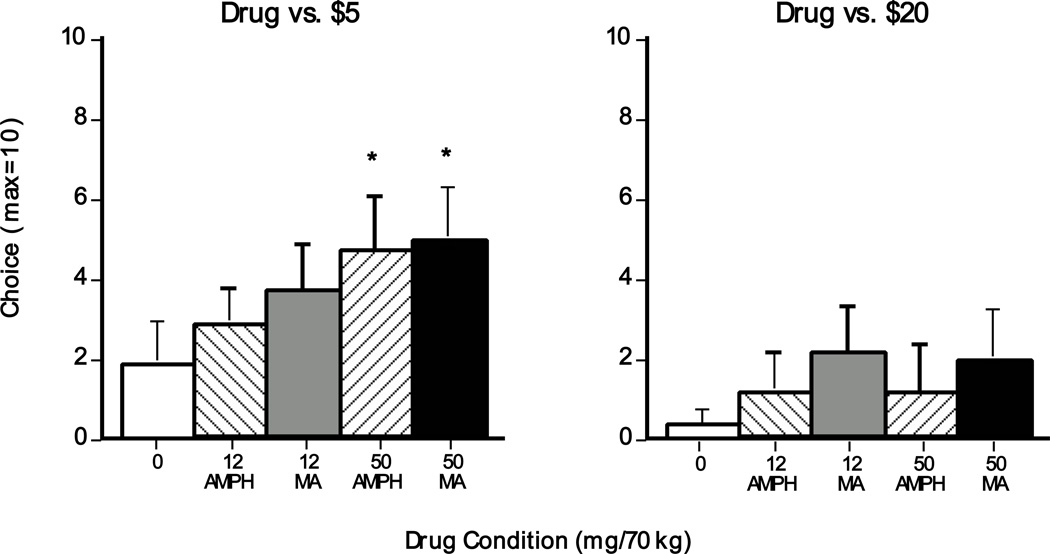
Figure 2 (left panel) shows that, when $5 was the alternative reinforcer, participants selected a greater number of 50-mg methamphetamine and 50-mg d-amphetamine options compared to placebo (p<0.05); there was no significant difference between methamphetamine and d-amphetamine. In contrast, when $20 was the alternative reinforcer, participants overwhelmingly chose the monetary option and no significant dose effects were noted (Figure 2; right panel).
Figure 2.
Number of selected drug options during the choice session as a function of drug dose ($5 group: N = 7; $20 group: N = 6). Error bars represent one SEM. An * indicates significantly different from placebo (p<0.05). A § indicates significantly different from 12 mg (p<0.05). A † indicates significantly different from 50 mg d-amphetamine (p<0.05).
Cardiovascular Effects
Acute Effects
Figure 1 (top right and bottom panels) displays cardiovascular measures as a function of dosing condition and time. Relative to placebo and the 12-mg doses, both 50-mg doses significantly increased heart rate (HR), systolic pressure (SP), and diastolic pressure (DP: p<0.01 for all comparisons). Regarding HR, methamphetamine produced greater increases than d-amphetamine (p<0.05). In contrast to peak drug plasma concentrations, which occurred hours after drug administration, peak cardiovascular effects occurred within 15 min.
Residual Effects
Both methamphetamine doses and the large d-amphetamine dose caused baseline HR on choice days to remain significantly increased 24 hrs after their administration compared to placebo (0 mg: 76.8 ± 2.3 vs. 12 mg MA: 86.1 ± 1.9; 50 mg AMPH: 90.5 ± 3.4; and 50 mg MA: 87.8 ± 2.3, p<0.01 for all comparisons). In addition, relative to placebo, 50 mg methamphetamine produced significantly elevated DP 24 hrs post drug administration (0 mg: 74.4 ± 2.2 vs. 50 mg MA: 78.4 ± 2.4 p<0.05).
Subjective Effects
Acute Effects
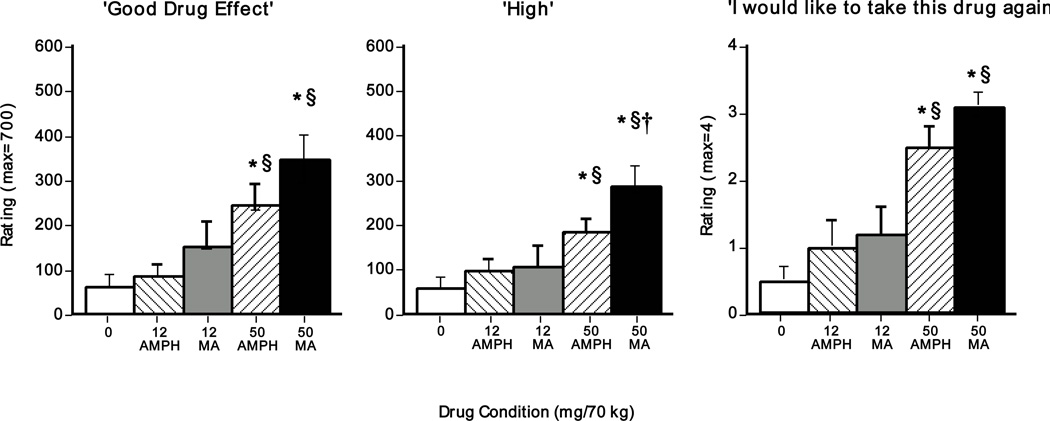
Figure 3 shows the effects of dosing condition on selected subjective-effect ratings summed across the entire sample session. Relative to placebo and the 12-mg amphetamine doses, both large doses significantly increased VAS ratings of ‘good drug effect’ and ‘high’ as well as DEQ ratings of ‘desire to take drug again’ (p<0.05 for all comparisons). Ratings between the two amphetamines on several subjective-effect items did not significantly differ, but some differences were observed. For example, the large methamphetamine dose significantly elevated ratings of ‘high’ compared to the large d-amphetamine dose (p<0.05). Additional statistically significant VAS and DEQ effects are summarized in Table 2.
Figure 3.
Selected subjective-effect ratings (summed across the session) as a function of drug dose (N = 13). Error bars represent one SEM. An * indicates significantly different from placebo (p<0.05). A § indicates significantly different from 12 mg (p<0.05). A † indicates significantly different from 50 mg d-amphetamine (p<0.05).
Table 2.
Sum of acute amphetamine-related effects on subjective-effect ratings
| Drug Conditions | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 12 mg AMPH | 12 mg MA | 50 mg AMPH | 50 mg MA | |||||||||
| Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | ||||
| VAS ratings (max=700) | |||||||||||||
| Alert | 274 | (64) | 353 | (75) | 389 | (74) | * | 419 | (81) | * | 446 | (69) | * |
| Energetic | 271 | (57) | 319 | (59) | 361 | (63) | * | 414 | (59) | *§ | 468 | (65) | *§ |
| Friendly | 324 | (58) | 359 | (64) | 375 | (70) | 434 | (66) | *§ | 463 | (70) | *§ | |
| Heart Pounding | 26 | (8) | 24 | (8) | 19 | (7) | 29 | (12) | 66 | (23) | *§† | ||
| Nose Burning | 37 | (10) | 48 | (19) | 41 | (12) | 102 | (19) | *§ | 107 | (25) | *§ | |
| Sleepy | 205 | (70) | 163 | (72) | 88 | (56) | * | 67 | (41) | * | 47 | (30) | * |
| Social | 314 | (54) | 346 | (59) | 367 | (64) | 448 | (46) | *§ | 454 | (64) | *§ | |
| Stimulated | 111 | (49) | 160 | (49) | 172 | (53) | 226 | (50) | *§ | 320 | (47) | *§† | |
| Talkative | 271 | (53) | 282 | (49) | 338 | (56) | 411 | (49) | *§ | 419 | (65) | *§ | |
| Tired | 224 | (74) | 176 | (71) | 127 | (63) | 68 | (39) | * | 56 | (21) | * | |
| DEQ ratings (max=4) | |||||||||||||
| Good Drug Effect | 0.7 | (0.2) | 0.8 | (0.3) | 1.6 | (0.4) | * | 2.3 | (0.3) | *§ | 3.1 | (0.2) | *§† |
| Like Drug | −0.1 | (0.4) | 0.7 | (0.4) | 1.1 | (0.4) | * | 2.1 | (0.4) | *§ | 2.8 | (0.3) | *§ |
| Drug Strength | 1.1 | (0.3) | 1.0 | (0.2) | 1.7 | (0.4) | 2.5 | (0.3) | *§ | 3.1 | (0.2) | *§† | |
p < .05, significantly different from placebo
p < .05, significantly different from 12 mg
p < .05, significantly different from 50 mg AMPH
Residual Effects
Relative to placebo and all other active drug conditions, under the 50-mg methamphetamine dose condition, ratings of ‘content’ were significantly lower approximately 24 hrs after drug administration (0 mg: 54.6 ± 8.6; 12 mg AMPH: 51.6 ± 9.6; 12 mg MA: 52.6 ± 10.3; 50 mg AMPH: 54.7 ± 10.8; and 50 mg MA: 40.7 ± 10.0, p<0.05 for all comparisons). No other significant subjective effects were observed.
Psychomotor Performance Effects
Acute Effects
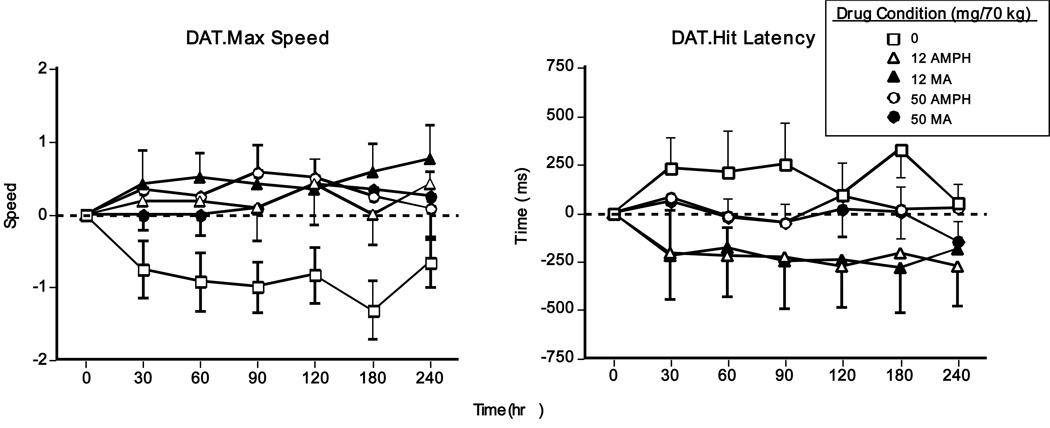
Figure 4 shows that both amphetamines improved performance on the DAT. Compared to placebo, all active doses increased maximum tracking speed, while the smaller methamphetamine and d-amphetamine doses decreased mean hit latency (p<0.05 for all comparisons). No other significant drug effects on performance were noted.
Figure 4.
DAT task performance (change from baseline) as a function of drug dose and time (N = 13). Error bars represent one SEM. Overlapping error bars were omitted for clarity.
Residual Effects
No significant residual performance effects were observed.
Discussion
The present findings show that when participants had the choice between the larger dose of drug and $5, methamphetamine and d-amphetamine (50 mg/70 kg) similarly increased the number of selected drug options. By contrast, when a higher magnitude monetary reinforcer ($20) was available, drug self-administration was significantly diminished. Consistent with these results, methamphetamine and d-amphetamine produced similar effects on the majority of physiological and behavioral measures. Both drugs enhanced ratings of euphoria and mood, increased cardiovascular activity, and improved psychomotor performance. Methamphetamine did, however, engender greater effects on some measures (e.g., heart rate and ratings of ‘high’). These data generally agree with previous studies that have compared low doses of oral methamphetamine and d-amphetamine [14,15,16]; they extend earlier findings by providing the first data directly comparing the reinforcing, subjective, and physiological effects of the two stimulants administered intranasally.
As predicted, methamphetamine and d-amphetamine (50 mg/70 kg) similarly increased drug self-administration; regardless of amphetamine, participants chose approximately 47–50% drug. This is consistent with results from the preclinical literature indicating that rats and rhesus monkeys self-administer both amphetamines at equivalent rates [12,13]. On the other hand, when $20 was the alternative reinforcer, both amphetamines were self-administered no more than placebo. Thus, amphetamine self-administration is malleable in the presence of higher and lower magnitude reinforcers and provides further evidence that monetary incentives may be particularly efficacious in substance abuse treatment programs that rely on alternative reinforcers to reduce problematic stimulant use [see ref. 29 for review>]. It is important to note, however, that it is unclear whether the current self-administration data would generalize to illicit methamphetamine use in the natural ecology. During the current study participants were given the opportunity to self-administer amphetamines in the morning and then were required to complete a four-hour work period consisting of computerized tasks. This procedure is quite different from recreational amphetamine-taking outside of the laboratory [17,30,31]. It is possible that we would have observed a different pattern of self-administration had we administered the drug during the evening, in a social setting with fewer work requirements. Nevertheless, the current data do not support the view that methamphetamine is a more potent reinforcer in humans compared with d-amphetamine.
We hypothesized that the drugs would produce equipotent mood effects. For many subjective-effects measures, this prediction was borne out. Methamphetamine and d-amphetamine increased ratings of ‘good drug effect’ and ‘social’ and decreased ratings of ‘tired’ to the same extent. On the other hand, methamphetamine-related effects were significantly greater for several other mood ratings. For instance, compared to d-amphetamine, the large methamphetamine dose produced greater increases in DEQ ratings of ‘good effects’ and ‘drug strength.’ Furthermore, between the smaller doses (12 mg), only methamphetamine increased several mood ratings including ‘alert,’ ‘energetic,’ and ‘good drug effect.’ These observations appear to be inconsistent with data from previous studies indicating that the drugs produced nearly identical subjective effects [16]. One explanation for this discrepancy is that the previous studies administered low oral doses (e.g., 10 mg). In this study, the drugs were given via the intranasal route, which is associated with a relatively faster onset of effects [26,32]. It is possible that some amphetamine-related effects are subtle and can only be detected following drug administration via a route associated with a rapid onset of effects. An alternative explanation is that the previous studies employed participants with no methamphetamine experience. By contrast, participants in the present study were current illicit methamphetamine users who used the drug regularly. Considering that acute drug effects can be influenced by use experience and learned associations [33,34], it is possible that the more prominent subjective responses caused by methamphetamine were partially due to a learned response to potentially subtle methamphetamine-related interoceptive cues. Of course, this possibility is speculative and highlights the importance of replication. Nevertheless, it is important to note that the current mood and self-administration data provide further evidence for the dissociation between drug-related reinforcing and subjective effects [20,21] and emphasize the importance of assessing multiple measures when evaluating the acute effects of drugs of abuse.
Concordant with the subjective effects findings, both amphetamines enhanced cardiovascular activity. The larger dose (50 mg) produced equivalent increases on blood pressure. Although both drugs increased heart rate, methamphetamine engendered greater sustained increases than d-amphetamine. Furthermore, approximately 24 hours after drug administration, heart rate remained elevated under both large amphetamine conditions and the 12-mg methamphetamine condition. In general, these results are consistent with previous findings from separate investigations of the acute cardiovascular effects of intranasal d-amphetamine [35] and the acute and residual effects of intranasal methamphetamine (26,36]. However, the current cardiovascular data should be interpreted within the context of a potential limitation: this study was conducted in an outpatient setting. Thus, several uncontrolled factors may have potentially influenced amphetamine-related residual cardiovascular effects. For instance, it is possible that participants may have consumed drugs outside of the laboratory that were undetected by standard urine toxicology. Future studies might investigate the relative residual effects of methamphetamine and d-amphetamine in an inpatient setting.
In conclusion, these results show that: 1) intranasal methamphetamine and d-amphetamine produced similar reinforcing effects in humans; and 2) amphetamine self-administration was malleable in the presence of an alternative monetary reinforcer. Additionally, both amphetamines similarly increased ratings of euphoria and cardiovascular activity and improved psychomotor performance. Although a nearly identical profile of effects was observed, methamphetamine-related effects were greater on some measures, including heart rate and several mood ratings. While it is possible that intranasal methamphetamine, given in relatively large doses, is more potent than d-amphetamine on these measures, an alternative explanation is related to the extensive methamphetamine experience of the participants. It is possible that a methamphetamine user’s response to her/his drug of choice is partially based on learned associations. Of course, this speculation relies on the prediction that methamphetamine and d-amphetamine are distinguishable by humans under appropriate conditions; future studies might address this issue by investigating the discriminative stimulus effects of the amphetamines employing a route of administration and dose levels commonly associated with abuse. Overall, these data provide support for the research strategy of administering d-amphetamine in order to investigate the behavioral and pharmacological variables that contribute to abuse of other amphetamines, including methamphetamine. Finally, given that methamphetamine is more likely than d-amphetamine to be abused in the natural ecology, the present data showing that these amphetamines produced predominately similar effects suggest that methamphetamine abuse is likely influenced by non-pharmacological factors.
Acknowledgements
The medical and nursing assistance of Drs. Elias Dakwar, Soteri Polydorou, and Audrey Perez, RN and technical assistance of Drew Thurmond, Michaela Bamdad, Maris Schwartz, and Marc Scullin are gratefully acknowledged. This research was supported by grant number DA-19559 awarded to Dr. Hart from the National Institute on Drug Abuse.
Footnotes
Declaration of interest: This research was funded by a grant awarded to Dr. Hart from the National Institute on Drug Abuse. The authors declare that except for the income received from our primary employer no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. There are no constraints on publishing these data.
References
- 1.Bartholow M. Top 200 prescription drugs of 2009. [Accessed July 12, 2011];Pharmacy Times Web site. 2010 www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. [Google Scholar]
- 2.Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 3.Grelotti DJ, Kanayama G, Pope HG., Jr Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: presentation of a case and review of the literature. Am J Psychiatry. 2010;167:17–23. doi: 10.1176/appi.ajp.2009.08111695. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set (TEDS) Highlights - - 2007 National Admissions to Substance Abuse Treatment Services. Rockville, MD: 2009. OAS Series #S-45, HHS Publication No. (SMA) 09-4360. [Google Scholar]
- 5.Gulaboski R, Cordeiro MN, Milhazes N, Garrido J, Borges F, Jorge M, et al. Evaluation of the lipophilic properties of opioids, amphetamine-like drugs, and metabolites through electrochemical studies at the interface between two immiscible solutions. Anal Biochem. 2007;361:236–243. doi: 10.1016/j.ab.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Nichols DE. Medicinal chemistry and structure–activity relationships. In: Cho AK, Segal DS, editors. Amphetamine and its Analogs. San Diego, CA: Academic Press; 1994. pp. 3–41. [Google Scholar]
- 7.Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- 8.Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology. 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- 9.Peachey JE, Rogers B, Brien JF. A comparative study of the behavioural responses induced by chronic administration of methamphetamine and amphetamine in mice. Psychopharmacology. 1977;51:137–140. doi: 10.1007/BF00431729. [DOI] [PubMed] [Google Scholar]
- 10.Hall DA, Stanis JJ, Marquez Avila H, Gulley JM. A comparison of amphetamine-and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology. 2008;195:469–478. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn DM, Appel JB, Greenberg I. An analysis of some discriminative properties of d-amphetamine. Psychopharmacologia. 1974;39:57–66. doi: 10.1007/BF00421458. [DOI] [PubMed] [Google Scholar]
- 12.Balster RL, Schuster CR. A comparison of d-amphetamine l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- 13.Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]
- 14.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 15.Lamb RJ, Henningfield JE. Human d-amphetamine drug discrimination: methamphetamine and hydromorphone. J Exp Anal Behav. 1994;61:169–180. doi: 10.1901/jeab.1994.61-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative stimulus and subject-rated effects of methamphetamine d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- 18.de Wit H, Dudish S, Ambre J. Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology. 1993;112:324–330. doi: 10.1007/BF02244928. [DOI] [PubMed] [Google Scholar]
- 19.Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276:1580–1588. [PubMed] [Google Scholar]
- 20.Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- 21.Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- 22.Nader MA, Woolverton WL. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 1992;108:295–300. doi: 10.1007/BF02245115. [DOI] [PubMed] [Google Scholar]
- 23.Campbell UC, Carroll ME. Reduction of drug self-administration by an alternative non-drug reinforcer in rhesus monkeys: magnitude and temporal effects. Psychopharmacology. 2000;147:418–425. doi: 10.1007/s002130050011. [DOI] [PubMed] [Google Scholar]
- 24.Hatsukami DK, Pentel PR, Glass J, Nelson R, Brauer LH, Crosby R, et al. Methodological issues in the administration of multiple doses of smoked cocaine-base in humans. Pharmacol Biochem Behav. 1994;47:531–540. doi: 10.1016/0091-3057(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 25.Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- 26.Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, et al. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Instr Comp. 1982;14:463–466. [Google Scholar]
- 28.Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- 29.Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacol Biochem Behav. 1997;57:419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- 30.Halkitis PN, Green KA, Mourgues P. Longitudinal investigation of methamphetamine use among gay and bisexual men in New York City: findings from Project BUMPS. J Urban Health. 2005;82:18–25. doi: 10.1093/jurban/jti020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly BC, Parsons JT, Wells BE. Prevalence and predictors of club drug use among club-going young adults in New York city. J Urban Health. 2006;83:884–895. doi: 10.1007/s11524-006-9057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- 33.Alessi SM, Roll JM, Reilly MP, Johanson CE. Establishment of a diazepam preference in human volunteers following a differential-conditioning history of placebo versus diazepam choice. Exp Clin Psychopharmacol. 2002;10:77–83. [PubMed] [Google Scholar]
- 34.Kirk JM, de Wit H. Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- 35.Lile JA, Babalonis S, Emurian C, Martin CA, Wermeling D, Kelly TH. Comparison of the behavioral and cardiovascular effects of intranasal and oral d-amphetamine in healthy human subjects. J Clin Pharmacol. 2011;51:888–898. doi: 10.1177/0091270010375956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, et al. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2008;94:258–262. doi: 10.1016/j.drugalcdep.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]