Abstract
Epidemiological and laboratory studies link polychlorinated biphenyls and their metabolites to adverse neurodevelopmental outcomes. Several neurotoxic PCB congeners are chiral and undergo enantiomeric enrichment in mammalian species, which may modulate PCB developmental neurotoxicity. This study measures levels and enantiomeric enrichment of PCB 95 and its hydroxylated metabolites (OH-PCBs) in adult female C57Bl/6 mice following subchronic exposure to racemic PCB 95. Tissue levels of PCB 95 and OH-PCBs increased with increasing dose. Dose-dependent enantiomeric enrichment of PCB 95 was observed in brain and other tissues. OH-PCBs also displayed enantiomeric enrichment in blood and liver, but were not detected in adipose and brain. In light of data suggesting enantioselective effects of chiral PCBs on molecular targets linked to PCB developmental neurotoxicity, our observations highlight the importance of accounting for PCB and OH-PCB enantiomeric enrichment in the assessment of PCB developmental neurotoxicity.
Keywords: Atropisomers, cytochrome P-450 enzyme, dose-response, atropisomeric enrichment, hydroxylated PCB metabolites, polychlorinated biphenyls, qPCR
INTRODUCTION
Polychlorinated biphenyls (PCBs) remain environmental contaminants of concern to public health, more than 30 years after their production was banned in the United States. Their ubiquitous distribution and persistence in the environment result in constant, low-level human PCB exposure via inhalation of contaminated indoor air1 and dietary intake.2 A number of epidemiological studies demonstrate a correlation of maternal PCB levels with neurological deficits in children.3,4 Adverse effects reported in children include decreased IQ, impaired learning and memory, attention deficits, reduced reading comprehension, and altered psychomotor development. Similarly, laboratory animal studies consistently demonstrate adverse neurodevelopmental outcomes following gestational and lactational PCB exposure.3
Several mechanisms have been proposed to mediate PCB developmental neurotoxicity, including altered Ca2+ signaling, interference with thyroid hormone signaling and decreased dopamine content.3 Recently, PCBs and hydroxylated PCB metabolites (OH-PCBs) with > 2 ortho substitutions have been linked to disruptions in Ca2+ signaling and subsequent adverse effects on neurodevelopment and behavior.5-9 The most sensitive mechanism mediating effects of PCBs on neuronal Ca2+ signaling is ryanodine receptor (RyR) sensitization,10 and this molecular effect has been causally linked to changes in dendritic growth.11 Multiple-ortho substituted PCB congeners, such as PCB 95 and PCB 136, are the most potent sensitizers of RyR.9 Both of these PCBs are chiral congeners that exist as two stable rotational isomers called atropisomers (a subclass of enantiomers) that are non-superimposable mirror images of each other.12 Interestingly, the interaction of pure PCB 136 atropisomers with RyR is enantiospecific.13
PCBs cross the blood-brain barrier and can interact with targets, such as RyR, in the brain. They are found in the human brain14 and also in the brain of laboratory animals after acute exposure by different routes, including intraperitoneal injection,15,16 oral administration17-19 and inhalation.20 Significant enantiomeric enrichment of neurotoxic, chiral PCB congeners, including PCB 95 and PCB 136, has been reported in the brain of mice,15,17-19 whereas chiral PCBs were nearly racemic in human brain.14 The enantiomeric enrichment in mice is thought to be due to enantioselective metabolism of chiral PCBs by cytochrome P450 (P450) enzymes to OH-PCBs.21-23 OH-PCBs can be formed by P450 enzymes through direct insertion of oxygen into an aromatic C-H bond or via formation of a PCB epoxide that subsequently rearranges to an OH-PCB.24 The rearrangement of the PCB epoxide can result in an NIH-shift, a change in the chlorination pattern.24,25 The produced OH-PCBs are also chiral and, as several studies have demonstrated, are formed enantioselectively by P450 enzymes.21-23
Studies reporting levels of OH-PCBs in the brain from PCB-exposed laboratory animals or humans are scarce. A developmental disposition study demonstrated that 4-107 (2,3,3′,4′,5-pentachlorobiphenyl-4-ol), a OH PCB found in humans, can transfer across the placenta and the blood-brain barrier into the brain of rat fetuses in a fashion similar to PCBs.8 4-107 is retained in the brain of fetal rats, presumably due to the structural similarity between OH-PCBs and thyroxine.8 In the same study, the levels of 4-107 in brain decreased after weaning, although it was still present in the blood.26 This implies that the blood-brain barrier may not protect the developing brain from exposure to OH-PCBs and the consequent adverse effects of this exposure.26
Although the evidence suggests that enantioselective disposition of neurotoxic chiral PCBs may modulate functional outcomes in developmentally exposed individuals, many unanswered questions remain regarding the enantioselectivity of PCB disposition and toxicity. This study focuses on chiral PCB disposition, and reports novel data demonstrating dose-dependent changes in levels and chiral signatures of PCB 95, a highly neurotoxic PCB congener, and its potentially neurotoxic hydroxylated metabolites in adult mice subchronically exposed to racemic PCB 95. Our results show significant enantiomeric enrichment of PCB 95 in the brain and in other tissues. Furthermore, OH-PCBs display significant enantiomeric enrichment in several tissues. Overall, this study suggests an important, but currently overlooked, role of enantiomeric enrichment of OH PCBs in developmental neurotoxicity.
EXPERIMENTAL SECTION
Chemicals
Two surrogate standards, 2,3,4′,5,6-pentachlorobiphenyl (PCB 117; 99% purity) and 2′,3,3′,4′,5,5′-hexachlorobiphenyl-4-ol (4-159, 100% purity), as well as 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95; 99.7% purity), and an internal standard, 2,2′3,4,4′,5,6,6′-octachlorobiphenyl (PCB 204; 99.9% purity), were purchased from AccuStandard (New Haven, CT, USA). 3 Methoxy 2,2′,4,5′,6-pentachlorobiphenyl (3-103), 2,2′,3,5′,6-pentachlorobiphenyl-4-ol (4-95), 2,2′,3,5′,6-pentachlorobiphenyl-5-ol (5-95) and 4,5-dimethoxy-2,2′,3,5′,6-pentachlorobiphenyl (4,5-95) were synthesized at > 95% purity as described previously.27,28
Animals and PCB exposure
Animals were treated according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Twenty female C57Bl/6 mice of breeding age (7 weeks old) were obtained from Charles River Laboratory (Hollister, CA, USA) and housed individually in standard plastic shoebox cages with corncob bedding in a temperature controlled room (22±2°C) on a 12 h reverse light-dark cycle. PicoLab Mouse Diet 20 (PMI Nutrition International, Brentwood, MO, USA) and water were provided ad libitum. After a 7 day acclimation period, mice were dosed daily with PCB 95 at 0.1 (low), 1.0 (medium) or 6.0 (high) mg/kg body weight (b.w.)/day for 39 days via oral administration in peanut butter (see Supporting Information for detailed description). Similar subchronic exposures corresponding to the length of time of gestation and lactation are frequently employed in studies of PCB developmental neurotoxicity.29 Control animals received the vehicle (peanut butter) only. Mice were weighed daily and the amount of peanut butter was adjusted to maintain the dose level. Mice were watched carefully to ensure that all the peanut butter was consumed (typically within 2-3 min). Twenty four hours after the last treatment, mice were euthanized by cervical dislocation. Blood was collected by cardiac puncture and placed into glass vials; liver, brain and abdominal adipose were harvested, wrapped in aluminum foil, and snap frozen in liquid nitrogen. Blood and harvested tissues were stored at −80°C until analyzed.
RNA isolation
Total RNA was extracted from liver and brain using the Qiagen RNeasy Mini Kit (Germantown, MD, USA) according to the manufacturer’s instructions. Following digestion of samples with DNAseI (Invitrogen, Carlsbad, CA, USA), total RNA was reverse transcribed to cDNA (Superscript III First Strand Synthesis kit, Invitrogen) according to the manufacturer’s protocol.
qPCR assay
Primer and probe sets specific for CYP1A2, CYP2B10, CYP3A11 and CYP2S1 were designed using PrimerBlast from NCBI (Bethesda, MD, USA) and PrimerQuest software (IDT, Coralville, IA, USA). A table with the primer sequences is provided in Table S1, Supporting Information. For the reference gene (murine Pgk1), standard qPCR assays were purchased from IDT.30 Amplification efficiencies are presented in Table S2, Supporting Information. Messenger RNA transcript levels of the selected P450 enzymes were quantified by qPCR and relative expression levels in treated animals versus controls were calculated using the REST2009 software (Qiagen, Valencia, CA).31-33
Tissue extraction
Adipose (0.03-0.11 g), brain (0.08-0.14 g) or liver (0.15-0.29 g) samples were thoroughly mixed with 2 g of pre-extracted diatomaceous earth and placed in the 33 mL extraction cell over 12 g of pre-extracted Florisil. The cells were spiked with the surrogate standard (50 ng PCB 117 in 0.1 mL isooctane; 68.5 ng 4-159 in 50 μL isooctane). The samples were extracted with hexane:dichloromethane:methanol (48:43:9, v/v) at 100 °C, 1500 psi (10 MPa) with pre-heat equilibration of 6 min, 35% cell flush volume and 1 static cycle of 5 min using a pressurized solvent extraction system (ASE 200, Dionex, Sunnyvale, CA).34 The extracts were concentrated to approximately 1 mL in a TurboVap (43 °C, 5 psi; Caliper Life Sciences, Hopkinton, Massachusetts) and derivatized with diazomethane to convert hydroxylated PCBs (OH-PCBs) into methoxylated derivatives. Afterwards, samples were subjected to a sulfur clean-up step, as described previously.18,34
Whole blood (0.10-0.45 g) was spiked with the surrogate standard (50 ng PCB 117 in 0.1 mL isooctane; 68.5 ng 4-159 in 50 μL isooctane) and treated with hydrochloric acid to denature proteins.35 Samples were then extracted with 2-propanol and hexane-MTBE mixture (5 mL, 1:1, v/v). The organic extracts were washed with potassium chloride and the solvent was exchanged to hexane. Afterwards, the samples were derivatized with diazomethane and subjected to a sulfur clean-up step, as described previously.18,34
Gas chromatographic analysis
Tissue levels of PCB 95 and OH-PCB 95 metabolites were quantified using an Agilent 7870A gas chromatograph equipped with a 63Ni electron capture detector (μ-ECD) and a SPB 1 column (60 m, 0.25 mm ID, 0.25 μm film thickness; Supelco, St. Louis, MO, USA). The injector temperature was 280 °C, and the detector temperature was 300 °C. The following temperature program was employed to separate the analytes: 50 °C for 1 min, 30 °C/min to 200 °C, 1 °C/min to 240 °C, hold 4 min. Concentrations of PCB 95 and its hydroxylated metabolites were determined in a single analysis using PCB 204 as an internal standard.
The enantioselective analysis was performed on an Agilent 7890 gas chromatograph equipped with a 63Ni μ-ECD and several enantioselective columns: Chirasil Dex (CD, 2,3,6-tri-O-methyl-β-cyclodextrin, 30 m × 250 μm × 0.39 μm, Agilent, Santa Clara, CA), BGB-172 (BGB, 20% tert-butyldimethyl-silyl-β-cyclodextrin, 30 m × 250 μm × 0.25 μm, BGB Analytics, Boecten, Switzerland) and ChiralDex B-DM (BDM; 2,3-di-O-methyl-6-tert-butyl-silyl-β-cyclodextrin, 30 m × 250 μm × 0.12 μm, Supelco, St. Louis, MO). The resolution, or Rs, of the atropisomers was 0.53 ± 0.05 for PCB 95 and 0.91 ± 0.10 for 5-95 on the CD column. The BGB column separated 4-95 (Rs = 0.65 ± 0.09). The BDM column separated PCB 95 with a better resolution (Rs = 1.3 ± 0.2) than the CD column and also separated atropisomers of 4,5-95 (Rs = 0.94 ± 0.12). The analysis on both enantioselective columns were performed under the following conditions: 10 °C/min from 35 °C to 140 °C, hold for 420 min, 10 °C/min to 200 °C, hold for 15 min. No co-elution problems were encountered with either column under these conditions. The injector and detector temperature was 250°C and the helium flow was 3 mL/min. The enantiomeric fractions were calculated as EF = A1/(A1+A2) where A1 and A2 are the peak area of the first (E1) and the second eluting (E2) atropisomers, respectively, since the elution order of the atropisomers is not known.
Statistics
Animal body weights were analyzed by two-way repeated measures ANOVA using GraphPad Version 4.00 (GraphPad Software, La Jolla, CA). The differences between PCB 95 and OH-PCBs levels between doses and tissues were tested using two-way ANOVA with Tukey post-hoc test. Differences were considered statistically significant if p < 0.05. The R software (version 2.14.1; www.r-project.org) was used for these statistical analyses. Statistical significance in relative expression changes in P450 mRNA levels was determined by automated randomization and bootstrapping tests built into the REST2009 software (http://www.genequantification.de/REST_2009_Software_User_Guide.pdf).
PCB 95 level and EF value estimations
Tissue levels and EF values of PCB 95 were estimated with WINFUNFIT, an interactive Windows (Microsoft) version of the general nonlinear regression program FUNFIT,36 using published toxicokinetic data for C57Bl/6 mice.35
RESULTS AND DISCUSSION
General toxicity
Female C567Bl/6 mice were treated daily for 39 days with a low (0.1 mg/kg b.w./day), medium (1 mg/kg b.w./day) or high dose (6 mg/kg b.w./day) of PCB 95, a neurotoxic PCB congener.9 PCB 95 treatment had no effect on final body weight, growth rate or other signs of general toxicity at any dose level investigated (Fig. S1, Supplementary Data). This is in agreement with previously published studies with PCB 9537,38 and other chiral congeners in rodents.17,18,39
Liver mRNA levels
CYP1A2, CYP3A11, CYP2B10 and CYP2S1 mRNA levels were determined by qPCR analysis in liver and brain. These P450 enzymes are potentially involved in the metabolism of PCBs and/or are transcriptionally regulated by PCBs.40,41
In liver, there was a 1.9 and 2.2-fold induction of CYP1A2 mRNA compared to control in the low and medium dose treatment groups, respectively; however, CYP1A2 transcript levels were not changed relative to control in the high dose treatment group (Fig. 1; Table S3, Supporting Information). Similarly, there was a 2.4 and 2.3-fold increase in hepatic CYP3A11 levels compared to control in the low and medium dose treatment groups, respectively, but no change in the high dose treatment group. Hepatic CYP2B10 expression was increased 1.6 and 3.7-fold in the medium and high dose treatment groups, respectively; however, there was no change in CYP2B10 mRNA levels in the low dose treatment group. Hepatic CYP2S1 mRNA levels and brain P450 mRNA levels were not affected by PCB treatment (data not shown).
Fig. 1.
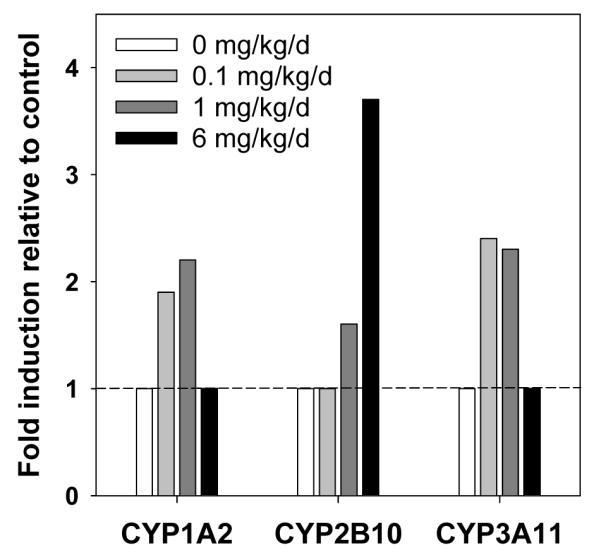
Increase in hepatic mRNA levels of CYP1A2, CYP2B10 and CYP3A11 in female C57Bl/6 mice orally exposed to PCB 95 for 39 days, relative to control animals (P450 mRNA levels normalized to mRNA levels of the reference gene Pgk1 in the same sample). Values above 1 indicate significant increase.
Exposure to certain PCB congeners can increase CYP1A2 and CYP3A11 protein levels and activities in some species,40,42 and both CYP1A and CYP3A enzymes have been shown to metabolize PCBs.43,44 The observation that subchronic exposure to PCB 95 significantly increases gene expression of CYP1A2 and CYP3A11 in the low and medium dose treatment group, but not in the high dose treatment group is consistant with earlier studies. For example, Rodman et al.45,46 showed that 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) and 2,2′,3,4,4′,6-heptachlorobiphenyl (PCB 139), cause non-monotonic dose-related effects of total cytochrome P450 concentration and ethoxyresorufin-O-deethylase (EROD) activity in chick embryo hepatocytes. Similarly, a study by Lai et al.47 showed a non-monotonic effect of PCB 126 on EROD activities in rats.
Chiral PCBs with multiple ortho substitutions not only induce CYP2B enzyme activities in rats,40,48 but are also oxidized by CYP2B enzymes.21-23,49 Chiral PCBs 139 and 197 are potent inducers of rat CYP2B (measured as aminopyrine N-demethylation activity), with PCB 139 being more potent than PCB 197.39 PCB 95 has also been shown to dose-dependently induce CYP2B activity, measured as pentoxyresorufin O-deethylase activity following sub-acute exposure of male Sprague Dawley rats, although this increase did not reach statistical significance.38 We observed that PCB 95 significantly increased CYP2B10 mRNA in mice in a dose-dependent manner. Although changes in mRNA levels can be transient and do not necessarily correlate with P450 enzyme activity in rodents exposed to PCBs,50 it seems probable that the dose-dependent increase in CYP2B10 mRNA levels correlates with increased CYP2B activity in mice treated with PCB 95. Since PCB 95 is metabolized by CYP2B enzymes to OH-PCBs,21,23 the induction of CYP2B10 is expected to modulate the levels and enantiomeric enrichment of both PCB 95 and/or its hydroxylated metabolites.
CYP2S1 is an “orphan” P450 found mostly in extra-hepatic tissues.51 Its physiological substrates are undetermined.52 Only limited information is currently available about the role of PCBs in the transcriptional regulation of CYP2S1 in rodents. A recent study by Wang et al.41 demonstrated that PCB 126 did not alter CYP2S1 mRNA levels in in male Sprague Dawley rats. In our study, mRNA levels in liver were not altered by exposure to PCB 95 at any dose level, which suggests that subchronic PCB 95 exposure does not alter CYP2S1 expression in mice.
PCB 95 levels in tissues
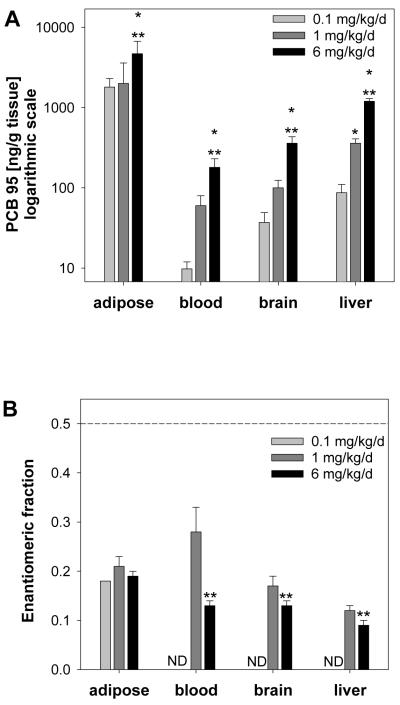
PCB 95 levels were measured in blood, adipose, brain and liver collected from C57Bl/6 female mice. The levels of PCB 95 were significantly higher in adipose than in the other tissues investigated and decreased in the rank order adipose > liver > brain > blood (Fig. 2A, Table S5). For all treatment groups, the adipose levels were 20 to 50 times higher than liver, 50 to 200 times higher than brain and 200 to 300 times higher than blood levels. These observations confirm previous reports indicating that the highest levels of chiral PCB congeners, including PCBs 95 and 136, are found in the adipose of mice, which is the major storage site for lipophilic compounds.17-19
Fig. 2.
PCB 95 levels (A) and enantiomeric fractions (B) in tissues of female C57Bl/6 mice orally exposed to PCB 95 daily for 39 days. The data shown in panel B were generated from the samples analyzed at 140 °C on a BDM column; the dashed line represents the EF of the racemic standard (EF = 0.50 ± 0.00). ND indicates levels were non-detectable. Data are presented as the mean ± SD. * Significantly different than low dose treatment group, p<0.05; ** significantly different than medium dose treatment group, p<0.05.
PCB 95 levels in all tissues increased with increasing dose, with the levels of PCB 95 in the high dose treatment group higher than the levels in either the low or medium dose treatment group. The 10-fold increase in dose from the low to the medium dose treatment group resulted in a corresponding 10-fold increase in adipose levels of PCB 95, but only a 3 to 6-fold increase in brain, liver and blood levels, respectively. The 6-fold dose increase from the medium to the high dose treatment group resulted in only 2.5 to 4-fold increase in tissue levels of PCB 95. Overall, the fold-increase in the PCB 95 tissue concentrations did not correspond to the fold-increase in dose. A similar observation has been reported in a study investigating the disposition of PCB 136 after administration of a single, oral PCB 136 dose.17 One potential explanation for the nonlinear increase in PCB 95 levels with increasing dose is a more rapid elimination of PCB 95 due to an increase in the activity of P450 enzymes following PCB treatment (see previous section).
The PCB 95 levels determined in the female mouse brain in this study (37-360 ng/g) are higher than the total PCB levels determined in human brain samples from Poland (1.9-7.0 ng/g)53 and Belgium (12 ng/g),14 but comparable to the values (64 and 84 ng/g) reported in two older British males54 and a Yucheng patient (80 ng/g).55 Although these studies did not specifically report PCB 95 levels, the fact that the levels found in the present study are comparable to brain PCB levels found in humans indicates that the PCB 95 doses used result in environmentally relevant brain PCB levels.
Enantiomeric fractions of PCB 95
To understand how the enantiomeric fractions change after repeated doses of PCB 95, the enantiomeric fractions of PCB 95 in different tissues were determined using a BDM (Fig. 2B) and a CD column (Table S6, Supporting Information). There was good agreement between the EF values determined with both enantioselective columns. The E2-PCB 95 was enriched in blood, brain, liver and adipose in all three treatment groups. The enrichment of E2-PCB 95 in adipose seemed to be less pronounced compared to other tissues. The average EF value in adipose across treatment groups was ≈ 0.19. The most pronounced enantiomeric enrichment was observed in the liver and in blood, with an average EF across treatment groups of approximately 0.10.
Similar to the present study, enantiomeric enrichment of E2-PCB 95 in adipose and blood was less pronounced (EF ≈ 0.30) than that observed in brain (EF ≈ 0.24) and liver (EF ≈ 0.22) in a toxicokinetic study with C57Bl/6 mice receiving a single dose of a synthetic PCB mixture containing PCB 95 and other chiral congeners.35 Similar trends were also observed by Milanowski et al.19 in FVB mice treated with the same PCB mixture, with EFs of 0.18 and 0.35 in the liver and adipose, respectively. In contrast, the E1-PCB 95 was enriched in adipose and liver of Sprague Dawley rats which received a single intraperitoneal dose of either Aroclor 1254 or an environmental PCB mixture (soil extract).16 E2-PCB 95 was enriched in blood from animals receiving the environmental mixture, but not in animals receiving Aroclor 1254. These emerging findings suggest pronounced species dependent differences in the disposition of neurotoxic PCB atropisomers in toxicologically relevant animal models.
An intriguing observation is the statistically significant increase in enantiomeric enrichment (i.e., a decrease in the EF value) in blood, brain and liver from animals in the high dose treatment groups compared to the medium dose treatment group (Fig. 2B), with a lack of difference in enantiomeric enrichment in adipose between treatment groups. The more pronounced enantiomeric enrichment at the higher dose treatment group is surprising because enantioselective biotransformation can become saturated at high doses,49 thus resulting in a less pronounced enantiomeric enrichment with increasing dose.17,18 Indeed, the expected inverse relationship between dose and EF values has been observed after administration of single, oral doses ranging from 2.5 to 50 mg/kg of PCB 136.17,18 Several factors (i.e., differences in dose, repeated versus single exposures, and differential induction of drug metabolizing enzymes) may explain the differences between observed and expected effects of dose on the extent of the enantiomeric enrichment in our study versus previously published studies.
To understand how subchronic exposure and dose influence PCB tissue levels and EF values, tissue levels (Table S7) and enantiomeric fractions (Table S8) of PCB 95 were estimated using published toxicokinetic parameters.35 These estimations assume linear, non-saturable kinetics. While the simulation accurately predicted the decrease in tissue PCB 95 levels in the order adipose > liver > brain > blood, the tissue levels were significantly overestimated compared to the experimental data. Furthermore, the simulation predicted that an increase in dose would result in a corresponding increase in the respective tissue levels, which also is in contrast with our results. The predicted enantiomeric fractions were underestimated and essentially independent of the dose.
The discrepancies between estimated and experimental tissue PCB 95 levels and EF values are likely due to the dose-dependent induction of CYP2B activity following subchronic PCB 95 exposure. This interpretation is supported by the dose-dependent increase in CYP2B10 gene expression. Consequently, the induction of CYP2B enzymes following subchronic PCB exposure apparently influences the enantiomeric enrichment of PCB 95 in mice in a dose-dependent manner. Since human CYP2B6 is a highly inducible enzyme that enantioselectively metabolizes chiral PCBs,23 induction of this P450 enzyme by environmental contaminants and drug molecules is also expected to alter the enantioselective disposition of chiral PCBs in humans, especially after repeated exposure to environmental PCBs. Such inter-individual differences in CYP2B6 activities may in part explain the variability of enantiomeric fractions of PCBs observed in human samples. Particularly in the case of developmental PCB exposures, altered CYP2B activity resulting from PCB-mediated enzyme induction and/or genetic polymorphisms would be expected to modulate the developmental neurotoxicity of PCBs in laboratory animals and humans by altering the levels of PCB atropisomers that cross the placenta during pregnancy and reach the developing brain.
OH-PCB levels in tissues
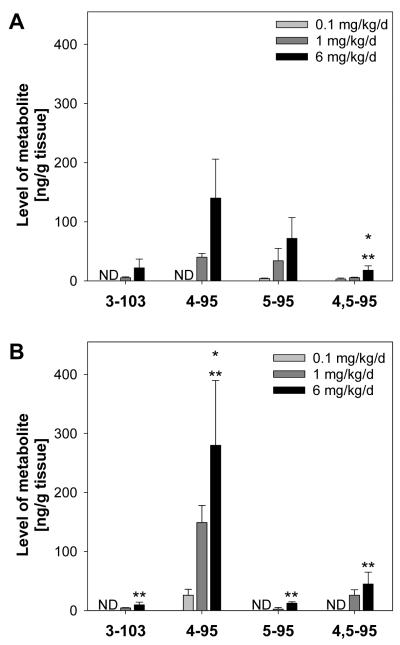
OH-PCB metabolites are potent RyR sensitizers and, thus, may also contribute to the developmental neurotoxicity of PCBs. Since little is known about the disposition of OH-PCBs in mice, especially after subchronic PCB treatment, the levels of 3-103 (NIH shift product,25), 4-95, 5-95 and 4,5-95 were determined in blood and liver using authentic standards (Fig. 3A and B, Table S9, Supporting Information). As reported by Sundström et al.,56 additional metabolites of PCB 95 may have been present, but these metabolites could not be identified or quantified due to the unavailability of analytical standards.
Fig. 3.
Levels of OH-PCB metabolites in (A) liver and (B) blood of female C57Bl/6 mice orally exposed to PCB 95 over 39 days. The levels in the low dose-treatment group were not different from the control animals (see Table S4), and levels in the higher dose treatment groups were dose dependent. Data are presented as the mean ± SD. * Significantly higher than low dose treatment group, p<0.05; ** significantly higher than medium dose treatment group, p<0.05.
The sum of OH-PCBs (ΣOH-PCBs) in blood (Table S9, Supporting Information) was 2 to 3 times higher than the concentration of PCB 95. In liver, the ΣOH-PCBs was 4 to 5 times lower than the levels of the parent compound, suggesting translocation of PCB metabolites from the liver into the blood circulation. The ΣOH-PCBs increased with increasing dose of PCB 95 (Fig. 3A and B; Table S9, Supporting Information). However, similar to PCB 95 levels, the ΣOH-PCBs did not correspond directly to the increase in dose. Specifically, the 10-fold increase in dose from low to medium dose treatment group resulted only in a 5-fold increase in the ΣOH-PCBs in both blood and liver. The 6-fold increase of the dose from the medium to high dose treatment group resulted in 2- and 3-fold higher levels of ΣOH-PCBs in blood and liver, respectively. One possible explanation for the observed trends in ΣOH-PCB levels is dose-dependent induction of phase I and phase II enzymes, including CYP2B10, due to subchronic PCB 95 treatment (Figure 1).
Levels of 3-103, 4-95, 5-95 and 4,5-95 in adipose and brain samples were below their respective detection limits of 11 to 16 ng/g tissue (Table S4, Supporting Information). Parent PCB 95 levels in brain (360 ng/g) were 33 times higher compared to the corresponding detection limit (11 ng/g) in the high dose treatment group, which suggests that the levels of OH-PCBs in brain—if present just below limit of detection—were at least one order of magnitude lower compared to parent PCB levels. Consequently, the OH-PCBs investigated in this study may not make a major contribution to the neurotoxicity of PCB 95 in adult mice. However, Meerts and co-workers have shown that considerable levels (~1 μg/g tissue) of 4-107 can be detected in fetal brains after oral administration of 4-107 to pregnant Wistar rats.8 In the same study, this metabolite was below detection in post-weanling rats. Analogously, hydroxylated metabolites of PCB 95 formed in the dam may either cross the placenta and enter the fetal brain or be produced in the brain by local P450 activity, with adverse neurodevelopmental effects depending on exposure level and duration, and on vulnerability of the brain at the time of exposure. It is further expected that dose-dependent enantiomeric enrichment of the parent PCB and/or its metabolites in the dam would influence the levels of parent and/or metabolite enantiomers that reach the fetal brain at different levels of maternal PCB intake.
Levels of individual OH-PCB metabolites
Hydroxylated metabolites levels decreased in the order 4-95 > 4,5-95 ~ 3-103 ~ 5-95 in blood at all PCB 95 doses (Fig. 3B and Table S9, Supporting Information). In liver, 4-95 was the major metabolite in the high dose treatment group. In the medium dose treatment group, 4-95 and 5-95 were the major metabolites and were present at similar concentrations. In the low dose treatment group, metabolite levels followed the order 5-95 > 3-103 ~ 4,5-95, with 4-95 being below the detection limit. In addition, a minor metabolite with the hydroxyl group in the lower chlorinated phenyl ring, X’-95, was present in both blood and liver samples at levels similar to 5-95 (data not shown). This mono-hydroxylated metabolite has been detected in an in vitro metabolism study with rat microsomes prepared from phenobarbital (PB)-treated rats21 and was identified in the present study based on its retention time. The slight differences in the OH-PCB profile may be due to dose-dependent changes in PCB 95 metabolism resulting from the induction of P450 or other, phase II metabolism enzymes.
There are only a few reports on the formation of OH-PCBs from PCB 95. In an in vitro study with rat liver microsomes prepared from PB-treated rats, 5-95 was formed as the major metabolite.21 As mentioned above, X’-95 was also detected. In contrast to the current study, 4-95 was not observed in those incubations with rat liver microsomes. Another in vitro study using purified rat CYP2B1 reported the formation of two mono-hydroxylated metabolites, but identification of either metabolite was not possible due to the unavailability of authentic standards.23 In contrast, 5-95 was reported by Sundström et al. as the major metabolite in feces from PCB 95-treated mice and rats, with 3′-95, 4′-95 and 4-95 being minor metabolites.56 The different metabolite profiles in the present study and the work by Sundström et al.56 may be due to differences in experimental parameters between studies, including the dose, length of exposure, gender, strain and the matrix investigated (feces versus liver and blood).
Enantiomeric fractions of OH-PCBs
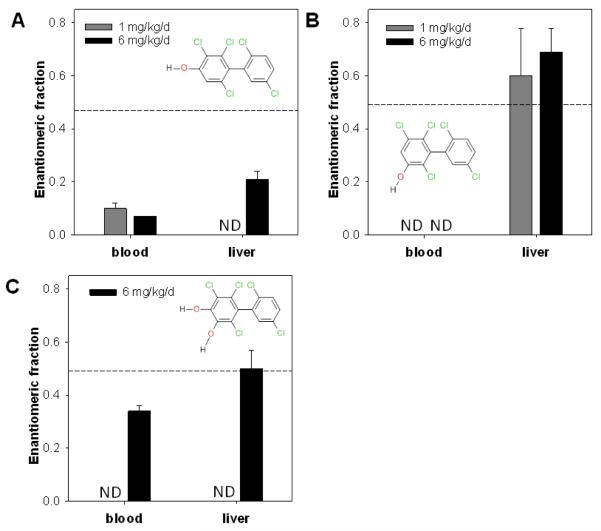
The present study is the first systematic study of the enantiomeric enrichment of potentially neurotoxic OH-PCBs in mice after subchronic, oral PCB administration. The EF values of OH-PCB metabolites could only be determined in samples from the high dose treatment group, with the exception of 5-95 and 4-95 in blood and 5-95 in the liver of mice from the medium dose treatment group (Fig. 4 and Table S10, Supporting Information). The OH-PCB metabolites in all other samples were below the detection limit in the enantioselective analysis.
Fig. 4.
Enantiomeric fractions of hydroxylated metabolites in blood and liver from female C57Bl/6 mice orally exposed to PCB 95 over 39 days. The samples were analyzed at 140°C on a BGB column (A, 4-95), CD column (B, 5-95) or BDM column (C, 4,5-95). The dashed line represents the EF of the respective racemic standard. Data are presented as the mean ± SD. ND: Below detection limit (for detection limits and background levels in control animals, see Table S4, Supporting information).
4-95, the major hydroxylated metabolite of PCB 95, showed the most pronounced enantiomeric enrichment among the studied metabolites (Fig. 4 and Table S10, Supporting Information). In blood, E2-4-95 was enriched, with an EF ≤ 0.1. Although a statistical analysis of the data was not possible due to small sample number, there seemed to be no difference in the extent of enantiomeric enrichment between the medium and high dose treatment groups in blood. A less pronounced enrichment of E2 4-95 was observed in the liver in the high dose treatment group, with an EF of 0.21. The extent of enantiomeric enrichment of 4-95 was similar to the enrichment of the parent compound in blood (EF = 0.13 and 0.07, respectively, for the high dose treatment group). In liver, PCB 95 (EF = 0.10) exhibited more enantiomeric enrichment than 4-95 (EF = 0.21) in the high dose treatment group.
E1-5-95 was enriched in liver samples from the high dose treatment group. However, no consistent enantiomeric enrichment was observed in the medium dose treatment group. One liver sample (out of 3 samples where an EF determination was possible) displayed an enrichment of E2-5-95. The enantiomeric enrichment of 5 95 was less pronounced compared to PCB 95 and 4-95 in the liver. E2-5-95 was also enriched in incubations of racemic PCB 95 with hepatic microsomes obtained from PB-treated rats, with an EF value of approximately 0.34.21 In contrast to the present study, the extent of the enantiomeric enrichment of PCB 95 and 5-95 was similar in a metabolism study with hepatic microsomes obtained from PB-treated rats.21 A less pronounced enrichment (EF = 0.38) has been reported for 5 136, a metabolite of PCB 136, in rat liver.27 Since the in vitro metabolism studies described above focus solely on microsomal metabolism, with no phase II metabolism or organ organ interactions, this by itself may account for the observed differences in those studies.
It was also possible to determine the EF values of 4,5-95, a minor metabolite, in blood and liver from the high dose treatment group. There was no enantiomeric enrichment observed in the liver, with an EF of 0.50. In blood, E2-4,5-95 was moderately enriched, with an EF of 0.34. There are two possible ways by which 4,5-95 may be formed. Similar to the formation of 4,5-136 from the corresponding OH-PCB 136 metabolites,57 4,5-95 may be formed by oxidation of 4-95 and/or 5-95 by CYP2B enzymes. Alternatively, 4,5-95 may be derived from a PCB 95 epoxide via the dihydrodiol, as reported previously for 4-chlorobiphenyl.43 The racemic signature (i.e. lack of enantiomeric enrichment) of 4,5-95 in the liver suggests that the enantiomerically enriched OH-PCB metabolites may not be the source of this metabolite. Alternatively, the racemic signature of this metabolite may be due to enantioselective processes (e.g., enantioselective phase II metabolism) that cause an enantiomeric enrichment opposite from the one observed for the “parent” OH-PCBs and, thus, result in an apparent, racemic signature.
Although the levels of OH-PCBs in brain of adult mice were below the detection limit of our analytical methods in this study, the levels and enantiomeric enrichment of OH-PCBs observed in the blood and liver have implications for understanding the developmental neurotoxicity of PCBs. Particularly intriguing is the earlier finding that 4-136, a PCB metabolite structurally similar to 5-95, is a potent sensitizer of RyR.13 Since PCB 136 enantiospecifically sensitizes RyR, it is likely that 4-136 and structurally related OH PCBs also sensitize RyR in an enantiospecific manner. This, combined with documented transplacental transport of these compounds to the developing fetus,8,26 suggests that OH PCB enantiomeric enrichment may play an important, but currently overlooked role in adverse neurodevelopmental outcomes following PCB exposure. Overall, our results emphasize the need for additional studies to assess levels and EF values of chiral OH PCBs in the fetal brain throughout sensitive developmental periods and to investigate the enantiospecific toxicity of OH PCB atropisomers.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIEHS grants ES05605, ES013661, ES014901 and ES017425. Marianna Stamou was supported by a fellowship from the UC Davis Superfund Basic Research Program, NIH grant ES04699. Christopher Barnhart was supported by an NIEHS training grant T32-ES007059-32 and an ARCS Fellowship.
Footnotes
SUPPORTING INFORMATION PARAGRAPH
Preparation of peanut butter; animal body weight changes during PCB exposure; quality assurance/quality control, amplification efficiencies of selected CYPs; primer sequences and nomenclature; relative mRNA levels of selected P450 enzymes in exposed animals compared to controls; calibration curve parameters (r2 and calibration range); instrument detection limit, method detection limit and background levels for the analysis of PCB 95 and its hydroxylated metabolites; levels of PCB 95 and its metabolites in tissues; enantiomeric fraction of PCB 95 and its metabolites in tissues; estimated levels and enantiomeric fractions of PCB 95 in tissues at different doses predicted based on published pharmacokinetic parameters. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Harrad S, Ibarra C, Robson M, Melymuk L, Diamond M, Douwes J. Polychlorinated biphenyls in indoor dust from Canada, New Zealand, United Kingdom and United States: Implications for human exposure. Chemosphere. 2009;76(2):232–238. doi: 10.1016/j.chemosphere.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated compounds, polychlorinated biphenyl, and organochlorine pesticide contamination in composite food samples from Dallas, Texas. Environ. Health Perspect. 2010;118(6):796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodavanti PR. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose Response. 2005;3(3):273–305. doi: 10.2203/dose-response.003.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 2010;118(12):1654–67. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117(3):426–35. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, Ledoux V, Pessah IN, Lein PJ. PCB 95 modulates calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ. Health Perspect. 2012;120:1003–1009. doi: 10.1289/ehp.1104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodavanti PR, Ward TR, Derr Yellin EC, McKinney JD, Tilson HA. Increased [3H]phorbol ester binding in rat cerebellar granule cells and inhibition of 45Ca(2+) buffering in rat cerebellum by hydroxylated polychlorinated biphenyls. Neurotoxicology. 2003;24(2):187–98. doi: 10.1016/S0161-813X(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 8.Meerts IA, Assink Y, Cenijn PH, Van Den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol. Sci. 2002;68(2):361–71. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- 9.Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem. Res. Toxicol. 2006;19(1):92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- 10.Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 2010;125(2):260–85. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun DA, Pessah IN, Lein PJ. PCB 95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ. Health Perspect. 2012;120:997–1002. doi: 10.1289/ehp.1104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmler HJ, Harrad SJ, Hühnerfuss H, Kania-Korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism and distribution: A review. Environ. Sci. Technol. 2009;44(8):2757–2766. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pessah IN, Lehmler HJ, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (−) 2,2′,3,3′,6,6′-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem. Res. Toxicol. 2009;22(1):201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu S, Covaci A, Schepens P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 2003;93:167–176. doi: 10.1016/s0013-9351(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 15.Lehmler H-J, Price DJ, Garrison AW, Birge WJ, Robertson LW. Distribution of PCB 84 enantiomers in C57Bl/6 mice. Fresenius’ Environ. Bull. 2003;12:254–260. [Google Scholar]
- 16.Kania-Korwel I, Garrison AW, Avants JK, Hornbuckle KC, Robertson LW, Sulkowski WW, Lehmler H-J. Distribution of chiral PCBs in selected tissues in the laboratory rat. Environ. Sci. Technol. 2006;40:3704–3710. doi: 10.1021/es0602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler H-J. Dose-dependent enantiomeric enrichment of 2,2′,3,3′,6,6′ hexachlorobiphenyl in female mice. Environ. Toxicol. Chem. 2008;27(2):299–305. doi: 10.1897/07-359R.1. [DOI] [PubMed] [Google Scholar]
- 18.Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler HJ. Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′ hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- 19.Milanowski B, Lulek J, Lehmler HJ, Kania-Korwel I. Assesment of disposition of chiral polychlorinated biphenyls in female mdr 1a/b knockout versus wild-type mice using multivariate analyses. Environ. Int. 2010;36(8):884–892. doi: 10.1016/j.envint.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Adamcakova Dodd A, Lehmler H-J, Hu D, Kania-Korwel I, Hornbuckle KC, Thorne PS. Time course of congener uptake and elimination in rats after short term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ. Sci. Technol. 2010;44(17):6893–900. doi: 10.1021/es101274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kania-Korwel I, Duffel MW, Lehmler HJ. Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ. Sci. Technol. 2011;45(22):9590–6. doi: 10.1021/es2014727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Pramanik A, Duffel MW, Hrycay EG, Bandiera SM, Lehmler HJ, Kania-Korwel I. 2,2′,3,3′,6,6′ Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem. Res. Toxicol. 2011;24(12):2249–57. doi: 10.1021/tx200360m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warner NA, Martin JW, Wong CS. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 2009;43:114–121. doi: 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- 24.James MO. Polychlorinated biphenyls: Metabolism and metabolites. In: Larry W, Robertson LGH, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. The University Press of Kentucky; Lexington, KY: 2001. pp. 35–46. [Google Scholar]
- 25.Guroff G, Daly JW, Jerina DM, Renson J, Witkop B, Udenfriend S. Hydroxylation-induced migration: The NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967;157:1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- 26.Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicol. Appl. Pharmacol. 1996;136(2):269–79. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- 27.Kania-Korwel I, Vyas S, Song Y, Lehmler HJ. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomer. J. Chromatogr. A. 2008;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi SN, Vyas SM, Duffel MW, Parkin S, Lehmler HJ. Synthesis of sterically hindered polychlorinated biphenyl derivatives. Synthesis. 2011;7:1045–1054. doi: 10.1055/s-0030-1258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 2009;117(3):426–35. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelissen K, Smeets K, Mulder M, Hendriks JJ, Ameloot M. Selection of reference genes for gene expression studies in rat oligodendrocytes using quantitative real time PCR. J. Neurosci. Methods. 2010;187(1):78–83. doi: 10.1016/j.jneumeth.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for groupwise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC, Lehmler HJ. Simultaneous extraction and clean-up of polychlorinated biphenyls and their metabolites from small tissue samples using pressurized liquid extraction. J. Chromatogr. A. 2008;1214:37–46. doi: 10.1016/j.chroma.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kania-Korwel I, El-Komy MHME, Veng-Pedersen P, Lehmler HJ. Clearance of polychlorinated biphenyl atropisomers is enantioselective in female C57Bl/6 mice. Environ. Sci. Technol. 2010;44(8):2828–2835. doi: 10.1021/es901781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veng-Pedersen P. Curve fitting and modeling in pharmacokinetics and some practical experiences with NONLIN and a new program, FUNFIT. J. Pharm. Biopharm. 1977;5:513–531. doi: 10.1007/BF01061732. [DOI] [PubMed] [Google Scholar]
- 37.Khan MA, Hansen LG. Ortho-substituted polychlorinated biphenyl (PCB) congeners (95 or 101) decrease pituitary response to thyrotropin releasing hormone. Toxicol. Lett. 2003;144(2):173–82. doi: 10.1016/s0378-4274(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 38.Martin L, Klaassen CD. Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol. Sci. 2010;117(1):36–44. doi: 10.1093/toxsci/kfq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Püttmann M, Mannschreck A, Oesch F, Robertson L. Chiral effects in the induction of drug-metabolizing enzymes using synthetic atropisomers of polychlorinated biphenyls (PCBs) Biochem. Pharmacol. 1989;38(8):1345–1352. doi: 10.1016/0006-2952(89)90342-0. [DOI] [PubMed] [Google Scholar]
- 40.Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J. Biol. Chem. 1983;258(9):5967–5976. [PubMed] [Google Scholar]
- 41.Wang B, Robertson LW, Wang K, Ludewig G. Species difference in the regulation of cytochrome P450 2S1: lack of induction in rats by the aryl hydrocarbon receptor agonist PCB126. Xenobiotica. 2011;41(12):1031–43. doi: 10.3109/00498254.2011.603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol. Pharmacol. 1998;54(6):1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 1996;9(1):158–64. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Boon JP, Lewis WE, van den Berg M, Nyman M, Letcher RJ. Hepatic microsomal cytochrome P450 enzyme activity in relation to in vitro metabolism/inhibition of polychlorinated biphenyls and testosterone in Baltic grey seal (Halichoerus grypus) Environmental toxicology and chemistry / SETAC. 2003;22(3):636–44. [PubMed] [Google Scholar]
- 45.Rodman LE, Shedlofsky SI, Mannschreck A, Puttmann M, Swim AT, Robertson LW. Differential potency of atropisomers of polychlorinated biphenyls on cytochrome P450 induction and uroporphyrin accumulation in the chick embryo hepatocyte culture. Biochem. Pharmacol. 1991;41(6/7):915–922. doi: 10.1016/0006-2952(91)90196-c. [DOI] [PubMed] [Google Scholar]
- 46.Rodman LE, Shedlofsky SI, Swim AT, Robertson LW. Effects of polychlorinated biphenyls on cytochrome P450 induction in the chick embryo hepatocyte culture. Arch Biochem Biophys. 1989;275(1):252–62. doi: 10.1016/0003-9861(89)90371-8. [DOI] [PubMed] [Google Scholar]
- 47.Lai IK, Chai Y, Simmons D, Watson WH, Tan R, Haschek WM, Wang K, Wang B, Ludewig G, Robertson LW. Dietary selenium as a modulator of PCB 126-induced hepatotoxicity in male Sprague-Dawley rats. Toxicol Sci. 2011;124(1):202–14. doi: 10.1093/toxsci/kfr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denomme MA, Bandiera S, Lambert I, Copp L, Safe L, Safe S. Polychlorinated biphenyls as phenobarbitone-type inducers of microsomal enzymes: Structure-activity relationship for a series of 2,4-dichloro-substituted congeners. Biochem. Pharmacol. 1983;32(19):2955–2964. doi: 10.1016/0006-2952(83)90402-1. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Wong CS. Factors affecting phase I stereoselective biotransformation of chiral polychlorinated biphenyls by rat cytochrome P450 2B1 isozyme. Environmental science & technology. 2011;45(19):8298–305. doi: 10.1021/es200673q. [DOI] [PubMed] [Google Scholar]
- 50.Staskal DF, Diliberto JJ, Devito MJ, Birnbaum LS. Inhibition of human and rat CYP1A2 by TCDD and dioxin-like chemicals. Toxicol. Sci. 2005;84(2):225–31. doi: 10.1093/toxsci/kfi090. [DOI] [PubMed] [Google Scholar]
- 51.Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414(1):91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 52.Nishida CR, Lee M, de Montellano PR. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Molecular pharmacology. 2010;78(3):497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szafran-Urbaniak B. Application of validated method for determination of selected polychlorinated biphenyls in human adipose tissue samples. Environ. Toxicol. Pharmacol. 2008;25(2):131–5. doi: 10.1016/j.etap.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Corrigan FM, French M, Murray L. Organochlorine compounds in human brain. Hum. Exp. Toxicol. 1996;15(3):262–4. doi: 10.1177/096032719601500314. [DOI] [PubMed] [Google Scholar]
- 55.Chen PH, Hites RA. Polychlorinated biphenyls and dibenzofurans retained in the tissues of a deceased patient with Yucheng in Taiwan. Chemosphere. 1983;12(11/12):1507–1516. [Google Scholar]
- 56.Sundström G, Jansson B. The metabolism of 2,2′,3,5′,6 pentachlorobiphenyl in rats, mice and quails. Chemosphere. 1975;4(6):361–370. [Google Scholar]
- 57.Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2′,3,3′,6,6′ hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem. Res. Toxicol. 1999;12(8):690–699. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.