Abstract
Regulation of apoplastic NH4+ concentration in leaves of oilseed rape (Brassica napus L.) was studied using a vacuum-infiltration technique that allowed controlled manipulations of the apoplastic solution. In leaves infiltrated with NH4+-free solution, the apoplastic NH4+ concentration returned in less than 1.5 min to the preinfiltration level of 0.8 mm. Infiltrated 15NH4+ was rapidly diluted by 14NH4+/14NH3 effluxed from the cell. The exchange rate of 15N/14N over the apoplast due to combined 14N efflux from the symplast and 15N influx from the apoplastic solution was 29.4 μmol g−1 fresh weight h−1 between 0 and 5 min after infiltration. The net uptake of NH4+ into the leaf cells increased linearly with apoplastic NH4+ concentrations between 2 and 10 mm and could be partially inhibited by the channel inhibitors La3+ and tetraethylammonium and by Na+ and K+. When apoplastic pH increased from 5.0 to 8.0, the steady-state apoplastic NH4+ concentration decreased from 1.0 to 0.3 mm. Increasing temperature increased the rate of NH4+ net uptake and reduced the apoplastic steady-state NH4+ concentration. We conclude that the apoplastic solution in leaves of oilseed rape constitutes a highly dynamic NH4+ pool.
NH4+ is constantly generated in large quantities in plant leaves by processes such as photorespiration, nitrate reduction, protein turnover, and lignin biosynthesis (Joy, 1988; Leegood et al., 1995). Refixation of NH4+ takes place mainly in the chloroplasts and is catalyzed by the chloroplastic isoform of Gln synthetase, GS2 (Leegood et al., 1995). In addition to being a central metabolic intermediate, NH4+ may be translocated to the leaves from the roots (Cramer and Lewis, 1993; Mattsson and Schjoerring, 1996).
The rapid turnover of NH4+ in plant leaves leads to the establishment of a finite NH4+ concentration in the leaf apoplastic solution (Husted and Schjoerring, 1995). This concentration and the concentration of H+ determines the size of the NH3 compensation point (i.e. the NH3 mole fraction in the air within the substomatal cavities; Farquhar et al., 1980; Husted and Schjoerring, 1996). The NH3 compensation point ranges between 0.1 and 20 nmol mol−1 air and is thus of the same order of magnitude as the naturally occurring atmospheric NH3 concentration (Sutton et al., 1994). At an NH3 compensation point of 5 nmol mol−1, for example, this would under conditions of equilibrium correspond to an apoplastic NH4+ concentration of 1 mm at 20°C and pH 5.8 (Husted and Schjoerring, 1996). The existence of an NH3 compensation point implies that vegetation has a major influence on the transport and budgets of atmospheric NH3, a pollutant with damaging environmental impacts (Langford and Fehsenfeld, 1992; Dentener and Crutzen, 1994; Sutton et al., 1995).
The concentration of NH4+ in the leaf apoplastic solution is very sensitive to leaf N status and external N supply. Therefore, the apoplastic NH4+ concentration may be about 10 times higher in oilseed rape (Brassica napus L.) plant leaves treated with high N than in leaves treated with low N (Husted and Schjoerring, 1996). Barley plants having access to NH4+ in the root medium have higher apoplastic NH4+ concentrations than plants absorbing NO3−, and the leaf apoplastic NH4+ concentration increases with the NH4+ concentration in the root medium (Mattsson and Schjoerring, 1996). Inhibition of Gln synthetase leads to a rapid and very substantial increase in apoplastic NH4+ (Husted and Schjoerring, 1995), and barley mutants with reduced Gln synthetase activity have increased apoplastic NH4+ relative to wild-type plants (Mattsson et al., 1997).
Despite the importance of leaf apoplastic NH4+ concentration in NH4+ recovery and plant-atmosphere NH3 exchange, very little information is available concerning the transport of NH4+ between the leaf apoplast and symplast. In leaf discs of bean, Raven and Farquhar (1981) observed that uptake of methylammonium (an NH4+ analog) could not be accounted for by passive diffusion but seemed to be mediated by some kind of energy-requiring transport system. In roots of various plant species as well as in Chara corallina, a high-affinity transport system showing Michaelis-Menten kinetics with a Km of approximately 15 to 40 μm and a low-affinity transport system showing a linear response to external NH4+ have been demonstrated (Ritchie, 1987; Glass et al., 1997). Considering the relatively high concentrations of NH4+ (0.5–1.5 mm) frequently encountered in the leaf apoplastic solution of oilseed rape plants, the low-affinity system appears to be central in NH4+ transport. In roots the low-affinity transport system has been proposed to be a uniport, with fluxes driven by the electrochemical gradient across the plasma membrane (Wang et al., 1994). This uniport may be a specific NH4+ channel, a K+ channel, or a shared cation channel (e.g. a K+/NH4+ channel, as indicated by Avery et al. [1992] and Schachtman et al. [1992]). Ninnemann et al. (1994) isolated and characterized a gene for a high-affinity NH4+ transporter that was highly expressed in both roots and leaves of Arabidopsis.
A further complicating factor concerning NH4+ transport in leaves relative to that in roots is the possible existence of a large efflux component due to diffusion of dissolved NH3. Even under conditions in which the intracellular NH4+ concentration is 10 to 100 times lower than the extracellular concentration, a high pH in the cytoplasm (7.0–7.5; Martin et al., 1982) and in the chloroplasts (approximately 8.0 in light) relative to that in the apoplastic solution (approximately 6.0) may maintain a gradient of dissolved NH3 directed toward the apoplast.
The objective of the present work was to investigate the response of NH4+ transport between the apoplast and symplast of leaf cells in oilseed rape to variations in apoplastic NH4+ concentration. The hypothesis tested was that the apoplastic NH4+ concentration is highly regulated and rapidly attains a steady-state level under changing conditions. A vacuum-infiltration method was used to manipulate the apoplastic NH4+ concentration and to introduce various transport inhibitors into the apoplast. Effects of controlled changes in different parameters such as temperature and pH on the steady-state NH4+ concentration and NH4+ net transport were elucidated. Finally, the stable isotope 15N was used to assess the contribution of bidirectional NH3/NH4+ transport over the plasma membrane to the maintenance of apoplastic NH4+ homeostasis.
MATERIALS AND METHODS
Seeds of oilseed rape (Brassica napus L. cv Global) were germinated in the dark on wet filter paper for 4 d prior to planting in 0.0025-m3 self-watering pots (four plants per pot). The pots were filled with a growth medium consisting of a 1:1 mixture of soil to sand and containing 0.15 mol NH4NO3 per pot, supplemented with additional nutrients as described by Husted and Schjoerring (1995). Plants were grown in a greenhouse at a day/night temperature cycle of approximately 18°C/14°C (70% ± 5% RH) under a 16-h photoperiod with a PPFD > 400 μmol m−2 s−1.
Before experiments, fully developed green leaves were cut off at the stem and their petioles were cut with a sharp blade under deionized water. Leaves were thereafter transferred to a growth chamber 1 h before experiments to allow adjustment to the environmental conditions under which the experiments were later carried out. Unless otherwise specified, the growth chamber had 70% ± 5% RH, a temperature of 20°C ± 1°C, and a PPFD of 475 ± 5 μmol m−2 s−1.
Extraction and Analysis of Apoplastic Solution
A leaf disc of approximately 1.0 g was washed in deionized water and infiltrated with different solutions adjusted to 350 mosmol with sorbitol. Infiltration was performed in a 50-mL syringe mounted in a hydraulic infiltrator designed in our laboratory. The infiltrator was programmed to expose the leaf disc to 5 atm of pressure for 8 s, followed by vacuum, and this procedure was repeated three times. The leaf disc was then blotted dry with thin paper tissues, and the apoplastic solution was collected in microcentrifuge vials by centrifuging the leaf disc at 2000g for 10 min at 5°C. Cytoplasmic contamination of the apoplast during the extraction procedure was between 0.1% and 0.7%, as assessed on the basis of measurements of the activity of the marker enzyme malate dehydrogenase (EC 1.1.1.37; Husted and Schjoerring, 1995).
Apoplastic air volume was determined by infiltrating the leaf disc with a high-viscosity silicone fluid (polydimethylsiloxane: viscosity, 5 centistoke, density, 0.904 g cm−3; Dow Corning, Poole, UK). The air volume was calculated as the increase in weight of the leaf disc after infiltration, corrected for the density of the silicone oil. The fraction of leaf apoplastic solution in the extracellular space of the leaf disc was determined by infiltrating the apoplast with indigo carmine (50 μm indigo-5,5′disulfonic acid, Sigma) dissolved in 50 mm phosphate buffer at pH 6.2 and adjusted to 350 mosmol with sorbitol. After the dye had infiltrated, the apoplastic solution was isolated by centrifugation, and the dilution of the indigo carmine solution was determined spectrophotometrically at 610 nm.
Cation concentrations in the apoplastic extracts were measured by isocratic HPLC using an IC-Pak C column (Waters-Millipore) at 30°C with a flow rate of 1.0 mL min−1 and an eluent containing 0.1 mm EDTA, 2.5 mm 18-crown-6-ether (Sigma), and 4.0 mm HNO3.
Measurements of pH in apoplastic extracts were conducted in a microcentrifuge tube using a microelectrode (ΦSmart ISFET Micro Probe, Beckman).
The osmolality of solutions used for infiltration was measured on a cryoscopic osmometer (Osmomat 030, Gonotec, Berlin, Germany). All solutions were prepared from ultrapure water (Milli-Q, Millipore) with an 18.2 mΩ resistance.
Apoplastic NH4+ Homeostasis
The steady-state apoplastic NH4+ concentration was determined after infiltration with either 0 or 0.8 mm NH4Cl in 150 mm Mes buffer, pH 6.20. The apoplastic solution was extracted 1.5, 2.5, and 4.0 min after infiltration.
To assess the bidirectional transport of NH3/NH4+ over the plasma membrane, leaf discs were infiltrated with 1 mm 15NH4Cl (98% 15N) in an unbuffered solution containing 280 mm sorbitol (350 mosmol). The leaf discs were then incubated for 5, 10, 15, or 25 min before extraction of the apoplastic solution. During the incubation period the leaf discs were placed on a soaked piece of filter paper in a zippered plastic bag to avoid evaporation of leaf water. The plastic bags were placed in a growth chamber (same conditions as described above).
The 15N abundance in the extracted apoplastic solution was determined by the Dumas combustion method in a system consisting of an elemental analyzer (ANCA-SL, Europa Scientific, Crewe, UK) coupled to a mass spectrometer (20–20 tracer, Europa Scientific). Because of the low quantity of apoplastic solution obtained from the leaf disc, it was necessary to spike the samples with 10 μg of N in the form of (NH4)2SO4 to facilitate analysis within the detection limit of the mass spectrometer. Samples and spiking solution were mixed in tin capsules and freeze-dried prior to analysis.
NH4+ Net Transport at Different NH4+ Concentrations in the Apoplastic Solution
Leaves were infiltrated with 150 mm Mes buffer, pH 6.20, containing NH4Cl in concentrations between 1 and 80 mm. The duration of the incubation was 4 min.
Influence of Inhibitors on NH4+ Net Uptake
NH4+ net uptake was investigated in the presence of 100 μm of the ATPase inhibitor DES and 20 μm of the protonophore CCCP (both from Sigma). The inhibitors were dissolved in ethanol in a 100× stock solution and added to a buffer solution to give a final ethanol concentration of 1%. The buffer contained 150 mm Mes, pH 6.2, and 10 mm NH4Cl. Controls received 1% ethanol without inhibitors. Leaves were pretreated for 1 h by petiole feeding in the presence of 100 μm DES, 20 μm CCCP, or control solution prior to the experiments.
The influence of the two channel blockers, La3+ and TEA-Cl, on the net uptake of NH4+ was tested by infiltration with 150 mm Mes buffer, pH 6.2, containing either 10 mm NH4Cl and 10 mm LaCl3 or 10 mm NH4Cl and 10 mm TEA-Cl. Control leaves were infiltrated with 10 mm NH4Cl only. Leaves were not pretreated with the inhibitors.
Effects of K+ and Na+ on NH4+ Net Uptake
Leaf discs were infiltrated and incubated for 4 min with a solution containing 150 mm Mes buffer, pH 6.2, 10 mm NH4Cl, and 0, 50, or 100 mm of KCl or NaCl.
Influence of pH and Net H+ Transport between Apoplast and Symplast on Steady-State NH4+ Concentration in the Apoplastic Solution
The effect of pH in the apoplastic solution on the steady-state concentration of NH4+ was investigated by infiltrating leaf discs with 150 mm Mes buffer adjusted to pH 5.0 and 6.0, or 150 mm Tes buffer adjusted to pH 7.0 and 8.0, followed by incubation of the leaf discs for 4 min.
The net H+ transport during the period of infiltration was determined by measuring the pH in the infiltration solution before and after infiltration (maximum difference 0.4 pH unit; Fig. 5b). The amount of protons required to cause this pH change was subsequently quantified by titration of 100 mL of the buffered infiltration solution, corrected for the dilution with solution already present in the apoplast before the infiltration. The buffer capacity of the latter solution was negligible relative to that of the infiltration buffer solution (data not shown).
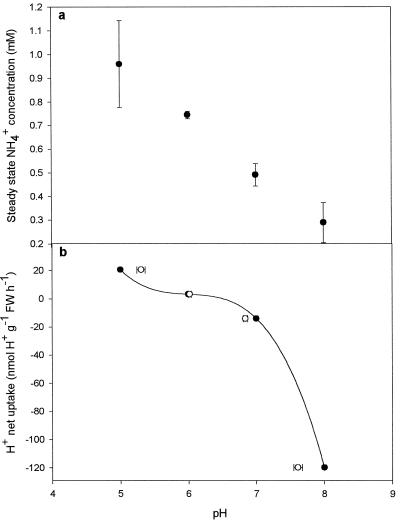
Figure 5.
Effect of apoplastic pH on apoplastic NH4+ concentration and H+ net uptake from the apoplastic solution in leaf discs of oilseed rape. a, Steady-state apoplastic NH4+ concentration at different pH values in the apoplastic solution. Leaf discs were infiltrated for 4 min with 150 mm Mes buffer, pH 5.0 and 6.0, or 150 mm Tes buffer, pH 7.0 and 8.0, adjusted to an osmotic potential of 350 mosmol with sorbitol. b, H+ net uptake from the apoplastic solution during the infiltrations described in a. •, pH in the infiltration solution prior to infiltration; ○, pH in the solution extracted after 4 min. Values are the means ± se of four replicates. FW, Fresh weight.
Effect of Temperature on Steady-State Apoplastic and Symplastic NH4+ Concentrations and Net NH4+ Transport between Apoplast and Symplast
Leaves were preincubated at temperatures ranging from 5°C to 35°C for 1 to 2 h in a growth chamber with a RH of 70% ± 5% and a PPFD of 475 ± 5 μmol m−2 s−1. The leaves were then infiltrated with a solution containing 150 mm Mes buffer, pH 6.2, and either 0 or 10 mm NH4Cl and incubated for 4 min at the same temperature used during the preincubation. Infiltration with 0 mm NH4+ was used to determine the steady-state apoplastic NH4+ concentration, and infiltrations with 10 mm NH4+ were used to determine the net uptake of NH4+. The solutions were adjusted to the respective temperature prior to infiltration, and all experimental work was carried out at the specified temperature (except centrifugation, which took place at 5°C). Tissue NH4+ was measured after 1.0 g of leaf material was ground in an ice-cooled mortar in the presence of 8 mL of 0.1 m H2SO4 and acid-washed sand. The extract was shaken for 30 min on ice and then centrifuged at 30,000g for 10 min at 5°C. The supernatant was collected, pH adjusted to 6.0 with 0.2 m KHCO3, and filtered on a 0.45-μm polysulfone filter. The filtered extract was analyzed for NH4+ concentration by HPLC.
Calculations
The NH4+ concentration in the apoplast immediately after infiltration was calculated by the following equation:
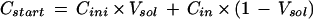 |
1 |
where Dini is the molar NH4+ concentration in the apoplastic solution prior to infiltration, Vsol is the volume fraction (L L−1) of apoplastic solution in the extracellular space, and Cin is the molar NH4+ concentration in the infiltration solution.
To obtain the net uptake of NH4+ over the plasma membrane per unit leaf fresh weight, the volume of extracellular space (Vapo, L g−1), including both apoplastic water and air, was calculated as:
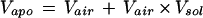 |
2 |
where Vair is the volume fraction (L g−1) of the extracellular air space. Finally, the net uptake (FNH4+) was calculated following the equation:
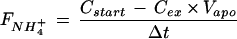 |
3 |
where Cex is the molar NH4+ concentration in the apoplastic solution at the conclusion of the experiment and Δt is the duration in hours of the experimental period. The experimental period was defined as starting at infiltration and ending at the start of centrifugation. The shortest possible duration of the experimental period was 1.5 min, which corresponds to the minimum time required to infiltrate and transfer the leaf disc to the centrifuge.
RESULTS
The volume of apoplastic air in the leaves ranged from 0.20 to 0.25 mL g−1 fresh weight. The corresponding range for apoplastic water was 0.06 to 0.10 mL g−1 fresh weight. The apoplastic NH4+ concentration in newly sampled leaves was approximately 0.8 mm.
NH4+ Homeostasis in Leaf Apoplastic Solution
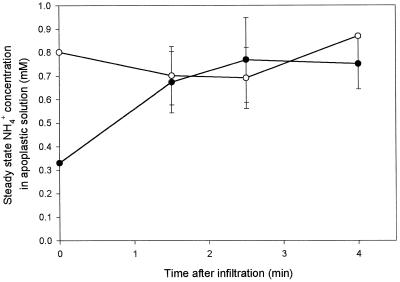
The apoplastic solution in leaves infiltrated with an NH4+-free solution attained in less than 1.5 min an NH4+ concentration of 0.8 mm (Fig. 1). The apoplastic NH4+ concentration remained at this level throughout the rest of the 4-min experimental period. No changes in apoplastic NH4+ concentration were observed upon infiltration with a solution containing 0.8 mm NH4+ (Fig. 1).
Figure 1.
Time course of apoplastic NH4+ concentration in leaf discs of oilseed rape infiltrated at 20°C with either 0 mm (•) or 0.8 mm (○) NH4Cl in a 150 mm Mes buffer solution, pH 6.2, adjusted to 350 mosmol with sorbitol. The concentration at time 0 after infiltration with 0 mm NH4Cl was corrected for the NH4+ already present in the apoplast. Values are the means ± se of four replicates.
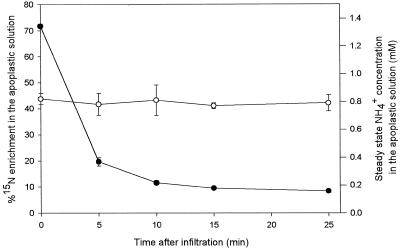
Introduction of a solution containing 1.0 mm 15N-enriched NH4+ into the apoplast, resulting in an initial 15N excess of 72 atom % in the apoplastic NH4+ pool, was followed by a very rapid dilution of the 15N with 14N (Fig. 2). After 5 min the 15N excess was reduced to 20.0 atom %, and after 25 min it was reduced to 8.4 atom %. During the same period the apoplastic NH4+ concentration remained constant at 0.8 mm (Fig. 2), and total apoplastic N, including all organic and inorganic N compounds, remained at 7.1 mm (data not shown). In the time intervals 0 to 5, 5 to 10, 10 to 15, and 15 to 25 min after infiltration, the decline in 15N atom % excess amounted to 75%, 41%, 18%, and 12%, respectively, when expressed relative to the 15N excess at the start of each period. The corresponding exchange rate of 15N/14N over the apoplast due to the combined 14N efflux from the symplast and 15N uptake from the apoplastic solution was 29.4 μmol g−1 fresh weight h−1 between 0 and 5 min after infiltration and 3.9 ± 0.7 μmol g−1 fresh weight h−1 between 15 and 25 min after infiltration (Table I).
Figure 2.
Time course of 15N excess (•) and NH4+ concentration (○) in the apoplastic solution of leaf discs of oilseed rape infiltrated with 1.0 mm 15NH4Cl solution adjusted to an osmotic potential of 350 mosmol with sorbitol. Values are the means ± se of six replicates.
Table I.
Exchange rate of 15N/14N over the plasma membrane determined by measuring the dilution of 15N with 14N (n = 6)
| Interval | Exchange Rate of 15N/14N |
|---|---|
| min | μmol g−1 fresh wt h−1 |
| 0–5 | 29.42 ± 3.95 |
| 5–10 | 19.87 ± 4.35 |
| 10–15 | 6.22 ± 1.79 |
| 15–20 | 3.86 ± 0.68 |
Exchange rates were calculated on the basis of the 15N-enrichment measurements illustrated in Figure 2.
Response of NH4+ Net Uptake to Increasing Apoplastic NH4+ Concentration
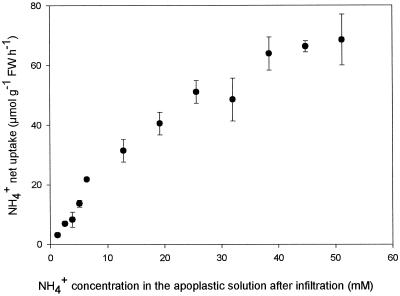
The net uptake of NH4+ over a 4-min experimental period responded linearly to increasing NH4+ concentrations up to about 10 mm (Fig. 3). At higher concentrations the NH4+ net uptake started to saturate, becoming close to saturation at concentrations greater than approximately 40 mm NH4+ (Fig. 3).
Figure 3.
Net uptake of NH4+ from the apoplastic solution in leaf discs of oilseed rape infiltrated for 4 min at 20°C with 150 mm Mes buffer solutions, pH 6.2, adjusted to an osmotic potential of 350 mosmol with sorbitol and different concentrations of NH4Cl. Values are the means ± se of four replicates. FW, Fresh weight.
Effect of Inhibitors and Competing Cations on NH4+ Net Uptake
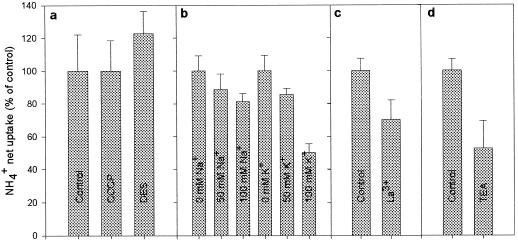
Neither the ATPase inhibitor DES nor the protonophore CCCP had any effect (P > 0.05) on the net NH4+ uptake (Fig. 4a). Conversely, the unspecific channel-blocker La3+ and the specific K+-channel-blocker TEA-Cl reduced (P < 0.05) the NH4+ net uptake (Fig. 4, c and d). The reduction caused by La3+ was 30%, and that of TEA-Cl was 47%.
Figure 4.
Effect of different inhibitors on NH4+ uptake from the apoplastic solution in leaf discs of oilseed rape. a, 20 μm CCCP (a protonophore) and 100 μm DES (an ATPase inhibitor) supplied by petiole feeding during a 1-h pretreatment period and subsequently added to the infiltration solution. b, KCl and NaCl added in increasing concentrations to the infiltration solution. c, 10 mm of the nonspecific channel blocker La3+ (LaCl3). d, 10 mm specific K+-channel blocker TEA-Cl. In addition to the specified inhibitor the infiltration solution contained 150 mm Mes, pH 6.2, and 10 mm NH4Cl and was adjusted to an osmotic potential of 350 mosmol with sorbitol. The experiments were carried out at 20°C with a incubation period of 4 min. Values are the means ± se of four replicates.
Increasing the concentrations of K+ or Na+ resulted in a decrease in NH4+ net uptake (Fig. 4b). The inhibition caused by K+ was 50% at 100 mm KCl in the infiltration solution. A similar concentration of NaCl resulted in only a 20% decline of NH4+ net uptake.
Effect of Apoplastic pH on Steady-State Apoplastic NH4+ Concentration and Proton Flux
The steady-state concentration of NH4+ in the apoplastic solution decreased with increasing pH between 5.0 and 8.0 (Fig. 5a). For each pH increment the NH4+ concentration declined by approximately 30% (P < 0.01).
Apoplastic pH also affected the net transport of H+ between the apoplast and the symplast (Fig. 5b). At approximately pH 6.5 the net transport of H+ was zero. Below this pH value, the leaf cells had a net uptake of H+, whereas at higher pH values the net H+ transport was in the opposite direction. The magnitude of the net H+ transport was 20.4 nmol g−1 fresh weight h−1 at pH 5.0 and −120 nmol g−1 fresh weight h−1 at pH 8.0 (Fig. 5b).
Effect of Temperature on NH4+ Net Uptake and Apoplastic NH4+ Concentration
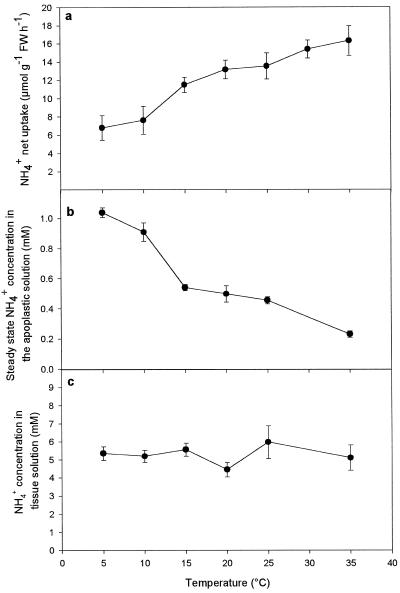
The net uptake of NH4+ from the apoplastic solution and into the symplast increased almost 3-fold with temperature in the interval from 5°C to 35°C (Fig. 6a). Over the same range of temperatures the steady-state concentration of NH4+ in the apoplastic solution decreased from approximately 1.0 to 0.2 mm (Fig. 6b), while the tissue NH4+ concentration remained almost constant at about 5.3 mm (Fig. 6c). The Q10 value (the ratio of rates at temperatures differing by 10°C) for net NH4+ uptake was 1.69 between 5°C and 15°C and decreased to about 1.2 between 25°C and 35°C (Table II). The greatest sensitivity of NH4+ net uptake and steady-state NH4+ concentration to changing temperature was observed between 10°C and 15°C (Fig. 6, a and b).
Figure 6.
The temperature response of NH4+ net uptake from the apoplastic solution (a), steady-state apoplastic NH4+ concentration (b), and tissue-water NH4+ concentration in leaf discs of oilseed rape (c). Leaf discs were infiltrated for 4 min at the specified temperature with 150 mm Mes buffer, pH 6.2, containing 10 mm NH4Cl and adjusted to an osmotic potential of 350 mosmol. Prior to the experiments both leaves and infiltration solutions were placed for 1 h at the same temperature as that used during the infiltration. Values are the means ± se of four replicates. FW, Fresh weight.
Table II.
Q10 values for NH4+ net flux at 10°C intervals between 5°C and 35°C (n = 4)
Q10 values were calculated on the basis of the results shown in Figure 6a.
| Temperature Range | Q10 |
|---|---|
| °C | |
| 5–15 | 1.69 |
| 10–20 | 1.49 |
| 15–25 | 1.20 |
| 20–30 | 1.38 |
| 25–35 | 1.22 |
DISCUSSION
NH4+ Homeostasis in Leaf Apoplastic Solution
Both the rapid dilution of infiltrated 15NH4+ with 14NH4+ (Fig. 2) and the rapid adjustment to steady-state NH4+ concentration after infiltration with NH4+-free solution (Fig. 1) suggest a substantial efflux of NH3/NH4+ from the leaf cells into the apoplastic solution. However, since no increase in steady-state apoplastic NH4+ concentration occurred over time, the NH3/NH4+ effluxed from the cell was recirculated back into the cell. The apparent decrease in NH4+ recirculation rate over time (Table I) was due to the dilution of 15NH4+ with existing 14NH4+ in the leaf plus incorporation of 15N into the organic pool. Substantial amounts of NH3/NH4+ are generated in photorespiration and during lignin biosynthesis. In the latter process NH3/NH4+ is released directly in the apoplast (Nakashima et al., 1997), and photorespiratory NH3/NH4+ is released in the mitochondria (Leegood et al., 1995). Since biomembranes are highly permeable to NH3 (Kleiner, 1981), the dissolved NH3 may escape to the apoplast on its way back to the chloroplasts for reassimilation. A higher pH in cytoplasm, mitochondria, and chloroplasts than in the apoplast (Kurkdjian and Guern, 1989) would sustain an outwardly directed gradient of dissolved NH3 even in cases in which the NH4+ concentration in the extracellular solution was higher than that in the cytoplasm or organelles. In root cells cytoplasmic NH4+ concentration can be up to 40 mm (Wang et al., 1993a), and leaf cells of oilseed rape can achieve high NH4+ concentrations (Finnemann and Schjoerring, 1998).
NH4+ Uptake from Leaf Apoplastic Solution
The net uptake of NH4+ from the leaf apoplastic solution into the mesophyll cells of oilseed rape increased linearly with apoplastic NH4+ concentration up to approximately 10 mm (Fig. 3). Because of the substantial efflux component (Fig. 2), the actual NH4+ influx was considerably higher than the recorded NH4+ net uptake. Influx rates of NH4+ in both Lemna gibba and rice roots were smaller than those observed in the present study for leaf cells and did not show any sign of saturation at external NH4+ concentrations even up to 40 mm (Ullrich et al., 1984; Wang et al., 1993b). The much higher NH4+ uptake in leaf cells may be related to the requirement for a rapid retrieval of effluxed NH3 originating from photorespiration and from NH4+ liberated in the apoplast during lignin biosynthesis.
NH4+ uptake in roots takes place via both a high- and a low-affinity transport system, with the former saturating at less than 1 mm (Glass et al., 1997). In the present study it was not possible to investigate NH4+ uptake below 0.8 mm, because apoplastic NH4+ rapidly attained a steady-state concentration of 0.8 mm, even after infiltration with an NH4+-free solution (Fig. 1). Although high-affinity NH4+ transport was not investigated, high levels of mRNA from the AMT1 gene, which codes for a high-affinity NH4+ transporter, were found in leaves of Arabidopsis (Ninnemann et al., 1994), suggesting that a high-affinity NH4+-transport system may also be present in the closely related oilseed rape species.
Neither the ATPase inhibitor DES nor the protonophore CCCP affected the net uptake of NH4+ (Fig. 4a), suggesting that NH4+ uptake via the low-affinity system in leaf cells of oilseed rape is independent of both plasma membrane ATPase activity and the establishment of a proton gradient. Tyerman et al. (1995) and Mouritzen and Rosendahl (1997) found that a channel-like transporter on the symbiotic interface of N2-fixing pea transported NH4+ independently of a proton gradient. In contrast, CCCP inhibited low-affinity NH4+ influx in rice roots by approximately 30% (Wang et al., 1993b). The inhibition of the low-affinity NH4+ uptake by K+ and the inhibitors La3+ and TEA-Cl (Fig. 3) indicates that the NH4+ transport took place via a K+ channel or a specific NH4+ channel closely related to a K+ channel, as was previously proposed by Ketchum and Poole (1990), Schatchtman et al. (1992), Terry et al. (1992), and Wegner et al. (1994). The fact that a 10-fold excess in apoplastic K+ concentration over that of NH4+ inhibited NH4+ net uptake by only approximately 50% (Fig. 3b) suggests a higher affinity for NH4+ relative to K+. Since the K+ concentration in the leaf apoplastic solution was typically more than 10 times higher than the concentration of NH4+ (data not shown), a relatively high affinity for NH4+ would be required for efficient NH4+ retrieval. A close relationship between an NH4+ channel and a K+ channel would also be expected. Uozumi et al. (1995) showed that only minor site mutations in the P-domain of the inward-rectifying K+ channel (KAT1) from Arabidopsis expressed in yeast increased the NH4+ conductance of the channel to 1 order of magnitude higher than that of K+.
Effect of Apoplastic pH and Temperature
The increase in steady-state apoplastic NH4+ concentration (Fig. 5a) and the decrease in net NH4+ uptake at decreasing pH in the apoplastic solution (Munn and Jackson, 1978; Vessey et al., 1990; Dyhr-Jensen and Brix, 1996) most likely reflects a depolarization of the membrane potential and closing of channels following increased net uptake of H+ (Fig. 4b; Poole, 1978; Kurkdjian and Guern, 1989; Seto-Young and Perlin, 1991; Yan et al., 1992). Enhanced release of cell wall-bound NH4+ following increases in H+ concentration did not contribute significantly to the higher steady-state NH4+ concentration at decreasing pH, because only a very small amount of NH4+ was bound to the cell walls (data not shown; Husted and Schjoerring, 1995).
The highest sensitivity of net NH4+ uptake to temperature change was observed at temperatures from 10°C to 15°C (Fig. 6a), which is in agreement with results previously reported for roots of oilseed rape and rice (Macduff et al., 1987; Wang et al., 1993b). The observed increase in net NH4+ uptake at increasing temperature was far too high to be caused solely by an effect on NH4+ diffusion, indicating that temperature affected the transport mechanism by opening and/or closing channels (Colombo and Cerana, 1993). In accordance with the stimulating effect of temperature on NH4+ net uptake, the steady-state apoplastic NH4+ concentration declined (Fig. 6).
In conclusion, our data show that the apoplastic solution in leaves of oilseed rape constitutes a highly dynamic NH4+ pool. NH4+ is constantly added to this pool via NH3 efflux from the mesophyll cells. The efflux of NH3 imposes requirements for an efficient NH4+-retrieval system in the leaf cell plasma membrane. This retrieval system includes a transporter with channel-like properties and is able to respond very rapidly to perturbations in apoplastic NH4+ concentration, thereby maintaining apoplastic NH4+ homeostasis. Documentation of the rapid NH4+/NH3 recirculation over the plasmalemma of mesophyll cells is a new contribution to understanding the dynamics and physiological implications of NH3/NH4+ transport and turnover in plants. The discovery of channel-mediated NH4+ transport in leaves calls for further investigation of the genetic and molecular basis for this transport.
Abbreviations:
- CCCP
carbonylcyanide-m-chlorophenylhydrazone
- DES
diethylstilbestrol
- TEA-Cl
tetraethylammonium chloride
Footnotes
This work was supported by grants from the Danish Agricultural and Veterinary Research Council and The Strategic Environmental Research Program II to J.K.S.
LITERATURE CITED
- Avery SV, Codd GA, Gadd GM. Caesium transport in the cyanobacterium Anabaena variabilis: kinetics and evidence for uptake via ammonium transport systems. FEMS Microbiol Lett. 1992;95:253–258. [Google Scholar]
- Colombo R, Cerana R. Physiol Plant. 1993;87:118–124. [Google Scholar]
- Cramer MD, Lewis OAM. The influence of nitrate and ammonium nutrition on growth of wheat (Triticum aestivum L.) and maize (Zea mays L.) plants. Ann Bot. 1993;72:359–365. [Google Scholar]
- Dentener FJ, Crutzen PJ. A three dimensional model of the global ammonia cycle. J Atmos Chem. 1994;19:331–369. [Google Scholar]
- Dyhr-Jensen K, Brix H. Effect of pH on ammonium uptake by Typha latifolia L. Plant Cell Environ. 1996;19:1431–1436. [Google Scholar]
- Farquhar GD, Firth PM, Wetselaar R, Weir B. On the gaseous exchange of ammonia between leaves and the environment. Determination of the ammonia compensation point. Plant Physiol. 1980;66:710–714. doi: 10.1104/pp.66.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann J, Schjoerring JK. Glutamine synthetase activity in Brassica napus L. leaves with different levels of free ammonium and amides. Plant Physiol Biochem. 1998;36:339–346. [Google Scholar]
- Glass ADM, Erner Y, Kronzucker H, Schjoerring JK, Siddiqi MY, Wang M-Y. Ammonium fluxes into plant roots: energetics, kinetics and regulation. J Plant Nutr Soil Sci. 1997;160:261–268. [Google Scholar]
- Husted S, Schjoerring JK. Apoplastic pH and ammonium concentration in leaves of Brassica napus L. Plant Physiol. 1995;109:1453–1460. doi: 10.1104/pp.109.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S, Schjoerring JK. Ammonia flux between oilseed rape plants and the atmosphere in response to changes in leaf temperature, light intensity and air humidity. Interactions with stomatal conductance and apoplastic NH4+ and H+ concentrations. Plant Physiol. 1996;112:67–74. doi: 10.1104/pp.112.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy KW. Ammonia, glutamine, and asparagine: a carbon-nitrogen interface. Can J Bot. 1988;66:2103–2109. [Google Scholar]
- Ketchum KA, Poole RJ. Pharmacology of the Ca2+-dependent K+ channel in corn protoplast. FEBS Lett. 1990;274:115–118. doi: 10.1016/0014-5793(90)81343-m. [DOI] [PubMed] [Google Scholar]
- Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta. 1981;639:41–52. doi: 10.1016/0304-4173(81)90004-5. [DOI] [PubMed] [Google Scholar]
- Kurkdjian A, Guern J. Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:271–303. [Google Scholar]
- Langford AO, Fehsenfeld FC. Natural vegetation as a source or sink for atmospheric ammonia: a case study. Science. 1992;255:581–583. doi: 10.1126/science.255.5044.581. [DOI] [PubMed] [Google Scholar]
- Leegood RC, Lea PJ, Adcock MD, Häusler RE. The regulation and control of photorespiration. J Exp Bot. 1995;46:1397–1414. [Google Scholar]
- MacDuff JH, Hopper MJ, Wild A. The effect of root temperature on growth and uptake of ammonium and nitrate by Brassica napus L. cv. Bien venu in flowing solution culture. J Exp Bot. 1987;38:53–66. [Google Scholar]
- Martin J-B, Bligny R, Rebeille F, Douce R, Leguay JJ, Mathiey Y, Guern J. A 31P nuclear magnetic resonance study of intracellular pH of plant cells cultivated in liquid medium. Plant Physiol. 1982;70:1156–1161. doi: 10.1104/pp.70.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson M, Häusler RE, Leegood RC, Lea P, Schjoerring JK. Leaf-atmosphere ammonia exchange in barley mutants with reduced activities of glutamine synthetase. Plant Physiol. 1997;114:1307–1312. doi: 10.1104/pp.114.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson M, Schjoerring JK. Ammonia emission from young barley plants: influence of N source, light/dark cycles, and inhibition of glutamine synthetase. J Exp Bot. 1996;47:477–484. [Google Scholar]
- Mouritzen P, Rosendahl L. Identification of a transport mechanism for NH4+ in the symbiosome membrane of pea root nodules. Plant Physiol. 1997;115:519–526. doi: 10.1104/pp.115.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DA, Jackson WA. Nitrate and ammonium uptake by rooted cuttings of sweet potato. Agron J. 1978;70:312–316. [Google Scholar]
- Nakashima J, Awano T, Takabe K, Fujita M, Saiki H. Immunocytochemical localization of phenylalanine ammonia-lyase and cinnamyl alcohol dehydrogenase in differentiating tracheary elements derived from Zinnia mesophyll cells. Plant Cell Physiol. 1997;38:113–123. [Google Scholar]
- Ninnemann O, Jauniaux J-C, Frommer WB. Identification of a high affinity NH4+ transporter from plants. EMBO J. 1994;103:3464–3471. doi: 10.1002/j.1460-2075.1994.tb06652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RJ. Energy coupling for membrane transport. Annu Rev Plant Physiol. 1978;29:437–460. [Google Scholar]
- Raven JA, Farquhar GD. Methylammonium transport in Phaseolus vulgaris leaf slices. Plant Physiol. 1981;67:859–863. doi: 10.1104/pp.67.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RJ. The permeability of ammonia, methylamine and ethylamine in the charophyte Chara corallina (C. australis) J Exp Bot. 1987;38:67–76. [Google Scholar]
- Schatchtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inwardly-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Seto-Young D, Perlin DS. Effect of membrane voltage on the plasma membrane H+-ATPase of Saccharomyces cerevisiae. J Biol Chem. 1991;266:1383–1389. [PubMed] [Google Scholar]
- Sutton M, Asman WAH, Schjoerring JK. Dry deposition of reduced nitrogen. Tellus. 1994;46:255–273. [Google Scholar]
- Sutton M, Schjoerring JK, Wyers P. Plant-atmosphere exchange of ammonia. Philos Trans R Soc Lond-Biol Sci. 1995;351:261–278. [Google Scholar]
- Terry BR, Findlay GP, Tyerman SD. Direct effects of the Ca2+-channel blockers on plasma membrane cation channels of Amaranthus tricolor protoplast. J Exp Bot. 1992;43:1457–1473. [Google Scholar]
- Tyerman SD, Whitehead LF, Day DA. A channel-like transporter for NH4+ on the symbiotic interface of N2-fixing plants. Nature. 1995;378:629–632. [Google Scholar]
- Ullrich WR, Larsson M, Larsson C-M, Lesch S, Novacky A. Ammonium uptake in Lemna gibba G 1, related membrane potential changes and inhibition of anion uptake. Physiol Plant. 1984;61:369–376. [Google Scholar]
- Uozumi N, Gassmann W, Cao Y, Schroeder JI. Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutant selection in yeast. J Biol Chem. 1995;270:24276–24281. doi: 10.1074/jbc.270.41.24276. [DOI] [PubMed] [Google Scholar]
- Vessey JK, Henry LT, Chaillou S, Raper CD., Jr Root-zone acidity affects relative uptake of nitrate and ammonium from mixed nitrogen sources. J Plant Nutr. 1990;13:95–116. doi: 10.1080/01904169009364061. [DOI] [PubMed] [Google Scholar]
- Wang MY, Glass ADM, Shaff JE, Kochian LV. Ammonium uptake by rice roots. III. Electrophysiology. Plant Physiol. 1994;104:899–906. doi: 10.1104/pp.104.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi Y, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. I. Fluxes and subcellular distribution of 13NH4+ Plant Physiol. 1993a;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi Y, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. II. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiol. 1993b;103:1259–1267. doi: 10.1104/pp.103.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner LH, De Boer AH, Raschke K. Properties of the K+ inward rectifier in the plasma membrane of xylem parenchyma cells from barley roots: effect of TEA+, Ca2+, Ba2+ and La3+ J Membr Biol. 1994;142:363–378. doi: 10.1007/BF00233442. [DOI] [PubMed] [Google Scholar]
- Yan F, Schubert S, Mengel K. Effects of low root medium pH on net proton release, root respiration, and root growth of corn (Zea mays L.) and broad bean (Vicia faba L.) Plant Physiol. 1992;99:415–421. doi: 10.1104/pp.99.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]