Abstract
IL-1β is a prominent proinflammatory cytokine that mediates degradation of extracellular matrix proteins through increased expression of matrix metalloproteinases, which involves a signaling pathway in adherent cells that is restricted by focal adhesions. Currently, the mechanism by which IL-1β affects cell adhesion to matrix proteins is not defined, and it is not known whether degraded matrix proteins affect IL-1β signaling. We examined adhesion-related IL-1β signaling in fibroblasts attaching to native or MMP3-degraded fibronectin. IL-1β increased cell attachment, resistance to shear force and the numbers of focal adhesions containing activated β1 integrins. IL-1β-enhanced attachment required FAK, kindlins 1/2, and talin. MMP3-degraded fibronectin-inhibited IL-1β-enhanced cell adhesion and promoted spontaneous ERK activation that was independent of IL-1β treatment. We conclude that IL-1β enhances the adhesion of anchorage-dependent cells to MMP3-degraded fibronectin, which, in turn, is associated with deregulated cellular responses to IL-1β. These data point to a novel role of IL-1β as a proadhesive signaling molecule in inflammation that employs kindlins and talin to regulate adhesion.—Rajshankar, D., Downey, G. P., McCulloch, C. A. IL-1β enhances cell adhesion to degraded fibronectin.
Keywords: integrins, talins, kindlins, MMP
Interleukin-1β (IL-1β) is a multifunctional, proinflammatory cytokine (1, 2) that is released as the first line of defense against external challenges (e.g., pathogenic microorganisms, environmental irritants, injury) and is critically involved in innate immunity (3). In adherent cells, such as fibroblasts, IL-1β signaling mediates the release of matrix metalloproteinases (MMPs; ref. 4), enzymes that remodel the extracellular matrix (5, 6) and facilitate tissue repair after short-term perturbations of connective tissue homeostasis, such as acute injury (7–10). However, in chronic inflammatory lesions, persistently high levels of IL-1β can lead to alterations of normal tissue architecture and a net loss of connective tissue matrix (11–15). Indeed, IL-1β is known to play a central role in the tissue destruction observed in a wide variety of chronic inflammatory diseases, such as rheumatoid arthritis, pulmonary and cardiac fibrosis, periodontitis, inflammatory bowel diseases, and type 2 diabetes (16–18).
In adherent cells, such as fibroblasts, the signal-generating IL-1β receptor (type I, IL-1β receptor) is enriched in focal adhesions (19). In fibroblasts, synoviocytes, and chondrocytes, the formation of focal adhesions is required for IL-1β-induced signaling events, such as tyrosine phosphorylation and activation of focal adhesion kinase (20), Ca2+ release from the endoplasmic reticulum (21, 22), and activation of extracellular signal-regulated kinases (23, 24), which are involved in the increased expression of MMPs (4, 25).
IL-1β can alter the composition of focal adhesions as shown earlier for the enrichment of talin (26), α-actinin (27), paxillin, vinculin (28), and protein tyrosine phosphatase-α in the focal adhesions of cultured cells after IL-1β treatment (4, 28). IL-1β also enhances spatial clustering of the endoplasmic reticulum with focal adhesions, as illustrated by the abundance of the ER-associated proteins calnexin and inositol triphosphate receptor-1 in purified focal adhesion preparations (29). Recent studies also suggest a possible role for IL-1β in providing resistance to anoikis in metastatic cancer cells (30, 31). Accordingly, we considered the hypothesis that IL-1β signaling may affect the adhesive strength of anchorage-dependent cells to matrix proteins in such a way that cells can survive in environments with MMP-degraded matrices while simultaneously enabling tissue remodeling. As the underlying mechanism of this phenomenon is undefined, we considered that it may involve regulation of adhesion systems that preserve cell attachment to extracellular matrices in the face of rapid matrix degradation. We examined here the β1 integrin-dependent mechanisms by which IL-1β promotes adhesion to fibronectin (FN), a prominent adhesive extracellular matrix protein. Further, we determined whether IL-1β signaling in fibroblasts is affected by adhesion to degraded FN.
MATERIALS AND METHODS
Materials
Recombinant human IL-1β (catalog no. 201-LB; ED50<12 pg/ml as measured by cell proliferation assay using D10.G4.1 mouse helper T cells), recombinant mouse MMP3, and goat polyclonal antibody (pAb) against human talin-2, and rabbit monoclonal antibody (mAb) against caspase-3 were obtained from R&D Systems (Minneapolis, MN, USA). Mouse mAb targeting talin-1 and mAb targeting β1 integrin (clone BV7; blocking antibody) were from Abcam (Cambridge, MA, USA). Mouse mAb against kindlin-2 and those against paxillin were from Millipore (Billerica, MA, USA). Mouse mAb to vinculin (clone hVIN-1), talin (clone 8d4), and bovine plasma FN, poly-l-lysine (PLL), bovine serum albumin (BSA), puromycin, and ferric oxide beads were from Sigma-Aldrich (St. Louis, MO, USA). Mouse mAb anti-α5β1 integrin (clone JB1A) was purchased from Chemicon (Temecula, CA, USA). In flow cytometry experiments, total β1 integrin in human cells was immunostained with mouse mAb (clone 4B4; Beckman-Coulter, Miami, FL, USA). The active conformation of β1 integrin was detected on human cells with mouse mAb (clone 12G10; Millipore, Billerica, MA, USA) and on mouse cells with rat mAb (clone 9EG7; BD Biosciences, Mississauga, ON, Canada). Rabbit mAb against extracellular signal-regulated kinase (ERK), mouse mAb against phospho-ERK, rabbit pAb against p38, and rabbit mAb against phospho-p38 were purchased from Cell Signaling Technologies (Boston, MA, USA). The rabbit mAb that recognizes vimentin was obtained from Epitomics (Burlingame, CA, USA). The smart pool siRNAs targeting talin-1, talin-2, and kindlin-2, as well as Dharmafect Reagent 1, BCA assay kit, and Zeba spin desalting columns were obtained from Thermo Scientific (Lafayette, CO, USA). Versene was purchased from Life Technologies (Invitrogen, Burlington, ON, Canada). The inhibitors to block the activity of the kinases rho-associated protein kinase (ROCK; Y-27632), p38 (SB 203580), and MEK (UO126), as well as the cyclic peptide Arg-Gly-Asp-Phe-Val (EMD 66203), which blocks αvβ3 integrin, were obtained from EMD Millipore Chemicals (Darmstadt, Germany). The tetrapeptide RGDS (H-Arg-Gly-Asp-Ser-OH; H-1155), which blocks β1 integrin attachment to FN, and the control peptide RGES (H-Arg-Gly-Glu-Ser-OH; H-7745) were from Bachem (Torrance, CA, USA).
Cell culture
Human gingival fibroblasts (HGFs; ScienCell, Carlsbad, CA, USA) were cultured in minimal essential medium (α-MEM) containing 10% fetal bovine serum (FBS) and were used between passages 5–12 in all experiments (28, 32). NIH 3T3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum. Murine embryonic fibroblasts (MEFs) expressing focal adhesion kinase (FAK+/+) or FAK-knockout (FAK−/−) cells (obtained from American Type Culture Collection, Manassas, VA, USA) were grown in DMEM containing 5% FBS. All media used during passaging were also supplemented with 146 U/ml penicillin G, 50 μg/ml gentamicin sulfate, and 0.25 μg/ml amphotericin B (Fungizone).
Cell adhesion assay
HGFs were spread on FN (10 μg/ml)-coated 24-well plates for 30 or 120 min in the presence or absence of IL-1β at 20 ng/ml in 2% serum medium. To determine the adhesion strength, cells were subjected to a jet wash assay using increasing numbers of washes with phosphate-buffered saline (PBS; 20 to 24 washes) with a repeating pipettor, as described previously (33). As described by Phares et al. (34), the shear force applied by this axisymmetric jet stream can be equated to wall shear stress, which varies radially outward from the point of impact, where the jet initially hits the cell surface to a maximum value of τmax, governed by the fluid density (ρ), the exit velocity of the jet (uo), the jet Reynold's number (Reo), the height of the jet stream from the planar surface (H), and the diameter of the pipette tip (D) by the following formula: τmax = 44.6ρuo2Reo−1/2 (H/D)−2.
The jet Reynold's number is Reo = uoD/ν, where ν is the kinematic viscosity. In our experiments, the diameter of the pipette tip D was 0.8 mm, the volumetric flow rate Q was estimated at 250 μl/s, the jet height H was ∼10 mm above the dish, and the fluid properties were almost identical to those of water. Since the exit velocity uo is a function of flow rate Q and the cross-sectional area of the pipette tip A, as in the equation uo = Q/A, the value of uo in our experiments was ∼ 0.5 m/s, and Reo can be estimated as ∼400. As calculated by Phares et al. (34), the maximum shear stress is experienced at a radial distance rmax = 0.009 H from the jet axis. Hence, maximum shear force at rmax ∼ 0.9 mm can be derived as ∼3.5 Pa (or 35 dyn/cm2). Cells were fixed with 4% paraformaldehyde and stained with DAPI, and the number of cells that remained attached to the substrate was estimated in 5 separate fields per well. The mean numbers of attached cells were calculated from values obtained from 4 wells.
For cell adhesion assays, a colorimetric method was also used (35). Briefly, 96-well tissue-culture-treated microtiter plates (Costar, Cambridge, MA, USA) were coated with native or MMP-3-degraded FN (100 μg/ml) for 60 min at room temperature and then blocked with heat-inactivated BSA (10 mg/ml) for 30 min at room temperature. HGFs were plated at a density of 2.5 × 104 cells/well in medium containing 1% FBS along with IL-1β (20 ng/ml) or with vehicle (PBS). In experiments with inhibitors, cells were pretreated with inhibitors (30 min at 37°C) prior to plating. Inhibitors were used at the following concentrations: ROCK inhibitor (Y-27632) at 500 nM; MEK inhibitor (U0126) at 10 μM; p38 inhibitor (SB 203580) at 1 μM; RGES peptide (H-7745) at 500 nM; RGDS peptide (H-1155) at 500 nM; and β1 integrin blocking antibody (clone BV7) at 1 μg/ml. Following cell attachment at 37°C for 30 or 120 min, cells were washed 3× to remove nonadherent or loosely attached cells and fixed with 5% (w/v) glutaraldehyde for 20 min at room temperature. After washing off the fixative with water, the adherent cells were stained with 0.1% (w/v) crystal violet in 200 mM 2-(N-morpholino) ethanesulfonic acid (MES) for 1 h at room temperature. Following a thorough wash with water to remove unbound stain, cells were solubilized with 10% (v/v) acetic acid for 5 min at room temperature, and the number of adherent cells was estimated from absorbance at 540 nm.
Flow cytometry
Cells were allowed to spread on FN for specified length of time and then stimulated with IL-1β (20 or 100 ng/ml) or with vehicle control in serum-free medium for varying time periods prior to harvesting in ice-cold versene and fixation in ice-cold 1% paraformaldehyde. Suspended cells were immunostained with rhodamine-conjugated anti-β1 integrin (4B4) antibody that recognizes both active and inactive forms of β1 integrin. Cell fluorescence was analyzed by flow cytometry (Beckman-Coulter). In some experiments, ligand-bound β1 integrin was quantified using neoepitope antibodies (12G10 for human cells, ref. 36; 9EG7 for mouse cells, ref. 37) prior to flow cytometric analysis.
Immunoblotting
Following treatments, cells were washed with PBS and extracted with lysis buffer. BCA assays used BSA as a standard for determining protein concentrations. Equal amounts of proteins were separated on 8 or 10% SDS-polyacrylamide gels under reducing conditions (except for β1 integrin immunoblotting) and transferred to polyvinylidene fluoride (PVDF) membranes. Blots were probed with appropriate primary and corresponding HRP-conjugated secondary antibodies. Immunoblots were developed by chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ, USA).
Focal adhesion isolation
Ferrous/ferric oxide beads (<5 μm diameter) were coated with FN (10 μg/ml solution) and incubated with cell cultures (37°C for 30 min). After incubation, beads were pelleted, washed with PBS, resuspended in PBS, and sonicated for 10 s, just before adding to the cells. HGFs were plated on PLL-coated dishes for 4 h in the presence of 2% serum and incubated with FN-coated beads with or without IL-1β (40 ng/ml) for 5, 15, 30, or 60 min. At the endpoints, cells were washed twice with ice-cold PBS to remove loosely bound beads. Cells and bound beads were scraped with cytoskeletal extraction buffer (0.5% Triton X-100, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM Na3VO4, 1 mM phenymethylsulfonyl fluoride, 1 μM phalloidin, and 10 mM PIPES, at pH 6.8, with Sigma Protease inhibitor cocktail at a ratio of 1:50). Pelleted beads were washed with ice-cold cytoskeletal extraction buffer 3 times and resuspended in 2× nonreducing SDS sample buffer, and bead-associated proteins were released by boiling the sample for 5 min. Equal amounts of bead-bound proteins were immunoblotted for talin, vinculin, paxillin, and β1 integrin as described previously (29).
Immunocytochemistry and total internal reflection fluorescence (TIRF) analysis
Cells were plated on FN-coated glass coverslips (MatTek dishes; MatTek Corp., Ashland, MA, USA) and treated with or without IL-1β (40 ng/ml in 2% serum-containing medium) for varying periods between 30 and 120 min. After fixation with 4% paraformaldehyde for 10 min, cells were permeabilized with 0.2% Triton-X-100 and blocked for 1 h in 0.2% BSA. Cells were incubated with primary antibody and FITC-conjugated secondary antibody for 1 h each. Immunostained cells were visualized by total internal reflection fluorescence (TIRF) microscopy. The mean number of focal adhesions per cell and the mean length of focal adhesions in the periphery (<5 μm from the cell membrane) and in the cell body (>5 μm from the cell membrane) were quantified with Leica MetaMorph software (>10 cells/condition; Leica Microsystems, Wetzlar, Germany).
MMP3 digestion of FN
Recombinant mouse MMP3 was activated with p-aminophenylmercuric acetate (APMA) as per the manufacturer's instructions and purified on a Zeba desalting column. MMP3 was incubated with FN (100 μg/ml) overnight at 37°C at an enzyme-substrate ratio of 1:10 or 1:100. For the undigested control, FN (100 μg/ml) was incubated overnight with TCNB (50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 0.05% Brij-35, pH 7.5) buffer alone. HGFs were plated for 3 h on native or degraded FN, and cell lysates were collected after treatment with or without IL-1β (20 ng/ml) for 15 or 30 min. Cells were immunoblotted for phospho-ERK and total ERK (p42/44), as described previously (28, 29, 32).
siRNA transfection
Cells were plated at ∼30% confluence 24 h prior to transfection. Cells were transfected with 10 μM scrambled siRNA or 5 μM each of talin-1 and talin-2 siRNA or 10 μM kindlin-2 siRNA using DharmaFect Reagent 1 for 48 h, as outlined in the manufacturer's guidelines. Cells were trypsinized and replated on FN (10 μg/ml) for 3 h in medium containing 2% serum. After stimulation with 20 ng/ml, IL-1β or vehicle control in serum-free medium for various times, whole cell lysates were collected and immunoblotted to estimate the level of protein knockdown and IL-1β-induced ERK activation.
Statistical analyses
All experiments were conducted in quadruplicate and were repeated ≥3 times. For continuous variables, means ± sd were computed. For 2-sample comparisons, Student's t test was conducted, and statistical significance was set at a type I error rate of P < 0.05. Analysis of variance was used for multiple comparisons, and Tukey's post hoc test was used for identifying differences between groups.
RESULTS
IL-1β promotes cell adhesion to FN
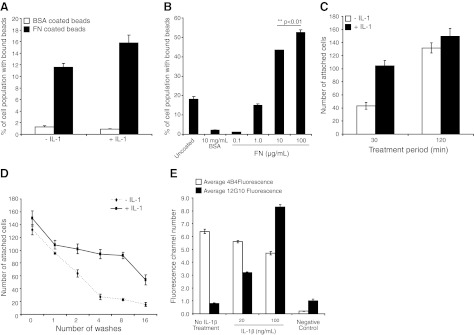
We examined whether IL-1β treatment affects cell adhesion to FN. HGFs were plated (1 h in regular medium and then in serum-free medium for 1 h) on a substrate (PLL, 1 mg/ml) that does not permit the formation of focal adhesions on the ventral cell surface. Cells were stimulated with IL-1β (20 ng/ml) in serum-free medium for 30 min prior to the addition of FN- or BSA-coated green fluorescence beads (2 μm diameter; refs. 33, 38). Under these conditions, beads attach to the dorsal surface of cells (24). Notably, longer bead incubations (i.e., >30 min) were not used because of bead internalization, which would confound the analysis of adhesion. Flow cytometry analyses of cell suspensions showed that IL-1β enhanced the binding of FN-coated beads by >30% (P<0.05; Fig. 1A) but did not affect the very low percentage of cells with bound BSA-coated beads. We determined whether the concentration of FN used for bead coatings influenced bead binding to cells and found that bead binding increased in a FN dose-dependent manner (Fig. 1B). Thus, the bead-binding assay is sensitive to changes in FN density on the bead surface. For the experiments reported here, FN coatings of 10 or 100 μg /ml were used.
Figure 1.
A) HGFs plated on PLL-coated dishes were treated with vehicle or with IL-1β (20 ng/ml) for 30 min prior to incubation with BSA- or FN-coated fluorescent beads, and the percentage of cells with bound beads was analyzed by flow cytometry. B) HGFs plated on FN (30 or 120 min in the presence or absence of IL-1β at 20 ng/ml) were washed and fixed, and the number of cells that remained attached to FN-coated dishes was determined by counting DAPI-stained nuclei. C) HGFs were plated on FN for 120 min with vehicle or IL-1β (20 ng/ml) and subjected to shear force by repeated jet washes, and DAPI-stained nuclei of cells remaining attached were counted. D) HGFs that were grown for 3 h in regular medium and serum starved overnight were treated with vehicle or with IL-1β (20 or 100 ng/ml) for 180 min. Total surface β1 integrins were immunolabeled with 4B4 (total β1 integrin); activated β1 integrins were immunolabeled with 12G10 antibody; stained cells were analyzed by flow cytometry.
We studied whole-cell attachment to FN-coated glass after IL-1β treatment (30 or 120 min treatment). IL-1β markedly increased cell attachment after 30 min (2.5-fold increase; P<0.001; Fig. 1C) but there was no significant difference of the number of attached cells after 120 min (P>0.2). We wondered whether the strength of the adhesions that formed after 120 min was affected by IL-1β. Accordingly, HGFs that had been stimulated with IL-1β for 120 min were subjected to shear force (35 dyn/cm2) by repeated jet washing, as described in Materials and Methods. There was increased resistance to shear-induced detachment of cells that had been treated with IL-1β (for all of 2-16 washes; P<0.001; Fig. 1D), indicating that for cells that attached and spread, IL-1β enhanced the adhesive strength to FN.
The β1 integrin subunit is the major FN receptor expressed in HGFs (39–43). Immunostaining and flow cytometry analysis of surface expression of FN-binding integrin subunits (α5, αv, β1, β3) showed that only α5 and β1 subunits were expressed at levels above background (data not shown). Further, incubation of cells with cyclic peptides that specifically block αvβ3 did not change IL-1β-induced cell resistance to shear stress (data not shown). Accordingly, we focused subsequent experiments on IL-1β-induced activation of β1 integrins in cells attaching to FN-coated substrates. HGFs were plated on PLL-coated substrates and stimulated with IL-1β (20 or 100 ng/ml) or with vehicle in serum-free medium for 180 min prior to the addition of BSA- or FN-coated beads. Cells were very quickly detached from the substrate with versene (<30 s; refs. 44, 45), fixed with 4% paraformaldehyde to maintain integrin activation status, immunostained with antibodies that recognize activated β1 integrins (clone 12G10) or total β1 integrins (clone 4B4) at the cell membrane, and analyzed by flow cytometry. We found that IL-1β treatment slightly reduced 4B4 staining of total β1 integrins (5% reduction at 20 ng/ml IL-1β, P>0.2; 16% reduction at 100 ng/ml IL-1β, P<0.05) but greatly increased staining for activated β1 integrins (3-fold increase at 20 ng/ml; >10-fold increase at 100 ng/ml; both treatments: P<0.001; Fig. 1E).
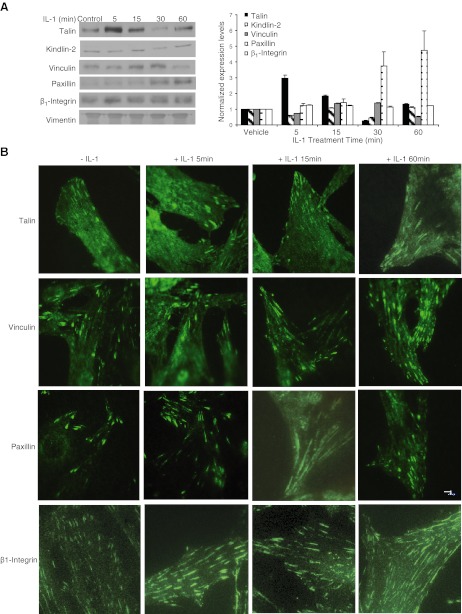
As cell adhesion to FN was evidently affected by IL-1β, we examined whether IL-1β-induced activation of β1 integrins was associated with changes of the protein composition of focal adhesions. Immunoblotting of FN-coated bead-associated proteins (4, 46) showed that following IL-1β treatment, there were time-dependent variations of the relative amounts of talin, kindlin-2, paxillin, vinculin, and β1 integrin when adjusted for vimentin (Fig. 2A), which has been shown to be enriched in these types of focal adhesion fractions (44, 45) and which is reasonably constantly expressed under these experimental conditions. There were particularly marked increases of talin in the focal adhesion fractions 5 min after IL-1β treatment and of paxillin 30 min after IL-1β treatment. TIRF microscopy of these same immunostained proteins in the focal adhesions of cells attached to FN-coated glass validated these biochemical observations as the relative abundance of immunostained talin was markedly increased at 5-min and paxillin-stained adhesions were more abundant at 60 min (Fig. 2B).
Figure 2.
A) HGFs were plated on PLL for 4 h, incubated with FN-coated magnetite beads, and treated with vehicle or with IL-1β (40 ng/ml) for 5, 15, 30, or 60 min. Left panel: magnetically isolated bead-associated proteins were immunoblotted for talin, kindlin-2, vinculin, paxillin, and β1-integrin, and the relative abundance of protein as measured by densitometry was normalized to vimentin. Right panel: normalized blot densities are plotted in the histogram. Data represent means ± sd of normalized expression levels to vimentin (n=3 separate experiments). B) HGFs were plated on FN for 4 h and treated with IL-1β for the indicated times and then immunostained for talin, vinculin, and paxillin and imaged by TIRF.
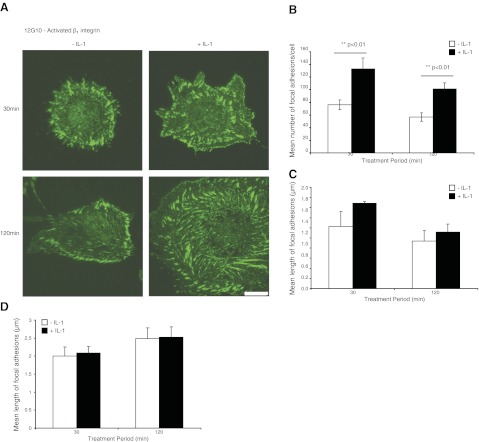
The flow cytometry approach described in Fig. 1E above provides estimates of β1 integrin abundance and activation for the whole plasma membrane surface in cells that are fixed immediately (45) after detachment from the matrix. To estimate the relative abundance of activated β1 integrins in focal adhesions of cells in situ, HGFs were plated on FN-coated glass coverslips (30–120 min), treated with vehicle or IL-1β (40 ng/ml in 2% serum-containing medium), fixed, immunostained for activated β1 integrin (clone 12G10), and imaged by TIRF microscopy (Fig. 3A). IL-1β enhanced the number of 12G10-stained focal adhesions per cell (P<0.01; Fig. 3B) but did not affect the length of focal adhesions (P>0.1; Fig. 3C, D).
Figure 3.
A) HGFs plated on FN-coated glass coverslips (30 or 120 min) and treated with vehicle or with IL-1β (40 ng/ml) were immunostained for activated β1 integrin (12G10 antibody) and visualized by TIRF microscopy. Scale bar = 10 μm. B) Mean ± sd numbers of focal adhesions per cell that were immunostained with 12G10. Data are from measurements of >10 cells/group. C) Mean ± sd length of focal adhesions immunostained with 12G10 in the cell body (>5 μm from the cell membrane; >10 cells/group). D) Mean ± sd length of focal adhesions in the cell periphery (<5 μm from the cell membrane; >10 cells/group). Focal adhesions were quantified with MetaMorph.
Effect of talin and kindlin knockdown on IL-1β-induced ERK activation and cell adhesion
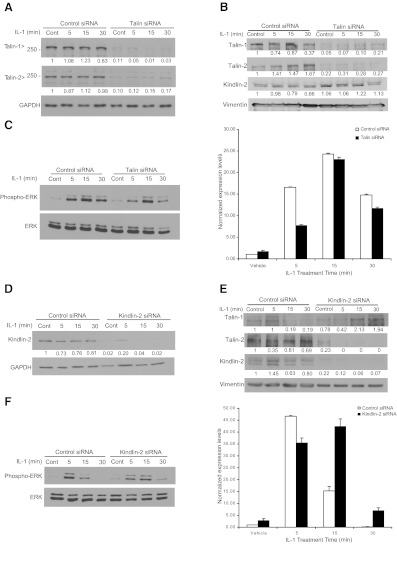
We examined the mechanism by which IL-1β may promote β1 integrin activation by first determining whether proteins implicated in integrin activation, namely talins (47) and kindlins (48), are involved in IL-1β signaling to ERK. As talin localizes rapidly to focal adhesions after IL-1β treatment (ref. 26 and Fig. 2A), we determined whether talin knockdown would affect IL-1β-induced ERK activation and focal adhesion formation. Because there are two isoforms of talins present in fibroblasts, siRNAs were used to knock down both isoforms. After verifying the efficacy of talin knockdown (∼90% for talin-1 and talin-2; Fig. 4A), HGFs treated with control siRNA or with talin 1- or talin 2-knockouts were replated on FN-coated substrates for 3 h and treated with vehicle or with IL-1β for 5, 15, or 30 min. We verified that the knockdown of these proteins was also detectable in the focal adhesion preparations (Fig. 4B).When whole-cell lysates were immunoblotted for ERK and phospho-ERK, we observed that IL-1β signaling to ERK (measured by ERK phosphorylation) was reduced by talin depletion, particularly at 5 min (Fig. 4C; densitometry performed from triplicate experiments).
Figure 4.
A–C) HGFs were transfected with control (scrambled) siRNA or talin-1 and talin-2 siRNAs for 48 h, as outlined in Materials and Methods. Cells were trypsinized and replated on FN for 3 h in medium containing 2% serum. After treatment with vehicle or with IL-1β (20 ng/ml) in serum-free medium for 5, 15, or 30 min, cell lysates were immunoblotted for talin-1, talin-2, and GAPDH (A); for the indicated proteins in focal adhesion preparations (B); and for phospho-ERK and total ERK (C, left panel). Histogram (C, right panel) shows densitometry data from immunoblots of C. D) NIH 3T3 cells were transfected with control (scrambled) or kindlin-2 siRNA using DharmaFect Reagent 1 for 48 h, as outlined in Materials and Methods. Cells were replated on FN for 3 h in medium containing 2% serum. After treatment of cells with vehicle or IL-1β (20 ng/ml; 5, 15, or 30 min) in serum-free medium, cell lysates were immunoblotted for kindlin-2 and GAPDH. E) Focal adhesion proteins from the experiments in D were prepared and immunoblotted for the indicated proteins. F) In the kindling-2-knockdown experiments, phospho-ERK and ERK were immunoblotted (left panel), and the densitometry results were quantified (right panel).
We examined the effect of kindlin knockdown on IL-1β-induced ERK activation. Although there are three known kindlin isoforms, in fibroblasts, the major isoform that is expressed is kindlin-2 (49). NIH 3T3 cells were used to study kindlin-2 siRNA knockdown (>90% knockdown in cell lysates and focal adhesion preparations; Fig. 4D, E). When control and kindlin-2-knockdown cells were treated with or without IL-1β, as described above and analyzed for ERK activation, depletion of kindlin-2 inhibited IL-1β-induced ERK activation at 5 min but increased ERK activation at 15 and 30 min (Fig. 4F).
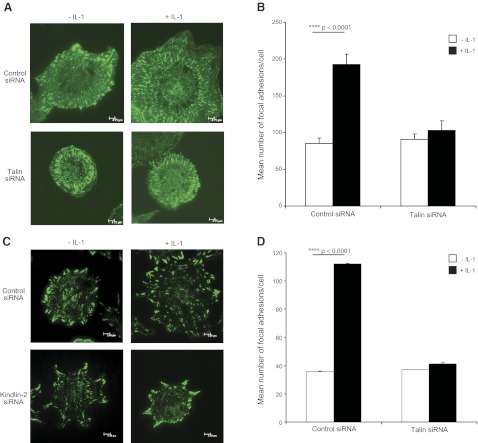
We determined whether IL-1β-enhanced cell adhesion was mediated by talins and kindlins. HGFs with talin or control knockdown were plated on FN-coated substrates and treated with vehicle or IL-1β for 60 min and then immunostained for activated β1 integrins with the 12G10 antibody. Cells that were treated with control siRNA exhibited ∼2.5-fold increases in the number of focal adhesions in the presence of IL-1β (P<0.001; Fig. 5A, B), while in talin-depleted cells, IL-1β did not increase the number of focal adhesions (P>0.2). Similarly, kindlin-2 or control siRNA-transfected NIH3T3 cells that were seeded on FN in the presence or absence of IL-1β were assessed for activated β1 integrins by immunostaining with 9EG7, a neoepitope antibody that recognizes activated mouse β1 integrins (37). Compared to controls, which exhibited a 3-fold increase of the number of focal adhesions after IL-1β treatment (P<0.001; Fig. 5C, D), cells with kindlin-2 knockdown showed no increase of IL-1β-induced increase in focal adhesion numbers (P>0.2).
Figure 5.
A) Talin-1 and talin-2 were both knocked down in HGFs, plated on FN in the presence or absence of IL-1β for 60 min, and fixed and immunostained for activated β1-integrin (12G10). B) Mean± sd numbers of focal adhesions were computed with MetaMorph. C) NIH 3T3 cells treated with siRNA to kindlin-2 were spread on FN with vehicle or IL-1β for 60 min, fixed, and immunostained for activated β1-integrin (9EG7). D) Metamorph was used to quantify mean ± sd numbers of focal adhesions per cell.
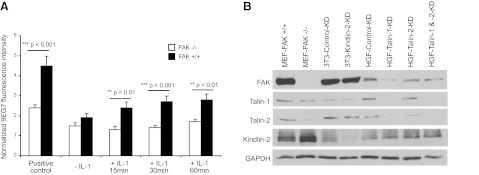
As integrin activation is regulated in part by FAK (50), we examined the role of FAK in IL-1β-induced β1 integrin activation. Wild-type or FAK-null MEFs were plated on FN, treated with manganese chloride (1 mM) as a positive control for β1 integrin activation (37) or with IL-1β (0-60 min), and immunostained for activated β1 integrin (44). The positive controls (manganese chloride group) exhibited very large increases of 9EG7 staining compared with controls, and the fluorescence signal for activated β1 integrin was nearly 2-fold higher in the FAK-expressing cells than the FAK-null cells (P<0.05; Fig. 6A). FAK-null cells showed no increase of activated β1 integrin after IL-1β treatment, while FAK-expressing cells exhibited 60% higher fluorescence than the FAK-null cells 60 min after IL-1β treatment.
Figure 6.
A) FAK−/− and FAK+/+ mouse embryonic cells were attached overnight to FN and treated with vehicle or with IL-1β (20 ng/ml) in 2% serum-containing medium for 15, 30, or 60 min. β1-Integrin activation was assessed by immunostaining with the 9EG7 neoepitope antibody and quantification of whole-cell fluorescence by flow cytometry. B) Proteins were separated from cell lysates of the various cell types under study and immunoblotted for the indicated proteins.
As FAK, talins, and kindlin-2 are likely to affect the activation status of β1 integrin in response to environmental ligands and the formation of focal adhesions, we determined whether the down-regulation of one of these proteins would affect the expression levels of the others in the 3T3 cells and the HGFs (Fig. 6B). In FAK-null MEFs, the relative expression levels of talins were reduced, whereas kindlin-2 expression was up-regulated. In 3T3 cells, when kindlin-2 was depleted by siRNA knockdown, no changes in the other proteins were observed. In HGFs, depletion of talin-1 resulted in reduction of FAK.
Role of FN degradation status on cell adhesion
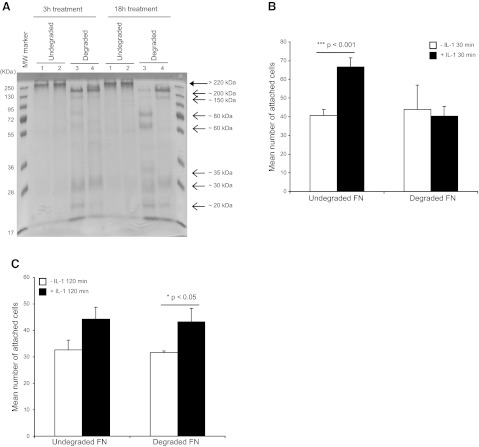
IL-1β induces ERK-dependent MMP3 release in HGFs (4, 25), which, in turn, promotes matrix remodeling (51–53) and, in particular, FN degradation (54). We considered that FN degradation could impair cell attachment, which, in view of our findings above, may be compensated by IL-1β-enhanced β1 integrin activation and focal adhesion maturation in order to maintain cell attachment to the matrix. Accordingly, we examined whether IL-1β enhances the adhesion of fibroblasts to degraded FN. First, FN (100 μg/ml) was attached overnight to culture plates and incubated with control buffer (for native FN) or with APMA-activated MMP3 (at 1:10 or 1:100 enzyme-substrate ratio, for production of degraded FN). Digestions (3 and 18 h) of FN were conducted in situ on the plates. The proteins were removed from the plates with SDS, separated by PAGE, and visualized with Coomassie brilliant blue. The stained gel of the protein eluted from the plates showed marked FN degradation after MMP3 treatments, particularly after 18 h digestions at a 1:10 enzyme-substrate ratio (Fig. 7A).
Figure 7.
A) FN was attached to plates and incubated with buffer (undegraded) or MMP3 at 1:10 or 1:100 enzyme-FN substrate ratios for 3 or 18 h (degraded). Native FN or FN-degradation fragments were separated by SDS-PAGE. Native FN (large arrow; >220 kDa) and degraded FN fragments (small arrows: ∼200-20 kDa) were stained with Coomassie blue. Lane 1, untreated FN; lane 2, native FN incubated with buffer alone; lane 3, FN digested with MMP3 at enzyme-substrate ratio of 1:10; lane 4, FN digested with MMP3 at enzyme-substrate ratio of 1:100. B, C) HGFs were plated on native FN or MMP3-digested FN (at 1:10 ratio) for 18 h and treated with vehicle or with IL-1β (20 ng/ml) for 30 min (B) or 120 min (C). FN was washed with PBS before cell adhesion assays. Adherent cells were counted by enumeration of DAPI-stained nuclei in 100-μm2 grids. Mean ± sd numbers of attached cells were calculated from values obtained from 4 wells.
We examined adhesion of HGFs to native or degraded FN (18 h digestions) after treatment with vehicle or with IL-1β (20 ng/ml; 30 or 120 min). Nonadherent cells were removed by washing, and the number of attached cells was estimated by counting DAPI-stained nuclei in grids of defined size (100-μm2 grids). In contrast to cells plated on native FN, cells plated on degraded FN were not influenced by IL-1β treatment during early attachment (30 min; Fig. 7B). At 120 min after plating, however, there was restoration of the effect of IL-1β-enhanced attachment (Fig. 7C).
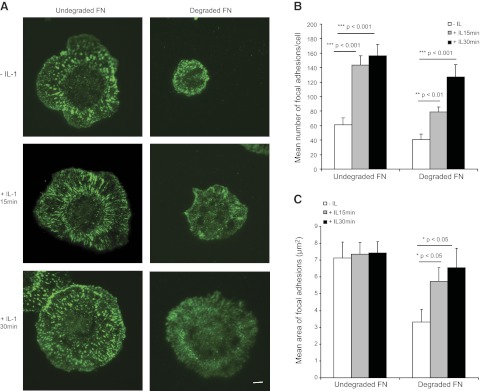
These observations led us to wonder whether plating cells on degraded FN influenced the ability of IL-1β to enhance β1 integrin activation. HGFs were plated on native FN or MMP3-degraded FN and treated with vehicle or IL-1β for 30 min. Activated β1 integrins were analyzed by TIRF microscopy in 12G10-stained cells (Fig. 8A). Cells on degraded FN did not spread as well as cells on native FN. IL-1β enhanced the spreading of cells plated on degraded FN. Quantification of the numbers and areas of focal adhesions showed that the numbers of focal adhesions were markedly increased after IL-1β treatment in cells plated on both native and degraded FN (Fig. 8B). Notably, the areas of the FAs did not change with IL-1β treatment in cells plated on native FN, while IL-1β treatment strongly increased the areas of focal adhesions in cells plated on degraded FN (Fig. 8C).
Figure 8.
A) Cells were plated overnight on vehicle or MMP3-degraded FN (1:10 ratio) and treated for 15 or 30 min with vehicle or with IL-1β (20 ng/ml). After fixation and immunostaining with 12G10 antibody, cells were visualized with TIRF microscopy. B, C) MetaMorph software was used to estimate mean ± sd numbers of focal adhesions per cell (B) and mean± sd area of focal adhesions in the cell body (C); n = 15 cells/group).
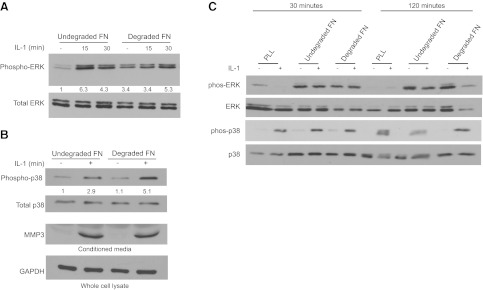
In view of the effect of IL-1β on the number of focal adhesions in cells plated on FN, we determined whether IL-1β signaling to ERK was also influenced by degradation of FN. HGFs were plated for 3 h on native FN (100 μg/ml) or on MMP3-degraded FN (18 h digestion) and were treated with vehicle or IL-1β (20 ng/ml; 15 or 30 min). Cell lysates were immunoblotted for phospho-ERK and total ERK. Cells plated on native FN showed robust ERK activation in response to IL-1β treatment. In contrast, for cells plated on MMP3-degraded FN, the basal levels of activated ERK were elevated, even in the absence of IL-1β treatment and ERK activation was not increased further by IL-1β treatment (Fig. 9A).
Figure 9.
A, B) HGFs plated for 3 h on native FN or MMP3-degraded FN (18 h of FN degradation prior to cell plating) were treated with IL-1β (20 ng/ml) for 15 or 30 min. Lysates were immunoblotted for phospho-ERK and total ERK (A) and phospho-p38, total p38, and GAPDH (B). In addition, conditioned media were collected from the cell preparations in A, concentrated 15×, and immunoblotted for MMP3. Whole-cell lysates were immunoblotted for GAPDH to provide loading controls. C) Cells were plated for indicated times on PLL or native or MMP3-degraded FN and then treated with vehicle (−) or with IL-1β (+) for 15 min. Cell lysates were immunoblotted for indicated proteins; densitometry analyses were adjusted for conditions on extreme left lane for each protein.
When cell lysates were immunoblotted for the stress-activated mitogen-activated protein kinase p38, we found that IL-1β increased p38 phosphorylation by 3-fold above basal levels on intact FN and ∼5-fold on degraded FN (Fig. 9B). Since IL-1β enhances the secretion of MMP3 (4), we assessed the effect of MMP3-degraded FN on IL-1β-induced MMP3 release. Conditioned media were collected from cells that were treated as described above; the media were concentrated 15 times by Centricon filters, and the levels of MMP3 were assessed by immunoblotting of the concentrated medium (4). Analysis of medium that was incubated overnight with MMP3-degraded FN and in the absence of cells showed no detectable MMP3 (data not shown), which was the same result as medium obtained from cells that were treated with vehicle (Fig. 9B). These findings indicate that the MMP3 measured in the conditioned medium originated from the cells and not from the a priori MMP3 treatment of the FN. Notably, we found that a priori FN degradation by MMP3 did not affect the relative abundance of IL-1β induced MMP3 release (Fig. 9B).
As we found that the time of plating strongly affected IL-1β-induced adhesion effects on cells, we examined ERK and p38 activation in more detail in cells plated on PLL or native or degraded FN at 30 and 120 min after plating (instead of overnight plating as above). These data showed that IL-1β signaling to ERK was dependent on cells being plated on FN and not PLL, whereas activation of p38 occurred in cells plated on both of FN and PLL (Fig. 9C). Further, cells plated on both native and degraded FN exhibited strong ERK phosphorylation independent of IL-1β treatment. In contrast, IL-1β treatment was required for p38 phosphorylation under all of these experimental conditions.
We determined whether IL-1β-induced increases of cell adhesion were dependent on specific adhesion receptors and signaling pathways (Fig. 10). Cell adhesion was measured using a colorimetric assay in HGFs plated on native or degraded FN for 30 or 120 min, as described in the Materials and Methods. IL-1β-induced enhancement of cell adhesion was dependent on β1 integrins, as the BV7 antibody blocked increased cell attachment attributable to IL-1β (Table 1). We also observed a similar dependence on β1 integrin for IL-1β-induced adherence based on data using the RGDS blocking peptide in contrast with the control RGES peptide (Table 1).
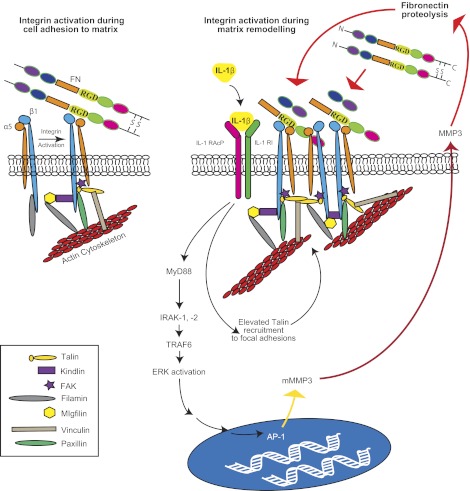
Figure 10.
Proposed model of β1 integrin activation in response to IL-1β stimulation, showing the role of adhesion regulatory molecule in affecting attachment to degraded matrix.
Table 1.
Regulation of IL-1β-induced adhesion of human gingival fibroblasts to native and degraded FN
| Treatment | 30 min |
120 min |
||||||
|---|---|---|---|---|---|---|---|---|
| Native FN |
Degraded FN |
Native FN |
Degraded FN |
|||||
| Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | |
| Control mAb or β1 blocking antibody (BV7) | 0.07 ± 0.01 | 0.06 ± 0.002* | 0.02 ± 0.004 | 0.015 ± 0.004* | 0.25 ± 0.05 | −0.03 ± 0.03** | 0.02 ± 0.02 | 0.01 ± 0.01 |
| RGES control or RGDS (experimental) | 0.17 ± 0.02 | 0.12 ± 0.003* | 0.2 ± 0.001 | −0.002 ± 0.001*** | 0.52 ± 0.02 | 0.26 ± 0.08** | 0.52 ± 0.01 | 0.25 ± 0.02*** |
| Vehicle control or ROCK inhibitor (experimental) | 0.034 ± 0.008 | 0.025 ± 0.004 | 0.025 ± 0.007 | 0.011 ± 0.002 | 0.037 ± 0.008 | 0.036 ± 0.01 | 0.026 ± 0.008 | −0.007 ± 0.002* |
| Vehicle control or MEK inhibitor (experimental) | 0.034 ± 0.008 | 0.01 ± 0.007* | 0.025 ± 0.007 | −0.002 ± 0.0004** | 0.037 ± 0.008 | 0.022 ± 0.009 | 0.026 ± 0.008 | 0.013 ± 0.002* |
| Vehicle control or p38 inhibitor (experimental) | 0.034 ± 0.008 | −0.01 ± 0.01* | 0.025 ± 0.007 | 0.012 ± 0.007 | 0.037 ± 0.008 | 0.002 ± 0.009* | 0.026 ± 0.008 | −0.008 ± 0.002** |
| Control siRNA or talin-1 siRNA | 0.025 ± 0.002 | 0.004 ± 0.004* | 0.02 ± 0.004 | 0.045 ± 0.008* | 0.045 ± 0.003 | 0.003 ± 0.0005** | 0.022 ± 0.004 | 0.023 ± 0.005 |
| Control siRNA or talin-2 siRNA | 0.025 ± 0.002 | 0.004 ± 0.004* | 0.02 ± 0.004 | 0.0 ± 0.002* | 0.045 ± 0.003 | 0.017 ± 0.004** | 0.022 ± 0.004 | 0.015 ± 0.016 |
| Control or talin-1/2 siRNA | 0.025 ± 0.002 | −0.02 ± 0.002** | 0.02 ± 0.004 | −0.008 ± 0.002** | 0.045 ± 0.003 | −0.016 ± 0.015 | 0.022 ± 0.004 | −0.06 ± 0.002*** |
| Control or kindlin-2 siRNA | 0.052 ± 0.011 | 0.056 ± 0.001 | 0.055 ± 0.015 | 0.03 ± 0.006* | 0.029 ± 0.02 | −0.015 ± 0.02 | 0.01 ± 0.008 | −0.002 ± 0.01 |
| Control (FAK+/+) or FAK−/− cells | 0.052 ± 0.005 | −0.13 ± 0.002** | 0.03 ± 0.007 | 0.002 ± 0.006 | 0.025 ± 0.04 | 0.27 ± 0.02 | −0.07 ± 0.006 | −0.065 ± 0.04 |
Experimental (Exp) groups are compared to vehicle controls (Ctrl) on native or MMP3-degraded FN for indicated times of adhesion. Data show the increase of absorbance units at 540 nm attributable to IL-1β-enhanced cell adhesion.
P < 0.05,
P < 0.01,
P < 0.001 vs. corresponding control.
With the use of inhibitors of the ROCK, ERK, and p38 pathways, we found that the IL-1β-induced increase of cell adhesion was strongly reduced by blockade of the ERK and p38 pathways but much less so by ROCK inhibition (Table 1).
We used these same colorimetric cell adhesion assays to examine the effect of talins, kindlin-2, and FAK, to complement the focal adhesion assays reported above. These data showed that talin-1 and talin-2, as well as kindlin-2 and FAK, were important for IL-1β-enhanced cell adhesion (Table 1), which was consistent with the data on focal adhesions (Fig. 5).
DISCUSSION
Our major finding is that IL-1β promotes initial cell attachment and sustained adhesive strength through β1-integrin activation (Fig. 8). Conceivably, IL-1β may increase the adhesive strength of anchorage-dependent cells to matrix proteins in such a way that cells can remain attached, even in inflammatory lesions with MMP-degraded matrices. Therefore IL-1β, in addition to its role as a prototypical proinflammatory cytokine, may be an important survival factor that preserves the function of connective tissue cells in inflammatory lesions and thereby enables ongoing matrix remodeling by anchorage-dependent cells.
In cells that were depleted of talin-1 or talin-2 by siRNA, we found that IL-1β-enhanced adhesion and β1 integrin activation were blocked. Talin has been implicated in the activation of β1 integrins as a result of its ability to displace covalent interactions between the cytoplasmic tails of α and β integrins (47, 55, 56). However, more recent evidence suggests that talin may not be required for the initial cell attachment to FN but instead is required for sustained cell spreading and the mechanical linkage between extracellular matrix-bound integrins and the actin cytoskeleton, processes which are required for transmission of outside-in integrin signals (57). Indeed, the rapidity of IL-1β signaling events may depend in part on the phosphorylation of the actin-binding protein talin and its recruitment to focal adhesions (26). Our new data extend these insights by demonstrating the importance of talin in mediating the IL-1β-enhanced adhesion to FN.
In addition to talins, kindlins are a family of proteins that are involved in the regulation of integrin activation (48, 56, 58). Among the three known different kindlin isoforms, kindlin-2 is expressed ubiquitously, whereas kindlin-1 expression is restricted to epidermal and dermal tissues, while kindlin-3 is expressed in the spleen, thymus, and lung tissues (49). After depletion of kindlin-2, we found that the ability of IL-1β to increase cell adhesion and β1 integrin was inhibited. Kindlin-2-knockout mice exhibit very early lethality (peri-implantation), which is reminiscent of the β1 integrin-knockout phenotype (59). Further, kindlin-2 can recruit migfilin, which disrupts the interaction between β1 integrin and filamin, thereby facilitating talin binding and β1 integrin activation (60, 61). Our decision to focus on β1 integrin activation in the context of cell adhesion to FN was based on previous data showing that this integrin is the principal β-integrin subunit in the FN receptors of human gingival fibroblasts (39–43), as well as on our analyses of surface expression levels of α5, β1, αv, and β3 integrin subunits (data not shown) and on resistance to shear stress in cells incubated with cyclic peptides that specifically block αvβ3.
IL-1β signaling to ERK activation in anchorage-dependent cells, such as fibroblasts, chondrocytes, and synoviocytes, requires the formation of focal adhesions to FN or collagen matrices (22, 23). We found here that after knockdown of either talin-1/2 or kindlin-2, IL-1β-induced ERK activation was affected in complex ways. These data indicate that IL-1β promotes β1 integrin activation and adhesion to FN in a process that influences IL-1β signaling to ERK. Notably, careful examination of the cells with talin-1/2 or kindlin-2 depletion showed that these cells exhibited focal adhesions, albeit of smaller size and of fewer number. However, the number of focal adhesions that are required for generation of the IL-1β signal is very small and can be induced with a few FN-coated beads (24). Even small focal adhesions can sequester Ras (62) and other critical signaling molecules (29) in sufficient abundance to allow IL-1β signals to be transmitted. This contention is supported by our finding that when cells were plated on MMP3-degraded FN, which reduces the numbers of focal adhesions, cells treated with IL-1β exhibited similar levels of MMP3 release as did cells plated on native FN. Further, cells plated on MMP3-degraded FN exhibited markedly increased basal levels of ERK phosphorylation, even without IL-1β treatment. Thus IL-1β can promote matrix remodeling through release of MMP3, even when the FN matrix is itself partially degraded by MMP3, and IL-1β signaling to ERK is perturbed. We interpret these data on the ability of IL-1β to promote cell adhesion of anchorage-dependent cells to native and degraded FN, and to maintain matrix remodeling activities (i.e., release of MMP3), as indicating that while IL-1β is a proinflammatory cytokine, it also helps to preserve cell function and conceivably to conserve overall matrix integrity in inflammatory lesions.
Acknowledgments
This study is supported by Canadian Institutes of Health Research operating grant MOP 84254 to C.A.M., who is also supported by a Canada Research Chair (Tier 1). This work was also supported, in part, by U.S. National Institutes of Health grant HL090669 (to G.P.D.).
Footnotes
- APMA
- p-aminophenylmercuric acetate
- BSA
- bovine serum albumin
- ERK
- extracellular signal-regulated kinase
- FAK
- focal adhesion kinase
- FBS
- fetal bovine serum
- FN
- fibronectin
- HGF
- human gingival fibroblast
- IL-1β
- interleukin-1β
- mAb
- monoclonal antibody
- MEF
- murine embryonic fibroblast
- MMP
- matrix metalloproteinase
- pAb
- polyclonal antibody
- PBS
- phosphate buffered saline
- PLL
- poly-l-lysine
- ROCK
- rho-associated protein kinase
- TIRF
- total internal reflection fluorescence
REFERENCES
- 1. Dinarello C. A. (1984) Interleukin-1 and the pathogenesis of the acute-phase response. N. Engl. J. Med. 311, 1413–1418 [DOI] [PubMed] [Google Scholar]
- 2. Dinarello C. A. (1996) Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 [PubMed] [Google Scholar]
- 3. Auron P. E. (1998) The interleukin 1 receptor: ligand interactions and signal transduction. Cytokine Growth Factor Rev. 9, 221–237 [DOI] [PubMed] [Google Scholar]
- 4. Wang Q., Rajshankar D., Laschinger C., Talior-Volodarsky I., Wang Y., Downey G. P., McCulloch C. A. (2010) Importance of protein-tyrosine phosphatase-alpha catalytic domains for interactions with SHP-2 and interleukin-1-induced matrix metalloproteinase-3 expression. J. Biol. Chem. 285, 22308–22317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Page-McCaw A., Ewald A. J., Werb Z. (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yong V. W. (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 6, 931–944 [DOI] [PubMed] [Google Scholar]
- 7. Zhang H., Chang M., Hansen C. N., Basso D. M., Noble-Haeusslein L. J. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics 8, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szabo K. A., Ablin R. J., Singh G. (2004) Matrix metalloproteinases and the immune response. Clin. Appl. Immunol. Rev. 4, 295, 319 [Google Scholar]
- 9. Franchi L., Munoz-Planillo R., Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rathinam V. A., Vanaja S. K., Fitzgerald K. A. Regulation of inflammasome signaling. Nat. Immunol. 13, 333–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinarello C. A. (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 [DOI] [PubMed] [Google Scholar]
- 12. Chung K. F. (2005) Inflammatory mediators in chronic obstructive pulmonary disease. Curr. Drug Targets Inflamm. Allergy 4, 619–625 [DOI] [PubMed] [Google Scholar]
- 13. Han Y. P. (2006) Matrix metalloproteinases, the pros and cons, in liver fibrosis. J. Gastroenterol. Hepatol. 21(Suppl, 3), S88–S91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Maitre C. L., Pockert A., Buttle D. J., Freemont A. J., Hoyland J. A. (2007) Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 35, 652–655 [DOI] [PubMed] [Google Scholar]
- 15. Maini R. N., Taylor P. C. (2000) Anti-cytokine therapy for rheumatoid arthritis. Annu. Rev. Med. 51, 207–229 [DOI] [PubMed] [Google Scholar]
- 16. Boch J. A., Wara-aswapati N., Auron P. E. (2001) Interleukin 1 signal transduction–current concepts and relevance to periodontitis. J. Dental Res. 80, 400–407 [DOI] [PubMed] [Google Scholar]
- 17. McCulloch C. A., Downey G. P., El-Gabalawy H. (2006) Signalling platforms that modulate the inflammatory response: new targets for drug development. Nat. Rev. Drug Discov. 5, 864–876 [DOI] [PubMed] [Google Scholar]
- 18. Dinarello C. A. (2011) A clinical perspective of IL-1ββ as the gatekeeper of inflammation. Eur. J. Immunol. 41, 1203–1217 [DOI] [PubMed] [Google Scholar]
- 19. Qwarnstrom E. E., Page R. C., Gillis S., Dower S. K. (1988) Binding, internalization, and intracellular localization of interleukin-1β in human diploid fibroblasts. J. Biol. Chem. 263, 8261–8269 [PubMed] [Google Scholar]
- 20. Arora P. D., Ma J., Min W., Cruz T., McCulloch C. A. (1995) Interleukin-1-induced calcium flux in human fibroblasts is mediated through focal adhesions. J. Biol. Chem. 270, 6042–6049 [DOI] [PubMed] [Google Scholar]
- 21. Lo Y. Y., Luo L., McCulloch C. A., Cruz T. F. (1998) Requirements of focal adhesions and calcium fluxes for interleukin-1-induced ERK kinase activation and c-fos expression in fibroblasts. J. Biol. Chem. 273, 7059–7065 [DOI] [PubMed] [Google Scholar]
- 22. Luo L., Cruz T., McCulloch C. (1997) Interleukin 1-induced calcium signalling in chondrocytes requires focal adhesions. Biochem. J. 324, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacGillivray M. K., Cruz T. F., McCulloch C. A. (2000) The recruitment of the interleukin-1 (IL-1β) receptor-associated kinase (IRAK) into focal adhesion complexes is required for IL-1ββ -induced ERK activation. J. Biol. Chem. 275, 23509–23515 [DOI] [PubMed] [Google Scholar]
- 24. Wang Q., Downey G. P., Choi C., Kapus A., McCulloch C. A. (2003) IL-1β induced release of Ca2+ from internal stores is dependent on cell-matrix interactions and regulates ERK activation. FASEB J. 17, 1898–1900 [DOI] [PubMed] [Google Scholar]
- 25. Reunanen N., Westermarck J., Hakkinen L., Holmstrom T. H., Elo I., Eriksson J. E., Kahari V. M. (1998) Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J. Biol. Chem. 273, 5137–5145 [DOI] [PubMed] [Google Scholar]
- 26. Qwarnstrom E. E., MacFarlane S. A., Page R. C., Dower S. K. (1991) Interleukin 1β induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc. Natl. Acad. Sci. U. S. A. 88, 1232–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herrera Abreu M. T., Wang Q., Vachon E., Suzuki T., Chow C. W., Wang Y., Hong O., Villar J., McCulloch C. A., Downey G. P. (2006) Tyrosine phosphatase SHP-2 regulates IL-1β signaling in fibroblasts through focal adhesions. J. Cell. Physiol. 207, 132–143 [DOI] [PubMed] [Google Scholar]
- 28. Wang Q., Herrera Abreu M. T., Siminovitch K., Downey G. P., McCulloch C. A. (2006) Phosphorylation of SHP-2 regulates interactions between the endoplasmic reticulum and focal adhesions to restrict interleukin-1-induced Ca2+ signaling. J. Biol. Chem. 281, 31093–31105 [DOI] [PubMed] [Google Scholar]
- 29. Wang Q., Rajshankar D., Branch D. R., Siminovitch K. A., Herrera Abreu M. T., Downey G. P., McCulloch C. A. (2009) Protein-tyrosine phosphatase-alpha and Src functionally link focal adhesions to the endoplasmic reticulum to mediate interleukin-1-induced Ca2+ signaling. J. Biol. Chem. 284, 20763–20772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas P., Forse R. A., Bajenova O. Carcinoembryonic antigen (CEA) and its receptor hnRNP M are mediators of metastasis and the inflammatory response in the liver. Clin. Exp. Metastasis 28, 923–932 [DOI] [PubMed] [Google Scholar]
- 31. Zhong X., Rescorla F. J. Cell surface adhesion molecules and adhesion-initiated signaling: understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell. Signal. 24, 393–401 [DOI] [PubMed] [Google Scholar]
- 32. Wang Q., Downey G. P., Herrera-Abreu M. T., Kapus A., McCulloch C. A. (2005) SHP-2 modulates interleukin-1-induced Ca2+ flux and ERK activation via phosphorylation of phospholipase Cgamma1. J. Biol. Chem. 280, 8397–8406 [DOI] [PubMed] [Google Scholar]
- 33. Chong S. A., Lee W., Arora P. D., Laschinger C., Young E. W., Simmons C. A., Manolson M., Sodek J., McCulloch C. A. (2007) Methylglyoxal inhibits the binding step of collagen phagocytosis. J. Biol. Chem. 282, 8510–8520 [DOI] [PubMed] [Google Scholar]
- 34. Phares D. J., Smedley G. T., Flagan R. C. (2000) The wall shear stress produced by the normal impingement of a jet on a flat surface. J. Fluid Mech. 418, 351–375 [Google Scholar]
- 35. Humphries M. J. (2009) Cell adhesion assays. Methods Mol. Biol. 522, 203–210 [DOI] [PubMed] [Google Scholar]
- 36. Mould A. P., Garratt A. N., Askari J. A., Akiyama S. K., Humphries M. J. (1995) Identification of a novel anti-integrin monoclonal antibody that recognises a ligand-induced binding site epitope on the β1 subunit. FEBS Lett. 363, 118–122 [DOI] [PubMed] [Google Scholar]
- 37. Lenter M., Uhlig H., Hamann A., Jeno P., Imhof B., Vestweber D. (1993) A monoclonal antibody against an activation epitope on mouse integrin chain β1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc. Natl. Acad. Sci. U. S. A. 90, 9051–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhide V. M., Laschinger C. A., Arora P. D., Lee W., Hakkinen L., Larjava H., Sodek J., McCulloch C. A. (2005) Collagen phagocytosis by fibroblasts is regulated by decorin. J. Biol. Chem. 280, 23103–23113 [DOI] [PubMed] [Google Scholar]
- 39. Hakkinen L., Heino J., Koivisto L., Larjava H. (1994) Altered interaction of human granulation-tissue fibroblasts with fibronectin is regulated by alpha 5 beta 1 integrin. Biochim. Biophys. Acta 1224, 33–42 [DOI] [PubMed] [Google Scholar]
- 40. Bolcato-Bellemin A. L., Elkaim R., Abehsera A., Fausser J. L., Haikel Y., Tenenbaum H. (2000) Expression of mRNAs encoding for alpha and beta integrin subunits, MMPs, and TIMPs in stretched human periodontal ligament and gingival fibroblasts. J. Dent. Res. 79, 1712–1716 [DOI] [PubMed] [Google Scholar]
- 41. Van der Pauw M. T., Everts V., Beertsen W. (2002) Expression of integrins by human periodontal ligament and gingival fibroblasts and their involvement in fibroblast adhesion to enamel matrix-derived proteins. J. Periodontal Res. 37, 317–323 [DOI] [PubMed] [Google Scholar]
- 42. Lallier T. E., Spencer A., Fowler M. M. (2005) Transcript profiling of periodontal fibroblasts and osteoblasts. J. Periodontol. 76, 1044–1055 [DOI] [PubMed] [Google Scholar]
- 43. Heng E. C., Huang Y., Black S. A., Jr., Trackman P. C. (2006) CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J. Cell. Biochem. 98, 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim H., Nakamura F., Lee W., Hong C., Perez-Sala D., McCulloch C. A. (2010) Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp. Cell. Res. 316, 1829–1844 [DOI] [PubMed] [Google Scholar]
- 45. Kim H., Nakamura F., Lee W., Shifrin Y., Arora P., McCulloch C. A. (2010) Filamin A is required for vimentin-mediated cell adhesion and spreading. Am. J. Physiol. Cell Physiol. 298, C221–C236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Glogauer M., Ferrier J. (1998) A new method for application of force to cells via ferric oxide beads. Pflügers Arch. 435, 320–327 [DOI] [PubMed] [Google Scholar]
- 47. Calderwood D. A. (2004) Talin controls integrin activation. Biochem. Soc. Trans. 32, 434–437 [DOI] [PubMed] [Google Scholar]
- 48. Larjava H., Plow E. F., Wu C. (2008) Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ussar S., Wang H. V., Linder S., Fassler R., Moser M. (2006) The Kindlins: subcellular localization and expression during murine development. Exp. Cell Res. 312, 3142–3151 [DOI] [PubMed] [Google Scholar]
- 50. Zhao J., Guan J. L. (2009) Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 28, 35–49 [DOI] [PubMed] [Google Scholar]
- 51. Boyle D. L., Han Z., Rutter J. L., Brinckerhoff C. E., Firestein G. S. (1997) Posttranscriptional regulation of collagenase-1 gene expression in synoviocytes by adenosine receptor stimulation. Arthritis Rheum. 40, 1772–1779 [DOI] [PubMed] [Google Scholar]
- 52. Van den Berg W. B. (1999) The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z. Rheumatol. 58, 136–141 [DOI] [PubMed] [Google Scholar]
- 53. Brauchle M., Gluck D., Di Padova F., Han J., Gram H. (2000) Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp. Cell Res. 258, 135–144 [DOI] [PubMed] [Google Scholar]
- 54. Forsyth K. D., Levinsky R. J. (1990) Fibronectin degradation; an in-vitro model of neutrophil mediated endothelial cell damage. J. Pathol. 161, 313–319 [DOI] [PubMed] [Google Scholar]
- 55. Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003) Talin binding to integrin beta tails: a final common step in integrin activation. Science 302, 103–106 [DOI] [PubMed] [Google Scholar]
- 56. Kim C., Ye F., Ginsberg M. H. (2012) Regulation of integrin activation. Annu. Rev. Cell. Dev. Biol. 3, 67–70 [DOI] [PubMed] [Google Scholar]
- 57. Zhang X., Jiang G., Cai Y., Monkley S. J., Critchley D. R., Sheetz M. P. (2008) Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 10, 1062–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meves A., Stremmel C., Gottschalk K., Fassler R. (2009) The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 19, 504–513 [DOI] [PubMed] [Google Scholar]
- 59. Montanez E., Ussar S., Schifferer M., Bosl M., Zent R., Moser M., Fassler R. (2008) Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ithychanda S. S., Das M., Ma Y. Q., Ding K., Wang X., Gupta S., Wu C., Plow E. F., Qin J. (2009) Migfilin, a molecular switch in regulation of integrin activation. J. Biol. Chem. 284, 4713–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tu Y., Wu S., Shi X., Chen K., Wu C. (2003) Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47 [DOI] [PubMed] [Google Scholar]
- 62. Wang Q., Downey G. P., McCulloch C. A. (2011) Focal adhesions and Ras are functionally and spatially integrated to mediate IL-1β activation of ERK. FASEB J. 25, 3448–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]