Abstract
CD36 has been linked to the etiology of insulin resistance and inflammation. We explored its function in regulating adipose tissue lipolysis, which influences fat accumulation by liver and muscle and overall metabolism. Knockdown of CD36 in differentiated 3T3-L1 adipocytes decreased lipolysis in response to 10 μM of the β-adrenergic agonist isoproterenol (by 42%), 10 μM of the adenyl cyclase activator forskolin (by 32%), and 500 μM of the phosphodiesterase (PDE) inhibitor isobutylmethylxanthine (by 33%). All three treatments in the knockdown adipocytes were associated with significant decreases of cAMP levels and of the hormone-sensitive lipase (HSL) and perilipin phosphorylation. An important role for PDE was supported by the lack of inhibition of the lipolysis induced by the poorly hydrolyzable dibutyryl cAMP analog. An additional contributory mechanism was diminished activation of the Src-ERK1/2 pathway. Regulation of lipolysis and lipolytic signaling by CD36 was reproduced with adipose tissue from CD36−/− mice. The importance of surface CD36 in this regulation was suggested by the finding that the plasma membrane-impermeable CD36 inhibitor sulfo-N-succinimidyl oleate (20 μM) decreased lipolysis. Interestingly, isoproterenol induced CD36 internalization, and this process was blocked by HSL inhibition, suggesting feedback regulation of adipocyte lipolysis via CD36 trafficking.—Zhou, D., Samovski, D., Okunade, A. L., Stahl, P. D., Abumrad, N. A., Su, X.. CD36 level and trafficking are determinants of lipolysis in adipocytes.
Keywords: triacylglycerol, lipid metabolism, protein kinase A, adipose triacylglycerol lipase, hormone-sensitive lipase
Adipose tissue stores excess nutrients in the form of triacylglycerol (TAG), which is hydrolyzed during fasting or exercise to release free fatty acids (FFAs) and glycerol (1). The released FFAs in the bloodstream are taken up and utilized by peripheral tissues for energy production. TAG is sequentially hydrolyzed by adipose TAG lipase (ATGL), hormone sensitive lipase (HSL), and monoacylglycerol lipase (2, 3). Perilipin proteins surrounding the lipid droplets protect TAG against breakdown by limiting access of HSL to the lipids and by binding to the ATGL activator CGI-58 (4, 5). Complex signal transduction cascades are involved in lipolytic regulation in adipocytes, and the cAMP-dependent protein kinase A (PKA) pathway has been the most studied (6). Catecholamines stimulate the activation of adenylyl cyclase and production of cAMP through binding to the G-protein-coupled β-adrenergic receptors (βARs). The increase in intracellular cAMP levels leads to the activation of PKA and subsequent phosphorylation of HSL and perilipin, resulting in TAG hydrolysis (7). In addition to the cAMP/PKA cascade, pathways involving protein kinase C (PKC), Src kinases and extracellular signal-regulated kinase (ERKs), TNF-α, and cGMP-dependent protein kinase contribute to activation of adipocyte lipolysis (reviewed in ref. 6).
CD36 is a 472-aa transmembrane protein with extensive glycosylation (88 kDa) that has a predicted hairpin membrane topology with 2 transmembrane regions and a large extracellular loop (8). It is a multifunctional scavenger receptor involved in immunity, behavior, angiogenesis, and metabolism through binding to a variety of ligands [e.g., long-chain fatty acids (FAs), thrombospondin 1, oxidized low-density lipoprotein (OxLDL); ref. 9]. CD36 is highly expressed in metabolic tissues and regulates lipid uptake and metabolism, in particular, that of FAs (10). Adipocytes from the CD36-null mouse (11) or from the spontaneously hypertensive rat with mutations in the CD36 gene (12) exhibit a significant decrease in FA transport and incorporation into TAG. In humans, CD36 protein content was independently related to palmitate storage rates in abdominal adipose tissue (13). However, adipose tissue CD36 protein content was decreased in subjects with high intrahepatic TAG content and insulin resistance (14), suggesting that some metabolic functions of CD36 in adipose tissue are independent of its activity in regulating FA uptake. Since adipose CD36 expression is highly regulated in diabetic mouse models (15) and humans with diabetes (16), understanding its roles in TAG storage and turnover may help discover new pathways leading to insulin resistance and diabetes in vivo.
CD36 has been shown to localize at the plasma membrane (PM) and in intracellular compartments (17). Activity in FA uptake correlated with PM localization during insulin stimulation or contraction and could be completely blunted by a specific membrane-impermeable inhibitor, sulfo-N-succinimidyl oleate (SSO; ref. 18). Several intracellular signaling pathways downstream of CD36 have been demonstrated to induce a macrophage proinflamatory response, promote platelet granule secretion, and release neurotransmitters (9). Although the precise CD36-mediated signaling pathways vary from one cell type to another, signal transduction is usually initiated via activation of Src family kinases and ERK (19, 20).
In adipocytes, there is little information related to the role of CD36 in the regulation of FA metabolism aside from the documented effect of CD36 deletion to reduce FA uptake and incorporation into TAG. A previous report using myocytes suggested a potential effect of CD36 expression on triglyceride mobilization (21). Because of its function in a broad array of signaling pathways relevant to energy metabolism, including the Src/ERK pathway, which regulates lipolysis (22), we explored whether CD36 could influence adrenergic signaling and lipolysis in adipocytes. We further examined the regulation of CD36 internalization on adrenergic stimulation and identified a novel negative feedback mechanism regulating adrenergic stimulation of lipolysis in adipocytes.
MATERIALS AND METHODS
Reagents
Fetal calf serum (FCS), calf serum (CS), DMEM, Lipofectamine RNAiMAX, and siRNA against mouse CD36 (5′-AAACCCAGATGACGTGGCAAA-3′) were purchased from Invitrogen (Grand Island, NY, USA). Antibodies for phospho-ERK1/2, total ERK1/2, phospho-HSL (Ser660), total HSL, adipocyte differentiation-related protein (ADRP), insulin receptor β subunit, and tubulin were ordered from Cell Signaling Technology (Beverly, MA, USA). Phospho-perilipin (Ser522) and total perilipin antibodies were obtained from Vala Sciences (San Diego, CA, USA). CD36 antibody was from Abcam (Cambridge, MA, USA). CAY10499 was from Cayman Chemical (Ann Arbor, MI, USA). (SSO was synthesized and purified as described previously (23). Other reagents were from Sigma (St. Louis, MO, USA).
Cell culture of 3T3-L1 cells and differentiation into adipocytes
3T3-L1 cells (from American Type Culture Collection, Manassas, VA, USA) were cultured to confluence in DMEM containing 20% CS, as described previously (24). At 2 d after confluence, cells were incubated with differentiation medium [500 μM isobutylmethylxanthine (IBMX), 0.25 μM dexamethasone, and 4 μg/ml insulin in DMEM containing 10% FBS]. After 2 d, IBMX and dexamethasone were removed, and insulin (4 μg/ml) was maintained for another 2 d. Thereafter, cells were grown in DMEM containing 10% FBS with media replaced every 2 d. The DMEM used for culture and differentiation of 3T3-L1 cells has a high concentration of glucose (4.5 mg/ml).
siRNA transfection of adipocytes
Transfection of siRNA (10 nM final concentration) in adipocytes 8 d postdifferentiation was performed using Lipofectamine RNAiMAX, as we have recently described (25). A scrambled siRNA (Invitrogen) was used as a negative control. Most of the experiments were performed 3 d after transfection.
Protein extraction, SDS-PAGE, and Western blot analysis
To obtain cell lysates, monolayers were washed with PBS and lysed in ice-cold lysis buffer (50 mM Tris·HCl, pH 7.6; 10% glycerol; 1% Triton X-100; 0.1% SDS; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; and 1% protease and phosphatase inhibitor cocktail solution; Fisher, Pittsburgh, PA, USA). The lysates were clarified by centrifugation, and proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes (26). The membranes were blocked in Odyssey blocking buffer (Li-COR, Lincoln, NE, USA) and incubated with primary antibodies overnight at 4°C and then with infrared fluorophore-coupled secondary antibodies. The proteins were visualized using direct infrared fluorescence detection (Odyssey Imaging System; Li-COR).
Measurement of glycerol and FFA release
Stimulation of adipocytes with isoproterenol (ISO; 10 μM), forskolin (10 μM), and IBMX (500 μM) were performed as described previously (25). Prior to lipolytic stimulation for assaying glycerol release, adipocytes were serum starved for 4 h. Lipolysis was assessed from the release of glycerol in the culture medium using free glycerol reagent (Sigma). FFAs in the culture medium were assayed according to the manufacturer's instructions using NEFA measuring kits (Wako, Richmond, VA, USA).
cAMP measurements
cAMP concentrations in adipocytes were measured according to the manufacturer's instructions using an enzyme immunoassay kit (Cayman).
SSO treatment
Adipocytes were serum starved for 4 h, washed with cold PBS buffer 3 times, and incubated with freshly prepared buffer containing 20 μM SSO (predissolved in DMSO) or DMSO for 20 min. At the end of the incubation, the cells were washed 3 times with PBS buffer and then with DMEM containing 0.2% FA-free BSA to remove any unbound SSO.
Glycerol release from ex vivo adipose tissue cultures
Epididymal fat pads from wild-type (WT) and CD36-null mice were cut into small pieces (<4 mm), washed, and immediately transferred to 12-well plates containing KRH buffer (129 mM NaCl, 5 mM NaHCO3, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgCl2, 2.8 mM glucose, and 10 mM HEPES, pH 7.4), which was equilibrated in the cell culture incubator for 1 h. Released glycerol in the culture medium was measured using free glycerol reagent (Sigma).
Quantitative gas chromatography (GC) analysis of TAG
Lipids were extracted by the method of Bligh-Dyer in the presence of an internal standard (T21:0 TAG, 10 nmol/mg protein) and separated on silica gel 60-Å plates that were developed with a nonpolar acidic mobile phase (70:30:1, v/v/v, hexane/ethyl ether/acetic acid). Spots corresponding to TAG were visualized with 0.01% rhodamine 6G and identified with TAG standard. The bands were scraped and extracted, and FA methyl esters of the TAG fractions were prepared as described previously (25). Quantitative GC analysis was conducted (Hewlett-Packard 5890 GC; Hewlett-Packard, Palo Alto, CA, USA) with a 30-m × 0.32 mm Omegawax 250 column (Sigma) and a flame ionization detector. Instrument response was calibrated using the Supelco 37 Component FAME Mix (Sigma) as described previously (25).
Biotinylation of cell surface proteins
Cell surface proteins were labeled with biotin using EZ-Link Sulfo-NHS-SS-Biotin (Fisher), according to the manufacturer's instructions. Cells were extracted using lysis buffer, and labeled membrane proteins were purified using streptavidin beads (Fisher).
Preparation and analysis of adipose PM fraction
Adipose PM fraction was prepared as described previously (27). CD36 levels were analyzed and normalized to insulin receptor levels in the same fraction.
Immunofluorescent microscopy
Cells grown on coverslips were fixed with 3% paraformaldehyde, quenched with 50 mM ammonium chloride, permeabilized, and then blocked with goat serum. Following incubation with CD36 antibody and then Alexa Fluor 488-conjugated secondary antibodies, the coverslips were mounted as described previously (24). Confocal microscopy was performed using a ×63 oil objective on a Zeiss LSM 510 laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). Images were processed using ImageJ software (U.S. National Institutes of Healtth, Bethesda, MD, USA).
CD36 translocation assay
CD36 surface translocation was measured as described previously (28). Briefly, cells seeded in 24-well plates were serum starved for 6 h and treated with different stimulants. Cells were then fixed, blocked, and incubated with monoclonal rat anti-mouse CD36 antibody (Abd Serotec, Raleigh, NC, USA). After incubation with secondary HRP-conjugated anti-rat antibody, cells were then extensively washed and incubated with 3,3′,5,5′-tetramethylbenzidine (Sigma). The reaction was stopped by addition of 1 N NaOH, and the absorbance at 450 nm was recorded. To normalize for well-to-well variations, cells were incubated for 30 min with wheat germ agglutinin Alexa Fluor 680 conjugate (Invitrogen) and read at 700 nm on Odyssey Imaging system (Li-COR), as described previously (28).
Statistical analysis
All experiments presented were repeated ≥3 times. The data are shown as means ± sd. Student's t test was performed to calculate statistical significance (P<0.05).
RESULTS
CD36 regulation of β-adrenergic stimulation of lipolysis in 3T3-L1 adipocytes and in mouse adipose tissue
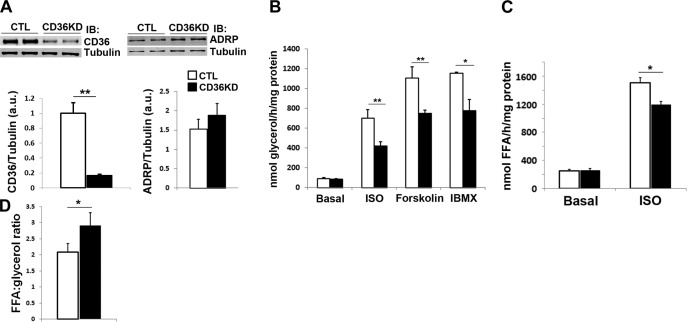
To determine whether CD36 plays a role in adrenergic stimulation of lipolysis in adipocytes, differentiated 3T3-L1 adipocytes were transfected with siRNA against CD36 (25), resulting in >80% decrease in CD36 protein levels (Fig. 1A). The ADRP level was not changed by CD36 knockdown (KD; Fig. 1A), arguing against its effect on adipocyte dedifferentiation. As shown in Fig. 1B, under basal conditions, there was no difference between adipocytes treated with negative control siRNA and siRNA targeting CD36. However, with ISO stimulation (10 μM, 1 h), CD36-KD adipocytes had significantly (45%) lower glycerol release than control cells (P<0.01; Fig. 1B). Similar effects were observed when the cells were stimulated with ISO for 0.5 or 2 h (data not shown). Similar to ISO stimulation, glycerol release stimulated by an adenylyl cyclase activator forskolin or the phosphodiesterase (PDE) inhibitor IBMX was also inhibited in CD36-KD cells (Fig. 1B).
Figure 1.
Regulation of adrenergic signaling-stimulated lipolysis by CD36 levels in 3T3-L1 adipocytes. 3T3-L1 adipocytes were treated with negative control (CTL) siRNA or with siRNA recognizing CD36 (CD36KD). A) Whole cell lysates were prepared and analyzed by immunoblotting (IB) using antibodies recognizing CD36 and tubulin. Signals were quantified by densitometry, and CD36/tubulin ratios are presented. B) Cells were serum starved for 4 h and stimulated with 10 μM ISO, 10 μM forskolin, or 500 μM IBMX for 1 h. Glycerol release was normalized to total protein. C) FA release with or without 10 μM ISO stimulation for 1 h, normalized to total protein. D) Ratio of FFAs to glycerol in the medium. Data represent means ± sd of 3 independent experiments. *P < 0.05, **P < 0.01.
In agreement with results from cultured adipocytes, ex vivo adipose tissue cultures from CD36-null mice had 50% lower rate of glycerol release in the presence of ISO for 60 or 120 min (P<0.01; Fig. 2A), whereas basal glycerol release was unaltered. Consistent with this, plasma glycerol concentration after overnight food withdrawal was lower in null as compared to WT mice (P<0.05; Fig. 2B), suggesting impaired white adipose tissue (WAT) lipolysis in vivo.
Figure 2.
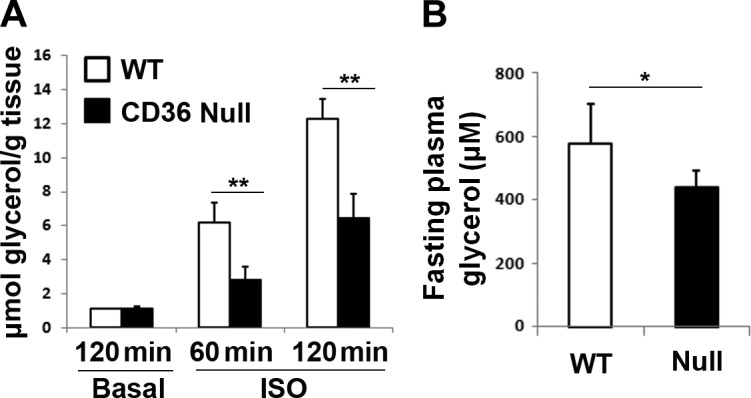
Adipose tissue of CD36-KD mice has decreased lipolytic activity. A) Epididymal fat pads cut into small pieces were washed and incubated in KRH buffer for 20 min to reduce residual hormones. The tissue was then incubated in fresh buffer for 4 h and stimulated with or without 10 μM ISO for 60 or 120 min, and glycerol release was assayed and normalized to tissue weight. B) Plasma glycerol levels of WT and CD36-null mice after 24 h without food at room temperature. *P < 0.05, **P < 0.01.
Regulation of FFA reuptake by CD36 during lipolysis
CD36 KD decreases lipolysis and would also be expected to inhibit FFA reuptake. As shown in Fig. 1C, the FFA released into the medium was significantly lower in the CD36-KD adipocytes stimulated with ISO for 1 h, as compared to control cells (P<0.05). However, the inhibitory effect on FFA release (∼20% decrease) is less profound than on glycerol release (42% decrease; Fig. 1B). In theory, in the absence of FA reuptake, 1 molecule of TAG would release 1 molecule of glycerol and 3 molecules of FFA. The ratio of FFA to glycerol was close to 2 in control adipocytes, suggesting the presence of FFA reuptake. However, this ratio was increased and close to 3 in the CD36-KD cells (P<0.05; Fig. 1D). In agreement with a role of adipocyte CD36 in FFA uptake (11), incorporation of exogenous [3H] oleic acid into TAG was decreased in CD36-KD adipocytes under basal or ISO-stimulated conditions (data not shown).
TAG accumulation in CD36-KD adipocytes
We determined the effect of CD36 KD on TAG accumulation measured by quantitative GC. In contrast to the smaller fat pad in CD36-null mice (29), the total TAG content in cultured adipocytes was significantly increased by CD36 KD (P<0.01; Fig. 3A) and an increase in total content of palmitoleate, an FA mainly from lipogenesis, was observed (P<0.01; Fig. 3B). The TAG FA compositions of adipocytes (WT and CD36 KD) and adipose tissues (WT and CD36 null) are presented in Supplemental Table S1. These results demonstrate that the inhibitory effect of CD36 KD on lipolysis is not due to lower availability of TAG.
Figure 3.
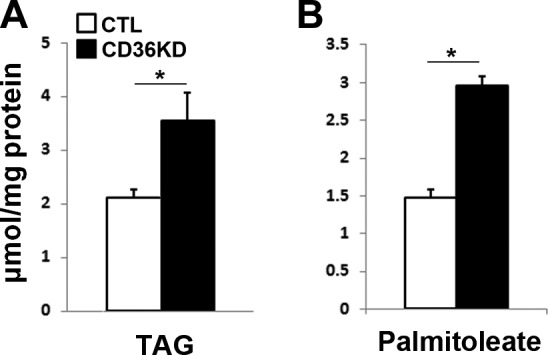
CD36 KD increases TAG accumulation in 3T3-L1 adipocytes. Total lipids were extracted, and TAG was separated by TLC. Fatty methyl esters were prepared and analyzed by quantitative GC. Total TAG (A) and palmitoleate content in TAG (B) were measured and normalized to total proteins. *P < 0.01.
Regulation of cAMP/PKA signaling by CD36 in adipocytes and in mouse adipose tissue
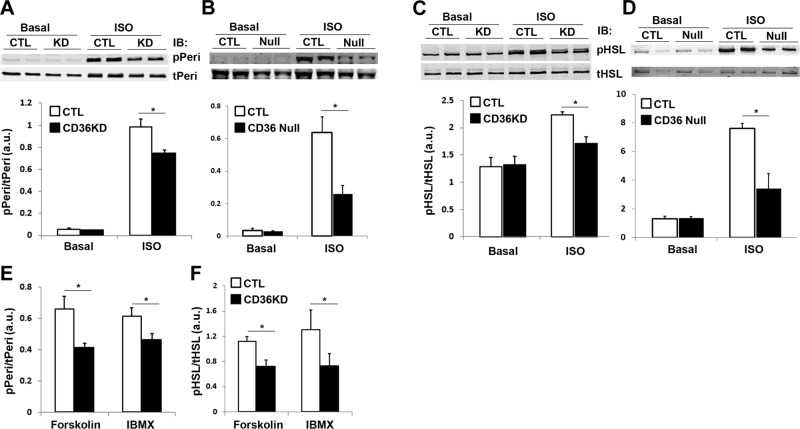
ISO, forskolin, and IBMX all lead to increased cellular cAMP levels. Our results demonstrated a comparable increase in cAMP levels following the different stimulations (Fig. 4A), and these increases were significantly blunted by CD36 KD (Fig. 4A). We asked whether CD36 regulates lipolysis stimulated by ISO, forskolin, and IBMX via its effect on cAMP turnover. Differential regulation of lipolysis by the hydrolysable cAMP analog, 8-bromo-cAMP, as compared to the poorly hydrolysable dibutyryl-cAMP (30), can inform about potential involvement of PDE regulation (31). CD36 KD impaired lipolysis stimulated by 8-bromo-cAMP, but not by dibutyryl-cAMP (Fig. 4B), supporting a potential role of CD36 in PDE regulation. We next examined PKA activation by measuring the phosphorylation of its targets perilipin and HSL. As anticipated, a dramatic increase of perilipin and HSL phosphorylation was observed after ISO treatment in control adipocytes (Fig. 5A, C). However, this was significantly attenuated in CD36-KD cells (Fig. 5A, C). Similarly, ISO-stimulated phosphorylation, but not basal perilipin and HSL phosphorylation, was impaired in ex vivo adipose tissue explants from CD36-null mice (Fig. 5B, D). Induction of perilipin and HSL phosphorylation by forskolin and IBMX was also attenuated in CD36-KD cells (Fig. 5E, F), consistent with the earlier observation of decreased cAMP levels in CD36-KD cells after stimulation with forskolin and IBMX (Fig. 4A).
Figure 4.
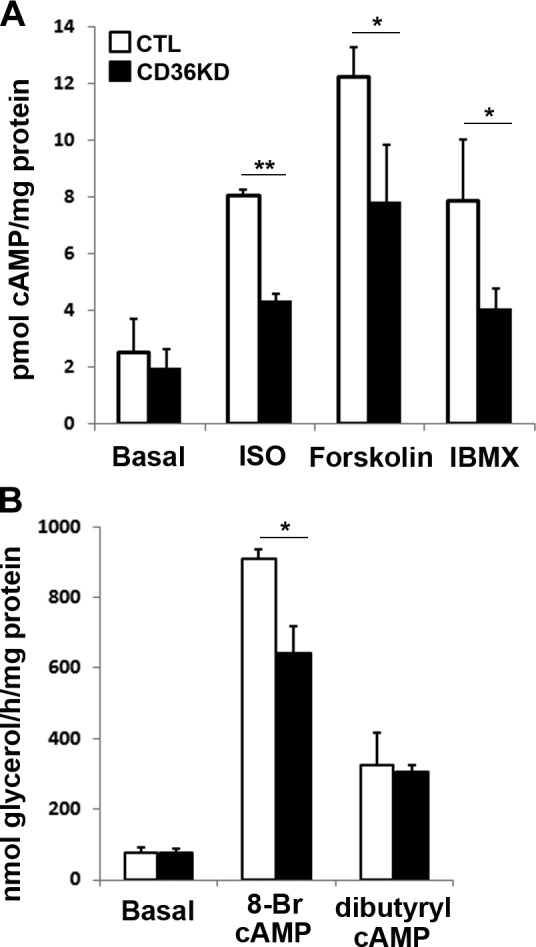
CD36 KD regulates cAMP turnover in 3T3-L1 adipocytes. A) Adipocytes were treated with negative control (CTL) siRNA or with siRNA recognizing CD36 (CD36KD) for 3 d, serum starved, and stimulated with 10 μM ISO, 10 μM forskolin, or 500 μM IBMX for 30 min. cAMP concentrations were measured and normalized to total protein. B) Cells were serum starved and stimulated with 1 mM 8-bromo-cAMP or dibutyryl-cAMP for 1 h. Glycerol release was assayed and normalized to total protein. Data represent means ± sd of 3 independent experiments. *P < 0.05, **P < 0.01.
Figure 5.
CD36 regulates perilipin and HSL phosphorylation stimulated by adrenergic signaling in 3T3-L1 adipocytes and adipose tissues. A, C, E, F) 3T3-L1 adipocytes were treated with negative control (CTL) siRNA or with siRNA recognizing CD36 (CD36KD). The cells were serum starved and stimulated with 10 μM ISO (A, C), 10 μM forskolin, or 500 μM IBMX (E, F) for 1 h. Whole-cell lysates were prepared and analyzed by immunoblotting (IB) using indicated antibodies. B, D) Epididymal fat pads cut into small pieces were washed and incubated in KRH buffer for 20 min to reduce residual hormones. The tissue was then incubated in fresh buffer for 4 h and stimulated with or without 10 μM ISO for 1 h, and lysates were prepared analyzed by IB using indicated antibodies. A, B, E) Phospho-perilipin A (pPeri) and total perilipin A (tPeri). C, D, F) Phospho-HSL (pHSL) and total HSL (tHSL). Signals were quantified by densitometry; pPeri/tPeri and pHSL/tHSL ratios are presented. Data are means ± sd from 3 independent experiments. *P < 0.05.
Regulation of Src/ERK pathway by CD36 KD in adipocytes
As shown in Fig. 1B, inhibition of lipolysis by CD36 KD in response to ISO (42%) is consistently more profound than that induced by forskolin (32%) or IBMX (33%), indicating that CD36 KD might regulate adrenergic stimulation of lipolysis via signaling pathways both dependent and independent of cAMP/PKA. In addition to PKA, βARs also activate ERK1/2 of MAPK by direct recruitment and activation of Src kinases. Pharmaceutical inhibition of Src or ERK1/2 impairs adrenergic stimulation of lipolysis (22). We examined whether CD36 regulates ERK activation in adipocytes. As shown in Fig. 6, CD36 KD attenuates both basal and ISO-stimulated ERK phosphorylation.
Figure 6.
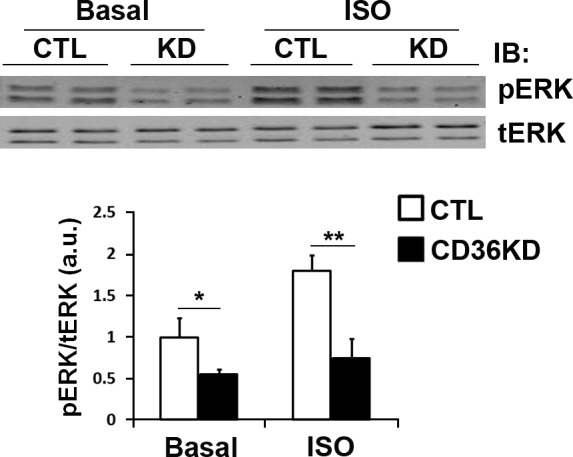
CD36 KD attenuates isoproterenol-stimulated ERK activation in 3T3-L1 adipocytes. Cells were treated with negative control (CTL) siRNA or siRNA recognizing CD36 (CD36KD), serum starved for 4 h, and stimulated with 10 μM ISO for 1 h. Whole-cell lysates were prepared and analyzed by Western blot for phospho-ERK1/2 (pERK) and total ERK1/2 (tERK). Signals were quantified by densitometry; pERK/tERK ratios are presented. Data are means ± sd from 3 independent experiments. *P < 0.05, **P < 0.01.
Regulation of lipolysis by PM localization of CD36
It has been demonstrated that CD36 is localized in intracellular compartments and at the PM (17). We investigated the relationship between PM localization of CD36 and its lipolytic effects using SSO, a membrane-impermeable CD36 inhibitor (23). Consistent with the results in CD36-KD cells, inhibition of membrane CD36 by SSO impaired stimulated but not basal lipolysis (Fig. 7A, B). SSO also inhibits ISO-stimulated lipolysis in adipose tissue cultures from CD36-null mice (data not shown). We recently demonstrated a role of ADP-ribosylation factor 6 (Arf6) in adrenergic stimulation of lipolysis (25). Because Arf6 regulates trafficking of a variety of membrane proteins, we examined whether Arf6 regulates PM localization of CD36. Arf6 KD significantly decreased PM localization of CD36 in adipocytes (Fig. 8A). Although Arf6 level is reduced in adipose tissue from CD36-null mice (25), its levels in WT and CD36 KD adipocytes are the same (Fig. 8B).
Figure 7.
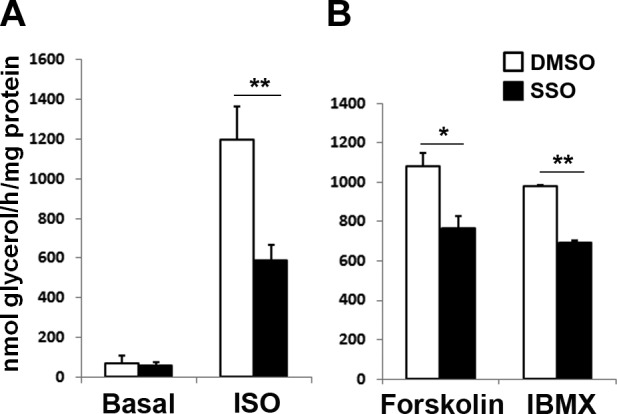
Inhibition of membrane CD36 by SSO attenuates adrenergic stimulation of lipolysis in 3T3-L1 adipocytes. Cells were serum starved for 4 h, washed with PBS 3 times, and incubated with DMSO or 20 μM SSO for 20 min. The SSO was removed, and the cells were washed with PBS 3 times before treatments. A) ISO (10 μM) in DMEM for 1 h. B) Forskolin (10 μM) or IBMX (500 μM) in DMEM for 1 h. Glycerol release was assayed and normalized to total protein. Data are means ± sd from 3 independent experiments. *P < 0.05, **P < 0.01.
Figure 8.
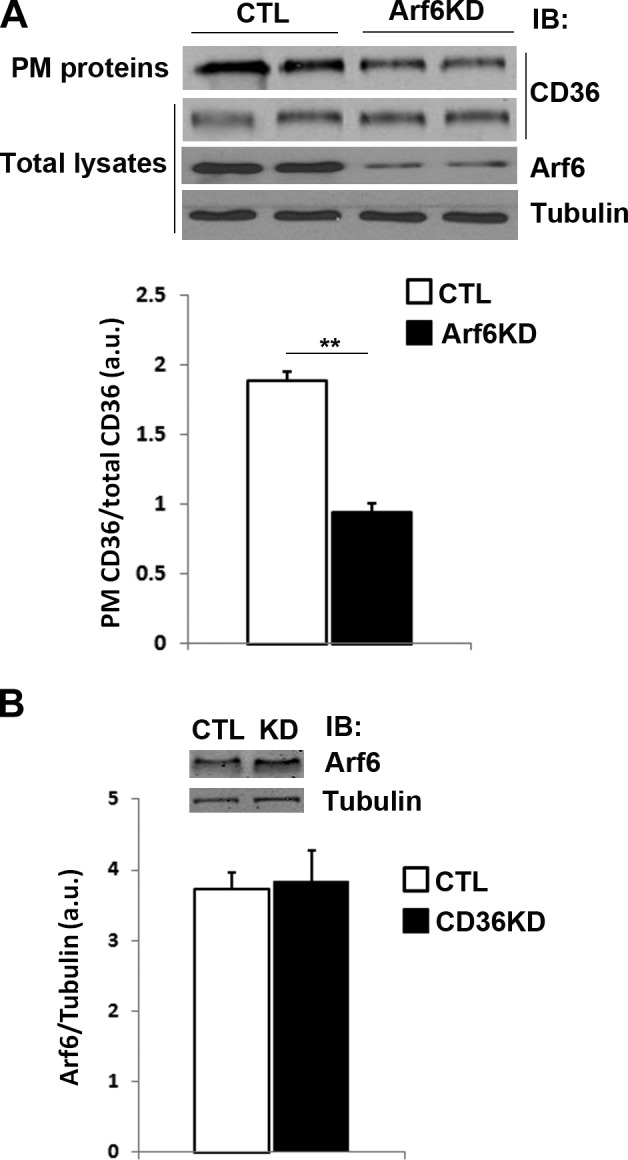
Arf6 knockdown decreases PM levels of CD36 in 3T3-L1 adipocytes. A) Cells were treated with negative control (CTL) siRNA or with siRNA recognizing Arf6 (Arf6KD). PM proteins were purified by biotinoylation as described in Materials and Methods, and labeled proteins and total cell lysates were analyzed by IB. B) Arf6 levels in 3T3-L1 adipocytes treated with negative control siRNA or siRNA recognizing CD36 (CD36KD). **P < 0.01.
CD36 trafficking during lipolysis
Because PM CD36 is important for adipocyte lipolysis and FA can promote CD36 internalization (10), we examined whether lipolysis can feed back to regulate PM CD36, thus preventing excessive TAG depletion. As shown in Fig. 9A, ISO stimulation caused the internalization of CD36 by immunofluorescence microscopy. Interestingly, this was reversed by including the HSL inhibitor CAY10449. The inhibitory effect of CAY10449 on lipolysis was confirmed by measuring glycerol release (Fig. 9B). Similar to the immunofluorescence microscopy results, the more quantitative CD36 translocation assay demonstrated that ISO decreased CD36 PM localization, and this effect depended on HSL activity and FFA release (Fig. 9C). The effects of ISO and CAY10449 were also replicated in adipose tissue (Fig. 9D). Total amounts of CD36 protein did not change in all conditions (data not shown).
Figure 9.
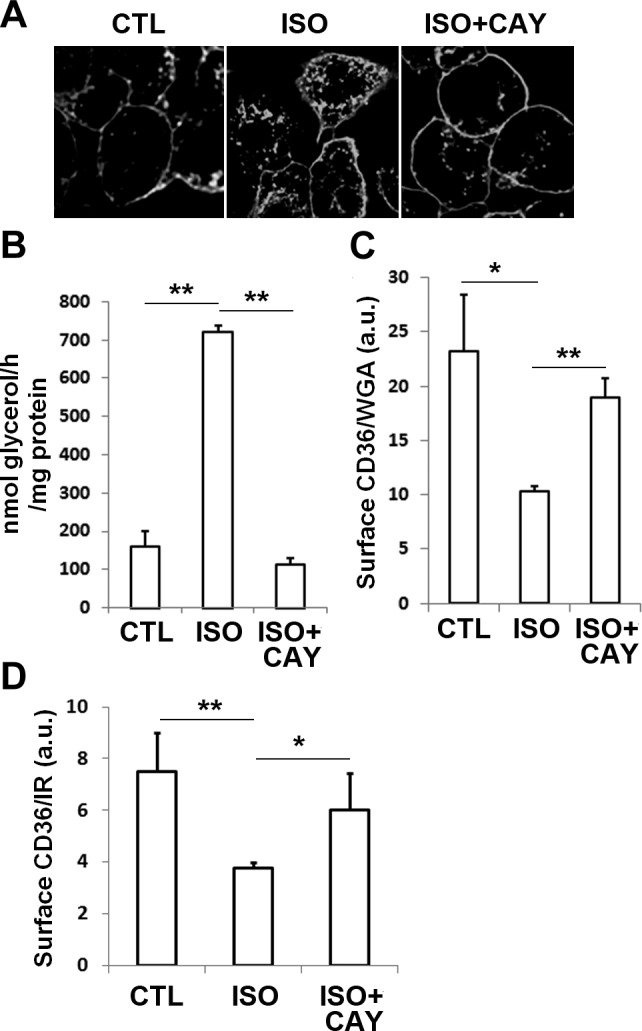
Internalization of CD36 by isoproterenol stimulation in 3T3-L1 adipocytes and adipose tissues. A–C) 3T3-L1 adipocytes were starved and treated with DMSO or 1 μM CAY10499 (CAY) for 5 min prior to ISO stimulation for 1 h. A) Cells fixed and immunostained for CD36. B) Glycerol release normalized to total protein. C) Cells seeded in 24-well plates were fixed and incubated with CD36 antibody and HRP-conjugated secondary antibody. HRP activity was measured and normalized to Alexa 680-WGA. D) Epididymal fat pads cut into small pieces were washed and incubated in KRH buffer for 20 min to reduce residual hormones. The tissue was then incubated in fresh buffer for 4 h and treated with DMSO or 1 μM CAY10499 (CAY) for 5 min prior to ISO stimulation for 1 h. PM fractions were prepared, and CD36 and insulin receptor (IR) levels were quantified by Western blot analysis. Data represent means ± sd of 3 independent experiments. *P < 0.05, **P < 0.01.
DISCUSSION
CD36 is a multifunctional membrane receptor that recognizes a broad array of lipid (e.g., long-chain FAs and OxLDL) and nonlipid (e.g., thrombospondin, collagen) ligands (9). In adipocytes, CD36 is a key facilitator of FA uptake and subsequent TAG storage. Functions of CD36 are regulated by cycling between the PM and intracellular compartments (10). Furthermore, CD36 initiates transduction of a number of signaling pathways that were shown to play a regulatory role in intracellular lipid metabolism (10, 32, 33). In this study, we documented a novel function of CD36 in adrenergic signaling-stimulated lipolysis in adipocytes. CD36 was required for optimal stimulation of lipolysis by ISO in both cultured 3T3-L1 adipocytes and ex vivo in adipose tissue cultures. The effect reflected altered signaling of the cAMP/PKA and Src/ERK pathways. Consistent with these alterations in signal transduction, CD36 localization at the PM was shown to be critical for its effect on stimulated lipolysis. In addition, we showed that as lipolysis proceeded, CD36 was internalized, and this process was blocked by HSL inhibition. This suggested a negative feedback loop by lipolytic products to induce CD36 internalization, which would act to prevent excessive TAG hydrolysis.
CD36-mediated lipolytic signaling
Ligand binding to the βARs leads to activation of adenylyl cyclase, increases cellular cAMP, and enhances PKA activity. Down-regulation of CD36 level impairs lipolysis stimulated by ISO, the adenylyl cyclase activator forskolin, or the PDE inhibitor IBMX. This suggests that CD36 is not required for βAR activation at the PM. However, it interacts with the adrenergic pathway via cAMP turnover and PKA activation, as shown by the reduced cAMP levels in CD36 KD cells (Fig. 4A). Reduced PKA activation was also evidenced from the decreased phosphorylation of the PKA targets perilipin and HSL in CD36-KD cells. CD36 KD attenuated stimulated lipolysis in response to the hydrolyzable 8-bromo-cAMP, but not to the poorly hydrolyzable butyryl analog (ref. 30 and Fig. 4B), suggesting that it interfered with PDE activity. This is not inconsistent with the finding that CD36 KD did impair lipolysis stimulated by the PDE inhibitor IBMX. There are several PDE isoforms, and PDE activity cannot be completely inhibited by IBMX, as previously shown (31), and agents that activate PDE, such as intracellular calcium (34), and the antidiabetic drug berberine (31) also inhibit lipolysis stimulated by IBMX and 8-bromo-cAMP, but not by butyryl-cAMP.
The classical activation of HSL by PKA is complicated by the participation of other protein kinases, such as Src and ERK, that can also activate HSL by serine phosphorylation. Although PKA phosphorylation of perilipin and HSL is a decisive event in adrenergic lipolysis, maximal adrenergic regulation involves activation of the Src/ERK pathway (6). Interaction between βARs and Src has been demonstrated to be subtype and agonist dosage dependent (35). The C-terminal tail of CD36 is the site of interaction with the Src-family kinases that initiate most of CD36 signaling (36) and would be required for optimal activation of Src/ERK by adrenergic stimulation. Our results document CD36 regulation of Src/ERK signaling, demonstrating that cAMP turnover is not the sole mechanism potentially mediating CD36 regulation of lipolysis. Moreover, contribution of additional pathways, including regulation of intracellular calcium concentration and production of PGE2 (32) to the CD36 effects on lipolysis, is also possible and will need to be explored in the future.
CD36 trafficking and regulation of lipolysis
CD36 cycles between the plasma membrane and intracellular compartments, and CD36 recruitment to the plasma membrane was shown to be important for many of its effects (10). In muscle, insulin and contraction acutely translocate CD36 from intracellular storage compartments to the plasma membrane and enhance FA uptake (17, 37) but have different influences on intracellular FA processing. Contraction increases FA uptake and oxidation, but not lipid synthesis, whereas insulin targets FAs to lipid esterification away from oxidation (10). Our data show that CD36 PM localization was important for its effect on ISO-induced lipolysis. As lipolysis proceeds, lipolytic products induce CD36 internalization in a feedback loop that might protect against excessive depletion of intracellular TAG. Internalization of CD36 seems to be a common way for the cell to terminate the metabolic events initiated via the CD36 pathway. CD36 is ubiquitinated on 2 terminal carboxyl lysines, and the ubiquitination is stimulated by FAs (38) and diacylglycerols (33), which are products of TAG lipolysis. In muscle cells (38) and in enterocytes (33), FAs induce CD36 internalization and ultimate degradation. Thus, CD36 trafficking is intimately linked to both initiation and termination of its functions.
Our current study demonstrated that Arf6 KD significantly decreases PM localization of CD36 in adipocytes, suggesting that part of the Arf6 effect on lipolysis may be due to altered CD36 trafficking. Both Arf6 and CD36 KD inhibit ISO-stimulated lipolysis, but only CD36 KD inhibits lipolysis stimulated by forskolin and IBMX. One explanation is that Arf6 regulates lipolysis via βAR trafficking upstream of cyclase and PDE activities, whereas CD36 targets PDE and cAMP turnover.
Intracellular TAG levels in CD36-depleted adipocytes
We observed elevated intracellular TAG levels in cultured CD36-KD adipocytes, whereas adiposity is decreased in CD36-null mice (29). In cultured adipocytes, the availability of extracellular FA supply for TAG synthesis is limited and most FA in TAG is from lipogenesis (39, 40), especially when cells are cultured in medium with high glucose. This is also supported by the high level of the lipogenic marker palmitoleate in TAG in cultured adipocytes (Fig. 3B) and higher expression of lipogenic genes (data not shown). Enhanced lipogenesis together with decreased lipolysis are the likely causes of TAG accumulation in cultured CD36-KD adipocytes. In contrast, FA uptake, but not de novo lipogenesis, would be the major supplier of FA for lipid storage in mouse adipocytes in vivo, where deletion of CD36 associates with a 60% decrease in FA uptake (11). Lipogenesis is likely a minor contributor to adipose tissue mass in vivo, as indicated by low palmitoleate in tissues (Supplemental Table S1). Depletion of GLUT4 selectively in the adipose tissue does not result in decreased adipose mass or adipocyte size (41). In addition, WAT accounts for <10% of whole-body glucose uptake (42). Thus, decreased adipocyte FA uptake may represent a major contributor to the limited adiposity in CD36-null mice. In addition, systemic effects of enhanced leptin signaling may also play a role in vivo (29).
Physiological significance of CD36 regulation of lipolysis
Net lipolytic capacity of adipose tissues is balanced by both stimulatory and inhibitory regulating factors, which coexist in adipocytes under different physiological states (43). Our results suggest a positive role of CD36 on adrenergic stimulation of lipolysis, and this may play a role in overall homeostasis of adipose tissue. Defective PPARγ impairs adrenergically induced lipolysis, and this abnormal lipolytic response is exacerbated by a state of positive energy balance in ob/ob mice (44). The PPARγ-dependent CD36 levels (45) are decreased in WAT from ob/ob mice (15), which might contribute to this lipolytic regulation. Potential clinical significance of our findings is supported by studies that document importance of CD36 in adipocyte metabolism in humans. Our data showed an effect of CD36 on lipolysis and on the reuptake of the mobilized FA. In a recent study of subjects in the postabsorptive state (46), abdominal fat CD36 protein levels positively correlated with and independently predicted upper body subcutaneous free FA uptake similar to plasma palmitate concentrations. This suggested an important role of fat tissue CD36 in reuptake of FA released by lipolysis and in determining the resulting circulating FA concentrations. It was proposed that this pathway could offer a protective effect against excessive FA levels in obesity. Interestingly, there is a fat depot-specific regulation of CD36 level in humans. Subcutaneous adipose tissue CD36 expression is up-regulated in obesity and type 2 diabetes, but in visceral adipose tissues, CD36 levels are similar in lean, overweight, and obese subjects, and are only increased in subjects with type 2 diabetes (16). It appears that visceral adipose tissue CD36 may respond in a less dynamic manner to metabolic disturbances than subcutaneous fat CD36. It will be interesting to determine how alteration in CD36 expression in different fat depots in humans contributes to differential regulation of lipolysis and how it influences FA availability for nonadipose organs and consequently ectopic TAG accumulation.
Collectively, our findings demonstrated a lipolytic role of CD36 signaling and localization in adipocytes and identified CD36-mediated negative feedback regulation of lipolysis. Adipocyte CD36 levels and trafficking may represent novel targets to contain excess free FA release and to protect against insulin resistance in obesity.
Supplementary Material
Acknowledgments
This work was supported by a Scientist Development grant from the American Heart Association (835140N to X.S.) and by U.S. National Institutes of Health grants DK33301 and DK56351. This work was also supported by the core services of the Nutrition Obesity Research Center (P30DK56341) and, in particular, the Adipocyte Biology and Molecular Nutrition Core at Washington University School of Medicine.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- βAR
- β-adrenergic receptor
- ADRP
- adipocyte differentiation-related protein
- Arf6
- ADP-ribosylation factor 6
- ATGL
- adipose TAG lipase
- CS
- calf serum
- ERK
- extracellular signal-regulated kinase
- FA
- fatty acid
- FFA
- free fatty acid
- GC
- gas chromatography
- HSL
- hormone sensitive lipase
- IBMX
- isobutylmethylxanthine
- ISO
- isoproterenol
- KD
- knockdown
- OxLDL
- oxidized low-density lipoprotein
- PDE
- phosphodiesterase
- PKA
- protein kinase A
- PM
- plasma membrane
- SSO
- sulfo-N-succinimidyl oleate
- TAG
- triacylglycerol
- WAT
- white adipose tissue
- WT
- wild type
REFERENCES
- 1. Lafontan M. (2012) Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. Am. J. Physiol. Cell Physiol. 302, C327–C359 [DOI] [PubMed] [Google Scholar]
- 2. Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. (2007) Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lafontan M., Langin D. (2009) Lipolysis and lipid mobilization in human adipose tissue. Progr. Lipid Res. 48, 275–297 [DOI] [PubMed] [Google Scholar]
- 4. Ducharme N. A., Bickel P. E. (2008) Lipid droplets in lipogenesis and lipolysis. Endocrinology 149, 942–949 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y., Sullivan S., Trujillo M., Lee M. J., Schneider S. H., Brolin R. E., Kang Y. H., Werber Y., Greenberg A. S., Fried S. K. (2003) Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes. Res. 11, 930–936 [DOI] [PubMed] [Google Scholar]
- 6. Carmen G. Y., Victor S. M. (2006) Signalling mechanisms regulating lipolysis. Cell. Signal. 18, 401–408 [DOI] [PubMed] [Google Scholar]
- 7. Holm C. (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31, 1120–1124 [DOI] [PubMed] [Google Scholar]
- 8. Gruarin P., Thorne R. F., Dorahy D. J., Burns G. F., Sitia R., Alessio M. (2000) CD36 is a ditopic glycoprotein with the N-terminal domain implicated in intracellular transport. Biochem. Biophys. Res. Commun. 275, 446–454 [DOI] [PubMed] [Google Scholar]
- 9. Silverstein R. L., Febbraio M. (2009) CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su X., Abumrad N. A. (2009) Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 20, 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coburn C. T., Knapp F. F., Jr., Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. (2000) Defective uptake and utilization of long-chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 [DOI] [PubMed] [Google Scholar]
- 12. Hajri T., Ibrahimi A., Coburn C. T., Knapp F. F., Jr., Kurtz T., Pravenec M., Abumrad N. A. (2001) Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J. Biol. Chem. 276, 23661–23666 [DOI] [PubMed] [Google Scholar]
- 13. Ali A. H., Koutsari C., Mundi M., Stegall M. D., Heimbach J. K., Taler S. J., Nygren J., Thorell A., Bogachus L. D., Turcotte L. P., Bernlohr D., Jensen M. D. (2011) Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes 60, 2300–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fabbrini E., Magkos F., Mohammed B. S., Pietka T., Abumrad N. A., Patterson B. W., Okunade A., Klein S. (2009) Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. U. S. A. 106, 15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin J., Gao Z., He Q., Zhou D., Guo Z., Ye J. (2009) Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 296, E333–E342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonen A., Tandon N. N., Glatz J. F., Luiken J. J., Heigenhauser G. J. (2006) The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int. J. Obes. (Lond.) 30, 877–883 [DOI] [PubMed] [Google Scholar]
- 17. Bonen A., Luiken J. J., Arumugam Y., Glatz J. F., Tandon N. N. (2000) Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J. Biol. Chem. 275, 14501–14508 [DOI] [PubMed] [Google Scholar]
- 18. Harmon C. M., Abumrad N. A. (1993) Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J. Membr. Biol. 133, 43–49 [DOI] [PubMed] [Google Scholar]
- 19. Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. (1991) Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc. Natl. Acad. Sci. U. S. A. 88, 7844–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Yassimi A., Hichami A., Besnard P., Khan N. A. (2008) Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283, 12949–12959 [DOI] [PubMed] [Google Scholar]
- 21. Bastie C. C., Hajri T., Drover V. A., Grimaldi P. A., Abumrad N. A. (2004) CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes 53, 2209–2216 [DOI] [PubMed] [Google Scholar]
- 22. Robidoux J., Kumar N., Daniel K. W., Moukdar F., Cyr M., Medvedev A. V., Collins S. (2006) Maximal beta3-adrenergic regulation of lipolysis involves Src and epidermal growth factor receptor-dependent ERK1/2 activation. J. Biol. Chem. 281, 37794–37802 [DOI] [PubMed] [Google Scholar]
- 23. Harmon C. M., Luce P., Beth A. H., Abumrad N. A. (1991) Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J. Membr. Biol. 121, 261–268 [DOI] [PubMed] [Google Scholar]
- 24. Guo L., Zhou D., Pryse K. M., Okunade A. L., Su X. (2010) Fatty acid 2-hydroxylase mediates diffusional mobility of Raft-associated lipids, GLUT4 level, and lipogenesis in 3T3-L1 adipocytes. J. Biol. Chem. 285, 25438–25447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y., Zhou D., Abumrad N. A., Su X. (2010) ADP-ribosylation factor 6 modulates adrenergic stimulated lipolysis in adipocytes. Am. J. Physiol. Cell Physiol. 298, C921–C928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su X., Mancuso D. J., Bickel P. E., Jenkins C. M., Gross R. W. (2004) Small interfering RNA knockdown of calcium-independent phospholipases A2 beta or gamma inhibits the hormone-induced differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 279, 21740–21748 [DOI] [PubMed] [Google Scholar]
- 27. Nishiumi S., Ashida H. (2007) Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 71, 2343–2346 [DOI] [PubMed] [Google Scholar]
- 28. Samovski D., Su X., Xu Y., Abumrad N. A., Stahl P. D. (2012) Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J. Lipid Res. 53, 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hajri T., Hall A. M., Jensen D. R., Pietka T. A., Drover V. A., Tao H., Eckel R., Abumrad N. A. (2007) CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56, 1872–1880 [DOI] [PubMed] [Google Scholar]
- 30. Beebe S. J., Redmon J. B., Blackmore P. F., Corbin J. D. (1985) Discriminative insulin antagonism of stimulatory effects of various cAMP analogs on adipocyte lipolys is and hepatocyte glycogenolysis. J. Biol. Chem. 260, 15781–15788 [PubMed] [Google Scholar]
- 31. Zhou L., Wang X., Yang Y., Wu L., Li F., Zhang R., Yuan G., Wang N., Chen M., Ning G. (2011) Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochim. Biophys. Acta 1812, 527–535 [DOI] [PubMed] [Google Scholar]
- 32. Kuda O., Jenkins C. M., Skinner J. R., Moon S. H., Su X., Gross R. W., Abumrad N. A. (2011) CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J. Biol. Chem. 286, 17785–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tran T. T., Poirier H., Clement L., Nassir F., Pelsers M. M., Petit V., Degrace P., Monnot M. C., Glatz J. F., Abumrad N. A., Besnard P., Niot I. (2011) Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J. Biol. Chem. 286, 25201–25210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xue B., Greenberg A. G., Kraemer F. B., Zemel M. B. (2001) Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes. FASEB J. 15, 2527–2529 [DOI] [PubMed] [Google Scholar]
- 35. McGarrigle D., Huang X. Y. (2007) GPCRs signaling directly through Src-family kinases. Sci. STKE 2007, pe35. [DOI] [PubMed] [Google Scholar]
- 36. Silverstein R. L., Li W., Park Y. M., Rahaman S. O. (2010) Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans. Am. Clin. Climatol. Assoc. 121, 206–220 [PMC free article] [PubMed] [Google Scholar]
- 37. Glatz J. F., Bonen A., Luiken J. J. (2002) Exercise and insulin increase muscle fatty acid uptake by recruiting putative fatty acid transporters to the sarcolemma. Curr. Opin. Clin. Nutr. Metab. Care 5, 365–370 [DOI] [PubMed] [Google Scholar]
- 38. Smith J., Su X., El-Maghrabi R., Stahl P. D., Abumrad N. A. (2008) Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J. Biol. Chem. 283, 13578–13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roberts L. D., Virtue S., Vidal-Puig A., Nicholls A. W., Griffin J. L. (2009) Metabolic phenotyping of a model of adipocyte differentiation. Physiol. Genom. 39, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glenn K. C., Shieh J. J., Laird D. M. (1992) Characterization of 3T3-L1 storage lipid metabolism: effect of somatotropin and insulin on specific pathways. Endocrinology 131, 1115–1124 [DOI] [PubMed] [Google Scholar]
- 41. Abel E. D., Peroni O., Kim J. K., Kim Y. B., Boss O., Hadro E., Minnemann T., Shulman G. I., Kahn B. B. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 [DOI] [PubMed] [Google Scholar]
- 42. James D. E., Burleigh K. M., Kraegen E. W. (1985) Time dependence of insulin action in muscle and adipose tissue in the rat in vivo. An increasing response in adipose tissue with time. Diabetes 34, 1049–1054 [DOI] [PubMed] [Google Scholar]
- 43. Lafontan M. (2008) Advances in adipose tissue metabolism. Int. J. Obes. (Lond.) 32(Suppl. 7), S39–S51 [DOI] [PubMed] [Google Scholar]
- 44. Rodriguez-Cuenca S., Carobbio S., Velagapudi V. R., Barbarroja N., Moreno-Navarrete J. M., Tinahones F. J., Fernandez-Real J. M., Oresic M., Vidal-Puig A. (2012) Peroxisome proliferator-activated receptor gamma-dependent regulation of lipolytic nodes and metabolic flexibility. Mol. Cell. Biol. 32, 1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hwang H. H., Moon P. G., Lee J. E., Kim J. G., Lee W., Ryu S. H., Baek M. C. (2011) Identification of the target proteins of rosiglitazone in 3T3-L1 adipocytes through proteomic analysis of cytosolic and secreted proteins. Mol. Cells 31, 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koutsari C., Ali A. H., Mundi M. S., Jensen M. D. (2011) Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 60, 2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.