Abstract
Traditional bone regeneration strategies relied on supplementation of biomaterials constructs with stem or progenitor cells or growth factors. By contrast, cell homing strategies employ chemokines to mobilize stem or progenitor cells from host bone marrow and tissue niches to injured sites. Although silica-based biomaterials exhibit osteogenic and angiogenic potentials, they lack cell homing capability. Stromal cell-derived factor-1 (SDF-1) plays a pivotal role in mobilization and homing of stem cells to injured tissues. In this work, we demonstrated that 3-dimensional collagen scaffolds infiltrated with intrafibrillar silica are biodegradable and highly biocompatible. They exhibit improved compressive stress-strain responses and toughness over nonsilicified collagen scaffolds. They are osteoconductive and up-regulate expressions of osteogenesis- and angiogenesis-related genes more significantly than nonsilicified collagen scaffolds. In addition, these scaffolds reversibly bind SDF-1α for sustained release of this chemokine, which exhibits in vitro cell homing characteristics. When implanted subcutaneously in an in vivo mouse model, SDF-1α-loaded silicified collagen scaffolds stimulate the formation of ectopic bone and blood capillaries within the scaffold and abrogate the need for cell seeding or supplementation of osteogenic and angiogenic growth factors. Intrafibrillar-silicified collagen scaffolds with sustained SDF-1α release represent a less costly and complex alternative to contemporary cell seeding approaches and provide new therapeutic options for in situ hard tissue regeneration.—Niu, L.-N., Jiao, K., Qi, Y.-P., Nikonov, S., Yiu, C. K. Y., Arola, D. D., Gong, S.-Q., El-Marakby, A., Carrilho, M. R. O., Hamrick, M. W., Hargreaves, K. M., Diogenes, A., Chen, J.-H., Pashley, D. H., Tay, F. R. Intrafibrillar silicification of collagen scaffolds for sustained release of stem cell homing chemokine in hard tissue regeneration.
Keywords: chemotaxis, bone regeneration, osteogenesis, angiogenesis
Several complementary attributes are highly desirable for laboratory-based hard tissue regeneration strategies to be successful (1). They include the following: a highly porous, 3-dimensional scaffold with controlled biodegradability to favor tissue integration and vascularization and for natural tissues to eventually replace the scaffold; adequate biomechanical properties to match the intended site of implantation and handling; bioactivity to facilitate attachment to soft and hard tissues; biocompatibility that can be readily adopted by the organ system without any harmful effects; osteoinductivity and angiogenic potential to stimulate graft integration and establishment of a functional vascular supply; and capability for seeding of harvested stem or progenitor cells or supplementation with growth factors to augment their osteogenic and angiogenic potential. Although contemporary tissue engineering scaffolds may possess one or more of these properties, no scaffold to date exhibits all of these properties.
Stem cell-based therapy is one of the best-documented approaches in regenerative medicine (2). For hard tissue regeneration, conventional exogenous strategies that rely on ex vivo expansion of harvested stem or progenitor cells and in vivo delivery via cell seeding are restricted by the limited availability of stem cell sources, donor site morbidity, immune rejection of donor cells, potential tumorigenesis, commercialization cost, and difficulties in regulatory approval. By contrast, cell homing relies on the use of chemokines to mobilize and induce chemotaxis of stem or progenitor cells that are present in host bone marrow and tissue niches to injured sites (3, 4). Mesenchymal stem cells (MSCs) and lineage-committed endothelial progenitor cells (EPCs) can be actively attracted to the sites of injury, allowing osteogenesis and angiogenesis to occur within an unseeded scaffold.
A method for infiltrating type I collagen fibrils with intrafibrillar silica has recently been developed based on biomimetics inspired by biosilicification of diatoms and sponges (5). Based on this method, 3-dimensional silicified collagen scaffolds (SCSs) may be produced. Unlike previous collagen silicification techniques in which extrafibrillar silica phases are deposited in the vicinity of a collagen matrix (6–8), biomimetic analogs of diatom biosilicification proteins are employed to stabilize silicic acid in the form of liquid-like silica precursors for infiltration into the intrafibrillar milieu (i.e., gap zones and microfibrillar spaces) of polycation-enriched, reconstituted type I collagen scaffolds (CSs). Previous biosilicification attempts produced extrafibrillar silica uptake but not intrafibrillar silica uptake, causing the scaffolds to collapse after implantation. By contrast, collagen scaffolds containing intrafibrillar silica do not collapse on desiccation. This facile, highly predictable biosilicification process is reproducible in natural soft tissue collagen and completely demineralized hard tissue collagen matrices. Because of the amorphous nature of silica, SCSs are not as stiff as scaffolds that are mineralized by crystallite mineral phases, such as carbonated apatite or biphasic calcium phosphates. Nevertheless, the SCSs exhibit sufficient stiffness and resilience (5) to enable them to be compressed during insertion into a bony defect and expand to adapt to the conformation of the defect. Although calcium phosphate-containing scaffolds are osteoconductive and have been used for tissue engineering, they are brittle and cannot be compressed during insertion into bony defects. They also lack angiogenic potential without embodiment of copper ions and/or angiogenesis growth factors within the scaffolds for prolonged release (9, 10). By contrast, silicon-containing bioceramics and bioactive glass have been shown to be osteoinductive (11), as well as stimulate neovascularization in the absence of copper ions or growth factor supplements (12). Thus, we hypothesize that SCSs are osetoinductive and proangiogenic by up-regulating expressions of genes associated with osteogenesis and angiogenesis in MSCs and EPCs, which, in turn, results in better extrafibrillar mineralization and capillary formation.
Although silica-based biomaterials exhibit osteogenic (7, 13) and angiogenic potentials (8, 12), they lack cell homing capability. Stromal cell-derived factor-1α (SDF-1α), a ligand for the CXCR4 chemokine receptor, plays a pivotal role in mobilization and homing of stem cells to injured tissues (14–16). SDF-1α is a highly basic protein (pI 9.9–10.9, depending on species from which they are derived; ref. 17). At pH 7.4, positively charged SDF-1α molecules can be electrostatically bound to the negatively charged silanol groups on the surface of SCSs. As amorphous silica within the collagen fibrils of the SCSs slowly solubilizes into silicic acid in aqueous salt solutions (18), we further hypothesize that SDF-1α-loaded SCSs may function as sustained chemokine-releasing devices (19, 20) for mobilization and chemotaxis of MSCs and EPCs from their bone marrow or tissue niches to the porous scaffolds.
Here, we demonstrate that CSs infiltrated with intrafibrillar silica are biodegradable and highly biocompatible. They exhibit improved biomechanical properties and osteoconductivity. They also up-regulate expressions of osteogenesis- and angiogenesis-related genes more significantly than nonsilicified CSs, thereby exhibiting osteoinductive potential. In addition, these scaffolds reversibly bind SDF-1α to create cell homing tissue engineering constructs for sustained release of this chemokine. This approach abrogates the need for cell seeding in scaffolds before implantation or the supplementation of the scaffolds with osteogenic and angiogenic growth factors. Prolonging the release of SDF-1α from these scaffolds partially addresses the issue of chemokine loss via protease degradation (21, 22) and/or rapid diffusion away from its application site.
MATERIALS AND METHODS
Biodegradability of SCSs
Preparation of SCSs
A silicifying medium was prepared from a 3% silicic acid stock solution by mixing 40% hydrolyzed tetraethyl orthosilicate (Silbond 40; Silbond, Weston, MI, USA), absolute ethanol, water, and 37% HCl in the molar ratios of 1.875:396.79:12.03:0.0218 for 1 h. The 3% silicic acid solution was then mixed with 72 mM choline chloride (Sigma-Aldrich, St. Louis, MO, USA) in a 1:1 volume ratio to obtain 1.5% choline-stabilized silicic acid solution (5). Dehydrated CSs (5 mm diameter, 2 mm thick) were cut from reconstituted type I collagen tapes (Ace Surgical Supply, Brockton, MA, USA), rehydrated, treated with 6.67 × 10−4 M poly(allylamine) hydrochloride (PAH; Sigma-Aldrich) for 4 h, and placed in the silicifying medium for 4 d, with daily change of the medium to produce SCSs.
Release of silicic acid
The silicomolybdic acid spectrophotometric method was used for determining the release of silicic acid. The method is based on the ability of silicic acid to form silico-12-molybdic acid in the presence of acidified ammonium heptamolybdate: 7 Si(OH)4 + 12 H6Mo7O24 · 4H2O + 17 H2O ↔ 7 H4SiMo12O40 · 29H2O. Dried SCSs (100 mg each) were immersed in 10 ml of PBS that was adjusted to different pH values (4.5, 7.4, or 10). At designated time periods (0.25, 0.5, 1, 3, 5, 7, 12, 24, 72, 120, 168, 336, and 672 h), 400-μl aliquots of the orthosilicic acid-containing PBS were withdrawn from each solution and added to deionized water (4.6 ml). With continuous stirring, 2.5 ml of 1.0 N HCl, 2.5 ml of Na2EDTA (26.9 mM), and 2.5 ml of ammonium molybdate solution (42.1 mM, pH 8) were added to the orthosilicic acid-containing PBS solutions. After 5 min, 2.5 ml of tartaric acid solution (0.67 M) was added to the solutions. Then, 5 ml of sodium sulfite solution (1.35 M) was added and mixed. Absorbance of the final solution was recorded using a spectrophotometer (Synergy HT; BioTek Instruments, Winooski, VT, USA) at λ = 700 nm. The daily release profiles of silicic acid with daily change of PBS (pH 7.4) during the first 7 d were also determined using the same method (Supplemental Table S1).
Enzymatic degradation of collagen
Fifty-two milligrams of SCS (10 mg before silicification) or 10 mg of CSs was immersed in PBS containing 0.1 mg/ml collagenase derived from clostridium histolyticum (type I, C0130; Sigma-Aldrich). The scaffolds were digested for 0.25, 0.5, 1, 3, 5, 7, 12, 24, 72, 120, and 168 h at 37°C. The degradation product of collagen [cross-linked C-terminal telopeptides of type I collagen (ICTP)] in the solution was quantified using the an ICTP ELISA kit (Biotang, Waltham, MA, USA). Absorbance of the colored reaction products was spectrophotometrically measured at 450 nm, with 650 nm as the reference wavelength. Experiments were performed in triplicates.
Transmission electron microscopy (TEM)
SCSs after immersion in PBS only (pH 7.4) for 7 d or those that had been incubated in collagenase for 3 h were fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated in an ascending ethanol series (50–100%), immersed in propylene oxide, and embedded in epoxy resin. Sections (90 nm thick) were examined unstained using a JSM-1230 TEM (Jeol, Tokyo, Japan) at 110 kV.
Compressive stress-strain responses
Uniaxial compression testing was performed on the following: CSs (control); SCSs; SCSs after immersion in PBS (pH 7.4) for 28 d; and SCSs after immersion in PBS (pH 7.4) for 28 d followed by incubation in 0.1 mg/ml collagenase-containing PBS for 7 d (n=4). All specimens were hydrated in PBS immediately before testing. Testing was performed using a Bose ELF 3100 universal testing system (Bose, Eden Prairie, MN, USA) at a crosshead speed of 0.02 mm/s. Data acquisition was performed at 20 Hz. The load cell has a maximum capacity of 40 N. Testing was performed at constant displacement rate until reaching the limit of the load cell. The tangent modulus for the scaffolds was calculated from the stress-strain curves using the responses from 0 to 5% strain. Modulus of resilience was determined up to 55% strain, which represents the maximum strain achieved without failure when the maximum capacity of the load cell was reached. Data obtained for the tangent modulus and the modulus of resilience were analyzed separately. The CS control in each data set was excluded from the statistical analysis. Each data set was first evaluated for its normality and homoscedasticity assumptions before analysis with 1-way ANOVA and Holm-Sidak multiple comparison procedures. Statistical significance for all tests was preset at α = 0.05.
In vitro bioactivity
SCSs were soaked in simulated body fluid (142.0 mM Na+, 5.0 mM K+, 1.5 mM Mg2+, 2.5 mM Ca2+, 147.8 mM Cl−, 4.2 mM HCO3−, 1.0 mM HPO42−, and 0.5 mM SO42−) at 37°C for 48 h and then processed for TEM. Selected area electron diffraction was used to identify the potential crystallinity of intrafibrillar silica and extrafibrillar calcium phosphate phase. Elemental analysis was performed using scanning TEM–energy-dispersive X-ray (STEM-EDX) analysis. Analysis was performed on unstained TEM sections, using a Tecnai G2 scanning transmission electron microscope (FEI, Hillsboro, OR, USA) at 200 K. Spectrum acquisition and elemental mapping were conducted using an INCA x-sight detector (Oxford Instruments, Abingdon, UK), using a spot dwell time of 300 ms.
Cell viability evaluation
Cell culture
Two adult murine cell lines were used: MSCs (non-GFP-expressing strain C57Bl/6J; Texas A&M Health Science Center, Institute for Regenerative Medicine, College Station, TX, USA) for osteogenesis, and EPCs (BioChain Institute, Hayward, CA, USA) for angiogenesis. The murine EPCs employed are considered as late-stage EPCs since they are CD 133 negative. Before differentiation, the MSCs were cultured in 77% DMEM with 10% FBS, 10% horse serum, 1% l-glutamine, and 2% 100U/ml penicillin and 100 mg/ml streptomycin. The EPCs were cultivated in EPOC basal medium (BioChain) that was supplemented with EPOC growth medium supplement (BioChain), 2% 100 U/ml penicillin and 100 mg/ml streptomycin. Both cell types were incubated at 37°C with 5% CO2 and 95% air in a humidified incubator, with the corresponding culture medium replaced every 2 d. Except for the in vivo control cell seeding procedures described in In Vivo Cell Seeding vs. Cell Homing, all experiments were performed by culturing the MSCs and EPCs in different conditioned culture media; the stem or progenitor cells were never in direct contact with the SCSs.
MTT assay
MSCs or EPCs were plated in a 24-well format at a density of 1 × 104 cells/cm2, in 0.5 ml of the respective growth medium. The materials investigated included CSs and SCSs (dimensions after rehydration and biosilicification: 5 mm diameter, 5 mm thick). Teflon disks and freshly polymerized polymethyl methacrylate disks of the same dimensions served as the negative and positive controls, respectively. Twelve replicates were used for each material, and all materials were sterilized before testing. The specimens were placed individually in transwell inserts with 3 μm pore size (BD Falcon, Franklin Lakes, NJ, USA) to prevent direct contact of cells by the specimen. The cells were exposed to the materials for 72 h before evaluation. Succinic dehydrogenase (SDH) activity of the cells was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (n=12 culture wells). Briefly, retrieved cells were incubated in an MTT-succinate solution for 60 min and fixed with Tris-formalin. The purple MTT-formazan in the cells was dissolved in situ using DMSO-NaOH, and the optical density was measured using the spectrophotometer at 562 nm. The optical density of blank DMSO-NaOH was subtracted from all wells. The formazan content of each well was computed as a percentage of the mean of the Teflon controls, which was taken to represent 100% biocompatibilty. When either the normality assumption or the homoscedasticity assumption was violated for each data set (MSCs or EPCs), each data set was analyzed with Krusal-Wallis 1-way ANOVA and Dunn's multiple comparison procedures.
Flow cytometry
Additional MSCs or EPCs were exposed to the 4 materials for 72 h, detached from the culture wells using 0.25% trypsin, and resuspended at 2-3 × 104 cells/ml in 1× binding buffer from an Apoptosis and Necrosis Quantification Kit (Biotium, Haywood, CA, USA). The cells were stained with FITC-annexin V (AnV; λabs/λem=492/514 nm; green fluorescence) and ethidium homodimer III (Etd-III; λabs/λem=528/617 nm; red fluorescence) and sorted with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) to determine the percentage distribution of viable (AnV/Etd-III−), early apoptotic (AnV+, Etd-III−), late apoptotic (AnV/Etd-III+), and necrotic cells (AnV−, Etd-III+). Experiments were performed in triplicates. For each cell line, the data were analyzed using 1-way ANOVA and Holm-Sidak pairwise multiple comparison procedures after the normality and homoscedasticity assumptions were confirmed.
Real-time reverse transcription–polymerase chain reaction (RT-PCR)
MSCs were cultured in the DMEM/serum complete expansion medium for 4 d to become established. The expansion medium was replaced with bone differentiation medium (complete expansion medium supplemented with 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone; Sigma-Aldrich). Differentiated MSCs were exposed to CSs or SCSs for 14 d. Similarly, EPCs were cultured in EPOC growth medium and exposed to CSs or SCSs for 14 d. For both cell lines, cells cultured in medium and not exposed to CSs or SCSs were used as controls.
Quantitative RT-PCR (qRT-PCR) was used for examining the expression of osteogenic differentiation markers in MSCs [alkaline phosphatase (ALP), RUNX2, osteocalcin, DMP-1, dentin sialophosphoprotein, and bone sialoprotein II], and the expression of angiogenesis marker genes in EPCs [angiopoietin (Ang)1, Ang2, and vascular endothelial growth factor-A (VEGF-A)]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. RNA extraction and cDNA synthesis were performed using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), respectively. qRT-PCR was performed using FastStart Universal SYBR Green Master Kit (Roche Applied Science, Indianapolis, IN, USA), and forward and reverse primers for the aforementioned genes (Supplemental Table S2), in a 7300 RT-PCR system (Applied Biosystems). Experiments were conducted in sextuplicates (n=6). The amount of target cDNA, relative to GAPDH, was calculated using the formula 2−ΔΔCt. The results were calculated as the relative quantification of the target gene compared with the respective control, which was set at 1.
In vitro osteogenesis and proangiogenesis
Additional CSs and SCSs were placed in transwell inserts and exposed to differentiated MSCs (1×104 cells/cm2) for 7 and 14 d to examine the production of ALP, and for 21 d to induce formation of extracellular bone nodules. Teflon disks of similar dimensions were used as the negative control. Six replicates were used for each group. The bone differentiation medium was changed every 3 d. ALP activity was determined using a QuantiChrom ALP Assay Kit (BioAssay Systems, Hayward, CA, USA). After incubation for 7 or 14 d, the cells were lyzed with 0.2% Triton X-100, and the cell lysate was incubated with p-nitrophenyl phosphate at 37°C for 1 h. Absorbance of p-nitrophenol was determined spectrophotometrically at λ = 405 nm. The ALP activities were expressed in international units per liter.
Extracellular mineralization was determined using alizarin red-S assay. The cells were washed with PBS, fixed in 10% formaldehyde, and stained with alizarin red-S (40 mM solution, pH 4.2). The stained cells were incubated in 10% acetic acid for 30 min and neutralized with 10% ammonium hydroxide. Absorbance of the supernatants was determined at λ = 405 nm. The amounts of calcium extracted (in millimoles) were determined using a calibration curve correlating absorbance of alizarin red-S with known calcium concentrations.
Evaluation of tube formation by EPCs was performed using an In Vitro Angiogensis Assay Kit (ECM625; Millipore, Billerica, CA). Established EPCs were cultured on ECMatrix, a solid gel consisting of basement membrane proteins, growth factors, and proteolytic enzymes. Briefly, ECMatrix gel solution was thawed, mixed with diluent buffer, and placed in a precooled 96-well plate, at 37°C for 1 h to enable the matrix solution to gel. SCSs were placed in PBS (pH 7.4) for different time periods (1, 6, 12, or 24 h). Similarly, CSs and Teflon control disks of similar dimensions were immersed in PBS for 24 h. A 30 μl aliquot of PBS, containing extracts derived from the Teflon negative control, SCS, or CS, was mixed with 120 μl of EPC growth medium and supplements (i.e., a 5-fold dilution), and served as proangiogensis medium. The EPCs (1×104 cells/well) were suspended in the respective medium, seeded onto the surface of the gelled matrix, and incubated at 37°C for 6 h. The extent of proangiogenesis was quantified by counting the capillary tube branch points in 5 randomly selected fields (×100 magnification) for each well. Experiments were conducted in triplicates.
One-factor ANOVA and Holm-Sidak pairwise comparison procedures were used to evaluate the effect of materials on all parameters except for ALP activity after validation of the normality and homoscedasticity assumptions of the corresponding data sets. The corresponding nonparametric, rank-based versions of the analyzes were employed if either the normality or homoscedastic assumption of a data set was violated. Statistical analysis of ALP activity was conducted using 2-factor repeated measures ANOVA to examine the effects of materials and cell exposure time and the interaction of these 2 factors on ALP activity. For all tests, statistical significance was set at α = 0.05.
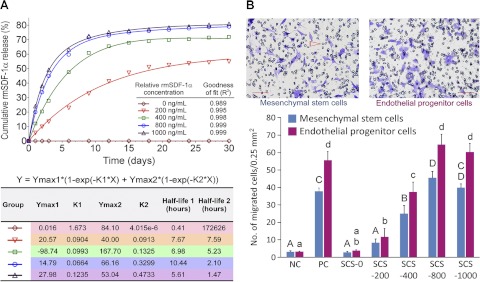
SDF-1α release kinetics
SCSs were loaded with 0, 200, 400, 800, or 1000 ng of recombinant murine CXCL12/SDF-1α (R&D Systems, Minneapolis, MN, USA; molecular mass 8 kDa) and lyophilized (n=3). The chemokine-loaded SCSs were placed in separate wells containing 1 ml of PBS containing 1 w/v% BSA. The supernatant was collected after 0, 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 d and replaced with equivalent amounts of PBS-1% BSA. The cumulative amount of rmSDF-1α released at each time point was determined using a mouse CXCL12/SDF-1α Quantikine ELISA Kit (R&D Systems; minimum detectable dose 0.044 ng/ml). Absorbance of the samples were read at λ = 450 nm. The amount of rmSDF-1α was determined from a calibration curve based on known concentrations of a serially diluted rmSDF-1α standard.
In vitro cell homing
Chemotaxis of MSCs or EPCs was evaluated using transwell (modified Boyden chamber) migration assay. Murine MSCs or EPCs (2×104 cells in 100 μl) were placed in the upper chamber of 24-well transwells with polycarbonate membrane containing 8-μm pores (Corning Costar; Corning Life Sciences, Lowell, MA, USA). SCSs loaded with 0, 120, 240, 480, or 600 ng of rmSDF-1α and 600 μl of DMEM supplemented with 1% BSA were placed in the lower chamber of each transwell. The relative concentrations of rmSDF-1α to the growth medium were 0, 200, 400, 800, or 1000 ng/ml, respectively, corresponding to the concentrations utilized in the aforementioned SDF-1α release test. The growth medium of the positive control consisted of 600 μl of DMEM, 1% BSA and 100 ng/ml of rmSDF-1α (i.e., no SCS). The negative control consisted of DMEM and 1% BSA only (i.e., no SCS and rmSDF-1α). After incubating at 37°C for 16 h, the cells were fixed with 10% formaldehyde for 30 min. Cells remaining on top of the polycarbonate membrane of each transwell were removed with cotton swabs. Cells that migrated to the lower chamber of the transwells were stained with crystal violet and counted in 5 randomly selected fields for each membrane at ×200 magnification (0.25 mm2). The mean cell count from the 5 fields was used to represent the number of migrated cells for that transwell. Experiments were conducted in triplicates for both MSCs and EPCs. Data obtained for the MSCs and EPCs were analyzed separately. Each data set consisted of the positive control, the negative control, and the 5 rmSDF-1α concentrations. For each cell type, the data set was first evaluated for its normality and homoscedasticity assumptions before analysis with 1-way ANOVA and Holm-Sidak post hoc multiple comparison procedures, with statistical significance preset at α = 0.05.
In vivo cell seeding vs. cell homing
For cell seeding, SCSs were immersed in culture medium overnight and seeded with 100 μl of undifferentiated MSC cell suspension (∼5×104 cells/scaffold). Cell suspensions were injected into the scaffolds with a pipette tip. Scaffolds were incubated at 37°C for 3 h, after which 2 ml of culture medium was added. Cells were cultured at 37°C, 5% CO2 for 3 d, with periodic change of the culture medium every other day. At d 3, the MSC-seeded SCSs were used for implantation. For cell homing, SCSs were loaded with 2 μg of rmSDF-1α and lyophilized.
Eight-week-old littermate male BalB/c (ν/ν) immunocompromized mice were obtained from the Laboratory Animal Research Center of the Fourth Military Medical University. Animal care and surgical procedures were approved by the Animal Experiment Administration Committee of Fourth Military Medical University, and performed according to institutional guidelines. The mice were anesthetized with 1.5–3% isoflurane. On disinfection with 10% povidone iodine and 70% ethanol, a linear incision of ∼10 mm was made in the dorsum of each mouse and blunt dissected to create a subcutaneous pocket. MSCs-seeded SCSs or rmSDF-1α-loaded SCSs (n=4) were implanted (1 implant/mouse), followed by wound closure. CSs seeded with MSCs and CSs loaded with a similar concentration of rmSDF-1α were also implanted as controls (n=4). At 6 wk after surgery, the mice from the 4 groups were euthanized. Ectopic ossicles, if present, were then harvested with the surrounding tissues. After fixation with 4% formaldehyde for 48 h, the extracted specimens were completely decalcified in 17% EDTA, dehydrated in a graded series of alcohol, and embedded in paraffin. Sections (6 μm thick) were prepared, stained with hematoxylin-eosin, and observed with a light microscope (Axioplan; Carl Zeiss Microscopy, Thornwood, NY, USA). For each implant, histomorphometry was performed on 2 sections taken from the middle of each implant. For each section, 5 fields at ×20 were used for analysis. For each field, the ratio of the area occupied by ectopic bone tissues to the total tissue area was calculated using a computer-assisted image analyzing system (Leica Qwin; Leica Microsystems, Bannockburn, IL, USA). The number of capillaries within each field was also counted. These two parameters were statistically analyzed using Student t tests after validation of the normality and homoscedasticity assumptions of the corresponding data sets. Statistical significance was preset at α = 0.05.
RESULTS AND DISCUSSION
Biodegradability
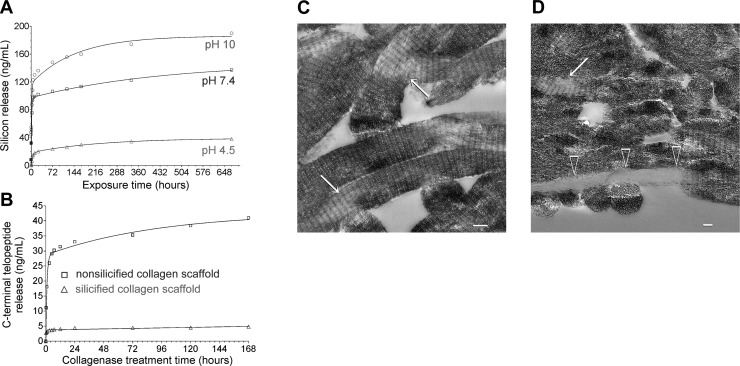
Scaffolds employed for tissue engineering have to be resorbable for natural tissues to eventually replace the scaffold and for vascularization. We prepared homogeneously silicified SCSs using choline-stabilized silicic acid and poly(allylamine)-enriched, porous CSs (5). For the release of silicic acid in PBS with different pH values, a higher release of silicic acid was found as the alkalinity of the medium increased (Fig. 1A). For each pH, silicic acid release from the homoegeneously silicified SCSs was characterized by an initial burst release, followed by a slower continuous release. These kinetics were expressed mathematically by a 2-phase exponential association repression model, with the general formula Y = Ymax1 * [1 − exp(−K1 * X)] + Ymax2 * [1 − exp(−K2 * X)]. For each model, the curve commences at 0 and ascends to Ymax1 and Ymax2, with rate constants K1 and K2. The corresponding half-lives are 0.69/K1 and 0.69/K2. The values for each pH-dependent silicic acid release profile are summarized in Supplemental Table S1. When the storage medium was replenished daily over a 7-d period, the SCSs showed a similar release profile for each day, with an initial burst release phase during the first 10 h, followed by a similar sustained release phase from 10 to 24 h (Supplemental Fig. S1).
Figure 1.
A) Effect of pH value of the incubating medium on the release of silicic acid from the SCSs. B) Effect of collagen silicification on the degradation of CSs. Each CS was immersed in 5.2 ml of PBS containing 0.1 mg/ml of collagenase derived from Clostridium histolyticum. C) Unstained TEM image of an SCS with localized loss of intrafibrillar silica (arrows) after immersion in PBS at pH 7.4. D) Unstained TEM image showing degradation of a collagen fibril (open arrowheads) along the periphery of a SCS. Note that part of the same fibril (right side of image) remained silicified. Partial loss of intrafibrillar silica can also be observed (arrow) in some collagen fibrils within the scaffold. Scale bars = 100 nm.
Bacterial collagenase cleaved type I collagen into cross-linked C-terminal telopeptides, which could be detected using enzyme-linked immunosorbent assay. CSs degraded too rapidly, whereas SCSs were only slowly degraded by bacterial collagenase (Fig. 1B). Similar to the release of silicic acid, 2-phase exponential association repression models provided excellent fits of the data obtained from collagen degradation (Supplemental Table S1). Both the loss of intrafibrillar silica (electron-lucent areas within the collagen fibrils in Fig. 1C) and degradation of collagen fibrils (open arrowheads in Fig. 1D) from SCSs could be morphologically identified using TEM.
Taken together, the data indicate that intrafibrillar biosilicification protects collagen fibrils from rapid degradation during the critical period when cell homing is initiated. Previous studies have shown that amorphous silica undergoes dissolution under a variety of electrolyte, temperature, and environmental conditions (18, 23). Sustained release of silicic acid from the silicified collagen fibrils at physiological pH allows the CS to be degraded gradually during osteogenesis and angiogenesis. This probably enables retention of some biomechanical properties of the supporting scaffold before the regerneration of enough bone tissues for support (see following section) and for cell homing chemokines that are electrostatically bound to the silicified collagen to be slowly released for continuous chemoattraction of stem/progenitor cells (see SDF-1α Release Kinetics).
Biomechanical properties
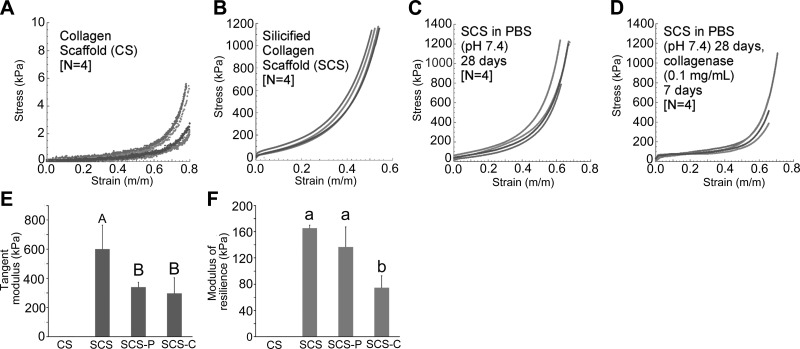
Changes in biomechanical properties of hydrated CS (control), SCS, SCS after immersion in PBS, and SCS after PBS immersion and collagenase treatment were evaluated by examining their compressive stress/strain responses (Fig. 2A–D). The tangent modulus values (kPa) of the different scaffolds from 0 to 5% strain are as follows: CS control, 0.80 ± 0.21; SCS, 599.8 ± 166.0; SCS in PBS, 340.3 ± 34.2; and SCS in PBS and collagenase, 296.5 ± 107.5. Figure 2E represents the results of the statistical analysis of the tangent moduli of SCSs before and after PBS immersion/collagenase treatment (control CS group not included in the analysis). Statistical significance was observed among the 3 groups (P=0.01). The tangent moduli of the SCSs after PBS immersion or collagenase treatment were significantly lower than untreated SCSs (P<0.05) but were not significantly different from each other. Nevertheless, those values were much higher than the tangent modulus of the CS control.
Figure 2.
Evaluation of biomechanical properties. A–D) Compressive stress-strain curves obtained for the 4 groups of CSs: CS (A), SCS (B), SCS in PBS for 28 d (SCS-P; C), and SCS in PBS for 28 d and collegenase for 7 d (SCS-C; D). E) Tangent moduli of the 4 groups of CSs. Groups with the same uppercase letter designations are not statistically significant (P>0.05). F) Modulus of resilience of the 4 groups of CSs. Groups with the same lowercase letter designations are not statistically significant (P>0.05). Note that data from the CS group were not included in the statistical analyzes.
The modulus of resilience values (kPa) of the different scaffolds are as follows: CS control, 0.18 ± 0.06; SCS, 165.3 ± 4.00; SCS in PBS, 136.8 ± 30.4; and SCS in PBS and collagenase, 74.5 ± 18.4. Figure 2F represents the results of the statistical analysis of the moduli of resilience of SCSs before and after PBS immersion/collagenase treatment (control CS group not included in the analysis). The modulus of toughness of the untreated SCSs and SCSs after PBS immersion only was significantly higher than that of collagenase-treated SCSs (P<0.05) but was not significantly different from each other. Even for the collagenase-treated SCSs, the modulus of toughness was much higher than for CS control.
In engineering terms, the tangent modulus is the slope of the compressive stress-strain curve at any specified strain. Below the proportional limit, the tangent modulus is equivalent to the Young's modulus, which is an indication of the stiffness of the scaffolds. The modulus of resilience is the area under the stress-strain curve. Modulus of resilience represents the energy consumed in deformation of the scaffolds to the designated strain and is commonly used as a measure of the toughness of materials under elastic deformation. The modulus of resilience of the untreated SCSs reflects the contribution of type I collagen to the toughness of mineralized tissues (24). The results of the compressive stress/strain responses indicate that even after partial-degradation, hydrated SCSs possess better biomechanical properties than CSs, thereby providing better mechanical support of the scaffolds during the course of MSC and EPC homing.
In vitro bioactivity
Although porous CSs containing extrafibrillar bioactive glass and silica sol-gel particles are rendered bioactive in simulated body fluids by the deposition of apatite on the scaffold surface (25), it is not known whether intrafibrillar silica-containing SCSs exhibit a similar phenomenon. When SCSs were immersed in simulated body fluid, extrafibrillar apatite deposition along the surface of collagen leaflets (i.e., aggregation of collagen fibrils into 3-dimensional sheets within the porous SCS) was observed by TEM imaging as early as 48 h. The presence of apatite was confirmed by selected area electron diffraction (Supplemental Fig.S2A) and X-ray diffraction (Supplemental Fig.S2B). Elemental analysis using STEM-EDX analysis further revealed the presence of hierarchical intrafibrillar Si that corresponded with the D spacing of collagen fibrils, and extrafibrillar Ca and P deposition along the surface of the collagen leaflets (Fig. 3). Thus, SCSs resemble class A bioactive materials in exhibiting osteoconductivity after their immersion in simulated body fluid (26). Potential bonding to soft and hard tissues may be achieved via hydrophillic silanol (Si-OH) groups present along the surface of the silicified leaflets. Deposition of apatite along the surface of the silicified fiber leaflets may also promote attachment of recruited stem/progenitor cells to the internal milieu of the highly porous SCS (27).
Figure 3.
In vitro bioactivity of an SCS after immersion in simulated body fluid, as demonstrated by STEM-EDX analysis. Top left panel: darkfield image of a collagen leaflet (consisting of multiple collagen fibrils) within the scaffold. Needle-shaped crystals were deposited along the periphery of the collagen leaflet. (High-resolution brightfield images can be found in Supplemental Fig. S2A). Top right, middle, and bottom left panels: elemental mappings of the distribution of oxygen (top right panel), silicon (middle left panel), calcium (middle right panel), and phosphorus (bottom left panel) within and around the collagen leaflet. Bottom right panel: bright magnification, merged distributions of calcium, phosphorus and silicon. Banded appearance of the collagen fibrils was reproduced by the hierarchical distribution of intrafibrillar silicon (arrow).
Cell viability
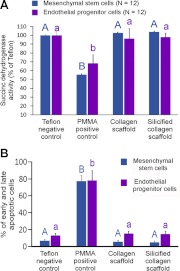
A major criterion for an ideal tissue engineering scaffold is that the material should be nontoxic to the stem or progenitor cells that are recruited to the injured site. The biocompatibility of SCSs to murine MSCs and murine EPCs was quantified using MTT assay and flow cytometry (Fig. 4). The MTT assay was used to evaluate the ability of the cells to provide energy for cell function and growth. This is because cellular damage by toxic biomaterials reduces the abiliy of the cells to metabolize tetrazolium salts via mitochondrial dehydrogenases involved in the citric acid cycle and the electron transport chain. Both CSs and SCSs demonstrated similar succinc dehydrogenase activities as the Teflon negative control (P>0.05; Fig. 4A). The results indicate that infiltration of CSs with intrafibrillar silica has no adverse effect on the viability of MSCs or EPCs.
Figure 4.
A) Results of MTT assays. Mitochondrial succinic dehydrogenase activities were normalized against the Teflon negative control, which was taken to be 100%. B) Summary of the combined percentage of early and late apoptotic cells in flow cytometry test. For the MSCs, groups identified with the same uppercase letters are not statistically significant (P>0.05). For the EPCs, groups identified with the same lowercase letters are not statistically significant (P>0.05). PMMA, polymethyl methacrylate.
Flow cytometry was used to examine the effect of the scaffolds on cell death-induced plasma membrane permeability to fluorescent dyes and DNA stains. This enabled the MSCs or EPCs to be classified as normal, early apoptotic, late apoptotic, and necrotic cells after exposure to the scaffolds for 72 h. Figure 4B summarizes the combined percentage of early and late apoptotic cells, which indicate that there is no significant differences among the CSs, SCSs, and the Teflon negative control group (P>0.05).
Although these 2 tests examined different aspects of cell viability, the results of the MTT assay and flow cytometry are complementary and indicate that infiltration of CSs with intrafibrillar silica has no adverse effects (apoptosis and necrosis) on the viability of MSCs or EPCs. The SCSs were as biocompatible as Teflon or CSs, and no statistical significance was observed in their combined percentage distribution of early and late apoptotic cells.
Changes in mRNA expressions in the presence of SCSs
Dissolution products from a bioactive tissue engineering scaffold should stimulate genes in the regenerating tissue to promote efficient cell differentiation and proliferation. As type I collagen has been shown to promote osteogenesis of MSCs (28) and angiogenesis of EPCs (29), it is unknown whether intrafibrillar biosilicification confers additional benefits to the osteoinductivity or angiogenic potential of CSs. Thus, mRNA expressions of biomarkers associated with osteogenesis and angiogenesis were examined, using qRT-PCR. The MSCs were cultured in bone differentiation medium, and EPCs were cultured in EPC growth medium containing growth factor supplements, respectively. Expressions of osteogenesis differentiation markers for MSCs and angiogenesis-related markers for EPCs are shown in Supplemental Fig. S3. Differentiated MSCs exposed to SCSs exhibit highly significant fold increases (P<0.001) in mRNA expressions of genes associated with osteogenesis, such as ALP, Runt-related transcription factor 2, osteocalcin, and bone sialoprotein II, when compared with those exposed to CSs or the differentiating medium control (Supplemental Fig. S3A). Similar to MSCs, EPCs exposed to SCSs exhibited highly significant up-regulation (P<0.001) in mRNA expressions of VEGF-A and Ang2 genes but not for Ang1 gene (Supplemental Fig. S3B). VEGF is a modulator of endothelial cell functions, after binding to a specific receptor kinase domain region. Ang1 and Ang2 bind competitively to their Tie-2 receptor, crosstalk with VEGF, and modulate its effects. Ang2 and VEGF contribute synergistically to the induction of angiogenesis, while Ang1 is responsible for the maintenance and stability of blood vessels (30).
In vitro osteogenesis and proangiogenesis
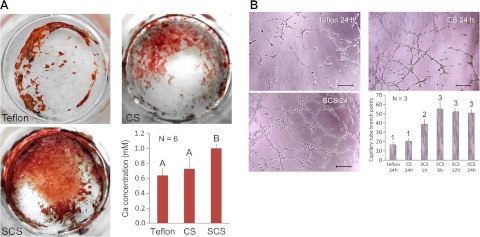
The results of mRNA uprgulation were further confirmed by examining ALP production and the formation of extracellular bone nodules by differentiated MSCs, and evaluation of capillary-like tube formation by EPCs. ALP activity of MSCs exposed to SCSs was found to increase significantly (P<0.001), both at 7 and 14 d after the addition of the differentiation medium, when compared with CSs or the Teflon negative control (Supplemental Fig. S4A). In vitro osteogenesis was performed on differentiated MSCs that had been exposed to SCSs, CSs, and the Teflon negative control for 21 d. Extracellular mineralization was determined using alizarin red-S assay. The latter stains calcium-rich deposits produced by cells in culture, and the dye can be extracted from the stained cell monolayer and assayed by spectrophotometry. Figure 5A shows representative images of calcium deposits produced by Teflon, CSs, and SCSs. The amount of extracted calcium for the SCS group was significantly higher (P<0.05) than the amounts extracted from Teflon or the CS groups (Fig. 5A, bottom right panel).
Figure 5.
A) Alizarin red S staining of extracellular bone nodules produced by differentiated MDCs after they were exposed to the Teflon control (top left panel), CSs (top right panel), and SCSs (bottom left panel) for 72 h. Bar chart (bottom right panel) summarizes the results of in vitro osteogenesis. Groups designated by the same uppercase letters are not statistically significant (P>0.05). B) Tube formation by EPCs represents a simple model of angiogenesis in which induction or inhibition of tube formation by exogenous signals may be monitored. Representative light microscopy images show EPCs after they were exposed to extracts derived from the Teflon control for 24 h (top left panel), CSs for 24 h (top right panel), and SCSs (bottom left panel) for 24 h. Scale bars = 100 μm. Bar chart (bottom right panel) summarizes the results of in vitro proangiogenesis. Extracts from SCSs were examined after the immersion of these scaffolds in phosphate-buffered saline (pH 7.4) for different time periods (1, 6, 12, and 24 h). Groups designated by the same numeric descriptors are not statistically significant (P>0.05).
In vitro proangiogenesis was performed by culturing EPCs on ECMatrix for 6 h to induce cell migration and alignment, followed by the development of capillary-like tubes, sprouting of new capillaries, and the formation of cellular networks. Figure 5B shows representative images of EPCs exposed to growth media containing Teflon, CSs, and 1-, 6-, 12-, and 24-h extracts from SCSs. The amounts of silicic acid present in the 1-, 6-, 12-, and 24-h SCS extracts, as analyzed by silicomolybdic acid assay, were 39, 80, 104, and 113 μg/ml, respectively. The effect of each material on the extent of proangiogenesis was quantified by counting the capillary tube branch points in the respective culture wells. The numbers of branched points induced by 1-, 6-, 12-, and 24-h SCS extracts were all significantly higher (P<0.05) than 24-h extracts derived from Teflon or CSs. The numbers of branched points induced by 6-, 12-, or 24-h SCS extracts were not significantly different from each other but were all higher than the 1-h SCS extract (Fig. 5B, bottom right panel).
Taken together, the up-regulation of osteogenic/angiogenic phenotypes and the enzymatic and morphological expressions of these intracellular events support the hypothesis that SCSs exhibit better osteoinductive potential in MSCs and angiogenic potential in EPCs than CSs.
SDF-1α release kinetics
Incorporation of biomolecules into 3-dimensional tissue engineering scaffolds to exert control over cell migration is challenging because of the fragile nature, chemical, and geometric complexity of these macromolecules (31). Peptides must bind nonspecifically to the tissue constructs, maintain their conformation, and be released in a manner that does not affect their biological functionality. Cumulative releases of rmSDF-1α from SCSs loaded with different concentrations of the chemokine are depicted in Fig. 6A. For each chemokine concentration, release profile is characterized by an initial phase of rapid release followed by a period of slow, sustained release, and was mathematically represented by a 2-phase exponential association model. SCDF-1α is a highly basic protein (pI 9.9; ref. 17). Thus, at pH 7.4, positively charged SDF-1α molecules can be electrostatically bound to the negatively charged silanol groups of silicified collagen fibrils within the SCS. It is pertinent to point out that the SDF-1α release profiles are similar to the silicic acid release profiles of SCSs. This suggests that dissociation of rmSDF-1α occurs with gradual biodegradation of the SCSs. The data confirm the hypothesis that SDF-1α-loaded SCSs function as sustained chemokine-releasing devices. The presence of a second, slow-releasing phase after the initial burst release phase improves the effectiveness of SDF-1α as a cell homing agent by reducing the need to continuously replenish SDF-1α that is lost by diffusion and/or degradation by exopeptidases and matrix metalloproteinases in an inflammatory environment (21).
Figure 6.
A) Release kinetics of rmSDF-1α after binding of different concentrations of the chemokine to silicified CSs. Two-phase exponential association models provided an excellent fit for the relationship between the cumulative rmSDF-1α release and rmSDF-1α concentration in each isotherm. Each model is characterized by an initial period of burst release (Ymax1, with rate constant K1) that is followed by a period of slow, sustained release (Ymax2, with rate constant K2). Values of these parameters and the corresponding half-lives are summarized in the bottom panel. B) Ability of rmSDF-1α-loaded SCSs to act as a chemoattractant for MSCs or EPCs. Representative crystal violet-stained images of MSCs (top left panel) and EPCs (top right panel) that migrated through the pores (arrowhead) of the transwell membrane to the bottom of the transwell. Scale bars = 100 μm. Bar chart (bottom panel) summarizes the in vitro cell homing results. NC, negative control (no SCS or rmSDF-1α); PC, positive control (no SCS, 100 ng/ml rmSDF-1α dissolved in culture medium); SCS-0 to SCS-1000, rmSDF-1α-loaded SCSs with relative concentrations of 0 to 1000 ng/ml. For MSCs, groups designated by the same uppercase letters are not statistically significant (P>0.05). For EPCs, groups designated by the same lowercase letters are not statistically significant (P>0.05).
In vitro cell homing
To demonstrate that the released SDF-1α still maintained its biological functionality for chemoattraction of stem or progenitor cells, in vitro homing of murine MSCs and EPCs by rmSDF-1α-loaded SCSs was evaluated separately with a modified Boyden chamber (transwell migration) assay, using relative rmSDF-1α concentrations that corresponded with those utilized in the aforementioned rmSDF-1α release experiment. The numbers of MSCs or EPCs that migrated from the top to the bottom of the transwells, caused by the chemotactic effect of rmSDF-1α released from chemokine-loaded SCSs (Fig. 6B, top panels), were compared with those that migrated in the presence of 100 ng/ml freshly prepared, unbound rmSDF-1α in solution. For both murine MSCs and EPCs, SCSs loaded with a minimum of 800 ng/ml of rmSDF-1α exhibited a homing effect that was equivalent to 100 ng/ml of free rmSDF-1α (Fig. 6B, bottom panel).
Apart from its effect on EPC homing, SDF-1 also exhibits proangiogenic effects via inducing heme oxygenase 1 expression in EPCs (32). To examine the proangiogenic role of SDF-1 in SCS, we added 100 and 200 ng/ml of rmSDF-1α to 6-h SCS extracts. Endothelial tube formation was compared with 6-h SCS extracts without rmSDF-1α. The results indicate that the presence of rmSDF-1α in extracts derived from SCSs further augments the role played by SCSs in promoting endothelial tube formation (Supplemental Fig. S4B).
In vivo cell homing vs. cell seeding
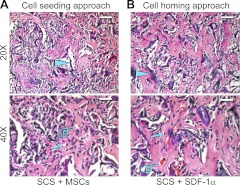
To confirm that SDF-1α released from the chemokine-loaded SCSs can mobilize and induce chemotaxis of stem or progenitor cells that are present in host bone marrow and tissue niches to injured sites, we compared the effects of cell homing vs. a cell seeding approach on ectopic bone formation in a mouse model. SCSs were either seeded with undifferentiated murine MSCs or loaded with rmSDF-1α, and implanted into subcutaneous pockets of BalB/c (ν/ν)-immunocompromized mice. Silicified collagen sponges without cell seeding or chemokine loading were used as blank controls and implanted similarly into additional mice. Undifferentiated MSCs were employed in the cell seeding approach, as no difference was observed between the use of undifferentiated MSCs and osteogenically induced MSCs in inducing ectopic bone formation in a immunodeficient mouse-human MSC xenotransplanation model (33). No additional growth factors, such as bone morphogenetic proteins (BMPs; ref. 34) or VEGFs (35) were used in either approach.
After 6 wk of in vivo implantation, implanted CSs (either seeded with MSCs or loaded with SDF-1α) completely degraded, and no ossicles were identified; those samples were not processed for histological examination. Rapid disintegration of CSs that were not infiltrated by intrafibrillar silica was in agreement with previous reports that intrafibrillar minerals protect collagen from degradation (36). For SCSs utilizing the cell seeding and cell homing approaches, the induced ossicles were encapsulated by a thin fibrous capsule (not shown). Figure 7 shows representative micrographs of ectopic bone formation within the center of MSC-seeded SCSs (Fig. 7A) and rmSDF-1α-loaded SCSs (Fig. 7B). Histological characteristics of the ectopic bone were similar between the cell seeding approach and the cell homing approach. In both approaches, remnant silicified collagen leaflets could still be identified after 6 wk. Osteocyte-like cells and capillaries containing red blood cells could be identified within the newly formed bone trabeculae. Histomorphometric analysis of the ratio of mineralized tissue area to total tissue area indicated that there is no difference (P=0.19) in the quantity of ectopic bone formed by the cell seeding approach (0.491±0.097) and by the cell homing approach (0.485±0.108). However, bone tissues formed by the cell homing approach have a significantly larger number of capillaries within the trabeculae (8.7±1.6; P<0.001), compared with those formed using the cell seeding approach (4.2±1.2). Neoangiogenesis plays an important role in successful healing of implanted tissue constructs (35). Inadequate vascularization may lead to nutritional limitations and necrosis of the newly formed bone. The less profuse distribution of new capillaries associated with the cell seeding approach in the present study may be due to the fact that only MSCs (and not cocultures of MSCs and EPCs) were seeded within the SCSs. By contrast, SDF-1α has the capacity to induce homing of host EPCs, as well as a stimulative effect on angiogenesis (see In Vitro Cell Homing). This may also have accounted for the larger number of capillaries in the cell homing group. It should be mentioned that the objective of the present in vivo work was to provide a proof of concept that SDF-1α-loaded SCSs can release SDF-1α without altering its biological functionality for chemoattraction of stem or progenitor cells. As such, these preliminary in vivo data do not permit us to definitively conclude that cell homing can replace cell seeding with the use of SCSs. Likewise, as there was no comparison between the efficacy of SDF-1α-loaded SCSs and the generally accepted scaffolds, such as those containing biphasic calcium phosphates, in inducing bone and vessel formation, we cannot conclude from the present in vivo data that SCSs are better than other commercially available or experimental scaffolds in hard tissue regeneration. Although rmSDF-1α had been incorporated in a variety of biomaterials for continuous release of the chemokine to induce homing of MSCs (19, 37), the efficacy of SDF-1α-loaded SCSs vs. those containing additional growth factors, such as BMP-2/7 or VEGF-A, is also unknown. These issues have to be investigated in future studies, using more clinically relevant orthotopic implantation animal models that contain critical-sized defects.
Figure 7.
Light microscopy images of hematoxylin-eosin-stained sections showing ectopic bone and blood capillary vessel formation in a mouse subcutaneous implantation model. A) Cell seeding approach using an SCS loaded with murine MSCs. B) Cell homing approach using an rmSDF-1α-loaded SCS. Top panels: ×20 view; scale bars =100 μm. Bottom panels: ×40 view; scale bars = 50 μm. Solid arrowheads indicate remnant silicified collagen leaflets derived from the implanted scaffolds. Arrows indicate osteocyte-like cells within newly formed bone trabeculae. Pointers indicate capillaries containing red blood cells.
CONCLUSIONS
Within the limits of the present study, it may be concluded that incorporation of intrafibrillar silica into highly porous CSs protects the collagen fibrils from rapid degradation but allows continuous release of silicic acid from the collagen matrix. This results in resorbable scaffolds with improvements in stiffness and resilience over the original CSs. Intrafibrillar silicification of collagen does not adversely affect the biocompatibility of the silicified scaffolds. Release of silicic acid from SCSs induces apatite deposition, as well as improvements in osteogenic and angiogenic potentials over the original CSs. The SCSs also function as sustained release devices for regulation of stem/progenitor cell homing via the SDF-1/CXCR4 system. They represent a less costly and less complex alternative to contemporary cell seeding approaches and have the potential to provide new therapeutic options for in situ hard tissue regeneration. These highly desirable qualities justify the use of SCSs loaded with recombinant human SDF-1α for evaluation of hard tissue regeneration using orthotopic implantation models in immunodeficient small animals.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institute of Dental and Craniofacial Research grant R01-DE-015306-06 (principal investigator D.H.P), Georgia Health Sciences University Extramural Success Award and Innovative Pilot Project in Regenerative Medicine (principal investigator F.R.T.), National Nature Science Foundation of China grant 81130078 (principal investigator J.-H.C.), and National Key Basic Research Program of China grant 2012CB526704 (principal investigator J.-H.C.). Some of the materials employed in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through the National Center for Research Resources, U.S. National Institutes of Health, grant P40-RR-017447.
The authors thank Michelle Barnes for secretarial support and Frankie Chan (University of Hong Kong) for help in STEM-EDX analysis.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Ang
- angiopoetin
- AnV
- annexin V
- ALP
- alkaline phosphatase
- CS
- collagen scaffold
- EPC
- endothelial progenitor cell
- Etd-III
- ethidium homoodimer III
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- MSC
- mesenchymal stem cell
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- qRT-PCR
- quantitative reverse transcription-polymerase chain reaction
- RT-PCR
- reverse transcription-polymerase chain reaction
- SCS
- silicified collagen scaffold
- SDF-1α
- stromal cell-derived factor-1α
- STEM-EDX
- scanning transmission electron microscopy– energy-dispersive X-ray
- TEM
- transmission electron microscopy
- VEGF-A
- vascular endothelial growth factor-A
REFERENCES
- 1. Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D. S. (2011) Polymeric scaffolds in tissue engineering application: a review. Int. J. Polym. Sci. 2011, 290602 [Google Scholar]
- 2. Teo A. K., Vallier L. (2010) Emerging use of stem cells in regenerative medicine. Biochem. J. 428, 11–23 [DOI] [PubMed] [Google Scholar]
- 3. Mao J. J., Stosich M. S., Moioli E. K., Lee C. H., Fu S. Y., Bastian B., Eisig S. B., Zemnick C., Ascherman J., Wu J., Rohde C., Ahn J. (2010) Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng. B Rev. 16, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen F. M., Wu L. A., Zhang M., Zhang R., Sun H. H. (2011) Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 32, 3189–3209 [DOI] [PubMed] [Google Scholar]
- 5. Niu L. N., Jiao K., Qi Y. P., Yiu C. K., Ryou H., Arola D. D., Chen J. H., Breschi L., Pashley D. H., Tay F. R. (2011) Infiltration of silica inside fibrillar collagen. Angew. Chem. Int. Ed. 50, 11688–11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desimone M. F., Hélary C., Rietveld I. B., Bataille I., Mosser G., Giraud-Guille M. M., Livage J., Coradin T. (2010) Silica-collagen bionanocomposites as three-dimensional scaffolds for fibroblast immobilization. Acta Biomater. 6, 3998–4004 [DOI] [PubMed] [Google Scholar]
- 7. Heinemann S., Heinemann C., Jäger M., Neunzehn J., Wiesmann H. P., Hanke T. (2011) Effect of silica and hydroxyapatite mineralization on the mechanical properties and the biocompatibility of nanocomposite collagen scaffolds. ACS Appl. Mater. Interfaces 3, 4323–4331 [DOI] [PubMed] [Google Scholar]
- 8. Alt V., Kögelmaier D. V., Lips K. S., Witt V., Pacholke S., Heiss C., Kampschulte M., Heinemann S., Hanke T., Thormann U., Schnettler R., Langheinrich A. C. (2011) Assessment of angiogenesis in osseointegration of a silica-collagen biomaterial using 3D-nano-CT. Acta Biomater. 7, 3773–3779 [DOI] [PubMed] [Google Scholar]
- 9. Barralet J., Gbureck U., Habibovic P., Vorndran E., Gerard C., Doillon C. J. (2009) Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. A 15, 1601–1609 [DOI] [PubMed] [Google Scholar]
- 10. Lindhorst D., Tavassol F., von See C., Schumann P., Laschke M. W., Harder Y., Bormann K. H., Essig H., Kokemüller H., Kampmann A., Voss A., Mülhaupt R., Menger M. D., Gellrich N. C., Rücker M. (2010) Effects of VEGF loading on scaffold-confined vascularization. J. Biomed. Mater. Res. A 95, 783–792 [DOI] [PubMed] [Google Scholar]
- 11. Mieszawska A. J., Fourligas N., Georgakoudi I., Ouhib N. M., Belton D. J., Perry C. C., Kaplan D. L. (2010) Osteoinductive silk-silica composite biomaterials for bone regeneration. Biomaterials 31, 8902–8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhai W., Lu H., Chen L., Lin X., Huang Y., Dai K., Naoki K., Chen G., Chang J. (2012) Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 8, 341–349 [DOI] [PubMed] [Google Scholar]
- 13. Wiens M., Wang X., Schröder H. C., Kolb U., Schlossmacher U., Ushijima H., Müller W. E. (2010) The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 31, 7716–7725 [DOI] [PubMed] [Google Scholar]
- 14. Petit I., Jin D., Rafii S. (2007) The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 28, 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitaori T., Ito H., Schwarz E. M., Tsutsumi R., Yoshitomi H., Oishi S., Nakano M., Fujii N., Nagasawa T., Nakamura T. (2009) Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 60, 813–823 [DOI] [PubMed] [Google Scholar]
- 16. Higashino K., Viggeswarapu M., Bargouti M., Liu H., Titus L., Boden S. D. (2011) Stromal cell-derived factor-1 potentiates bone morphogenetic protein-2 induced bone formation. Tissue Eng. A 17, 523–530 [DOI] [PubMed] [Google Scholar]
- 17. Kim C. H., Broxmeyer H. E. (1998) In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood 91, 100–110 [PubMed] [Google Scholar]
- 18. Dove P. M., Han N., Wallace A. F., De Yoreo J. J. (2008) Kinetics of amorphous silica dissolution and the paradox of the silica polymorphs. Proc. Natl. Acad. Sci. U. S. A. 105, 9903–9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thieme S., Ryser M., Gentsch M., Navratiel K., Brenner S., Stiehler M., Rölfing J., Gelinsky M., Rösen-Wolff A. (2009) Stromal cell-derived factor-1alpha-directed chemoattraction of transiently CXCR4-overexpressing bone marrow stromal cells into functionalized three-dimensional biomimetic scaffolds. Tissue Eng. C Methods 15, 687–696 [DOI] [PubMed] [Google Scholar]
- 20. Ratanavaraporn J., Furuya H., Kohara H., Tabata Y. (2011) Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials 32, 2797–2811 [DOI] [PubMed] [Google Scholar]
- 21. McQuibban G. A., Butler G. S., Gong J. H., Bendall L., Power C., Clark-Lewis I., Overall C. M. (2001) Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 276, 43503–43508 [DOI] [PubMed] [Google Scholar]
- 22. Davis D. A., Singer K. E., De La Luz Sierra M., Narazaki M., Yang F., Fales H. M., Yarchoan R., Tosato G. (2005) Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation. Blood 105, 4561–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Icenhower J. P., Dove P. M. (2000) The dissolution kinetics of amorphous silica into sodium chloride solutions: Effects of temperature and ionic strength. Geochim Cosmochim Acta 64, 4193–4203 [Google Scholar]
- 24. Wang X., Bank R. A., TeKoppele J. M., Agrawal C. M. (2001) The role of collagen in determining bone mechanical properties. J. Orthop. Res. 19, 1021–1026 [DOI] [PubMed] [Google Scholar]
- 25. Eglin D., Maalheem S., Livage J., Coradin T. (2006) In vitro apatite forming ability of type I collagen hydrogels containing bioactive glass and silica sol-gel particles. J. Mater. Sci. Mater. Med. 17, 161–167 [DOI] [PubMed] [Google Scholar]
- 26. Hench L. L. (1991) Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 74, 1487–1510 [Google Scholar]
- 27. Shirtliff V. J., Hench L. L. (2003) Bioactive materials for tissue engineering, regeneration and repair. J. Mater. Sci. 38, 4697–4707 [Google Scholar]
- 28. Tsai K. S., Kao S. Y., Wang C. Y., Wang Y. J., Wang J. P., Hung S. C. (2010) Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J. Biomed. Mater. Res. A 94, 673–682 [DOI] [PubMed] [Google Scholar]
- 29. Kuraitis D., Hou C., Zhang Y., Vulesevic B., Sofrenovic T., McKee D., Sharif Z., Ruel M., Suuronen E. J. (2011) Ex vivo generation of a highly potent population of circulating angiogenic cells using a collagen matrix. J. Mol. Cell. Cardiol. 51, 187–197 [DOI] [PubMed] [Google Scholar]
- 30. Visconti R. P., Richardson C. D., Sato T. N. (2002) Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc. Natl. Acad. Sci. U. S. A. 99, 8219–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim H. D., Peyton S. R. (2012) Bio-inspired materials for parsing matrix physicochemical control of cell migration: a review. Integr. Biol. (Camb.) 4, 37–52 [DOI] [PubMed] [Google Scholar]
- 32. Deshane J., Chen S., Caballero S., Grochot-Przeczek A., Was H., Li Calzi S., Lach R., Hock T. D., Chen B., Hill-Kapturczak N., Siegal G. P., Dulak J., Jozkowicz A., Grant M. B., Agarwal A. (2007) Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J. Exp. Med. 204, 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kasten P., Vogel J., Luginbühl R., Niemeyer P., Tonak M., Lorenz H., Helbig L., Weiss S., Fellenberg J., Leo A., Simank H. G., Richter W. (2005) Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials 26, 5879–5889 [DOI] [PubMed] [Google Scholar]
- 34. Kato M., Namikawa T., Terai H., Hoshino M., Miyamoto S., Takaoka K. (2006) Ectopic bone formation in mice associated with a lactic acid/dioxanone/ethylene glycol copolymer-tricalcium phosphate composite with added recombinant human bone morphogenetic protein-2. Biomaterials 27, 3927–3933 [DOI] [PubMed] [Google Scholar]
- 35. Santos M. I., Reis R. L. (2010) Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol. Biosci. 10, 12–27 [DOI] [PubMed] [Google Scholar]
- 36. Collins M. J., Nielsen-Marsh C. M., Hiller J., Smith C. I., Roberts J. P., Prigodich R. V., Wess T. J., Csapò J., Millard A. J., Turner-Walker G. (2001) The survival of organic matter in bone: a review. Archaeometry 44, 384–394 [Google Scholar]
- 37. Shen W., Chen X., Chen J., Yin Z., Heng B. C., Chen W., Ouyang H. W. (2010) The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 31, 7239–7249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.