Abstract
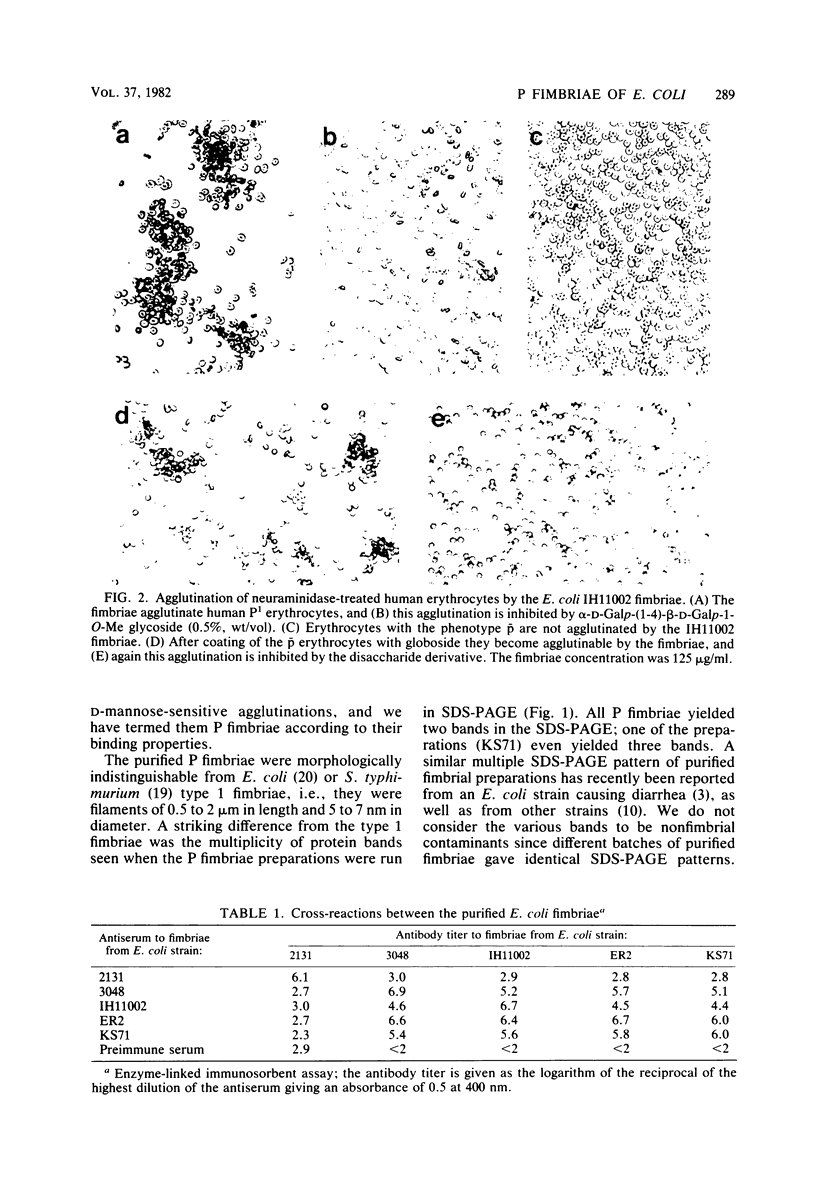
P-antigen-recognizing fimbriae (P fimbriae) from four pyelonephritogenic Escherichia coli strains and type 1 fimbriae from an E. coli strain and a Salmonella typhimurium strain were purified. The P fimbriae were morphologically similar to type 1 fimbriae. The purified P fimbriae agglutinated neuraminidase-treated human P1 and P2k erythrocytes but not p erythrocytes, which lack all P-blood group-specific glycosphingolipids. However, coating of neuraminidase-treated p erythrocytes with globoside rendered such erythrocytes agglutinable by the P fimbriae. The hemagglutinations were in all instances fully inhibited by the synthetic alpha-D-Galp-(1-4)-beta-D-Galp-1-O-Me glycoside. The binding specificity of the P fimbriae could also be demonstrated by using fimbriae coated onto latex particles and nontreated erythrocytes. It was thus concluded that the P fimbriae recognize and bind to the alpha-D-Galp-(1-4)-beta-D-Galp carbohydrate sequence occurring in the series of P-blood group antigen-specific glycosphingolipids. In contrast to both type 1 fimbriae, all four P fimbriae preparations showed multiple bands in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Antisera were raised in rabbits against the various E. coli fimbriae. In enzyme-linked immunosorbent assays each one of the antisera to the P fimbriae reacted to titers of log 4 to 7 with both the homologous and the heterologous P fimbriae, but not with the type 1 fimbriae of E. coli. In a reciprocal fashion, the antiserum to the type 1 fimbriae of one E. coli strain reacted only with the homologous type 1 but not with any of the P fimbriae preparations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigen-specific serotyping of Neisseria gonorrhoeae. I. Use of an enzyme-linked immunosorbent assay to quantitate pilus antigens on gonococci. J Infect Dis. 1978 Sep;138(3):319–315. doi: 10.1093/infdis/138.3.319. [DOI] [PubMed] [Google Scholar]
- Deneke C. F., Thorne G. M., Gorbach S. L. Attachment pili from enterotoxigenic Escherichia coli pathogenic for humans. Infect Immun. 1979 Oct;26(1):362–368. doi: 10.1128/iai.26.1.362-368.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Campbell I. Antigens of the type-1 fimbriae of salmonellae and other enterobacteria. J Med Microbiol. 1969 Nov 4;2(4):535–553. doi: 10.1099/00222615-2-4-535. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Eriksson B., Hanson L. A., Jodal U., Kaijser B., Janson G. L., Lindberg U., Olling S. Adhesion to normal human uroepithelial cells of Escherichia coli from children with various forms of urinary tract infection. J Pediatr. 1978 Sep;93(3):398–403. doi: 10.1016/s0022-3476(78)81145-7. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Clegg S., Pauley J. A. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Leffler H., Svanborg Edén C. Binding specificity of piliated strains of Escherichia coli and Salmonella typhimurium to epithelial cells, saccharomyces cerevisiae cells, and erythrocytes. Infect Immun. 1981 May;32(2):796–804. doi: 10.1128/iai.32.2.796-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Lounatmaa K., Ranta H., Kuusi N. Characterization of type 1 pili of Salmonella typhimurium LT2. J Bacteriol. 1980 Nov;144(2):800–805. doi: 10.1128/jb.144.2.800-805.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Winberg J., Hultberg H. Identification of a carbohydrate receptor recognized by uropathogenic Escherichia coli. Infection. 1980;8 (Suppl 3):288–293. doi: 10.1007/BF01639597. [DOI] [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Naiki M., Kundu S. K. Abnormalities in the glycosphingolipid content of human Pk and p erythrocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3263–3267. doi: 10.1073/pnas.73.9.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebli H. P., Roeder C., Tarcsay L. Reaction of lectins with human erythrocytes. III. Surface charge density and agglutination. Exp Cell Res. 1976 Mar 15;98(2):273–276. doi: 10.1016/0014-4827(76)90438-9. [DOI] [PubMed] [Google Scholar]
- Sedlock D. M., Bartus H. F., Zajac I., Actor P. Analysis of parameters affecting the hemagglutination activity of Escherichia coli possessing colonization factor antigens: improved medium for observing erythrocyte agglutination. J Clin Microbiol. 1981 Feb;13(2):301–308. doi: 10.1128/jcm.13.2.301-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]





