Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a debilitating side effect that occurs in many patients undergoing chemotherapy. It is often irreversible and frequently leads to early termination of treatment. In this study, we have identified two compounds, lithium and ibudilast, that when administered as a single prophylactic injection prior to paclitaxel treatment, prevent the development of CIPN in mice at the sensory-motor and cellular level. The prevention of neuropathy was not observed in paclitaxel-treated mice that were only prophylactically treated with a vehicle injection. The coadministration of lithium with paclitaxel also allows for administration of higher doses of paclitaxel (survival increases by 60%), protects against paclitaxel-induced cardiac abnormalities, and, notably, does not interfere with the antitumor effects of paclitaxel. Moreover, we have determined a mechanism by which CIPN develops and have discovered that lithium and ibudilast inhibit development of peripheral neuropathy by disrupting the interaction between paclitaxel, neuronal calcium sensor 1 (NCS-1), and the inositol 1,4,5-trisphosphate receptor (InsP3R) to prevent treatment-induced decreases in intracellular calcium signaling. This study shows that lithium and ibudilast are candidate therapeutics for the prevention of paclitaxel-induced neuropathy and could enable patients to tolerate more aggressive treatment regimens.—Mo, M., Erdelyi, I., Szigeti-Buck, K., Benbow, J. H., Ehrlich, B. E. Prevention of paclitaxel-induced peripheral neuropathy by lithium pretreatment.
Keywords: neuronal calcium sensor-1; inositol; 1,4,5 trisphosphate receptor; intracellular calcium signaling; ibudilast
Chemotherapy-induced peripheral neuropathy (CIPN) is an incapacitating side effect that results from the use of chemotherapeutic agents such as taxanes and vinca alkaloids (1). Approximately 1 million patients are at risk for developing chemotherapy-induced neuropathic pain (2), and it is estimated that >40% of patients undergoing chemotherapy treatment will eventually develop irreversible CIPN (1). This neuropathic pain, characterized by numbness, tingling, or shooting pain in the hands and feet, can begin weeks to months after initial treatment and is usually only partially reversible and, oftentimes, permanent (3).
Paclitaxel (Taxol) is a chemotherapeutic agent that is commonly used to treat solid tumors, such as breast, ovarian, and lung cancers (4, 5). Although paclitaxel is highly prescribed due to its efficacy and potency as a chemotherapeutic (6), it causes a high incidence of CIPN that can be dose limiting (7). Moreover, the sensory neuropathy that develops is often severe enough to lead to cessation of treatment (8). Despite ongoing efforts to understand how paclitaxel induces peripheral neuropathy, much is still unknown, and there is no standard treatment to prevent CIPN. Because of these factors, it is crucial to find compounds that can prevent paclitaxel-induced peripheral neuropathy (PIPN), as well as determine the mechanism by which it develops.
Previously, it was found that acute addition of paclitaxel to cultured neuroblastoma cells increases binding of neuronal calcium sensor 1 (NCS-1), a cytoplasmic calcium binding protein (9), to the inositol 1,4,5 trisphosphate receptor (InsP3R; ref. 10). This increased binding induces oscillatory changes in cytosolic calcium by increasing the open probability of the InsP3R and activating calpain, a calcium-dependent protease (10). Over time, prolonged exposure to paclitaxel leads to elevated calpain activity, eventual degradation of NCS-1 and other proteins by calpain (11), and the subsequent loss of intracellular calcium signaling due to decreased activity of the InsP3R (12). It was reported that symptoms of CIPN could be treated in mice by intrathecal administration of drugs that restore cytosolic calcium levels to normal (13, 14). As a result, we hypothesize that the initial increase in calcium due to interaction of paclitaxel with NCS-1, and the decrease in calcium signaling over time due to degradation of NCS-1, are contributing factors toward the development of PIPN.
Using a candidate approach, we identified two compounds, lithium and ibudilast, that bind to NCS-1. Lithium has also been demonstrated to inhibit sensitization of the InsP3R by NCS-1 (15), which is a critical component of the paclitaxel-dependent decrease in intracellular signaling (12). Recently, we have shown that the addition of these compounds to paclitaxel-treated neuroblastoma cells prevents the degradation of NCS-1 and the subsequent decrease in calcium signaling (16). Therefore, we propose that dysregulated calcium signaling, initiated on interaction of paclitaxel with NCS-1, leads to the eventual development of peripheral neuropathy. In addition, we hypothesize that lithium and ibudilast can prevent the development of PIPN by inhibiting the acute increase in calcium signaling (in addition to elevated calpain activity) and, later, the decrease in calcium signaling.
In this study, PIPN was inhibited in mice using a single prophylactic injection of lithium or ibudilast prior to treatment with paclitaxel. These compounds act by disrupting the interaction between paclitaxel, NCS-1, and the InsP3R. The chronic administration of paclitaxel activates the paclitaxel/NCS-1/InsP3R signaling pathway and leads to decreased intracellular calcium signaling. The disruption of the chemotherapy-induced calcium activation by paclitaxel allows signaling to remain at pretreatment levels. In addition, we show that pretreatment with lithium can increase the survival rate of mice receiving high-dose paclitaxel and can also protect against paclitaxel-induced cardiac abnormalities. Furthermore, in vivo and in vitro studies show that lithium does not interfere with the antitumor effect of paclitaxel, making it a promising therapeutic for the prevention of PIPN in patients.
MATERIALS AND METHODS
Animal use and treatment
This study was carried out in strict accordance with the recommendations in the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee at Yale University. All surgical procedures were performed under isoflurane anesthesia, and all efforts were made to minimize suffering.
Paclitaxel-induced model of peripheral neuropathy
Seven-week-old female C57BL/6 mice (Charles River, Wilmington, MA, USA) were separated into groups and were treated as described in Supplemental Fig. S1B. Depending on the treatment group, Li2CO3 (12.8 mg/kg in saline), ibudilast (10 mg/kg in 35% PEG-400), SB216763 (5 mg/kg in 50% DMSO), or saline was administered intraperitoneally an hour prior to injection of vehicle (20% 50:50 Cremophor EL:ethanol, 80% saline), or paclitaxel (4.5 mg/kg in 20% 50:50 Cremophor EL:ethanol, 80% saline to induce PIPN, or 25 mg/kg for xenograft studies). The concentration of high-dose paclitaxel used was 60 mg/kg. All compounds except for SB216763 (Tocris Biosciences, Minneapolis, MN, USA) were obtained from Sigma Aldrich (St. Louis, MO, USA). PIPN model was based on several studies (11, 17, 18). Lithium dosage was within therapeutic levels, as described previously (19, 20). Ibudilast dosage was established in previous mouse models (21). SB216763 dosage was used as described previously (22). Dosage of paclitaxel used in xenograft model was based on several studies (23, 24).
Sensory-motor, thermal algesia, and cardiac function tests
Mice were subjected to testing on a rotarod apparatus (IITC Life Sciences, Woodland Hills, CA, USA). Baseline was established prior to treatment, and 3 additional sessions were run following treatment. Habituation sessions were performed as described previously (11). Thermal algesia was tested in mice using a hot-plate apparatus (IITC Life Sciences). Mice were placed in a container with the hot-plate surface maintained at 52°C. Thermal latency was defined as the time in seconds elapsed from the time the mouse was placed on the hot-plate surface until the time the mouse either licked, shook, or lifted its right hind paw or jumped onto the Plexiglas wall around the metal hot-plate surface. For the cardiac function tests, mice were pretreated with lithium or saline and were briefly anesthetized and imaged 1 h after injection with paclitaxel or vehicle. Echocardiograms were obtained on lightly anesthetized mice (isoflurane inhalation via nosecone) by using a 40-MHz transducer and a Vevo 2100 console (VisualSonics, Toronto, ON, Canada). Zoomed 2D images were used to determine a short axis plane at the level of the papillary muscles and then M-mode was obtained at this level. Measurements were obtained using the Vevo 2100 analysis software (VisualSonics, Toronto, ON, Canada). Left ventricular (LV; minor axis) percentage fractional shortening (LV%FS), a measure of systolic function, was calculated as LVFS = [(LVIDd − LVIDs)/LVIDd] × 100.
Calcium imaging
L3, L4, and L5 dorsal root ganglia (DRGs) were isolated from mice and were cultured as described by Boehmerle et al. (12) for 18 h. Subsequently, DRGs were incubated for 1 h at 37°C in HEPES medium containing 5 μM Fluo-4/AM (Molecular Probes-Invitrogen, Carlsbad, CA, USA) with 0.1% Pluronic F-127 (Molecular Probes-Invitrogen). The HEPES medium contained 130 mM NaCl, 4.7 mM KCl, 1 mM MgSO4, 1.2 mM KH2PO4, 1.3 mM CaCl2, 20 mM HEPES, and 5 mM glucose (pH 7.4). A Zeiss LSM 710 Duo NLO microscope equipped with a Plan NeoFluar ×10 objective (Carl Zeiss, Oberkochen, Germany)was used to image the DRGs. After incubation in Fluo-4/AM dye, DRGs were washed once with HEPES medium, and the imaging chambers containing the DRGs were replaced with fresh medium. For the imaging experiment, DRGs were stimulated with 50 μM ATP after establishing a 100-s baseline. DRGs were then stimulated with 10 μM ionomycin and 1 M KCl, and those that did not respond were excluded from evaluation. Calcium-induced fluorescence intensity ratio F/F0 was plotted as a function of time, with F0 as an average of the first 10 points of the baseline.
Immunoblotting and Western blot analysis
DRGs were lysed in Cytobuster (Calbiochem, Billerica, MA, USA), and immunoblotting was performed as described previously (15). Antibodies used were NCS-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-actin (Abcam, Cambridge, MA, USA), cyclin D3 (Cell Signaling, Danvers, MA, USA), and anti-InsP3R type I (25). Blots were quantified by scanning densitometry using Un-Scan-It (Silk Scientific, Orem, UT, USA).
Calpain activity and cell viability assays
DRGs were lysed in Cytobuster and used for the calpain-Glo protease assay (Promega, Madison, WI, USA), according to manufacturer's protocol. Activity was measured with luminescence, and the relative luminescence was averaged over 10 s, background subtracted, and normalized to the amount of protein in the lysate. NCI-H1975 cells [American Type Culture Collection (ATCC), Manassas, VA, USA] were plated at an equal density in a 96-well plate and treated with various compounds, as described previously. Cell viability was assessed using the cell titer 96 nonradioactive cell proliferation assay kit (Promega, Madison, WI, USA) according to the manufacturer's protocol. Cells were pretreated with Li2CO3, followed by treatment with increasing concentrations of paclitaxel. Absorbance was measured at 490 nm, and background absorbance was subtracted.
Non-small-cell lung cancer xenograft model
H1975 cells (ATCC) were cultured and resuspended in Matrigel (BD Biosciences, San Diego, CA, USA) at a concentration of 1 × 106 cells/200 μl. Twenty NCr nude mice (Taconic, Hudson, NY, USA) were inoculated subcutaneously with the mixture. Approximately 2 wk later, mice bearing tumors with a mean volume of 500 mm3 were randomized to receive vehicle-saline, vehicle-lithium, paclitaxel-saline, or paclitaxel-lithium treatments, as described in Fig. 1. Tumor size and weight of the mouse were measured every other day. Tumor volume (cm3) was calculated using the formula 0.5 × a2 × b, where a is the smallest tumor diameter (cm) and b is the largest. Following treatment, all mice were euthanized, and tumors were removed for histopathological examination.
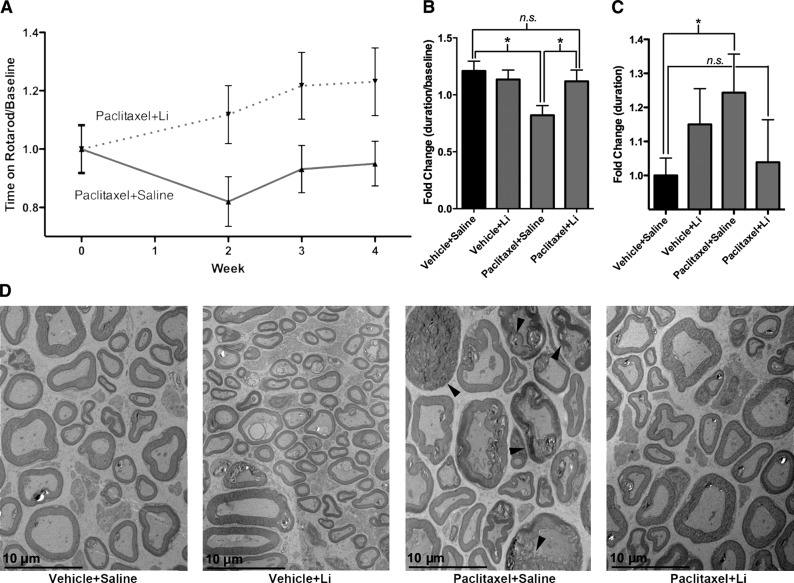
Figure 1.
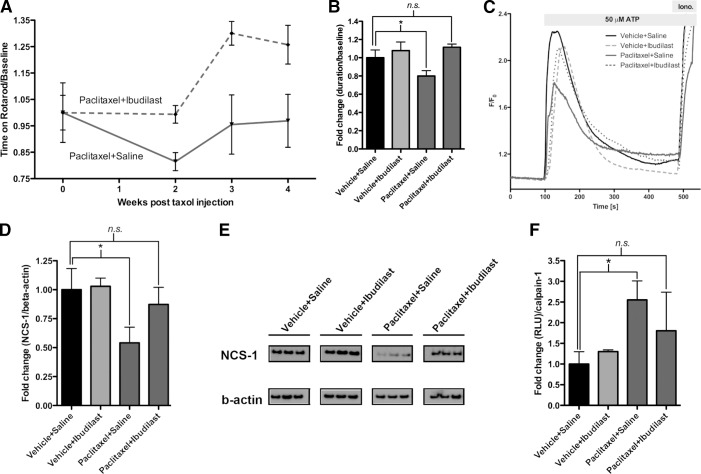
Pretreatment with lithium prevents mice from developing paclitaxel-induced neuropathy. A) Mice pretreated with lithium (paclitaxel-lithium-treated mice; n=10) do not develop PIPN at the sensory-motor level. However, mice that are only treated with paclitaxel (paclitaxel-saline-treated mice; n=10) develop PIPN. Symptoms of neuropathy begin at wk 2 and persist until mice are euthanized at wk 4. Data were normalized to baseline rotarod measurements. B) Week 2 data normalized to baseline rotarod measurements showed that the paclitaxel-saline-treated mice (n=10) experienced a significant drop in time spent on the rotarod compared to the vehicle-saline-treated mice, indicating development of neuropathy. There was no significant difference between paclitaxel-lithium-, vehicle-saline, or vehicle-lithium-treated mice (n=10/group). Data are expressed as (time on rotarod/baseline rotarod time) ± se. *P < 0.05. C) Hot-plate data from wk 2 show that the paclitaxel-saline-treated mice (n=9) stay on the hot plate for a longer duration when compared to the vehicle-saline (n=10)-, vehicle-lithium (n=10)-, or paclitaxel-lithium (n=10)-treated mice. This indicates that the paclitaxel-saline-treated mice develop neuropathy. Data are expressed as (duration on hot plate) ± se. *P < 0.05. D) TEM shows that the paclitaxel-saline-treated mice are experiencing macrophage-mediated demyelination. In some cases, axons are completely stripped of their myelin sheaths and surrounded by the cytoplasm of debris-filled phagocytes, as indicated by the arrows. Occasional fibers with myelin splitting and evidence of myelinated fiber loss are also present. This is not observed in the vehicle-saline-, vehicle-lithium-, or paclitaxel-lithium-treated mice.
Histopathology and transmission electron microscopy (TEM)
Tissues for histopathology were fixed in Bouin's fixative (Ricca Chemical, Arlington, TX, USA) and embedded in paraffin for histopathologic examination. Paraffin sections of the sciatic nerve and tumors were stained with hematoxylin and eosin (H&E), neurofilament, and Luxol fast blue for histopathologic examination by a certified pathologist (I.E.). Sciatic nerve was dissected and fixed in 4% paraformaldehyde with 0.5% glutaraldehyde before being embedded into blocks and sectioned for TEM analysis.
Statistical analysis
Data are expressed as means±se. Statistical analysis between 2 groups was performed using Student's t test for unpaired data (GraphPad Prism; GraphPad, San Diego, CA, USA); values of P < 0.05 were considered statistically significant.
RESULTS
Pretreatment with lithium prevents paclitaxel-induced neuropathy
To determine whether lithium protects against PIPN, we established a mouse model of PIPN using a commonly used model of neuropathy (ref. 26 and Supplemental Fig. S1A, B). Mice were given a single injection of lithium (or saline as a control) 1 h prior to each injection of paclitaxel or vehicle, for a total of 4 injections over 7 d. This protocol results in 4 groups: 2 control groups, vehicle-saline and vehicle-lithium; and 2 treatment groups, paclitaxel-saline and paclitaxel-lithium. Administration of lithium prior to injection with 4.5 mg/kg paclitaxel prevented mice from developing neuropathy when monitored with a sensory-motor test (performance on a rotarod) or a thermal algesia test (performance on a hot plate) or when sciatic nerve samples were examined by histopathology and TEM. Sensory-motor testing of the paclitaxel-saline-treated mice 1 wk after the last paclitaxel or vehicle injection showed that the mice developed neuropathy that persisted until the 4-wk time point, when they were euthanized (Fig. 1A). The paclitaxel-lithium-treated mice, however, did not develop neuropathy; their performance on the rotarod was indistinguishable from mice in the two control groups (Fig. 1B). At the 2-wk time point, paclitaxel-saline-treated mice were unable to remain on the rotarod as long as the mice in the control groups; the duration on the rotarod for the paclitaxel-saline-treated mice dropped to 67 ± 6% compared to vehicle-saline-treated mice, whereas the paclitaxel-lithium-treated mice only dropped by 5 ± 9% (Fig. 1B). The controls, vehicle-saline- and vehicle-lithium-treated mice, were not significantly different (Fig. 1B). Thermal algesia testing at the 2-wk time point demonstrated that the paclitaxel-saline-treated mice stayed on the hot plate 27 ± 12% longer than did the vehicle-saline-treated mice (Fig. 1C). The paclitaxel-lithium-treated mice only stayed on the hot plate 5 ± 14% longer than did the vehicle-saline-treated mice. The differences between the paclitaxel-lithium-, vehicle-saline-, and vehicle-lithium-treated mice were not statistically different. These results demonstrate that the paclitaxel-saline-treated mice develop neuropathy and that lithium prevents the development of PIPN.
Histopathology of the sciatic nerve by H&E staining, Luxol fast blue staining for myelin, and neurofilament staining for axons showed no obvious degenerative changes, such as axonal spheroids and myelin fragmentation.
However, an overall mild increase in axonal swelling, with slightly decreased neurofilament staining and myelin vacuolization, was observed in the paclitaxel-saline-treated mice (Supplemental Fig. S2A–C). Damage to the axons and myelin sheaths was clearly visible by TEM in the paclitaxel-saline-treated mice. Axonal swelling and demyelination were present (Fig. 1D), and in some cases, axons were completely stripped of their myelin sheaths and were surrounded by the cytoplasm of debris-filled phagocytes (Fig. 1D, arrows). The appearance of the damaged myelinated axons in the paclitaxel-saline-treated mice suggested classic Wallerian degeneration, in which destruction of the myelin sheath and digestion of the myelin by macrophage infiltration occurs. This type of degeneration was not observed in the vehicle-saline-, vehicle-lithium-, or paclitaxel-lithium-treated mice. The protection of the myelin sheaths in the paclitaxel-lithium-treated mice may also occur because lithium has been shown to enhance remyelination of peripheral nerves (27). In addition, examination of the C fibers, the unmyelinated axons, revealed that there was degeneration of these fibers in the paclitaxel-saline-treated mice. The vehicle-saline-, vehicle-lithium-, and paclitaxel-lithium-treated mice all had healthy appearing and distinct Remak bundle structures (clusters of C fibers), whereas these characteristic structures were absent or degenerated in the paclitaxel-saline-treated mice (Supplemental Fig. S3). These results confirm that paclitaxel is damaging peripheral nerves and show that lithium protects against paclitaxel-induced nerve damage.
Lithium prevents the decrease in calcium signaling observed in peripheral neurons of paclitaxel-treated mice
Dysregulation of calcium signaling has been linked to the development of peripheral neuropathy. In isolated DRGs, paclitaxel has been shown to diminish InsP3R-dependent signaling over time via calpain-mediated degradation of NCS-1 (12). The diminished calcium signaling observed in paclitaxel-treated cells can be prevented by pretreatment of neuroblastoma cells with lithium (16). However, it was necessary to test in vivo if paclitaxel treatment alters calcium signaling in peripheral neurons.
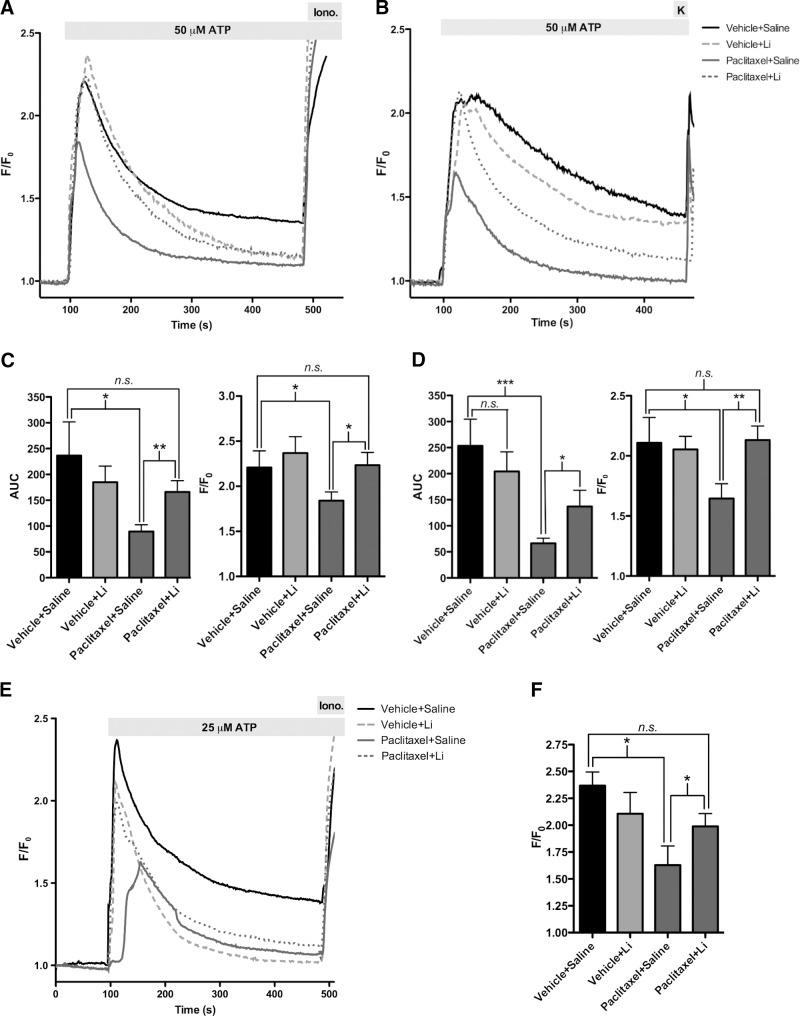
To determine whether the effect of paclitaxel on the peripheral nerve results from dysregulation of calcium, we examined intracellular calcium signaling in DRGs that were isolated from mice at the 2- and 4-wk time points. At the 2-wk time point, total calcium release from the DRGs of the paclitaxel-saline-treated group was only 38 ± 6% of the total calcium release of the DRGs from the vehicle-saline-treated mice (Fig. 2A, C). Total calcium release from DRGs isolated from the paclitaxel-lithium-treated mice, on the other hand, was 70 ± 9% of the total calcium release of DRGs isolated from the vehicle-saline-treated mice. Peak calcium release was 20 ± 4% less in paclitaxel-saline-treated mice compared to the vehicle-saline-treated mice; the differences between the vehicle-saline, vehicle-lithium, and paclitaxel-lithium groups were not significantly different (Fig. 2A, C). These comparisons indicate that lithium maintains pretreatment levels of calcium signaling.
Figure 2.
Pretreatment with lithium prevents mice from experiencing paclitaxel-mediated decrease in phosphoinositide calcium signaling. A) Calcium imaging of DRGs from paclitaxel-saline-treated mice showed a diminished response to 50 μM ATP 1 wk after paclitaxel treatment. n = 18-20 cells/group; 4 mice/group. B) Calcium imaging of DRGs from paclitaxel-saline-treated mice still show a diminished response to 50 μM ATP 3 wk after paclitaxel treatment. n = 20 cells/group; 4 mice/group. C) Total calcium release was diminished in the paclitaxel-saline-treated mice compared to vehicle-saline-treated mice 1 wk after paclitaxel treatment. Difference between vehicle-saline-, vehicle-lithium-, and paclitaxel-lithium-treated mice was not significant. Peak calcium release was also diminished in the paclitaxel-saline-treated mice. n = 20 cells/group. D) Total calcium release was diminished in the paclitaxel-saline-treated mice compared to the vehicle-saline-treated mice 3 wk after paclitaxel treatment. Difference between vehicle-saline-, vehicle-lithium-, and paclitaxel-lithium-treated mice were not significant. Peak calcium release was also diminished in the paclitaxel-saline-treated mice. n = 18–20 cells/group. E) Calcium imaging of DRGs from paclitaxel-saline-treated mice showed a diminished response to 25 μM ATP 18 h after the first paclitaxel or vehicle injection. n = 10–20 cells/group. F) Peak calcium release was diminished in the paclitaxel-saline-treated mice when compared to the vehicle-saline-treated mice 18 h after first paclitaxel or vehicle injection. n = 10–20 cells/group. Bar graph data are expressed as means ± se. *P < 0.05, **P < 0.01, ***P < 0.001.
Calcium signaling in the DRGs isolated at the 4-wk time point was similar to that at the 2-wk time point (Fig. 2B). DRGs isolated from the paclitaxel-saline-treated group showed a decrease of 66 ± 7% of the total calcium release when compared to the DRGs from the vehicle-saline-treated mice (Fig. 2B, D). In contrast, DRGs isolated from the paclitaxel-lithium-treated mice showed a partial recovery of 20 ± 12% of the total calcium release when compared to the paclitaxel-saline-treated mice. Peak calcium release as compared to the vehicle-saline group was 20 ± 6% less in the paclitaxel-saline-treated mice; the differences between the vehicle-saline, vehicle-lithium, and paclitaxel-lithium groups were not significantly different (Fig. 2B, D). These comparisons indicate that lithium pretreatment is still protective, even weeks after the initial treatment of mice with paclitaxel.
To determine whether paclitaxel-mediated calcium decrease occurs at a time point earlier than 2 wk and whether lithium is able to rescue this decrease, we examined intracellular calcium signaling in DRGs that were isolated from mice 18 h after their first injection with paclitaxel or vehicle. DRGs isolated from the paclitaxel-saline-treated group had a 54 ± 13% decrease in peak calcium release when compared to the vehicle-saline-treated mice; the differences between the vehicle-saline and vehicle-lithium groups were not statistically different (Fig. 2E, F). On the other hand, DRGs isolated from the paclitaxel-lithium-treated mice was 72 ± 8% of the peak calcium release of DRGs isolated from the vehicle-saline-treated mice (Fig. 2E, F). A similar trend among the treatment groups was observed for the total calcium release. These comparisons suggest that the paclitaxel-mediated decrease in calcium release occurs very soon after the first paclitaxel injection and persists up to 3 wk after the last paclitaxel injection. In addition, the data indicate that lithium is acting as early as several hours after the first paclitaxel injection to prevent the paclitaxel-mediated decrease in calcium release; these effects then persist up to 3 wk after the last lithium and paclitaxel injections.
Lithium prevents calpain-mediated degradation of NCS-1 by paclitaxel
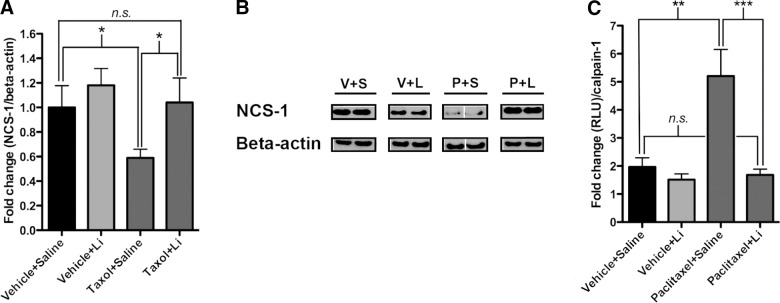
We previously reported that degradation of NCS-1 in DRGs occurs after treatment of mice with paclitaxel (12), and cell-based studies have shown that NCS-1 degradation is calpain mediated (12, 28). Using our model of peripheral neuropathy, we hypothesized that the activation of calpain and the subsequent degradation of NCS-1 over the course of the 4-wk study lead to the diminished calcium signaling observed and thus the development of neuropathy. Immunoblot analysis of NCS-1 protein levels in DRGs isolated from paclitaxel-saline-treated mice at wk 4 of the study, 3 wk after the last paclitaxel injection, showed a 59 ± 8% decrease in NCS-1 levels compared to the vehicle-saline-treated mice (Fig. 3A, B and Supplemental Fig. S4B). No decrease was observed in the paclitaxel-lithium-treated mice. Unlike NCS-1, levels of InsP3R type I (InsP3RI) were unchanged among groups (Supplemental Fig. S4A). As expected, levels of NCS-1 were not significantly different in the cerebral cortex due to low permeability of paclitaxel through the blood-brain barrier (Supplemental Fig. S4A).
Figure 3.
Pretreatment with lithium prevents mice from calpain-mediated degradation of NCS-1. A) Levels of NCS-1 were decreased in paclitaxel-saline-treated mice compared to vehicle-saline-treated mice. No such decrease in NCS-1 levels was observed in the paclitaxel-lithium-treated mice when compared to vehicle-saline-treated mice. Difference between vehicle-saline-, vehicle-lithium-, and paclitaxel-lithium-treated mice was not significant. n = 8 mice/group. B) Representative lanes from Western blot. Each lane represents an individual mouse. V+S, vehicle-saline; V+L, vehicle-lithium; P+S, paclitaxel-saline; P+L, paclitaxel-lithium. Note that the gap in the paclitaxel-saline-treated group indicates that the membrane was cut. C) Individual calpain activity was altered significantly in paclitaxel-saline-treated mice when compared to vehicle-saline-treated mice at the 4-wk time point. No significant difference was observed between vehicle-saline, vehicle-lithium-, or paclitaxel-lithium-treated mice. n = 8 mice/group. Bar graph data are expressed as means ± se. *P < 0.05, **P < 0.01.
Because NCS-1 levels in the paclitaxel-lithium-treated mice were comparable to control levels, it should follow that calpain activity in the paclitaxel-lithium-treated mice would be unchanged relative to control mice. Calpain activity assayed using DRG lysates from the paclitaxel-saline mice showed a 165 ± 51% increase in calpain activity compared to the vehicle-saline-treated mice, and a 201 ± 67% increase compared to the vehicle-lithium-treated mice (Fig. 3C). Activity of calpain in the paclitaxel-lithium-treated mice was not significantly different from the vehicle-saline- or vehicle-lithium-treated mice; calpain activity was actually decreased by 14 ± 10% compared to the vehicle-saline-treated mice (Fig. 3C).
Maintenance of pretreatment levels of calcium signaling by lithium does not occur through the glycogen synthase kinsase-3 (GSK-3) pathway
Lithium affects several cellular pathways in addition to the paclitaxel/NCS-1/InsP3R pathway. One of the predominant proteins whose activity is affected by lithium is GSK-3. GSK-3 is a serine/threonine protein kinase that is the only known protein kinase inhibited at therapeutically tolerated concentrations of lithium (0.5–2 mM; ref. 29). Inhibition of GSK-3 by lithium activates the Wnt signaling pathway by preventing phosphorylation of β-catenin and its subsequent degradation. It is possible that the maintenance of pretreatment levels of calcium signaling by lithium could be occurring through the GSK-3 pathway, as GSK-3 inhibition can enhance voltage-dependent calcium channel gating and can increase translation of the InsP3R (30).
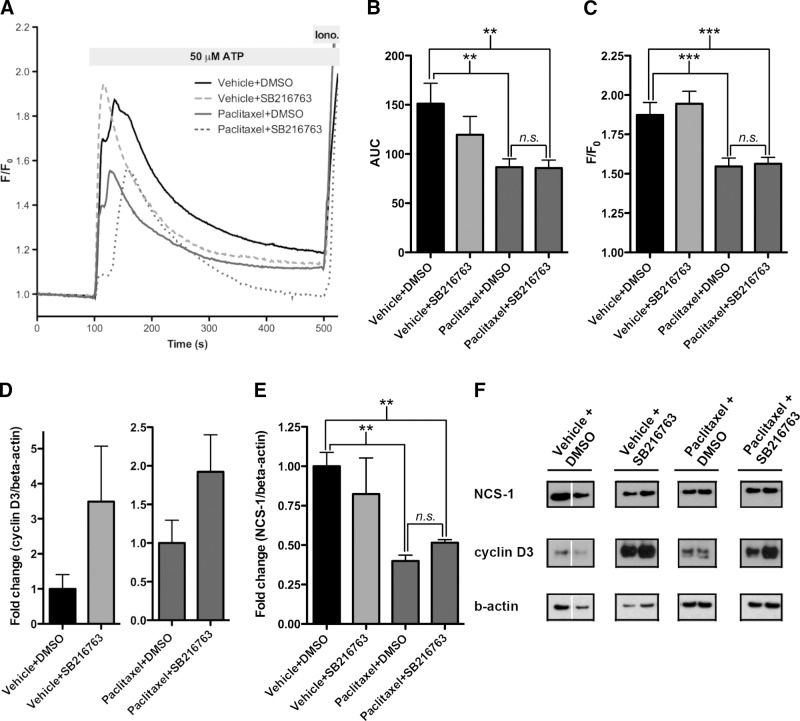
To test whether lithium prevents paclitaxel-induced diminished calcium release by inhibition of GSK-3, we injected a GSK-3 inhibitor (SB216763) into mice prior to treatment with paclitaxel. The dose of SB216763 used was within the therapeutic range used in mice to treat tumors (22, 31). DRGs were isolated from mice 6 h after injection with paclitaxel or vehicle and were subjected to examination of calcium transients and Western blot analysis. Unlike the paclitaxel-lithium-treated mice, calcium signaling remained attenuated in the paclitaxel-SB216763-treated mice when compared to the two control groups, vehicle-DMSO- and vehicle-SB216763-treated mice (Fig. 4A). Total calcium release decreased by 43 ± 6 and 43 ± 5% in DRGs isolated from paclitaxel-DMSO- and paclitaxel-SB216763-treated mice, respectively (Fig. 4B). Vehicle-DMSO and vehicle-SB216763 groups did not differ significantly from one another. In addition to the decrease in total calcium release observed in the paclitaxel-DMSO- and paclitaxel-SB216763-treated mice, peak calcium release was decreased by 19 ± 3 and 20 ± 2% in DRGs isolated from paclitaxel-DMSO- and paclitaxel-SB216763-treated mice, respectively (Fig. 4C). As with the total calcium release, no significant differences were observed in the vehicle-DMSO- or vehicle-SB216763-treated mice.
Figure 4.
Lithium prevents PIPN via the paclitaxel/NCS-1/InsP3R pathway and not through inhibition of GSK-3. A) Calcium imaging of DRGs from paclitaxel-DMSO- and paclitaxel-SB216763-treated mice showed a diminished response to 50 μM ATP 6 h after paclitaxel treatment. n = 60–70 cells/group; 3 mice/group. B) Total calcium release was diminished in both the paclitaxel-DMSO- and paclitaxel-SB216763-treated mice when compared to vehicle-saline-treated mice. Difference between vehicle-DMSO- and vehicle-SB216763-treated mice was not significant. n = 60–70 cells/group; 3 mice/group. C) Peak calcium release was diminished in the paclitaxel-DMSO- and paclitaxel-SB216763-treated mice. Vehicle-DMSO- and vehicle-SB216763-treated mice were not significantly different. n = 60–70 cells/group, 3 mice/group. D) Cyclin-D3 levels were elevated in vehicle-SB216763- and paclitaxel-SB216763-treated mice due to inhibition of GSK-3. No such elevation was observed in the vehicle-DMSO- or paclitaxel-DMSO-treated mice. n = 3 mice/group. E) Levels of NCS-1 were decreased in paclitaxel-DMSO- and paclitaxel-SB216763-treated mice compared to vehicle-DMSO-treated mice. Difference between vehicle-DMSO- and vehicle-SB216763-treated mice was not significant. n = 3 mice/group. F) Representative lanes from Western blot. Each lane represents an individual mouse. Note that the gap in the vehicle+DMSO-treated group indicates that the membrane was cut. Bar graph data are expressed as means ± se. **P < 0.01, ***P < 0.001.
To confirm that SB216763 was working to inhibit GSK-3, immunoblot analysis of cyclin-D3 levels was examined because cyclin-D3 is protected by GSK-3 inhibition (32). Levels of cyclin-D3 were decreased in the vehicle-DMSO- and paclitaxel-DMSO-treated mice, but not in the vehicle-SB216763- and paclitaxel-SB216763-treated mice (Fig. 4D, F and Supplemental Fig. S4D). Adding further support that lithium inhibition of GSK-3 does not interfere with the NCS-1/InsP3R/calcium pathway, immunoblot analysis of NCS-1 levels showed that levels of NCS-1 were decreased by 60 ± 4 and 49 ± 2% in paclitaxel-DMSO- and paclitaxel-SB216763-treated mice, respectively (Fig. 4E, F and Supplemental Fig. S4D). These results indicate that GSK-3 inhibition does not protect NCS-1 against degradation.
Treatment with ibudilast prevents paclitaxel-induced neuropathy in a manner similar to that of lithium
Ibudilast (21) was found to bind to NCS-1 and protect against the degradation of NCS-1 and decrease in calcium signaling observed in neuroblastoma cells treated with paclitaxel (16). Because ibudilast can interact with the paclitaxel/NCS-1/InsP3R pathway, it was tested as a candidate for the prevention of PIPN. Not only would this be an additional compound that could be used to prevent PIPN, but it would add further support to the suggestion that lithium acts primarily through the paclitaxel/NCS-1/InsP3R pathway and not through inhibition of GSK-3.
Pretreatment of mice with ibudilast prior to injection with 4.5 mg/kg paclitaxel prevented mice from developing neuropathy. Sensory-motor testing of the paclitaxel-saline-treated mice 1 wk after their last paclitaxel injection showed that mice developed neuropathy that persisted until the 4-wk time point, when the mice were euthanized (Fig. 5A). The paclitaxel-ibudilast-treated mice, however, did not develop neuropathy at the sensory-motor level. Time spent on the rotarod by the paclitaxel-saline-treated mice decreased by 18 ± 10% at the 3-wk time point (Fig. 5B) and 23 ± 8% at the 4-wk time point, when compared to vehicle-saline-treated mice. Time spent on the rotarod by the paclitaxel-ibudilast-treated mice actually increased by 12 ± 4% at the 3-wk time point and was not significantly different at the 4-wk time point, when compared to vehicle-saline-treated mice. Vehicle-ibudilast-treated mice were not significantly different from vehicle-saline-treated mice at the 3- or 4-wk time points.
Figure 5.
Ibudilast prevents PIPN through a pathway similar to that of lithium. A) Mice pretreated with ibudilast (paclitaxel-ibudilast-treated mice; n=5) do not develop PIPN at the sensory-motor level. However, mice that are only treated with paclitaxel (paclitaxel-saline-treated mice; n=5), develop PIPN. Symptoms of neuropathy begin at wk 2 and persist until mice are euthanized at wk 4. Data were normalized to baseline rotarod measurements. B) Week 2 data normalized to baseline rotarod measurements showed that the paclitaxel-saline-treated mice experienced a significant drop in time spent on the rotarod compared to the vehicle-saline-treated mice, indicating development of neuropathy. There was no significant difference between paclitaxel-ibudilast-, vehicle-saline-, or vehicle-ibudilast-treated mice. Data are expressed as (time on rotarod/baseline rotarod time) ± se. n =5/group. C) Calcium imaging of DRGs from paclitaxel-saline-treated mice showed a diminished response to 50 μM ATP 3 wk after paclitaxel treatment. No such decrease was observed in the paclitaxel-ibudilast-treated mice. n = 39–40 cells/group; 3–4 mice/group. D) Levels of NCS-1 were decreased in paclitaxel-saline-treated mice compared to vehicle-saline-treated mice. No such decrease was observed in the paclitaxel-ibudilast-treated mice. Difference between vehicle-saline, vehicle-ibudilast, and paclitaxel-ibudilast groups was not significant. n = 3–4 mice/group. E) Representative lanes from Western blot. Each lane represents an individual mouse. F) Calpain activity was elevated significantly in paclitaxel-saline-treated mice compared to vehicle-saline-treated mice. No significant difference was observed between vehicle-saline-, vehicle-ibudilast-, or paclitaxel-ibudilast-treated mice. n = 3 mice/group. Data are expressed as means ± se. *P < 0.05, **P < 0.01;
Maintenance of pretreatment levels of calcium signaling by lithium, observed at the 4-wk time point in the lithium-treated mice, also occurred in the ibudilast-treated mice. Total calcium release from DRGs isolated from the paclitaxel-saline-treated group decreased by 21 ± 8% compared to vehicle-saline-treated mice (Fig. 5C). DRGs isolated from the paclitaxel-ibudilast-treated mice had a nonsignificant decrease of 6 ± 13% when compared to the vehicle-saline-treated mice. Peak calcium release was diminished by 21 ± 5% in the paclitaxel-saline-treated mice compared to vehicle-saline-treated mice; the difference between the vehicle-saline, vehicle-ibudilast, and paclitaxel-ibudilast groups were not significantly different (Fig. 5C).
As observed in the lithium-treated group, NCS-1 protein levels in DRGs isolated from paclitaxel-saline-treated mice 4 wk after the last paclitaxel injection showed a 46 ± 14% decrease when compared to vehicle-saline-treated mice (Fig. 5D, E and Supplemental Fig. S4C). A nonsignificant decrease of only 13 ± 15% was observed in the paclitaxel-ibudilast-treated mice when compared to vehicle-saline-treated mice. These results indicate that ibudilast is able to prevent degradation of NCS-1.
Following the observations from the lithium-treated mice, levels of NCS-1 in the ibudilast-treated mice were likely to be protected due to the prevention of increased calpain activity. As expected, calpain activity increased significantly by 155 ± 80% in the paclitaxel-saline-treated mice compared to vehicle-saline-treated mice (Fig. 5F). Paclitaxel-ibudilast-treated mice only had a nonsignificant increase of 81 ± 161% compared to vehicle-saline-treated mice; indicating that ibudilast protects against elevated calpain activity (Fig. 5F).
Lithium does not interfere with the antitumor effect of paclitaxel
As indicated by the data presented here, lithium is promising in its ability to prevent the development of PIPN. However, if lithium is to be used clinically to prevent PIPN, it is critical to ensure that lithium does not interfere with the antitumor effect of paclitaxel. To confirm this, we conducted in vitro studies using cell death assays and in vivo studies using a xenograft tumor mouse model.
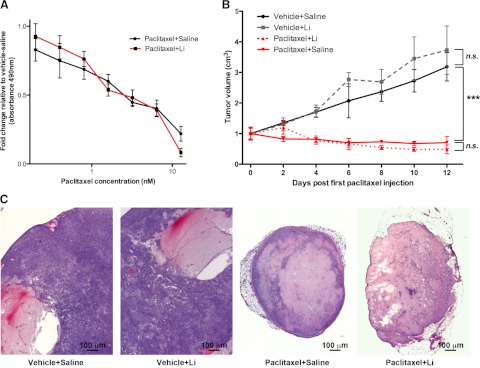
In vitro studies showed that both saline-pretreated and lithium-pretreated H1975 (human non-small-cell lung cancer) cells were equally susceptible to cell death, as indicated by decreased absorbance in a cell viability assay when cells were subjected to increasing concentrations of paclitaxel (Fig. 6A). On the basis of our in vitro data showing that lithium does not affect the antitumor effect of paclitaxel, we next conducted in vivo studies by injecting H1975 cells into nude mice. Tumors were visible 2 wk later, and mice were pretreated with lithium or saline, followed by an injection with vehicle or paclitaxel, as described in Supplemental Fig. S1B. The tumor growth curves show that paclitaxel was able to prevent growth of the tumors in the paclitaxel-saline- and paclitaxel-lithium-treated mice (Fig. 6B). Histology of the tumors showed that the vehicle-saline- and vehicle-lithium-treated mice had abundant growth of tumor cells around the cysts, whereas the paclitaxel-saline- and paclitaxel-lithium-treated mice had an almost complete recession of tumor growth. No difference was observed between the paclitaxel-saline- and paclitaxel-lithium-treated mice (Fig. 6C). These results show that lithium does not affect the antitumor ability of paclitaxel.
Figure 6.
Lithium does not interfere with the antitumor effect of paclitaxel. A) Cell viability assay of H1975 cells using increasing concentrations of paclitaxel did not show a significant difference between saline-treated cells or lithium-treated cells. Data are expressed as means ± se; n = 6/data point. B) H1975 NSCLC cells were injected into nude mice. Tumor growth was measured after 2 wk and normalized to baseline tumor measurements. No significant difference in tumor volume was observed between vehicle-saline- and vehicle-lithium-treated mice. No significant difference in tumor volume was observed between paclitaxel-saline- or paclitaxel-lithium-treated mice. However, tumor size was substantially different between vehicle-treated mice and paclitaxel-treated mice. Data are expressed as means ± se; n = 5 mice/group. ***P < 0.001. C) H&E staining of representative tumors removed from H1975 xenograft model. Lithium treatment does not appear to interfere with tumor growth or paclitaxel's ability to decrease tumor size. Both paclitaxel-treated groups have extensive recession of tumor growth.
Pretreatment with lithium prevents death associated with high-dose paclitaxel treatment
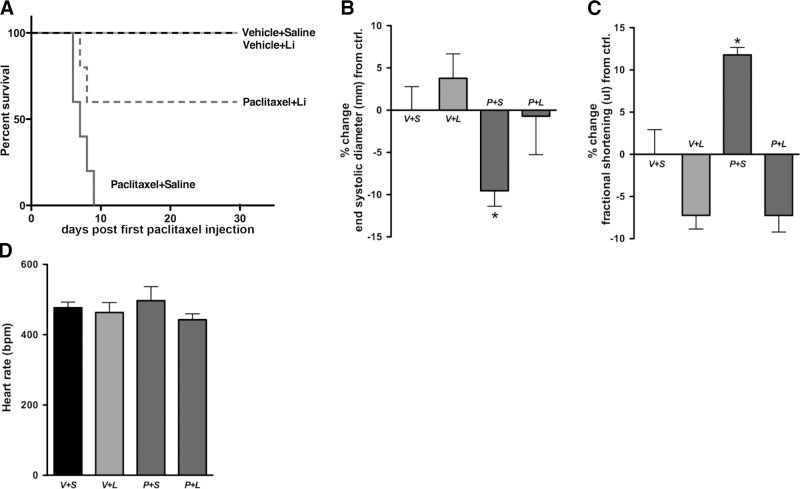
Lithium has also been shown to be beneficial to patients who are undergoing chemotherapy by preventing development of symptoms, such as neutropenia and leucopenia (33–35). Treatment of mice with high-dose paclitaxel (60 mg/kg) caused 100% of paclitaxel-saline-treated mice to die within 10 d of their last paclitaxel injection (Fig. 7A). However, 60% of paclitaxel-lithium-treated mice survived, indicating that lithium protects against the toxic effects of the high-dose paclitaxel treatment. None of the vehicle-saline- or vehicle-lithium-treated mice died.
Figure 7.
Pretreatment with lithium prevents death and cardiac dysfunction associated with the toxic effects of paclitaxel. A) No paclitaxel-saline-treated mice survived (n=5); 60% of paclitaxel-lithium-treated mice survived (n=5); 100% of vehicle-saline (n=5)- and vehicle-lithium (n=4)-treated mice survived. Note that 1 mouse in the vehicle-lithium-treated group died due to injection error rather than to effects of high-dose paclitaxel, and was not included in the data set. B, C) Echocardiograms were performed on H1975 xenograft mice. Paclitaxel-saline-treated mice showed a significant decrease in LV end-systolic diameter (B) and a significant increase in LVFS (C); this indicates increased cardiac contractility. No significant differences were observed between vehicle-saline-, vehicle-lithium-, or paclitaxel-lithium-treated mice. D) Heart rate was unchanged among all groups. V+S, vehicle-saline; V+L, vehicle-lithium; P+S, paclitaxel-saline; P+L, paclitaxel-lithium. Data are expressed as means ± se; n = 3 mice/group. *P < 0.05.
Lithium prevents increased cardiac contractility in paclitaxel-treated mice
Because of the increased survival rate observed in the paclitaxel-lithium-treated mice, we decided to look at one of the side effects associated with paclitaxel treatment. Patients treated with paclitaxel have been shown to develop arrhythmias and cardiac dysfunction that have been linked to dysregulated calcium signaling (36, 37). Cardiomyocytes isolated from mice treated with paclitaxel show an increase in the frequency of spontaneous calcium oscillations. These changes in cardiomyocytes result from the increased interaction of NCS-1 with the InsP3R (37).
On the basis of these observations in mice and humans, we tested whether paclitaxel alters cardiac function in the paclitaxel-saline-treated mice and whether lithium attenuates these changes. Echocardiograms conducted on paclitaxel-saline-treated mice showed that there was a significant decrease of 10 ± 2% in LV end-systolic diameter (Fig. 7B) and a 12 ± 3% increase in LVFS compared to vehicle-saline-treated mice (Fig. 7C). No significant differences were observed between the vehicle-saline-, vehicle-lithium-, or paclitaxel-lithium-treated mice. Heart rate was unchanged among all groups (Fig. 7D).
The increased FS in the paclitaxel-saline-treated mice is of note, as changes in FS have been shown to predict dysrhythmias and late cardiac decompensation in patients treated with anthracyclines, another chemotherapeutic agent known to cause cardiomyopathy (38). In addition, the increased FS observed in the paclitaxel-saline-treated mice can be compared with the observed increase in frequency of oscillations in paclitaxel-treated cardiomyocytes. Increased calcium oscillations due to paclitaxel-induced interaction of NCS-1 with the InsP3R would lead to increased cardiac contractility. These results suggest that lithium is acting to prevent cardiac abnormalities in paclitaxel-treated mice in a manner similar to that by which it prevents PIPN. By disrupting the excessive interaction of NCS-1 with the InsP3R, lithium prevents the increase in frequency of calcium oscillations and in this way attenuates the increase in cardiac contractility linked to paclitaxel treatment. These data provide further support that lithium acts on the paclitaxel/NCS-1/InsP3R pathway to prevent PIPN, and gives a first indication that lithium has the potential to prevent paclitaxel-induced cardiac abnormalities in patients.
DISCUSSION
The data presented here demonstrate that a single dose of lithium or ibudilast prior to treatment with paclitaxel prevents the development of PIPN by interfering with the paclitaxel/NCS-1/InsP3R pathway. By binding to NCS-1, lithium and ibudilast are able to prevent the diminished calcium signaling that occurs through the phosphoinositide pathway. The inhibition occurs at an early step in the process, before calcium signaling is enhanced and before calpain is activated to cleave cellular proteins. Here, we measured degradation of NCS-1, but it is assumed that NCS-1 is only one of the protein targets of calpain. The ability to measure protection of NCS-1 levels, as a proxy for calpain-induced protein cleavage, shows that it is possible to prevent the dysregulation of calcium signaling that leads to axonal degeneration in PIPN.
The identification of lithium and ibudilast as compounds that can prevent PIPN is novel, as there are currently no known pharmacological agents that have proven effective for the prevention of PIPN (1). Although lithium and ibudilast (20, 21, 39), in addition to other compounds, such as AK295 (11), glutathione (40, 41), carbamazepine (42), glutamine (43, 44), and calcium/magnesium infusions (45), have been used to reduce neurotoxic symptoms that occur after administration of paclitaxel, either their mechanism of action is unknown or the use of these compounds in patients has yet to be deemed effective. Remarkably, we have shown that lithium and ibudilast appear to be effective as a single prophylactic injection prior to treatment with paclitaxel.
In addition to the lack of treatments available to prevent PIPN, the mechanism by which the neuropathy develops is not well understood. It has been proposed that increased cytosolic calcium and increased calpain activity leads to PIPN due to neuronal toxicity, such as activation of caspases and degradation of proteins by the activated calpains (11, 13, 14). In this study, we found that lithium and ibudilast disrupt the paclitaxel/NCS-1/InsP3R pathway, and thereby prevent increased calpain activity and intracellular calcium signaling due to excessive interaction of NCS-1 with the InsP3R. In addition, lithium and ibudilast prevent the chronic decrease in calcium signaling due to calpain-mediated degradation of NCS-1. The effects of paclitaxel on calcium signaling in vivo occur soon after the first paclitaxel injection and persist up to 4 wk after the last paclitaxel injection. Lithium was able to prevent the alteration in calcium signaling at both the 18-h and 4-wk time points, indicating that the effects of lithium on paclitaxel occur at a very early stage. These data suggest that lithium prevents PIPN by interfering with the acute effects of paclitaxel (the enhancement of cytosolic calcium levels via a NCS-1/Ins3P3R-dependent mechanism).
These results also complement prior reports showing that NCS-1 is necessary for the regulation of voltage-gated calcium channels (46), where decreased NCS-1 will allow excess activation of these channels and lead to increased cytoplasmic calcium. By preserving NCS-1 levels, calcium homeostasis is maintained, and axonal degeneration is prevented (as reviewed in ref. 47).
It also was important to determine whether lithium acts primarily through the paclitaxel/NCS-1/InsP3R pathway to prevent PIPN. Although lithium is known to inhibit inositol monophosphatase (IMPase) and GSK-3 (48), we have demonstrated that these two pathways are unlikely to be involved in the prevention of PIPN. Inhibition of IMPase leads to decreased levels of PIP2 (49). When PIP2 is decreased, InsP3R calcium signaling is diminished (50). However, as described by the experiments here and in previous work, calcium signaling was maintained at baseline levels in cultured cells (16) and in isolated DRGs treated with lithium. These findings support the conclusion that prevention of PIPN does not occur through inhibition of IMPase. Inhibition of the GSK-3 pathway by lithium was explored in this study as well, and it was shown not to have an effect on preventing PIPN. When mice were treated with SB216763 (a GSK-3 inhibitor) and paclitaxel, the measured responses were similar to paclitaxel treatment alone, rather than treatment with paclitaxel and one of the protective agents. The SB216763-treated group showed increased degradation of NCS-1 and decreased calcium signaling, as observed in the paclitaxel-treated groups. These findings demonstrate that inhibiting the GSK-3 pathway does not prevent PIPN and that lithium must be acting elsewhere. Furthermore, the ability of ibudilast to prevent PIPN via the paclitaxel/NCS-1/InsP3R pathway adds additional support to the suggestion that lithium acts by the same pathway.
In summary, we have identified two compounds, lithium and ibudilast, that when administered prior to treatment with paclitaxel, prevent the development of PIPN in mice. We have shown that pretreatment with lithium increases the survival rate of mice given high doses of paclitaxel, appears to protect against paclitaxel-induced cardiac abnormalities, and does not interfere with the antitumor effect of paclitaxel. Furthermore, we have outlined a potential mechanism by which PIPN develops and have determined the manner by which lithium and ibudilast prevent PIPN. The understanding that PIPN can be prevented through the disruption of the paclitaxel/NCS-1/InsP3R pathway will allow for the development of better taxanes and encourage the discovery of additional compounds that act similarly to lithium and ibudilast. Moreover, the addition of protective agents, such as lithium and ibudilast, to treatment regimens will potentially enable patients to tolerate more aggressive rounds of chemotherapy, as well as protect patients against the cardiac side effects of paclitaxel. Overall, this study demonstrates for the first time that the development of PIPN can be prevented with a single prophylactic injection of lithium or ibudilast prior to paclitaxel administration. Because current clinical practices can only treat symptoms of PIPN, these results show that the disruption of the paclitaxel/NCS-1/InsP3R pathway prevents PIPN and that lithium and ibudilast have the potential to be used clinically to prevent PIPN in patients.
Supplementary Material
Acknowledgments
This study was supported by grants from the U.S. Department of Defense (W81XWH-10-1-0033) and the U.S. National Institutes of Health (DK 57751 and DK61747) and a National Science Foundation predoctoral fellowship.
The authors are grateful for the insightful conversations regarding this study with Ivana Kuo, Colleen Feriod, Brenda DeGray, and Ed Kaftan. The authors thank Gordon Terwilliger and Caroline Zeiss for their help and expertise in histological processing and analysis; Nikki Mikkush and Kerry Russell for their assistance and knowledge in performing and analyzing echocardiograms; and Jiang Zaoli and Tommy Cheng for their expertise and assistance in creating a xenograft mouse model.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CIPN
- chemotherapy-induced peripheral neuropathy
- DRG
- dorsal root ganglion
- FS
- fractional shortening
- GSK-3
- glycogen synthase kinase-3
- H&E
- hematoxylin and eosin
- IMPase
- inositol monophosphatase
- InsP3R
- inositol 1,4,5 trisphosphate receptor
- LV
- left ventricular
- LVFS
- left ventricular fractional shortening
- NCS-1
- neuronal calcium sensor 1
- PIPN
- paclitaxel-induced peripheral neuropathy
- TEM
- transmission electron microscopy
REFERENCES
- 1. Pachman D. R., Barton D. L., Watson J. C., Loprinzi C. L. (2011) Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin. Pharmacol. Ther. 90, 377–387 [DOI] [PubMed] [Google Scholar]
- 2. Lema M. J., Foley K. M., Hausheer F. H. (2010) Types and epidemiology of cancer-related neuropathic pain: the intersection of cancer pain and neuropathic pain. Oncologist 15(Suppl. 2), 3–8 [DOI] [PubMed] [Google Scholar]
- 3. Windebank A. J., Grisold W. (2008) Chemotherapy-induced neuropathy. J. Periph. Nerv. Syst. 13, 27–46 [DOI] [PubMed] [Google Scholar]
- 4. Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93, 2325–2327 [DOI] [PubMed] [Google Scholar]
- 5. Chang A. Y., Garrow G. C. (1995) Pilot study of vinorelbine (Navelbine) and paclitaxel (Taxol) in patients with refractory breast cancer and lung cancer. Sem. Oncol. 22, 66–70; discussion 70–61 [PubMed] [Google Scholar]
- 6. Baird R. D., Tan D. S., Kaye S. B. (2010) Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat. Rev. Clin. Oncol. 7, 575–582 [DOI] [PubMed] [Google Scholar]
- 7. Sarosy G. A., Hussain M. M., Seiden M. V., Fuller A. F., Nikrui N., Goodman A., Minasian L., Reed E., Steinberg S. M., Kohn E. C. (2010) Ten-year follow-up of a phase 2 study of dose-intense paclitaxel with cisplatin and cyclophosphamide as initial therapy for poor-prognosis, advanced-stage epithelial ovarian cancer. Cancer. 116, 1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowinsky E. K., Chaudhry V., Cornblath D. R., Donehower R. C. (1993) Neurotoxicity of Taxol. J. Natl. Cancer Inst. Monogr. 15, 107–115 [PubMed] [Google Scholar]
- 9. Weiss J. L., Hui H., Burgoyne R. D. (2010) Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell. Mol. Neurobiol. 30, 1283–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boehmerle W., Splittgerber U., Lazarus M. B., McKenzie K. M., Johnston D. G., Austin D. J., Ehrlich B. E. (2006) Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A. 103, 18356–18361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M. S., Davis A. A., Culver D. G., Wang Q., Powers J. C., Glass J. D. (2004) Calpain inhibition protects against Taxol-induced sensory neuropathy. Brain 127, 671–679 [DOI] [PubMed] [Google Scholar]
- 12. Boehmerle W., Zhang K., Sivula M., Heidrich F. M., Lee Y., Jordt S. E., Ehrlich B. E. (2007) Chronic exposure to paclitaxel diminishes phosphoinositide signaling by calpain-mediated neuronal calcium sensor-1 degradation. Proc. Natl. Acad. Sci. U. S. A. 104, 11103–11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernyhough P., Calcutt N. A. (2010) Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium 47, 130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siau C., Bennett G. J. (2006) Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 102, 1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlecker C., Boehmerle W., Jeromin A., DeGray B., Varshney A., Sharma Y., Szigeti-Buck K., Ehrlich B. E. (2006) Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J. Clin. Invest. 116, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benbow J. H., Mann T., DeGray B., Ehrlich B. E. (2012) Protection against paclitaxel induced decreases in calcium signaling. 286, 34575–34582 [Google Scholar]
- 17. Saha L., Hota D., Chakrabarti A. (2012) Evaluation of Lercanidipine in paclitaxel-induced neuropathic pain model in rat: a preliminary study. Pain Res. Treat. 2012, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahn E. J., Zvonok A. M., Thakur G. A., Khanolkar A. D., Makriyannis A., Hohmann A. G. (2008) Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J. Pharmacol. Exp. Ther. 327, 584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wood A. J., Goodwin G. M., De Souza R., Green A. R. (1986) The pharmacokinetic profile of lithium in rat and mouse; an important factor in psychopharmacological investigation of the drug. Neuropharmacology 25, 1285–1288 [DOI] [PubMed] [Google Scholar]
- 20. Pourmohammadi N., Alimoradi H., Mehr S. E., Hassanzadeh G., Hadian M. R., Sharifzadeh M., Bakhtiarian A., Dehpour A. R. (2012) Lithium attenuates peripheral neuropathy induced by Paclitaxel in rats. Basic Clin. Pharmacol. Toxicol. 110, 231–237 [DOI] [PubMed] [Google Scholar]
- 21. Ledeboer A., Liu T., Shumilla J. A., Mahoney J. H., Vijay S., Gross M. I., Vargas J. A., Sultzbaugh L., Claypool M. D., Sanftner L. M., Watkins L. R., Johnson K. W. (2006) The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shakoori A., Mai W., Miyashita K., Yasumoto K., Takahashi Y., Ooi A., Kawakami K., Minamoto T. (2007) Inhibition of GSK-3 beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci. 98, 1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma A., Straubinger R. M. (1994) Novel taxol formulations: preparation and characterization of taxol-containing liposomes. Pharm. Res. 11, 889–896 [DOI] [PubMed] [Google Scholar]
- 24. Shree T., Olson O. C., Elie B. T., Kester J. C., Garfall A. L., Simpson K., Bell-McGuinn K. M., Zabor E. C., Brogi E., Joyce J. A. (2011) Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 25, 2465–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johenning F. W., Zochowski M., Conway S. J., Holmes A. B., Koulen P., Ehrlich B. E. (2002) Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J. Neurosci. 22, 5344–5353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costa R., Motta E. M., Dutra R. C., Manjavachi M. N., Bento A. F., Malinsky F. R., Pesquero J. B., Calixto J. B. (2011) Anti-nociceptive effect of kinin B and B receptor antagonists on peripheral neuropathy induced by paclitaxel in mice. Brit. J. Pharmacol. 164, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Makoukji J., Belle M., Meffre D., Stassart R., Grenier J., Shackleford G., Fledrich R., Fonte C., Branchu J., Goulard M., de Waele C., Charbonnier F., Sereda M. W., Baulieu E. E., Schumacher M., Bernard S., Massaad C. (2012) Lithium enhances remyelination of peripheral nerves. Proc. Natl. Acad. Sci. U. S. A. 109, 3973–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benbow J. H., DeGray B., Ehrlich B. E. (2011) Protection of neuronal calcium sensor 1 protein in cells treated with paclitaxel. J. Biol. Chem. 286, 34575–34582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien W. T., Klein P. S. (2009) Validating GSK3 as an in vivo target of lithium action. Biochem. Soc. Trans. 37, 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wada A. (2009) GSK-3 inhibitors and insulin receptor signaling in health, disease, and therapeutics. Front. Biosci. 14, 1558–1570 [DOI] [PubMed] [Google Scholar]
- 31. Fishman P., Bar-Yehuda S., Ohana G., Barer F., Ochaion A., Erlanger A., Madi L. (2004) An agonist to the A3 adenosine receptor inhibits colon carcinoma growth in mice via modulation of GSK-3 beta and NF-κB. Oncogene 23, 2465–2471 [DOI] [PubMed] [Google Scholar]
- 32. Naderi S., Gutzkow K. B., Lahne H. U., Lefdal S., Ryves W. J., Harwood A. J., Blomhoff H. K. (2004) cAMP-induced degradation of cyclin D3 through association with GSK-3β. J. Cell Sci. 117, 3769–3783 [DOI] [PubMed] [Google Scholar]
- 33. Lee M., Hopkins L. E. (1980) Attenuation of chemotherapy-induced neutropenia with lithium carbonate. Am. J. Hosp. Pharm. 37, 1066–1071 [PubMed] [Google Scholar]
- 34. Lyman G. H., Williams C. C., Preston D. (1980) The use of lithium carbonate to reduce infection and leukopenia during systemic chemotherapy. N. Engl. J. Med. 302, 257–260 [DOI] [PubMed] [Google Scholar]
- 35. Lyman G. H., Williams C. C., Preston D., Goldman A., Dinwoodie W. R., Saba H., Hartmann R., Jensen R., Shukovsky L. (1981) Lithium carbonate in patients with small cell lung cancer receiving combination chemotherapy. Am. J. Med. 70, 1222–1229 [DOI] [PubMed] [Google Scholar]
- 36. Yeh E. T., Bickford C. L. (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 53, 2231–2247 [DOI] [PubMed] [Google Scholar]
- 37. Zhang K., Heidrich F. M., DeGray B., Boehmerle W., Ehrlich B. E. (2010) Paclitaxel accelerates spontaneous calcium oscillations in cardiomyocytes by interacting with NCS-1 and the InsP3R. J. Mol. Cell. Cardiol. 49, 829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinherz L. J., Steinherz P. G., Tan C. (1995) Cardiac failure and dysrhythmias 6–19 years after anthracycline therapy: a series of 15 patients. Med. Ped. Oncol. 24, 352–361 [DOI] [PubMed] [Google Scholar]
- 39. Hama A. T., Broadhead A., Lorrain D. S., Sagen J. (2012) The antinociceptive effect of the asthma drug ibudilast in rat models of peripheral and central neuropathic pain. J. Neurotrauma 29, 600–610 [DOI] [PubMed] [Google Scholar]
- 40. Colombo N., Bini S., Miceli D., Bogliun G., Marzorati L., Cavaletti G., Parmigiani F., Venturino P., Tedeschi M., Frattola L., Buratti C., Mangioni C. (1995) Weekly cisplatin ± glutathione in relapsed ovarian carcinoma. Intl. J. Gynecol. Cancer 5, 81–86 [DOI] [PubMed] [Google Scholar]
- 41. Smyth J. F., Bowman A., Perren T., Wilkinson P., Prescott R. J., Quinn K. J., Tedeschi M. (1997) Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann. Oncol. 8, 569–573 [DOI] [PubMed] [Google Scholar]
- 42. Lersch C., Schmelz R., Eckel F., Erdmann J., Mayr M., Schulte-Frohlinde E., Quasthoff S., Grosskreutz J., Adelsberger H. (2002) Prevention of oxaliplatin-induced peripheral sensory neuropathy by carbamazepine in patients with advanced colorectal cancer. Clin. Colorectal Cancer 2, 54–58 [DOI] [PubMed] [Google Scholar]
- 43. Amara S. (2008) Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann. Pharmacother. 42, 1481–1485 [DOI] [PubMed] [Google Scholar]
- 44. Wang W. S., Lin J. K., Lin T. C., Chen W. S., Jiang J. K., Wang H. S., Chiou T. J., Liu J. H., Yen C. C., Chen P. M. (2007) Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 12, 312–319 [DOI] [PubMed] [Google Scholar]
- 45. Grothey A., Nikcevich D. A., Sloan J. A., Kugler J. W., Silberstein P. T., Dentchev T., Wender D. B., Novotny P. J., Chitaley U., Alberts S. R., Loprinzi C. L. (2011) Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J. Clin. Oncol. 29, 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rousset M., Cens T., Gavarini S., Jeromin A., Charnet P. (2003) Down-regulation of voltage-gated Ca2+ channels by neuronal calcium sensor-1 is beta subunit-specific. J. Biol. Chem. 278, 7019–7026 [DOI] [PubMed] [Google Scholar]
- 47. Wang J. T., Medress Z. A., Barres B. A. (2012) Axon degeneration: molecular mechanisms of a self-destruction pathway. J. Cell. Biol. 196, 7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phiel C. J., Klein P. S. (2001) Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 41, 789–813 [DOI] [PubMed] [Google Scholar]
- 49. Agam G., Bersudsky Y., Berry G. T., Moechars D., Lavi-Avnon Y., Belmaker R. H. (2009) Knockout mice in understanding the mechanism of action of lithium. Biochem. Soc. Trans. 37, 1121–1125 [DOI] [PubMed] [Google Scholar]
- 50. Johenning F. W., Wenk M. R., Uhlen P., Degray B., Lee E., De Camilli P., Ehrlich B. E. (2004) InsP3-mediated intracellular calcium signalling is altered by expression of synaptojanin-1. Biochem. J. 382, 687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.