Abstract
Exercise-induced angiogenesis is a key determinant of skeletal muscle function. Here, we investigated whether the E3 ubiquitin ligase murine double minute-2 (Mdm2) exerts a proangiogenic function in exercised skeletal muscle. Mdm2 hypomorphic (Mdm2Puro/Δ7-9) mice have a 60% reduction in Mdm2 expression compared with that in wild-type animals. Capillary staining on muscle sections from Mdm2Puro/Δ7-9 sedentary mice with a wild-type or knockout background for p53 revealed that deficiency in Mdm2 resulted in 20% capillary regression independently of p53 status. In response to one bout of exercise, protein expression of the proangiogenic vascular endothelial growth factor-A (VEGF-A) was increased by 64% in muscle from wild-type animals, and endothelial cell outgrowth from exercised muscle biopsy samples cultured in a 3-dimensional collagen gel was enhanced by 37%. These proangiogenic responses to exercise were impaired in exercised Mdm2Puro/Δ7-9 mice. Prolonged exercise training resulted in increased Mdm2 protein expression (+49%) and capillarization (+24%) in wild-type muscles. However, exercise training-induced angiogenesis was abolished in Mdm2Puro/Δ7-9 mice. Finally, exercise training restored Mdm2, VEGF-A, and capillarization levels in skeletal muscles from obese Zucker diabetic fatty rats compared with those in healthy animals. Our results define Mdm2 as a crucial regulator of capillary maintenance and exercise-induced angiogenesis in skeletal muscle.—Roudier, E., Forn, P., Perry, M. E., Birot, O. Murine double minute-2 expression is required for capillary maintenance and exercise-induced angiogenesis in skeletal muscle.
Keywords: VEGF-A, endothelial cell, p53, physiology
The regular practice of physical exercise is well established as a preventive and therapeutic approach for many chronic metabolic and cardiovascular diseases. Skeletal muscles represent 40% of the body weight and play crucial roles in locomotion and metabolism (1). Angiogenesis, the formation of new capillaries, has tremendous implications in muscle health and disease because, through this process, the muscle capillary network adequately matches the metabolic needs of myofibers (1–4). This balance between blood supply and metabolic activity is, however, challenged during physical exercise, thus disrupting muscle homeostasis. The resulting physiological and metabolic stress is a strong proangiogenic stimulus in skeletal muscle, and its chronic repetition during prolonged exercise training ultimately leads to muscle capillary growth (1–3). Of importance, this proangiogenic effect of exercise is not restricted to healthy muscle tissue and can efficiently contribute to restore the muscle microcirculation in chronic diseases associated with capillary regression in skeletal muscles (5–7).
A few of the factors that regulate angiogenesis are known. By promoting endothelial cell survival, proliferation, and migration, vascular endothelial growth factor-A (VEGF-A) appears to be a master regulator of angiogenesis (4, 8). In contrast, the p53 tumor suppressor exerts an antiangiogenic effect both by stimulating the expression of multiple antiangiogenic factors and by inhibiting the expression of proangiogenic factors (9, 10–13). The E3 ubiquitin ligase murine double minute-2 (Mdm2) is well established as a major negative regulator of p53 (14, 15), and angiogenesis is increased in tumors overexpressing Mdm2 (9). Interestingly, mice that overexpress Mdm2 have a higher incidence of hemangiosarcoma, a malignant type of tumor due to uncontrolled and excessive endothelial cell proliferation, independent of their p53 status, suggesting also a p53-independent angiogenic role for Mdm2 (16). In vitro studies indicate that Mdm2 can regulate the expression of VEGF-A (17, 18). In particular, Mdm2 can enhance the expression of hypoxia-inducible factor-1α (HIF-1α), a transcriptional activator for VEGF-A (17, 19–23). The Mdm2 inhibitor Nutlin-3 inhibits HIF-1α function in a p53-independent manner (21) and prevents angiogenesis in the Matrigel assay (24). Because most of these in vitro studies were performed on tumor cells in which Mdm2 is amplified or overexpressed, the physiological relevance of such a regulatory effect of Mdm2 on VEGF-A and angiogenesis remains uncertain.
Here, we prove that Mdm2 is part of a key proangiogenic signaling pathway in both resting and exercised skeletal muscles. We found that prolonged exercise training strongly stimulates Mdm2 expression in rodent skeletal muscle under both physiological and pathological conditions. Depletion of Mdm2 protein results in capillary loss independently of p53 status and prevents exercise-induced VEGF-A expression and capillary formation in mouse skeletal muscle. Our results define Mdm2 as a key regulator of muscle angiogenesis. This discovery could broaden the utility of therapeutic agents being developed to modulate Mdm2 function (25, 26).
MATERIALS AND METHODS
Exercise protocols in rodents
All animal experiments were conducted according to the directives of the Canadian Council on Animal Care and the animal care and use committees of York University, the University of Montreal, and the U.S. National Cancer Institute. Female Sprague-Dawley rats (n=16, 180–200 g), 5-wk-old male lean rats (n=9), and Zucker diabetic fatty (ZDF) rats (n=22) were purchased from Charles River Laboratories (Saint-Constant, QC, Canada). Mdm2Puro/Δ7-9 mice, with or without a deletion for p53 gene, were F1 hybrids on a C57BL6/SV129 background and were bred at the animal facilities of the National Cancer Institute (Frederick, MD, USA). Characterization of these mice has been described previously (27). All animals were housed on a 12-h light-dark cycle, with controlled humidity and room temperature (20–23°C), and access to food and water ad libitum. Lean and ZDF rats were fed with Purina 5008 chow (Purina Mills, St. Louis, MO, USA).
Single bout of running exercise
Nine- to 11-wk-old Mdm2Puro/Δ7-9 and wild-type mice performed one bout of intense running exercise, as described previously in rodents (28). Animals were familiarized with the running treadmill (model CL; Omnitech, Columbus, OH, USA) by running at low speed (5 m/min, no incline for 10 min) for 3 d before performing exercise. The night before the exercise test, food was removed, so mice were starved for 12 h. Mice ran at 10 m/min for the first 10 min (10% incline) followed by a gradual increase of speed until exhaustion. The average time to exhaustion was 50-80 min, and the maximal speed was 30 m/min.
Endurance training
After acclimatization with the treadmill, female Sprague-Dawley rats were divided into sedentary or trained groups (n=8/group), as described previously (29). Endurance training consisted of a running exercise on a rodent treadmill (Quinton Instruments, Seattle, WA, USA) 5×/wk for 8 wk (60 min/d, 25 m/min, 4% incline).
After acclimatization, wild-type and Mdm2Puro/Δ7-9 mice were divided into sedentary (n=5 Mdm2Puro/Δ7-9 and n=6 wild-type) and trained groups (n=5 Mdm2Puro/Δ7-9 and n=7 wild type). Trained groups were exercised daily on a treadmill for 60 min at 25 m/min (no incline) for 7 wk.
After acclimatization, ZDF rats were assigned either to sedentary (n=11) or trained (n=11) groups, as described previously (29). Lean rats were kept sedentary. All animals were individually placed for 7 wk into wheel-running cages. Sedentary lean and ZDF rats had a locked wheel, whereas the trained ZDF group had a free wheel allowing spontaneous and voluntary activity.
In all protocols, muscles were harvested immediately after the completion of the last exercise and either immediately frozen for further analyses by Western blotting and histology or freshly used for 3-dimensional (3D) muscle angiogenesis assay.
3D muscle angiogenesis assay
Before surgery, all tools and solutions were sterilized. Plantaris muscles were carefully isolated from 9- to 11-wk-old wild-type and Mdm2Puro/Δ7-9 mice, either kept sedentary or after the completion of one bout of running exercise as described above. Each plantaris was divided into 4–6 fragments or biopsy samples of 3 × 3 mm in size. Biopsy samples were rinsed in cold sterile PBS and kept on ice for a few minutes. Then samples were rinsed 3 times with 5% FCS in Dulbecco's modified Eagle medium (DMEM; Gibco, Burlington, ON, Canada) and then embedded in type 1 collagen mix as described below. Four to 6 biopsy samples from each animal were embedded.
The embedding mix was prepared using type 1 collagen from calf skin (5 mg/ml in 0.1% acetic acid; Elastin Products Co., Owensville, MO, USA). For 1 ml of mix, 120 μl of type 1 collagen (5 mg/ml), 400 μl of 2.5× DMEM, 50 μl of 0.1 N NaOH, and 10 μl of penicillin/streptomycin (Gibco) were mixed together.
Collagen mix was kept on ice to avoid unwanted polymerization before being poured into precooled 24-well tissue culture plates maintained on ice (250 μl of mix per well). Plantaris biopsy samples were incubated for 1–2 min in cold collagen mix and then were placed at the center of the well. After polymerization (30 min at 37°C in CO2 incubator), DMEM containing 5% serum was added for culture. Medium was changed every other day.
Muscle biopsy samples were cultivated in medium with 5% FBS for different durations to allow the analysis of the successive steps of endothelial cell proliferation/migration into the collagen matrix until their assembly into primitive vascular tubes (see Fig. 4C). After 6 d of culture in our conditions, endothelial cells were still in a dynamic phase of migration. 3D collagen gels were then washed 3 times with cold PBS and fixed overnight at 4°C with 4% formalin in PBS. After further PBS washing (three 20-min washes), collagen gels were incubated overnight with biotinylated isolectin B4 (1:100 in PBS containing 1% goat serum, 1% Triton X-100, and 2.5% BSA). Gels were washed 3 times and then incubated with TRITC-conjugated streptavidin (016-020-084; Jackson ImmunoResearch, West Grove, PA, USA) in darkness for ≥2 h. After 3 washing steps with PBS, Vectashield DAPI mounting medium (Vector Laboratories, Burlingame, CA, USA) was added to each well. Images were acquired by epifluorescence on an inversed microscope (Leica DMIRB; Leica Microsystems, Richmond Hill, ON, Canada) using a Retiga EX-I camera (QImaging, Tucson, AZ, USA). Analysis was done using Northern Elite software (Empix Imaging Inc., Mississaugua, ON, Canada). Alternatively, endothelial cells were stained for alkaline phosphatase activity by incubation with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (FAST BCIP/NBT; Sigma-Aldrich, Oakville, ON, Canada) for 45 min at 37°C. Pictures were acquired using an Axio Imager (Zeiss, Jena, Germany) equipped with an AxioCam camera (Zeiss).
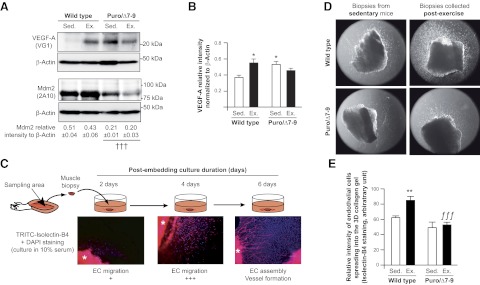
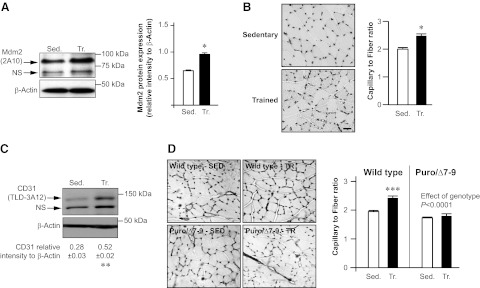
Figure 4.
Exercise-induced angiogenic activity is abolished in skeletal muscle from Mdm2-deficient mice. A, B) Representative immunoblots and ratio to β-actin of Mdm2 and VEGF-A in plantaris muscles from wild-type and Mdm2-deficient mice (Puro/Δ7-9) at rest [sedentary (Sed.); n=7] and after completion of one bout of running exercise (Ex.; n=9). Data are means ± se. A) Overall effect of genotype on Mdm2 expression. Mdm2 expression is not affected by one single bout of exercise. †††P ≤ 0.001. B) Significant differences from sedentary wild-type animals (wild-type Sed.) for VEGF-A expression. *P ≤ 0.05; 2-way ANOVA and post hoc comparison. C) Principle of the 3D muscle angiogenesis assay. Endothelial cells (EC) migrate out of collagen-embedded plantaris biopsies into the collagen gel, where they are detected by specific staining for isolectin B4 or by alkaline phosphatase activity. Asterisk identifies the muscle biopsy. D) Representative images of the 3D muscle angiogenesis assay used to estimate endothelial cell outgrowth from biopsy samples harvested from sedentary or exercised wild-type and Puro/Δ7-9 mice. E) Quantitative analysis of staining intensity in the 3D muscle angiogenic assay. Data represent means ± se. Value for each mouse is the average of 4–6 biopsy samples/ muscle, taken from wild-type (n=4) and Puro/Δ7-9 (n=3) mice for each condition (sedentary vs. exercise). Overall, both genotype (P≤0.05) and exercise stimulus (P≤0.001) had an effect (2-way ANOVA and post hoc comparison). **P ≤ 0.01 vs. sedentary wild-type mice; ƒƒƒP ≤ 0.001 vs. exercised wild-type mice.
The relative number of endothelial cells was quantified by measuring the staining intensity of the major invasive migration front from the muscle biopsy. The area of measurement was identical for all fragments. The value attributed to an animal is the average of 4–6 analyzed biopsy samples. The data show the staining intensity (arbitrary units) per pixel with n = 4 animals/group.
Western blotting
Immunoblotting was performed on protein extracts from rodent plantaris muscles as described previously (28–31). Proteins were extracted from muscle tissue using RIPA buffer containing 1 mg/ml PMSF, 1 mM Na3VO4, 1 mM NaF (Sigma-Aldrich), and 1× protease inhibitors (Complete Mini, Roche Diagnostics, Laval, QC, Canada). Twenty to 40 mg of frozen muscle was mixed at 4°C with RIPA buffer (15 vol RIPA/mg muscle). For each sample, extractions were performed using two stainless carbide beads (Retsch; Fisher Scientific, Montreal, QC, Canada) in the Retsch MM400 tissue lyser (30 pulses/s; Retsch GmbH, Haan, Germany). Denatured samples (20–30 μg/well) were subjected to SDS-PAGE and blotted onto nitrocellulose (Whatman BA95; Sigma-Aldrich) membranes. After blocking with 5% fat-free milk, the blots were probed using the following primary antibodies: antibodies against phospho-p53 and Forkhead box O-1 (FoxO1; C29H4) were from Cell Signaling Technology (Beverly, MA); antibodies against p21 (C19), p53 (FL-393), αβ-tubulin, and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); antibody against VEGF-A was either from Santa Cruz Biotechnology (sc-507) or Millipore (VG-1; Millipore, Etobicoke, ON, Canada); antibody against HIF-1α (NB100-105) was from Novus Biologicals (Littleton, CO, USA); antibodies against subunit IV of cytochrome c oxidase (COX-IV; clone 20E8C12) and thrombospondin-1 (TSP-1; clone A6.1) were from Invitrogen (Burlington, ON, Canada); antibody against CD31 (TLD-3A12) was from BD Pharmingen (Mississauga, ON, Canada); and antibody against Mdm2 (2A10) was either from Calbiochem (San Diego, CA, USA) or a supernatant from the hybridoma (32). Proteins were visualized using an enhanced chemiluminescence procedure (Santa Cruz Biotechnology) and a Kodak Imaging station 4000MM Pro (Eastman Kodak, Rochester, NY, USA). The Western blot results were analyzed with Image 1.62 (U.S. National Institutes of Health, Bethesda, MD, USA) and Carestream (Carestream Health, Rochester, NY, USA) software.
Muscle capillarization analysis
Transverse 10-μm cryosections were obtained in the midbelly of plantaris muscles. As described previously (29–31), capillaries were visualized after a brief fixation in cold acetone or 4% formaldehyde, followed either by staining for alkaline phosphatase activity using incubation with FAST BCIP/NBT for 45 min at 37°C or by incubation with biotin-conjugated isolectin B4 (L-2140; Sigma-Aldrich) and streptavidin-horseradish peroxidase solution (catalog 550946; BD Biosciences, Mississauga, ON, Canada) and with 3,3′-diaminobenzidine (D5905; Sigma-Aldrich). Images were acquired by epifluorescence on an inversed microscope using a Retiga EX-I camera or an Axio Imager equipped with an AxioCam camera. Analysis was done using Northern Elite or AxioVision 4.5 (Zeiss) software. The capillary/fiber ratio was determined after counting capillaries and myofibers on 3-5 cross sections from each muscle and in quintuplicate on each of these sections. Because the plantaris muscle is not homogeneous in its fiber-type composition, counting areas were chosen so they cover all the muscle cross section.
Mouse angiogenesis proteome profiler array
The assay was run according to the manufacturer's instructions (R&D Systems ARY015; Cedarlane Corp., Burlington, ON, Canada). In brief, plantaris muscle extracts from wild-type and Mdm2Puro/Δ7-9 mice were incubated with nitrocellulose membranes preprobed with primary antibodies against 53 positive and negative regulators of the angiogenic process, each antibody being spotted in duplicate on each membrane. Each membrane was incubated with 250 μg of total proteins from one plantaris muscle. Two plantaris muscles from each group, wild-type and Mdm2Puro/Δ7-9, were analyzed. Protein spots were visualized by incubating each membrane with a cocktail of biotinylated detection antibodies and streptavidin-horseradish peroxidase, and using an enhanced chemiluminescence procedure (Immobilon Western ECL; Cedarlane). Protein spots were quantified using a Kodak Imaging station 4000MM Pro with Carestream software.
Statistical analysis
Statistical analyses were performed using Student's t test, 1-way or 2-way ANOVA using Prism5 (GraphPad Software Inc., San Diego, CA, USA). For 1-way and 2-way ANOVA analyses, the Newman-Keuls multiple comparison test or Bonferroni posttest was used, respectively. The results were considered to be statistically significant at values of P < 0.05.
RESULTS
Mdm2 expression and markers of capillarization are well correlated in striated muscle
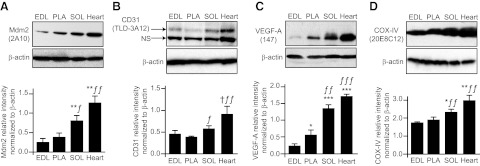
We compared the protein expression levels of Mdm2 and markers of capillarization between rat extensor digitorum longus, the plantaris, the soleus, and the myocardial muscles (Fig. 1A–C). Muscle capillarization was reflected by the protein levels of the endothelial marker CD31 and of the proangiogenic VEGF-A (Fig. 1B, C). Interestingly, the higher the level of capillarization, the higher was Mdm2 protein expression. Muscle microcirculation and oxidative metabolism are intimately linked, and Mdm2 and markers of capillarization levels were correlated with protein expression of COX-IV, a well-established indicator of oxidative metabolism (Fig. 1D and ref. 33).
Figure 1.
Mdm2 expression in striated muscles follows a pattern similar to that of markers for the vascular and oxidative phenotype. Protein levels of Mdm2 (A), CD31 (B), VEGF-A (C), and COX-IV (D) were determined by Western blot in rat striated muscles. Mdm2 is highly expressed in muscles presenting a high level of capillarization, as reflected by CD31 and VEGF-A expression, as well as a high oxidative phenotype, as reflected by COX-IV expression. Data from densitometry analysis are means ± se (n=4–7 rats). EDL, extensor digitorum longus; PLA, plantaris; SOL, soleus; NS, nonspecific. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. EDL; ƒP ≤ 0.05, ƒƒP ≤ 0.01, ƒƒƒP ≤ 0.001 vs. PLA; †P ≤ 0.05 vs. SOL.
Mdm2 protein expression is required for the maintenance of skeletal muscle capillarization
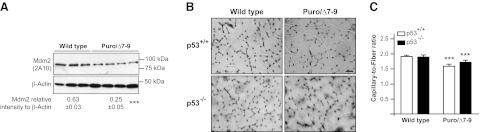
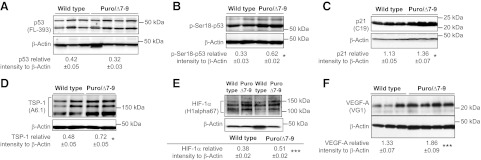
To assess the role of Mdm2 in regulating skeletal muscle capillarization and metabolism, we used hypomorphic Mdm2Puro/Δ7-9 mice that express ∼30% of the wild-type level of Mdm2 mRNA and protein in multiple tissues and present enhanced p53 activity (27). Mdm2Puro/Δ7-9 mice had 60% reduced expression of Mdm2 in the hindlimb plantaris muscle (Fig. 2A) that was associated with a 20% decrease in the level of muscle capillarization (Fig. 2B, C), indicating that Mdm2 expression is indispensable for the maintenance of skeletal muscle capillarization in sedentary animals. We investigated whether the decreased capillarization could be due to either changes in p53 or in HIF-1α, two proteins known to interact with Mdm2 and to have an effect on angiogenesis (17, 34, 35). Whereas Mdm2Puro/Δ7-9 mice presented no significant change in p53 levels (Fig. 3A), they did show increased levels of p53 phosphorylation on serine 18 (Fig. 3B) and of the p53 transcriptional target p21 (Fig. 3C). TSP-1 protein, a well-established antiangiogenic factor in skeletal muscle (36) that is also a p53 target (37, 38), was up-regulated by 50% in muscles from Mdm2Puro/Δ7-9 mice (Fig. 3D). These results suggested that increased p53 activity could lead to enhanced TSP-1 expression in skeletal muscle of Mdm2Puro/Δ7-9 mice. However, knocking out p53 gene expression in Mdm2Puro/Δ7-9 mice did not restore the capillary/fiber ratio to the wild-type ratio (Fig. 2B, C), indicating that the mechanism responsible for capillary regression in Mdm2Puro/Δ7-9 mice is p53 independent. This result is in line with a recent study from Park et al. (39) showing no difference in skeletal muscle capillarization between wild-type and p53-knockout mice.
Figure 2.
Mdm2 is required for the maintenance of capillaries in skeletal muscle. A) Representative immunoblots and densitometry analysis of Mdm2 protein expression in plantaris muscle from wild-type and Mdm2-deficient mice (Puro/Δ7-9). Data are means ± se (n=7 wild-type and n=9 Puro/Δ7-9 mice). β-Actin was used as a loading control. B, C) Visualization of capillaries in plantaris muscle cross sections from wild-type and Puro/Δ7-9 mice that are deficient or not for p53 (p53−/− and p53+/+, respectively; B), and determination of the capillary/fiber ratio (C). Scale bar = 50 μm; ×200 view. Data are means ± se (n=5 mice/group). ***P ≤ 0.001 vs. wild-type.
Figure 3.
Determination of the consequence of Mdm2 deficiency on the expression levels of proteins involved in p53 and VEGF-A angio-adaptive pathways. Representative immunoblots and densitometry analyses of protein expression are shown for p53 (A), the phosphorylated form of p53 on serine 18 (B), p21 (C), TSP-1 (D), HIF-1α (E), and VEGF-A (F) in plantaris muscles from wild-type and Mdm2-deficient mice (Puro/Δ7-9). Data are means ± se (n=4 for p53, p-Ser-18-p53, TSP-1, HIF-1α, and VEGF-A; n=8 for p21). β-Actin was used as a loading control. *P ≤ 0.05, ***P ≤ 0.001 vs. wild-type.
Aside from its action on p53, Mdm2 has been shown to enhance HIF-1α stability and transcriptional activity in tumor cells, increasing in turn expression of VEGF-A independently of p53 (19, 20). VEGF-A plays a key role in maintaining established capillaries and promoting angiogenesis in skeletal muscle (40, 41). Therefore, we analyzed HIF-1α and VEGF-A expression in Mdm2Puro/Δ7-9 mice. Unexpectedly, we observed that both HIF-1α and VEGF-A protein expression were increased in Mdm2Puro/Δ7-9 mice (Fig. 3E, F). Therefore, neither enhanced p53 activity nor decreased VEGF expression can explain the capillary rarefaction observed in Mdm2Puro/Δ7-9 mice.
Taken together, these findings suggest that Mdm2 depletion results in a reduced level of skeletal muscle capillarization, independent of p53 status. We therefore next determined whether Mdm2 exerts a proangiogenic role in exercised skeletal muscle.
Mdm2 is required for the proangiogenic response of the skeletal muscle to exercise stimulus
One single bout of exercise induces an important cellular stress that represents a strong proangiogenic stimulus in skeletal muscle (1–3). Because Mdm2 plays a key role in sensing some cellular stresses (42), we postulated that Mdm2 itself is required for effective signaling of proangiogenic pathways in exercised skeletal muscle. Despite a higher VEGF-A basal level in skeletal muscles from sedentary Mdm2Puro/Δ7-9 compared with that of their littermates (Fig. 3F), no further increase in VEGF-A occurs in Mdm2Puro/Δ7-9 mice when challenged by one bout of intense running exercise, indicating a loss of the adaptive response to exercise present in wild-type animals (Fig. 4A, B). The inability of Mdm2Puro/Δ7-9 mice to increase VEGF-A protein levels in response to exercise extends in vitro observations suggesting that Mdm2 regulates VEGF-A expression (17, 19–21, 43).
Previous studies using myofiber-specific VEGF-A-deficient mice have demonstrated that the increase in VEGF-A expression in response to an exercise stimulus was indispensable for skeletal muscle angiogenesis to occur (40, 41). To test whether the level of Mdm2 expression affects the angiogenic capacity of skeletal muscle endothelial cells, we performed an ex vivo 3D muscle angiogenic assay in which we analyzed the migratory activity of endothelial cells from plantaris muscle biopsy samples obtained from sedentary or exercised mice and embedded into a 3D collagen gel (Fig. 4C). Whereas a modest endothelial cell outgrowth into the collagen matrix was observed from biopsy samples taken from sedentary mice, outgrowth was strongly increased from biopsy samples from exercised wild-type mice (+37%, P≤0.01) but not from biopsy samples from exercised Mdm2Puro/Δ7-9 mice (Fig. 4D, E). Thus, our data indicate that Mdm2 expression is required for muscle angiogenic activity in response to physical exercise.
Muscle angiogenesis in response to exercise training is Mdm2-dependent
Taken together, our results suggest that Mdm2 is a critical effector of the proangiogenic signal during exercise. To confirm the angiogenic role of Mdm2 in promoting capillary growth in skeletal muscle, we used the model of prolonged exercise training (i.e., one single bout of exercise repeated daily during several weeks) to stimulate angiogenesis in plantaris muscle. Concomitantly with muscle angiogenesis, our training program enhanced Mdm2 protein expression in trained rat plantaris muscles (Fig. 5A–C). Interestingly, whereas muscle angiogenesis occurred in wild-type mice trained for 7 wk, no capillary growth was observed in trained Mdm2Puro/Δ7-9 mice, thus confirming that Mdm2 is indispensable for physiological muscle angiogenesis (Fig. 5D).
Figure 5.
Mdm2 is indispensable for exercise-induced angiogenesis in skeletal muscle. A) Representative immunoblots and densitometry analysis of the ratio of Mdm2 to β-actin in plantaris muscles from sedentary (Sed.) and endurance-trained (Tr.) female Sprague-Dawley rats (n=8/group). B) Detection of capillaries after alkaline phosphatase staining on plantaris muscle cross sections from rats described in A and determination of the capillary/fiber ratio. Scale bar = 50 μm. C) Representative immunoblots for CD31 (endothelial marker) and β-actin and ratio of CD31 to β-actin in plantaris muscles from rats described in A, B. β-Actin was used as a loading control. Data are means ± se (n=8 rats/group). *P ≤ 0.05; **P ≤ 0.01. D) Staining of capillaries with isolectin B4 on plantaris muscle cross sections from sedentary (Sed.) and trained (Tr.) wild-type and Mdm2-deficient (Puro/Δ7-9) mice, and determination of the capillary/fiber ratio. Data are means ± se (n=5 mice/group). NS, nonspecific. Two-way ANOVA shows an overall effect of the genotype. ***P ≤ 0.001 vs. sedentary wild-type mice; 2-way ANOVA and post hoc comparison.
Exercise training increases Mdm2 protein expression in diseased skeletal muscle
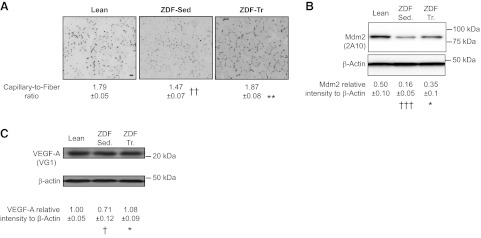
Obesity and type 2 diabetes are chronic diseases often associated with capillary regression in skeletal muscle (5–7, 29). Because our results demonstrated that Mdm2 was required for maintaining muscle capillarization as well as for exercise-induced angiogenesis, we investigated whether the stimulatory effect of exercise training on Mdm2 expression was preserved in diseased skeletal muscles. Whereas the levels of capillarization and protein expression for Mdm2 and VEGF-A were decreased in skeletal muscle from sedentary ZDF rats, voluntary exercise training efficiently restored all these parameters to physiological levels (Fig. 6).
Figure 6.
Exercise training increases Mdm2 expression levels in diseased skeletal muscles. A) Detection of capillaries after alkaline phosphatase staining on plantaris muscle cross sections from sedentary lean and sedentary ZDF (ZDF-Sed) and trained obese ZDF (ZDF-Tr) rats. Scale bar = 50 μm. Capillary/fiber ratio was also determined. Data are means ± se (n=3 sections/animal, n=5 rats/group). B, C) Representative immunoblots and densitometry analyses of Mdm2 and VEGF-A protein expression in plantaris muscles from sedentary lean (n=9), ZDF-Sed (n=11), and ZDF-Tr (n=11) rats. β-Actin was used as a loading control. *P ≤ 0.05, **P ≤ 0.01 vs. ZDF-Sed.; †P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001 vs. lean.
Mdm2 modulates the expression of various antiangiogenic molecules
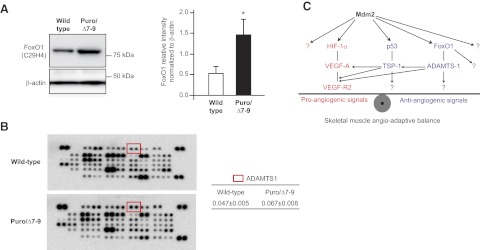
In addition to its effect on TSP-1 expression, we investigated whether Mdm2 could modulate the expression of other antiangiogenic molecules in skeletal muscle. Conditional deletion of the FoxO1 transcription factor in the vascular compartment induces a strong hemangiomatous phenotype in the skeletal muscle, suggesting a strong antiangiogenic function of endothelial FoxO1 in the muscle (44). The expression of FoxO1 protein was increased 2.76-fold more in plantaris muscles from Mdm2Puro/Δ7-9 mice than from wild-type mice (1.49±0.42 vs. 0.54±0.17, respectively; P≤ 0.05; Fig. 7A). Analysis of the mouse angiogenesis proteome profiler array indicated a 43% increase in a desintegrin and metalloproteinase with thrombospondin motif 1 (ADAMTS-1) protein expression in plantaris muscles from Mdm2Puro/Δ7-9 mice (Fig. 7B).
Figure 7.
Mdm2 modulates the expression of various antiangiogenic molecules and exerts a complex control of the angioadaptive balance in rodent skeletal muscle. A) Representative immunoblots and ratio to β-actin of FoxO1 protein in plantaris muscles from wild-type and Mdm2-deficient (Puro/Δ7-9) mice (n=5 animals/group). Data are means ± se. *P ≤ 0.05 vs. wild-type. B) Representative images of two membranes from the mouse angiogenesis proteome profiler array incubated with protein extracts from wild-type and Mdm2Puro/Δ7-9 plantaris muscles, respectively. Red squares on membranes localize the duplicate spots corresponding to the antiangiogenic ADAMTS-1 protein. At right are means ± se from ADAMTS-1 in wild-type and Mdm2Puro/Δ7-9 plantaris muscles (n=2 muscles analyzed in duplicate per group). C) Hypothetical schematic diagram of the complex regulatory role of Mdm2 on the skeletal muscle angioadaptive balance. Question marks indicate potential and unknown signaling pathways.
DISCUSSION
Here, we showed that the E3 ubiquitin ligase Mdm2, most well known as an oncoprotein, is a key regulator of exercise-induced angiogenesis in skeletal muscle. By using exercise as a physiological and metabolic stress, we demonstrated that prolonged exercise training increased the Mdm2 protein level. Mdm2 depletion in skeletal muscles from Mdm2Puro/Δ7-9 mice resulted in a reduced basal level of capillarization and a loss of the angiogenic response to exercise. This result clearly demonstrated that Mdm2 expression is indispensable for the maintenance of existing capillaries as well as for exercise-induced angiogenesis in healthy skeletal muscle. Although it would be reasonable to expect that these effects are due to enhanced antiangiogenic p53 activity in Mdm2Puro/Δ7-9 mice, we showed that p53 deficiency did not rescue the reduced muscle capillarization observed. Thus, Mdm2 is required for the maintenance of established capillaries independent of p53 status.
In addition to its action on the tumor suppressor p53, Mdm2 interacts with other proteins to eventually ubiquitinate them, thus involving Mdm2 in many important cellular processes (for reviews, see refs. 15, 45, 46). HIF-1α has been identified as a potential target for Mdm2. Initially, Mdm2 was thought to mediate the p53-induced degradation of HIF-1α in tumor cells (47). However, there is now increasing evidence that Mdm2 might promote HIF-1α stabilization in response to hypoxia or growth factors in a p53-independent way in tumors. By regulating HIF-1α, Mdm2 could then govern the expression level of the proangiogenic VEGF-A protein in cancer cells (18). Overexpression of Mdm2 increases the level of HIF-1α protein and HIF-1α activity toward the VEGF-A promoter (19, 20). LaRusch et al. (21) reported that the inhibition of the Mdm2-HIF-1α interaction by Nutlin-3 reduced HIF-1α stability, resulting in decreased levels of VEGF-A expression. Mdm2 might also regulate VEGF-A expression via the stabilization of its mRNA (48).
In our study, we observed that Mdm2Puro/Δ7-9 mice that express only 40% of the wild-type level of Mdm2 protein in plantaris muscle have higher basal levels of HIF-1α and VEGF-A proteins. This observation, which could suggest that Mdm2 triggers HIF-1α degradation in healthy skeletal muscle, appears to be contradictory to much of what has been shown recently. However, we cannot exclude the possibility of compensatory mechanisms in this transgenic animal model. An alternative hypothesis is that the decreased capillarization in Mdm2Puro/Δ7-9 mice leads to hypoxia, which then causes stabilization of HIF-1α. In line with this, the reduced level of muscle capillarization in Mdm2Puro/Δ7-9 mice despite a 40% increase in VEGF-A basal level is intriguing. However, in addition to the 40% increase in VEGF-A expression in skeletal muscle, Mdm2Puro/Δ7-9 mice also presented 50% increased expression of the antiangiogenic TSP-1. Depending on the complex nature of its cleavage, TSP-1 protein could modulate VEGF-A receptor-2 signaling either negatively or positively (49). Other molecules involved in the regulation of pro- and antiangiogenic pathways, such as the FoxO1 transcription factor known to exert antiangiogenic effects (31) and to be negatively regulated by Mdm2, might be under the control of Mdm2 (50). We observed that skeletal muscles from Mdm2Puro/Δ7-9 mice express 2.76-fold more FoxO1 protein than the wild-type muscles. Interestingly, it was recently suggested that FoxO1 could be a regulator of the expression of ADAMTS-1 (51), a protein enhancing TSP-1 cleavage and antiangiogenic activity (52). Results from our mouse proteome profiler array showed a 43% increased expression of ADAMTS-1 protein in skeletal muscles from Mdm2Puro/Δ7-9 mice. From these results, Mdm2 revealed itself as an important regulator of skeletal muscle angioadaptation, exerting various and complex roles on both sides of the angioadaptive balance (Fig. 7C).
Matching the blood supply to the metabolic demand of active myofibers is crucial for muscle function, having important repercussions for metabolic homeostasis. Alteration of skeletal muscle metabolism is commonly observed in many chronic diseases, such as obesity and diabetes, in which capillary rarefaction occurs in peripheral skeletal muscles. Physical exercise is well established as an efficient preventive and rehabilitating approach to treat these diseases, and one of its beneficial effects resides in promotion of skeletal muscle angiogenesis (6). Thus, understanding the molecular events that regulate the exercise-induced angiogenic process could have major therapeutic implications. In this context, our discovery that Mdm2 expression is regulated by physical activity and required for muscle angiogenesis brings new perspectives on its physiological functions. In particular, it opens the door to exciting questions such as what could be the stimuli triggering this increase in Mdm2 expression in response to exercise training. Recent evidence suggests that the metabolic/energy stress could lead to Mdm2 phosphorylation on residue serine 166 (53), a modification associated with increased stability of Mdm2 protein (54). We have also previously noted that shear stress can induce this phosphorylation of Mdm2 on serine 166 in skeletal muscle endothelial cells (31). Therefore, we could hypothesize that several exercise-induced stimuli, such as metabolic, mechanical, or hemodynamic stresses, could act in concert to enhance Mdm2 expression. Whether they are all involved and through which mechanisms should be worthy of investigation in the future.
In the context of cancer, the use of Mdm2 antagonists, such as Nutlins, to restore p53 function in cancer cells or to inhibit Mdm2-mediated angiogenesis in tumors is very attractive (9, 25). In fact, clinical trials of Mdm2 antagonists are in place, and one inhibitor has already shown promising results (26). Of high interest, the ability to enhance Mdm2 expression and activity in muscle tissue by exercise training might represent a practical approach to counteract potential side effects in healthy muscle tissue of anti-Mdm2 strategies currently under development for cancer therapy (25, 26).
In summary, our results show that Mdm2 is a critical factor in skeletal muscle angiogenesis. Such a discovery provides new insight into the mechanisms by which physical activity improves muscle function. Further assessment of the physiological functions of Mdm2 will illuminate the spectrum of disorders for which Mdm2 could be an attractive therapeutic target to modulate angiogenesis.
Acknowledgments
The authors thank Nicole Morris [National Cancer Institute, U.S. National Institutes of Health (NIH)] for maintaining the Mdm2puro/Δ7-9 mice, and Dr. Raynald Bergeron (University of Montreal) for his help in the ZDF mouse conditioning.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC discovery grant 341258 to O.B.) and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (M.E.P.).
E.R. and O.B. conceived the study; E.R., M.E.P., and O.B. designed experiments; E.R., P.F., and O.B. performed experiments and analyzed data; M.E.P. supplied Mdm2puro/Δ7-9 mice; E.R., M.E.P., and O.B. participated in the redaction of the manuscript.
Footnotes
- 3D
- 3-dimensional
- ADAMTS-1
- a desintegrin and metalloproteinase with thrombospondin motif 1
- BCIP/NBT
- 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
- COX-IV
- subunit IV of cytochrome c oxidase
- DMEM
- Dulbecco's modified Eagle medium
- FoxO1
- Forkhead box O-1
- HIF-1α
- hypoxia-inducible factor-1α
- Mdm2
- murine double minute-2
- Mdm2Puro/Δ7-9
- Mdm2 deficient
- TSP-1
- thrombospondin-1
- VEGF-A
- vascular endothelial growth factor-A
- ZDF
- Zucker diabetic fatty.
REFERENCES
- 1. Hudlicka O., Brown M., Egginton S. (1992) Angiogenesis in skeletal and cardiac muscle. Physiol. Rev. 72, 369–417 [DOI] [PubMed] [Google Scholar]
- 2. Bloor C. M. (2005) Angiogenesis during exercise and training. Angiogenesis 8, 263–271 [DOI] [PubMed] [Google Scholar]
- 3. Egginton S. (2009) Invited review: activity-induced angiogenesis. Pflügers Arch. 457, 963–977 [DOI] [PubMed] [Google Scholar]
- 4. Carmeliet P., Jain R. K. (2011) Molecular mechanisms and clinical implications of angiogenesis. Nature 473, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hiatt W. R., Regensteiner J. G., Hargarten M. E., Wolfel E. E., Brass E. P. (1990) Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation 81, 602–609 [DOI] [PubMed] [Google Scholar]
- 6. Gustafsson T., Kraus W. E. (2001) Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front. Biosci. 6, 75–89 [DOI] [PubMed] [Google Scholar]
- 7. Yang H. T., Prior B. M., Lloyd P. G., Taylor J. C., Li Z., Laughlin M. H., Terjung R. L. (2008) Training-induced vascular adaptations to ischemic muscle. J. Physiol. Pharmacol. 59, 57–70 [PMC free article] [PubMed] [Google Scholar]
- 8. Lee S., Chen T. T., Barber C. L., Jordan M. C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K. P., Iruela-Arispe M. L. (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shangary S., Wang S. (2008) Targeting the MDM2-p53 interaction for cancer therapy. Clin. Cancer Res. 14, 5318–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouvet M., Ellis L. M., Nishizaki M., Fujiwara T., Liu W., Bucana C. D., Fang B., Lee J. J., Roth J. A. (1998) Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 58, 2288–2292 [PubMed] [Google Scholar]
- 11. Zhang L., Yu D., Hu M., Xiong S., Lang A., Ellis L. M., Pollock R. E. (2000) Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 60, 3655–3661 [PubMed] [Google Scholar]
- 12. Qin G., Kishore R., Dolan C. M., Silver M., Wecker A., Luedemann C. N., Thorne T., Hanley A., Curry C., Heyd L., Dinesh D., Kearney M., Martelli F., Murayama T., Goukassian D. A., Zhu Y., Losordo D. W. (2006) Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc. Natl. Acad. Sci. U. S. A. 103, 11015–11020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshioka Y., Shimizu S., Ito T., Taniguchi M., Nomura M., Nishida T., Sawa Y. (2012) p53 inhibits vascular endothelial growth factor expression in solid tumor. J. Surg. Res. 174, 291–297 [DOI] [PubMed] [Google Scholar]
- 14. Prives C. (1998) Signaling to p53: breaking the Mdm2-p53 circuit. Cell 95, 5–8 [DOI] [PubMed] [Google Scholar]
- 15. Manfredi J. J. (2010) The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 24, 1580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones S. N., Hancock A. R., Vogel H., Donehower L. A., Bradley A. (1998) Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 95, 15608–15612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skinner H. D., Zheng J. Z., Fang J., Agani F., Jiang B. H. (2004) Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1α, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J. Biol. Chem. 279, 45643–45651 [DOI] [PubMed] [Google Scholar]
- 18. Carroll V. A., Ashcroft M. (2008) Regulation of angiogenic factors by HDM2 in renal cell carcinoma. Cancer Res. 68, 545–552 [DOI] [PubMed] [Google Scholar]
- 19. Bardos J. I., Chau N. M., Ashcroft M. (2004) Growth factor-mediated induction of HDM2 positively regulates hypoxia-inducible factor 1α expression. Mol. Cell. Biol. 24, 2905–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nieminen A. L., Qanungo S., Schneider E. A., Jiang B. H., Agani F. H. (2005) Mdm2 and HIF-1α interaction in tumor cells during hypoxia. J. Cell. Physiol. 204, 364–369 [DOI] [PubMed] [Google Scholar]
- 21. LaRusch G. A., Jackson M. W., Dunbar J. D., Warren R. S., Donner D. B., Mayo L. D. (2007) Nutlin-3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1α and Hdm2. Cancer Res. 67, 450–454 [DOI] [PubMed] [Google Scholar]
- 22. Lee Y. M., Lim J. H., Chun Y. S., Moon H. E., Lee M. K., Huang L. E., Park J. W. (2009) Nutlin-3, an Hdm2 antagonist, inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated inactivation of HIF-1α. Carcinogenesis 30, 1768–1775 [DOI] [PubMed] [Google Scholar]
- 23. Park J. H., Lee J. Y., Shin D. H., Jang K. S., Kim H. J., Kong G. (2011) Loss of Mel-18 induces tumor angiogenesis through enhancing the activity and expression of HIF-1α mediated by the PTEN/PI3K/Akt pathway. Oncogene 30, 4578–4589 [DOI] [PubMed] [Google Scholar]
- 24. Secchiero P., Corallini F., Gonelli A., Dell'Eva R., Vitale M., Capitani S., Albini A., Zauli G. (2007) Antiangiogenic activity of the MDM2 antagonist nutlin-3. Circ. Res. 100, 61–69 [DOI] [PubMed] [Google Scholar]
- 25. Vassilev L. T. (2007) MDM2 inhibitors for cancer therapy. Trends Mol. Med. 13, 23–31 [DOI] [PubMed] [Google Scholar]
- 26. Millard M., Pathania D., Grande F., Xu S., Neamati N. (2011) Small-molecule inhibitors of p53-MDM2 interaction: the 2006-2010 update. Curr. Pharm. Des. 17, 536–559 [DOI] [PubMed] [Google Scholar]
- 27. Mendrysa S. M., McElwee M. K., Michalowski J., O'Leary K. A., Young K. M., Perry M. E. (2003) Mdm2 is critical for inhibition of p53 during lymphopoiesis and the response to ionizing radiation. Mol. Cell. Biol. 23, 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Birot O., Koulmann N., Peinnequin A., Bigard X. A. (2003) Exercise-induced expression of vascular endothelial growth factor mRNA in rat skeletal muscle is dependent on fibre type. J. Physiol. 552, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roudier E., Chapados N., Decary S., Gineste C., Le Bel C., Lavoie J. M., Bergeron R., Birot O. (2009) Angiomotin p80/p130 ratio: a new indicator of exercise-induced angiogenic activity in skeletal muscles from obese and non-obese rats? J. Physiol. 587, 4105–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roudier E., Gineste C., Wazna A., Dehghan K., Desplanches D., Birot O. (2010) Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J. Physiol. 588, 4579–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milkiewicz M., Roudier E., Doyle J. L., Trifonova A., Birot O., Haas T. L. (2011) Identification of a mechanism underlying regulation of the anti-angiogenic forkhead transcription factor FoxO1 in cultured endothelial cells and ischemic muscle. Am. J. Pathol. 178, 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen J., Marechal V., Levine A. J. (1993) Mapping of the p53 and mdm-2 interaction domains. Mol. Cell. Biol. 13, 4107–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uguccioni G., Hood D. A. (2011) The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Endocrinol. Metab. 300, 361–371 [DOI] [PubMed] [Google Scholar]
- 34. Teodoro J. G., Evans S. K., Green M. R. (2007) Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J. Mol. Med. 85, 1175–1186 [DOI] [PubMed] [Google Scholar]
- 35. Assadian S., Teodoro J. G. (2008) Regulation of collagen-derived antiangiogenic factors by p53. Expert Opin. Biol. Ther. 8, 941–950 [DOI] [PubMed] [Google Scholar]
- 36. Malek M. H., Olfert I. M. (2009) Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp. Physiol. 94, 749–760 [DOI] [PubMed] [Google Scholar]
- 37. Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. (1994) Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 265, 1582–1584 [DOI] [PubMed] [Google Scholar]
- 38. Su F., Pascal L. E., Xiao W., Wang Z. (2010) Tumor suppressor U19/EAF2 regulates thrombospondin-1 expression via p53. Oncogene 29, 421–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park J. Y., Wang P. Y., Matsumoto T., Sung H. J., Ma W., Choi J. W., Anderson S. A., Leary S. C., Balaban R. S., Kang J. G., Hwang P. M. (2009) p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ. Res. 105, 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olfert I. M., Howlett R. A., Tang K., Dalton N. D., Gu Y., Peterson K. L., Wagner P. D., Breen E. C. (2009) Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J. Physiol. 587, 1755–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olfert I. M., Howlett R. A., Wagner P. D., Breen E. C. (2010) Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am. J. Physiol. 299, 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perry M. E. (2010) The regulation of the p53-mediated stress response by Mdm2 and Mdm4. Cold Spring Harb. Perspect. Biol. 2, a000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alam H., Weck J., Maizels E., Park Y., Lee E. J., Ashcroft M., Hunzicker-Dunn M. (2009) Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology 150, 915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paik J. H., Kollipara R., Chu G., Ji H., Xiao Y., Ding Z., Miao L., Tothova Z., Horner J. W., Carrasco D. R., Jiang S., Gilliland D. G., Chin L., Wong W. H., Castrillon D. H., DePinho R. A. (2007) FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ganguli G., Wasylyk B. (2003) p53-independent functions of MDM2. Mol. Cancer Res. 1, 1027–1035 [PubMed] [Google Scholar]
- 46. Marine J. C., Lozano G. (2010) Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 17, 93–102 [DOI] [PubMed] [Google Scholar]
- 47. Ravi R., Mookerjee B., Bhujwalla Z. M., Sutter C. H., Artemov D., Zeng Q., Dillehay L. E., Madan A., Semenza G. L., Bedi A. (2000) Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 14, 34–44 [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou S., Gu L., He J., Zhang H., Zhou M. (2011) MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol. Cell. Biol. 31, 4928–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang X., Kazerounian S., Duquette M., Perruzzi C., Nagy J. A., Dvorak H., Lawler J. (2009) Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 23, 3368–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu W., Ma Q., Chen L., Li P., Zhang M., Ramamoorthy S., Nawaz Z., Shimojima T., Wang H., Yang Y., Shen Z., Zhang Y., Zhang X., Nicosia S. V., Zhang Y., Pledger J. W., Chen J., Bai W. (2009) MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J. Biol. Chem. 284, 13987–14000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hohberg M., Knockel J., Hoffmann C. J., Chlench S., Wunderlich W., Alter A., Maroski J., Vorderwulbecke B. J., Da Silva-Azevedo L., Knudsen R., Lehmann R., Fiedorowicz K., Bongrazio M., Nitsche B., Hoepfner M., Styp-Rekowska B., Pries A. R., Zakrzewicz A. (2011) Expression of ADAMTS1 in endothelial cells is induced by shear stress and suppressed in sprouting capillaries. J. Cell. Physiol. 226, 350–361 [DOI] [PubMed] [Google Scholar]
- 52. Lee N. V., Sato M., Annis D. S., Loo J. A., Wu L., Mosher D. F., Iruela-Arispe M. L. (2006) ADAMTS1 mediates the release of anti-angiogenic polypeptides from TSP-1 and 2. EMBO J. 25, 5270–5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Z., Li B. (2010) Mdm2 links genotoxic stress and metabolism to p53. Protein Cell 1, 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feng J., Tamaskovic R., Yang Z., Brazil D. P., Merlo A., Hess D., Hemmings B. A. (2004) Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 279, 35510–35517 [DOI] [PubMed] [Google Scholar]