Abstract
Mutations in fibulin proteins that cause cellular secretion deficiencies are linked to a variety of diseases, ranging from retinopathies to cutis laxa (CL). One secretion-deficient fibulin mutant, R345W fibulin-3, causes the macular dystrophy malattia leventinese by increased endoplasmic reticulum retention and/or extracellular misfolding. Herein, we report that small-molecule activation of the PERK arm of the unfolded protein response partially rescues R345W secretion deficiencies through translational attenuation mediated by eIF2α phosphorylation. Enhanced mutant fibulin-3 secretion can also be achieved by activation of a PERK-independent eIF2α kinase through arsenite treatment and is independent of activating transcription factor 4 signaling and protein translation. However, this translational attenuation strategy was unsuccessful for enhancing the secretion deficiencies of fibulin-5 mutants associated with age-related macular degeneration or CL. While lowered growth temperature enhanced the secretion of mutants associated with CL (C217R and S227P), these effects were not mediated through translational attenuation. In stark contrast to the situation with fibulin-3, protein translation was required for efficient wild-type and mutant fibulin-5 secretion. These data suggest that alteration of specific cellular signaling pathways and proteostasis network components can differentially influence fibulin fate, a hypothesis that could be exploited as a therapy for fibulin-related diseases.—Hulleman, J. D., Balch, W. E., Kelly, J. W. Translational attenuation differentially alters the fate of disease-associated fibulin proteins.
Keywords: eIF2α, age-related macular degeneration, cutis laxa, malattia leventinese, proteostasis
The fibulin protein family has at least 8 individual members that are minimally defined by the presence of tandem calcium-binding epidermal growth factor (cbEGF)-like domains followed by a unique C-terminal fibulin domain (reviewed in ref. 1). All fibulins are naturally secreted, disulfide-rich glycoproteins that bind to a diverse assortment of ligands and function as intermolecular bridges responsible for stabilizing the extracellular matrix (ECM). The tissue distribution, precise function, and domain organization of each fibulin appears to be unique; thus, the individual proteins are important in the regulation and organization of the ECM of different organs ranging from the eye to the heart to the skin (reviewed in ref. 2).
The rare macular dystrophy, malattia leventinese (ML), is caused by a single Arg345Trp (R345W) mutation in fibulin-3 (a.k.a. EFEMP1, S1-5) (3). Observations in R345W knock-in mice (4, 5) suggest that ML is characterized by many of the phenotypes associated with age-related macular degeneration (AMD), the leading cause of human blindness in industrialized nations (6–8). The involvement of fibulins in retinal pathogenesis is strengthened by the finding that fibulin-1 and fibulin-4 genes appear to cause retinopathies (9) and by the identification of multiple mutations in fibulin-5 linked to AMD (10, 11). Many of the mutations in fibulin-5 cause secretion deficiencies, analogous to those exhibited by R345W fibulin-3 (10, 12).
Fibulin-3 and fibulin-5 have the same domain organization and exhibit high sequence homology (see Fig. 1A and ref. 1). Both proteins are thought to be critical for the maintenance of the ECM and the regulation of elastogenesis and angiogenesis (13–18). The mutations in fibulin-3 and fibulin-5 that have been linked to ML, cutis laxa (CL), and AMD apparently cause fibulin misfolding, possibly by compromising disulfide bonding (12, 19). This results in their inefficient secretion and increased intracellular steady-state concentrations, presumably as misfolded conformations within the endoplasmic reticulum (ER; refs. 12, 19–21). The accumulation of misfolded or aggregated proteins within the ER normally causes the dissociation of the heat-shock protein 70 chaperone, binding immunoglobulin protein (BiP), from the ER luminal domains of one or more of three distinct transmembrane stress sensor proteins: inositol-requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-like ER kinase (PERK), thereby activating the unfolded protein response (UPR; reviewed in ref. 22). Activation of each of these distinct stress sensors results in unique downstream signaling that is intended to either rectify the proteostasis imbalance or, if the stress cannot be remedied, to trigger programmed cell death (reviewed in ref. 22).
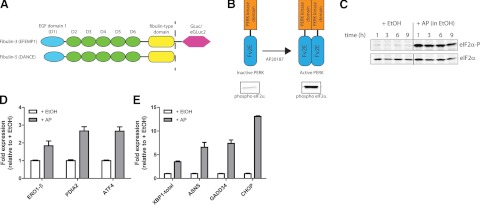
Figure 1.
Activation of Fv2E-PERK with the small molecule, AP20187 (AP), induces eIF2α phosphorylation, leading to translational attenuation and transcriptional up-regulation of ATF4-dependent genes. A) Comparison of fibulin-3 and fibulin-5 domain organization. Fibulin-5 bears >50% sequence similarity to fibulin-3. B) Schematic of AP-induced homodimerization and subsequent activation of PERK. C) Time course of AP-induced eIF2α phosphorylation in Fv2E-PERK-293 cells monitored by Western blotting using a phospho-specific (serine 51) eIF2α (eIF2α-P) antibody, and a total eIF2α antibody. D, E) Fv2E-PERK-293 cells were treated with 10 nM AP for 9 h, and the transcription of select genes was compared to EtOH-treated Fv2E-PERK-293 cells by qPCR (mean±95% CI). Data are representative of 3 independent experiments.
Adenovirus-mediated overexpression of R345W fibulin-3 [and, to a lesser extent, wild-type (WT) fibulin-3] in retinal pigmented epithelium (RPE) cells increased levels of BiP and spliced X-box binding protein 1 (XBP1s), indicating activation of the ATF6 and IRE1 arms of the UPR, respectively (the PERK arm was not investigated; ref. 18). An S227P mutation in fibulin-5, which causes CL, also appears to increase certain ER chaperones [BiP, protein disulfide isomerase (PDI) and calreticulin] relative to the levels in cells expressing WT fibulin-5, potentially indicating UPR activation (23). Thus, it would appear that modulation of the UPR is important for optimizing the ER folding and secretion capacity, as well as the ER-associated degradation (ERAD) of fibulins.
Activation of the third protein misfolding sensor in the ER, PERK, causes downstream signaling distinct from ATF6 and XBP1s, resulting in translational attenuation and/or apoptosis if proteostasis cannot be restored (24). Activation of the PERK stress-responsive signaling pathway by dissociation of BiP from its ER luminal domain results in the dimerization and autophosphorylation of PERK kinase domains, which, in turn, phosphorylate eukaryotic initiation factor 2α (eIF2α). Alternatively, eIF2α can also be phosphorylated in a PERK-independent manner following exposure to diverse cellular insults, including arsenite treatment [most likely through heme-regulated inhibitor eIF2α kinase (HRI); ref. 25]. Production of phosphorylated eIF2α (eIF2α-P) globally attenuates translation throughout the cell by binding to eIF2B, which prevents the transfer of methionyl-initiator tRNAMet to the small ribosomal subunit (26). Paradoxically, eIF2α-P induces reinitiation of activating transcription factor 4 (ATF4) translation through upstream open reading frames unique to a subset of mRNAs (27). ATF4 translocation to the nucleus up-regulates genes involved in UPR signaling, cellular redox maintenance, amino acid transport, and growth arrest. ATF4 nuclear localization also up-regulates growth arrest and DNA damage-inducible protein 34 (GADD34), a phosphatase regulatory subunit that negatively regulates the effects of eIF2α-P by dephosphorylating it (28).
Aside from the ATF4-mediated transcriptional effects, translational attenuation mediated by the production of eIF2α-P allows the cell to prevent the further accumulation of misfolded proteins by reducing synthesis and favoring an increase in the chaperone to misfolded protein ratio, thus favoring refolding or targeting the malfolded proteins to ERAD or lysosomal degradation (29). Consistent with this notion, PERK-knockout cells experience more ER stress when exposed to protein folding perturbations (30), and mice lacking PERK or bearing human loss-of-function mutations in PERK display a variety of defects in secretory cells, resulting in permanent neonatal diabetes, impaired bone development, and pancreatic dysfunction (31–34). However, it is important to note that while inactivation of PERK is detrimental to cellular homeostasis, conversely, sustained PERK activation is also unfavorable, as it can trigger a cellular signaling switch from prosurvival to proapoptosis when CCAAT/enhancer-binding protein-homologous protein (CHOP) expression is prolonged (reviewed in ref. 35). Overall, these studies underscore the importance of balancing PERK signaling in the maintenance of proteostasis and cellular homeostasis.
In this study, we explored whether translational attenuation (mediated by eIF2α-P or pharmacologic means) enhanced R345W fibulin-3 secretion and disease-associated mutant fibulin-5 secretion. Activation of the PERK arm of the UPR, without triggering protein misfolding or protein aggregation, enhanced mutant fibulin-3 proteostasis, leading to an increase in its cellular secretion by nearly three-fold. Utilization of pharmacologic and genetic experiments, revealed that enhanced R345W fibulin-3 secretion is due to translational attenuation via eIF2α-P and is independent of ATF4 signaling. In stark contrast, translational attenuation through eIF2α-P or cycloheximide (CHX) treatment of cells expressing mutant fibulin-5 did not lead to the enhanced secretion of disease-associated variants. Instead, our results indicate that translational attenuation significantly impairs fibulin-5 secretion even when translational attenuation is brief, suggesting that fibulin-5 requires the synthesis of additional proteins (or of additional fibulin-5) for its efficient secretion. These results begin to elucidate the differential influence of translational control on very similar proteins within the same family, and they suggest that it is possible to alter specific pathways within the protein homeostasis network of a cell to differentially affect the proteostasis of closely related disease-associated variants of a given protein.
MATERIALS AND METHODS
Construct generation
Fibulin-3-Gaussia luciferase (GLuc) constructs in the pcDNA-DEST40 destination vector (Life Technologies, Carlsbad, CA, USA) driven by the CMV promoter were generated and prepared as described previously (19). An enhanced GLuc (eGLuc2), which provided more “glow-like” luminescence characteristics than GLuc (36, 37), was generated by mutating oxidation-prone methionine 60 and methionine 127 both to isoleucine (M60I and M127I, equivalent to M43I and M110I in numbering without the signal sequence) via QuickChange mutagenesis (Agilent, Santa Clara, CA, USA). A carboxy-terminal eGLuc2 fusion to fibulin-3/5 was generated by PCR amplification using primers that eliminated the natural stop codon of fibulin-3/5 and inserted a flexible, 16-residue linker (sequence: FEGSAGSAAGSGEFEA; ref. 38). This gene was then ligated to eGLuc2 (without a start codon or signal sequence). Fusion genes were inserted into the pENTR1A Dual Selection plasmid (Life Technologies) using the DraI and XhoI restriction sites. These genes were then shuttled into the pLenti4/TO tetracycline/doxycycline-regulated vector or the pcDNA40-DEST vector (Life Technologies) by LR clonase II recombination. To generate fibulin-5 mutants, site-directed mutagenesis was performed using the QuickChange method (Agilent). All constructs were sequenced to verify their identity.
Cell culture
Human embryonic kidney (HEK) 293T cells (Life Technologies), HEK cells expressing the Fv2E-PERK fusion (Fv2E-PERK-293; gift of Dr. Jonathan Lin, University of California, San Diego, CA, USA), and HEK-293 cells stably expressing the tet repressor (TREx-293; Life Technologies) were maintained at 37°C (unless otherwise noted) in high-glucose DMEM (Cellgro; Mediatech, Manassas, VA, USA) supplemented with 10% FBS (Omega Scientific, Tarzana, CA, USA), glutamine, and 1% penicillin/streptomycin (Cellgro). Stable, heterogeneous pools of TREx-293 cells, which express WT or R345W fibulin-3 fused to eGLuc2 when tetracycline or doxycycline is present, were generated by lentiviral infection followed by selection in medium containing 5 μg/ml blasticidin and 200 μg/ml zeocin. Expression of fibulin-3 was induced only during individual experiments and never while propagating the TREx-293 cell lines. Stable pools were propagated in medium supplemented with 5 μg/ml blasticidin and 100 μg/ml zeocin. Selective antibiotics were excluded in wells used for experiments.
For PERK activation experiments, cells were transfected with GLuc or eGLuc2 constructs using FuGene6 or X-tremeGene9 (250 ng DNA and 1.5 μl of transfection reagent/well of a 24-well plate; Roche, Nutley, NJ, USA). Cells were transfected for 24 h, then replated into 24-well plates and allowed to attach overnight. Activation of PERK in Fv2E-PERK-293 cells was achieved with 10 nM of AP20187 (AP; Ariad, Cambridge, MA, USA), for up to 24 h.
For experiments involving arsenite, TREx-293 cells stably expressing WT or R345W fibulin-3-eGLuc2 were plated at a density of 15,000 cells/well of a 96-well plate in the presence of doxycycline (1 μg/ml) for 48 h to induce fibulin-3 expression. Cells were then treated with 0–100 μM sodium arsenite (Sigma, St. Louis, MO, USA) for up to 9 h. For experiments using a C-terminal variant (lacking residues 1–179) of GADD34 harboring a C-terminal FLAG tag or for experiments using green fluorescent protein (GFP) as a control, TREx-293 cells were plated at a density of 300,000–400,000 cells/well of a 6-well plate. Cells were transfected for 24 h, followed by replating in doxycycline-containing medium for an additional 48 h prior to arsenite treatment.
GLuc assay to quantify intracellular vs. secreted levels of fibulin-3 and fibulin-5
Secreted and intracellular GLuc activity was monitored as described previously (19). Briefly, secretion of fibulin-3/5-GLuc (or eGLuc2) was monitored by reacting 45-μl aliquots of conditioned medium (∼1/6 to 1/10 of the total medium volume) with 50 nl substrate diluted in 10 μl of neat GLuc buffer [BioLux Gaussia Luciferase kit, New England Biolabs (NEB), Ipswich, MA, USA]. Immediately after mixing, luminescence was measured in Costar flat-bottomed black assay plates (Corning, Corning, NY, USA) in a Safire II microplate reader (Tecan, Uppsala, Sweden) using a 100-ms integration time. Cell lysates were prepared by freeze/thaw (≥4×) in a dry ice/ethanol bath. An aliquot (25–40 μl, typically 1/2 to 1/4 of the total cell lysate) was used for the GLuc assay, employing the same substrate-buffer ratio as described for secreted GLuc.
Western blot analysis
For experiments measuring secreted fibulin, equal volumes (25–50 μl) of conditioned medium aliquots were denatured in reducing Laemmli buffer (unless otherwise indicated) by boiling at 95°C for 5 min, followed by loading onto a 4–20% Tris-glycine SDS-PAGE gel. eIF2α phosphorylation, GADD34, and GFP were monitored by Western blot analysis by loading 40–60 μg of protein in reducing SDS-PAGE sample buffer also on a 4–20% Tris-glycine SDS-PAGE. For blots probing for ATF4 and matrin-3, 30–50 μg of nuclear isolates were loaded. Proteins were transferred onto nitrocellulose membranes and blocked in 5% milk dissolved in PBS with Tween. Primary antibody dilutions were as follows: rabbit anti-GLuc (1:2000; NEB), mouse anti-eIF2α (total, 1:3000; Abcam, Cambridge, MA, USA), rabbit anti-eIF2α-P (phosphorylated at serine 51, 1:1000; Cell Signaling, Danvers, MA, USA), rabbit anti-ATF4 (1:500; Santa Cruz, Santa Cruz, CA, USA), rabbit anti-matrin-3 (1:5000; Bethel, Montgomery, TX, USA), or mouse anti-FLAG M2 (for CT-GADD34, 1:500; Agilent). GFP was visualized using a Typhoon Trio Variable Mode Imager (488-nm laser, 520-nm BP 40 emission filter; GE Healthcare, Waukesha, WI, USA). Blots were imaged on a LI-COR Odyssey infrared imager (LI-COR, Lincoln, NE, USA) using goat anti-rabbit IRDye 800-nm CW or goat anti-mouse IRDye 680-nm secondary antibodies (1:10,000; LI-COR).
Real-time quantitative PCR analysis
Cells were harvested by trypsinization and washed once in Dulbecco's PBS (Gibco; Life Technologies). RNA was extracted from the cells using the RNeasy kit (Qiagen, Germantown, CA, USA), according to the manufacturer's protocol. RNA (500 ng) was reverse transcribed using the Quantitect reverse transcription kit (Qiagen), as described previously (19).
qPCR primers were selected using Primer3 software (http://frodo.wi.mit.edu/primer3/) and were optimized for amplification of 130- to 180-bp amplicons with annealing temperatures centered around 62.5°C. qPCR primers were purchased from Integrated DNA Technologies (IDT; Coralville, IA, USA). cDNA was amplified using FastStart Universal SYBR Green Master Mix (Roche) on an ABI 7900 fast real-time PCR machine (ABI, Foster City, CA, USA), as described previously (19). The resultant data were analyzed using the relative quantification (RQ) Manager (ABI) followed by analysis in DataAssist (ABI). Transcripts were normalized to the housekeeping gene with primers targeting human RPLP2.
Small-interfering RNA (siRNA) transfection
Introduction of siRNAs into Fv2E-PERK-293 cells was achieved by Fast-Forward transfection using HiPerFect (Qiagen), according to the manufacturer's protocol. Briefly, 30 min prior to transfection, 60,000 cells were seeded in a 48-well plate and were transfected with 50 nM (final concentration) siRNA using 1.5 μl HiPerFect in 50 μl OptiMEM (Gibco; Life Technologies). Knockdown efficiency was assessed by qPCR and Western blot analysis between 24 and 48 h after transfection. For Western blot analysis, siRNA transfections were scaled to 6-cm dishes, according to Qiagen's Fast-Forward protocol. siRNAs were purchased from Dharmacon (Lafayette, CO, USA); siATF4 (On-Target Plus, J-005125-13-0005), siGAPDH (siGENOME, D-001140-01-05), and siGLO cyclophilin B control (D-001610-01-05).
RESULTS
The small-molecule AP20187 causes eIF2α phosphorylation and increases transcription of ATF4-dependent genes in Fv2E-PERK-293 cells
The hypothesis that up-regulation of the proteostasis capacity of the secretory pathway would increase mutant fibulin folding and secretion, taken together with our previous observation that reduced growth temperatures enhance fibulin-3 secretion, at least partially through translational attenuation (19), led us to first explore the effect of activation of the PERK arm of the UPR on the secretion of fibulin proteins. To achieve selective activation of the PERK arm of the UPR in the absence of triggering protein misfolding or aggregation in the ER (which could detrimentally affect fibulin secretion), we utilized the HEK-293-based cell culture system previously described by Lin et al. (39). This system uses the small-molecule AP20187 (AP) to induce the dimerization of an FK506-binding protein-derived Fv2E domain, which is fused to the PERK kinase domain (Fig. 1B). Dimerization of the Fv2E domain facilitated by AP causes the transautophosphorylation of the PERK kinase domains, thereby activating PERK (Fig. 1B). Treatment of Fv2E-PERK-293 cells with low levels of AP (10 nM) caused strong and sustained phosphorylation of eIF2α over a period of 9 h (Fig. 1C). Furthermore, AP treatment of Fv2E-PERK-293 cells also caused the transcriptional up-regulation of genes typically associated with increased ATF4 expression, including endoplasmic oxidoreductin-1-like-β (ERO1-β), PDIA2, ATF4, total XBP1 (XBP1-total), asparagine synthetase (ASNS), GADD34, and CHOP (Fig. 1D, E), but did not increase the ATF6 or XBP1s-dependent genes (i.e., BiP or ERdj4, respectively; Supplemental Fig. S1A).
PERK activation enhances mutant fibulin-3 secretion
To examine the effect of PERK activation on mutant fibulin-3 secretion, Fv2E-PERK-293 cells were transfected with either WT or R345W fibulin-3-GLuc followed by treatment with AP for up to 24 h. Without AP-mediated PERK activation, R345W was secreted at levels similar to those observed previously in HEK-293T cells (∼15% relative to WT; Fig. 2A; 19). AP-mediated PERK activation significantly increased R345W secretion after 3 h of treatment, raising the medium levels of the mutant relative to WT fibulin-3 from 13 ± 2.7 to 19 ± 3% (Fig. 2A). The enhanced secretion of R345W fibulin-3 was most pronounced at 9 h after AP treatment (15±4% with vehicle vs. 44±12% of WT fibulin-3 levels with AP, Fig. 2A). The beneficial influence of PERK activation on R345W fibulin-3 secretion, as measured by the GLuc luminescence assay, was also verified by Western blot analysis of conditioned medium from transfected cells (Fig. 2B). The increased pool of secreted R345W protein resulting from AP-mediated PERK activation appears to be properly folded, as ascertained by comparisons to WT fibulin-3. Secreted WT and R345W migrate analogously in a nonreducing SDS-PAGE gel, with or without AP treatment (Fig. 2C). Moreover, >77% of the enhanced secreted R345W pool comigrates with the major WT species on a nonreducing SDS-PAGE gel (Fig. 2C and Supplemental Fig. S1B). In addition, by using a protein backbone chemical cleavage strategy (BNPS skatole, which cleaves the backbone C-terminally to Trp residues; ref. 19), we demonstrate that the amount of R345W fibulin-3 that is secreted (and the enhanced pool of secreted R345W after PERK activation) appears to exhibit a similar disulfide bonding pattern when compared to secreted WT fibulin-3 (Supplemental Fig. S1C, D). However, BNPS skatole cleavage of intracellular R345W fibulin-3 reveals that it has defects in its ability to form native disulfide bonds relative to WT fibulin-3 (Supplemental Fig. 1C, D). Collectively, these observations support the hypothesis that PERK activation enhances the secretion of correctly folded R345W fibulin-3.
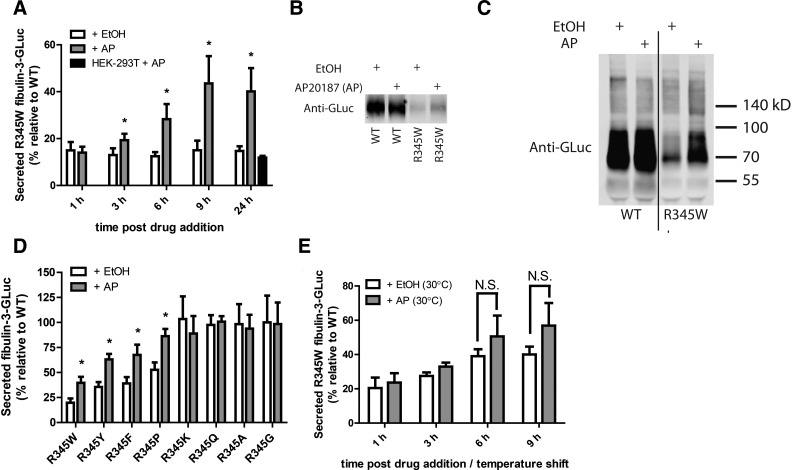
Figure 2.
Activation of Fv2E-PERK by AP treatment enhances R345W fibulin-3-GLuc secretion. A) Transfected Fv2E-PERK-293 cells were treated with 10 nM AP or vehicle, ethanol (EtOH), for up to 24 h. AP-mediated PERK activation enhances R345W secretion, whereas AP has no effect on R345W secretion in HEK-293T cells lacking dimerizable PERK (n≥3±sd). *P < 0.05; t test. B) Enhanced R345W secretion confirmed by Western blotting. Aliquots of conditioned medium from transfected, AP- or EtOH-treated (9 h) Fv2E-PERK-293 cells were probed using an anti-GLuc antibody. Data are representative of 3 independent experiments. C) Pool of secreted R345W enhanced as a consequence of AP treatment migrates analogously to WT under nonreducing conditions. Aliquots of conditioned medium from transfected, AP- or EtOH-treated (9 h) Fv2E-PERK-293 cells were probed using an anti-GLuc antibody. Data are representative of 3 independent experiments. D) PERK activation enhances the secretion of other poorly secreted fibulin-3 position 345 mutants. Transfected Fv2E-PERK-293 cells were treated with AP or EtOH for 9 h (n≥3±sd). *P < 0.05, t test. E) Temperature reduction and PERK activation do not synergistically enhance R345W secretion. Transfected Fv2E-PERK-293 cells were grown at 30°C for up to 24 h in the presence of 10 nM AP or EtOH (n≥3±sd, N.S., not significant). GLuc luminescence assay was used to monitor fibulin-3 secretion (A, D, E).
AP treatment of transfected HEK-293T cells lacking the Fv2E-PERK construct did not alter the relative secretion of R345W vs. WT when compared to vehicle-treated cells, demonstrating the dependence on PERK activation for increased secretion (Fig. 2A). Interestingly, cellular activation of PERK with AP (10 nM) for only 1 h followed by a medium change was sufficient to generate enhanced secretion effects for up to 24 h (Supplemental Fig. S1E). The absolute (not relative) levels of secreted WT fibulin-3 were not detrimentally affected by AP treatment for up to 9 h of AP incubation but were impaired after 24 h of sustained AP treatment (Supplemental Fig. S1F). In contrast, absolute medium levels of R345W fibulin-3 continued to increase over the 24-h time course of cellular exposure to AP (10 nM; Supplemental Fig. S1F). Because of the detrimental effect of sustained PERK activation on WT fibulin-3 secretion over 24 h, we chose to monitor fibulin-3 secretion after 9 h of AP cellular exposure, as only a moderate decrease in cellular growth/viability after 9 h of AP administration was observed (Supplemental Fig. S2A). To exclude the possibility that AP could be altering fibulin protein levels by changing its transcript levels, we analyzed fibulin and GLuc mRNA levels with and without AP treatment and found no significant differences in WT or R345W fibulin-3 GLuc mRNA levels (Supplemental Fig. S2B). In addition, enhanced secretion effects observed after PERK activation are most likely not due to global UPR activation, since treatment of HEK-293T cells with low levels of the ER SERCA pump inhibitor, thapsigargin, significantly decreased WT and R345W fibulin-3 secretion (Supplemental Fig. S2C), consistent with the observation that the GLuc protein alone could be used as a sensor of global UPR activation (40).
In addition to the enhanced secretion of the pathogenic R345W mutation, AP-mediated PERK activation was also able to significantly increase the relative medium concentrations of other poorly secreted fibulin-3 variants including R345Y (36±5 to 63±6%), R345F (39±6 to 68±10%), and R345P (53±7 to 86±7%) (percentage relative to WT; Fig. 2D). Previously, we showed that a cell growth temperature reduction to 30°C enhanced the secretion of these mutant fibulin-3 proteins. However, the combination of a permissive temperature and AP-mediated PERK activation was not additive in increasing R345W secretion relative to WT (Fig. 2E), indicating that the two treatments are most likely acting through the same or an overlapping mechanism (e.g., translational attenuation).
ATF4 is not required for enhanced fibulin-3 secretion
Because the activation of PERK leads to the production of ATF4 and its downstream transcriptome and proteome (41), we sought to determine the effect of the downstream ATF4 transcriptional program after PERK activation in the context of fibulin-3 secretion using siRNA to reduce cellular ATF4 levels in Fv2E-PERK-293 cells (Fig. 3A, B). The mRNA levels of ATF4 were reduced by >62% in both WT and R345W fibulin-3-expressing cells 24–48 h after transfection with siRNA against ATF4 (siATF4; Fig. 3A). Moreover, nuclear extracts from Fv2E-PERK cells treated with ATF4 siRNA showed lower levels of ATF4 protein after 9 h of AP treatment, indicating successful knockdown at the protein level (Fig. 3B). ATF4 knockdown prevented the transcriptional up-regulation of select ATF4 downstream genes, including XBP1-total and ASNS after 9 h of AP treatment (Fig. 3A), indicating that the transcription of these genes is most likely strictly ATF4-dependent in the context of PERK activation. Surprisingly, some transcripts, such as CHOP, were not dramatically reduced in ATF4-knockdown cells (Fig. 3A), suggesting that their induction could be originating from a yet to be uncovered ATF4-independent, PERK-dependent pathway (42). Notably, knockdown of ATF4 in Fv2E-PERK-293 cells did not prevent the AP-triggered PERK-mediated enhancement of R345W fibulin-3 secretion when compared to untransfected cells or cells transfected with a control siRNA against GAPDH (siGAPDH, Fig. 3C). These data suggest that the PERK-mediated enhanced R345W fibulin-3 secretion is most likely independent of ATF4 signaling.
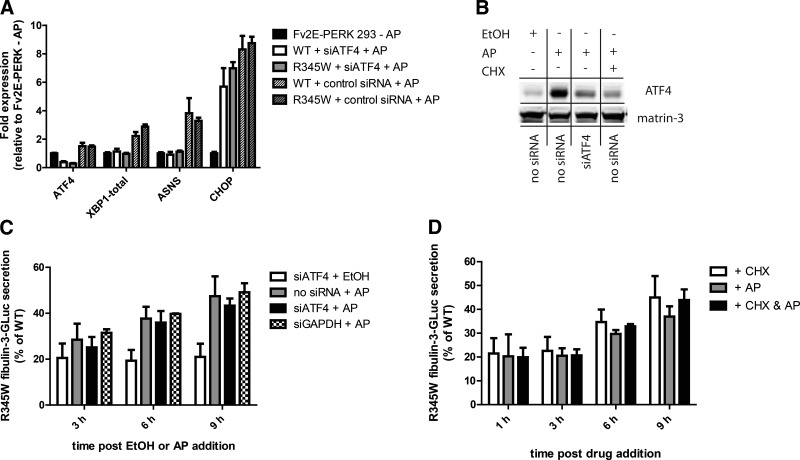
Figure 3.
ATF4-dependent genes are not required for enhanced R345W secretion. A) Confirmation of ATF4 knockdown by qPCR and by transcript analysis of ATF4 target genes (mean±95% CI). Data are representative of 3 independent experiments. B) siRNA against ATF4 or CHX treatment reduces AP-induced ATF4 protein levels. Fv2e-PERK cells were untransfected or transfected with siRNA against ATF4 for 48 h, followed by treatment with AP for 9 h. C) Knockdown of ATF4 has no effect on AP-mediated R345W enhanced secretion. Fv2E-PERK-293 cells were transfected with fibulin-3 constructs for 24 h, followed by transfection of the indicated siRNAs for an additional 24–48 h. Cells were treated with AP (10 nM) for up to 9 h (n≥3±sd; n=2±sd for siGAPDH and siATF4+EtOH). D) Nonspecific inhibition of translation using CHX has no effect on AP-enhancement of R345W secretion. Transfected Fv2E-PERK-293 cells were treated with either AP (10 nM), CHX (25 μM), or the combination thereof for up to 9 h (n≥3±sd). GLuc assay was used to monitor fibulin-3 secretion (C, D).
As a complementary approach to the siRNA knockdown of PERK-mediated ATF4 signaling, we also used CHX to prevent the translation of any new proteins, including ATF4 (Fig. 3B). Similar to what we observed with ATF4 knockdown, R345W fibulin-3 secretion was still enhanced when Fv2E-PERK cells were cotreated with AP and 25 μM CHX (Fig. 3D). These results indicate that translation is not required for increased R345W secretion after AP treatment. Moreover, CHX treatment alone enhances mutant fibulin-3 secretion (Fig. 3D). Collective consideration of these data and the ATF4 knockdown data suggest that the mechanism behind the PERK activation-mediated increase in R345W fibulin-3 secretion is likely due to the established translational attenuation effects of eIF2α-P.
PERK-independent eIF2α phosphorylation by way of arsenite treatment also increases R345W fibulin-3 secretion
eIF2α can be phosphorylated by at least three other kinases in addition to PERK, one of which is the HRI kinase (25). This kinase can be activated in nonerythroid cells through a heme-independent mechanism via the disruption of a repressive chaperone complex, potentially though oxidative stress (25). We explored the possibility that mutant fibulin-3 secretion could also be enhanced by UPR-independent means of generating eIF2α-P, namely, through HRI activation by the chemical perturbant, arsenite (24, 43). Treatment of TREx-293 cells with 100 μM arsenite increased the phosphorylation of eIF2α within 1 h of arsenite addition (Fig. 4A), and levels of eIF2α-P remained elevated for up to 3 h, after which eIF2α-P levels returned to baseline (Fig. 4A), most likely because of the expression of the stress-inducible eIF2α phosphatase regulatory subunit, GADD34 (44). Arsenite treatment (25–100 μM) of TREx-293 cells expressing a doxycycline-inducible WT or R345W fibulin-3 fused to an eGLuc2 (36, 37) increased the secretion of R345W fibulin-3 to levels up to 35% above those of untreated R345W-expressing cells at 9 h, based on luminescence measurements (Fig. 4B). In contrast, the secretion of WT fibulin-3 decreased by >40% relative to untreated WT-expressing cells after arsenite treatment (100 μM; Fig. 4B). Similar trends in R345W and WT fibulin-3 secretion were observed at earlier timepoints, peaking at 6 h after initial administration of arsenite (100 μM; Fig. 4C).
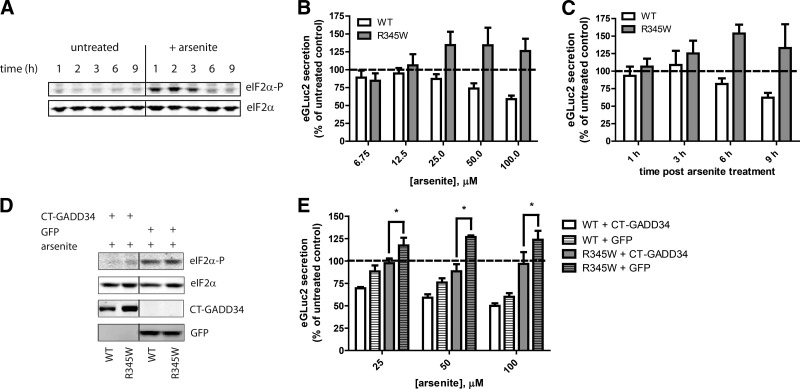
Figure 4.
Induction of eIF2α phosphorylation through arsenite treatment enhances R345W fibulin-3-eGLuc2 secretion. A) TREx-293 cells were treated with 100 μM arsenite for up to 9 h, and cell lysates were probed for eIF2α-P and total eIF2α by Western blot analysis. Data are representative of 3 independent experiments. B) TREx-293 cells expressing WT or R345W fibulin-3-eGLuc2 were treated with increasing concentrations of arsenite (6.75-100 μM) for 9 h (n≥3±sd). C) Time course of the effect of arsenite (100 μM) on fibulin-3 secretion in TREx-293 cells expressing WT or R345W fibulin-3-eGLuc2 (n≥3±sd). D) TREx-293T cells expressing WT or R345W fibulin-3 eGLuc2 were transfected with CT-GADD34 or GFP and treated with 100 μM arsenite for 3 h. Lysates were analyzed by Western blot analysis as described above. Data are representative of 3 independent experiments. E) Expression of CT-GADD34 eliminates arsenite-enhanced R345W secretion. Transfected cells were treated with 25-100 μM arsenite for 9 h (n≥3±sd), as indicated. *P < 0.05, t test. GLuc luminescence assay was used to monitor fibulin-3 secretion (B, C, E).
To demonstrate that the arsenite-mediated influence on fibulin-3 secretion was dependent on eIF2α phosphorylation and not a nonspecific effect, such as altering oxidative stress (45), we overexpressed a constitutively active C-terminal variant of GADD34 [CT-GADD34, described previously in (28) in TREx-293 cells prior to arsenite treatment. Overexpression of CT-GADD34, but not GFP, significantly lowered the amount of eIF2α-P after arsenite treatment, reducing levels to those observed in untreated cells (cf. Fig. 4A, D). Expression of CT-GADD34 significantly mitigated the arsenite-mediated increase in R345W fibulin-3 secretion (compared to GFP-transfected cells) at all arsenite concentrations used, reducing R345W secretion by 20, 38, and 27% at arsenite concentrations of 25, 50, and 100 μM, respectively (Fig. 4E). These results reaffirm the involvement of eIF2α-P in influencing the ability of cells to secrete mutant fibulin-3 and demonstrate that multiple pathways, which culminate in eIF2α-P-linked translational attenuation can significantly alter the fate of mutant fibulin-3, in this case, ultimately increasing its secretion efficiency.
Fusion of fibulin-5 to GLuc allows for its sensitive luminescence detection, and this fusion recapitulates mutant-associated secretion defects of untagged fibulin-5
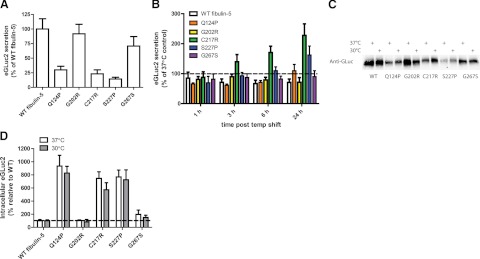
Because the domain organization and sequence composition of fibulin-5 is analogous to that of fibulin-3 (Fig. 1A), we hypothesized that the same GLuc fusion-based strategy used to monitor the secretion of fibulin-3 could also be applied to accurately quantify secreted and intracellular concentration differences between WT and AMD- or CL-associated mutations in fibulin-5 (10, 21). Indeed, by quantifying GLuc luminescence, we determined that the medium concentrations of two mutants of fibulin-5 associated with the development of AMD (Q124P and G267S; ref. 10) were reduced to 30 ± 6.3 and 71 ± 16% of WT fibulin-5 levels, respectively (Fig. 5A). Two fibulin-5 mutants linked to CL, C217R (21, 23) and S227P (46), also displayed dramatic cellular secretion deficits (23±7 and 14±3% of WT levels, respectively, Fig. 5A). As a demonstration that this assay most likely accurately reflects changes in fibulin-5 secretion efficiencies, a nondisease-associated missense mutation in fibulin-5 (G202R) had no detrimental effect on fibulin-5 secretion (91±16% of WT levels), in accordance with previous studies (10, 12, 23).
Figure 5.
Fibulin-5-eGLuc2 fusion proteins recapitulate secretion defects caused by disease-associated mutants. A) HEK-293T cells were transfected with WT fibulin-5-eGLuc2 or fibulin-5 variants associated with AMD (Q124P, G267S), CL (C217R, S227P), or a nondisease-associated G202R mutation. At 72 h post-transfection, fibulin-5-eGLuc2 secretion was measured by GLuc luminescence assay (n≥3±sd). B) HEK-293T cells were transfected with the indicated variants for 48 h, followed by temperature reduction to 30°C for up to 24 h (n≥3±sd). C) Medium aliquots from HEK-293T cells transfected for 48 h, followed by incubation at 37°C or 30°C for an additional 24 h. Blots were probed with an anti-GLuc antibody. Note the slight increase in apparent molecular weight of C217R and S227P. Data are representative of 3 independent experiments. D) Poorly secreted fibulin-5 variants have high intracellular steady state levels. HEK-293T cells were transfected with the indicated constructs for 48 h, followed by an additional 24-h incubation at 30 °C or 37°C (n≥3±sd).
Because disease-associated mutations in fibulin-5 caused secretion defects analogous to those observed with R345W fibulin-3, we rationalized that conditions that enhance mutant fibulin-3 secretion may also rescue the secretion defects caused by fibulin-5 mutations. Therefore, we explored whether a permissive growth temperature and/or translational attenuation would enhance mutant fibulin-5 secretion. Growth of fibulin-5-transfected HEK-293T cells at subphysiological temperatures (30°C) for 24 h resulted in the enhanced secretion of the CL-related mutants, C217R and S227P, increasing their medium concentrations to 227 ± 38 and 162 ± 30%, respectively, relative to identically transfected cells grown at 37°C (Fig. 5B). Surprisingly, C217R fibulin-5 was most amenable to the influence of a permissive temperature, at odds with our previous observation that any mutation that eliminated a Cys in domain 6 of fibulin-3 prevented its secretion at either 37°C or 30°C (19). The increased levels of secreted C217R fibulin-5 were apparent after only 3 h of temperature reduction and continued to increase over the course of the experiment (Fig. 5B). The S227P mutant, however, required 24 h of reduced growth temperature to observe dramatically enhanced secretion (Fig. 5B). Interestingly, the two AMD-linked mutants, Q124P and G267S, were relatively unaffected by up to 24 h of incubation at what is a permissive temperature for the other variants (Fig. 5B). The secretion of WT fibulin-5 and the similarly secreted mutant, G202R, were detrimentally affected by a reduced growth temperature, decreasing their medium levels after 24 h by 27 and 39%, respectively (Fig. 5B). To ensure that the GLuc assay was measuring total fibulin-5 in the medium and not just folded fibulin-5, we assessed GLuc-fusion protein levels in the conditioned medium of transfected cells by Western blot analysis (Fig. 5C). As expected, the GLuc and Western blot analysis results were in agreement (cf. Fig. 5A, C), with the luciferase activity and medium fibulin-5 concentration exhibiting a linear correlation (Supplemental Fig. S3A; see Supplemental Fig. S3B for 30°C band intensity quantification). A closer look at fibulin-5 after Western blotting reveals a slight increase in the apparent molecular weight of CL-associated variants when compared to WT or AMD-linked fibulin-5 proteins (Fig. 5C). This molecular weight difference is also apparent under nonreducing conditions (Supplemental Fig. S3C), suggesting that this change most likely does not arise from aberrant disulfide bonding. Transfection of WT and C217R fibulin-5 constructs into HEK-293S cells that are deficient in N-acetylglucosaminyltransferase I activity (which prevents the remodeling of N-linked glycans in the Golgi) suggests that this modification is also not due to excessive glycosylation (Supplemental Fig. S3D). This apparent post-translational modification or altered protein structure, while currently undetermined, merits consideration when probing the etiology of CL.
To account for the total distribution of fibulin-5 protein, we also assessed the intracellular levels of the eGLuc2 fusion protein after transfection. Similar to what we, and other groups, had observed for mutant fibulin-3 (19, 20), mutations in fibulin-5 that cause inefficient secretion also exhibit higher intracellular steady state levels of fibulin-5, suggesting increased ER accumulation (Fig. 5D). However, one key distinction is that intracellular levels of fibulin-5 mutants are much higher (relative to WT fibulin-5) than those of fibulin-3 (19). Whereas R345W fibulin-3 accumulated inside of cells up to 2 times WT levels, mutations that caused severe defects in fibulin-5 secretion (Q124P, C217R, S227P) amassed intracellularly at levels 7.47–9.34 times those of WT fibulin-5 (Fig. 5D). The G267S mutation, which modestly reduced fibulin-5 secretion, also accumulated intracellularly, albeit to a lesser extent (1.98±0.7-fold more than WT; Fig. 5D). The G202R mutation did not significantly affect fibulin-5 intracellular levels relative to WT fibulin-5 (Fig. 5D), as expected.
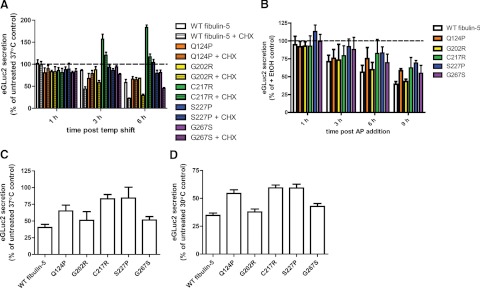
Translational attenuation initiated by PERK activation or chemical means does not enhance secretion of inefficiently secreted fibulin-5 mutants
To assess whether the enhanced secretion of C217R fibulin-5 at a lower, or “permissive,” growth temperature was due to translational attenuation, we performed two types of follow-up experiments. First, we treated fibulin-5-transfected HEK-293T cells with high levels of CHX, and second, fibulin-5-transfected Fv2E-PERK-293 cells were treated with AP to activate PERK. Surprisingly, in contrast to our observations that general translational attenuation enhances R345W fibulin-3 secretion, high amounts of CHX actually nullified the enhanced fibulin-5 secretion mediated by permissive growth temperatures (Fig. 6A). The secreted levels of C217R after 3 h of temperature reduction decreased from 157 ± 19% of 37°C levels to 120 ± 12% with addition of 25 μM CHX (Fig. 6A). This decrease was even more pronounced after 6 h of CHX treatment, at which time levels of C217R in the medium were reduced from 184 ± 10% without CHX to 117 ± 11% with CHX treatment (Fig. 6A). The secretion of all other fibulin variants except for Q124P were also detrimentally affected by the combination of temperature reduction and CHX treatment, by an average of 34% relative to untreated cells grown at 37°C (Fig. 6A). Interestingly, while the total levels of secreted Q124P were reduced by growth at 30°C, these levels were not further reduced by CHX addition (Fig. 6A). These results suggest that the folding landscape and secretion of Q124P at 30°C may be governed differently than the other fibulin-5 variants. Consistent with this idea, Q124P secreted from cells grown at 30°C for 24 h clearly forms disulfide-linked aggregates more so than any other fibulin-5 variant based on nonreducing SDS-PAGE analysis (Supplemental Fig. S3C).
Figure 6.
New translation is required for enhanced secretion of C217R and S227P fibulin-5-eGLuc2. A) Translational attenuation via CHX negates the enhanced secretion mediated by temperature reduction. Transfected HEK-293T cells were incubated at 30°C without or with 25 μM CHX for up to 6 h (n≥3±sd). B) Fv2E-PERK cells were transfected with fibulin-5-eGLuc2 variants for 48 h, followed by treatment with AP (10 nM) for up to 9 h (n≥3±sd). C, D) CHX (1 μM, 24 h) treatment does not enhance mutant fibulin-5 secretion at 37°C (C) or 30°C (D). Fibulin-5 secretion was measured by the GLuc luminescence assay in all panels (n≥3±sd).
Other mechanisms of translational attenuation, such as PERK activation (Fig. 6B) or more gentle pharmacological translational attenuation (1 μM CHX) for a longer time (24 h; Fig. 6C, D), also reduced fibulin-5 secretion progressively over the course of the experiment and did not enhance the secretion of the temperature-sensitive mutants, C217R or S227P (Fig. 6B–D). Intriguingly, the secretion of fibulin-5 variants, which were poorly secreted at 37°C and retained intracellularly, were less affected by temperature reduction or translational attenuation by PERK activation or CHX (Fig. 6). These results suggest that the enhanced secretion of C217R and S227P at permissive temperature is not due to translational attenuation, but instead may be due to other effects that temperature reduction has on the cell or fibulin-5 protein folding.
DISCUSSION
Mutations leading to folding and secretion deficiencies in fibulin proteins targeted to the ECM cause a variety of organismal problems and diseases, including hemorrhaging, herniation, loose skin (CL), and retinopathies (ML and AMD; reviewed in ref. 2). To date, there are no known pharmacological agents or treatments that can be used for fibulin-associated diseases. Toward this end, we have demonstrated that translational attenuation, either through eIF2α-P (mediated by AP or arsenite) or CHX treatment, improves the secretion of a disease-associated fibulin-3 mutant (R345W) and inhibits the secretion of WT and mutant fibulin-5 proteins. Interestingly, secretion defects caused by certain mutations in fibulin-3 (R345 mutations) and fibulin-5 (C217R, S227P) proteins can be partially rescued by reduced growth temperatures, yet the mechanisms behind this enhancement are distinct for each protein. Enhanced mutant fibulin-3 secretion is nearly entirely dependent on translational attenuation, whereas mutation-specific enhanced fibulin-5 secretion may be a result of the “cold shock” transcriptional program, consistent with the requirement for protein synthesis to observe enhanced secretion at 30°C.
The reasons why the fates of these two highly homologous proteins are differently affected by translational attenuation is still unclear. However, we speculate that one key factor that likely contributes to this effect is the kinetics by which fibulin-3 and fibulin-5 fold and are secreted. Quickly folding, efficiently secreted proteins are likely to be adversely affected by translational attenuation since there is less of an intracellular steady-state pool of protein that can fold and be secreted once translation is halted. On the other hand, slowly folding proteins have higher steady-state intracellular levels, and translational attenuation would be predicted to have less of an effect on their secretion, since the intracellular reserves can still eventually fold and be secreted through increased chaperone and folding enzyme stoichiometry within the ER (47). In agreement with this idea, a direct comparison between the absolute secretion and intracellular accumulation of WT fibulin-3 and WT fibulin-5 shows that fibulin-3 is less efficiently secreted from HEK-293T cells than fibulin-5 (Supplemental Fig. S4A). Conversely, the intracellular levels of fibulin-3 at 37°C were substantially higher than those of fibulin-5 (Supplemental Fig. S4B). Thus, fibulin-3 seems to fold more slowly and has a much larger intracellular protein pool when compared to that of fibulin-5. The differences in the steady-state levels of intracellular fibulin could explain why growth at reduced temperatures and gentle translational attenuation have no apparent effect on WT fibulin-3 secretion, but reduce WT fibulin-5 by >30%. However, it is important to note that intracellular buildup of fibulin-5 mutants does not guarantee that the alterations to the proteostasis network that we have performed (temperature reduction and/or translational attenuation) will enhance the secretion of variants that are largely unfoldable (e.g., Q124P).
Another aspect that may influence the different response of fibulin-3 and fibulin-5 mutants to permissive temperatures is the quaternary structure of the secreted protein. WT and R345W fibulin-3 are predicted to exist as monomers under physiological conditions (1, 20), whereas fibulin-5 has been demonstrated to form dimers in solution (48, 49). While the Q124P and G267S mutations did not significantly affect fibulin-5 dimerization in a previous study, C217R and S227P were shown to dimerize more readily than WT fibulin-5 (48). Assuming that dimerization of fibulin-5 may be necessary for secretion, it is possible that growth at lower temperatures may shift the dimerization equilibrium toward folded dimer for certain fibulin-5 variants (i.e., C217R and S227P), while shifting the equilibrium toward monomer for other fibulin-5 mutants (i.e., WT and AMD-related mutants). A similar phenomenon has been observed with the temperature-sensitive mutant capsid proteins of phage P22 (50, 51).
Our experiments demonstrating that arsenite treatment can also enhance fibulin-3 secretion, while strictly proof of principle, provide compelling data that the common downstream effector, eIF2α-P, can enhance fibulin-3 folding, most likely through indirect means (i.e., by shifting the chaperone to protein stoichiometry in the ER). These results also suggest that in addition to ER-specific manipulation of the proteostasis network (i.e., PERK activation), compounds that target cytosolic components (i.e., arsenite) can also dramatically alter the fate of fibulin-3, as has been demonstrated with other ECM proteins, such as matrix metalloproteinase-2 and -9 (52). While mutant fibulin-3 secretion was increased with arsenite, WT fibulin-3 secretion was dramatically reduced, by nearly 40%. Given that an increase in oxidative stress has been broadly implicated in the progression of AMD (reviewed in ref. 53), and that arsenite has been shown to increase vascular endothelial growth factor levels (54), it is intriguing to speculate that exposure to arsenite (or other environmental toxins) could also affect AMD progression by altering the secretion of fibulin-3 or other critical ECM proteins.
PERK activation or arsenite treatment as ways to increase mutant fibulin-3 secretion are unlikely to be employed to fight ML due to the potential for apoptosis and toxicity. However, our results suggest that slowing the rate of protein synthesis and/or increasing the stoichiometry of proteostasis network components relative to challenging-to-fold client proteins may translate into a therapeutic strategy to enhance the proper folding and secretion of aggregation and/or misfolding-prone fibulin proteins (55, 56). This may be achieved through genetic manipulation of proteins that interact with nascent fibulin-3 or are involved in new protein translocation into the ER, such as Sec61, the ER translocon; BiP, a regulator of ER entry; signal recognition particles; or even oligosaccharyltransferase (reviewed in ref. 57). We anticipate that alteration of one or more of these components may yield an enhancement of mutant fibulin-3 secretion on par with translational attenuation, but without the detrimental global attenuation of protein synthesis.
Our data provide the first evidence that the proteostasis network can be adapted to partially rescue the folding and secretion of CL-associated fibulin-5 mutants. Although there currently is no definitive activity assay for evaluating whether R345W fibulin-3 is active after its secretion, fibulin-5 activity can be tested by measuring its binding to elastic fiber components, such as lysyl oxidase (58) or elastin (59). Since C217R and S227P fibulin-5 display partial tropoelastin binding when compared to WT fibulin-5 (23), it is possible that by increasing the medium concentrations of these variants, one might delay the onset or severity of CL, assuming this is a loss-of-function disease.
The success of applying the Fv2E-PERK system to activate PERK signaling to enhance the folding and secretion of mutant fibulin-3 leads us to hypothesize that the selective activation of one or more of the other two arms of the UPR (IRE1 and/or ATF6 pathways) may also alter mutant fibulin proteostasis. Given the differences among the downstream gene targets of each of the arms, it is feasible to imagine that individual activation of each of the UPR signaling pathways may alter fibulin fate differentially, potentially targeting disease-causing mutants toward degradation, which could be useful if ML turns out to be a gain-of-toxic-function disease associated with extracellular mutant fibulin-3 misfolding and/or aggregation. Overall, these data suggest that alteration of the proteostasis network capacity via specific stress-responsive signaling pathways or by more targeted approaches can be employed to selectively increase or decrease mutant fibulin folding and secretion, a hypothesis that we intend to exploit to identify practical therapies for mutant fibulin-associated diseases.
Supplementary Material
Acknowledgments
The authors thank R. Luke Wiseman for his insightful review of the manuscript.
This work was supported by the Lita Annenberg Hazen Foundation, the Skaggs Institute for Chemical Biology, grant UL1 RR025774 from the Scripps Translational Science Institute (J.W.K.), and grant AG018917 from the U.S. National Institutes of Health (J.W.K.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AMD
- age-related macular degeneration
- AP
- AP20187
- ATF4
- activating transcription factor 4
- ATF6
- activating transcription factor 6
- ASNS
- asparagine synthetase
- BiP
- binding immunoglobulin protein
- cbEGF
- calcium-binding epidermal growth factor
- CL
- cutis laxa
- CHOP
- CCAAT/enhancer-binding protein-homologous protein
- CHX
- cycloheximide
- ECM
- extracellular matrix
- eIF2α
- eukaryotic initiation factor 2α
- eIF2α-P
- phosphorylated eukaryotic initiation factor 2α
- eGLuc2
- enhanced Gaussia luciferase 2
- ER
- endoplasmic reticulum
- ERAD
- endoplasmic reticulum-associated degradation
- ERO1-β
- endoplasmic oxidoreductin 1β
- GADD34
- growth arrest and DNA damage-inducible protein 34
- GFP
- green fluorescent protein
- GLuc
- Gaussia luciferase
- HEK
- human embryonic kidney
- HRI
- heme-regulated inhibitor eIF2α kinase
- IRE1
- inositol-requiring enzyme 1
- ML
- malattia leventinese
- PDI
- protein disulfide isomerase
- PERK
- PKR-like endoplasmic reticulum kinase
- siRNA
- small interfering RNA
- XBP1
- X-box binding protein 1
- XBP1s
- spliced X-box binding protein 1
- UPR
- unfolded protein response
- WT
- wild type
REFERENCES
- 1. Zhang Y., Marmorstein L. Y. (2010) Focus on molecules: fibulin-3 (EFEMP1). Exp. Eye Res. 90, 374–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Argraves W. S., Greene L. M., Cooley M. A., Gallagher W. M. (2003) Fibulins: physiological and disease perspectives. EMBO Rep. 4, 1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stone E. M., Lotery A. J., Munier F. L., Heon E., Piguet B., Guymer R. H., Vandenburgh K., Cousin P., Nishimura D., Swiderski R. E., Silvestri G., Mackey D. A., Hageman G. S., Bird A. C., Sheffield V. C., Schorderet D. F. (1999) A single EFEMP1 mutation associated with both malattia leventinese and Doyne honeycomb retinal dystrophy. Nat. Genet. 22, 199–202 [DOI] [PubMed] [Google Scholar]
- 4. Marmorstein L. Y., McLaughlin P. J., Peachey N. S., Sasaki T., Marmorstein A. D. (2007) Formation and progression of sub-retinal pigment epithelium deposits in Efemp1 mutation knock-in mice: a model for the early pathogenic course of macular degeneration. Hum. Mol. Genet. 16, 2423–2432 [DOI] [PubMed] [Google Scholar]
- 5. Fu L., Garland D., Yang Z., Shukla D., Rajendran A., Pearson E., Stone E. M., Zhang K., Pierce E. A. (2007) The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Hum. Mol. Genet. 16, 2411–2422 [DOI] [PubMed] [Google Scholar]
- 6. Tielsch J. M., Javitt J. C., Coleman A., Katz J., Sommer A. (1995) The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N. Engl. J. Med. 332, 1205–1209 [DOI] [PubMed] [Google Scholar]
- 7. Attebo K., Mitchell P., Smith W. (1996) Visual acuity and the causes of visual loss in Australia. The Blue Mountains Eye Study. Ophthalmology 103, 357–364 [DOI] [PubMed] [Google Scholar]
- 8. Klaver C. C., Wolfs R. C., Vingerling J. R., Hofman A., de Jong P. T. (1998) Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch. Ophthalmol. 116, 653–658 [DOI] [PubMed] [Google Scholar]
- 9. Weigell-Weber M., Sarra G. M., Kotzot D., Sandkuijl L., Messmer E., Hergersberg M. (2003) Genomewide homozygosity mapping and molecular analysis of a candidate gene located on 22q13 (fibulin-1) in a previously undescribed vitreoretinal dystrophy. Arch. Ophthalmol. 121, 1184–1188 [DOI] [PubMed] [Google Scholar]
- 10. Lotery A. J., Baas D., Ridley C., Jones R. P., Klaver C. C., Stone E., Nakamura T., Luff A., Griffiths H., Wang T., Bergen A. A., Trump D. (2006) Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Human Mutation 27, 568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stone E. M., Braun T. A., Russell S. R., Kuehn M. H., Lotery A. J., Moore P. A., Eastman C. G., Casavant T. L., Sheffield V. C. (2004) Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 351, 346–353 [DOI] [PubMed] [Google Scholar]
- 12. Schneider R., Jensen S. A., Whiteman P., McCullagh J. S., Redfield C., Handford P. A. (2010) Biophysical characterisation of fibulin-5 proteins associated with disease. J. Mol. Biol. 401, 605–617 [DOI] [PubMed] [Google Scholar]
- 13. McLaughlin P. J., Bakall B., Choi J., Liu Z., Sasaki T., Davis E. C., Marmorstein A. D., Marmorstein L. Y. (2007) Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 16, 3059–3070 [DOI] [PubMed] [Google Scholar]
- 14. Drewes P. G., Yanagisawa H., Starcher B., Hornstra I., Csiszar K., Marinis S. I., Keller P., Word R. A. (2007) Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am. J. Pathol. 170, 578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rahn D. D., Acevedo J. F., Roshanravan S., Keller P. W., Davis E. C., Marmorstein L. Y., Word R. A. (2009) Failure of pelvic organ support in mice deficient in fibulin-3. Am. J. Pathol. 174, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura T., Lozano P. R., Ikeda Y., Iwanaga Y., Hinek A., Minamisawa S., Cheng C. F., Kobuke K., Dalton N., Takada Y., Tashiro K., Ross J., Jr., Honjo T., Chien K. R. (2002) Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415, 171–175 [DOI] [PubMed] [Google Scholar]
- 17. Sullivan K. M., Bissonnette R., Yanagisawa H., Hussain S. N., Davis E. C. (2007) Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab. Invest. 87, 818–827 [DOI] [PubMed] [Google Scholar]
- 18. Roybal C. N., Marmorstein L. Y., Vander Jagt D. L., Abcouwer S. F. (2005) Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest. Ophthalmol. Vis. Sci. 46, 3973–3979 [DOI] [PubMed] [Google Scholar]
- 19. Hulleman J. D., Kaushal S., Balch W. E., Kelly J. W. (2011) Compromised mutant EFEMP1 secretion associated with macular dystrophy remedied by proteostasis network alteration. Mol. Biol. Cell 22, 4765–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marmorstein L. Y., Munier F. L., Arsenijevic Y., Schorderet D. F., McLaughlin P. J., Chung D., Traboulsi E., Marmorstein A. D. (2002) Aberrant accumulation of EFEMP1 underlies drusen formation in malattia leventinese and age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 99, 13067–13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Claus S., Fischer J., Megarbane H., Megarbane A., Jobard F., Debret R., Peyrol S., Saker S., Devillers M., Sommer P., Damour O. (2008) A p.C217R mutation in fibulin-5 from cutis laxa patients is associated with incomplete extracellular matrix formation in a skin equivalent model. J. Invest. Dermatol. 128, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 22. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 23. Hu Q., Loeys B. L., Coucke P. J., De Paepe A., Mecham R. P., Choi J., Davis E. C., Urban Z. (2006) Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum. Mol. Genet. 15, 3379–3386 [DOI] [PubMed] [Google Scholar]
- 24. Harding H. P., Zhang Y., Ron D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 [DOI] [PubMed] [Google Scholar]
- 25. Lu L., Han A. P., Chen J. J. (2001) Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 21, 7971–7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krishnamoorthy T., Pavitt G. D., Zhang F., Dever T. E., Hinnebusch A. G. (2001) Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 21, 5018–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu P. D., Harding H. P., Ron D. (2004) Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novoa I., Zeng H., Harding H. P., Ron D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 153, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spriggs K. A., Bushell M., Willis A. E. (2010) Translational regulation of gene expression during conditions of cell stress. Mol. Cell. 40, 228–237 [DOI] [PubMed] [Google Scholar]
- 30. Harding H. P., Zhang Y., Bertolotti A., Zeng H., Ron D. (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 5, 897–904 [DOI] [PubMed] [Google Scholar]
- 31. Zhang W., Feng D., Li Y., Iida K., McGrath B., Cavener D. R. (2006) PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 4, 491–497 [DOI] [PubMed] [Google Scholar]
- 32. Delepine M., Nicolino M., Barrett T., Golamaully M., Lathrop G. M., Julier C. (2000) EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet. 25, 406–409 [DOI] [PubMed] [Google Scholar]
- 33. Harding H. P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., Sabatini D. D., Ron D. (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell. 7, 1153–1163 [DOI] [PubMed] [Google Scholar]
- 34. Zhang P., McGrath B., Li S., Frank A., Zambito F., Reinert J., Gannon M., Ma K., McNaughton K., Cavener D. R. (2002) The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walter P., Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 36. Maguire C. A., Deliolanis N. C., Pike L., Niers J. M., Tjon-Kon-Fat L. A., Sena-Esteves M., Tannous B. A. (2009) Gaussia luciferase variant for high-throughput functional screening applications. Anal. Chem. 81, 7102–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Welsh J. P., Patel K. G., Manthiram K., Swartz J. R. (2009) Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochem. Biophys. Res. Commun. 389, 563–568 [DOI] [PubMed] [Google Scholar]
- 38. Waldo G. S., Standish B. M., Berendzen J., Terwilliger T. C. (1999) Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol. 17, 691–695 [DOI] [PubMed] [Google Scholar]
- 39. Lin J. H., Li H., Zhang Y., Ron D., Walter P. (2009) Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4, e4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Badr C. E., Hewett J. W., Breakefield X. O., Tannous B. A. (2007) A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS One 2, e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., Ron D. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 42. Ma Y., Hendershot L. M. (2004) Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J. Biol. Chem. 279, 13792–13799 [DOI] [PubMed] [Google Scholar]
- 43. McEwen E., Kedersha N., Song B., Scheuner D., Gilks N., Han A., Chen J. J., Anderson P., Kaufman R. J. (2005) Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 280, 16925–16933 [DOI] [PubMed] [Google Scholar]
- 44. Kojima E., Takeuchi A., Haneda M., Yagi A., Hasegawa T., Yamaki K., Takeda K., Akira S., Shimokata K., Isobe K. (2003) The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 17, 1573–1575 [DOI] [PubMed] [Google Scholar]
- 45. Qin X. J., Hudson L. G., Liu W., Ding W., Cooper K. L., Liu K. J. (2008) Dual actions involved in arsenite-induced oxidative DNA damage. Chem. Res. Toxicol. 21, 1806–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loeys B., Van Maldergem L., Mortier G., Coucke P., Gerniers S., Naeyaert J. M., De Paepe A. (2002) Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11, 2113–2118 [DOI] [PubMed] [Google Scholar]
- 47. Tsaytler P., Harding H. P., Ron D., Bertolotti A. (2011) Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 [DOI] [PubMed] [Google Scholar]
- 48. Jones R. P., Ridley C., Jowitt T. A., Wang M. C., Howard M., Bobola N., Wang T., Bishop P. N., Kielty C. M., Baldock C., Lotery A. J., Trump D. (2010) Structural effects of fibulin 5 missense mutations associated with age-related macular degeneration and cutis laxa. Invest. Ophthalmol. Vis. Sci. 51, 2356–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones R. P., Wang M. C., Jowitt T. A., Ridley C., Mellody K. T., Howard M., Wang T., Bishop P. N., Lotery A. J., Kielty C. M., Baldock C., Trump D. (2009) Fibulin 5 forms a compact dimer in physiological solutions. J. Biol. Chem. 284, 25938–25943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galisteo M. L., Gordon C. L., King J. (1995) Stability of wild-type and temperature-sensitive protein subunits of the phage P22 capsid. J. Biol. Chem. 270, 16595–16601 [DOI] [PubMed] [Google Scholar]
- 51. Greene B., King J. (1999) Folding and stability of mutant scaffolding proteins defective in P22 capsid assembly. J. Biol. Chem. 274, 16141–16146 [DOI] [PubMed] [Google Scholar]
- 52. Kojima C., Ramirez D. C., Tokar E. J., Himeno S., Drobna Z., Styblo M., Mason R. P., Waalkes M. P. (2009) Requirement of arsenic biomethylation for oxidative DNA damage. J. Natl. Cancer Inst. 101, 1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kinnunen K., Petrovski G., Moe M. C., Berta A., Kaarniranta K. (2011) Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 90, 299–309 [DOI] [PubMed] [Google Scholar]
- 54. Roybal C. N., Hunsaker L. A., Barbash O., Vander Jagt D. L., Abcouwer S. F. (2005) The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J. Biol. Chem. 280, 20331–20339 [DOI] [PubMed] [Google Scholar]
- 55. Siller E., DeZwaan D. C., Anderson J. F., Freeman B. C., Barral J. M. (2010) Slowing bacterial translation speed enhances eukaryotic protein folding efficiency. J. Mol. Biol. 396, 1310–1318 [DOI] [PubMed] [Google Scholar]
- 56. Gupta R., Lakshmipathy S. K., Chang H. C., Etchells S. A., Hartl F. U. (2010) Trigger factor lacking the PPIase domain can enhance the folding of eukaryotic multi-domain proteins in Escherichia coli. FEBS Lett. 584, 3620–3624 [DOI] [PubMed] [Google Scholar]
- 57. Chevet E., Cameron P. H., Pelletier M. F., Thomas D. Y., Bergeron J. J. (2001) The endoplasmic reticulum: integration of protein folding, quality control, signaling and degradation. Curr. Opin. Struct. Biol. 11, 120–124 [DOI] [PubMed] [Google Scholar]
- 58. Liu X., Zhao Y., Gao J., Pawlyk B., Starcher B., Spencer J. A., Yanagisawa H., Zuo J., Li T. (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 36, 178–182 [DOI] [PubMed] [Google Scholar]
- 59. Yanagisawa H., Davis E. C., Starcher B. C., Ouchi T., Yanagisawa M., Richardson J. A., Olson E. N. (2002) Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415, 168–171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.