Abstract
The axotomy-inducible enzyme Nna1 defines a subfamily of M14 metallocarboxypeptidases, and its mutation underlies the Purkinje cell degeneration (pcd) mouse. However, the relationship among its catalytic activity, substrate specificities, and the critical processes of neurodegeneration/axon regeneration is incompletely understood. Here we used a transgenic rescue strategy targeting expression of modified forms of Nna1 to Purkinje cells in pcd mice to determine structure-activity relationships for neuronal survival and in parallel characterized the enzymatic properties of purified recombinant Nna1. The Nna1 subfamily uniquely shares conserved substrate-determining residues with aspartoacylase that, when mutated, cause Canavan disease. Homologous mutations (D1007E and R1078E) inactivate Nna1 in vivo, as does mutation of its catalytic glutamate (E1094A), which implies that metabolism of acidic substrates is essential for neuronal survival. Consistent with reports that Nna1 is a tubulin glutamylase, recombinant Nna1—but not the catalytic mutants—removes glutamate from tubulin. Recombinant Nna1 metabolizes synthetic substrates with 2 or more C-terminal glutamate (but not aspartate) residues (Vmax for 3 glutamates is ∼7-fold higher than 2 glutamates although KM is similar). Catalysis is not ATP/GTP dependent, and mutating the ATP/GTP binding site of Nna1 has no effect in vivo. Nna1 is a monomeric enzyme essential for neuronal survival through hydrolysis of polyglutamate-containing substrates.—Wu, H.-Y., Wang, T., Li, L., Correia, K., Morgan, J. I. A structural and functional analysis of Nna1 in Purkinje cell degeneration (pcd) mice.
Keywords: CCP, enzyme kinetics, neurodegeneration, transgenic rescue, deglutamylation
ATP/GTP binding protein 1 (Agtpbp1) was identified as a gene induced in spinal motor neurons undergoing axon regeneration following axotomy and encodes the metallocarboxypeptidase Nna1 [a.k.a. cytosolic carboxypeptidase 1 (CCP1); ref. 1]. Subsequently, Agtpbp1 (named for the presence of a predicted ATP/GTP binding site) was found to be the gene mutated in the recessive Purkinje cell degeneration (pcd) mutant mouse (2). Thus, enhanced Nna1 expression is associated with the response to axonal damage, whereas loss of function in pcd mice leads to selective neuronal degeneration in cerebellum, thalamus, olfactory bulb, and retina (refs. 3–6; reviewed in ref. 7). However, the precise contribution of Nna1 to the biology of axon regeneration or neuronal survival/degeneration remains to be fully elucidated.
Nna1 belongs to the M14 family of metallocarboxypeptidases (1, 8). Indeed, Nna1 defines a 6-member (CCP1–CCP6) subfamily of enzymes in mice (8, 9) that are distinguished from other M14 carboxypeptidases by having a phenylalanine (F1067) instead of a tyrosine in the S1′ substrate-binding pocket (8). Furthermore, whereas most M14 proteases are secreted or associated with the secretory pathway (10, 11), Nna1 and its subfamily members lack signal sequences and are predominantly cytoplasmic proteins (1, 9). Nna1 has been variously reported to be an α-tubulin tyrosine carboxypeptidase (9), a protease that cleaves proteasome-generated peptides (12), and a contributor to autophagy (12–14), ciliogenesis (15), mitophagy, and mitochondrial function (14, 16). Although an enzyme related to CCP6 from Caenorhabditis elegans has a nucleoside triphosphate-dependent activity against a spectrum of carboxypeptidase substrates (17) our initial experiments failed to reveal enzymatic activity in preparations of recombinant Nna1, suggesting it either required a cofactor or metabolized atypical substrates (8). In the absence of overt catalytic activity, we developed an in vivo functional assay to investigate both the substrate specificity of Nna1 and its relevance to neuronal survival (8). Wild-type or mutant forms of Nna1 were expressed in cerebellar Purkinje cells of pcd3J mice using the L7 promoter (18), and Purkinje cell survival was determined. Whereas wild-type Nna1 rescued Purkinje cell degeneration, Nna1 harboring mutations of two substrate-binding site residues conserved in M14 enzymes did not (8).
Here we extend the structure-activity relationship for Nna1. We noted that Nna1 and its subfamily members uniquely shared several catalytic site residues with the succinylglutamate desuccinylase (ASTE)–aspartoacylase (ASPA) family of enzymes, which includes ASPA, the enzyme defective in the recessive neurodegenerative disorder Canavan disease (19, 20). Moreover, two of the conserved residues are considered determinants of acidic substrate specificity, and their mutation causes Canavan disease (21). This finding suggested that Nna1 metabolized acidic substrates. Therefore, we designed additional mutations of Nna1 both to test this hypothesis and to assess whether the conserved substrate binding site F1067, catalytic center E1094, or putative ATP/GTP binding site were essential for biological activity. We complemented our in vivo structure-activity studies with an in vitro analysis of the enzymatic properties of purified recombinant Nna1 using model synthetic substrates. During this study, two reports appeared demonstrating that Nna1, agbl1 (a.k.a. CCP4), agbl4 (a.k.a. CCP6; ref. 22), and agbl5 (a.k.a. CCP5; refs. 22, 23) possessed a glutamylase activity and were involved in the metabolism of the polyglutamate side-chains of tubulin (22, 23). Therefore, we also assessed whether recombinant Nna1, as well as catalytic site mutants, removed glutamate from tubulin.
MATERIALS AND METHODS
Animals
pcd3J+/−, FVB/NJ, and C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Animals were maintained on a 12-h light-dark cycle with free access to food and water. All studies were approved by the St. Jude Children's Research Hospital (SJCRH) Animal Care and Use Committee and complied with the standards set forth in the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
Histology and immunohistochemistry
The procedures for histological analyses of cerebella were as described previously (8, 24). To visualize Purkinje cells, calbindin D-28K antibody (Sigma-Aldrich, St. Louis, MO, USA) was used at a dilution of 1:1000, and immune complexes were revealed using a peroxidase-conjugated anti-rabbit kit and diaminobenzidine tetrahydrochloride (DAB) substrate (Dako, Carpenteria, CA, USA). After immunostaining, sections were counterstained with hematoxylin (Vector Laboratories, Burlingame, CA, USA). The presence or absence of Purkinje cells was assessed in a minimum of 10 coronal sections spanning from anterior to posterior cerebellum and sampling all folia.
Mutagenesis of Nna1
The coding sequence of Nna1 was mutated in the context of the L7-Nna1 construct in the pBluescript KS vector (L7-Nna1/pBKS) as described previously (8) using a Quickchange XL site-directed mutagenesis kit (Stratagene Corp., La Jolla, CA, USA). For the E1094A mutation, the forward and reverse primers were GAGGAGCTACACCATGGCGAGTACTTTATGTGGC and GCCACATAAAGTACTCGCCATGGTGTAGCTCCTC, respectively. For the F1067Y/A mutation, the forward and reverse primers were GCATGAGCAGTTGTAGCTATGTGGTGGAAAAATCTAAAG/GCATGAGCAGTTGTAGCGCTGTGGTGGAAAAATCTAAAG and CTTTAGATTTTTCCACCACATAGCTACAACTGCTCATGC/CTTTAGATTTTTCCACCACAGCGCTACAACTGCTCATGC, respectively. For the K816N mutation, the forward and reverse primers were GGCGGACAGAAGGGCAACTCCTACTACACCATC and GATGGTGTAGTAGGAGTTGCCCTTCTGTCCGCC, respectively. After mutations were confirmed, a 1.8-kb fragment was generated by partial digestion with PshA1/Xcm1 and exchanged with the corresponding fragment from L7-Nna1/pBKS to produce final constructs. To generate R1078E and D1007E mutants, a 1.7-kb fragment containing the targeted sites was released from L7-Nna1/pBKS by BamH1 digestion. This fragment was subcloned into a pBKS vector and used as template for mutagenesis with a Quickchange XL site-directed mutagenesis kit. Primers for the R1078E mutation were CTAAAGAATCCACAGCTGAGGTTGTCGTGTGGCGGG and CCCGCCACACGACAACCTCAGCTGTGGATTCTTTAG. Primers for the D1007E mutation were CCTCTGGTTTATTGTGAATACCATGGCCATTCTCG and CGAGAATGGCCATGGTATTCACAATAAACCAGAGG. After mutagenesis was confirmed, BamH1 fragments were inserted into the parental L7-Nna1/pBKS vector following digestion with BamH1.
Generation of transgenic mice
The procedures used to generate and genotype wild-type and transgenic alleles of Nna1 and mutant Nna1 was as described previously (2, 8, 18, 25). We characterized ≥2 independent lines for each mutant construct. Subsequently, all lines of transgenic mice were crossed repeatedly with pcd3J+/− mice to produce pcd3J−/−/Tg mice and all intermediate genotypes.
RT-PCR analysis of transgene expression
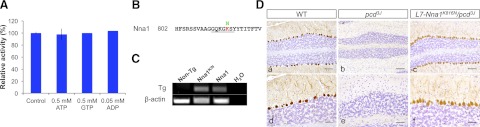
The level of L7-Nna1 chimeric mRNAs in transgenic mice was monitored by RT-PCR as described previously (8). Primers were 5′-GGCTTCTTCAACCTGCTGAC-3′ and 5′-GTCCCACAACCCGAGAACTA-3′. Length of the PCR product was 471 bp. β-Actin was amplified using primers 5′-AGGATGGCGTGAGGGAGAGC-3′ and 5′-ATATCGCTGCGCTGGTCGTC-3′. For comparison, total RNA from an L7-Nna1 line that rescues the pcd3J phenotype (8) was included. Mutant lines with the highest levels of transcript that were equivalent to or greater than the L7-Nna1 line were chosen for further analysis.
Generation of myc-tagged Nna1 and Nna1 mutants
DNA encoding Nna1 and its mutants was amplified using primers 5′-CCGTCGACCATGAGCAAGCTAAAAGTGGTGG-3′ and 5′-CCGCGGCCGCAGCTGTCTAAATGCTTGTCCCTCTCAAAC-3′ from the L7-Nna1/pBKS or L7-Nna1mut/pBKS plasmids. Amplified fragments were digested with SalI and NotI and cloned into the pCMV-Myc vector (Clontech, Mountain View, CA, USA).
Preparation of recombinant mouse Nna1
A recombinant mouse Nna1 construct containing a 6-histidine tag at its N terminus was introduced into a Baculovirus expression vector (pFastBac-HT-B) and used for large-scale protein production in insect cells within the Protein Production Facility at SJCRH. The recombinant protein was purified to near homogeneity by sequential nickel chelate and Sephacryl S200 fast protein liquid chromatography (FPLC). Purity was monitored by Coomassie staining and immunoblotting.
Recombinant protein stability assay
To assess the stability of recombinant proteins, we used a cycloheximide (CHX) chase procedure (26). HEK293 cells were transfected with plasmids expressing Nna1 or its mutants. After 48 h, cells were treated with CHX (50 μg/ml) and harvested in electrophoresis sample buffer at 6-h intervals over an 18-h period. The level of Nna1 and its mutants was analyzed by immunoblotting using an Nna1 antibody.
Protein electrophoresis and immunoblotting
Recombinant Nna1 or Nna1 derived from cerebellar or cell lysates were separated using a Criterion XT precast gel (4–12% Bis-Tris; Bio-Rad, Hercules, CA, USA). After electrophoresis, proteins were transferred onto a nitrocellulose membrane using the Criterion Blotter (Bio-Rad). Membranes were incubated with rabbit anti-Nna1 (1:500; ref. 7), mouse anti-polyglutamate (B3; 1:2000; Sigma) or rabbit anti-α-tubulin (EP1332Y; 1:3000; Millipore, Billerica, MA, USA) antibodies. Immunoreactive protein bands were visualized with Supersignal West Pico Chemiluminescence Substrate (Thermo, Rockford, IL, USA) following incubation with horseradish peroxidase-labeled sheep anti-mouse (1:2000, Sigma) or donkey anti-rabbit (1:10,000, Sigma) antisera.
Preparative glycerol gradient ultracentrifugation
A gradient was constructed manually by layering 100-μl aliquots of 45.0, 42.5, 40.0, 37.5, 35.0, 32.5, 30.0, 27.5, 25.0, 22.5, 20.0, 17.5, and 15% glycerol in 20 mM Tris (pH 7.5) containing protease inhibitors (Complete Mini EDTA-free protease inhibitor cocktail tablets; Roche Diagnostics, Mannheim, Germany; 1 tablet/10 ml final concentration) in an 11- × 34-mm centrifuge tube (Beckman Coulter, Fullerton, CA, USA). The resulting gradient spans 15–45% glycerol and occupies a column height of 3 cm. BALB/c wild-type cerebella were homogenized in 200 μl buffer containing 10 mM sodium phosphate (pH 7.2), 150 mM NaCl, 1 mM CHAPS, and protease inhibitors and were centrifuged at 15,000 g and 4°C for 30 min. Supernatant (40 μl) was loaded onto a 15–45% glycerol gradient and covered with 50 μl silicone fluid. Centrifugation was at 55,000 rpm at 4°C for 270 min in an Optima TLX preparative ultracentrifuge using a swinging bucket TLS-55 rotor (Beckman Coulter). Molecular weight standard proteins were centrifuged in parallel under identical conditions. The tube contents were fractionated into 24 (45 μl each) fractions and a bottom fraction of 200 μl using a Brandel Microfractionator equipped with FR-HA 1.0 block assembly (Brandel, Gaithersburg, MD, USA). Nna1 was detected by immunoblotting.
S200 gel filtration
Lysis buffer (5 ml) comprising 0.5% Nonidet P-40, 0.1 M potassium phosphate buffer (KH2PO4-K2HPO4, pH 7.4), and 2% protease inhibitor cocktail (Sigma) was used to homogenize ten 2-mo-old C57BL6 mouse cerebella. The lysate was centrifuged at 17,000 g for 25 min, and the supernatant was loaded onto an S200 gel filtration column (HiPrep 26/60 Sephacryl S-200HR; GE Healthcare, Piscataway, NJ, USA) using a P-920 Äkta pump with a UPC-900 monitor (GE Healthcare). The running buffer was 0.1 M potassium phosphate buffer (KH2PO4-K2HPO4, pH 7.4), and flow speed was 2 ml/min. Fractions (5 ml) were collected, and proteins were monitored at 280 nm. A 1-ml quantity of each fraction was precipitated with 250 μl 100% (w/v) trichloroacetic acid (TCA); the pellet was redissolved in 100 μl sodium dodecyl sulfate (SDS) sample buffer, boiled for 10 min, and 45 μl run on SDS-polyacrylamide gel electrophoresis (PAGE). Nna1 was detected by immunoblotting.
Determination of protein-bound zinc
The zinc content of the recombinant enzyme was determined by inductively coupled plasma optical emission spectroscopy (ICP OES) using a Varian 715-ES device (Agilent Technologies, Santa Clara, CA, USA) at 202.6 nm. Different concentrations of ZnCl2 solution were used to construct a standard curve, and purified carboxypeptidase A (from bovine pancreas; Sigma), another zinc metallocarboxypeptidase, was used as a control zinc-containing protein.
Assay of Nna1 carboxypeptidase activity
The synthetic carboxypeptidase substrates hippuryl-l-arginine (Hipp-l-Arg), hippuryl-l-phenylalanine (Hipp-l-Phe), and N-acetyl-glutamic acid (N-Ace-Glu) were purchased from Sigma. N-benzyloxycarbonyl (Z) derivatives Z-Glu-Tyr, Z-Gly-Asp, Z-Gly-Glu, furyl-acryloyl-glutamyl-glutamatic acid (FA-Glu-Glu), and Asp-Asp-Asp-Asp-OH were purchased from Bachem (Torrance, CA, USA). N-acetyl-aspartic acid (N-Ace-Asp) was from Fluka. Biotin-Glu-Glu-OH (biotin-2E) and biotin-Glu-Glu-Glu-OH (biotin-3E) were synthesized in the Hartwell Center for Bioinformatics and Biotechnology at SJCRH. The Department of Chemical Biology and Therapeutics at SJCRH performed the synthesis of hippuryl-l-aspartic acid (Hipp-l-Asp) and hippuryl-l-glutamic acid (Hipp-l-Glu). To examine the activity of recombinant Nna1 on synthetic substrates, a 500-μl reaction mixture comprising 4.5 μg purified Nna1, 100 mM NaCl, and 0.5 mM substrates in 25 mM HEPES-K buffer (pH 7.4) was incubated for 5 h at 37°C. Heat-denatured enzyme was incubated under identical conditions as control. Reactions were terminated with 1 ml ninhydrin-CdCl2 reagent, and released amino acids were detected as described previously (ref. 27, method C) and quantified using a standard curve of aspartic (Sigma) or glutamic acid (Sigma).
Tubulin glutamylase assay
To determine Nna1 activity toward the polyglutamate component of tubulin, a 20 μl reaction mixture containing 2 μg Nna1 and 2 μg porcine tubulin (Cytoskeleton Inc., Denver, CO, USA) in PBS was incubated at 37°C. Heat-denatured Nna1 was used as a negative control. Samples were incubated for various times and the reaction terminated with 4× sample buffer. Subsequently samples were subjected to immunoblot analysis using B3 (anti-polyglutamate) or anti-α-tubulin antibodies.
HEK293 cells were transfected with plasmids expressing Nna1 or its mutants. After 40 h, cells were washed twice with prechilled PBS and lysed in PBS containing 0.2% Nonidet P-40. After centrifugation for 20 min at 50,000 g at 4°C, 20 μl of supernatant was incubated with 2 μg porcine tubulin for 5 h at 37°C. The reaction was stopped and processed as above.
Influence of nucleoside triphosphates and carboxypeptidase inhibitors on recombinant Nna1
Biotin-3E was used as substrate to assess the dependence of Nna1 on zinc and nucleotides as well as its sensitivity to carboxypeptidase inhibitors. A 500-μl reaction mixture containing 3 μg Nna1, 100 mM NaCl, and 40 μM biotin-3E in 25 mM HEPES-K buffer (pH7.4) was supplemented with various concentrations of ZnCl2, EDTA, 1,10-phenanthroline (OP), or nucleotides. To offset the potential of nucleoside triphosphates to chelate zinc, the reaction mixture was supplemented with 5 mM MgCl2. The reaction was incubated at 37°C for 30 min, and enzymatic activities were determined as described above.
Enzyme kinetics
To determine KM and Vmax of recombinant Nna1, 40, 100, 150, and 200 μM biotin-2E or 20, 40, 100, 150, and 200 μM biotin-3E was incubated with 3 μg enzyme in a 500-μl reaction containing 25 mM HEPES-K and 100 mM NaCl at 37°C. The reactions were terminated at 15 min with ninhydrin-Cd and initial rate of reaction determined as described above. Reactions were run in triplicate. Linear regression of the double-reciprocal plots of initial rates against substrate concentrations were generated using Microsoft Excel (Microsoft, Redmond, WA, USA). The KM and Vmax values were calculated according to Mathews et al. (28).
Liquid chromatography-mass spectrometry (LC-MS)
Biotin-2E (40 μM) or biotin-3E (20 μM) was incubated with 3 μg recombinant Nna1 in a 500 μl reaction buffered with 25 mM HEPES-K in the presence of 100 mM NaCl at 37°C. The reaction was terminated with an equal volume of methanol containing 0.2% formic acid. Samples (5 μl) were analyzed with a UPLC-MS system (Waters Acquity UPLC, SQD mass detector; Waters Corp., Milford, MA, USA) equipped with a Waters BEH C18 UPLC column (2.1×50 mm, 1.7 μm). Column temperature was 40°C, and mobile phase was a gradient from 20% methanol and 80% 0.05% formic acid/water to 95% methanol and 5% 0.05% formic acid/water at a flow rate of 0.5 ml/min. The detection acquisition range was for ES+ scan from 110 to 800 m/z.
Statistical methods
Student's t test was used to compare two independent samples for statistical significance. Significance was set at P < 0.05. Lineweaver-Burk plots for Nna1 were generated by linear regression analysis using Microsoft Excel.
RESULTS
Transgenic mouse strategy to dissect Nna1 structure-function activity in vivo
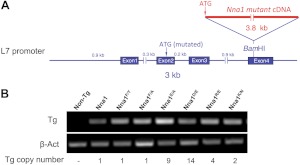
To establish the contribution of particular residues within the catalytic domain of Nna1 to biological activity in vivo, we generated transgenic mice expressing wild-type or mutant Nna1 under the control of the Purkinje cell specific L7 promoter (18) using the strategy outlined in Fig. 1A. Multiple lines with the highest levels of transgene mRNA expression were crossed to pcd3J mice, and Purkinje cell survival was monitored across all cerebellar folia, as described previously (8). Figure 1B shows the copy number and comparative mRNA expression levels of the transgenic mouse lines depicted in subsequent figures. Expression of all mutant Nna1 transgenes was higher than an L7-Nna1 line that rescues Purkinje cell degeneration (8).
Figure 1.
Construction of Nna1 and mutant Nna1 transgenic mice. A) Schematic representation of L7-Nna1 transgenes. Respective mutant Nna1 cDNA was inserted into a unique BamHI site in the fourth exon of the L7 gene. B) Comparison of mRNA expression levels in cerebellum of transgenic mice. Total RNA from cerebellum of the mouse strains depicted in Figs. 3, 4, and 9, as well as from wild-type (non-Tg, nontransgenic) and transgenic mice expressing wild-type Nna1 (Nna1) at a level that rescues Purkinje cell degeneration (8) was analyzed in parallel by RT-PCR with β-actin as control. Expression of all mutant transgenes was equivalent to or higher than the effective L7-Nna1 line. Also shown are the transgene (Tg) copy numbers for the respective strains.
Role of the catalytic center and conserved S1′ site phenylalanine in Nna1
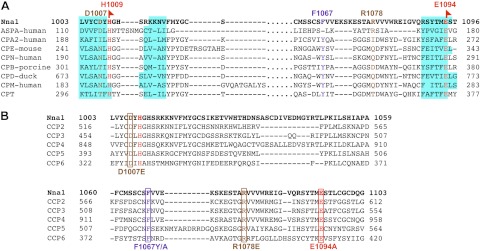
To identify amino acids within Nna1 informative for structure-function and substrate specificity, alignments were performed between the catalytic domains of Nna1 and other vertebrate M14 carboxypeptidases for which crystal structures are available (Fig. 2A; see also ref. 8). The zinc carboxypeptidase catalytic triad of Nna1 consists of Arg962, Glu1094, and an atom of zinc coordinated by His912, Glu915, and His1009 (8). Phe1067 lies in the S1′ substrate binding pocket and is conserved among the Nna1 subfamily (Fig. 2A, B), whereas most M14 enzymes have a tyrosine in the homologous position (Fig. 2A and ref. 8).
Figure 2.
Conservation of critical amino acids between the carboxypeptidase domains of the Nna1 family and aspartoacylase suggest acidic substrates. A) Amino acid alignment of carboxypeptidase domain of Nna1 with human ASPA and other M14 enzymes was modeled based on crystallographic data, as described previously (8, 21). Amino acids in red (H1009 and E1094) are conserved among all enzymes, those common to ASPA and Nna1 (D1007 and R1078) are in brown, and those unique to the Nna1 subfamily (F1067) are in blue. Cyan boxes highlight β sheets in the secondary structures. B) Amino acid alignment of carboxypeptidase domains of all Nna1 family members (Nna1 and CCP2-CCP6). Boxes highlight amino acids mutated in this study that are variously unique to the Nna1 subfamily (F1067Y/A) or shared with ASPA (D1007E, R1078E and E1094A). Amino acid color-coding scheme is same as panel A. Note the D1007E and R1078E mutants in Nna1 mimic Canavan disease mutations in human ASPA.
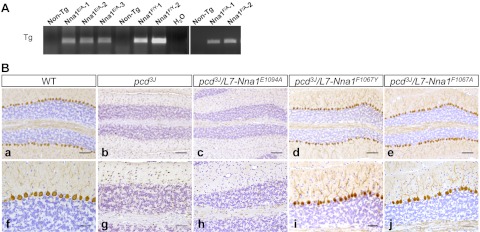
To establish the relevance of the catalytic activity of Nna1 in the pcd neurodegenerative phenotype and the role of F1067, we generated transgenic mice expressing mutations of the catalytic glutamate (E1094A) and S1′ site phenylalanine (F1067Y, mimicking the residue in other M14 enzymes and F1067A). Nna1 harboring a mutation of E1094 failed to rescue Purkinje cell degeneration (Fig. 3Ba–c, f–h). In contrast, Nna1 harboring F1067A or F1067Y mutations spared Purkinje cell loss (Fig. 3Bd, e, i, j).
Figure 3.
The catalytic site glutamate (E1094) in Nna1 is essential for activity, whereas the subfamily specific phenylalanine (F1067) in the S1′ site of Nna1 is not. A) Representative RT-PCR of total RNA from cerebellum of wild-type (non-Tg, nontransgenic) and mutant Nna1 transgenic lines. Letters indicate mutations of E1094 (Nna1E/A) and F1067 (Nna1F/Y and Nna1F/A). B) Calbindin D-28K immunohistochemistry and hematoxylin counterstaining of 2-mo-old cerebellum from wild-type (a, f), pcd3J−/− (b, g), pcd3J−/− harboring an L7-Nna1E1097A transgene (c, h), pcd3J−/− harboring an L7-Nna1F1067Y transgene (d, i), and pcd3J−/− harboring an L7-Nna1F1067A transgene (e, j) mice. Note loss of calbindin-positive Purkinje neurons in pcd3J−/− (b, g) and pcd3J−/− mice harboring an L7-Nna1E1097A transgene (c, h), whereas both the L7-Nna1F1067Y (d, i) and L7-Nna1F1067A (e, j) transgenes rescue Purkinje cell degeneration. Scale bars = 100 μm (a–e); 50 μm (f–j).
Structural similarity between Nna1 and ASPA
A motif scan using the PROSITE database (29) indicated that the carboxypeptidase domain of Nna1 shares sequence similarity with the ASTE-ASPA family of zinc carboxypeptidases (30). Moreover, mutations of ASPA cause Canavan disease (19, 20) and provide additional structure-activity insights among this class of enzymes. When human ASPA (Fig. 2A) was included in the alignment, we noted that D114 and R168 of ASPA were conserved with the Nna1 subfamily, but not other M14 enzymes examined (Fig. 2A, B).
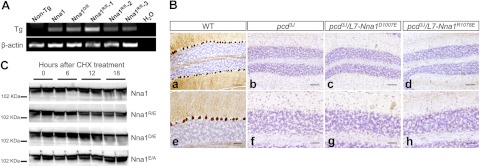
D114 in ASPA is equivalent to D1007 in Nna1 and to I194 in human CPA (Fig. 2A). In ASPA and other zinc carboxypeptidases, the amino acid at this position participates in formation of the hydrogen bond network of the active site (21). As a D114E mutation in ASPA causes Canavan disease (21), we generated L7-Nna1D1007E transgenic mice (Fig. 4A). When the L7-Nna1D1007E line was crossed onto a homozygous pcd3J background, it did not rescue Purkinje cell death (Fig. 4Bc, Bg). Therefore, D1007 is required for Nna1 activity in vivo and suggests some common functional characteristic between Nna1 and ASPA.
Figure 4.
Two amino acids uniquely conserved between the Nna1 family and ASPA are essential for Nna1 activity. A) Representative RT-PCR of total RNA from cerebellum from wild-type (non-Tg, nontransgenic) and transgenic mice harboring mutations of D1007 (Nna1D/E) and R1078E (Nna1R/E) as well as transgenic mice expressing wild-type Nna1 (Nna1) at a level that rescues Purkinje cell degeneration. Identical mutations in ASPA homologous to D1007 and R1078 cause Canavan disease. B) Calbindin D-28K immunohistochemistry and hematoxylin counterstaining of cerebellum from 2-mo-old wild-type mice (a, e), pcd3J mice (b, f), and pcd3J/L7-Nna1D1007E (c, g) and pcd3J/L7-Nna1R1078E (d, h) transgenic mice reveals that neither mutant rescues Purkinje cell degeneration, which suggests that D1007 and R1078 are essential for activity of Nna1 in vivo and that Nna1 and ASPA have similar catalytic properties. Scale bars = 100 μm (a–d); 50 μm (e–h). C) HEK293 cells were transfected with plasmids expressing Nna1 or the indicated mutants for 40 h and then treated with CHX (50 μg/ml). Levels of Nna1 and its mutants were determined at various times following CHX addition by immunoblotting using an Nna1 antibody. Following protein synthesis, inhibition levels of wild-type Nna1 and Nna1 mutants declined at comparable rates.
R168 in ASPA is equivalent to R1078 in Nna1 and I255 in CPA (Fig. 2A), and the residue at this position is a major determinant of substrate-specificity in the zinc carboxypeptidase family (21, 31). Familial mutations of R168 in ASPA (e.g., R168E) cause Canavan disease (21). To test the contribution of R1078, we generated L7-Nna1R1078E transgenic mice (Fig. 4A). When the L7-Nna1R1078E line was crossed onto a pcd3J homozygous background, it failed to rescue Purkinje cell death (Fig. 4Bd, h). Thus, R1078 is essential for Nna1 activity in vivo, suggesting it metabolizes an acidic substrate.
It is possible that lack of activity of the mutant proteins is due to instability. As no antisera are useful for immunohistochemical detection of Nna1, and levels of proteins expressed in Purkinje cells of transgenic animals are below the reliable detection threshold for immunoblotting, we assessed their stability in culture using a CHX chase paradigm (26). When transfected into HEK293 cells, all constructs were expressed at similar levels, and the proteins showed comparable decay kinetics (Fig. 4C). Therefore, the mutants are stable proteins with similar turnover rates to Nna1.
Wild-type Nna1, but not catalytic site mutants, metabolize the polyglutamate side-chains of tubulin
Recent studies showed that lysates from cells transfected with Nna1 removed glutamate from the polyglutamate side-chain of tubulin (22, 23). Therefore, we determined whether purified recombinant Nna1, as well as lysates containing wild-type and catalytic site mutants of Nna1, possessed tubulin glutamylase activities. Purified recombinant mouse histidine-tagged Nna1 was generated as described in Materials and Methods. Coomassie blue staining following SDS-PAGE showed a single major band at ∼140 kDa, the predicted size of Nna1, which was immunoreactive (Fig. 5A) with an Nna1-specific polyclonal antiserum (7). Immunoblotting of cerebellar extracts also detected a band of Nna1-like immunoreactivity at ∼140 kDa in wild-type but not pcd3J mice (Fig. 5A).
Figure 5.
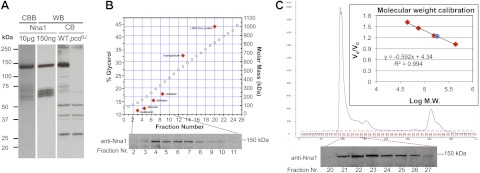
Physical characterization of Nna1. A) SDS-PAGE reveals that purified recombinant Nna1 runs as a major band of ∼140 kDa when visualized by Coomassie brilliant blue (CBB) staining (left lane) or Western blotting (WB; middle lane). In cerebellar lysates, a band of equivalent molecular mass to recombinant Nna1 is detected in wild-type (WT) but not pcd3J mice (right panel). In both purified recombinant Nna1 preparations and cerebellar extracts from wild-type but not pcd mice, several lower-molecular-mass bands of Nna1-like immunoreactivity are detected. B) Determination of molecular mass of native Nna1 using glycerol gradient preparative ultracentrifugation. Cerebellar lysate from adult wild-type mice was subjected to centrifugation on a calibrated glycerol gradient and fractions collected and Nna1 detected by immunoblotting. Percentage (%) of glycerol (open circle) of each fraction was determined by measuring refractive index. Standard proteins (solid red diamond) are ovalbumin (43 kDa), bovine serum albumin (67 kDa), aldolase (158 kDa), catalase (232 kDa), and thyroglobulin (669 kDa). Position of standard proteins postcentrifugation in the gradient was determined by SDS-PAGE. Positions for the standard proteins were simulated with the program SEDFIT (http://www.analyticalultracentrifugation.com) and coincided with the positions determined via experimentation. The position of a 1000-kDa protein was calculated with SEDFIT, assuming a compact globular protein. Nna1-immunoactivity peaks among fractions 4–7 (lower panel), consistent with a molecular mass of ∼140 kDa. C) Determination of the molecular mass of native Nna1 using gel filtration. A cerebellum lysate was subjected to FPLC gel filtration analysis with a S200 column. Inset: calibration curve using standard proteins (red diamonds): thyroglobulin (669 kDa), ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), and ovalbumin (44 kDa). Nna1-immunoactive signal appeared between fractions 21 to 26, peaking in fraction 23. Molecular mass of Nna1 (blue circle) calculated from the calibration curve is 177 kDa, suggesting that native Nna1 behaves as a monomer. Ve, elution volume; V0, void volume.
As the N-terminal region of Nna1 contains features suggestive of protein–protein association interfaces (7), it may exist in heteromeric complexes in vivo that cannot be recapitulated by recombinant Nna1. To test this theory, cerebellar extracts were subjected to analytical ultracentrifugation (Fig. 5B) or Sephacryl S200 size-exclusion FPLC (Fig. 5C). Using both methods, we found that Nna1 had the predicted mass of a monomer (Figs. 5B, C). While we cannot exclude the possibility that native Nna1 is associated with low-molecular-mass cofactors, it behaves as a monomeric enzyme.
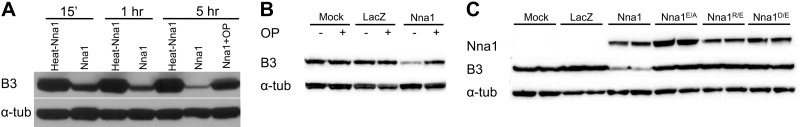
To study polyglutamylation, we used Western blotting with an antibody (B3) that detects tubulin side-chains with >2 glutamate residues (32, 33). When incubated with porcine tubulin, recombinant Nna1 caused the time-dependent loss of B3-immunoreactivity without a loss of total α-tubulin (Fig. 6A). Heat-denatured Nna1 was inactive, and OP, a general metallocarboxypeptidase inhibitor, blocked Nna1 activity (Fig. 6A).
Figure 6.
Nna1 but not Nna1 mutants degrade polyglutamate chain of tubulin. A) Recombinant Nna1 or heat-denatured recombinant Nna1 were incubated for the indicated times with porcine tubulin, and polyglutamylation levels were monitored by immunoblotting with the B3 antibody. Presence of Nna1 (but not heat-denatured Nna1) caused a time-dependent loss of B3-like immunoreactivity, indicating progressive degradation of polyglutamate chains. Addition of 5 mM OP inhibited the activity of Nna1. Levels of α-tubulin served as control for overall tubulin loading and stability and were not affected by Nna1. B) Incubation of tubulin with lysates from HEK293 cells transfected with an Nna1 expression plasmid also reduced B3 immunoreactivity in an OP-inhibitable fashion. C) Catalytic site mutants of Nna1 fail to decrease polyglutamylation level of tubulin. Lysates from HEK293 cells transfected with expression plasmids for Nna1 or the indicated mutants were incubated with tubulin, and B3 immunoreactivity was assessed 5 h later; whereas Nna1 reduced B3 immunoreactivity, the mutants were inactive. Levels of recombinant enzymes were monitored by immunoblotting for Nna1 and were roughly equivalent. Levels of α-tubulin served as loading and integrity control.
Nna1 and Nna1 catalytic site mutants were expressed in HEK293 cells (Fig. 6B, C). Like recombinant Nna1, lysates from Nna1-transfected cells removed the polyglutamate chains of tubulin in an OP-inhibitable manner (Fig. 6B). However, extracts from cells expressing the three catalytic site mutants (Nna1E/A, Nna1R/E, and Nna1D/E) were inactive at equivalent or higher levels of enzyme input to Nna1 (Fig. 6C and data not shown). Therefore, the various catalytic site mutants of Nna1 are stable proteins, but they have undetectable tubulin glutamylase activity, consistent with their lack of biological activity in vivo.
Characterization of enzymatic properties of recombinant Nna1 using synthetic substrates
The tubulin glutamylase assay is not suited for a more extensive characterization of the catalytic properties of recombinant Nna1. Therefore, we developed a simple and sensitive in vitro assay based on synthetic substrates. Although we detected weak activity of Nna1 toward FA-Glu-Glu, we did not detect activity against an array of typical substrates for CPA (Hipp-l-Phe and Z-Glu-Tyr) or CPB (Hipp-l-Arg) (Table 1). In addition, Nna1 did not metabolize substrates with single acidic amino acids (Hipp-l-Glu/Asp or Z-Gly-Glu/Asp), and, despite sequence similarities to ASPA, Nna1 did not cleave either N-Ace-Asp or N-Ace-Glu (Table 1). Therefore, we synthesized additional potential substrates.
Table 1.
Activity of purified Nna1 toward synthetic substrates
| Substrate | Activity of Nna1 |
|---|---|
| Hipp-l-Arg | − |
| Hipp-l-Phe | − |
| Z-Glu-Tyr | − |
| Hipp-l-Asp | − |
| Z-Gly-Asp | − |
| Hipp-l-Glu | − |
| Z-Gly-Glu | − |
| N-Ace-Asp | − |
| N-Ace-Glu | − |
| FA-Glu-Glu-OH | + |
| Asp-Asp-Asp-Asp-OH | − |
+, 0.78 ± 0.03% of substrate cleaved and P < 0.001 vs. control reactions with the denatured enzyme (Student's t test); −, ≤0.1% of substrates cleaved (P>0.5).
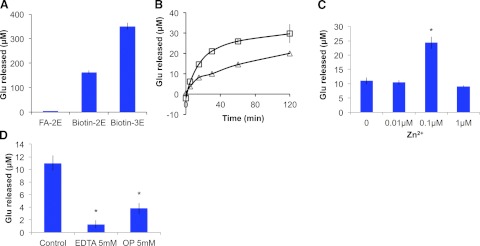
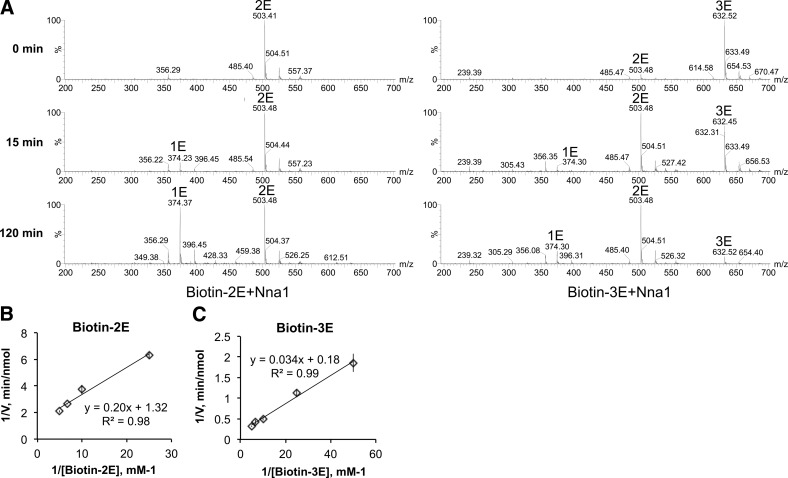
As shown in Fig. 7A, biotin-2E and biotin-3E are better substrates for Nna1 than FA-Glu-Glu. Temporal analysis of conversion of biotin-2E and biotin-3E by Nna1 reveals that biotin-3E is a better substrate than biotin-2E, even when substrate concentrations are adjusted to compensate for the additional glutamate in biotin-3E (Fig. 7B). Furthermore, not all glutamate is released from either biotin-2E or biotin-3E (Fig. 7B). Mass spectrometry revealed that a substantial fraction of biotin-3E is converted to biotin-2E within 15 min of incubation, whereas only a small fraction of biotin-2E is converted to biotin-1E (Fig. 8A). Only at 120 min do significant quantities of biotin-1E appear, and biotin is never observed.
Figure 7.
Characterization of the catalytic properties of recombinant Nna1. A) Nna1 catalyzes glutamate release from synthetic polyglutamate-containing substrates. Recombinant Nna1 was incubated with 0.5 mM FA-Glu-Glu (FA-2E), biotin-2E, or biotin-3E at 37°C for 5 h, and glutamate release was assessed using ninhydrin-Cd. Both biotin-2E and biotin-3E were much better substrates for Nna1 than FA-2E. Bars represent means ± se of triplicate determinations. B) Time courses of glutamate release from synthetic substrates. Nna1 (6 μg/ml) was incubated with either 40 μM biotin-2E (triangles) or 20 μM biotin-3E (squares) for the indicated times, and glutamate release was determined. Data are means ± se of triplicate determinations. Both substrates were metabolized in a time-dependent manner, with the initial reaction rates being higher for biotin-3E compared to biotin-2E. C) Zinc stimulates Nna1 activity. Nna1 and biotin-3E (40 μM) were incubated for 30 min with various concentrations of zinc chloride, and glutamate released was measured. Data are means ± se of triplicate determinations. Nna1 activity approximately doubled in the presence of 0.1 μM Zn2+ but was inhibited by higher concentrations. *P < 0.05. D) Nna1 is inhibited by zinc chelating agents. Nna1 was incubated with either 5 mM EDTA or OP and 40 μM biotin-3E for 30 min, and glutamate release was assessed. Both agents significantly reduced Nna1 activity. *P < 0.05.
Figure 8.
Kinetic analysis of recombinant Nna1. A) Time course of Nna1 catalysis of biotin-2E and biotin-3E, assessed by mass spectrometry. Masses corresponding to biotin-1E (1E), biotin-2E (2E), and biotin-3E (3E) are indicated. A substantial fraction of biotin-3E is converted to biotin-2E within 15 min, and biotin-3E is no longer detectable by 120 min of incubation with recombinant Nna1. In contrast, conversion of biotin-2E to biotin-E is insignificant at 15 min and is only marked at 120 min, indicating that the terminal glutamate in biotin-3E is more efficiently removed than the terminal glutamate in biotin-2E. The reaction conditions were as for Fig. 5B. B, C) Lineweaver-Burk plot for Nna1 with biotin-2E (B) or biotin-3E (C) as substrates. Increasing concentrations of biotin-2E or biotin-3E were incubated with 3 μg Nna1 for 15 min, and glutamate released was measured. All reactions were performed in triplicate. Double reciprocal plots were generated, and KM and Vmax were calculated by the method of Matthews et al. (27). For biotin-2E, Vmax is 0.76 nmol/min, and KM is 0.15 mM. For biotin-3E, Vmax is 5.42 nmol/min, and KM is 0.18 mM. Binding affinity of Nna1 for substrates with 2 or 3 glutamate residues is roughly the same, but the reaction rate for removing the third glutamate is 7 times faster than removal of the second glutamate.
Michaelis-Menten kinetic analysis was performed using biotin-E2 and biotin-E3. Whereas the two substrates had similar KM values (biotin-2E, 0.15mM; biotin-3E, 0.18 mM), the Vmax values of Nna1 toward biotin-2E and biotin-3E were 0.76 nmol/min and 5.42 nmol/min, respectively (Fig. 8B, C). Overall, these results indicate that Nna1 has a preference for a substrate with >2 glutamate residues; Nna1 rapidly removes the terminal glutamate residue from substrates with 3 (and potentially more) glutamates; and Nna1 slowly metabolizes the penultimate glutamate residue from biotin-3E.
Purified recombinant Nna1 is activated by zinc and inhibited by zinc chelators
ICP emission spectroscopy showed purified recombinant Nna1 contained bound zinc. However, assuming a molar ratio of 1:1 for Nna1 and zinc, the theoretical zinc value of Nna1 should be 69.4 ppb, whereas we observed 21.7 ppb, which suggests that some zinc was lost during purification. As zinc is essential for catalysis (11), we assessed the effects of Zn2+ on the activity of Nna1. Addition of 0.1 μM Zn2+ led to an approximate doubling in enzyme activity, whereas 1 μM Zn2+ was inhibitory (Fig. 7C). This biphasic property is observed in other Zn2+-dependent enzymes (34, 35). Zinc carboxypeptidases are inhibited by divalent cation chelators (36, 37), and both EDTA and OP, inhibitors of zinc carboxypeptidases (37, 38), reduce the activity of Nna1 (Fig. 7D).
The putative ATP/GTP binding site of Nna1 is not essential for in vivo activity
Nna1 contains a putative ATP/GTP binding motif (1, 7) and ATP and ADP stimulate the activity of an Nna1-related enzyme from C. elegans (17). However, inclusion of ATP, ADP, or GTP at physiologically relevant concentrations did not alter the catalytic activity of Nna1 (Fig. 9A), and neither did higher concentrations (data not shown). In addition, recombinant Nna1 did not bind to ATP-Sepharose (data not shown).
Figure 9.
Nna1 activity is not nucleotide-dependent. A) Relative activity of recombinant Nna1 toward biotin-3E in the presence of physiological concentrations of ATP, ADP, and GTP. None of the nucleotides affected Nna1 activity at the indicated or higher concentrations (data not shown). Bars represent means ± se of triplicate determinations. B) Site of lysine to asparagine mutation (K816N) in the putative ATP/GTP binding motif of Nna1. Walker A-box type (GXXGKS) motif is underscored. C) RT-PCR of total RNA from cerebellum of wild type (non-Tg, nontransgenic) L7-Nna1K816N (Nna1K/N) and L7-Nna1 (Nna1) transgenic mice. Expression level of mRNA for L7-Nna1K816N is comparable to that in the active L7-Nna1 line. D) ATP/GTP binding motif is not essential for Nna1 activity. Calbindin D-28K immunohistochemistry and hematoxylin counterstaining in sections of cerebellum from 2 mo-old wild-type (WT; a, d), pcd3J (b, e), and pcd3J/L7-Nna1K816N mice (c, f) show preservation of Purkinje cells in the mutant transgenic line. Scale bars = 100 μm (a–c); 50 μm (d–f).
To test the relevance of the ATP/GTP site, we generated transgenic mice expressing a mutant of Nna1 (L7-Nna1K816N) in which lysine-816 in the binding motif was mutated to asparagine (Fig. 9B, C). This mutation was selected, as an equivalent mutation in another ATP binding protein causes loss of function without destabilizing protein structure (39). L7-Nna1K816N rescued the pcd3J phenotype (Fig. 9D), indicating that this motif is not essential for biological activity.
DISCUSSION
The predicted structure of the catalytic domain of Nna1 was initially derived through modeling based on the tertiary structures of zinc carboxypeptidases (8). Expanding this alignment to include the ASTE-ASPA family permitted us to identify additional residues in Nna1 that may be informative in terms of substrate specificity, regulation, and biological activity (Fig. 2). The role of these and additional conserved residues within the catalytic domain of Nna1 has been systematically assessed through mutation analysis in vivo and enzymatic analysis in vitro using tubulin and model synthetic substrates.
Catalytic site glutamate (E1094) in Nna1 is essential for in vivo activity and deglutamylation of tubulin
Nna1 belongs to the M14 family of metallocarboxypeptidases (1, 8), and mutation of its catalytic glutamate residue (E1094A) eliminates its ability to rescue the pcd3J phenotype (Fig. 3). This finding implies that it is an enzyme whose catalytic activity is essential for neuronal survival, which complements studies showing that critical substrate-binding (8) and zinc-binding (40) residues are also essential for activity. Based on the structure of its substrate-binding pocket, Nna1 was predicted to have broad substrate specificity (9, 17) and a recombinant Nna1-related protein from C. elegans has enzymatic activity against a spectrum of substrates (17). However, purified recombinant Nna1 failed to metabolize a wide variety of standard carboxypeptidase substrates (Table 1). In contrast, recombinant Nna1 removed glutamate from the polyglutamate chains of tubulin and mutation of E1094 abrogated this activity (Fig. 6C). These data confirm and extend the findings that Nna1 is a tubulin glutamylase that is essential for Purkinje cell survival (22, 23).
Subfamily specific phenylalanine (F1067) in the S1′ substrate binding site of Nna1 is not essential in vivo
The S1′ substrate binding site of M14 enzymes contains several conserved critical amino acids, which in carboxypeptidase A include N144, R145, and Y248 (31, 41–43). We showed previously that the homologous N971 and R972 in Nna1 were essential for in vivo activity (8). However, Nna1 and its subfamily members lack a tyrosine equivalent to Y248 and instead harbor a phenylalanine (F1067 in Nna1), suggesting that it may be a determinant of substrate specificity (8). The role of tyrosine in the S1′ site of M14 carboxypeptidases is a matter of debate (41, 42, 44, 45). Structural studies of CPA, which show that the hydroxyl group of this tyrosine forms a hydrogen bond with the terminal carboxyl group of the substrate (46), serve as evidence that this is an important functional interaction. However, mutation of this tyrosine in various CPAs to either phenylalanine (preserving aromatic nature) or alanine led to conflicting results. In some enzymes, the mutation has no effect on catalytic activity (44), whereas in others, the tyrosine is essential (41). When we tested the in vivo activity of Nna1 harboring F1067Y and F1067A mutations, both rescued the pcd3J phenotype (Fig. 3). Therefore, despite being a canonical feature of the Nna1 subfamily, F1067 probably does not play a prominent role in substrate specificity.
Two amino acids uniquely conserved between the Nna1 family and ASPA are essential for Nna1 activity in vivo and vitro
The recognition that Nna1 and its subfamily members shared unique structural similarities with ASPA (Fig. 2) provided further insight into potential substrates. R168 in ASPA is equivalent to R1078 in Nna1, and the residue at this position is a major determinant of substrate specificity in the zinc carboxypeptidase family (21, 31). When the residue is hydrophobic, as in CPA (Fig. 2A), the enzymes prefer substrates with a hydrophobic C-terminal residue (31, 47). When the enzyme has a negatively charged amino acid, as in CPB, it preferentially hydrolyzes positively charged residues (48, 49). In CPO and ASPA, the substrate-determining residue is arginine and has a preference for acidic C-terminal residues (21, 50, 51). Moreover, in an engineered human CPB, where the substrate-determining aspartate was mutated to a basic amino acid, its substrate preference was changed from basic to acidic amino acids (52). The presence of an arginine at position 1078 suggested that Nna1 cleaves acidic substrates. To establish that R1078 was critical in Nna1, we mutated it to glutamate, mimicking the mutation in ASPA (R168E) that causes Canavan disease (21). Nna1R1078E failed to rescue Purkinje cell degeneration (Fig. 4B), confirming that it is essential for Nna1 function. Furthermore, the same mutation eliminates Nna1's ability to degrade the polyglutamate chain of tubulin (Fig. 6C). In ASPA, Arg168 provides the specificity for binding of the β-carboxyl group of N-acetyl-aspartate (21), and presumptively Arg1078 in Nna1 is involved in binding the equivalent carboxyl group of an acidic (glutamate) substrate.
In human CPA2, I194, I243, S253, I255, and T268 (and equivalent residues in other M14 enzymes) are important in defining the substrate pocket (17, 31). In ASPA and the Nna1 subfamily, but not M14 enzymes examined, the residue equivalent to I194 is always an aspartic acid (Fig. 2A, B). This finding indicated some common functional characteristic of the substrate pocket in the Nna1 subfamily and ASPA. Therefore, we mutated D1007 to glutamate, mimicking a mutation in ASPA (D114E) that causes Canavan disease (21). Nna1D1107E failed to rescue Purkinje cell degeneration (Fig. 4B) and abrogates tubulin glutamylase activity (Fig. 6C), indicating that this residue is critical to Nna1 function. D114 in ASPA forms hydrogen bonds with the adjacent H116 and participates in catalysis (21). Therefore, D1007 in Nna1 may form hydrogen bonds with H1009 (see Fig. 2) and influence catalysis of polyglutamate substrates.
Kinetic analysis of recombinant Nna1
Some proteins containing a glutamate rich sequence may undergo a mode of post-translational modification termed polyglutamylation (53). Initially, a glutamate is covalently coupled via an amide linkage between its amino group to the γ-carboxyl group of a primary chain glutamate. Subsequently, additional glutamates are added to the branching glutamate via a peptide bond with the α-carboxyl of the preceding glutamyl unit (54). Protein polyglutamylation is a reversible process regulated by two sets of enzymes. Several tubulin-tyrosine ligase-like (TTLL) family members catalyze glutamylation (reviewed in ref. 54), whereas Nna1 and several other family members remove glutamate residues (22, 23). In particular, Nna1, CCP4, and CCP6 trim the glutamate chains, and CCP5 uniquely removes the branch point glutamate (22). The best-studied proteins subjected to polyglutamylation are α- and β-tubulins (55–58).
We developed an in vitro assay using biotin-based substrates that permitted us to perform kinetic analyses on Nna1. These analyses demonstrate that Nna1 is a selective C-terminal glutamylase that does not metabolize polyaspartate substrates (Table 1). Nna1 activity requires another glutamate in the −1 position, and an additional glutamate at the −2 position greatly enhances degradation (Fig. 7A, B). The Vmax for Nna1 to catalyze the C-terminal glutamate in biotin-3E is 7 times higher than for biotin-2E although KM values are similar (Fig. 8B, C). This finding indicates that Nna1 more efficiently catalyzes the degradation of chains containing ≥3 glutamate residues. The similar KM values for biotin-2E and biotin-3E suggest that Nna1 has a similar affinity to the C-terminal glutamate without distinguishing the length of the chain. Such a property could provide a mechanism for the processive catabolism of polyglutamate chains, with the enzyme attaching to and metabolizing a single chain rather than jumping from chain to chain.
Endogenous Nna1 is monomeric
A number of M14 enzymes either assemble into multisubunit complexes (e.g., CPN; ref. 59) or are generated as zymogens that require proteolytic processing (e.g., CPA/B; reviewed in ref. 60). Compared to M14 enzymes, Nna1 is large and has structural features in its N-terminal region that are characteristic of protein-protein interaction motifs (1, 7). Indeed, an Nna1-like enzyme from C. elegans has a consensus leucine zipper motif (1, 7). However, both analytical ultracentrifugation and size-exclusion chromatography of cerebellar extracts indicated that Nna1 behaved as a monomeric protein. We do see smaller (60- to 70-kDa) Nna1-immunoreactive bands in the purified recombinant protein preparation (Fig. 5A). Furthermore, lower-molecular-mass (70- to 80-kDa) Nna1-like bands appear in cerebellar extracts from wild-type mice but not pcd3J mice (Fig. 5A). The Nna1 antiserum recognizes an epitope in the C terminus of Nna1, and based on the sizes of the fragments on SDS-PAGE, they must contain all or part of the catalytic domain. Therefore, whereas Nna1 does not appear to be a multisubunit protein, it remains to be determined whether these fragments are enzymatically active and biologically relevant.
Nna1 activity is not nucleotide dependent
The genetic nomenclature of Nna1 (agtpbp1) reflects the presence of a putative ATP/GTP binding motif of the Walker A-box type (GXXGKS) conserved in sequence and location in mammals, amphibians and fish (1, 7). In addition, ATP and ADP stimulate the catalytic activity of an Nna1-related enzyme from C. elegans (17). However, the binding consensus sequence is not conserved in birds or invertebrates, nor is it present in the other Nna1 subfamily members (7), raising questions of its functional relevance. We show that transgenic mice expressing a mutant form of Nna1 in which the critical Lys816 in the binding motif was mutated to asparagine (L7-Nna1K816N) rescues Purkinje cell degeneration (Fig. 9D). In addition, the activity of Nna1 is not influenced by ATP, ADP, or GTP (Fig. 9A), and purified Nna1 does not bind to ATP-Sepharose (data not shown). Thus, the ATP/GTP binding motif is not essential for biological activity, raising the question of whether Nna1 is an authentic nucleoside triphosphate binding protein whose activity is regulated by these molecules.
Of the 6 Nna1 subfamily members, a number undergo extensive alternative splicing (9). Whereas CCP5 appears to be the only enzyme that removes the branch point glutamate (22), Nna1, CCP4, and CCP6 apparently catalyze the same reaction (22), which begs the question of the need for such a diverse array of proteins. Although the tubulins are the most studied substrates in terms of polyglutamylation (54), other proteins may undergo this form of post-translational modification (53). Thus, different family members may exhibit further aspects of substrate specificity. The in vitro and in vivo strategies described here can be applied to assess whether other members are biologically redundant with Nna1 and influence neuronal survival.
Acknowledgments
The authors thank Drs. Jaeki Min and Fangyi Zhu (Department of Chemical Biology and Therapeutics, St. Jude Children's Research Hospital) and Drs. Parmil Bansal, Roberto Pattarini, and Hong Guo (Department of Developmental Neurobiology, St. Jude Children's Research Hospital) for assistance with chemical synthesis, mass spectrometry, and FPLC analysis, as well as the Hartwell Center for Bioinformatics and Biotechnology and Protein Production Facility (St. Jude Children's Research Hospital) for molecular biology and proteomics support.
This work was supported in part by U.S. National Cancer Institute cancer center support grant CA 21765, U.S. National Institutes of Health grant NS051537 to J.I.M., and American Lebanese Syrian Associated Charities (ALSAC).
The authors declare no conflicts of interest.
Footnotes
- Agtpbp1
- ATP/GTP binding protein 1
- ASPA
- aspartoacylase
- ASTE
- succinylglutamate desuccinylase
- biotin-2E
- biotin-Glu-Glu-OH
- biotin-3E
- biotin-Glu-Glu-Glu-OH
- CCP
- cytosolic carboxypeptidase
- CHX
- cycloheximide
- FA-Glu-Glu
- furyl-acryloyl-glutamyl-glutamatic acid
- FPLC
- fast protein liquid chromatography
- Hipp-l-Arg
- hippuryl-l-arginine
- Hipp-l-Asp
- hippuryl-l-aspartic acid
- Hipp-l-Glu
- hippuryl-l-glutamic acid
- Hipp-l-Phe
- hippuryl-l-phenylalanine
- ICP OES
- inductively coupled plasma optical emission spectroscopy
- LC-MS
- liquid chromatography-mass spectrometry
- N-Ace-Asp
- N-acetyl-aspartic acid
- N-Ace-Glu
- N-acetyl-glutamic acid
- OP
- 1,10-phenanthroline
- pBKS
- pBluescript KS
- pcd
- Purkinje cell degeneration
- SDS-PAGE
- sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- Z
- N-benzyloxycarbonyl
REFERENCES
- 1. Harris A., Morgan J. I., Pecot M., Soumare A., Osborne A., Soares H. D. (2000) Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Mol. Cell. Neurosci. 16, 578–596 [DOI] [PubMed] [Google Scholar]
- 2. Fernandez-Gonzalez A., La Spada A. R., Treadaway J., Higdon J. C., Harris B. S., Sidman R. L., Morgan J. I., Zuo J. (2002) Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science 295, 1904–1906 [DOI] [PubMed] [Google Scholar]
- 3. Blanks J. C., Spee C. (1992) Retinal degeneration in the pcd/pcd mutant mouse: accumulation of spherules in the interphotoreceptor space. Exp. Eye Res. 54, 637–644 [DOI] [PubMed] [Google Scholar]
- 4. Greer C. A., Shepherd G. M. (1982) Mitral cell degeneration and sensory function in the neurological mutant mouse Purkinje cell degeneration (PCD). Brain Res. 235, 156–161 [DOI] [PubMed] [Google Scholar]
- 5. Mullen R. J., Eicher E. M., Sidman R. L. (1976) Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl. Acad. Sci. U. S. A. 73, 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Gorman S., Sidman R. L. (1985) Degeneration of thalamic neurons in “Purkinje cell degeneration” mutant mice. I. Distribution of neuron loss. J. Comp. Neurol. 234, 277–297 [DOI] [PubMed] [Google Scholar]
- 7. Wang T., Morgan J. I. (2007) The Purkinje cell degeneration (pcd) mouse: an unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 1140, 26–40 [DOI] [PubMed] [Google Scholar]
- 8. Wang T., Parris J., Li L., Morgan J. I. (2006) The carboxypeptidase-like substrate-binding site in Nna1 is essential for the rescue of the Purkinje cell degeneration (pcd) phenotype. Mol. Cell. Neurosci. 33, 200–213 [DOI] [PubMed] [Google Scholar]
- 9. Kalinina E., Biswas R., Berezniuk I., Hermoso A., Aviles F. X., Fricker L. D. (2007) A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 21, 836–850 [DOI] [PubMed] [Google Scholar]
- 10. Arolas J. L., Vendrell J., Aviles F. X., Fricker L. D. (2007) Metallocarboxypeptidases: emerging drug targets in biomedicine. Curr. Pharm. Des. 13, 349–366 [DOI] [PubMed] [Google Scholar]
- 11. Vendrell J., Aviles F. X., Fricker L. D. (2004) Metallocarboxypeptidases. In Handbook of Metalloproteins (Messerschmidt A., Bode W., Cygler M., eds) pp. 167–192, John Wiley & Sons, Chichester, UK [Google Scholar]
- 12. Berezniuk I., Sironi J., Callaway M. B., Castro L. M., Hirata I. Y., Ferro E. S., Fricker L. D. (2010) CCP1/Nna1 functions in protein turnover in mouse brain: Implications for cell death in Purkinje cell degeneration mice. FASEB J. 24, 1813–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berezniuk I., Fricker L. D. (2010) A defect in cytosolic carboxypeptidase 1 (Nna1) causes autophagy in Purkinje cell degeneration mouse brain. Autophagy 6, 558–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chakrabarti L., Eng J., Ivanov N., Garden G. A., La Spada A. R. (2009) Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol. Brain 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J., Lee J. E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J. G. (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chakrabarti L., Zahra R., Jackson S. M., Kazemi-Esfarjani P., Sopher B. L., Mason A. G., Toneff T., Ryu S., Shaffer S., Kansy J. W., Eng J., Merrihew G., MacCoss M. J., Murphy A., Goodlett D. R., Hook V., Bennett C. L., Pallanck L. J., La Spada A. R. (2010) Mitochondrial dysfunction in NnaD mutant flies and Purkinje cell degeneration mice reveals a role for Nna proteins in neuronal bioenergetics. Neuron 66, 835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez de la Vega M., Sevilla R. G., Hermoso A., Lorenzo J., Tanco S., Diez A., Fricker L. D., Bautista J. M., Aviles F. X. (2007) Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 21, 851–865 [DOI] [PubMed] [Google Scholar]
- 18. Oberdick J., Smeyne R. J., Mann J. R., Zackson S., Morgan J. I. (1990) A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science 248, 223–226 [DOI] [PubMed] [Google Scholar]
- 19. Kaul R., Gao G. P., Balamurugan K., Matalon R. (1993) Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat. Genet. 5, 118–123 [DOI] [PubMed] [Google Scholar]
- 20. Matalon R., Michals K., Sebesta D., Deanching M., Gashkoff P., Casanova J. (1988) Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am. J. Med. Genet. 29, 463–471 [DOI] [PubMed] [Google Scholar]
- 21. Bitto E., Bingman C. A., Wesenberg G. E., McCoy J. G., Phillips G. N., Jr. (2007) Structure of aspartoacylase, the brain enzyme impaired in Canavan disease. Proc. Natl. Acad. Sci. U. S. A. 104, 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogowski K., van Dijk J., Magiera M. M., Bosc C., Deloulme J. C., Bosson A., Peris L., Gold N. D., Lacroix B., Grau M. B., Bec N., Larroque C., Desagher S., Holzer M., Andrieux A., Moutin M. J., Janke C. (2010) A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 143, 564–578 [DOI] [PubMed] [Google Scholar]
- 23. Kimura Y., Kurabe N., Ikegami K., Tsutsumi K., Konishi Y., Kaplan O. I., Kunitomo H., Iino Y., Blacque O. E., Setou M. (2010) Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs). J. Biol. Chem. 285, 22936–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei P., Blundon J. A., Rong Y., Zakharenko S. S., Morgan J. I. (2011) Impaired locomotor learning and altered cerebellar synaptic plasticity in pep-19/PCP4-null mice. Mol. Cell. Biol. 31, 2838–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rong Y., Wang T., Morgan J. I. (2004) Identification of candidate Purkinje cell-specific markers by gene expression profiling in wild-type and pcd(3J) mice. Brain Res. Mol. Brain Res. 132, 128–145 [DOI] [PubMed] [Google Scholar]
- 26. Cullen D. A., Leigh P. N., Gallo J. M. (2004) Degradation properties of polyglutamine-expanded human androgen receptor in transfected cells. Neurosci. Lett. 357, 175–178 [DOI] [PubMed] [Google Scholar]
- 27. Doi E., Shibata D., Matoba T. (1981) Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 118, 173–184 [DOI] [PubMed] [Google Scholar]
- 28. Mathews C. K., van Holde K. E., Ahern K. C. (2000) Enzymes: biological catalysis. In Biochemistry pp. 375–395, Addison Wesley Longman, San Francisco [Google Scholar]
- 29. Falquet L., Pagni M., Bucher P., Hulo N., Sigrist C. J., Hofmann K., Bairoch A. (2002) The PROSITE database, its status in 2002. Nucleic Acids Res. 30, 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makarova K. S., Grishin N. V. (1999) The Zn-peptidase superfamily: functional convergence after evolutionary divergence. J. Mol. Biol. 292, 11–17 [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Saez I., Reverter D., Vendrell J., Aviles F. X., Coll M. (1997) The three-dimensional structure of human procarboxypeptidase A2. Deciphering the basis of the inhibition, activation and intrinsic activity of the zymogen. EMBO J. 16, 6906–6913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gagnon C., White D., Cosson J., Huitorel P., Edde B., Desbruyeres E., Paturle-Lafanechere L., Multigner L., Job D., Cibert C. (1996) The polyglutamylated lateral chain of alpha-tubulin plays a key role in flagellar motility. J. Cell Sci. 109(Pt. 6), 1545–1553 [DOI] [PubMed] [Google Scholar]
- 33. Ikegami K., Heier R. L., Taruishi M., Takagi H., Mukai M., Shimma S., Taira S., Hatanaka K., Morone N., Yao I., Campbell P. K., Yuasa S., Janke C., Macgregor G. R., Setou M. (2007) Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. U. S. A. 104, 3213–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirose J., Ando S., Kidani Y. (1987) Excess zinc ions are a competitive inhibitor for carboxypeptidase A. Biochemistry 26, 6561–6565 [DOI] [PubMed] [Google Scholar]
- 35. Le Coq J., An H. J., Lebrilla C., Viola R. E. (2006) Characterization of human aspartoacylase: the brain enzyme responsible for Canavan disease. Biochemistry 45, 5878–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith E. L. (1949) The mode of action of the metal-peptidases. Proc. Natl. Acad. Sci. U. S. A. 35, 80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vallee B. L., Neurath H. (1955) Carboxypeptidase, a zinc metalloenzyme. J. Biol. Chem. 217, 253–261 [PubMed] [Google Scholar]
- 38. Coombs T. L., Felber J. P., Vallee B. L. (1962) Metallocarboxpeptidases: mechanism of inhibition by chelating agents, mercaptans, and metal ions. Biochemistry 1, 899–905 [DOI] [PubMed] [Google Scholar]
- 39. Rozen F., Pelletier J., Trachsel H., Sonenberg N. (1989) A lysine substitution in the ATP-binding site of eucaryotic initiation factor 4A abrogates nucleotide-binding activity. Mol. Cell. Biol. 9, 4061–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chakrabarti L., Eng J., Martinez R. A., Jackson S., Huang J., Possin D. E., Sopher B. L., La Spada A. R. (2008) The zinc-binding domain of Nna1 is required to prevent retinal photoreceptor loss and cerebellar ataxia in Purkinje cell degeneration (pcd) mice. Vision Res. 48, 1999–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho J. H., Kim D. H., Lee K. J., Choi K. Y. (2001) The role of Tyr248 probed by mutant bovine carboxypeptidase A: insight into the catalytic mechanism of carboxypeptidase A. Biochemistry 40, 10197–10203 [DOI] [PubMed] [Google Scholar]
- 42. Christianson D. W., Lipscomb W. N. (1986) X-ray crystallographic investigation of substrate binding to carboxypeptidase A at subzero temperature. Proc. Natl. Acad. Sci. U. S. A. 83, 7568–7572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lipscomb W. N. (1980) Carboxypeptidase A mechanisms. Proc. Natl. Acad. Sci. U. S. A. 77, 3875–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gardell S. J., Craik C. S., Hilvert D., Urdea M. S., Rutter W. J. (1985) Site-directed mutagenesis shows that tyrosine 248 of carboxypeptidase A does not play a crucial role in catalysis. Nature 317, 551–555 [DOI] [PubMed] [Google Scholar]
- 45. Rees D. C., Lipscomb W. N. (1981) Binding of ligands to the active site of carboxypeptidase A. Proc. Natl. Acad. Sci. U. S. A. 78, 5455–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Christianson D. W., Lipscomb W. N. (1986) Structure of the complex between an unexpectedly hydrolyzed phosphonamidate inhibitor and carboxypeptidase A. J. Am. Chem. Soc. 108, 545–546 [DOI] [PubMed] [Google Scholar]
- 47. Faming Z., Kobe B., Stewart C. B., Rutter W. J., Goldsmith E. J. (1991) Structural evolution of an enzyme specificity. The structure of rat carboxypeptidase A2 at 1.9-A resolution. J. Biol. Chem. 266, 24606–24612 [PubMed] [Google Scholar]
- 48. Eaton D. L., Malloy B. E., Tsai S. P., Henzel W., Drayna D. (1991) Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma. J. Biol. Chem. 266, 21833–21838 [PubMed] [Google Scholar]
- 49. Skidgel R. A. (1988) Basic carboxypeptidases: regulators of peptide hormone activity. Trends Pharmacol. Sci. 9, 299–304 [DOI] [PubMed] [Google Scholar]
- 50. Lyons P. J., Fricker L. D. (2011) Carboxypeptidase O is a glycosylphosphatidylinositol-anchored intestinal peptidase with acidic amino acid specificity. J. Biol. Chem. 286, 39023–39032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei S., Segura S., Vendrell J., Aviles F. X., Lanoue E., Day R., Feng Y., Fricker L. D. (2002) Identification and characterization of three members of the human metallocarboxypeptidase gene family. J. Biol. Chem. 277, 14954–14964 [DOI] [PubMed] [Google Scholar]
- 52. Edge M., Forder C., Hennam J., Lee I., Tonge D., Hardern I., Fitton J., Eckersley K., East S., Shufflebotham A., Blakey D., Slater A. (1998) Engineered human carboxypeptidase B enzymes that hydrolyse hippuryl-L-glutamic acid: reversed-polarity mutants. Protein Eng. 11, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 53. Van Dijk J., Miro J., Strub J. M., Lacroix B., van Dorsselaer A., Edde B., Janke C. (2008) Polyglutamylation is a post-translational modification with a broad range of substrates. J. Biol. Chem. 283, 3915–3922 [DOI] [PubMed] [Google Scholar]
- 54. Janke C., Rogowski K., van Dijk J. (2008) Polyglutamylation: a fine-regulator of protein function? Protein Modifications: Beyond the Usual Suspects review series. EMBO Rep. 9, 636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alexander J. E., Hunt D. F., Lee M. K., Shabanowitz J., Michel H., Berlin S. C., MacDonald T. L., Sundberg R. J., Rebhun L. I., Frankfurter A. (1991) Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 88, 4685–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Edde B., Rossier J., Le Caer J. P., Prome J. C., Desbruyeres E., Gros F., Denoulet P. (1992) Polyglutamylated alpha-tubulin can enter the tyrosination/detyrosination cycle. Biochemistry 31, 403–410 [DOI] [PubMed] [Google Scholar]
- 57. Redeker V., Melki R., Prome D., Le Caer J. P., Rossier J. (1992) Structure of tubulin C-terminal domain obtained by subtilisin treatment. The major alpha and beta tubulin isotypes from pig brain are glutamylated. FEBS Lett. 313, 185–192 [DOI] [PubMed] [Google Scholar]
- 58. Rudiger M., Plessman U., Kloppel K. D., Wehland J., Weber K. (1992) Class II tubulin, the major brain beta tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett. 308, 101–105 [DOI] [PubMed] [Google Scholar]
- 59. Skidgel R. A., Erdos E. G. (2007) Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator. Int. Immunopharmacol. 7, 1888–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reznik S. E., Fricker L. D. (2001) Carboxypeptidases from A to Z: implications in embryonic development and Wnt binding. Cell. Mol. Life Sci. 58, 1790–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]