Abstract
The molecular mechanisms of neuronal morphology and synaptic vesicle transport have been largely elusive, and only a few of the molecules involved in these processes have been identified. Here, we developed a novel morphology-based gene trap method, which is theoretically applicable to all cell lines, to easily and rapidly identify the responsible genes. Using this method, we selected several gene-trapped clones of rat pheochromocytoma PC12 cells, which displayed abnormal morphology and distribution of synaptic vesicle-like microvesicles (SLMVs). We identified several genes responsible for the phenotypes and analyzed three genes in more detail. The first gene was BTB/POZ domain-containing protein 9 (Btbd9), which is associated with restless legs syndrome. The second gene was cytokine receptor-like factor 3 (Crlf3), whose involvement in the nervous system remains unknown. The third gene was single-stranded DNA-binding protein 3 (Ssbp3), a gene known to regulate head morphogenesis. These results suggest that Btbd9, Crlf3, and Ssbp3 regulate neuronal morphology and the biogenesis/transport of synaptic vesicles. Because our novel morphology-based gene trap method is generally applicable, this method is promising for uncovering novel genes involved in the function of interest in any cell lines.—Hashimoto, Y., Muramatsu, K., Kunii, M., Yoshimura, S., Yamada, M., Sato, T., Ishida, Y., Harada, R., Harada, A. Uncovering genes required for neuronal morphology by morphology-based gene trap screening with a revertible retrovirus vector.
Keywords: PC12, synaptic vesicles
Neurons extend two different processes, axons and dendrites, whose functions are essential for signal transmission (1–3). This neuronal polarity is generated and maintained by the selective transport of proteins and lipids (4). The distribution of synaptic vesicles to the tip of axons is a good indicator of selective transport. In PC12 cells, which is a pheochromocytoma-derived cell line used as a typical model system of neurons, acetylcholine (ACh) and monoamines are transported through different pathways. The transport proteins responsible for packaging these neurotransmitters sort to distinct vesicular compartments: The vesicular ACh transporters (VAChTs) localize to synaptic vesicle-like microvesicles (SLMVs), and the vesicular monoamine transporters (VMATs) localize to large dense core vesicles (LDCVs) (5). However, only a few of the genes involved in these trafficking systems have been identified, and thus, the molecular mechanism of selective transport in neurons remains elusive (6–8).
As a method to identify novel genes involved in neuronal transport and morphology, we employed a gene trap method to screen PC12 cells. To date, chemical mutagens, such as N-ethyl-N-nitrosourea (ENU), have been used to obtain mutant clones in CHO cells for the identification of genes involved in the trafficking of LDL receptors (9) or the biogenesis of peroxisomes (10). However, chemical mutagenesis has some drawbacks. First, it is a labor-intensive method to identify the genes responsible for the mutant phenotype because it requires phenotype rescue by introducing a cDNA library into the mutant clones, the identification of rescued cells, and the identification of responsible cDNAs from the rescued cells. Second, it is difficult to distinguish between the phenotypes caused by spontaneous genetic instability and the genuine phenotypes by chemical mutagen-induced point mutations. Thus, it is almost impossible to identify genes by chemical mutagenesis in most of the differentiated cell lines.
Gene trap, a method widely used to study insertional mutagenesis, has been developed as a powerful technique to efficiently identify unknown genes (11–14). In mouse embryonic stem cells (ESCs), it allows for gene mutation and the simultaneous generation of the corresponding mutant mice. Gene trap has several variations, including enhancer, promoter, exon (or gene) and poly(A) traps. Recently, the exon trap method, which contains a strong splice acceptor (SA) sequence upstream of a promoterless reporter, has been widely used. The selectable reporter of this vector is not expressed unless integration into the intron of an expressed gene occurs. Exon trap vectors are highly efficient in mutating genes, and the mutated genes can be easily identified using the 5′ rapid amplification of cDNA ends (5′RACE) method or other methods.
In the present study, we modified the removable exon trap (RET) retrovirus vector (14). Using this vector, we excised most of the inserted vector using the Cre-loxP technology to obtain revertants; the clones that displayed no phenotype reversion on expressing Cre recombinase were considered as consequences of spontaneous genetic instability irrespective of vector integration. We applied this modified RET retrovirus vector to PC12 cells to identify the genes involved in neuronal morphology and synaptic vesicle transport. The infected PC12 cells were screened using two methods: aerolysin and morphology screening and morphology-based screening.
Using these methods, we successfully identified clones that were defective in cellular morphology and/or distribution of SLMVs in PC12 cells. Moreover, we could revert the phenotypes of some of these PC12 clones by expressing Cre recombinase, and the genes responsible for the phenotypes were identified using 5′RACE, inverse PCR, or splinkerette (SPLK) PCR methods. These genes are likely involved in neuronal morphology and/or the biogenesis and transport of synaptic vesicles. In addition, the screening strategy used here is easy, requires less time and labor, and is theoretically applicable to all cell lines. Thus, we believe this strategy will be useful to identify the genes involved in any desired function in any cell lines.
MATERIALS AND METHODS
Constructing the gene trap vector and preparing retrovirus-producing cells
A 7.15-kb gene trap vector, SA-IRES-Puro-pA-PGK-βgeo-pA, containing an SA followed by an internal ribosomal entry site (IRES) joined in-frame with the puromycin resistance (Puro) and β-geo sequences, the PGK promoter and a poly(A) (pA) signal, was constructed. Briefly, the pBluescript vector pBS-PGK-Puro-pA (a kind gift from Dr. P. W. Laird, University of Southern California, Los Angeles, CA, USA) was digested with HindIII/SalI and subcloned into pBC (Agilent Technologies, Palo Alto, CA, USA). Subsequent to digesting pBS-SA-IRES (14) with NotI and EcoRI, an SA-IRES fragment was generated. This SA-IRES fragment was inserted into the NotI/EcoRI site of the pBC-PGK-Puro-pA vector to obtain pBC-SA-IRES-PGK-Puro-pA. This plasmid was digested with SalI, treated with Klenow DNA polymerase to create blunt ends, and further digested with XhoI.
Finally, to obtain the pBC-SA-IRES-Puro-pA-PGK-βgeo-pA plasmid, the HindIII-blunt end/XhoI fragment of PGK-βgeopA derived from pBS-PGK-βgeo-pA was inserted into the SalI-blunt end/XhoI site of pBC-SA-IRES-PGK-Puro-pA. To generate the retroviral vector pGen-loxP-SA-IRES-Puro-pA-PGK-βgeo-pA, the gene trap cassette described above was digested using NotI and XhoI, and was inserted into a NotI/XhoI site in the pGen-loxP retrovirus vector (14). To generate retrovirus-producing cells, pGen-loxP-SA-IRES-Puro-pA-PGK-βgeo-pA was transfected into PlatE cells using FuGene 6 (Roche, Basel, Switzerland). After 48 h of incubation, the virus-containing supernatant was harvested, passed through a 0.45-μm filter, and stored at −80°C until further use.
Generating PC12 cell lines
PC12 cells were routinely maintained in Dulbecco's modified minimal Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) at 37°C in a humidified 5% CO2/95% air atmosphere. To induce neurite outgrowth, PC12 cells were cultured in the differentiation medium containing 50 ng/ml of nerve growth factor (NGF;Sigma-Aldrich, St. Louis, MO, USA) and 1 mM dibutyryl-cAMP (Wako, Osaka, Japan). When the PC12 cells were exposed to differentiation medium, drugs, or retrovirus, the dishes and coverslips were coated with 40 μg/ml of calfskin collagen (Calbiochem, San Diego, CA, USA).
To create infectable PC12 cells, the parental PC12 cells were transfected with the murine cationic amino acid transporter 1 (mCAT1; ref. 15) fused with a zeocin resistance gene (Life Technologies, Carlsbad, CA, USA) using Lipofectamine 2000 (Life Technologies). The cell line, named PC12-mCAT1, was isolated with 100 μg/ml of zeocin (Life Technologies), and subcloned using limiting dilution in 96-well dishes. PC12-mCAT1 displayed high retrovirus infectability, constant drug sensitivity to puromycin and aerolysin, and a typical PC12 morphology.
To establish a PC12 line stably expressing VAChT fused to enhanced green fluorescent protein (EGFP), PC12-mCAT1 cells were transfected with VAChT-EGFP (kindly provided by Dr. M. Itakura, Tokyo Institute of Technology, Yokohama, Japan) and the hygromycin resistance gene (Life Technologies) using Lipofectamine 2000. After selection using 75 μg/ml of hygromycin (Life Technologies), the cells were trypsinized and plated in 96-well dishes for limiting dilution, and this cell line, PC12-VAChT, was isolated for further experiments.
Aerolysin and morphology screening of PC12-mCAT1 and antibody staining
For retrovirus infection, PC12-mCAT1 cells were plated in 100-mm dishes. At 80% confluence, the medium was replaced with a retrovirus-containing medium. After 48 h, 700 μg/ml of G418 (Nacalai Tesque, Kyoto, Japan), 0.3 μg/ml of puromycin (Nacalai Tesque) and 0.8–1.2 nM aerolysin (Protox Biotech, Victoria, BC, Canada) were added to the medium. After 3 wk of selection, we picked up the drug-resistant colonies under a microscope; the colonies were trypsinized and plated in 96-well dishes. The cells were passaged in 24-well dishes, frozen, and stored until further use.
For antibody staining, selected PC12 lines were differentiated on collagen-coated coverslips in differentiation medium containing NGF and dibutyryl-cAMP. After 3 d, the PC12 cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4), permeabilized in 0.05% saponin in PBS for 5 min at room temperature, and incubated with the mouse anti-synaptophysin monoclonal antibody (Millipore, Billerica, MA, USA), followed by incubation with Alexa 488-conjugated anti-mouse IgG antibody (Life Technologies). The cells were observed using a laser scanning confocal microscope (model MRC-1024; Zeiss, Oberkochen, Germany), and the confocal images were processed digitally using Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Morphology-based screening of PC12-VAChT
PC12-VAChT cells were plated in 100-mm dishes, and at 80% confluence, the medium was replaced with a retrovirus-containing medium. After 48 h, G418 (700 μg/ml) and puromycin (0.3 μg/ml) were added to the medium, and after 4 wk of selection, the colonies were picked up under a microscope. Each individual colony was trypsinized and divided in two: half of the colony was plated in a 96-well dish containing growth medium (DMEM+10% FCS), and the other half was subcultured in glass-bottom 96-well collagen-coated dishes in differentiation medium containing NGF and dibutyryl-cAMP. The cells were cultured for 2–5 d, and the differentiated cells in the glass-bottom dishes were observed using a laser scanning confocal microscope. The cells that showed changes in morphology and vesicle distribution/amount were selected and passaged from the 96-well dishes with the growth medium.
Cre/loxP-mediated excision using adenovirus
Adenovirus encoding Cre recombinase was prepared using an adenovirus Cre/loxP kit, version 2.0 (Takara, Otsu, Japan), according to the manufacturer's instructions. For the adenovirus infection, PC12 cells were plated in 24-well dishes containing coverslips. At 30–50% confluence, the cells were incubated with the adenovirus-containing medium at 37°C for 7 h, and subsequently, the virus-containing medium was replaced with the differentiation medium containing NGF and dibutyryl-cAMP. At 3 d after infection, the cells were fixed and examined with a laser scanning confocal microscope.
RNA isolation, 5′RACE, inverse PCR, and SPLK PCR
Total RNA was extracted from the PC12 cell clones using the phenol-chloroform extraction procedure, RNAiso (Takara, Otsu, Japan). Total RNA (3 μg) was primed with oligo(dT) to synthesize first-strand cDNA using reverse transcriptase.
The 5′RACE reactions were conducted using a 5′RACE kit (Life Technologies) according to the manufacturer's instructions. The primers for the first RACE reactions were SA377 (5′-CATGTTGACTTCACTTGTGG-3′) and AAP primer (Life Technologies), and the primers for the second RACE reaction were SA285 (5′-CCAAACTGAGCAGAGTCTTC-3′) and AUAP primer (Life Technologies). The 5′RACE products were subcloned into the TA cloning vector (Promega, Madison, WI, USA) for subsequent sequencing steps.
For inverse PCR, genomic DNA was extracted from the PC12 cell clones and digested with HindIII or XbaI at 37°C overnight. The digested genomic DNA fragments were incubated with T4 DNA ligase (Promega, Madison, WI, USA) at 4°C overnight for self-circulation. The self-circularized DNA was used directly for PCR, and the amplification products were subcloned into the TA cloning vector (Promega) for subsequent sequencing steps. The primers used for inverse PCR were vec3′ forward (5′-AGCTAGCTTGCCAAACCTAC-3′), PGK promoter reverse (5′-CTAAAGCGCATGCTCCAGAC-3′), and poly(A) reverse (5′-AGATGGCTGGCAACTAGAAGGC-3′).
The SPLK PCR was performed with standard protocols (16). Genomic DNA was digested with ApoI, and the primers used were first PCR lox5′ reverse (5′-GGTCAGGAACAGATGGAACA-3′), first PCR lox3′ out forward (5′-CGATCCCGATCTGAACTTCT-3′), second PCR lox5′ far reverse (5′-TGTGGTCTCGCTGTTCCTTG-3′), and vec3′ 65 forward (5′-AGCTAGCTTGCCAAACCTAC-3′).
Genomic Southern blotting and quantitative RT-PCR
Genomic DNAs from PC12 clones digested with several restriction enzymes were analyzed using Southern blotting in accordance with standard protocols (17). A probe (ca. 300 bp) from the puromycin resistance gene was amplified by a forward primer (5′-TCACCGAGCTGCAAGAACTC-3′) and a reverse primer (5′-AAGCCGAGCCGCTCGTAGAA-3′) using the gene trap vector pGen-loxP-SA-IRES-Puro-pA-PGK-βgeo-pA as a template.
Quantitative RT-PCR was performed as described previously (18). To evaluate the mRNA levels in the trapped clones, we used the Universal Probe Library (Roche, Basel, Switzerland): probe 68 for BTB/POZ domain-containing protein 9 (Btbd9) 5′ primers (upstream of insertion) and probe 84 for Btbd9 3′ primers (downstream of insertion) for Btbd9 (clone A61), probe 17 for myocardial zonula adherens protein (Myzap; clone A64), probe 55 for T-complex 11-like 1 (clone P05), probe 46 for cdc16 (clone P12), probe 50 for Btf3l4 (clone P26), probe 66 for Crlf3 (clone P173), probe 63 for single-stranded DNA-binding protein 3 (Ssbp3) 3′ primers (downstream of insertion) and probe 17 for Ssbp3 flanking primers (flanking insertion) (clone P207), probe 63 for β-actin, and probe 83 for rat glucose 6-phosphate dehydrogenase X-linked (rG6PDX).
The following primers were used for the quantitative RT-PCR: Btbd9 sense 5′ primer (upstream of insertion; 5′-GCTTCCTCTCTCTGTCCAAGAC-3′), Btbd9 antisense 5′ primer (upstream of insertion; 5′-TGCATTGTGCTTACACCAGTT-3′); Btbd9 sense 3′ primer (downstream of insertion; 5′-ATCGTCGGAACGCACAAC-3′), Btbd9 antisense 3′ primer (downstream of insertion; 5′-TCACTGCTGTCTTCCTTCTGG-3′) for clone A61; Myzap sense primer (5′-GAGAAGGAACGGCATCAACT-3′), Myzap antisense primer (5′-CCCCGACATTTCTGTCTCAT-3′) for clone A64; T-complex 11-like 1 sense primer (5′-AGCTGCCTCTCCATGTGTG-3′), T-complex 11-like 1 antisense primer (5′-CCCTTGAGCACAGTCTCCTT-3′) for clone P05; Cdc16 sense primer (5′-GAGAGCGAGCATGACCAAG-3′), Cdc16 antisense primer (5′-AGCATTGGCAAGTGACACC-3′) for clone P12; Btf3l4 sense primer (5′-TGGGTTGAAAATGTCCTGAGA-3′), Btf3l4 antisense primer (5′-ACACCAAAGGTGCCTGACTT-3′) for clone P26; Crlf3 sense primer (5′-TAACCGGGAGGTGGTGTTT-3′), Crlf3 antisense primer (5′-TCCGAAGTAGAGAGAGCCACA-3′) for clone P173; Ssbp3 sense 3′ primer (downstream of insertion; 5′-CGATCAGAATGGGAAACCAG-3′), Ssbp3 antisense 3′ primer (downstream of insertion; 5′-ATCCATGGAATTGGGCATC-3′); Ssbp3 sense flanking primer (flanking insertion; 5′-TGGTGTGTATTTTGGGACCTT-3′), Ssbp3 antisense flanking primer (flanking insertion; 5′-TGCTTCACTTGAATGTTCACAAG-3′) for clone P207; β-actin sense primer (5′-CTGGCTCCTAGCACCATGA-3′), β-actin antisense primer (5′-TAGAGCCACCAATCCACACA-3′); or rG6PDX sense primer (5′-AAGGTGTCTTCGGGTAGAAGG-3′), rG6PDX antisense primer (5′-TTATCATCATGGGTGCATCG-3′) as a standard for quantitation.
Knockdown and overexpression analysis
To knock down the Btbd9 gene (for clone A61) in PC12 cells, we used ON-TARGETplus SMARTpool, Rat Btbd9 (294318; Thermo Fisher Scientific, Lafayette, CO, USA). This siRNA was introduced into the parental PC12-mCAT1 cell line with Cy3-labeled negative control siRNA (AM4621; Life Technologies) by Nucleofector (Lonza, Cologne, Germany) for identification of transfectants. To knock down the Ctrf3 (Cytor4) gene (for clone P173), we used Accell SMARTpool, Rat Cytor4 (54395; Thermo Fisher Scientific, Lafayette, CO, USA). We introduced the siRNA into the parental PC12-VAChT cell line together with Accell Red Nontargeting siRNA for identification of transfectants and as a negative control (when used without siRNA against rat Cytor4). Introduction of Accell SMART pool was performed according to the manufacturer's instruction. By both methods, siRNAs were introduced efficiently and mRNAs were efficiently knocked down. To express truncated genes, we produced DNA fragments of a truncated Btbd9 gene (exon1–6) and a truncated Ctrf3 gene (exon1) by PCR. These genes were cloned into the N-terminally Flag tagging mammalian expression vector, pcDNA5 (Life Technologies). These constructs were introduced into the parental PC12 cells by Nucleofector (Lonza, Cologne, Germany) with pcDNA4-Cherry (Life Technologies) for identification of transfectants.
Karyotype analysis
PC12 cells were passaged so that cells should be in a stage of active cell division when initiating the karyotyping procedure. Colcemid solution (1 mg/ml; Sigma-Aldrich) was added to the culture medium to the final (0.05 μg/ml) concentration. Cells were incubated 30 min at 37°C. Culture medium was transferred to a 15-ml tube. PBS was added to the cells, and, after swirling the dishes, it was transferred to the same 15-ml tube. Cells were trypsinized, transferred to the same 15-ml tube, and centrifuged 5 min at 1500 rpm. Supernatant was removed and pellet was resuspended in 5 ml of 0.075 M KCl aq. After standing tubes for 15 min at room temperature, 5 ml of Carnoy's fixative (mixture of acetic acid/methanol, 1:3) was added, pipetted again, and centrifuged 3 min at 1500 rpm. Cells were resuspended in 10 ml of Carnoy's fixative and centrifuged again. The same process was repeated once again. Finally, Carnoy's fixative was added to the pellet for making cell suspension. Cells were aspired by a glass pipette and dropped onto the clean, wet, and chilled slides from 20–30 cm above the slides. Dry slides were set 2–3 cm above the water bath at 75°C. After drying, chromosomes were stained by Quinacrine (Sigma-Aldrich) by standard procedures.
Bioinformatics
Vector integration sites were determined by BLAST analysis using the databases of the U.S. National Center for Biotechnology Information (NCBI; http://blast.ncbi.nlm.nih.gov/) and Ensembl Rat (http://www.ensembl.org/). Homology searches were performed using ClustalW (http://clustalw.ddbj.nig.ac.jp/) through the DNA Data Bank of Japan (DDBJ).
RESULTS
Constructing the gene trap retrovirus and developing screening methods
Retrovirus vectors have been used for trapping genes in murine ESCs (11–13). They are known to cause fewer chromosomal aberrations than plasmid vectors when inserted into the genome of its host. To enable gene trapping and identification in any desired cell lines, we modified the RET retrovirus vector established previously (14). We applied this method to identify novel genes involved in the morphology of neurons and the distribution of synaptic vesicles.
To identify the genes expressed, we introduced SA-IRES-Puro-pA in the retrovirus vector. The IRES increases gene trapping efficiency by enabling the expression of downstream genes irrespective of the open reading frames of the preceding genes (19). In the latter half of the vector, we introduced the PGK promoter-driven β-geo to confirm the insertion of the vector through resistance to G418 and blue X-gal staining of the trapped cells. In addition, we added loxP sequences to the long terminal repeats (LTRs) flanking the insert of the gene trap vector, which allows the excision of most of the inserted fragment out of the genome. This technique enables us to easily confirm that the mutated phenotype is created by gene trapping through phenotypic reversion using Cre/loxP-mediated recombination.
We infected PC12 cells with this retrovirus vector (pGen-loxP-SA-IRES-Puro-pA-PGK-βgeo-pA) and selected the infected clones with G418 and puromycin. Subsequently, we screened the clones using two different methods. First, we selected clones using aerolysin, which kills cells by binding to their surface GPI-anchored proteins (20), to select clones that had defects in the transport of GPI-anchor proteins to the plasma membrane. The aerolysin-resistant clones were further examined, and the clones with morphological abnormalities were selected for further experiments. We called this method aerolysin and morphology screening.
As a second method to select clones, we used a PC12 cell line stably expressing VAChT fused with EGFP (VAChT-EGFP). We used VAChT as a marker of SLMVs, and in parental PC12 cells, it is predominantly targeted to SLMVs and clustered at the nerve terminals (5, 6). After retroviral infection, we performed visual screening under the fluorescent microscope to select clones that showed defects in the amount or distribution of VAChT-EGFP. We called this method morphology-based screening (Fig. 1).
Figure 1.
Schematic representation of the gene trap retrovirus and the method for morphology-based screening. The gene trap retrovirus contains a SA and IRES upstream of the puromycin resistance and β-geo genes. Productive integration occurs in an intron of an expressed gene downstream of coding exons such that the puromycin resistance gene is spliced into the gene transcript. After selection with G418 and puromycin, the mutant clones were morphologically examined under a fluorescent microscope. The abnormal phenotype should be reverted to normal through Cre-mediated excision if the phenotype is due to the provirus insertion.
Identifying clones obtained by aerolysin and morphology screening
To generate PC12 cells infectable by a retrovirus, we established a PC12 cell line, PC12-mCAT1, which stably expressed mCAT1, a cellular receptor for ecotropic retrovirus (15). We introduced the gene trap retrovirus into the PC12-mCAT1 cells and selected 69 colonies using G418, puromycin, and aerolysin. For each clone, we examined the branching of neurites after NGF stimulation under a fluorescence microscope as well as the intensity and distribution of the SLMVs, which were stained using an antibody against synaptophysin. We selected 10 clones that showed morphology or synaptophysin staining different from parental PC12-mCAT1. We prepared mRNA and genomic DNA from each clone and examined the integration sites using 5′RACE or inverse PCR. The morphological phenotypes of the selected clones and the identified genes are summarized in Table 1.
Table 1.
Genes identified by aerolysin and morphology screening
| Clone | Identifying method | Clone phenotype | Identified gene | Accession no. | Chr no. | Revert |
|---|---|---|---|---|---|---|
| A18 | Inverse PCR | Thin and long neurites | Thyroid adenoma associated (Thada) | NM_001191769.1 | 6q12 | |
| A21 | Inverse PCR | Small cell body | Similar to glyceraldehyde-3-phosphate dehydrogenase | NM_001037190.1 | 10q26 | |
| A28 | Inverse PCR | Short and multiply-branched neurites | Growth associated protein 43 (Gap43) | NM_017195.3 | 11q21 | |
| A38 | Inerse PCR | Thin and long neurites | G-protein-coupled receptor 19 (Gpr19) | NM_080579.1 | 4q43 | |
| A44 | Not identified | Flat shape | ||||
| A46 | Not identified | Thin and long neurites | ||||
| A56 | Inverse PCR | Few neurites | MICAL C-terminal like (Micalcl) | NM_182669.2 | 1q33 | |
| A61 | Inverse PCR | Weak staining of synaptophysin | BTB (POZ) domain containing 9 (Btbd9) | NM_001013073.1 | 20p12 | + |
| A63 | Not identified | Few small neurites | ||||
| A64 | 5′ RACE | Small cell body | Myocardial zonula adherens protein (Myzap) | NM_001014211.1 | 8q24 | + |
One of these clones, A61, displayed a significant decrease of synaptophysin staining (Fig. 2A). We identified the trapped gene of this clone as Btbd9 (Table 1), a gene associated with restless legs syndrome (21).
Figure 2.
Features of clone A61. A) Parental PC12-mCAT1 cells (left panel), A61 cells (middle panel), and A61 cells after Cre/loxP-mediated recombination (right panel) were stained using an antibody against synaptophysin. A61 displayed weak expression of synaptophysin, but this phenotype recovered after infection with the adenovirus-encoding Cre. B) A61 cells were resistant to aerolysin but became sensitive to aerolysin after Cre/loxP-mediated recombination. Scale bars = 100 μm.
Cre/loxP-mediated excision of the integrated provirus
To examine whether the phenotype was a consequence of provirus insertion, Cre recombinase was transiently expressed by adenovirus infection to remove the integrated provirus from the trapped gene. At 3 d after infection with the adenovirus encoding Cre, the infected cells were fixed and examined under a fluorescence microscope. We observed that 2 of these clones, A61 and A64, became indistinguishable from parental PC12-mCAT1 cells after Cre/loxP-mediated recombination, while the other 8 clones were not reverted (Table 1 and Fig. 2A). These cells were sensitive to aerolysin again when Cre was expressed in this clone (Fig. 2B): the reverted cells were rounded and detached from the substrate when exposed to aerolysin. These results showed that the phenotype of the trapped gene was restored using the Cre/loxP-mediated excision of the integrated provirus.
Identifying clones obtained by morphology-based screening
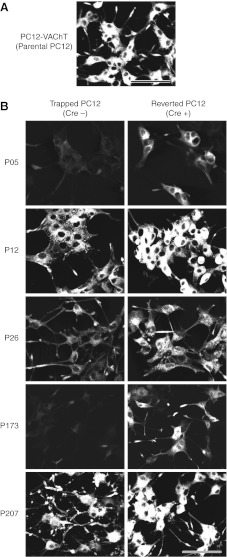
The VAChT is expressed on SLMVs in PC12 cells. Thus, for the morphology-based screening, we established a PC12 cell line, PC12-VAChT, which stably expressed VAChT-EGFP in addition to mCAT1 (Fig. 3A). Table 2 shows the summary of these results. We introduced the gene trap retrovirus into the PC12-VAChT cell line and selected 180 colonies using G418 and puromycin. For each clone, we examined the branching of neurites after NGF stimulation as well as the intensity and distribution of VAChT-EGFP using a fluorescence microscope. A total of 24 clones that displayed abnormal morphologies and/or abnormal VAChT distribution were selected for further experiments. The typical clones are shown in the left panels of Fig. 3B. In some cases (clones P05, P26, and P173), the amount of SLMVs was reduced; in other cases (clones P12 and P207), the distribution pattern of SLMVs or the morphology of PC12 was changed.
Figure 3.
Morphological features of mutant clones selected using morphology-based screening. Expression of VAChT-EGFP in the parental PC12-VAChT cells (A), mutant clones (B, left panels), and reverted clones (B, right panels). Fluorescence of VAChT-EGFP is diminished (P26), almost absent (P05 and P173), or aggregated in the cell body (P12). Clone P207 has multibranched neurites and intense VAChT-EGFP fluorescence at the tips of these neurites. After Cre/loxP-mediated recombination (B, right panels), these mutant clones became indistinguishable from the parental PC12 cells (PC12-VAChT). Scale bars = 100 μm.
Table 2.
Summary of morphology-based screening
| Characteristic | n |
|---|---|
| Clones picked up | 180 |
| Clones with abnormal morphologies | 24 |
| Clones reverted by Cre/loxP-mediated excision | 14 |
| Clones whose responsible genes are identified | 5 |
We tested whether the phenotypes could be reverted through the deletion of the viral insertion using Cre recombinase (Fig. 3B, right panels). We confirmed that the phenotypes of 14 of the 24 clones were reverted on infection with the Cre-encoding adenovirus (Table 2). The cells successfully reverted to the normal status were used to identify the inactivated genes. We attempted the 5′RACE method first because the mRNA upstream of the inserted site was expected to be trapped by the splice acceptor localized at the beginning of the gene trap vector. We also performed inverse PCR and SPLK PCR to identify the insertion sites. Table 3 shows the genes trapped using the retrovirus vectors and the methods used to identify the genes. Among these mutated clones, two clones were selected for further analysis (Figs. 3 and 4 and Table 4). Clone P173 displayed weak VAChT-EGFP fluorescence in the cell body and neurites. The trapped gene, Crlf3, is a cytokine receptor-like factor, and at present, its association with biogenesis or VAChT trafficking is unknown. Clone P207 had multibranched neurites and intense VAChT-EGFP fluorescence at the tip of the neurites. The responsible gene, Ssbp3 (also known as Ssdp1), has been reported to regulate head morphogenesis in mice (22).
Table 3.
Genes identified by morphology-based screening
| Clone | Identifying method | Clone phenotype | Identified gene | Accession no. | Chr no. |
|---|---|---|---|---|---|
| P05 | Inverse PCR | VAChT very weak expression | T-complex 11 like 1 (Tcp11l1) | NM_001109202 | 3q32 |
| P12 | SPLK PCR | VAChT aggregated in cell body | Cell division cycle 16 homolog (S. cerevisiae) (Cdc16) | NM_001024744.1 | 16q 12.5 |
| P26 | Inverse PCR | VAChT weak expression | Basic transcription factor 3-like 4, transcript variant 3 (Btf3l4) | XM_345561.5 | 5q35 |
| P173 | 5′ RACE and inverse PCR | VAChT very weak expression | Cytokine receptor-like factor 3 (Crlf3) (Also known as Cytor4) | NM_001168612.1 | 10q25 |
| P207 | 5′ RACE and SPLK PCR | Branched neurites/VAChT at neurite tip | Single stranded DNA binding protein 3 (Ssbp3) | NM_053358.1 | 5q34 |
Figure 4.
Identification of the proviral integration sites of clones A61, P173, P207, and P26. Insertion sites in Btbd9, Crlf3, Ssbp3, and Btf3l4 genes are illustrated by red triangles. Exons are represented as vertical black bars with the exon number indicated above. Primers used for quantitative RT-PCR are indicated by blue arrows. See Table 4 for nucleotide sequences at the junction of the endogenous gene and vector DNA.
Table 4.
Nucleotide sequences at the junction of the endogenous gene and vector DNA
| Cell line (gene) | Method | Inserted DNA sequence and vector sequence (underscored) |
|---|---|---|
| A61 (Btbd9) | Inverse PCR (5′ side) | CGCACACTTTGGGAGAGTAAGCTT–GATATCGAATTCTACCGGG |
| Inverse PCR (3′ side) | TACAGGTGGGGTCTTTCA–ACCTAAAAAAGAGAACCTTGCATC | |
| P173 (Crlf3) | 5′ RACE | GGCTGCGGGAGGCGCAGAGGCAG–GATGCCTTTGTGGAACTG |
| Inverse PCR (3′ side) | TACAGGTGGGGTCTTTCA–TGAGGAAGAATTCGGGGAGAGTTT | |
| P207 (Ssbp3) | 5′ RACE | GAAGCAAAAGCCTTTCATGATAC–GATGCTTTGTGGAACTGT |
| SPLK PCR | GTCAGCGGGGGTCTTTCA–CTACCTAAAGCCTCTGCCCTGGC | |
| P26 (Btf3l4) | Inverse PCR (3′ side) | TACAGGTGGGGTCTTTCA–GTATGTGATGGTATATTTTTCTTAAT |
Vector DNA sequences are underscored. Fig. 4 illustrates insertion sites.
Southern blot analysis of the trapped clones
To examine the number of inserted vectors in the genome, we performed genomic Southern blot of these clones using probes within the gene trap vector (Fig. 5A). All clones had 1 vector/cell except P05, which appeared to have 2 bands by genomic Southern blot, and thus appeared to have 2 vectors/cell.
Figure 5.
Southern blot analysis to confirm the integration of the gene trap vector and the integration sites in the genome. A) Genomic DNAs extracted from parental and mutant PC12 cells were digested with a restriction enzyme, ApaI. They were then probed with a puromycin probe (left panel) and an IRES probe (right panel). Bands are indicated by asterisks. Weak nonspecific bands (indicated by left arrowhead) were observed in all of the clones including the parental PC12. B) Genomic DNA from nonrevertant clones (A44, A28, A18, and A38) was purified before (Cre−), 1 d after (1D), and 3 d after (3D) the treatment Cre recombinase, digested by ApaI, and probed with a puromycin probe. Bands are indicated by white asterisks. C) Genomic DNAs extracted from the parental and mutant PC12 cells were digested with various restriction enzymes. A61 and P173 displayed two bands; P207 and P26 displayed one band. Genomic probes used are indicated in panel D. D) Genomic regions around the integration sites. Gene trap vectors are shown in red; probes in blue.
To confirm whether the floxed regions of the trap vector were excised by Cre recombinase even in the nonrevertant clones, we expressed Cre recombinase by adenovirus in nonrevertant clones (Fig. 5B). The nonrevertant clones completely lost puromycin-resistant genes in their inserted vectors 3 d after Cre expression. This observation suggests that nonrevertants cannot revert to the normal phenotypes not because of inability to excise their insertions by Cre recombinase.
Next, we performed Southern blot analysis for A61, P173, and P207 to confirm the provirus integration sites determined by 5′RACE, inverse PCR or SPLK PCR. Genomic DNAs extracted from these clones were digested with several restriction enzymes, and the DNA fragments were detected with each probe near the integration site on the Btbd9, Crlf3 and Ssbp3 genes. As shown in Fig. 5C, A61 and P173 gave two bands: one band was derived from the parental clone, and the other was derived from the trapped allele. However, P207 gave only a single band, which differed from the parental band and suggests that this clone had only one allele of the Ssbp3 gene. These results indicate that the proviral integration occurred in one allele in A61, P173, and P207, and in P207, the other allele might have already been deleted in the parental PC12 cell line.
To determine the amount of mRNA from the trapped genes, we used real-time PCR. In two cell lines (A61 and P173), approximately half of the mRNA from the trapped genes remained (Fig. 6A). When we measure the amount of mRNA of A61, we used primers and probes for real-time PCR at both upstream (5′ primers in Fig. 4) and downstream of the inserted site (3′ primers in Fig. 4) and found a reduction of mRNA by both primers and probes (Fig. 6A). We could not find splicing from the upstream exons (exons 1 and 2) to the splice acceptor of the gene trap vector (Fig. 6B), which suggests that the reduced amount of mRNA in the upstream of the inserted site was not because of the skipping of the upstream probe located at exons 3 and 4.
Figure 6.
Trapped clones expressing detectable amounts of mRNA exhibit the phenotype by their reduced amount of mRNA rather than expression of truncated products. A) Quantitative RT-PCR of A61 and P173. Expression of Btbd9 mRNA in clone A61 was 70.7 ± 4.5% (n=3) by 5′ primers [A61(5′)] and 34.7 ± 4.8% (n=3) by 3′ primers [A61(3′)], compared with the parental PC12 clones. Expression of Crlf3 mRNA in clone P173 was 54.0 ± 11.0% (n=2). Data represent averages ± sem. B) PCR from exons 1, 2, and 6 to IRES in the gene trap vector. PCR products were not detectable between exons 1/2 and IRES, while PCR product between exon 6 and IRES was clearly detected (an arrow). C) Knockdown of Btbd9 and Crlf3 in parental PC12 clones (middle panels) displayed reduction in the staining of synaptophysin similar to A61 (top right panel), and that of VAChT-EGFP similar to P173 (bottom right panel), respectively. D) Overexpression of truncated Btbd9 (tr-Btbd9) and Crlf3 (tr-Crlf3) does not change the phenotype of parental PC12. Transfected cells (arrows) and nontransfected cells (arrowheads) displayed similar staining of synaptophysin (left panels) and VAChT-EGFP (right panels), respectively. Cherry staining was used as a marker for transfected cells. Scale bars = 100 μm.
In these cell lines, two possibilities were considered for their phenotypes. First, the levels of Btbd9 and Crlf3 expression were not sufficient to permit normal function. Second, truncated gene products may have dominant negative effects. To distinguish these possibilities, we first tried to reduce the amount of Btbd9 and Crlf3 by siRNA in parental clones. After reduction by siRNA, the staining of VAChT-EGFP of parental PC12 (PC12-VAChT) appeared similar to the trapped clone (P173; Fig. 6C). Similarly, the staining of synaptophysin of parental PC12 (PC12-mCAT1) resembled the trapped clone (A61; Fig. 6C). In contrast, expression of truncated cDNA in the parental PC12 cells did not cause the phenotypes of the trapped clones (Fig. 6D), excluding the latter possibility.
In contrast, in clone P207, we could hardly detect Ssbp3 mRNA (Fig. 7A, left bars). This result is consistent with the genomic Southern blot results showing that P207 had only one Ssbp3 allele. Using primers that flanked the insertion site (Fl primers in Fig. 4), we could hardly detect the PCR product (Fig. 7A, middle panel). Thus, the efficiency of gene trap was almost complete, which confirmed the result from the previous study (14).
Figure 7.
Trapped clones with small amounts of mRNA from the trapped loci frequently have monosomy of chromosome 5. A) Quantitative RT-PCR of P207 and P26. Expression of Ssbp3 mRNA in clone P207 was 1.4 ± 0.7% (n=3) by 3′ primers [P207(3′)] and 0.2 ± 0.01% (n=3) by flanking primers [P207(Fl)], compared with the parental PC12. The expression of P26 was 1.6 ± 0.3% (n=3). Data represent averages ± sem. B) Karyotype analysis of parental PC12 (PC12-mCAT1 and PC12-VAChT). Markers correspond to structurally abnormal chromosomes. C) Frequency of monosomy in each chromosome of PC12-mCAT1 and PC12-VAChT. Results are expressed as percentage of cells with monosomy at the indicated chromosome number. Five cells were counted from each parental cell line.
To find out how many trapped alleles were haploids, we examined the level of mRNA for the rest of the trapped cell lines (P05, P12, P26, and A64) and discovered that only one of these cell lines, P26, had only a trace amount of mRNA (Fig. 7A, right bars). We performed genomic Southern blot using a probe near the insertion site of P26 (Fig. 5C). Only one band was detected with this probe, supporting that the locus was haploid. To know whether the absence of mRNA in P207 and P26 resulted from monosomy of chromosome 5, on which both Ssbp3 (P207) and Btf3l4 (P26) genes were localized, we performed karyotype analysis of the parental PC12 clones (PC12-mCAT1 and PC12-VAChT; Fig. 7B, C). In PC12-VAChT, the parental line of both P207 and P26, only one chromosome 5 was observed in 80% of the PC12 cells. In contrast, clone PC12-mCAT1 had two chromosomes 5 in most cases.
DISCUSSION
There are a number of established mammalian cell lines that reflect the nature of the tissues of their origin. However, mammalian cell lines are generally not considered to be suitable for somatic cell mutagenesis for two reasons. First, they are (more than) diploid. Thus, we are only able to observe phenotypes when both alleles are simultaneously mutated. However, considering the low frequency of mutagenesis, mutations in the same gene do not frequently occur at one time. Second, many cell lines are prone to genetic instability. CHO cells are known to have a functional haploid genome and a stable phenotype over many generations. Therefore, CHO cells are frequently used for somatic mutagenesis to uncover genetic factors that cause various phenotypes (9, 10). However, CHO cells are not suitable for identifying genes expressed in highly differentiated cells, such as neurons or lymphocytes.
Here, we present a novel gene trap method that can theoretically be applied to any cell line. We could easily obtain revertants using this trap vector and confirm whether the observed phenotypes originated from the gene trap events. In our experiments, 16 of the 34 clones (2 clones from 10 clones screened by aerolysin and 14 clones from 24 clones screened by their morphology) reverted to normal phenotypes on infection with an adenovirus encoding Cre (Tables 1 and 2). The nonreverted clones were considered to have phenotypes that were not caused by the trapped and inactivated genes. Araki et al. (23) used a Cre expression vector for gene trap mutagenesis in mouse ESCs and reported a recombination frequency of 10–70%. As we have obtained 47.1% (16/34) recombination frequency, this retrovirus vector will be efficient enough for gene trap screening.
Recently, genome-wide RNAi screening has been frequently used to identify genes involved in specific cellular phenotypes (24). Although this method is powerful, it also has some disadvantages. For example, some cell lines have low transfection efficiencies and are not suitable for RNAi screening. In contrast, when a retrovirus receptor (mCAT1, for example) is successfully introduced to the cell lines, our gene trap retrovirus could easily be infected into any cell line. Another disadvantage is that some RNAi oligonucleotides, which have off-target effects, may cause false positive phenotypes. Our gene trap retroviral vector can easily be removed on the transient expression of Cre recombinase to exclude these false positive phenotypes.
In the present work, we identified a number of candidate genes for neuronal cell morphogenesis or the amount/distribution of SLMVs. Using this method, only one allele would be inactivated or lost in diploid cells. In case of Ssbp3, although only one allele was lost, the mutant clone did not express the mRNA at all. We suspected that the parental PC12 cell line had already lost one of the Ssbp3 alleles. This inference was supported by genomic Southern blot and karyotype analyses (Figs. 5 and 7).
In contrast, in the other two cases, the remaining allele was intact, and approximately half of the mRNA was still expressed. The phenotypes of the mutant clones were mimicked not by overexpression of the truncated gene products, but by reducing the product by siRNA. Thus, it is more likely that these phenotypes are caused by the inactivation of the trapped genes. In these cases, the reduction of mRNA/protein expression by half seemed to be sufficient to cause the phenotypes of the cells. This phenomenon, called haploinsufficiency, is sometimes found in human hereditary diseases and might be a possible explanation of our result (25).
Previously, novel genes potentially involved in neuronal wiring have been identified by a gene trap method in the ESCs (26). The gene trap vector used in this experiment was constructed so that the molecules which had two characteristics would be easily identified. First, only molecules which had secretory signals would be enriched. Second, when heterozygous mutants were generated from the trapped ESCs, axons of the neurons which had this trap vector could be visualized by human placental alkaline phosphatase (PLAP) driven by the endogenous promoter. The staining pattern of PLAP gives information on when and where the molecules would be expressed in the nervous system. Thus, enrichment of molecules which have secretary signal and visualization of the axonal pathways are two major merits of this system.
However, this previous gene trap system has four disadvantages. First, because ESCs are usually diploid, only one insertion of a gene trap vector is insufficient to observe phenotypes in the ESCs. In many cases, phenotypes will be masked by the remaining allele. In our system, we could identify genes which lost their products in the trapped PC12 cells because the chromosomes which have the trap vectors are haploid. Second, if a gene of potential interest is expressed in a strictly neuron-specific manner, it will not be expressed in the ESCs and thus the trapped ESCs will be killed by the drugs during the screening procedure. However, as we used PC12 cells, frequently used as a model cell for neurons, we have more chance to identify neuron-specific genes. Third, this method only enriches the genes that have secretory signals. This finding indicates some important genes involved in biogenesis and localization of synaptic vesicles will not be discovered if they do not have secretory signals. In our system, we screened PC12 cells based on their morphology and the amount/localization of synaptic vesicles. Using this system, we might be able to identify genes involved in neuronal polarity or biogenesis of synaptic vesicles independent of their characters. Fourth, to know the expression pattern in the nervous system, their method required to make chimeric or heterozygous mice, which requires cost and labor. In contrast, we only use PC12 cells in our system, which requires less cost and labor.
More recently, a technique that allows biallelic disruption has been developed in ESCs by regulated deletion of the Bloom's syndrome gene alleles (27, 28). More mutant clones might be obtained through the disruption of both alleles in any cell line by combining our gene trap vector with the knockdown of the Bloom's syndrome gene product.
Among the genes we identified, Btbd9 and Ssbp3 have been previously reported to have important roles in neurons. Recently, a locus in Btbd9 was associated with restless legs syndrome, which is a common sleep disorder also associated with periodic limb movements during sleep (21, 29). The second trapped gene, Ssbp3, is a component of the LIM domain-binding protein 1-associated transcriptional complex. The Ssbp3-deficient mouse was previously generated and displayed developmental defects resulting in the hypogenesis or agenesis of the head, but the mechanism behind these defects has not been revealed yet (22). Thus, our finding that these novel genes are involved in SLMV distribution will lead to the further elucidation of neuronal morphology, the biogenesis or transport of the synaptic vesicles, and the pathogenesis of restless legs syndrome.
Acknowledgments
The authors thank M. Takano, A. Goto, H. Togawa, and A. Watanabe for assistance with cell culture and immunostaining.
The funding for the open access charge was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan [Grant-in-Aid for Scientific Research (B) 21390050 to A.H.; Grant-in-Aid for Scientific Research (C) 21590210 to R.H.]; grants from Kato Memorial Bioscience Foundation to T.S.; and grants from the Mishima Kaiun Memorial Foundation to R.H.
Footnotes
- 5′RACE
- 5′ rapid amplification of cDNA ends
- ACh
- acetylcholine
- Btbd9
- BTB/POZ domain-containing protein 9
- Crlf3
- cytokine receptor-like factor 3
- DMEM
- Dulbecco's modified minimal Eagle's medium
- EGFP
- enhanced green fluorescent protein
- ENU
- N-ethyl-N-nitrosourea
- ESC
- embryonic stem cell
- FCS
- fetal calf serum
- IRES
- internal ribosomal entry site
- LDCV
- large dense core vesicle
- LTRs
- long terminal repeats
- mCAT1
- murine cationic amino acid transporter 1
- Myzap
- myocardial zonula adherens protein
- NGF
- nerve growth factor
- pA
- polyA
- PLAP
- human placental alkaline phosphatase
- Puro
- puromycin resistance
- RET
- removable exon trap
- rG6PDX
- rat glucose 6-phosphate dehydrogenase X-linked
- SA
- splice acceptor
- SLMV
- synaptic vesicle-like microvesicle
- SPLK PCR
- splinkerette PCR
- Ssbp3
- single-stranded DNA-binding protein 3
- VAChT
- vesicular ACh transporter
- VAChT-EGFP:
- VAChT fused with EGFP
- VMAT
- vesicular monoamine transporter
REFERENCES
- 1. Black M. M., Baas P. W. (1989) The basis of polarity in the neuron. Trends Neurosci. 12, 211–214 [DOI] [PubMed] [Google Scholar]
- 2. Barnes A. P., Polleux F. (2009) Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rasband M. N. (2010) The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11, 552–562 [DOI] [PubMed] [Google Scholar]
- 4. Burack M. A., Silverman M. A., Banker G. (2000) The role of selective transport in neuronal protein sorting. Neuron 26, 465–472 [DOI] [PubMed] [Google Scholar]
- 5. Liu Y., Edwards R. H. (1997) Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J. Cell Biol. 139, 907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weihe, Tao-Cheng E. J. H., Schafer M. K., Erickson J. D., Eiden L. E. (1996) Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 93, 3547–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Itakura M., Misawa H., Sekiguchi M., Takahashi S., Takahashi M. (1999) Transfection analysis of functional roles of complexin I and II in the exocytosis of two different types of secretory vesicles. Biochem. Biophys. Res. Commun. 265, 691–696 [DOI] [PubMed] [Google Scholar]
- 8. Fei H., Grygoruk A., Brooks E. S., Chen A., Krantz D. E. (2008) Trafficking of vesicular neurotransmitter transporters. Traffic 9, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krieger M., Brown M. S., Goldstein J. L. (1981) Isolation of Chinese hamster cell mutants defective in the receptor-mediated endocytosis of low density lipoprotein. J. Mol. Biol. 150, 167–184 [DOI] [PubMed] [Google Scholar]
- 10. Tsukamoto T., Yokota S., Fujiki Y. (1990) Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J. Cell Biol. 110, 651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gossler A., Joyner A. L., Rossant J., Skarnes W. C. (1989) Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science 244, 463–465 [DOI] [PubMed] [Google Scholar]
- 12. Stanford W. L., Cohn J. B., Cordes S. P. (2001) Gene-trap mutagenesis: past, present and beyond. Nat. Rev. Genet. 2, 756–768 [DOI] [PubMed] [Google Scholar]
- 13. Yamamura K., Araki K. (2008) Gene trap mutagenesis in mice: new perspectives and tools in cancer res. Cancer Sci. 99, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishida Y., Leder P. (1999) RET: a poly A-trap retrovirus vector for reversible disruption and expression monitoring of genes in living cells. Nucleic Acids Res. 27, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albritton L. M., Scadden L. T. D., Cunningham J. M. (1989) A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57, 659–666 [DOI] [PubMed] [Google Scholar]
- 16. Horn C., Hansen J., Schnutgen F., Seisenberger C., Floss T., Irgang M., De-Zolt S., Wurst W., von Melchner H., Noppinger P. R. (2007) Splinkerette PCR for more efficient characterization of gene trap events. Nat. Genet. 39, 933–934 [DOI] [PubMed] [Google Scholar]
- 17. Harada A., Oguchi K., Okabe S., Kubo J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. (1994) Altered microtubule organization in small-caribre axons of mice lacking tau protein. Nature 369, 488–491 [DOI] [PubMed] [Google Scholar]
- 18. Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A., Harada R., Harada A. (2007) The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366–369 [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury K., Bonaldo P., Torres M., Stoykova A., Gruss P. (1997) Evidence for the stochastic integration of gene trap vectors into the mouse germline. Nucleic Acids Res. 25, 1531–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diep D. B., Nelson K. L., Raja S. M., Pleshak E. N., Buckley J. T. (1998) Glycosyl- phosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J. Biol. Chem. 23, 2355–2360 [DOI] [PubMed] [Google Scholar]
- 21. Winkelmann J., Schormair B., Lichtner P., Ripke S., Xiong L., Jalilzadeh S., Fulda S., Pütz B., Eckstein G., Hauk S., Trenkwalder C., Zimprich A., Stiasny-Kolster K., Oertel W., Bachmann C. G., Paulus W., Peglau I., Eisensehr I., Montplaisir J., Turecki G., Rouleau G., Gieger C., Illig T., Wichmann H. E., Holsboer F., Müller-Myhsok B., Meitinger T. (2007) Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat. Genet. 39, 1000–1006 [DOI] [PubMed] [Google Scholar]
- 22. Nishioka N., Nagano S., Nakayama R., Kiyonari H., Ijili T., Taniguchi K., Shawlot W., Hayashizaki Y., Westphal H., Behringer R. R., Matsuda Y., Sakoda S., Kondoh H., Sasaki H. (2005) Ssdp1 regulates head morphogenesis of mouse embryos by activating the Lim1-Ldb1 complex. Development 132, 2535–2546 [DOI] [PubMed] [Google Scholar]
- 23. Araki K., Imaizumi T., Sekimoto T., Yoshinobu K., Yoshimuta J., Akizuki M., Miura K., Araki M., Yamamura K. (1999) Exchangeable gene trap using the Cre/mutated lox system. Cell. Mol. Biol. 45, 737–750 [PubMed] [Google Scholar]
- 24. Root D. E., Hacohen N., Hahn W. C., Lander E. S., Sabatini D. M. (2006) Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat. Methods 3, 715–719 [DOI] [PubMed] [Google Scholar]
- 25. Judge D. P., Biery N. J., Keene D. R., Geubtner J., Myers L., Huso D. L., Sakai L. Y., Dietz H. C. (2004) Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Invest. 14, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leighton P. A., Mitchell K. J., Goodrich L. V., Lu X., Pinson K., Scherz P., Skarnes W. C., Tessier-Lavigne M. (2001) Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature 410, 174–179 [DOI] [PubMed] [Google Scholar]
- 27. Guo G., Wang W., Bradley A. (2004) Mismatch repair genes identified using genetic screens in Blm-deficient embryonic stem cells. Nature 429, 891–895 [DOI] [PubMed] [Google Scholar]
- 28. Yusa K., Horie K., Kondoh G., Kouno M., Maeda Y., Kinoshita T., Takeda J. (2004) Genome-wide phenotype analysis in ES cells by regulated disruption of Bloom's syndrome gene. Nature 429, 896–899 [DOI] [PubMed] [Google Scholar]
- 29. Stefansson H., Rye D. B., Hicks A., Petursson H., Ingason A., Thorgeirsson T. E., Palsson S., Sigmundsson T., Sigurdsson A. P., Eiriksdottir I., Soebech E., Bliwise D., Beck J. M., Rosen A., Waddy S., Trotti L. M., Iranzo A., Thambisetty M., Hardarson G. A., Kristjansson K., Gudmundsson L. J., Thorsteinsdottir U., Kong A., Gulcher J. R., Gudbjartsson D., Stefansson K. (2007) A genetic risk factor for periodic limb movements in sleep. N. Engl. J. Med. 357, 639–647 [DOI] [PubMed] [Google Scholar]