Abstract
We previously demonstrated that isoflurane targets lymphocyte function-associated antigen-1 (LFA-1), a critical adhesion molecule for leukocyte arrest. However, it remains to be determined how isoflurane interacts with the full ectodomain LFA-1 and modulates its conformation and function. Isoflurane binding sites on the full ectodomain LFA-1 were probed by photolabeling using photoactivatable isoflurane (azi-isoflurane). The adducted residues were determined by liquid chromatography/mass spectrometry analysis. Separately, docking simulations were performed to predict binding sites. Point mutations were introduced around isoflurane binding sites. The significance of isoflurane's effect was assessed in both intracellular adhesion molecule-1 (ICAM-1) binding assays and epitope mapping of activation-sensitive antibodies using flow cytometry. Two isoflurane binding sites were identified using photolabeling and were further validated by the docking simulation: one at the hydrophobic pocket in the ICAM-1 binding domain (the αI domain); the other at the βI domain. Mutagenesis of the α′1 helix showed that isoflurane binding sites at the βI domain were significantly important in modulating LFA-1 function and conformation. Epitope mapping using activation-sensitive antibodies suggested that isoflurane stabilized LFA-1 in the closed conformation. This study suggested that isoflurane binds to both the αI and βI domains allosteric to the ICAM-1 binding site, and that isoflurane binding stabilizes LFA-1 in the closed conformation.—Yuki, K., Bu, W., Xi, J., Sen, M., Shimaoka, M., Eckenhof, R.G. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1.
Keywords: immunomodulation, photolabeling, rigid docking, volatile anesthetics
Isoflurane perturbs leukocyte-endothelial cell interactions and suppresses leukocyte accumulation at inflammatory sites both in vitro and in vivo (1–4). We showed previously that isoflurane directly interacts with lymphocyte function-associated antigen-1 (LFA-1; αLβ2), the major leukocyte adhesion molecule that mediates leukocyte arrest, a critical step for leukocyte recruitment. We further suggested that this isoflurane/LFA-1 interaction is one of the underlying mechanisms for the suppression of leukocyte recruitment (5). A structural understanding of isoflurane/LFA-1 interaction will not only enhance our knowledge of the still unresolved mechanism of volatile anesthetics but also provide the foundation for redesigning new anesthetic drugs without immunomodulation.
LFA-1 is a heterodimeric adhesion molecule that consists of the α subunit, which has an intercellular adhesion molecule-1 (ICAM-1) binding domain called the αI domain, and the noncovalently associated β subunit (6–8). Each subunit consists of a large extracellular segment, a single transmembrane segment, and a short cytoplasmic tail. The αI domain adopts an α/β Rossman fold with a metal ion-dependent adhesion site (MIDAS; the αI MIDAS) on the “top” of the domain. The αI MIDAS serves as the ICAM-1 binding site; its ability to bind to ICAM-1 is regulated by a large conformational change in LFA-1, as described below. Like the α subunit, the β2 subunit contains the βI domain, which also adopts an α/β Rossman fold with a MIDAS (the βI MIDAS) on the top of the domain. The βI domain is considered to function as a regulatory domain that relays conformational signals to the αI domain.
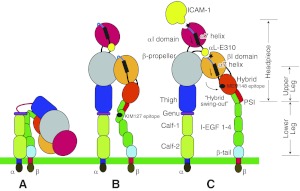
Delineation of the LFA-1 structure has been the target of considerable investigation. Negative-stain electron microscopy has shed light on the global structure and has demonstrated the existence of three different conformations (ref. 9 and Fig. 1). In the resting state, LFA-1 is in a bent (or closed) conformation in which α- and β-cytoplasmic tails associate with one another and the headpiece contacts the “legs.” In this state, ICAM-1 binds to the αI MIDAS with a low affinity (Fig. 1A). Once LFA-1 is activated through intracellular signaling induced by other receptors (e.g., GPCRs; refs. 10, 11), a separation of the cytoplasmic tails is induced (12). This cytoplasmic separation is translated to a separation at the transmembrane and extracellular levels, which destabilizes the headpiece-leg interface and induces an extension of the legs (leg-extended conformation with a closed headpiece; Fig. 1B; ref. 13). Then the α7 helix of the βI domain is pulled down, and the hybrid domain swings out (14). This latter motion opens up the headpiece and alters the geometry of the βI MIDAS (leg-extended conformation with an open headpiece; Fig. 1C; refs.15, 16). Now, αL-Glu-310 at the end of the C-terminal α7 helix acts as an internal ligand and binds to the βI MIDAS (17). Presumably, this is how the conformational change of the βI domain is transmitted to the αI domain (Fig. 1C; refs. 14, 17). Following this event, the C-terminal α helix of the αI domain is pulled down, which finally triggers the structural alteration of the αI MIDAS into the high-affinity conformation for ICAM-1 (14).
Figure 1.
Schema of LFA-1 conformation. A) LFA-1 in a resting condition. Legs are in a bent conformation. B) Leg-extended conformation with a closed headpiece; the cytoplasmic domains are separated. KIM127 epitope is shown as a black circle. C) Leg-extended conformation with an open headpiece. Arrows next to the α7 helices in the αI and βI domains indicate that both helices are pulled down. Both the αI and βI MIDAS are shown as black stars. MEM148 epitope is shown as a black circle.
Our previous nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography of the isolated αI domain demonstrated that isoflurane binds to the hydrophobic pocket underneath the α7 helix (5, 18). This pocket has been termed the lovastatin site, because it binds αI allosteric antagonists such as lovastatin and BIRT377 (19). The C-terminal α7 helix of the αI domain is extremely flexible (20). Simulation studies suggest that αI allosteric antagonists work by disrupting the intrinsic dynamics coupled to the requisite downward traction of the C-terminal α7 helix of the αI domain, thereby stabilizing the αI domain in the closed conformation (21, 22). Since isoflurane shares the binding site with αI allosteric antagonists, we suspect that it interacts with the αI domain similarly. However, small molecules like isoflurane are promiscuous; it cannot be concluded that this is the sole isoflurane binding site without testing the full LFA-1 ectodomain. The αI and βI domains are structurally homologous (Fig. 2), and it may be possible that isoflurane also binds outside the αI domain. In addition, the aforementioned, isolated αI domain was expressed bacterially and lacked glycosylation. Human LFA-1 has multiple N-glycosylation sites (23), which could have an effect on the overall structural conformation and response to ligands. Therefore, it is extremely important to validate our previous finding with mammalian expressed LFA-1. However, the high-resolution crystal structure of the intact LFA-1 molecule has not been reported, and it is beyond the size amendable to NMR (∼250 kDa). Here we attempted to delineate the interaction of isoflurane with the mammalian expressed, full-length LFA-1 ectodomain using a novel isoflurane photolabel called azi-isoflurane (24). We further examined how isoflurane binding affects LFA-1 conformation and function.
Figure 2.
Structure-based sequence alignment of the αL and β2 I domains. α Helices and β strands are underscored. Secondary structure assignment was by the DSSP algorithm (50) from the structure coordinates.
MATERIALS AND METHODS
Cells
HEK293T cells were cultured in DMEM with HEPES modification, 10% FBS at 37°C in 5% CO2.
Antibodies
Monoclonal antibodies TS2/4, TS1/18, KIM127, and MEM148 (Immune Disease Institute, Boston, MA, USA) have been previously described (25, 26).
Protein expression and purification
Soluble LFA-1 protein was expressed and purified from CHO Lec 3.2.8.1 cells stably transfected with αL and β2 plasmids, as previously described (9). The protein yield was ∼1 mg/L.
Photolabeling experiments
Azi-isoflurane has been described previously (24). This compound was produced by incorporating a diazrinyl moiety (CHN2, 40 Da) into isoflurane. LFA-1 protein incubated with or without 1 mM azi-isoflurane in quartz cuvettes was exposed to 300 nm illumination at ∼1 cm distance for 15 min. The protein was separated from unreacted compounds and other contaminates using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Excised bands were trypsinized and submitted for nano–liquid chromatography (LC)/mass spectrometry (MS) analysis. LC was performed using a 10 cm C18 capillary column run at 200 nl/min for 60 min with gradient elution. MS-detected peptides were searched for adducts of the appropriate mass (196 Da) and then further fragment patterns (MS/MS) were searched using Sequest software (Thermo Finnigan, San Jose, CA, USA) to determine the adduct attachment sites. Mass spectrometry work was performed at the University of Pennsylvania Proteomics Core Facility.
Rigid docking
Currently, the complete high-resolution structure of the full ectodomain LFA-1 is not available for docking simulations. We postulated that isoflurane binding sites are localized within the αΙ and βI domains, as suggested by the results of photolabeling experiment. The αΙ and βI domains were docked separately as follows. The structure of the αI domain subunit was obtained from the Protein Data Bank (PDB) ID numbers 1ZOO (27), 1MQ8 (28), and 1T0P (29). Both integrin αXβ2 and LFA-1 share the same β subunit (β2 subunit). Also, the α subunits of αXβ2 and LFA-1 are highly homologous (30). Therefore, the structure of the βI domain of LFA-1 was extrapolated from the βI domain structure of integrin αXβ2 in the closed conformation (PDB 3K6S; ref. 31). The program Glide (Schrodinger, Cambridge, MA, USA) was used to perform molecular rigid docking of isoflurane with the αΙ or βI domain (32). Isoflurane binding position was sought with the grid size of 20 × 20 × 20 Å within the αI domain and 23 × 23 × 23 Å within the βI domain. Both grid sizes are substantially larger than the size of isoflurane (144 Å3; ref. 33). No positional constraint was applied. This program searches positions, orientations, and conformations of the ligand at the binding sites on the receptor using a series of hierarchical filters (32). Once a small number of candidate sites are selected, a Monte Carlo procedure (34) is applied to minimize the energy. Glide has a scoring system, called glidescore, that ranks docked pairs based on predicted affinity. The one with the most negative glidescore is considered to have the highest affinity. For each I domain, we selected the docked pair with the most negative glidescore.
Mutagenesis and transfection
Site-directed mutagenesis was performed using Quikchange XL kit (Stratagene, La Jolla, CA, USA), and DNA sequences were confirmed. Plasmids were transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
Head opening and leg extension epitope analysis
KIM127 and MEM148 are activation-sensitive antibodies that recognize leg extension and headpiece opening, respectively (35–37). HEK293T cells were cotransfected with αL and β2 plasmids 48 h prior to the experiment. Cells were harvested in HEPES-buffered saline (HBS) containing 10 mM EDTA and washed with HBS 3 times. Cells were suspended in HBS and stained with KIM127 or MEM148 in the presence of 1 mM MgCl2/CaCl2 or 1 mM MnCl2 for 30 min at room temperature. Goat anti-mouse IgG-FITC was used as a secondary antibody. After washing, analysis was performed using a FACScan flow cytometer (BD Bioscience, San Jose, CA, USA).
ICAM-1 binding assay
Flow cytometry-based soluble ICAM-1:LFA-1 binding assays were performed as described previously, with minor modification (5). Briefly, cells were cotransfected with αL wild-type (WT) and β2 WT or mutant plasmids 48 h before the experiment. Cells were harvested in HBS containing 10 mM EDTA and washed 3 times with HBS. Cells (5×105) were incubated with 10 μg/ml ICAM-1-IgA fusion protein in the presence of 1 mM MgCl2/CaCl2 or 1 mM MnCl2 for 60 min at room temperature. For some of the experiments, cells were also coincubated with isoflurane (Baxter, Deerfield, IL, USA) or LFA-1 allosteric antagonists (BIRT377, XVA143; Immune Disease Institute) (38). Isoflurane was administered to the cells in a closed chamber using a Fluotec vaporizer (Cyprane Ltd., Keighley, UK). The concentration of isoflurane was measured using Ultima infrared spectroscopy (Datex Instrument Corp., Helsinki, Finland). FITC-conjugated goat anti-human IgA antibody (Pierce, Rockford, IL, USA) was used as a secondary antibody. After washing, bound ICAM-1 was detected using FACScan flow cytometry.
Statistical analysis
Data were analyzed using an analysis of variance (ANOVA) with Tukey post hoc pairwise comparisons or Student's t test. Statistical significance was defined as P < 0.05. All statistical calculations were performed using Prism 5 software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Conformational modulation of LFA-1 by isoflurane
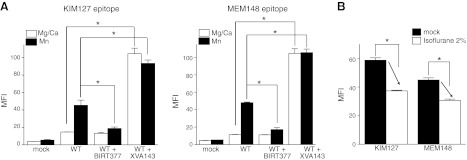
The conformation of LFA-1 was mapped using activation-sensitive antibodies KIM127 and MEM148. KIM127 is mapped to EGF-2 domain and recognizes extension of the legs (ref. 35 and Fig. 1B). MEM148 has been mapped to the hybrid domain junction with the βI domain and probes opening of the headpiece (refs. 36, 37 and Fig. 1C). Two classes of small molecule LFA-1 antagonists are reported: αI allosteric antagonists and α/β allosteric antagonists. αI allosteric antagonists, such as BIRT377 and lovastatin, bind underneath the α7 helix of the αI domain (19, 38–40) to prevent the downward movement of this helix (21, 22). The effect of BIRT377 on leg extension and headpiece opening was examined in 1 mM Mg2+/Ca2+ (resting condition) or 1 mM Mn2+(activating condition). BIRT377 stabilized LFA-1 in the bent conformation, as the exposure of the KIM127 epitope was diminished in Mn2+ condition in the presence of this compound (Fig. 3A), compatible with a previous report (38). In addition, BIRT377 keeps LFA-1 in a closed headpiece conformation, as the exposure of the MEM148 epitope was inhibited (Fig. 3A). On the contrary, α/β allosteric antagonists, such as XVA143, presumably bind to the βI MIDAS and competitively inhibit the binding to its intrinsic ligand αL-Glu-310. Due to the loss of the interaction between αL-Glu-310 and the βI MIDAS, the αI domain stays in the default, closed conformation (16). Since these antagonists are analogous to the intrinsic ligand for the βI MIDAS, they induced leg extension and headpiece opening, as shown by an increase in the exposure of the KIM127 and MEM148 epitopes even in Mg2+/Ca2+ conditions, respectively (Fig. 3A). Isoflurane behaved similarly to αI allosteric antagonists, as demonstrated by impaired exposure of the KIM127 and MEM148 epitopes in Mn2+ conditions (Fig. 3B).
Figure 3.
Effect of known LFA-1 allosteric antagonists or isoflurane on KIM127 and MEM148 epitope exposure. A) KIM127 and MEM148 epitope exposure in the presence of BIRT377 (10 μM) or XVA143 (10 μM). MFI, mean fluorescence intensity. One-way ANOVA with Tukey post hoc analysis was used to compare the data within Mg2+/Ca2+ or Mn2+ group. *P < 0.05 vs. WT. B) KIM 127 and MEM 148 epitope exposure at 2% isoflurane in 1 mM Mn2+. *P < 0.05 vs. mock-treated samples; Student's t test. Data are shown as means ± se of 3 independent experiments of triplicates.
Azi-isoflurane binds to both αI and βI domains
Azi-isoflurane was used to reveal the residual-level binding sites of isoflurane on the full ectodomain LFA-1. Our previous experiment using the isolated αI domain demonstrated that azi-isoflurane was cross-linked to Tyr-257, residue that was found to interact with isoflurane in our previous NMR and crystallographic studies of the same αI domain (24). This experiment provided confidence that azi-isoflurane will reliably report isoflurane's equilibrium binding sites. In our experiment using full LFA-1, 64% of the sequence of the α subunit, as well as 52% of the β subunit, was covered by peptides detected in the mass spectra following photolabeling. Three peptides in the αI domain and one peptide in the βI domain were found to contain adducted residues in the LC/MS. MS/MS approaches revealed the adducted αI domain residues as Tyr-257, Leu-302, Lys-304, and Lys-305 (Table 1 and Supplemental Fig. S1). These residues are known to be the lining residues in the previously identified isoflurane binding cavity, which corresponds to the lovastatin site (18), as mentioned before. Three residues (Leu-302, Lys-304, and Lys-305) were located in the α7 helix, whereas Tyr-257 was in the β5 strand. The previous crystallographic study of the isolated αI domain showed that isoflurane interacts with Ile-235, Tyr-257, Ile-259, Lys-287, Leu-298, Glu-301, and Leu-302 of the αI domain (18). This result gave further confidence that azi-isoflurane will also reliably report adducted residues within the βI domain. Two residues (Leu-135 and Glu-137) in the βI domain were photolabeled, both of which were on the α′1 helix (Table 1 and Supplemental Fig. S1). The α′1 helix faces the α7 helix, which plays an important role in swing-out of the hybrid domain.
Table 1.
Photolabeled residues of LFA-1 by azi-isoflurane
| Subunit | Sequence coverage | Residues |
|---|---|---|
| α | 64.3% | Y257, L302, K304, K305 |
| β | 54.2% | L135, E137 |
Rigid docking supports azi-isoflurane's binding sites
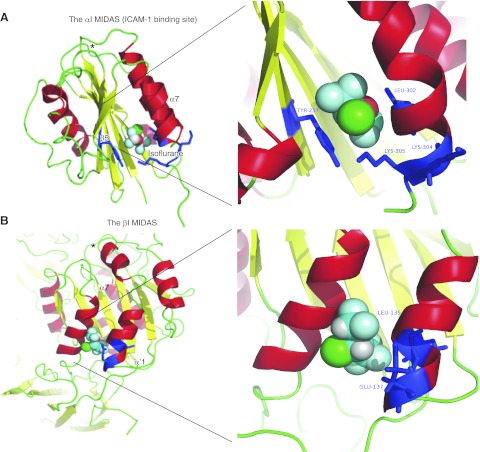
Rigid docking was performed to test the binding site candidates among the αΙ and βI domains. The docking site with the highest calculated affinity was chosen from each domain. The result of azi-isoflurane photolabeling was in excellent agreement with the docking simulations using the αI domain structure in the low-affinity conformation (PDB 1ZOO; glidescore −4.49; Fig. 4A). Docking with the αI domain in the high-affinity conformation (PDB 1MQ8 and 1T0P, respectively) was unsuccessful, as predicted, due to the disruption of the lovastatin site (data not shown). This finding lends further support to the conclusion that isoflurane binds to the αI domain in the low-affinity, closed conformation but not in the high-affinity conformation. All adducted residues except Lys-304 are within 4 Å from the docked isoflurane (Supplemental Fig. S1). Also, the residues found to interact with isoflurane in the previous crystal structure are within 4 Å from docked isoflurane (Supplemental Fig. S1).
Figure 4.
Docking of isoflurane on the αI and βI domains using Glide. A) Docking of isoflurane to the αI domain in the closed conformation (PBD 1ZOO). B) Docking of isoflurane to the βI domain of β2 subunit in the closed conformation (PBD 3K6S). Residues photolabeled by azi-isoflurane are shown in blue. Right panels show blowup of the docking site. Asterisks indicate αI MIDAS. In isoflurane: red, oxygen; green, chloride; light blue, fluoride.
We docked isoflurane to the βI domain in the closed conformation. The best docking site (glidescore −2.37) is shown in Fig. 4B. The trifluoromethyl head of isoflurane formed hydrophobic interactions with Ile-138, Ile-334, and Tyr-338. Another set of interactions was suggested by the proximity of the difluoromethyl fluorine atoms with Val-331 and Lys-335. The two photolabeled residues, Ile-135 and Glu-137, were adjacent to each other and to the lining residues of the putative isoflurane binding cavity; both were within 4 Å from the docked isoflurane (Supplemental Fig. S1). Notably, we did not implement positional constraints in either docking simulation. In addition, from the sequence alignment between the αΙ and βI domains, the isoflurane binding pockets of the two domains are aligned well (Fig. 2 and Supplemental Fig. S1). This agreement between domain results, and between photolabeling and unbiased docking results, lends further confidence to the interpretation that isoflurane favors the intact LFA-1 in the closed, low-affinity conformation.
Role of α′1 helix
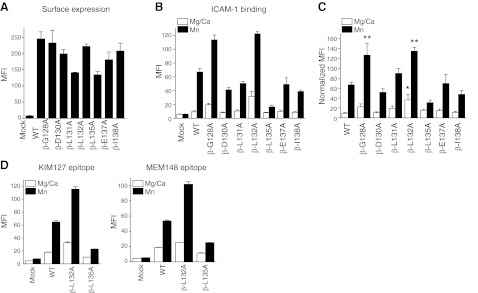
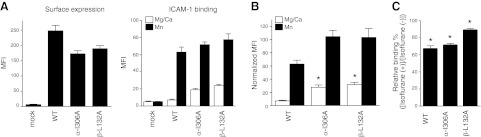
The functional role of the α′1 helix in the β2 I domain has not yet been delineated. To appreciate the functional importance of isoflurane binding sites, alanine-scanning mutagenesis of residues in the α′1 helix was performed. Mutants with >50% surface expression relative to WT LFA-1, as probed by TS2/4 antibody, were tested for ICAM-1 binding. As shown in Fig. 5B, C, β- G128A and L132A mutants bound more to ICAM-1 than WT, while β-L135A mutant diminished ICAM-1 binding. The results of KIM127 and MEM148 epitope exposure suggested that β-L132A was extended with an open headpiece, while β-L135A was bent with a closed headpiece (Fig. 5D). This finding suggests that the mobility of the α′1 helices in the βI domain may affect the α7 helix movement and the activation of the βI domain (the degree of leg extension and head opening), and, in turn, the activation of the αI domain (the degree of ICAM-1 binding).
Figure 5.
Functional role of the α′1 helix of the βI domain. A) Surface expression of alanine-scanning mutant in the α′1 helix probed by TS2/4 antibody. MFI; mean fluorescence intensity. B) ICAM-1 binding of mutants in 1 mM Mg2+/Ca2+ or 1 mM Mn2+. C) ICAM-1 binding of mutants normalized with surface expression. Normalized MFI of ICAM-1 binding was calculated as [MFI of ICAM-1 binding of mutant] × {[(MFI of surface expression of WT) − (MFI of surface expression of mock transfectant)]/[(MFI of surface expression of mutant) − (MFI of surface expression of mock transfectant)]}. One-way ANOVA with Tukey post hoc was performed to compare data within Mg2+/Ca2+ or Mn2+ group. *P < 0.05 vs. WT Mg2+/Ca2+, **P < 0.05 vs. WT Mn2+. D) Leg extension and headpiece opening in β-L132A and β-L135A mutants probed by KIM127 and MEM148 antibodies, respectively. Data are shown as means ± se of 3 independent experiments of triplicates.
Effect of isoflurane on activating mutants
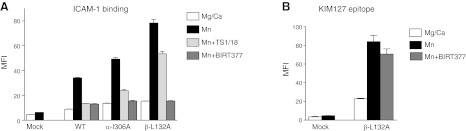
The effect of isoflurane was further tested using αΙ- and βI-activating mutants. As an α I mutant, α-I306A was used (41). An invariant isoleucine (αL-I306) is located in the second half of the α7 helix and intercalates deeply into the hydrophobic pocket in which isoflurane binds, thus providing the stability needed for the low-affinity conformation. This α-I306A mutant may alter the structure of this hydrophobic pocket, destabilize the low-affinity state, and thereby mildly activate LFA-1 even under resting conditions (Mg2+/Ca2+) relative to the WT (Fig. 6A, B). However, BIRT377 significantly abolished the binding to ICAM-1, suggesting that this hydrophobic pocket was still available. TS1/18 is a LFA-1 blocking antibody that binds to the βI domain. TS1/18 abolished only 50% of ICAM-1 binding, suggesting that the mutation from Ile to Ala stabilizes the ICAM-1 binding site in an intermediate affinity conformation (Fig. 7A).
Figure 6.
Effect of isoflurane on activating mutants. A) Surface expression and ICAM-1 binding to α-I306A and β-L132A mutants. LFA-1 surface expression was probed by TS2/4 antibody. Data are shown as means ± se of 3 independent experiments of triplicates. MFI, mean fluorescence intensity. B) ICAM-1 binding of mutants normalized with surface expression. Normalized MFI of ICAM-1 binding was calculated as [MFI of ICAM-1 binding of mutant] × {[(MFI of surface expression of WT) − (MFI of surface expression of mock transfectant)]/[(MFI of surface expression of mutant) − (MFI of surface expression of mock transfectant)]}. One-way ANOVA with Tukey post hoc test was performed to compare data within Mg2+/Ca2+ or Mn2+ group. *P < 0.05 vs. WT Mg2+/Ca2+. C) ICAM-1 binding was performed at 1 mM Mn2+ with or without 2% isoflurane. Relative binding (%) was defined as [(MFI of ICAM-1 binding with 2% isoflurane) − (MFI of mock transfected sample)]/[(MFI of ICAM-1 binding without isoflurane) − (MFI of mock transfected sample)] × 100 (%). Samples that did not get exposed to isoflurane were considered 100% binding (control sample). Data are shown as means ± se of 3 independent experiments, each with triplicates. Statistical analysis was done using 1-way ANOVA with Tukey post hoc analysis. *P < 0.05 vs. control.
Figure 7.
Effect of BIRT377 and TS1/18 on αI and βI mutants. A) Soluble ICAM-1 binding assay. TS1/18 (10 μg/ml) or BIRT377 (10 μM) was used with 1 mM Mg2+/Ca2+ or 1 mM Mn2+. MFI, mean fluorescence intensity. B) KIM127 epitope exposure of β-L132A mutant in the presence of BIRT377 (10 μM). One-way ANOVA with Tukey post hoc analysis was performed. No statistical significance was found between Mn2+ and Mn2++BIRT377 of β-L132A. Data are shown as means ± se of 3 independent experiments of triplicates.
In the case of βI mutant, the β-L132A mutant was chosen for the study. BIRT377 significantly decreased binding to ICAM-1 with no evidence of loss of leg extension (Fig. 7A, B). This finding suggests that ICAM-1 binding remains low as long as the αI domain stays in the low-affinity conformation, irrespective of leg extension in the β subunit. TS1/18 inhibited only ∼25% of binding, suggesting that this mutant prefers to be in the extended conformation, which destabilizes the αI domain low-affinity state. The effect of isoflurane on ICAM-1 binding was tested in the WT and these two mutants. Isoflurane impaired ICAM-1 binding to all mutants, but to WT LFA-1 the most, and β-L132A mutant the least (Fig. 6C).
DISCUSSION
In this study, we demonstrated that isoflurane binds to both αI and βI domains of LFA-1 using a photolabel mimic of the commonly used anesthetic isoflurane. This binding event stabilizes LFA-1 in the closed conformation. The binding site on the αI domain, when using full ectodomain LFA-1, was compatible with our previous results using the isolated αI domain and is located in the known binding pocket of αI allosteric inhibitors. The presence of N-glycosylation did not affect the location of isoflurane binding sites, which further supports the validity of this binding. Another binding site was also noted on the α′1 helix of the βI domain. The importance of this α′1 helix from the perspective of ICAM-1 binding was shown by the mutagenesis study. Our result suggests that binding of isoflurane on both subunits is functionally important to keep LFA-1 in the closed conformation.
The αI domain assumes at least three different conformations; low-affinity, intermediate-affinity, and high-affinity forms (28). During the shift from low-affinity to high-affinity conformation, the β6-α7 loop of the αI domain is pulled downward (28), obliterating the hydrophobic pocket underneath the C-terminal helix. The conformation of the intermediate-affinity state is in between that of the low-affinity and high-affinity states and still possesses the hydrophobic pocket. In the previous study, we used the engineered disulfide bond LFA-1 mutant (α-K287C/K294C), which disrupts the pocket underneath the α7 helix, locking the mutant in a high-affinity state (Table 2 and refs. 28, 40, 42). Therefore, only direct competitors, not αI allosteric antagonists (such as BIRT377), can block ICAM-1 binding. Isoflurane did not affect the binding of ICAM-1 to this mutant (5). On the contrary, this study showed that the α-I306A mutant acts as an intermediate affinity conformer (Table 2). The fact that isoflurane impaired the binding of ICAM-1 to α-I306A mutation, but not α-K287C/K294C mutation, further supports the idea that the binding of isoflurane to the pocket underneath the α7 helix of the αI domain prevents the downward translation of the C-terminal helix (19).
Table 2.
Various LFA-1 mutants and presumed status of the lovastatin site
| Genotype | Site status | Mutant classification |
|---|---|---|
| Wild-type | Available | Not applicable |
| α-K287C/K294C | Disrupted | High affinity |
| α-I306A | Altered, but available | Intermediate affinity |
| β-L132A | Available | Intermediate affinity |
Availability of the lovastatin site was assessed based on whether αI allosteric antagonists inhibit ICAM-1 binding. Three mutants bound to ICAM-1 higher than wild-type in a resting condition (1 mM Mg2+/Ca2+). Regarding α-K287C/K294C, the data using K562 transfectants were considered (42). Because no increase occurred in ICAM-1 binding by Mn2+, α-K287C/K294C was classified as high-affinity mutant. However, two other mutants showed enhanced ICAM-1 binding in Mn2+.
The conversion of the βI domain into the leg-extended and open-headpiece conformation is triggered by the downward shift of the C-terminal α7 helix, which is induced by the outward swing of the hybrid domain. Thus, small molecules or antibodies that stabilize the βI domain in the bent form could inhibit ICAM-1 binding to LFA-1. Antibody TS1/18, the epitopes of which map to adjacent residues in the α′1 and α7 helices of the βI domain (Arg-133 and Gln-332, respectively; ref. 43), was found to inhibit β2 integrin activation. These residues overlap with isoflurane binding sites on the βI domain, which supports the idea that the binding of isoflurane on the βI domain helps to stabilize the β subunit in the closed conformation. While intermediate affinity α-I306A and β-L132A mutants presumably retain the hydrophobic pocket in the αI domain, the β-L132A mutant prefers to be leg extended, which possibly alters the pocket between the α7 and α′1 helices of the βI domain. The fact that isoflurane inhibits ICAM-1 binding of α-I306A mutant to a greater degree than that of β2-L132A mutant suggests that the binding of isoflurane to the βI domain is functionally important. Based on our docking simulation, the affinity of isoflurane to the lovastatin site is predicted to be stronger than to the pocket between the α7 and α′1 helices of the β I domain. However, our docking simulation on the βI domain was done using the βI domain structure of integrin αXβ2. The local environment of the area around the α′1 and α7 helices may be subtly different between LFA-1 (αLβ2) and αXβ2. For example, the quaternary structure of the βI domain of LFA-1 has been found to be different from that of αMβ2, other integrin possessing β2 subunit (44). In our previous NMR study using the isolated αI domain, the estimated KD for isoflurane binding is ∼800 μM. Although within a clinically relevant range, isoflurane cannot be considered a strong α I antagonist. Unfortunately, the isolated β2 I domain cannot be expressed (45), and thus it is not possible to perform a similar binding study to determine KD of the β2 I domain. The presence of two sites on LFA-1 raises the possibility of cooperative interactions, which might enhance the functional importance of isoflurane binding.
Azi-isoflurane is a powerful tool to explore the binding sites of isoflurane on proteins. In particular, it is useful for proteins such as LFA-1, which lacks the fully defined structure by X-ray crystallography, and is not appropriate for NMR due to its size. In addition it should be noted that photolabeling can be extremely useful for proteins that may exist in various conformations. By testing azi-isoflurane onto the same protein in different conformations induced by various conditions (e.g., mutations), this method can identify the condition at which isoflurane can bind. However, it has been kept in mind that the identification of binding sites can be meaningful only when we can understand the structure and function of binding sites in the context of the structure of the target protein and its conformers. When the structure of full LFA-1 is solved, our understanding of the interaction between isoflurane and LFA-1 will be enhanced further. It needs to be noted that the presence of residual binding sites cannot be ruled out completely if full coverage of protein sequence is not achieved. Finally, azi-isoflurane is not isoflurane, so the sites occupied might be different. However, the identity of the sites in the isolated LFA-1 αI domain and in apoferritin (24) suggests that sufficient physicochemical similarity exists for azi-isoflurane to reliably report isoflurane binding sites.
How significant the interaction between isoflurane and LFA-1 is in the clinical setting remains to be answered. It is well known that surgical stress causes a systemic proinflammatory response. If exaggerated, the systemic inflammatory stress response ensues, an example of which occurs in cardiac surgery (46, 47). In this context, the effect of isoflurane on LFA-1 may be helpful to diminish the exaggerated immune response in the perioperative period. However, the anti-inflammatory effect by isoflurane can be a double-edged sword. In the study of long-term alcoholic patients who underwent abdominal surgery, isoflurane anesthesia increased postoperative tracheobronchitis and pneumonia over propofol anesthesia (48), suggesting that a putative anti-inflammatory effect might be detrimental, especially in immunocompromised patients. For those patients, immunomodulation-free anesthetics might be desirable. Previously, we demonstrated that another halogenated volatile anesthetic sevoflurane also binds to the αI domain using NMR (49). It would be particularly interesting to see if whether any difference can be found in the interaction with LFA-1 among contemporary available anesthetics. This will provide key information to lead into designing immunomodulation-free anesthetics.
In summary, we have demonstrated that isoflurane stabilizes LFA-1 in the closed conformation by binding to both αI and βI domain allosteric to ICAM-1 binding site and suggest that this may lead to impaired leukocyte arrest and thus anti-inflammatory actions in patients.
Supplementary Material
Acknowledgments
The authors thank W. P. Dailey (University of Pennsylvania) for providing azi-isoflurane, and T. A. Springer (Immune Disease Institute) for providing reagents and insightful comments.
This work was supported by grants from the U.S. National Institutes of Health, P01GM55876 (R.G.E.) and K08GM101345 (K.Y.).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- HBS
- HEPES-buffered saline
- ICAM-1
- intercellular adhesion molecule-1
- LC
- liquid chromatography
- LFA-1
- lymphocyte function-associated antigen-1
- MIDAS
- metal ion-dependent adhesion site
- MS
- mass spectrometry
- NMR
- nuclear magnetic resonance
- PDB
- Protein Data Bank
- WT
- wild type
REFERENCES
- 1. Mobert J., Zahler S., Becker B. F., Conzen P. F. (1999) Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology 90, 1372–1381 [DOI] [PubMed] [Google Scholar]
- 2. Kowalski C., Zahler S., Becker B. F., Flaucher A., Conzen P. F., Gerlach E., Peter K. (1997) Halothane, isoflurane, and sevoflurane reduce postischemic adhesion of neutrophils in the coronary system. Anesthesiology 86, 188–195 [DOI] [PubMed] [Google Scholar]
- 3. Shayevitz J. R., Rodriguez J. L., Gilligan L., Johnson K. J., Tait A. R. (1995) Volatile anesthetic modulation of lung injury and outcome in a murine model of multiple organ dysfunction syndrome. Shock 4, 61–67 [DOI] [PubMed] [Google Scholar]
- 4. Reutershan J., Chang D., Hayes J. K., Ley K. (2006) Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology 104, 511–517 [DOI] [PubMed] [Google Scholar]
- 5. Yuki K., Astrof N. S., Bracken C., Yoo R., Silkworth W., Soriano S. G., Shimaoka M. (2008) The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 22, 4109–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimaoka M., Takagi J., Springer T. A. (2002) Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 [DOI] [PubMed] [Google Scholar]
- 7. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 8. Humphries M. J. (2000) Integrin structure. Biochem. Soc. Trans. 28, 311–339 [PubMed] [Google Scholar]
- 9. Nishida N., Xie C., Shimaoka M., Cheng Y., Walz T., Springer T. A. (2006) Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity 25, 583–594 [DOI] [PubMed] [Google Scholar]
- 10. Lollo B. A., Chan K. W., Hanson E. M., Moy V. T., Brian A. A. (1993) Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J. Biol. Chem. 268, 21693–21700 [PubMed] [Google Scholar]
- 11. Constantin G., Majeed M., Giagulli C., Piccio L., Kim J. Y., Butcher E. C., Laudanna C. (2000) Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 13, 759–769 [DOI] [PubMed] [Google Scholar]
- 12. Kim M., Carman C. V., Springer T. A. (2003) Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725 [DOI] [PubMed] [Google Scholar]
- 13. Kim M., Carman C. V., Yang W., Salas A., Springer T. A. (2004) The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2. J. Cell Biol. 167, 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo B. H., Carman C. V., Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao T., Takagi J., Coller B. S., Wang J. H., Springer T. A. (2004) Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimaoka M., Springer T. A. (2003) Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2, 703–716 [DOI] [PubMed] [Google Scholar]
- 17. Yang W., Shimaoka M., Salas A., Takagi J., Springer T. A. (2004) Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc. Natl. Acad. Sci. U. S. A. 101, 2906–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang H., Astrof N. S., Liu J. H., Wang J. H., Shimaoka M. (2009) Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J. 23, 2735–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weitz-Schmidt G., Welzenbach K., Brinkmann V., Kamata T., Kallen J., Bruns C., Cottens S., Takada Y., Hommel U. (2001) Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 7, 687–692 [DOI] [PubMed] [Google Scholar]
- 20. Legge G. B., Kriwacki R. W., Chung J., Hommel U., Ramage P., Case D. A., Dyson H. J., Wright P. E. (2000) NMR solution structure of the inserted domain of human leukocyte function associated antigen-1. J. Mol. Biol. 295, 1251–1264 [DOI] [PubMed] [Google Scholar]
- 21. Nam K., Maiorov V., Feuston B., Kearsley S. (2006) Dynamic control of allosteric antagonism of leukocyte function antigen-1 and intercellular adhesion molecule-1 interaction. Proteins 64, 376–384 [DOI] [PubMed] [Google Scholar]
- 22. Gaillard T., Martin E., San Sebastian E., Cossio F. P., Lopez X., Dejaegere A., Stote R. H. (2007) Comparative normal mode analysis of LFA-1 integrin I-domains. J. Mol. Biol. 374, 231–249 [DOI] [PubMed] [Google Scholar]
- 23. Gahmberg C. G. (1997) Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 9, 643–650 [DOI] [PubMed] [Google Scholar]
- 24. Eckenhoff R. G., Xi J., Shimaoka M., Bhattacharji A., Covarrubias M., Dailey W. P. Azi-isoflurane, a photolabel analog of the commonly used inhaled general anesthetic isoflurane. ACS Chem. Neurosci. 1, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. (1982) Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc. Natl. Acad. Sci. U. S. A. 79, 7489–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Springer T., Galfre G., Secher D. S., Milstein C. (1979) Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur. J. Immunol. 9, 301–306 [DOI] [PubMed] [Google Scholar]
- 27. Qu A., Leahy D. J. (1996) The role of the divalent cation in the structure of the I domain from the CD11a/CD18 integrin. Structure 4, 931–942 [DOI] [PubMed] [Google Scholar]
- 28. Shimaoka M., Xiao T., Liu J. H., Yang Y., Dong Y., Jun C. D., McCormack A., Zhang R., Joachimiak A., Takagi J., Wang J. H., Springer T. A. (2003) Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song G., Yang Y., Liu J. H., Casasnovas J. M., Shimaoka M., Springer T. A., Wang J. H. (2005) An atomic resolution view of ICAM recognition in a complex between the binding domains of ICAM-3 and integrin alphaLbeta2. Proc. Natl. Acad. Sci. U. S. A. 102, 3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnaout M. A. (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75, 1037–1050 [PubMed] [Google Scholar]
- 31. Xie C., Zhu J., Chen X., Mi L., Nishida N., Springer T. A. Structure of an integrin with an alphaI domain, complement receptor type 4. EMBO J. 29, 666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friesner R. A., Banks J. L., Murphy R. B., Halgren T. A., Klicic J. J., Mainz D. T., Repasky M. P., Knoll E. H., Shelley M., Perry J. K., Shaw D. E., Francis P., Shenkin P. S. (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 [DOI] [PubMed] [Google Scholar]
- 33. Jenkins A., Greenblatt E. P., Faulkner H. J., Bertaccini E., Light A., Lin A., Andreasen A., Viner A., Trudell J. R., Harrison N. L. (2001) Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J. Neurosci. 21, RC136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hart T. N., Read R. J. (1992) A multiple-start Monte Carlo docking method. Proteins 13, 206–222 [DOI] [PubMed] [Google Scholar]
- 35. Lu C., Ferzly M., Takagi J., Springer T. A. (2001) Epitope mapping of antibodies to the C-terminal region of the integrin beta 2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 166, 5629–5637 [DOI] [PubMed] [Google Scholar]
- 36. Drbal K., Angelisova P., Cerny J., Hilgert I., Horejsi V. (2001) A novel anti-CD18 mAb recognizes an activation-related epitope and induces a high-affinity conformation in leukocyte integrins. Immunobiology 203, 687–698 [DOI] [PubMed] [Google Scholar]
- 37. Tang R. H., Tng E., Law S. K., Tan S. M. (2005) Epitope mapping of monoclonal antibody to integrin alphaL beta2 hybrid domain suggests different requirements of affinity states for intercellular adhesion molecules (ICAM)-1 and ICAM-3 binding. J. Biol. Chem. 280, 29208–29216 [DOI] [PubMed] [Google Scholar]
- 38. Shimaoka M., Salas A., Yang W., Weitz-Schmidt G., Springer T. A. (2003) Small molecule integrin antagonists that bind to the beta2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity 19, 391–402 [DOI] [PubMed] [Google Scholar]
- 39. Kallen J., Welzenbach K., Ramage P., Geyl D., Kriwacki R., Legge G., Cottens S., Weitz-Schmidt G., Hommel U. (1999) Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J. Mol. Biol. 292, 1–9 [DOI] [PubMed] [Google Scholar]
- 40. Lu C., Shimaoka M., Ferzly M., Oxvig C., Takagi J., Springer T. A. (2001) An isolated, surface-expressed I domain of the integrin alphaLbeta2 is sufficient for strong adhesive function when locked in the open conformation with a disulfide bond. Proc. Natl. Acad. Sci. U. S. A. 98, 2387–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huth J. R., Olejniczak E. T., Mendoza R., Liang H., Harris E. A., Lupher M. L., Jr., Wilson A. E., Fesik S. W., Staunton D. E. (2000) NMR and mutagenesis evidence for an I domain allosteric site that regulates lymphocyte function-associated antigen 1 ligand binding. Proc. Natl. Acad. Sci. U. S. A. 97, 5231–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu C., Shimaoka M., Zang Q., Takagi J., Springer T. A. (2001) Locking in alternate conformations of the integrin alphaLbeta2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl. Acad. Sci. U. S. A. 98, 2393–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang C., Zang Q., Takagi J., Springer T. A. (2000) Structural and functional studies with antibodies to the integrin beta 2 subunit. A model for the I-like domain. J. Biol. Chem. 275, 21514–21524 [DOI] [PubMed] [Google Scholar]
- 44. Dahms N. M., Hart G. W. (1986) Influence of quaternary structure on glycosylation. Differential subunit association affects the site-specific glycosylation of the common beta-chain from Mac-1 and LFA-1. J. Biol. Chem. 261, 13186–13196 [PubMed] [Google Scholar]
- 45. Takagi J., DeBottis D. P., Erickson H. P., Springer T. A. (2002) The role of the specificity-determining loop of the integrin beta subunit I-like domain in autonomous expression, association with the alpha subunit, and ligand binding. Biochemistry 41, 4339–4347 [DOI] [PubMed] [Google Scholar]
- 46. Hall R. I., Smith M. S., Rocker G. (1997) The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth. Analg. 85, 766–782 [DOI] [PubMed] [Google Scholar]
- 47. Wan S., LeClerc J. L., Vincent J. L. (1997) Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 112, 676–692 [DOI] [PubMed] [Google Scholar]
- 48. Von Dossow V., Baur S., Sander M., Tonnesen H., Marks C., Paschen C., Berger G., Spies C. D. (2007) Propofol increased the interleukin-6 to interleukin-10 ratio more than isoflurane after surgery in long-term alcoholic patients. J. Int. Med. Res. 35, 395–405 [DOI] [PubMed] [Google Scholar]
- 49. Yuki K., Astrof N. S., Bracken C., Soriano S. G., Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology 113, 600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kabsch W., Sander C. (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.