Abstract
Autophagy, the cytoprotection mechanism that takes place under metabolic impairment, has been implicated in the pathogenesis of autoimmunity. Here, we investigated the spontaneous and induced autophagic behavior of T lymphocytes from patients with systemic lupus erythematosus (SLE) compared with that of T lymphocytes from healthy donors by measuring the autophagy marker microtubule-associated protein 1 light chain 3 (LC3)-II. No significant differences in spontaneous autophagy were found between T lymphocytes from patients with SLE and from healthy donors, apart from CD4+ naive T cells from patients with SLE in which constitutively higher levels of autophagy (P<0.001) were detected. At variance, whereas treatment of T lymphocytes from healthy donors with serum IgG from patients with SLE resulted in a 2-fold increase in LC3-II levels (P<0.001), T lymphocytes from SLE patients were resistant to autophagic induction and also displayed an up-regulation of genes negatively regulating autophagy, e.g., α-synuclein. These findings could open new perspectives in the search for pathogenetic determinants of SLE progression and in the development of therapeutic strategies aimed to recover T-cell compartment homeostasis by restoring autophagic susceptibility.—Alessandri, C., Barbati, C., Vacirca, D., Piscopo, P., Confaloni, A., Sanchez, M., Maselli, A., Colasanti, T., Conti, F., Truglia, S., Perl, A., Valesini, G., Malorni, W., Ortona, E., Pierdominici, M. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy.
Keywords: autoimmunity, cell fate, autoantibodies
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown etiology characterized by the production of antinuclear autoantibodies and by a broad range of clinical presentations involving almost all organ systems. Alterations in T-lymphocyte homeostasis have been suggested to play a key role in the pathogenesis of SLE (1–3).
Apoptosis has long been known to orchestrate the maintenance of peripheral lymphocyte homeostasis, avoiding unregulated clonal expansion of autoreactive immune cells (4). Abnormal death of T lymphocytes has been extensively documented in SLE. The enhanced spontaneous apoptosis of circulating T cells has been linked to chronic lymphopenia (5) and compartmentalized release of autoantigens (6), whereas the decreased T-cell death induced by activation has been suggested to contribute to persistence of autoreactive cells (7).
Besides the crucial role of apoptosis, growing evidence supports the importance of autophagy in the maintaining of lymphocyte homeostasis (8–11). Autophagy is a lysosome-mediated catabolic process involved both in basal turnover of cellular components and in response to stressful conditions (e.g., nutrient depletion and oxidative stress; refs. 12, 13). The extremity of this catabolic process may paradoxically lead to cell death by self-cannibalism (14, 15). During autophagy, portions of cytoplasm are sequestered by double-membrane vesicles, the autophagosomes, and degraded after fusion with lysosomes for subsequent recycling. Like apoptosis, autophagy is a genetically programmed process that requires the activity of autophagy-related gene (Atg) proteins. Among these is the microtubule-associated protein 1 light chain 3 (LC3), a mammalian homolog of yeast Atg8 (16). Endogenous LC3 is post-transcriptionally processed into LC3-I, which is found in the cytosol. LC3-I is, in turn, lipidated to LC3-II, which associates with autophagosome membranes. Therefore, the level of this form of LC3 is a good quantification for autophagy (17).
Double-membrane autophagosomes have been identified in both human and murine T lymphocytes (10, 18–20), and a complex function for autophagy in T-lymphocyte development, survival, and proliferation has been demonstrated (20, 21). However, its precise role in cell fate remains controversial, partly because of the complex functional and molecular intersection of autophagy with the apoptotic pathway (8, 22).
Aberrant regulation of autophagy has been implicated in an increasing number of human diseases including autoimmune disorders (13, 23). The involvement of the lysosomal compartment in autoimmunity was suggested for the first time in 1964 by a pioneering study that associated the lysosomal function with SLE (24). More recently, genetic studies have linked mutations in autophagy regulators and SLE (25–29), and a deregulation of autophagy has been described either in T cells from lupus-prone mice or in a small number of patients with SLE (30). In addition, the activation of the mammalian target of rapamycin (mTOR), a metabolic regulator of autophagy, has been documented in SLE T cells (2, 31), and its blockade with rapamycin improved disease activity in patients with SLE (32). In this work, our interest was to extensively examine the autophagic behavior of peripheral T lymphocytes from patients with SLE. Furthermore, because serum autoantibodies purified from patients with active SLE have been shown to induce autophagy (33), we also tested the hypothesis that serum autoimmune factors may contribute to the dysregulation of lymphocyte homeostasis in SLE via a pathway involving modulation of autophagy.
MATERIALS AND METHODS
Patients
Thirty-four consecutive patients with SLE (32 women and 2 men) attending the Lupus Clinic of Sapienza University of Rome were enrolled in this study. All patients fulfilled the American College of Rheumatology revised criteria for the classification of SLE (34). Current SLE disease activity was measured using the SLE Disease Activity Index (SLEDAI; ref.35). Five patients with the diagnosis of rheumatoid arthritis (RA) were also included in the study. Steroids or various other disease-modifying antirheumatic drugs (hydroxychloroquine, methotrexate, azathioprine, and mycophenolate mofetil) were discontinued at least 24 h before venipuncture. Thirty-four age- and sex-matched healthy donors served as controls. Sera were collected and frozen at −70°C until used. Sera were heat-inactivated at 56°C for 30 min and centrifuged before use. Informed consent was obtained from all participants, and the local ethics committee approved the study. Experiments were designed such that cells from at least one control subject were always studied at the same time as cells from patients.
Indirect immunofluorescence and ELISAs for autoantibody reactivity
All sera were blindly analyzed for antinuclear antibodies and anti-double-stranded DNA IgG Abs by indirect immunofluorescence on Hep2 cells (serum dilution 1:80) and Crithidia luciliae (serum dilution 1:10), respectively. Commercially available ELISAs were used to measure anti-cardiolipin and anti-β2-glycoprotein I Abs (Diamedix, Miami, FL, USA). Results are expressed as international units according to the manufacturer's instructions. A positive control and normal human sera were run in all assays to confirm the specificity of the results.
Purification of IgG from patient sera
IgG were purified from sera of 10 patients with SLE, arbitrarily chosen as representative of the whole series, using Dynabeads Protein G (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instruction. In brief, serum (300 μl) was incubated for 40 min at room temperature with Dynabeads Protein G-conjugated. The beads were then removed with a magnet, and the effluent (serum without IgG) was collected; the IgG bound to the beads was eluted using 0.1 M citrate (pH 2). The eluted IgG was immediately neutralized with 1 M Tris-HCl (pH 8) and dialyzed against PBS. The purity of each fraction was assayed by SDS-PAGE followed by Coomassie Blue staining. Purified IgG was stored at −70°C until use. Endotoxin contamination of IgG was determined by the quantitative chromogenic Limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD, USA). The protein concentration of purified IgG was determined by the Bradford assay (Bio-Rad Laboratories, Richmond, CA, USA).
Cell cultures
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density-gradient centrifugation. Cells were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY, USA), supplemented with 2 mM glutamine (Sigma-Aldrich, St. Louis, MO, USA) and 50 μg/ml gentamicin (Sigma-Aldrich), and subjected to different treatments for 48 h: 1) complement-inactivated SLE serum (1:10 dilution), RA serum (1:10 dilution), or normal serum (human AB serum, 1:10 dilution; Gemini Bioproducts, Woodland, CA, USA); 2) complement-inactivated SLE serum without IgG (1:10 dilution); 3) IgG from serum of patients with SLE [50 μg/ml; an equal amount of Abs from a preparation of intravenous IgG (IVIG), precipitated by saturated ammonium sulfate solution and dialyzed against PBS, was used as a control]; and 4) anti-human CD3 Ab (clone UCHT1, 50 μg/ml; R&D Systems, Minneapolis, MN, USA) or normal murine IgG (50 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA, USA). For serum-starvation experiments, cells were cultured in RPMI 1640 medium (Gibco BRL) supplemented with 1% FBS (EuroClone, Pero, Milan, Italy) for 4 h (time chosen on the basis of preliminary time course experiments for 2–24 h; data not shown). Where indicated, cells were treated in the presence of lysosomal inhibitors E64d and pepstatin A (both at 10 μg/ml; Sigma-Aldrich) for 2 h before the end of culture. Separation of untouched T cells from PBMCs was performed by immunomagnetic-based depletion of non-T cells using the Pan T Cell Isolation Kit II (Miltenyi Biotec, Bergisch-Gladbach, Germany).
Flow cytometry and cell sorting
Surface phenotyping of freshly isolated PBMCs was performed with combinations of mAbs conjugated with FITC, phycoerythrin, peridinin chlorophyll protein, or allophycocyanin as described before (36). Conjugated mAbs against human CD3, CD4, CD8, CD45RA, CD62L, CD95, and HLA-DR and control mouse IgG1 (all from BD Immunocytometry Systems, San Jose, CA, USA) were used. The naive subset was defined as CD45RA+CD62L+, whereas the remaining cells comprised the memory subsets (CD45RA−CD62L+, central memory subset; CD45RA+CD62L− and CD45RA−CD62L−, effector memory subset; ref. 36). For CD4+ and CD8+ T-lymphocyte subsets, data are expressed as the percentage of each subset within the CD4+ or CD8+ population considered as 100%. Human IgG purified from sera of patients (1 μg/1×106 cells) and the appropriate FITC-conjugated secondary Ab (Pierce, Rockford, IL, USA) were used for staining the lymphocyte surface. Equal amounts of IVIG were used as negative controls. Apoptosis was measured immediately after lymphocyte separation and after 48 h of culture using FITC-conjugated annexin V (AV) and a propidium iodide (PI) apoptosis detection kit (Marine Biological Laboratory, Woods Hole, MA, USA) according to the manufacturer's protocol. Reported data are referred to AV-positive apoptotic cells. Acquisition was performed on a FACSCalibur cytometer (BD Immunocytometry Systems), and 50,000 events/sample were run. Data were analyzed using CellQuest Pro software (BD Immunocytometry Systems). Immunomagnetically preenriched CD3+ T cells were sorted into CD4 and CD8 naive (CD45RA+CD62L+) and memory (CD45RA−CD62L+, CD45RA+CD62L−, and CD45RA−CD62L−) T-cell subsets, based on CD4, CD8, CD45RA, and CD62L molecule expression, using a BD FACSAria cell sorter (BD Immunocytometry Systems). Dead cells were excluded with Sytox Blue (Invitrogen) staining. Purity of the enriched populations was greater than 99% in all experiments. After cell sorting, purified T-cell subsets were lysed and run in SDS-PAGE as described below.
SDS-PAGE and Western blot
Purified T lymphocytes were lysed in RIPA buffer (100 mM Tris-HCl, pH 8; 150 mM NaCl; 1% Triton X-100; 1 mM MgCl2; and 25 mM Na3VO4) in the presence of complete protease-inhibitor mixture (Sigma-Aldrich). Protein content was determined by the Bradford assay (Bio-Rad Laboratories). The samples were loaded onto a 12 or 15% SDS-polyacrylamide gel and, after electrophoresis, proteins were transferred onto nitrocellulose membrane (Amersham Hybond-ECL; GE Healthcare Europe, Munich, Germany) by means of a Trans-Blot transfer cell (Bio-Rad Laboratories). Regarding α-synuclein (SNCA), because this protein as monomer tends to easily detach from blotted membranes, a mild fixation of blotted membranes with PBS containing 0.4% paraformaldehyde was applied to the Western blot method as described previously (37). The membranes were then blocked in 5% skim milk for 1 h at room temperature, rinsed, and incubated with the intended Abs in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and 5% BSA. As primary Abs, rabbit anti-human LC3B Ab (Cell Signaling Technology, Beverly, MA, USA) and mouse anti-SNCA mAb (Sigma-Aldrich) were used at a dilution of 1:1000. Excess primary Ab was removed by washing the membrane in TBS-T. The membranes were then incubated with peroxidase-conjugated anti-rabbit IgG Ab (Bio-Rad Laboratories) or anti-mouse IgG Ab (Jackson ImmunoResearch, West Grove, PA, USA), and the reaction was developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce). To ensure the presence of equal amounts of protein, the membranes were reprobed with a rabbit anti-human β-actin Ab (Amersham, Gent, Belgium) or a rabbit anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Ab (Sigma-Aldrich). Quantification of protein expression was performed by densitometry analysis of the autoradiograms (GS-700 Imaging Densitometer; Bio-Rad Laboratories).
RNA isolation and quantitative real-time PCR
PBMCs obtained from 10 patients with SLE and 10 healthy donors, arbitrarily chosen as representative of the whole series, were treated with sera of patients with SLE. After 48 h of culture, T lymphocytes were purified by an immunomagnetic method (see above) and pooled. Total RNA was extracted (RNeasy Mini Kit; Qiagen, Valencia, CA, USA) and retrotranscribed in cDNA. For first-strand cDNA synthesis, we performed a reverse transcription of 1 μg of total RNA of each sample using an RT2 First Strand kit (SABioscience, Frederick, MD, USA) according to the manufacturer's protocol.
To perform gene expression profiling, we used SYBR Green based-technology and the RT2 Profiler Autophagy PCR array (SABioscience) that analyzes simultaneously the expression of 84 genes involved in autophagy. Each focused array includes specific validated primer sets and PCR master mixes (SABioscience). Real-time PCRs were performed in 96-well plate format using the ABI 7000 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The parameters for PCR amplification were 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. Fold changes of autophagic gene expression in experimental samples relative to the control samples were calculated using the ΔCt method (38). The ΔCt value of each sample was normalized by up to a total of 5 housekeeping genes (β2-microglobulin, hypoxanthine phosphoribosyltransferase 1, ribosomal protein L13a, GAPDH, and β-actin).
Statistical analysis
Data are expressed as means ± sd. Results were analyzed with SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Qualitative differences between subgroups were analyzed by χ2 and Fisher exact tests. The Mann-Whitney unpaired test was used to compare quantitative variables in different groups. The Spearman rank correlation coefficient was applied for calculation of the correlation between parallel variables in single samples. Values of P < 0.05 were considered statistically significant.
RESULTS
Clinical, serological, and immunophenotypic characteristics of patients with SLE
The clinical and serological features of the patients studied are summarized in Table 1. The peripheral distribution of the main T-cell subpopulations was evaluated by flow cytometry. As shown in Fig. 1A, the percentage of effector memory T cells was significantly higher in patients with SLE than in healthy donors both in CD4+ (13±8 vs. 9±5%, P=0.03) and CD8+ (28±15 vs. 21±9%, P=0.02) T cells. A significantly higher percentage of HLA-DR-positive cells was also detected in CD4 (7±4 vs. 4±2%, P=0.001) and CD8 (16±13 vs. 9±6%, P=0.01) subsets. According to previous literature data (39, 40), the altered distribution of lymphocyte subsets was independent of disease activity and organ manifestation (data not shown). The distribution of the other T-cell subsets investigated (i.e., naive, central memory, and CD95-positive T cells) fell within the normal range.
Table 1.
Clinical and serological characteristics of the study sample
| Characteristic | Value |
|---|---|
| Women/men | 32/2 |
| Age, median and range (yr) | 35 (20–74) |
| Disease duration, median and range (yr) | 6.6 (0.42–29) |
| Arthritis [n (%)] | 12 (35.2) |
| Cutaneous manifestations [n (%)] | 8 (23.5) |
| Serositis [n (%)] | 1 (2.9) |
| Cytopenia [n (%)] | 7 (20.5) |
| Renal manifestations [n (%)] | 3 (8.8) |
| NPSLE [n (%)] | 3 (8.8) |
| Arterial thrombosis [n (%)] | 2 (5.8) |
| Venous thrombosis [n (%)] | 2 (5.8) |
| Pregnancy morbidity [n (%)] | 6 (17.6) |
| SLEDAI, mean and range | 3 (0–12) |
| ANA [n (%)] | 34 (100) |
| Anti-dsDNA IgG [n (%)] | 25 (73.5) |
| aCL IgG/IgM [n (%)] | 11 (32.3) |
| Anti-β2GPI IgG/IgM [n (%)] | 4 (11.7) |
NPSLE, neuropsychiatric lupus; ANA, anti-nuclear antibodies; anti-dsDNA, anti-double-stranded DNA; aCL, anti-cardiolipin; anti-β2GPI, anti-β2-glycoprotein I.
Figure 1.
Flow cytometric immunophenotyping and Western blot analysis of the autophagy marker LC3-II in freshly isolated T cells from patients with SLE and from healthy donors. A) Flow cytometry analysis of distribution of T-cell subsets. Data are represented as box plots (white and gray box plots for healthy donors and patients with SLE, respectively) displaying medians, 25th and 75th percentiles as boxes, and 10th and 90th percentiles as whiskers. *P < 0.05 vs. normal cells. B) LC3-II Western blot analysis of T-cell lysates (30 μg/lane) from 6 healthy donors and from 6 patients with SLE. Blots shown are representative of independent experiments performed in T cells from healthy donors (n=34) and from patients with SLE (n=34). Quantification of LC3-II levels relative to β-actin in normal and SLE T cells is also shown (mean with range is presented). C) LC3-II Western blot analysis of cell lysates obtained from purified CD4+ and CD8+ naive and memory T lymphocytes. Blots shown are representative of independent experiments performed in T cells from 10 healthy donors and from 10 patients with SLE, arbitrarily chosen as representative of the whole series. Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. *P < 0.05.
Autophagy levels in freshly isolated T cells from patients with SLE and healthy donors
To evaluate whether autophagy was detectable in freshly isolated peripheral T lymphocytes, we examined these cells for the presence of the autophagosomal marker LC3-II by Western blot. The time required for isolation of lymphocytes was constant (2–3 h) in all subjects, and biological samples were isolated and studied immediately after blood drawing. As shown in Fig. 1B, a great interindividual variability for LC3-II levels was present in T lymphocytes from both patients with SLE and healthy donors, and no significant differences in the level of LC3-II were found between these two groups. Because some patients with SLE were treated with hydroxychloroquine (see Materials and Methods) and this drug was demonstrated to be a potent inhibitor of autophagic flux (41), we also evaluated our results in light of this issue. However, no difference was found between patients treated with or without this drug. In addition, no significant correlations between autophagy levels and the clinical features of patients were observed (data not shown).
Previous studies have clearly shown that autophagy plays an essential role in T-cell activation and proliferation (8). Therefore, we explored the possible relationship between the LC3-II level and the activation state of T cells to unveil differences between patients with SLE and healthy donors. To this aim, we analyzed the LC3-II protein expression in purified CD4 and CD8 T-cell subsets. Two T-cell subsets were considered within the CD4+ and CD8+ T lymphocytes, i.e., naive and memory T cells. As shown in Fig. 1C, in healthy donors, CD4+ and CD8+ memory T cells showed a 4-fold increase in LC3-II levels compared with that in naive T cells (P=0.001 and P=0.0002 for CD4+ and CD8+ T cells, respectively). In patients with SLE, this difference was observed in the CD8 subset (memory vs. naive T cells, P=0.0008) but not in the CD4 subset, in which naive and memory T cells showed comparable LC3-II levels. Of note, CD4+ naive T cells from patients with SLE showed significantly higher LC3-II levels than those from healthy donors (P=0.0002; Fig. 1C).
Apoptosis levels in freshly isolated T cells from patients with SLE and healthy donors
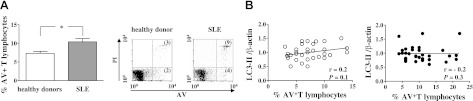
On the basis of the well-known role of apoptosis in the maintenance of peripheral lymphocyte homeostasis (4), lymphocytes were also analyzed for this parameter. In line with previous published data (5), freshly isolated T lymphocytes from patients with SLE showed significantly higher spontaneous apoptosis compared with those from healthy donors (10±5 vs. 7±3%, P=0.001; Fig. 2A). The percentage of apoptotic cells was not related to drug therapy and did not correlate with disease activity as measured by the SLEDAI (data not shown). In both healthy donors and patients with SLE, there was no significant correlation between apoptosis and autophagy levels (Fig. 2B).
Figure 2.
Apoptosis detection in freshly isolated T cells from patients with SLE and from healthy donors. A) Flow cytometry analysis of lymphocyte apoptosis. Data are referred to AV-positive cells and are presented as means ± sd of independent experiments performed in T lymphocytes from healthy donors (n=34) and from patients with SLE (n=34). *P < 0.05. Representative dot plots of flow cytometry analysis (PI on y axis vs. AV on x axis) are also shown. Numbers reported in bottom and top right quadrants represent percentages of AV single-positive cells and AV/PI double-positive cells, respectively. B) Correlation and linear regression analysis of apoptosis and LC3-II levels in T cells from healthy donors (○) and patients with SLE (●).
Susceptibility to autophagy induction in T cells from patients with SLE and healthy donors
Because SLE sera were reported to induce autophagy in human neuroblastoma SH-SY5Y cells (33), we asked whether, in our experimental conditions, sera from patients with SLE could modulate autophagy also in primary T lymphocytes. To this aim, peripheral T lymphocytes from healthy donors and patients with SLE were cultured in the presence or absence of SLE sera. As shown in Fig. 3A, treatment of normal T cells with SLE sera for 48 h significantly increased the level of LC3-II compared with treatment with normal sera (LC3-II/β-actin ratio, 2±0.3 vs. 1±0.5, respectively, P=0.0006). Interestingly, in the same experimental conditions, autophagy induction was not detectable in T lymphocytes from patients with SLE treated with their own serum (LC3-II/β-actin ratio, 0.9±0.2 vs. 1±0.2, P> 0.05; Fig. 3B). It is well known that LC3-II can accumulate because of increased upstream autophagosome formation or impaired downstream autophagosome-lysosome fusion (17). To distinguish between these two possibilities, we assayed LC3-II in the presence of the lysosomal proteases E64d and pepstatin A, which block LC3-II/autophagosome degradation. This analysis allowed us to assess the rate of autophagosome formation (i.e., autophagic flux). In the presence of these protease inhibitors, SLE sera further increased LC3-II levels of normal T lymphocytes, indicating that autophagic flux occurred and that the observed increase in LC3-II was not due to an aberration in lysosomal degradation (Fig. 3A).
Figure 3.
Effects of sera from patients with SLE on T-lymphocyte autophagy and apoptosis. A) LC3-II Western blot analysis of T-cell lysates (30 μg/lane) from one representative healthy donor (of the 34 analyzed). Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. Where indicated, cells were treated with the lysosomal inhibitors E64d and pepstatin A (Pep A). Statistically significant differences are indicated. B) LC3-II Western blot analysis of T-cell lysates from 3 representative patients with SLE (of the 34 analyzed). Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. C) Flow cytometry analysis of lymphocyte apoptosis. Data are referred to AV-positive apoptotic cells and are expressed as the mean ± sd of independent experiments performed in T cells from patients with SLE (n=34) and from healthy donors (n=34). Representative dot plots of flow cytometry analysis (PI on y axis vs. AV on x axis) are also shown. Numbers reported in bottom and top right quadrants represent percentages of AV single-positive cells and AV/PI double-positive cells, respectively. D) LC3-II Western blot analysis of T-cell lysates from one representative healthy donor (of 10 analyzed) after treatment with normal serum, IgG from serum of patients with SLE (50 μg/ml), serum of patients with SLE without IgG, and IVIG (50 μg/ml). Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. Statistically significant differences are indicated in the figure. E) Reactivity of human IgG purified from healthy donors (dotted line) and from sera of patients with SLE (black line) on lymphocyte surface. A representative histogram plot of flow cytometry analysis is shown.
Differently from autophagy, apoptosis was not modulated by the treatment with SLE sera either in T cells from patients with SLE or in those from healthy donors (Fig. 3C). However, SLE T cells showed higher levels of apoptosis than normal T cells, independently of the influence of serum soluble factors (P=0.001 for both treatments).
In sum, these in vitro experiments suggested that SLE sera were capable of promoting autophagy of lymphocytes from healthy donors but not of those from patients with SLE. Furthermore, SLE sera did not influence the apoptotic rate of lymphocytes from healthy donors or patients with SLE, these latter showing high levels of spontaneous apoptosis.
To test the hypothesis that autoantibodies present in the sera of patients with SLE could induce autophagy, normal T lymphocytes were treated with IgG purified from sera of 10 patients with SLE, arbitrarily chosen as representative of the whole series. As shown in Fig. 3D, SLE-purified IgG significantly increased LC3-II levels compared with those for normal serum (P=0.004), SLE serum without IgG (P=0.007), and IVIG, i.e., IgG from healthy donors (P=0.003). Of note, sera of patients with SLE deprived of IgG did not show any effect on autophagy; this result suggested that serum antibodies constitute the only factor responsible for the observed autophagy induction.
Because it is firmly established that a population of serum autoantibodies from patients with SLE can react with lymphocyte membrane proteins, including the CD3 molecule (42, 43), we tested the ability of the purified IgG from sera of patients with SLE to bind to the lymphocyte surface. Flow cytometry analysis, performed on intact nonpermeabilized T lymphocytes, showed that purified IgG from patients with SLE, but not that from healthy donors, specifically reacted with the lymphocyte surface (Fig. 3E). Furthermore, to assess whether antibodies directed against CD3 could exert the same effect observed with SLE IgG, i.e., induction of autophagy, we treated T lymphocytes from healthy donors with antibodies against CD3. However, no signs of autophagy induction were detected (Supplemental Fig. S1).
In summary, antilymphocyte antibodies from sera of patients with SLE could act as autophagy inducers in lymphocytes from healthy donors. On the contrary, lymphocytes from patients with SLE appeared resistant to proautophagic ignition.
To further confirm this hypothesis, cells were cultured under growth factor deficiency (serum starvation), a condition that is known to induce metabolic impairment and to trigger autophagy (ref. 44 and Fig. 4A). Normal T cells cultured in 1% FBS showed increased levels of LC3-II compared with those cultured in 10% FBS (LC3-II/β-actin ratio, 1.7±0.2 vs. 0.8±0.1, respectively, P=0.005). In contrast, no significant changes in LC3-II levels were detectable under serum starvation in T lymphocytes from patients with SLE. In particular, when the CD4+ T-cell subset was considered, memory, but not naive, T lymphocytes appeared to be responsible for the resistance to autophagy induction described above (Supplemental Fig. S2).
Figure 4.
Susceptibility of T lymphocytes to proautophagic stimuli. A) LC3-II Western blot analysis of T-cell lysates (30 μg/lane) from one healthy donor and from one patient with SLE after treatment with 10 or 1% FBS for 4 h. Blots shown are representative of independent experiments performed in T cells from 10 healthy donors and from 10 patients with SLE, arbitrarily chosen as representative of the whole series. Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. Statistically significant differences are indicated. B) LC3-II Western blot analysis of T-cell lysates from one healthy donor and from one patient with RA after treatment with normal serum or RA serum for 48 h. Blots shown are representative of independent experiments performed in T cells from 5 healthy donors and from 5 patients with RA. Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. Statistically significant differences are indicated in the figure. C) LC3-II Western blot analysis of T-cell lysates from one healthy donor and from one patient with RA after treatment with 10 or 1% FBS for 4 h. Blots shown are representative of independent experiments performed in T cells from 5 healthy donors and from 5 patients with RA. Densitometry analysis of LC3-II levels relative to β-actin is also shown. Values are expressed as means ± sd. Statistically significant differences are indicated.
To define whether the deregulation of the autophagy level was a general feature shared by other autoimmune diseases, we tested five patients with RA as a disease control population. Interestingly, as observed with sera from patients with SLE, sera from patients with RA were equally able to induce autophagy in T cells from healthy donors after 48 h of treatment (P=0.026; Fig. 4B). However, differently from T lymphocytes from patients with SLE, those from patients with RA were not resistant to autophagy induction by using RA serum (P=0.021; Fig. 4B) or serum starvation (P=0.01; Fig. 4C).
Altered autophagy-related gene expression in T lymphocytes from patients with SLE
To identify genes potentially involved in the autophagy resistance in SLE, we performed an array profile of autophagy-related genes of T lymphocytes from 10 patients with SLE and from 10 healthy donors under treatment with an autophagic stimulus, i.e., sera from patients with SLE. After 48 h of culture, purified T lymphocytes were pooled, and the expression of 84 genes involved in the autophagy pathway was analyzed using the RT2 Profiler Autophagy PCR array system.
Differences in gene expression of ≥1.5-fold for SLE T cells vs. normal T cells were considered (Table 2). Six genes, i.e., v-akt murine thymoma viral oncogene homolog 1 (Akt1), B-cell lymphoma 2 (Bcl2), cyclin-dependent kinase inhibitor 2A (CDKN2A), interferon-γ (IFNγ), nuclear factor of κ light polypeptide gene enhancer in B-cells 1 (NFkB1), and α-synuclein (SNCA), were up-regulated, and two genes, i.e., hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) and tumor necrosis factor (ligand) superfamily, member 10 (TNFSF10), were down-regulated. All the proteins coded by these genes are known to exert regulatory roles in apoptosis and/or autophagy pathways. In particular, SNCA showed the most significant difference between SLE and normal T cells with a fold change of >5. Hence, we analyzed by Western blot the protein expression level of SNCA in T lymphocytes from patients with SLE and healthy donors. We found significantly higher levels of SNCA in SLE T cells than in normal T cells (SNCA/β-actin ratio, 1.5±0.4 vs. 0.8±0.3, respectively, P=0.0007; Supplemental Fig. S3).
Table 2.
Changes in the expression of autophagy-related genes in peripheral T lymphocytes from patients with SLE compared with healthy donors
| Symbol | Description | Fold change | GenBank |
|---|---|---|---|
| Akt1 | v-akt murine thymoma viral oncogene homolog 1 | +1.53 | NM_005163 |
| Bcl2 | B-cell lymphoma 2 integral outer mitochondrial membrane protein | +1.5 | NM_000633 |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A | +1.9 | NM_000077 |
| IFNγ | Interferon-γ | +1.61 | NM_000619 |
| NFkB1 | Nuclear factor of κ light polypeptide gene enhancer in B-cells 1 | +1.5 | NM_003998 |
| SNCA | α-Synuclein | +5.32 | NM_000345 |
| HRS | Hepatocyte growth factor-regulated tyrosine kinase substrate | −1.5 | NM_004712 |
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 | −1.79 | NM_003810 |
DISCUSSION
In this study, we extensively addressed for the first time to our knowledge the autophagic behavior of T lymphocytes from patients with SLE and from healthy donors. Our results pointed out no significant differences in spontaneous autophagy in T lymphocytes from patients with SLE and from healthy donors (apart from CD4+ naive T cells from patients with SLE, for which constitutively higher levels of autophagy were detected) and a significant disparity in autophagic propensity between T lymphocytes from healthy donors and patients with SLE, the latter being resistant to autophagy induction.
Spontaneous autophagy
In freshly isolated CD4+ and CD8+ T lymphocytes from healthy donors, higher autophagy levels were observed in memory than in naive subsets. This result is consistent with previous studies suggesting a positive, cytoprotective, and homeostatic role for autophagy in rapidly proliferating, activated T cells (10, 21, 45). In fact, autophagy may be critically important in T cells for several reasons associated with their activation (8). For example, T-cell receptor stimulation leads to an oxidative burst that may produce massive levels of toxic reactive oxygen species (ROS; ref. 46). In this context, autophagy may be used to limit the production of ROS, whose overload triggers apoptotic cell death (46). A further explanation for the autophagy requirement in memory T cells is its accepted function in nutrient-deprivation conditions. In fact, autophagy may support the early stages of T-cell activation, when extracellular nutrient is limited, but metabolic reprogramming for proliferation has already begun (8, 47). A comparison between T lymphocytes from healthy donors and from patients with SLE showed no significant differences in LC3-II levels within the CD8 subsets. Conversely, CD4+ naive T cells from patients with SLE showed higher constitutive autophagy levels than those from healthy donors. This result could explain the heterogeneous distribution of autophagic structures that is more pronounced in certain T cells and was recently observed in SLE (30). We could hypothesize that in SLE proliferating CD4+ naive T cells undergo enhanced autophagy to supply their metabolic needs. In fact, peripheral post-thymic expansion of CD4+ naive T cells has been hypothesized to be driven by self-peptides for the maintenance of T-cell immunity in adults (48, 49), and an unregulated expansion of these autoreactive T cells has been suggested to contribute to the pathogenesis of autoimmune diseases (49). Further studies on the autophagic behavior of purified naive and memory T cells will help to understand their differential contribution to the pathogenesis of SLE.
Autophagic susceptibility
In this study, we also analyzed the susceptibility to autophagy induction of T lymphocytes from patients with SLE and from healthy donors. Sera from patients with SLE were able to induce autophagy in T lymphocytes from healthy donors, and this effect resided within the IgG fraction. Conversely, T lymphocytes from patients with SLE were resistant to autophagy induction. Of note, sera from patients with RA also induced autophagy in T lymphocytes, suggesting that the presence of serum factors modulating autophagy may be a hallmark of distinct autoimmune disorders. Conversely, different from the case with SLE, susceptibility to autophagy of T lymphocytes from patients with RA was similar to that of healthy donors.
It is well known that the majority of patients with SLE develop autoantibodies to lymphocyte surface antigens, able to inhibit T-cell activation and proliferation, although few data are so far available on the antigenic target of these antibodies (42, 50). Here, we suggest a role for antilymphocyte antibodies in inducing autophagy in T lymphocytes. In the same manner, Towns et al. (33) previously demonstrated that sera from patients with SLE induced autophagy in the human neuroblastoma cell line SH-SY5Y, suggesting that serum autoantibodies may influence the autophagic cell behavior. However, in this study, we found that antilymphocyte antibodies were unable to induce autophagy in T lymphocytes from patients with SLE. This result suggests that chronic exposure to specific autoantibodies, as occurs in SLE, could lead to the selection of autophagy-resistant T lymphocytes. In this context, the failure of autophagy induction could result in an overload of damaged mitochondria, release of apoptogenic factors from these organelles, and excessive ROS production, events that are commonly observed in SLE (3). Supporting this observation, Fernandez et al. (32) found that rapamycin, a well-known autophagy inducer (51), reduced disease activity and normalized T-cell activation in patients with SLE, probably restoring the autophagic response. The failure of autophagy induction was also suggested by gene expression analyses. In fact, we found that T lymphocytes from patients with SLE, cultivated in the presence of their sera, showed overexpression of genes specific to proteins that are negative regulators of autophagy, i.e., Akt1, Bcl2, NFkB1, and SNCA (52–55). Among these genes, the most prominent appeared to be SNCA, whose overexpression has recently been demonstrated to inhibit autophagy by reducing autophagosome formation at a very early stage (55). Akt1 and Bcl2 are well-known oncogenes with multiple functions in the regulation of cell metabolism, including autophagy (53). In particular, these two genes suppress autophagy: Bcl-2 by inhibiting the autophagy-regulatory gene Beclin1 (53) and Akt1 by activating mTOR, an upstream inhibitor of autophagy (52). Fittingly, NFkB1, a transcription factor that is considered to be the first responder to harmful cellular stimuli, has been demonstrated to suppress autophagy by activating the mTOR pathway (54). Other genes we found up-regulated were CDKN2A and IFNγ, which are coregulators of apoptosis and autophagy (56, 57), even if their precise role in the apoptosis/autophagy balance remains to be elucidated. In our gene expression analysis of T lymphocytes, two genes appeared down-regulated in SLE samples: HRS, which was demonstrated to exert a proautophagic role (58), and TNFSF10, which was suggested to exert a complex regulatory function on T cells, inhibiting Th1 cells and promoting the expansion of regulatory T lymphocytes (59).
In summary, our data, in line with the key role of lysosomal compartment defects in the cytopathology of SLE hypothesized since 1964 (24), clearly suggest that an autophagic resistance can take place in T cells from patients with SLE. This may result in the increased apoptosis detected in patients with SLE (this work and ref. 5) and could be associated with the defective removal of apoptotic bodies, typical of SLE, favoring the persistence of autoimmune phenomena (6). Overall, this study opens new perspectives for therapeutic strategies targeted to induce autophagy. In fact, on the basis of the literature (2, 32), we can suggest a reappraisal of well-known drugs that have recently been recognized to ignite autophagic flux by inhibiting inositol 1,4,5-trisphosphate-dependent calcium signals, i.e., glucocorticoids (60). More intriguingly, we cannot rule out the possibility that drugs that are capable of inducing autophagy, e.g., rapamycin and its analogs such as everolimus, could lead, in the long run, to new therapeutic strategies in the clinical management of SLE.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Ministero della Salute (W.M. and E.O.), Associazione Italiana Ricerca Cancro (AIRC) MCO-9998 (W.M.), Programma di Ricerca di Rilevante Interesse Nazionale (PRIN) 2007 (G.V.), the U.S. National Institutes of Health (AI048079 and AI072648), the Alliance for Lupus Research, and the Central New York Community Foundation.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Akt1
- v-akt murine thymoma viral oncogene homolog 1
- AV
- annexin V
- Atg
- autophagy-related gene
- Bcl2
- B-cell lymphoma 2
- CDKN2A
- cyclin-dependent kinase inhibitor 2A
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- HRS
- hepatocyte growth factor-regulated tyrosine kinase substrate
- IFNγ
- interferon-γ
- IVIG
- intravenous IgG
- LC3
- microtubule-associated protein 1 light chain 3
- mTOR
- mammalian target of rapamycin
- NFkB1
- nuclear factor of κ light polypeptide gene enhancer in B-cells 1
- PBMC
- peripheral blood mononuclear cell
- PI
- propidium iodide
- RA
- rheumatoid arthritis
- ROS
- reactive oxygen species
- SLE
- systemic lupus erythematosus
- SLEDAI
- SLE Disease Activity Index
- SNCA
- α-synuclein
- TNFSF10
- tumor necrosis factor (ligand) superfamily, member 10
REFERENCES
- 1. Crispin J. C., Liossis S. N., Kis-Toth K., Lieberman L. A., Kyttaris V. C., Juang Y. T., Tsokos G. C. (2010) Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol. Med. 16, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez D., Perl A. (2010) mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov. Med. 9, 173–178 [PMC free article] [PubMed] [Google Scholar]
- 3. Moulton V. R., Tsokos G. C. (2011) Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res. Ther. 13, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krammer P. H., Arnold R., Lavrik I. N. (2007) Life and death in peripheral T cells. Nat. Rev. Immunol. 7, 532–542 [DOI] [PubMed] [Google Scholar]
- 5. Emlen W., Niebur J., Kadera R. (1994) Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J. Immunol. 152, 3685–3692 [PubMed] [Google Scholar]
- 6. Gaipl U. S., Munoz L. E., Grossmayer G., Lauber K., Franz S., Sarter K., Voll R. E., Winkler T., Kuhn A., Kalden J., Kern P., Herrmann M. (2007) Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 28, 114–121 [DOI] [PubMed] [Google Scholar]
- 7. Kovacs B., Vassilopoulos D., Vogelgesang S. A., Tsokos G. C. (1996) Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF-α. Clin. Immunol. Immunopathol. 81, 293–302 [DOI] [PubMed] [Google Scholar]
- 8. Walsh C. M., Edinger A. L. (2010) The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunol. Rev. 236, 95–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine B., Mizushima N., Virgin H. W. (2011) Autophagy in immunity and inflammation. Nature 469, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C., Capan E., Zhao Y., Zhao J., Stolz D., Watkins S. C., Jin S., Lu B. (2006) Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J. Immunol. 177, 5163–5168 [DOI] [PubMed] [Google Scholar]
- 11. Pua H. H., He Y. W. (2007) Maintaining T lymphocyte homeostasis: another duty of autophagy. Autophagy 3, 266–267 [DOI] [PubMed] [Google Scholar]
- 12. Klionsky D. J. (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 [DOI] [PubMed] [Google Scholar]
- 13. Levine B., Kroemer G. (2008) Autophagy in the pathogenesis of disease. Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galluzzi L., Vitale I., Abrams J. M., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., Dawson T. M., Dawson V. L., El-Deiry W. S., Fulda S., Gottlieb E., Green D. R., Hengartner M. O., Kepp O., Knight R. A., Kumar S., Lipton S. A., Lu X., Madeo F., Malorni W., Mehlen P., Nunez G., Peter M. E., Piacentini M., Rubinsztein D. C., Shi Y., Simon H. U., Vandenabeele P., White E., Yuan J., Zhivotovsky B., Melino G., Kroemer G. (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scarlatti F., Granata R., Meijer A. J., Codogno P. (2009) Does autophagy have a license to kill mammalian cells? Cell Death Differ. 16, 12–20 [DOI] [PubMed] [Google Scholar]
- 16. Tanida I., Ueno T., Kominami E. (2004) LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 36, 2503–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., Bamber B. A., Bassham D. C., Bergamini E., Bi X., Biard-Piechaczyk M., Blum J. S., Bredesen D. E., Brodsky J. L., Brumell J. H., Brunk U. T., Bursch W., Camougrand N., Cebollero E., Cecconi F., Chen Y., Chin L. S., Choi A., Chu C. T., Chung J., Clarke P. G., Clark R. S., Clarke S. G., Clave C., Cleveland J. L., Codogno P., Colombo M. I., Coto-Montes A., Cregg J. M., Cuervo A. M., Debnath J., Demarchi F., Dennis P. B., Dennis P. A., Deretic V., Devenish R. J., Di Sano F., Dice J. F., Difiglia M., Dinesh-Kumar S., Distelhorst C. W., Djavaheri-Mergny M., Dorsey F. C., Droge W., Dron M., Dunn W. A., Jr., Duszenko M., Eissa N. T., Elazar Z., Esclatine A., Eskelinen E. L., Fesus L., Finley K. D., Fuentes J. M., Fueyo J., Fujisaki K., Galliot B., Gao F. B., Gewirtz D. A., Gibson S. B., Gohla A., Goldberg A. L., Gonzalez R., Gonzalez-Estevez C., Gorski S., Gottlieb R. A., Haussinger D., He Y. W., Heidenreich K., Hill J. A., Hoyer-Hansen M., Hu X., Huang W. P., Iwasaki A., Jaattela M., Jackson W. T., Jiang X., Jin S., Johansen T., Jung J. U., Kadowaki M., Kang C., Kelekar A., Kessel D. H., Kiel J. A., Kim H. P., Kimchi A., Kinsella T. J., Kiselyov K., Kitamoto K., Knecht E., Komatsu M., Kominami E., Kondo S., Kovacs A. L., Kroemer G., Kuan C. Y., Kumar R., Kundu M., Landry J., Laporte M., Le W., Lei H. Y., Lenardo M. J., Levine B., Lieberman A., Lim K. L., Lin F. C., Liou W., Liu L. F., Lopez-Berestein G., Lopez-Otin C., Lu B., Macleod K. F., Malorni W., Martinet W., Matsuoka K., Mautner J., Meijer A. J., Melendez A., Michels P., Miotto G., Mistiaen W. P., Mizushima N., Mograbi B., Monastyrska I., Moore M. N., Moreira P. I., Moriyasu Y., Motyl T., Munz C., Murphy L. O., Naqvi N. I., Neufeld T. P., Nishino I., Nixon R. A., Noda T., Nurnberg B., Ogawa M., Oleinick N. L., Olsen L. J., Ozpolat B., Paglin S., Palmer G. E., Papassideri I., Parkes M., Perlmutter D. H., Perry G., Piacentini M., Pinkas-Kramarski R., Prescott M., Proikas-Cezanne T., Raben N., Rami A., Reggiori F., Rohrer B., Rubinsztein D. C., Ryan K. M., Sadoshima J., Sakagami H., Sakai Y., Sandri M., Sasakawa C., Sass M., Schneider C., Seglen P. O., Seleverstov O., Settleman J., Shacka J. J., Shapiro I. M., Sibirny A., Silva-Zacarin E. C., Simon H. U., Simone C., Simonsen A., Smith M. A., Spanel-Borowski K., Srinivas V., Steeves M., Stenmark H., Stromhaug P. E., Subauste C. S., Sugimoto S., Sulzer D., Suzuki T., Swanson M. S., Tabas I., Takeshita F., Talbot N. J., Talloczy Z., Tanaka K., Tanida I., Taylor G. S., Taylor J. P., Terman A., Tettamanti G., Thompson C. B., Thumm M., Tolkovsky A. M., Tooze S. A., Truant R., Tumanovska L. V., Uchiyama Y., Ueno T., Uzcategui N. L., van der Klei I., Vaquero E. C., Vellai T., Vogel M. W., Wang H. G., Webster P., Wiley J. W., Xi Z., Xiao G., Yahalom J., Yang J. M., Yap G., Yin X. M., Yoshimori T., Yu L., Yue Z., Yuzaki M., Zabirnyk O., Zheng X., Zhu X., Deter R. L. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Espert L., Denizot M., Grimaldi M., Robert-Hebmann V., Gay B., Varbanov M., Codogno P., Biard-Piechaczyk M. (2006) Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J. Clin. Invest. 116, 2161–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerland L. M., Genestier L., Peyrol S., Michallet M. C., Hayette S., Urbanowicz I., Ffrench P., Magaud J. P., Ffrench M. (2004) Autolysosomes accumulate during in vitro CD8+ T-lymphocyte aging and may participate in induced death sensitization of senescent cells. Exp. Gerontol. 39, 789–800 [DOI] [PubMed] [Google Scholar]
- 20. Pua H. H., Dzhagalov I., Chuck M., Mizushima N., He Y. W. (2007) A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 204, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pua H. H., He Y. W. (2009) Autophagy and lymphocyte homeostasis. Curr. Top. Microbiol. Immunol. 335, 85–105 [DOI] [PubMed] [Google Scholar]
- 22. Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, 741–752 [DOI] [PubMed] [Google Scholar]
- 23. Lleo A., Invernizzi P., Selmi C., Coppel R. L., Alpini G., Podda M., Mackay I. R., Gershwin M. E. (2007) Autophagy: highlighting a novel player in the autoimmunity scenario. J. Autoimmun. 29, 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weissmann G. (1964) Lysosomes, autoimmune phenomena, and diseases of connective tissue. Lancet 2, 1373–1375 [DOI] [PubMed] [Google Scholar]
- 25. Harley J. B., Alarcon-Riquelme M. E., Criswell L. A., Jacob C. O., Kimberly R. P., Moser K. L., Tsao B. P., Vyse T. J., Langefeld C. D., Nath S. K., Guthridge J. M., Cobb B. L., Mirel D. B., Marion M. C., Williams A. H., Divers J., Wang W., Frank S. G., Namjou B., Gabriel S. B., Lee A. T., Gregersen P. K., Behrens T. W., Taylor K. E., Fernando M., Zidovetzki R., Gaffney P. M., Edberg J. C., Rioux J. D., Ojwang J. O., James J. A., Merrill J. T., Gilkeson G. S., Seldin M. F., Yin H., Baechler E. C., Li Q. Z., Wakeland E. K., Bruner G. R., Kaufman K. M., Kelly J. A. (2008) Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gateva V., Sandling J. K., Hom G., Taylor K. E., Chung S. A., Sun X., Ortmann W., Kosoy R., Ferreira R. C., Nordmark G., Gunnarsson I., Svenungsson E., Padyukov L., Sturfelt G., Jonsen A., Bengtsson A. A., Rantapaa-Dahlqvist S., Baechler E. C., Brown E. E., Alarcon G. S., Edberg J. C., Ramsey-Goldman R., McGwin G., Jr., Reveille J. D., Vila L. M., Kimberly R. P., Manzi S., Petri M. A., Lee A., Gregersen P. K., Seldin M. F., Ronnblom L., Criswell L. A., Syvanen A. C., Behrens T. W., Graham R. R. (2009) A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han J. W., Zheng H. F., Cui Y., Sun L. D., Ye D. Q., Hu Z., Xu J. H., Cai Z. M., Huang W., Zhao G. P., Xie H. F., Fang H., Lu Q. J., Li X. P., Pan Y. F., Deng D. Q., Zeng F. Q., Ye Z. Z., Zhang X. Y., Wang Q. W., Hao F., Ma L., Zuo X. B., Zhou F. S., Du W. H., Cheng Y. L., Yang J. Q., Shen S. K., Li J., Sheng Y. J., Zuo X. X., Zhu W. F., Gao F., Zhang P. L., Guo Q., Li B., Gao M., Xiao F. L., Quan C., Zhang C., Zhang Z., Zhu K. J., Li Y., Hu D. Y., Lu W. S., Huang J. L., Liu S. X., Li H., Ren Y. Q., Wang Z. X., Yang C. J., Wang P. G., Zhou W. M., Lv Y. M., Zhang A. P., Zhang S. Q., Lin D., Low H. Q., Shen M., Zhai Z. F., Wang Y., Zhang F. Y., Yang S., Liu J. J., Zhang X. J. (2009) Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat. Genet. 41, 1234–1237 [DOI] [PubMed] [Google Scholar]
- 28. Perl A. (2010) Systems biology of lupus: mapping the impact of genomic and environmental factors on gene expression signatures, cellular signaling, metabolic pathways, hormonal and cytokine imbalance, and selecting targets for treatment. Autoimmunity 43, 32–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou X. J., Lu X. L., Lv J. C., Yang H. Z., Qin L. X., Zhao M. H., Su Y., Li Z. G., Zhang H. (2011) Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann. Rheum. Dis. 70, 1330–1337 [DOI] [PubMed] [Google Scholar]
- 30. Gros F., Arnold J., Page N., Decossas M., Korganow A. S., Martin T., Muller S. (2012) Macroautophagy is deregulated in murine and human lupus T lymphocytes [E-pub ahead of print]. Autophagy, doi:10.4161/auto.20275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pierdominici M., Vomero M., Barbati C., Colasanti T., Maselli A., Vacirca D., Giovannetti A., Malorni W., Ortona E. (2012) Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J. 26, 1400–1412 [DOI] [PubMed] [Google Scholar]
- 32. Fernandez D., Bonilla E., Mirza N., Niland B., Perl A. (2006) Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 2983–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Towns R., Kabeya Y., Yoshimori T., Guo C., Shangguan Y., Hong S., Kaplan M., Klionsky D. J., Wiley J. W. (2005) Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitochondria in SY5Y cells. Autophagy 1, 163–170 [DOI] [PubMed] [Google Scholar]
- 34. Hochberg M. C. (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725. [DOI] [PubMed] [Google Scholar]
- 35. Mosca M., Bombardieri S. (2006) Assessing remission in systemic lupus erythematosus. Clin. Exp. Rheumatol. 24, S-99–104 [PubMed] [Google Scholar]
- 36. Giovannetti A., Pierdominici M., Mazzetta F., Marziali M., Renzi C., Mileo A. M., De Felice M., Mora B., Esposito A., Carello R., Pizzuti A., Paggi M. G., Paganelli R., Malorni W., Aiuti F. (2007) Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J. Immunol. 178, 3932–3943 [DOI] [PubMed] [Google Scholar]
- 37. Lee B. R., Kamitani T. (2011) Improved immunodetection of endogenous alpha-synuclein. PLoS One 6, e23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 39. Spronk P. E., Horst G., Van Der Gun B. T., Limburg P. C., Kallenberg C. G. (1996) Anti-dsDNA production coincides with concurrent B and T cell activation during development of active disease in systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 104, 446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fritsch R. D., Shen X., Illei G. G., Yarboro C. H., Prussin C., Hathcock K. S., Hodes R. J., Lipsky P. E. (2006) Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 54, 2184–2197 [DOI] [PubMed] [Google Scholar]
- 41. Amaravadi R. K., Yu D., Lum J. J., Bui T., Christophorou M. A., Evan G. I., Thomas-Tikhonenko A., Thompson C. B. (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 117, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Minota S., Winfield J. B. (1987) IgG anti-lymphocyte antibodies in systemic lupus erythematosus react with surface molecules shared by peripheral T cells and a primitive T cell line. J. Immunol. 138, 1750–1756 [PubMed] [Google Scholar]
- 43. Juang Y. T., Wang Y., Solomou E. E., Li Y., Mawrin C., Tenbrock K., Kyttaris V. C., Tsokos G. C. (2005) Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J. Clin. Invest. 115, 996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hubbard V. M., Valdor R., Patel B., Singh R., Cuervo A. M., Macian F. (2010) Macroautophagy regulates energy metabolism during effector T cell activation. J. Immunol. 185, 7349–7357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hildeman D. A., Mitchell T., Teague T. K., Henson P., Day B. J., Kappler J., Marrack P. C. (1999) Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10, 735–744 [DOI] [PubMed] [Google Scholar]
- 47. Powell J. D., Delgoffe G. M. (2010) The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Viret C., Wong F. S., Janeway C. A., Jr. (1999) Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10, 559–568 [DOI] [PubMed] [Google Scholar]
- 49. Kohler S., Thiel A. (2009) Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood 113, 769–774 [DOI] [PubMed] [Google Scholar]
- 50. Liao J. J., Huang M. C., Fast K., Gundling K., Yadav M., Van Brocklyn J. R., Wabl M. R., Goetzl E. J. (2009) Immunosuppressive human anti-lymphocyte autoantibodies specific for the type 1 sphingosine 1-phosphate receptor. FASEB J. 23, 1786–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubinsztein D. C., Gestwicki J. E., Murphy L. O., Klionsky D. J. (2007) Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 6, 304–312 [DOI] [PubMed] [Google Scholar]
- 52. Ciuffreda L., Di Sanza C., Incani U. C., Milella M. (2010) The mTOR pathway: a new target in cancer therapy. Curr. Cancer Drug Targets 10, 484–495 [DOI] [PubMed] [Google Scholar]
- 53. Levine B., Sinha S., Kroemer G. (2008) Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4, 600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Djavaheri-Mergny M., Amelotti M., Mathieu J., Besancon F., Bauvy C., Souquere S., Pierron G., Codogno P. (2006) NF-κB activation represses tumor necrosis factor-α-induced autophagy. J. Biol. Chem. 281, 30373–30382 [DOI] [PubMed] [Google Scholar]
- 55. Winslow A. R., Chen C. W., Corrochano S., Acevedo-Arozena A., Gordon D. E., Peden A. A., Lichtenberg M., Menzies F. M., Ravikumar B., Imarisio S., Brown S., O'Kane C. J., Rubinsztein D. C. (2010) α-Synuclein impairs macroautophagy: implications for Parkinson's disease. J. Cell Biol. 190, 1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balaburski G. M., Hontz R. D., Murphy M. E. (2010) p53 and ARF: unexpected players in autophagy. Trends Cell Biol. 20, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tu S., Quante M., Bhagat G., Takaishi S., Cui G., Yang X. D., Fox J. G., Pritchard M., Wang T. (2011) Interferon-γ inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T cell apoptosis. Cancer Res. 71, 4247–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tamai K., Tanaka N., Nara A., Yamamoto A., Nakagawa I., Yoshimori T., Ueno Y., Shimosegawa T., Sugamura K. (2007) Role of Hrs in maturation of autophagosomes in mammalian cells. Biochem. Biophys. Res. Commun. 360, 721–727 [DOI] [PubMed] [Google Scholar]
- 59. Ikeda T., Hirata S., Fukushima S., Matsunaga Y., Ito T., Uchino M., Nishimura Y., Senju S. (2010) Dual effects of TRAIL in suppression of autoimmunity: the inhibition of Th1 cells and the promotion of regulatory T cells. J. Immunol. 185, 5259–5267 [DOI] [PubMed] [Google Scholar]
- 60. Harr M. W., McColl K. S., Zhong F., Molitoris J. K., Distelhorst C. W. (2010) Glucocorticoids downregulate Fyn and inhibit IP(3)-mediated calcium signaling to promote autophagy in T lymphocytes. Autophagy 6, 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.