Abstract
Cortisol has one of the most distinct and fascinating circadian rhythms in human physiology. This is regulated by the central clock located in the suprachiasmatic nucleus of the hypothalamus. It has been suggested that cortisol acts as a secondary messenger between central and peripheral clocks, hence its importance in the synchronization of body circadian rhythms. Conventional immediate-release hydrocortisone, either at twice- or thrice-daily doses, is not capable of replicating physiological cortisol circadian rhythm and patients with adrenal insufficiency or congenital adrenal hyperplasia still suffer from a poor quality of life and increased mortality. Novel treatments for replacement therapy are therefore essential. Proof-of-concept studies using hydrocortisone infusions suggest that the circadian delivery of hydrocortisone may improve biochemical control and life quality in patients lacking cortisol with an impaired cortisol rhythm. Recently oral formulations of modified-release hydrocortisone are being developed and it has been shown that it is possible to replicate cortisol circadian rhythm and also achieve better control of morning androgen levels. These new drug therapies are promising and potentially offer a more effective treatment with less adverse effects. Definite improvements clearly need to be established in future clinical trials.
Keywords: central clock, circadian rhythm, cortisol, hypothalamo—pituitary—adrenal axis, modified release hydrocortisone
Introduction
Cortisol is an essential steroid hormone secreted by the adrenal gland and like many other physiological processes in the body has a circadian rhythm. This rhythm is distinct and is regulated by the main circadian oscillator (pacemaker) in the suprachiasmatic nucleus (SCN) which is located in the hypothalamus. Normal individuals, without disease of the hypothalamo—pituitary— adrenal (HPA) axis, at midnight, have very low or undetectable cortisol levels that build up overnight to peak first thing in the morning. Cortisol levels then decline slowly throughout the day [Debono et al. 2009; Krieger et al. 1971; Weitzman et al. 1971].
Patients who are deficient in cortisol are known to suffer from adrenal insufficiency. The condition typically presents insidiously and may be easily overlooked. Patients will die if adrenal insufficiency is not diagnosed promptly and treated effectively [Oelkers, 1996] and longevity may be reduced as a consequence of stress-induced crises [Arlt and Allolio, 2003]. All patients need to be on life-long glucocorticoid replacement treatment [Arlt and Allolio, 2003].
Management of adrenal insufficiency is one of the challenges that face every endocrinologist. Hydrocortisone, the generic pharmaceutical name of cortisol and the most commonly used drug for adrenal insufficiency, has a short plasma half-life and patients taking this tablet wake with undetectable cortisol levels only achieving peak cortisol levels an hour after taking their dose of hydrocortisone [Mah et al. 2004]. The pharmacokinetics of immediate-release hydrocortisone makes it impossible for physicians to replicate physiological cortisol release. A number of research studies have explored different hydrocortisone regimes to try and identify the best doses and patterns of treatment [Mah et al. 2004; Howlett, 1997; Groves et al. 1988]. Unfortunately, notwithstanding doctors' efforts, these regimens inevitably result in steroid over-replacement or under-replacement [Peacey et al. 1997]. Impaired general health and vitality perception [Bergthorsdottir et al. 2006; Mills et al. 2004], bone loss [Wichers et al. 1999] and abnormal glucose levels [al-Shoumer et al. 1995] are some of the consequences of these regimes.
Whilst adrenal insufficiency typically presents insidiously, there is a risk of death if it is not diagnosed and treated appropriately [Oelkers, 1996]. Patients with primary adrenal insufficiency, although on full replacement therapy, have a mortality rate which is twofold greater than that of the background population the greatest number of deaths occurring from cardiovascular, malignant, endocrine, respiratory and infectious diseases [Bergthorsdottir et al. 2006]. In a cohort of 6107 patients on pituitary-derived growth hormone providing 105,797 person-years of follow up the overall risk of death was four times the normal population. Importantly, one of the most striking risk factors was adrenal insufficiency. Eighty six per cent of subjects found dead or comatose by relatives probably died from adrenal insufficiency which was either mistreated or overlooked [Mills et al. 2004].
New formulations of hydrocortisone bearing modified-release characteristics with the aim of imitating the physiological cortisol circadian rhythm can hopefully reduce morbidity and mortality rates.
Synchronization of circadian rhythms
Humans exhibit daily physiological and behavioural rhythms with nearly all body functions showing significant daily variations; these include sleep, body temperature, plasma concentrations of cortisol and growth hormone, and urinary excretion of potassium [Moore-Ede et al. 1983]. These circadian rhythms are produced by endogenous processes referred to as circadian oscillators which coordinate and orchestrate molecular and physiological rhythms with changes in the environment [Dunlap, 1999]. The autonomic nervous system and endocrine signals are the principal mediators of this internal rhythmicity [Buijs and Kalsbeek, 2001].
Central and peripheral circadian oscillators
In the early 1970s, brain lesion experiments and metabolic and electrophysiologic studies indicated that in mammals, in the hypothalamic SCN, existed a central circadian oscillator (pacemaker) or central clock [Moore and Lenn, 1972]. The SCN, a cluster of around 10,000 neurones located on either side of the midline above the optic chiasma [Hastings, 1997], is subdivided into a ventral ‘core’ region, that receives information from the retina and brain stem and is responsible for entrainment, and a dorsal ‘shell’ region, which appears to be a primary pacemaker whose output drives behavioural and other rhythms.
The phase of a circadian rhythm can be synchronized to the phase of the day—night cycle to which it is exposed. This process is initiated by light stimulating a specialized group of retinal ganglion cells [Rollag et al. 2003]. Their unmyelinated axons form the retinohypothalamic tracts in the optic nerves and their transmitters synaptically affect the SCN clock cells, harbouring CLOCK genes. This leads to activation of proteins that reset the circadian pacemaker's core autoregulatory transcription—translation loop [Meijer and Schwartz, 2003].
In the early 1980s, CLOCK genes were identified in Drosophila, whilst homologue genes were identified in mammals, 10 years later [Vitaterna et al. 1994; Reddy et al. 1984]. Pacemaking neurons express clock-controlled genes and these cells within the shell region are postulated to synchronize with each other and communicate rhythmicity to distinct target tissues. Transcription of five clock genes Period (Per 1, 2), Cryptochrome (Cry 1, 2), and Reverbα are activated during the day when CLOCK and BMAL1 proteins bind to promoter sequences. Transcription of these genes continues until PER and CRY proteins accumulate to sufficient levels in the nucleus to repress CLOCK/BMAL1 activation. This occurs in the night. Transcription of the five daytime genes begins again in the early morning hours when PER/CRY protein levels begin to fall [Reppert and Weaver, 2001]. The pacemaking cells within the core synchronize with each other, communicate with pacemakers in the shell region, and drive rhythms outside the SCN [Hastings and Herzog, 2004]. These output pathways are likely to involve both humoral and nervous signals [Silver et al. 1996], which act as secondary messengers relaying information between clock cells in central and also peripheral oscillators; manifesting itself in circadian physiology and behaviour.
CLOCK genes have also been found to be expressed peripherally in different tissues in a circadian fashion. In several peripheral tissues circadian rhythms in RNA are evident for each of the PER genes [Zylka et al. 1998]. In these tissues, the oscillation of each PER gene is delayed by 3–9 h to the oscillation in the central pacemaker, suggesting that peripheral pacemakers are synchronized and regulated by the SCN. Circadian cycles can be entrained by serum components, highlighting the importance of chemical signal transduction for the coordination of circadian gene expression [Balsalobre et al. 1998]. Given that glucocorticoids do exhibit a circadian rhythm and glucocorticoid receptors are found in most peripheral cells and tissues but not in the SCN [Rosenfeld et al. 1993], glucocorticoids are highly likely candidates to act as secondary messengers or entraining signals [Balsalobre et al. 2000].
Physiology of cortisol circadian rhythm
Regulation of cortisol circadian rhythm
Cortisol has one of the most distinct and interesting circadian rhythms in the body. It is well established and has been analysed in fine detail [Weitzman et al. 1971], and is characterized by a constant and reproducible pattern under stable physiological conditions [Selmaoui and Touitou, 2003]. In 33 normal individuals who had 20-minute cortisol profiling over 24 h we have shown that cortisol levels reach lowest levels at around midnight, levels start to rise at around 02:00 to 03:00 and reach a peak at around 08:30. Cortisol levels then slowly decrease back to the nadir to complete the cycle over 24 h. The peak cortisol level attained was approximately 399 nmol/l, whilst the nadir cortisol was <50 nmol/l [Debono et al. 2009] (Figure 1).
Figure 1.
Circadian rhythm of cortisol in 33 individuals with 20-minute cortisol profiling. Peak cortisol levels are reached at around 08:30 and nadir cortisol levels at around midnight. The peaks of cortisol at noon and around 18:00 represent meal-induced cortisol stimulation. (Reproduced with permission from The Endocrine Society and Debono et al. [2009]).
The regulation of glucocorticoid, or cortisol release, is critically determined by the activity of the HPA axis. The HPA axis receives input from the central pacemaker which controls the circadian release of corticotrophin-releasing hormone (CRH) in the paraventricular nucleus, this also stimulated by physical and emotional stressors. CRH in turn stimulates release of adrenocorticotrophic hormones (ACTH) from the corticotroph cells in the anterior pituitary, and thence the glucocorticoid cortisol from the adrenal cortex. In turn, cortisol exerts inhibitory effects at pituitary and hypothalamic levels, in a classical negative feedback loop although there is no feedback on the SCN [Oster et al. 2006b].
The adrenal gland contains a circadian clock that sets specific time intervals during which the adrenal most effectively responds to ACTH. This is regulated via the splanchnic nerve [Jasper and Engeland, 1997]. Clock genes are expressed rhythmically in the zona glomerulosa and zona fasciculata, and entire pathways characteristic for the adrenal gland, such as steroid metabolism or catecholamine production, are transcriptionally regulated by the circadian clock [Oster et al. 2006a]. Expression of clock genes in the adrenal gland shows a 6-h phase delay relative to the SCN which is mainly induced via the SCN—sympathetic nervous system without accompanying activation of the HPA axis [Fahrenkrug et al. 2008; Ishida et al. 2005]. This gene expression accompanies the rhythmic secretion of plasma and brain cortisol.
Cortisol production rate
A number of cortisol secretory episodes occur during the 24 h of the day making it possible to describe four different unequal temporal phases. These phases are represented by a period of minimal secretory activity, during which cortisol secretion is negligible, and occurs 4 h prior to and 2 h after sleep onset, a preliminary nocturnal secretory episode at the third through fifth hours of sleep, a main secretory phase of a series of three to five episodes occurring during the sixth to eighth hours of sleep and continuing through the first hour of wakefulness and an intermittent waking secretory activity of four to nine secretory episodes found in the 2–12-h waking period [Weitzman et al. 1971]. Advances in the measurement of the total amount of cortisol produced in a day shows that this is around 5.7–7.4 mg/m2/day or 9.5–9.9 mg/day [Kerrigan et al. 1993; Esteban and Yergey, 1990; Linder et al. 1990] which is much less than previous estimates. Cortisol production rates in children and adolescents are very similar. These findings support regimes with lower oral daily hydrocortisone doses of 15–25 mg [Peacey et al. 1997].
Current hydrocortisone therapy in adrenal insufficiency
The importance of cortisol is especially evident when it becomes deficient, a state known as adrenal insufficiency. Thomas Addison described Addison's disease in 1855 recognizing the importance of the adrenal cortex for life and Brown Sequard in 1856 performed the first adrenalectomies to highlight this finding. Notwithstanding, it took years to confirm this theory in view of conflicting ideas especially when epinephrine was discovered in 1900. It was not until the 1930s that a good amount of work was done on cortical extracts. In 1936 Pfiffner, Reichstein and Kendall showed that a large number of steroids could be crystallized from the extract. A few years later ACTH was discovered by Li, Evans and Simpson in 1943 and cortisone by Sarett in 1946 [Savage, 1951]. Since the first published report of the efficacy of cortisone in the treatment of rheumatoid arthritis in 1949 [Rubin, 2007] and Hench, Kendall and Reichstein were awarded the Nobel prize in medicine, patients with adrenal insufficiency have been treated with glucocorticoid replacement, and apart from the introduction of fludrocortisone in the 1950s, replacement therapy has not changed [Lovas and Husebye, 2008]. Hydrocortisone is now used in most centres around the world although only cortisone acetate or synthetic glucocorticoids such as prednisolone are available in some European countries, and elsewhere such as Brazil.
Management of adrenal insufficiency is not so straightforward. This is because hydrocortisone has a short plasma half-life and patients taking this tablet wake with undetectable cortisol levels achieving peak cortisol levels an hour after taking their dose of hydrocortisone [Mah et al. 2004; Derendorf et al. 1991]. Low levels of cortisol are then present by mid-afternoon. The pharmacokinetics of immediate release hydrocortisone makes it impossible for physicians to replicate physiological cortisol release.
Identifying an optimal regime
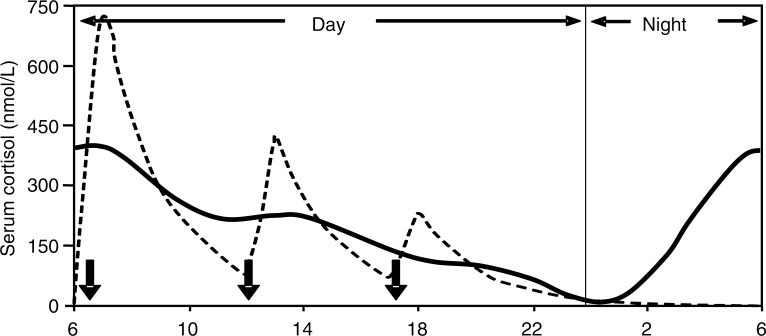
A number of research studies have explored different hydrocortisone regimes to try and identify the best doses and patterns of treatment. Patients on a thrice-daily regimen, monitored using cortisol day curves, showed a much more constant level then when compared with those on a twice-daily regimen, who had plasma cortisol levels falling to very low levels by 16:00 [Groves et al. 1988]. In 20 cortisol insufficient patients given oral immediate-release hydrocortisone in the fasted or fed state it was shown that weight-adjusted dosing decreased interpatient variability in maximum cortisol concentration from 31% to 7% when compared with the fixed dose, and reduced overexposure to <5%. Thrice-daily dosing before food was recommended as the preferred hydrocortisone regime [Mah et al. 2004] but although better this still was far from replicating physiological cortisol rhythm (Figure 2).
Figure 2.
Simulated cortisol profile for a patient (broken line) following thrice-daily hydrocortisone administration (10 mg at 06:00, 5 mg at 12:00 and 2.5 mg at 18:00, shown as solid arrows). (Reproduced with permission from John Wiley & Sons Ltd. and Mah et al. [2004]).
Limitations of current hydrocortisone replacement therapy
In general, inadequate replacement of glucocorticoids may lead to malaise, postural hypotension, diarrhoea, abdominal pain, weight loss, poor response to stress and electrolyte abnormalities. Conversely, excessive replacement may lead to Cushingoid features, glucose intolerance, hypertension and cardiovascular disease, neuro-psychiatric illness such as mania and depression, protein catabolism and osteoporosis [Arlt et al. 2006; Arlt and Allolio, 2003].
The subjective health status of patients with Addison's disease has been shown to be low when compared with normal individuals and one quarter of patients are out of work due to disability [Lovas et al. 2002]. A poor quality of life has also been revealed in patients with secondary adrenal insufficiency [Hahner et al. 2007]. Groves and colleagues revealed that well-being is lowest just before the first dose of steroid is taken in the morning, it then rises to a maximum at lunch time, and falls gradually toward evening [Groves et al. 1988]. When investigating the effects of different hydrocortisone regimes on quality of life, doses above 30 mg/day were associated with a worse health status and thrice-daily intake of hydrocortisone was not superior to twice-daily intake [Bleicken et al. 2010]. An explanation for such a significantly impaired quality of life may be related to the nonphysiological replacement of cortisol using conventional treatments. This highlights the benefits patients with cortisol deficiency may potentially receive from physiological cortisol replacement.
A bidirectional relationship exists between cortisol rhythmic activity and the sleep—wake cycle (SWC) although both systems have two separate generators in the SCN [Spath-Schwalbe et al. 1992]. Sleep disturbances associated with increased daytime fatigue have been reported for patients with adrenal insufficiency [Lovas et al. 2003]. Rapid eye movement (REM) sleep latency and time may vary in patients with adrenal insufficiency on conventional treatment this interrupting sleep continuity [Garcia-Borreguero et al. 2000]. Effects on the SWC are variable and may also be related to the dose of corticosteroids [Buckley and Schatzberg, 2005]. Physiological cortisol replacement could potentially achieve sleep indices closer to normal values in patients with adrenal insufficiency.
Bone loss secondary to depression of osteoblastic function, evident by lower osteocalcin levels, may also occur with increasing hydrocortisone doses [Peacey et al. 1997]. Whether effects on bone density do occur is debatable [Arlt et al. 2006; Zelissen et al. 1994]. The circadian variation in osteocalcin is under the control of the endogenous circadian variation in serum cortisol [Heshmati et al. 1998]. Replacement of conventional hydrocortisone by circadian cortisol therapy could possibly provide a treatment which interacts with bone physiology more effectively.
Patients with hypopituitarism on hydrocortisone equivalent doses greater than 20 mg/day have a higher body mass index (BMI), total cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglycerides [Filipsson et al. 2006]. Large cortisol peaks, as may occur with conventional hydrocortisone, may be associated with a reduction in insulin sensitivity that manifests itself 4–6 h after the cortisol elevation and may persist for more than 16h [Plat et al. 1996]. It is unlikely that patients on low-dose conventional therapy or those on physiological replacement develop steroid-induced diabetes, as very high peaks of cortisol should not occur, but clearly the risk increases with higher doses.
Conventional hydrocortisone replacement therapy has made it possible for patients with adrenal insufficiency to live a relatively normal life but it is evident that mortality and morbidity risks are higher than in the normal population.
Circadian hydrocortisone therapy: moving to improved replacement
The management of patients with adrenal insufficiency should be improved to ameliorate health-related quality of life, improve biochemical control and to reduce long-term adverse effects. Physiological hormone replacement, using sustained formulations of hydrocortisone, should be the safest and most effective and practical solution. Over the past few years interventions introducing circadian cortisol therapy, using hydrocortisone infusions and modified-release oral formulations, have shown that these treatments could potentially imitate physiological cortisol rhythm and hence result in more valuable options for patients with adrenal insufficiency.
Circadian hydrocortisone infusions
In two proof-of-concept studies using circadian intravenous and subcutaneous infusions of hydrocortisone, replicating the physiological cortisol circadian rhythm, it was shown that morning ACTH and 17OHP levels improved when compared with conventional hydrocortisone therapy [Lovas and Husebye, 2007; Merza et al. 2006] (Figure 3). These data support the notion that delivering physiological hydrocortisone replacement is likely to improve control in these patients. The problem with hydrocortisone infusions are their lack of practicality. An alternative regime is waking to take immediate-release hydrocortisone dose at 03:00, and such an approach resulted in a significant improvement in 17OHP, testosterone and individual urinary 17-ketosteroids in five patients with congenital adrenal hyperplasia. This was not achieved by giving doses which were either higher or taken later on in the evening [Moeller, 1985]. Although effective this strategy is not practical as this would mean interrupting patients' sleep and only extremely cooperative patients would benefit. Further, daytime fatigue may result from sleep fragmentation.
Figure 3.
Comparison of mean serum ACTH levels in Addison's and congenital adrenal hyperplasia patients during conventional replacement therapy and during circadian infusion of hydrocortisone (to convert values from ng/l to pmol/l × 0.22). (Reproduced with permission from John Wiley & Sons Ltd. and Merza et al. [2006]).
A more practical solution is the development of oral modified-release formulations of hydrocortisone.
Delayed and sustained release oral formulations of hydrocortisone
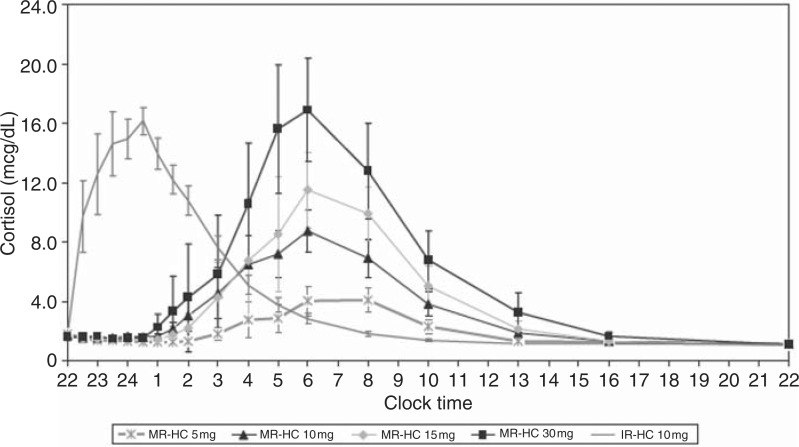
One approach is a modified-release hydrocortisone (MR-HC) tablet that can be taken late at night and then allow a delayed and then sustained release that can then simulate the cortisol circadian rhythm, by allowing a rise in circulating cortisol starting in the early hours of the morning and peaking at approximately 08:00. This consists of an insoluble barrier coat covering all but the upper surface of the tablet, where a layer dissolving slowly retards release from an inner drug-containing layer. When giving a once-daily MR-HC at different doses at 22:00 in dexamethasone-suppressed individuals, the 24-h cortisol profile at this once-daily dose showed an earlier peak level at 06:00 compared with the physiological peak at 08:32, and only maintained a physiological cortisol level for less than 12 h (Figure 4). By pharmacokinetic modelling we showed that taking 15–20 mg of MR-HC at 23:00 and 10 mg at 07:00 the drug could potentially reproduce physiological circadian cortisol levels [Debono et al. 2009] over 24 h.
Figure 4.
Concentration-time profiles for modified-release hydrocortisone (MR-HC) 5 mg, 10 mg, 15 mg and 30 mg compared with immediate-release hydrocortisone (IRHC). Graph showing delayed and sustained release characteristics of MR-HC (to convert values from mcg/dl to nmol/l × 27.59). (Reproduced with permission from The Endocrine Society and Debono et al. [2009]).
When compared with immediate-release hydrocortisone in patients with congenital adrenal hyperplasia those on MR-HC 30 mg at 22:00 had significantly lower 17OHP levels; highlighting this formulation's advantageous characteristics [Verma et al. 2010].
Another formulation with combined immediate-and extended-release characteristics has also been developed. In a study to determine single dose pharmacokinetics and dose proportionality it was shown that the time to reach a serum concentration of cortisol (>200 nmol/l) of clinical significance was within 25 minutes and a peak of 400–450 nmol/l was obtained within 50 minutes after the 20 mg tablet. Serum cortisol persisted above 200 nmol/l for around 6 h thereafter whereas all serum concentrations 18–24 h after intake were below 50 nmol/l [Johannsson et al. 2009]. This formulation was unable to fully replicate the physiological cortisol rhythm as taking the tablet at 07:00 misses the 03:00 cortisol rise. For this tablet to achieve near-normal circadian cortisol levels, it would have to be taken earlier as a once-daily dose raising compliance issues and causing sleep problems.
The hope is that these new drug-delivery technologies should improve and simplify glucocorticoid replacement therapy by being more effective, have less adverse effects and improve compliance. Despite these breakthrough discoveries of modified-release hydrocortisone that aim to replicate the 24-h physiological cortisol profile, further studies in patients with adrenal insufficiency are still needed. Further, these recommended regimens are not necessarily without adverse affects and their effects on symptoms, including quality of life, still need to be addressed.
Conclusion
The adrenal glucocorticoid, cortisol, is an essential stress hormone and deficiency leads to death. Cortisol levels are high early in the morning and low at time of sleep onset and loss of the cortisol circadian rhythm is associated with adrenal insufficiency. Unfortunately, conventional hydrocortisone replacement cannot reproduce this physiological rhythm so patients inadvertently are under- or over-replaced. This could possibly explain why patients with adrenal insufficiency suffer from a poor health-related quality of life, with an increased mortality risk, sleep disturbances, impaired psychological well being and also, at high doses, worsening of cardiovascular risk factors and defects in bone turnover.
Physiological cortisol replacement with improvements in biochemical control and quality of life has offered new prospects for patients on hydrocortisone replacement. Studies using hydrocortisone infusions have highlighted the efficacy of this therapy and oral formulations of modified release hydrocortisone are at advanced stages of development with initial data showing optimistic results. The future of hydrocortisone replacement lies in the use of physiological therapy for patients with adrenal insufficiency and congenital adrenal hyperplasia. Hopefully this should reduce adverse effects and improve quality of life.
Funding
This article received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- al-Shoumer K.A., Beshyah S.A., Niththyananthan R., Johnston D.G. (1995) Effect of glucocorticoid replacement therapy on glucose tolerance and intermediary metabolites in hypopituitary adults. Clin Endocrinol (Oxf) 42: 85–90 [DOI] [PubMed] [Google Scholar]
- Arlt W., Allolio B. (2003) Adrenal insufficiency. Lancet 361: 1881–1893 [DOI] [PubMed] [Google Scholar]
- Arlt W., Rosenthal C., Hahner S., Allolio B. (2006) Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin Endocrinol (Oxf) 64: 384–389 [DOI] [PubMed] [Google Scholar]
- Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M., et al. (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347 [DOI] [PubMed] [Google Scholar]
- Balsalobre A., Damiola F., Schibler U. (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937 [DOI] [PubMed] [Google Scholar]
- Bergthorsdottir R., Leonsson-Zachrisson M., Oden A., Johannsson G. (2006) Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab 91: 4849–4853 [DOI] [PubMed] [Google Scholar]
- Bleicken B., Hahner S., Loeffler M., Ventz M., Decker O., Allolio B., et al. (2010) Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 72: 297–304 [DOI] [PubMed] [Google Scholar]
- Buckley T.M., Schatzberg A.F. (2005) On the interactions of the hypothalamic—pituitary—adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90: 3106–3114 [DOI] [PubMed] [Google Scholar]
- Buijs R.M., Kalsbeek A. (2001) Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2: 521–526 [DOI] [PubMed] [Google Scholar]
- Debono M., Ghobadi C., Rostami-Hodjegan A., Huatan H., Campbell M.J., Newell-Price J., et al. (2009) Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 94: 1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derendorf H., Mollmann H., Barth J., Mollmann C., Tunn S., Krieg M. (1991) Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol 31: 473–476 [DOI] [PubMed] [Google Scholar]
- Dunlap J.C. (1999) Molecular bases for circadian clocks. Cell 96: 271–290 [DOI] [PubMed] [Google Scholar]
- Esteban N.V., Yergey A.L. (1990) Cortisol production rates measured by liquid chromatography/ mass spectrometry. Steroids 55: 152–158 [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J., Hannibal J., Georg B. (2008) Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J Neuroendocrinol 20: 323–329 [DOI] [PubMed] [Google Scholar]
- Filipsson H., Monson J.P., Koltowska-Haggstrom M., Mattsson A., Johannsson G. (2006) The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab 91: 3954–3961 [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D., Wehr T.A., Larrosa O., Granizo J.J., Hardwick D., Chrousos G.P., et al. (2000) Glucocorticoid replacement is permissive for rapid eye movement sleep and sleep consolidation in patients with adrenal insufficiency. J Clin Endocrinol Metab 85: 4201–4206 [DOI] [PubMed] [Google Scholar]
- Groves R.W., Toms G.C., Houghton B.J., Monson J.P. (1988) Corticosteroid replacement therapy: twice or thrice daily? J R Soc Med 81: 514–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahner S., Loeffler M., Fassnacht M., Weismann D., Koschker A.C., Quinkler M., et al. (2007) Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab 92: 3912–3922 [DOI] [PubMed] [Google Scholar]
- Hastings M.H. (1997) Central clocking. Trends Neurosci 20: 459–464 [DOI] [PubMed] [Google Scholar]
- Hastings M.H., Herzog E.D. (2004) Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms 19: 400–413 [DOI] [PubMed] [Google Scholar]
- Heshmati H.M., Riggs B.L., Burritt M.F., McAlister C.A., Wollan P.C., Khosla S. (1998) Effects of the circadian variation in serum cortisol on markers of bone turnover and calcium homeostasis in normal postmenopausal women. J Clin Endocrinol Metab 83: 751–756 [DOI] [PubMed] [Google Scholar]
- Howlett T.A. (1997) An assessment of optimal hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 46: 263–268 [DOI] [PubMed] [Google Scholar]
- Ishida A., Mutoh T., Ueyama T., Bando H., Masubuchi S., Nakahara D., et al. (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2: 297–307 [DOI] [PubMed] [Google Scholar]
- Jasper M.S., Engeland W.C. (1997) Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol 273: E363–E368 [DOI] [PubMed] [Google Scholar]
- Johannsson G., Bergthorsdottir R., Nilsson A.G., Lennernas H., Hedner T., Skrtic S. (2009) Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol 161: 119–130 [DOI] [PubMed] [Google Scholar]
- Kerrigan J.R., Veldhuis J.D., Leyo S.A., Iranmanesh A., Rogol A.D. (1993) Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 76: 1505–1510 [DOI] [PubMed] [Google Scholar]
- Krieger D.T., Allen W., Rizzo F., Krieger H.P. (1971) Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab 32: 266–284 [DOI] [PubMed] [Google Scholar]
- Linder B.L., Esteban N.V., Yergey A.L., Winterer J.C., Loriaux D.L., Cassorla F. (1990) Cortisol production rate in childhood and adolescence. J Pediatr 117: 892–896 [DOI] [PubMed] [Google Scholar]
- Lovas K., Husebye E.S. (2007) Continuous subcutaneous hydrocortisone infusion in Addison's disease. Eur J Endocrinol 157: 109–112 [DOI] [PubMed] [Google Scholar]
- Lovas K., Husebye E.S. (2008) Replacement therapy for Addison's disease: recent developments. Expert Opin Investig Drugs 17: 497–509 [DOI] [PubMed] [Google Scholar]
- Lovas K., Husebye E.S., Holsten F., Bjorvatn B. (2003) Sleep disturbances in patients with Addison's disease. Eur J Endocrinol 148: 449–456 [DOI] [PubMed] [Google Scholar]
- Lovas K., Loge J.H., Husebye E.S. (2002) Subjective health status in Norwegian patients with Addison's disease. Clin Endocrinol (Oxf) 56: 581–588 [DOI] [PubMed] [Google Scholar]
- Mah P.M., Jenkins R.C., Rostami-Hodjegan A., Newell-Price J., Doane A., Ibbotson V., et al. (2004) Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 61: 367–375 [DOI] [PubMed] [Google Scholar]
- Meijer J.H., Schwartz W.J. (2003) In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms 18: 235–249 [DOI] [PubMed] [Google Scholar]
- Merza Z., Rostami-Hodjegan A., Memmott A., Doane A., Ibbotson V., Newell-Price J., et al. (2006) Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 65: 45–50 [DOI] [PubMed] [Google Scholar]
- Mills J.L., Schonberger L.B., Wysowski D.K., Brown P., Durako S.J., Cox C., et al. (2004) Long-term mortality in the United States cohort of pituitary-derived growth hormone recipients. J Pediatr 144: 430–436 [DOI] [PubMed] [Google Scholar]
- Moeller H. (1985) Chronopharmacology of hydrocortisone and 9 alpha-fluorhydrocortisone in the treatment for congenital adrenal hyperplasia. Eur J Pediatr 144: 370–373 [DOI] [PubMed] [Google Scholar]
- Moore-Ede M.C., Czeisler C.A., Richardson G.S. (1983) Circadian timekeeping in health and disease. Part 1. Basic properties of circadian pacemakers. N Engl J Med 309: 469–476 [DOI] [PubMed] [Google Scholar]
- Moore R.Y., Lenn N.J. (1972) A retinohypothalamic projection in the rat. J Comp Neurol 146: 1–14 [DOI] [PubMed] [Google Scholar]
- Oelkers W. (1996) Adrenal insufficiency. N Engl J Med 335: 1206–1212 [DOI] [PubMed] [Google Scholar]
- Oster H., Damerow S., Hut R.A., Eichele G. (2006a) Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms 21: 350–361 [DOI] [PubMed] [Google Scholar]
- Oster H., Damerow S., Kiessling S., Jakubcakova V., Abraham D., Tian J., et al. (2006b) The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4: 163–173 [DOI] [PubMed] [Google Scholar]
- Peacey S.R., Guo C.Y., Robinson A.M., Price A., Giles M.A., Eastell R., et al. (1997) Glucocorticoid replacement therapy: are patients over treated and does it matter? Clin Endocrinol (Oxf) 46: 255–261 [DOI] [PubMed] [Google Scholar]
- Plat L., Byrne M.M., Sturis J., Polonsky K.S., Mockel J., Fery F., et al. (1996) Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol 270: E36–E42 [DOI] [PubMed] [Google Scholar]
- Reddy P., Zehring W.A., Wheeler D.A., Pirrotta V., Hadfield C., Hall J.C., et al. (1984) Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38: 701–710 [DOI] [PubMed] [Google Scholar]
- Reppert S.M., Weaver D.R. (2001) Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647–676 [DOI] [PubMed] [Google Scholar]
- Rollag M.D., Berson D.M., Provencio I. (2003) Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J Biol Rhythms 18: 227–234 [DOI] [PubMed] [Google Scholar]
- Rosenfeld P., van Eekelen J.A., Levine S., de Kloet E.R. (1993) Ontogeny of corticosteroid receptors in the brain. Cell Mol Neurobiol 13: 295–319 [DOI] [PubMed] [Google Scholar]
- Rubin R.P. (2007) A brief history of great discoveries in pharmacology: in celebration of the centennial anniversary of the founding of the American Society of Pharmacology and Experimental Therapeutics. Pharmacol Rev 59: 289–359 [DOI] [PubMed] [Google Scholar]
- Savage O. (1951) Cortisone and ACTH. Postgrad Med J 27: 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaoui B., Touitou Y. (2003) Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sci 73: 3339–3349 [DOI] [PubMed] [Google Scholar]
- Silver R., LeSauter J., Tresco P.A., Lehman M.N. (1996) A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382: 810–813 [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E., Scholler T., Kern W., Fehm H.L., Born J. (1992) Nocturnal adrenocorticotropin and cortisol secretion depends on sleep duration and decreases in association with spontaneous awakening in the morning. J Clin Endocrinol Metab 75: 1431–1435 [DOI] [PubMed] [Google Scholar]
- Verma S., Vanryzin C., Sinaii N., Kim M.S., Nieman L.K., Ravindran S., et al. (2010) A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 72: 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna M.H., King D.P., Chang A.M., Kornhauser J.M., Lowrey P.L., McDonald J.D., et al. (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman E.D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T.F., Hellman L. (1971). Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 33: 14–22 [DOI] [PubMed] [Google Scholar]
- Wichers M., Springer W., Bidlingmaier F., Klingmuller D. (1999) The influence of hydrocortisone substitution on the quality of life and parameters of bone metabolism in patients with secondary hypo-cortisolism. Clin Endocrinol (Oxf) 50: 759–765 [DOI] [PubMed] [Google Scholar]
- Zelissen P.M., Croughs R.J., van Rijk P.P., Raymakers J.A. (1994) Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann Intern Med 120: 207–210 [DOI] [PubMed] [Google Scholar]
- Zylka M.J., Shearman L.P., Weaver D.R., Reppert S.M. (1998) Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20: 1103–1110 [DOI] [PubMed] [Google Scholar]