Abstract
Hydroxyoctadecadienoic acids (HODEs) are stable oxidation products of linoleic acid, the generation of which is increased where oxidative stress is increased, such as in diabetes. In early atherosclerosis, 13-HODE is generated in macrophages by 15-lipoxygenase-1. This enhances protective mechanisms through peroxisome proliferator-activated receptor (PPAR)-g activation leading to increased clearance of lipid and lipid-laden cells from the arterial wall. In later atherosclerosis, both 9-HODE and 13-HODE are generated nonenzymatically. At this stage, early protective mechanisms are overwhelmed and pro-inflammatory effects of 9-HODE, acting through the receptor GPR132, and increased apoptosis predominate leading to a fragile, acellular plaque. Increased HODE levels thus contribute to atherosclerosis progression and the risk of clinical events such as myocardial infarction or stroke. Better understanding of the role of HODEs may lead to new pharmacologic approaches to modulate their production or action, and therefore lessen the burden of atherosclerotic disease in high-risk patients.
Keywords: atherosclerosis, diabetes, oxidative stress, oxidized lipids, G protein-coupled receptors
Introduction
Atherosclerosis is a leading cause of morbidity and premature mortality. Many of the processes involved in atherogenesis are now well understood, but there are considerable gaps in knowledge about how these are regulated. Lipid accumulation in the vascular wall and oxidative stress (both increased in diabetes) lead to the generation of oxidized lipids. The latter are involved in processes which lead to atheromatous plaque formation and rupture. Oxidized derivatives of linoleic acid, 9- and 13-hydroxyoctadecadienoic acid (9-HODE and 13-HODE), are abundant in atherosclerotic lesions and regulate cellular pathways through interaction with recently described cell surface receptors for long-chain fatty acids, and also through the peroxisome proliferator-activated receptor (PPAR) family of transcription factors. In this review, we summarize what is known about the actions of 9-HODE and 13-HODE, and how they may help explain the increased cardiovascular risk in patients with diabetes.
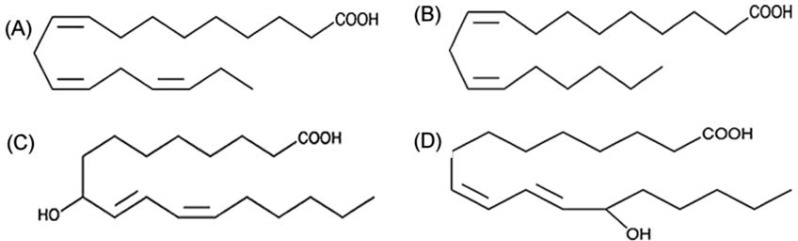
HODEs are stable oxidation products of linoleic acid (LA; C18:2, omega-6). LA is the most abundant fatty acid in atherosclerotic plaques, being seven times more abundant than arachidonic acid (AA). Oxidized lipids accumulate at sites of tissue injury, including atherosclerotic plaque. It was recognized more than 50 years ago that oxidized fatty acids accumulated in low-density lipoprotein (LDL) with age, particularly in individuals susceptible to atherosclerosis [Jira et al. 1998]. HODEs were 20 times more abundant in the LDL of patients with atherosclerosis compared with controls [Jira et al. 1998]. The structure of 9-HODE and 13-HODE, are shown in Figure 1, along with LA and the omega-3 fatty acid α-linolenic acid (ALA; C18:3, omega-3). Although the structures of these fatty acids differ only subtly, there is accumulating evidence that HODEs have distinct biological properties. Accumulation of HODEs in human atherosclerotic lesions was described nearly two decades ago [Kuhn et al. 1992], when they were shown to be components of both the cholesterol ester and phospholipid fractions. LA accounts for 40–45% of the polyunsaturated fatty acid (PUFA) in plaque, and 30% of PUFAs in plaque are oxidized. HODEs are the most abundant oxidation products in plaque, and they are present in all advanced lesions, although the quantity varies from patient to patient [Waddington et al. 2003, 2001].
Figure 1.
Structures of hydroxyoctadecadienoic acids (HODEs) and other C-18 polyunsaturated fatty acids: (A) α-linolenic acid; (B) linoleic acid; (C) 9-HODE; (D) 13-HODE.
The key enzyme for lipid oxidation in macrophages, both in animal models and in humans, is 15-lipoxygenase-1 (15-LOX-1) which is absent from normal vascular intima. With LA as substrate, 15-LOX-1 produces almost exclusively 13-HODE [Jostarndt et al. 2002; Kuhn et al. 1994]. By contrast, nonenzymatic oxidation of LA produces an approximately equal mixture of 9-HODE and 13-HODE [Upston et al. 1997]. Kuhn and colleagues studied progression of HODE deposition in the aorta of rabbits fed a cholesterol-rich diet, and compared this with composition of advanced human lesions [Kuhn et al. 1994]. In early (12-week) rabbit lesions, HODE deposition was predominantly of an enzymatic pattern while nonenzymatic generation of HODEs is the major mechanism in later lesions (26 weeks). Human lesions also showed a nonenzymatic pattern. Brinckmann and Kuhn later confirmed that enzymatic HODE generation predominated in early human lesions [Brinckmann and Kuhn, 1997]. Waddington and colleagues analysed carotid endarterectomy specimens: HODEs were the most abundant oxidised lipid and the pattern of isomers and enantiomers was consistent with nonenzymatic generation [Waddington et al. 2003, 2001]. The HODE content of lesions did not correlate with histopathological parameters studied, and there was no difference between symptomatic and asymptomatic lesions. HODEs have recently been recognized to occur in barley, malt and other plant products. It is not known whether dietary HODE is of any biological importance. Diets providing increased amounts of LA may increase 13-HODE synthesis in the vascular endothelium and thus decrease platelet adhesion and thrombogenicity [Bertomeu et al. 1990].
Whether produced enzymatically or nonenzymatically, the initial step in the synthesis of 13-HODE is formation of 13-hydroperoxy-oxydecadienoic acid (13-HPODE) which is unstable and quickly reduced to 13-HODE. A comparable step is involved in formation of 9-HODE. Recently, Muellner and colleagues reported that the gasotransmitter hydrogen sulphide (H2S) may be involved in converting HPODEs to HODEs, and was thus postulated to have a potentially atheroprotective effect [Muellner et al. 2009]. For 13-HODE, further reduction to 2,4-dienone, 13-oxooctadecadienoic acid (13-oxo-ODE) is catalysed by the enzyme 13-HODE dehydrogenase. This enzyme, which is expressed in leukocytes, is NAD-dependent and has a narrow range of substrate specificity (but including 9-HODE). The enzyme may play a role in partitioning cells between differentiated and proliferating phenotypes [Bull et al. 1993]. 13-oxo-ODE is a potent ligand for PPAR-γ. Higher HODE concentrations are an indication of oxidative stress in biological fluids and systems. F2-isoprostanes, more widely used as a marker of oxidative stress, are less abundant (as little as one twentieth) than are HODEs [Yoshida et al. 2008; Carpenter et al. 1995].
Effects of HODEs on monocytes and macrophages
Vessel wall-resident macrophages are derived from circulating monocytes following activation of the latter. Macrophage accumulation in the arterial wall and increased expression of macrophage 15-LOX-1 occur early in atherosclerosis [Wittwer and Hersberger, 2007; Zhao et al. 2004]. Lipoxygenases are nonhaem iron dioxygenases classified according to their positional specificity for AA oxidation. 5-lipoxygenase (5-LOX) and 15-lipoxygenase-1 (15-LOX-1) are involved in atherogenesis, by modulating production of inflammatory mediators, regulating cell trafficking and angiogenesis, and effects on lipid metabolism/transport. The products of 15-LOX-1 from AA are 12-hydroxyeicosatetrae-noic acid (12-HETE) and 15-hydroxyeicosate-traenoic acid (15-HETE). As noted above, the enzyme principally synthesizes 13-HODE from LA. The precise role of 15-LOX-1 in atherosclerosis has been controversial, partly because of the difficulty in extrapolating findings of animal studies to human disease [Wittwer and Hersberger, 2007; Zhao et al. 2004]. In animal studies, disruption of 12/15-LOX (mouse orthologue of 15-LOX-1) decreases lesion progression. Conversely, overexpression of the enzyme is associated with increased lesion size [Wittwer and Hersberger, 2007; Zhao et al. 2004]. Mild enzymatic oxidation of LDL particles leads to their uptake into macrophages through scavenger receptors (scavenger receptor [SR-A] and CD36) while 13-HODE produced by the action of 15-LOX-1 may also increase reverse cholesterol transport through a mechanism involving PPAR-α [Zhao et al. 2004]. Thus, although increased expression of 15-LOX-1 is a feature of early atherosclerosis, the effect of 13-HODE may actually be protective at this stage of the disease.
Accumulation of lipoprotein particles in the sub-endothelial space also occurs early in atherosclerosis. Under conditions of oxidative stress, which prevail in patients prone to atherosclerosis (including those with diabetes), LDL particles, in particular, undergo oxidation (oxLDL). To protect the vasculature from potentially damaging effects of the oxidized lipoproteins, macrophages undergo differentiation allowing them to assimilate extracellular lipid. When this initially adaptive response becomes overwhelmed, the macrophages become lipid-laden (foam cells), develop a pro-inflammatory phenotype, and become prone to apoptosis [Rusinol et al. 2000; Wintergerst et al. 2000]. The most significant components of the scavenger receptor family are CD36 and SR-A. Lipids taken up by macrophages are stored in cytoplasmic droplets as neutral lipids including triglycerides, phospholipids and cholesteryl esters. There is also a process of active cholesterol efflux, following which cholesterol is removed from the circulation by high-density lipoproteins. The major regulators of this latter process are CD36, (ATP)-binding cassette transporter-A1 (ABCA1) and scavenger receptor B1 (SR-B1). CD36 is expressed on macrophages, platelets, smooth muscle cells, endothelial cells and adipocytes. CD36 null mice accumulate less lipid and phospholipid microparticles in their macrophages, have less-advanced atherosclerosis, and also have decreased thrombus formation. As with other genes involved in lipid storage, expression of CD36 in macrophages is regulated by PPAR-γ [Lim et al. 2006; Jostarndt et al. 2004]. There is limited evidence that 13-HODE increases CD36 expression, by activating PPAR-γ [Jostarndt et al. 2002], although other mechanisms may also be involved.
Fatty acid binding protein 4 (FABP4, aP2) has a central role in adipocyte differentiation, during which its expression is regulated by PPAR-γ. FABP4 also regulates lipid accumulation in macrophages, and its expression is again controlled through PPAR-γ [Cabrero et al. 2003]. The latter transcription factor is thought to mediate the enhancing action of oxLDL on FABP4 expression and foam cell formation [Fu et al. 2000, 2002]. Fu and colleagues reported that FABP4 expression in THP1 cells was increased by 9-HODE, 13-HODE, 15-dPGJ2, and retinoic acid [Fu et al. 2002]. Overexpression of FABP4 led to marked increase in lipid accumulation. [Fu et al. 2006]
Increased susceptibility to apoptosis amongst vascular cells has been well described as a feature of developing atherosclerosis [Wintergerst et al. 2000; Hardwick et al. 1996]. Macrophage apoptosis is at least partly regulated by PPAR-γ [Chinetti et al. 1998]. A pro-apoptotic effect of HODEs has been described in nonvascular cells [Mahipal et al. 2007; Mani et al. 1998]. Two studies have reported that apoptosis of monocytic cells is increased on exposure to HODEs [Hampel et al. 2006; Jostarndt et al. 2002]. Jostarndt and colleagues investigated effects of enzymatically modified LDL on Mono Mac 6 monocytes [Jostarndt et al. 2002]. Oxidized LDL thus produced both induced apoptosis and also increased CD36 expression. The effects of oxLDL were replicated by 13-HODE.
The effect of 9-HODE was not studied. Hampel and colleagues investigated the effects of 9-HODE, 13-HODE and the PPAR-γ agonist ciglitazone on the human U937 line [Hampel et al. 2006]. Both 9-HODE and ciglitazone inhibited cell proliferation. 9-HODE increased the proportion of cells in G0/1 phase and also enhanced apoptosis. The latter action was not blocked by the PPAR-γ antagonist GW9662. There was no increase in PPAR-γ transcripts but 9-HODE specifically increased PPAR-γ2 transcripts fourfold. Using a PPRE-luciferase reporter, all three ligands increased PPAR-γ transactivation.
In summary, macrophage accumulation and increased expression of 15-LOX-1 are features of early atherosclerosis and associated with increased generation of 13-HODE. The latter (through PPAR-γ activation) increases CD36 and FABP4 expression and stimulates apoptosis. These processes are protective in early lesions leading to clearance of lipid and debris from the vascular wall, and removal of damaged or lipid-laden cells.
Effects on other vascular cells
The vascular endothelium forms an effective barrier between circulating blood and the subendothelial space. Damage to this barrier leads to activation of endothelial cells, with increased release of inflammatory, chemotactic, and pro-thrombotic mediators. Endothelial damage is also associated with increased permeability of the barrier allowing for migration of monocytes into the subendothelial space and increased deposition of lipoprotein particles in the vascular wall. HODEs have been reported to decrease platelet adhesion to endothelial cells [Haas et al. 1988, 1990]. 13-HODE may also increase synthesis of prostacyclin (PGI2) [Setty et al. 1987]. PPAR-γ is expressed in endothelial cells, and activation of this by 15-dPGJ2, 9-HODE or 13-HODE leads to increased secretion of plasminogen activator inhibitor type 1 (PAI-1) [Marx et al. 1999]. Increased levels of this fibrinolytic inhibitor predispose to vascular events and have been proposed to be a link between insulin resistant states and increased vascular risk. PPAR-γ activation in endothelial cells also leads to increased ICAM-1 expression, which, in turn, increases adhesion of monocytes. Endothelial cells have been shown to synthesise 13-HODE [Haas et al. 1990] and 9-HODE [Kaduce et al. 1989]. Synthesis of the latter is inhibited by aspirin and ibuprofen, suggesting that it may be catalysed by cyclo-oxygenase rather than lipoxygenases.
Vascular smooth muscle cells (VSMCs) are also involved in atherogenesis. Following damage to the endothelium, VSMCs proliferate, secrete increased amounts of extracellular matrix, and may differentiate into foam cells. VSMCs synthesize HODEs, predominantly 13-HODE, and this may be catalysed by the prostaglandin synthase complex [Daret et al. 1993]. VSMCs may also respond to HODEs generated by adjacent cells including platelets and macrophages. They respond particularly to 13-HODE [Stoll et al. 1994; Ramboer et al. 1992], with increased intracellular calcium, inositol trispho-sphate, and cyclic guanosine monophosphate (GMP). This release of intracellular messengers contributes to increased expression of VSMC differentiation molecules, increased cell mobility, and release of prostacyclin. Limor and colleagues have shown that VSMC express 12/15-LOX and are thus capable of synthesizing 12-HETE, 15-HETE and 13-HODE [Limor et al. 2008]. As discussed below, macrophages may be partly sequestered in the subendothelial space by downregulation of CCR2 in response to PPAR-γ agonists, while concomitant upregulation of the fractalkine (CXCCL1) receptor CX3CR1 enhances interaction between macrophages and VSMCs [Barlic and Murphy, 2007; Barlic et al. 2006]. VSMCs are responsible for the fibrous cap which protects against plaque rupture and thrombosis. Apoptosis of VSMCs may impair the integrity of the fibrous cap.
Platelets are a rich source of HODEs in vivo, possessing both 15-LOX-1 and prostaglandin synthase enzyme systems. Platelets produce predominantly 13-HODE, with LOX activity being the predominant source in the cytosolic fraction [Truitt et al. 1999; Daret et al. 1989]. In common with 8-epiPGF2α, another marker of oxidative stress, 13-HODE has potent platelet anti-aggregatory effects [Tloti et al. 1991; Coene et al. 1986]. Conflicting data have been published regarding the effects of 13-HODE on platelet adhesion to endothelial cells with one study reporting no effect when platelet adhesion was studied under flow conditions [de Graaf et al. 1989], while other studies have reported anti-aggregatory actions including decreased thrombin-induced platelet adherence with 13-HODE in vitro [Haas et al. 1990, 1988; Coene et al. 1986]. Damage to the vascular wall may decrease 13-HODE synthesis, and this may increase thrombogenicity [Weber et al. 1990].
Details of the effects of HODEs on nonvascular cells cannot be considered here in detail. They have effects on the functions of polymorphonu-clear leukocytes, and are involved in regulation of bronchial smooth muscle responsiveness. Potentially important roles in regulating reproductive function have also been described. HODEs are generated in gastrointestinal epithelial cells where they act as PPAR-γ agonists. They regulate cell proliferation and inflammation, and this may contribute to protective effects of the molecules in inflammatory bowel disease and large bowel neoplasia.
Mechanisms of action
PPAR nuclear transcription factors are activated by fatty acids and eicosanoids. PPAR-γ is involved in adipocyte differentiation, lipid storage and in regulating insulin sensitivity. It is principally associated with adipose tissue but is also expressed in vascular cells (endothelial, macrophages and VSMCs). PPAR-γ2, the predominant form in adipose tissue, has an additional 30 amino acids at the N-terminal compared with PPAR-γ1. PPARs regulate gene expression by forming heterodimers with the retinoic X receptor and binding to PPAR-response elements in the promoter region of target genes. PPAR-γ activation has effects on vascular cells which are protective against atherosclerosis. For example, although PPAR-γ can increase expression of molecules involved in lipid storage (including CD36), the net effect of increased PPAR-γ expression in macrophages is generally to decrease lipid accumulation [Bouhlel et al. 2008]. Furthermore, PPAR-α and PPAR-γ may increase cholesterol efflux by increasing expression of HDL receptor and SR-B1 [Bouhlel et al. 2008]. PPAR-γ activation also decreases levels of pro-inflammatory mediators. Some of the protective effect of PPAR-γ may be through enhancing differentiation of monocytes into alternatively activated M2 macrophages which have anti-inflammatory and other protective properties [Bouhlel et al. 2007]. The level of PPAR-γ expression in plaque correlates with the number of M2 macrophages [Bouhlel et al. 2007].
Of naturally occurring ligands for PPAR-γ, 15-deoxy-Δ12,14-prostaglandin J2 (15-dPGj2) and HODEs are the most significant. There is considerable evidence that HODEs activate PPAR-γ in macrophages leading to increased CD36 expression [Jostarndt et al. 2004; Nagy et al. 1998]. Nagy and colleagues demonstrated the capacity of HODEs to upregulate CD14, CD36 and SR-A (markers for macrophage maturation) [Nagy et al. 1998]. HODEs may also activate PPAR-α [Delerive et al. 2000] and may thus have further beneficial effects on lipid metabolism. In summary, HODEs are potent PPAR-γ agonists and may exert protective effects in atherogenesis by modulating macrophage lipid accumulation and inflammatory mediator generation.
GPR132 is a stress-inducible receptor, expression of which is increased on exposure of cells to DNA-damaging agents. Expression of GPR132 is associated with a block in cell cycle progression in the G2/M phase (G2A =G2 accumulation) [Weng et al. 1998]. GPR132 is highly expressed in macrophages in atherosclerotic plaque. GPR132 is a receptor for oxidized fatty acids with 9-HODE being the most potent ligand [Obinata et al. 2009; Yin et al. 2009]. 13-HODE is a weak ligand, and esterified HODEs do not activate the receptor. A recent study by Hattori and colleagues demonstrated that 9-HODE is a pro-inflammatory mediator in skin and that this action is GPR132-mediated [Hattori et al. 2008]. GPR132 is expressed in macrophages of atherosclerotic plaques, both in humans and in experimental rabbits [Rikitake et al. 2002]. Recent genetic manipulation studies in animals [Bolick et al. 2009, 2007; Parks et al. 2009; Parks et al. 2005] strongly support a role for GPR132 in pathogenesis of atherosclerosis. However, we do not know at present whether GPR132 generally mediates favourable or unfavourable effects in human vascular cells. Another potential mediator of HODE action is the testicular orphan nuclear receptor-4 (TR4), a nuclear transcription factor with involvement in diverse biological processes. In a very recent study, Xie and colleagues showed that TR4−/− mice had decreased macrophage expression of CD36 and reduced foam cell formation [Xie et al. 2009]. Expression of CD36 was stimulated by 13-HODE partly through the activation of TR4.
Comparison of 9-HODE and 13-HODE
Early in atherosclerosis, 13-HODE is the predominant HODE, while in advanced disease 9-HODE is at least as abundant. The known effects of 9-HODE and 13-HODE are compared in Table 1. Recently, increased 13-HODE levels have been reported in the circulation of patients with essential hypertension, presumably reflecting increased oxidative stress [Wang et al. 2009]. The best evidence that 9-HODE and 13-HODE have differing, indeed opposing effects comes from studies in the skin. Here, 9-HODE has pro-inflammatory actions mediated through GPR132 [Hattori et al. 2008]. By contrast, 13-HODE which accumulates in the cer-amides and phospholipids in the skin [Cho and Ziboh, 1994; Gron et al. 1993a] and has anti-inflammatory actions. 13-HODE also affects proliferation of keratinocytes: levels of PUFAs are decreased in hyperproliferating skin, and the increased proliferation is reversed by topical application of 13-HODE [Miller and Ziboh, 1990]. In psoriasis there is a decrease in 9-HODE, 13-HODE and 15-HETE [Gron et al. 1993b]. The anti-proliferative action of 13-HODE involves down-regulation of the onco-gene activator protein-1 (AP-1).
Table 1.
Actions of HODEs relevant to atherosclerosis.
| Effect | Cell type | 9-HODE | 13-HODE | |
|---|---|---|---|---|
| Present in early lesions | — | — | ++ | |
| Present in late lesions | — | ++ | ++ | |
| Marker of oxidative stress | — | ++ | ++ | |
| CD36 expression | Macro | NS | ↑ | |
| FABP4 expression | Macro | ↑↑ | ↑↑ | |
| Lipid accumulation | Macro | ↑↑ | ↑↑ | |
| Apoptosis | Macro | ↑ | ↑ | |
| Antithrombotic* | Endo | + | ++ | |
| Differentiation/mobility | VSMC | NS | ++ | |
| Aggregation | Platelet | NS | ↓↓ | |
| Inflammation | Skin/colon | ↑↑ | ↓↓ |
However, both 9-HODE and 13-HODE increase PAI-1 expression in endothelial cells [Marx et al. 1999]. Macro, macrophage; Endo, endothelial cell; VSMC, vascular smooth muscle cell; NS, not significant; HODE, hydroxyoctadecadienoic acid.
Conclusions
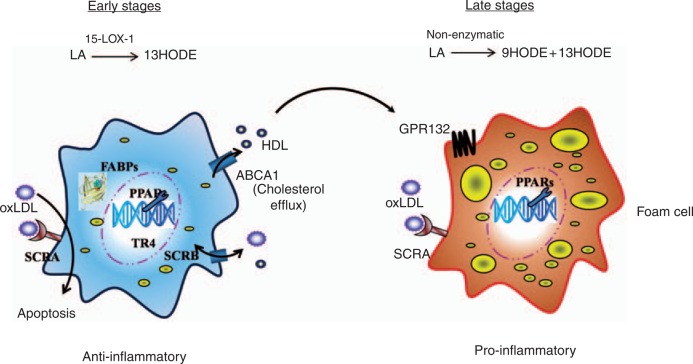
HODEs are markers of oxidative stress, and components of the oxidized lipid in atherosclerotic plaque. The major effects of HODEs in regulating macrophage functions are summarized in Figure 2. In early atherosclerosis, 13-HODE is generated in macrophages enzymatically by 15-LOX-1. This may be important in activating protective mechanisms which increase clearance of lipid and cellular debris from the vessel wall. Effects of 13-HODE are predominantly mediated through PPAR-γ, although a possible role for the nuclear transcription factor TR4 has been reported. In later atherosclerosis, nonenzymatic oxidation of LA generates a mixture of 9-HODE and 13-HODE. At this stage of the disease, and with the pro-inflammatory actions of 9-HODE acting through the GPR132 receptor, the effects of HODEs may be predominantly harmful rather than beneficial. Increased oxidative stress and enhanced monocyte activation in diabetes may increase generation of HODEs and this could partly explain the susceptibility of diabetic individuals to macrovascular disease. Clearer understanding of the mechanisms involved might lead to development of new atheroprotective agents, and thus help lessen the considerable health and health economic burden of macrovascular disease in high-risk conditions such as diabetes.
Figure 2.
Effects of HODEs on monocytes and macrophages. The contrasting effects of HODEs on the macrophage in early and late atherosclerosis. In early disease, 13-HODE is generated enzymatically by 15-lipoxygenase, and its actions are protective with increased lipid uptake, reverse cholesterol transport and enhanced apoptosis. In later disease, HODEs are synthesized nonenzymatically and pro-inflammatory actions of 9-HODE mediated by the GPR132 receptor contribute to lesion progression.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- Barlic J., Murphy P.M. (2007) An oxidized lipid-peroxisome proliferator-activated receptor gamma-chemokine pathway in the regulation of macrophage-vascular smooth muscle cell adhesion. Trends Cardiovasc Med 17: 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlic J., Zhang Y., Foley J.F., Murphy P.M. (2006) Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor gamma-dependent pathway. Circulation 114: 807–819 [DOI] [PubMed] [Google Scholar]
- Bertomeu M.C., Crozier G.L., Haas T.A., Fleith M., Buchanan M.R. (1990) Selective effects of dietary fats on vascular 13-HODE synthesis and platelet/vessel wall interactions. Thromb Res 59: 819–830 [DOI] [PubMed] [Google Scholar]
- Bolick D.T., Skaflen M.D., Johnson L.E., Kwon S.C., Howatt D., Daugherty A., et al. (2009) G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ Res 104: 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolick D.T., Whetzel A.M., Skaflen M., Deem T.L., Lee J., Hedrick C.C. (2007) Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta. Circ Res 100: 572–580 [DOI] [PubMed] [Google Scholar]
- Bouhlel M.A., Derudas B., Rigamonti E., Dievart R., Brozek J., Haulon S., et al. (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6: 137–143 [DOI] [PubMed] [Google Scholar]
- Bouhlel M.A., Staels B., Chinetti-Gbaguidi G. (2008) Peroxisome proliferator-activated receptors—from active regulators of macrophage biology to pharmacological targets in the treatment of cardiovascular disease. J Intern Med 263: 28–42 [DOI] [PubMed] [Google Scholar]
- Brinckmann R., Kuhn H. (1997) Regulation of 15-lipoxygenase expression by cytokines. Adv Exp Med Biol 400B: 599–604 [PubMed] [Google Scholar]
- Bull A.W., Branting C., Bronstein J.C., Blackburn M.L., Rafter J. (1993) Increases in 13-hydroxy-octadecadienoic acid dehydrogenase activity during differentiation of cultured cells. Carcinogenesis 14: 2239–2243 [DOI] [PubMed] [Google Scholar]
- Cabrero A., Cubero M., Llaverias G., Jove M., Planavila A., Alegret M., et al. (2003) Differential effects of peroxisome proliferator-activated receptor activators on the mRNA levels of genes involved in lipid metabolism in primary human monocyte-derived macrophages. Metabolism 52: 652–657 [DOI] [PubMed] [Google Scholar]
- Carpenter K.L., Taylor S.E., van der Veen C., Williamson B.K., Ballantine J.A., Mitchinson M.J. (1995) Lipids and oxidised lipids in human atherosclerotic lesions at different stages of development. Biochim Biophys Acta 1256: 141–150 [DOI] [PubMed] [Google Scholar]
- Chinetti G., Griglio S., Antonucci M., Torra I.P., Delerive P., Majd Z., et al. (1998) Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem 273: 25573–25580 [DOI] [PubMed] [Google Scholar]
- Cho Y., Ziboh V.A. (1994) Incorporation of 13-hydroxyoctadecadienoic acid (13-HODE) into epidermal ceramides and phospholipids: phospholipase C-catalyzed release of novel 13-HODE-containing diacylglycerol. J Lipid Res 35: 255–262 [PubMed] [Google Scholar]
- Coene M.C., Bult H., Claeys M., Laekeman G.M., Herman A.G. (1986) Inhibition of rabbit platelet activation by lipoxygenase products of arachidonic and linoleic acid. Thromb Res 42: 205–214 [DOI] [PubMed] [Google Scholar]
- Daret D., Blin P., Dorian B., Rigaud M., Larrue J. (1993) Synthesis of monohydroxylated fatty acids from linoleic acid by rat aortic smooth muscle cells and tissues: influence on prostacyclin production. J Lipid Res 34: 1473–1482 [PubMed] [Google Scholar]
- Daret D., Blin P., Larrue J. (1989) Synthesis of hydroxy fatty acids from linoleic acid by human blood platelets. Prostaglandins 38: 203–214 [DOI] [PubMed] [Google Scholar]
- de Graaf J.C., Bult H., de Meyer G.R., Sixma J.J., de Groot P.G. (1989) Platelet adhesion to subendothelial structures under flow conditions: no effect of the lipoxygenase product 13-HODE. Thromb Haemost 62: 802–806 [PubMed] [Google Scholar]
- Delerive P., Furman C., Teissier E., Fruchart J., Duriez P., Staels B. (2000) Oxidized phospholipids activate PPARalpha in a phospholipase A2-dependent manner. FEBS Lett 471: 34–38 [DOI] [PubMed] [Google Scholar]
- Fu Y., Luo N., Lopes-Virella M.F. (2000) Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res 41: 2017–2023 [PubMed] [Google Scholar]
- Fu Y., Luo N., Lopes-Virella M.F., Garvey W.T. (2002) The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 165: 259–269 [DOI] [PubMed] [Google Scholar]
- Fu Y., Luo L., Luo N., Garvey W.T. (2006) Lipid metabolism mediated by adipocyte lipid binding protein (ALBP/aP2) gene expression inhuman THP-1 macrophages. Atherosclerosis 188: 102–111 [DOI] [PubMed] [Google Scholar]
- Gron B., Iversen L., Ziboh V., Kragballe K. (1993a) Distribution of monohydroxy fatty acids in specific human epidermal phospholipids. Exp Dermatol 2: 38–44 [DOI] [PubMed] [Google Scholar]
- Gron B., Iversen L., Ziboh V., Kragballe K. (1993b) Monohydroxy fatty acids esterified to phospholipids are decreased in lesional psoriatic skin. Arch Dermatol Res 285: 449–454 [DOI] [PubMed] [Google Scholar]
- Haas T.A., Bastida E., Nakamura K., Hullin F., Admirall L., Buchanan M.R. (1988) Binding of 13-HODE and 5–, 12– and 15-HETE to endothelial cells and subsequent platelet, neutrophil and tumor cell adhesion. Biochim Biophys Acta 961: 153–159 [DOI] [PubMed] [Google Scholar]
- Haas T.A., Bertomeu M.C., Bastida E., Buchanan M.R. (1990) Cyclic AMP regulation of endothelial cell triacylglycerol turnover, 13-hydro-xyoctadecadienoic acid (13-HODE) synthesis and endothelial cell thrombogenicity. Biochim Biophys Acta 1051: 174–178 [DOI] [PubMed] [Google Scholar]
- Hampel J.K.A., Brownrigg L.M., Vignarajah D., Croft K.D., Dharmarajan A.M., Bentel J.M., et al. (2006) Differential modulation of cell cycle, apoptosis and PPARgamma2 gene expression by PPARgamma agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukot Essent Fatty Acids 74: 283–293 [DOI] [PubMed] [Google Scholar]
- Hardwick S.J., Hegyi L., Clare K., Law N.S., Carpenter K.L., Mitchinson M.J., et al. (1996) Apoptosis in human monocyte-macrophages exposed to oxidized low density lipoprotein. J Pathol 179: 294–302 [DOI] [PubMed] [Google Scholar]
- Hattori T., Obinata H., Ogawa A., Kishi M., Tatei K., Ishikawa O., et al. (2008) G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol 128: 1123–1133 [DOI] [PubMed] [Google Scholar]
- Jira W., Spiteller G., Carson W., Schramm A. (1998) Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids 91: 1–11 [DOI] [PubMed] [Google Scholar]
- Jostarndt K., Gellert N., Rubic T., Weber C., Kuhn H., Johansen B., et al. (2002) Dissociation of apoptosis induction and CD36 upregulation by enzymatically modified low-density lipoprotein in monocytic cells. Biochem Biophys Res Commun 290: 988–993 [DOI] [PubMed] [Google Scholar]
- Jostarndt K., Rubic T., Kuhn H., Anthosen M.W., Andera L., Gellert N., et al. (2004) Enzymatically modified low-density lipoprotein upregulates CD36 in low-differentiated monocytic cells in a peroxisome proliferator-activated receptor-gamma-dependent way. Biochem Pharmacol 67: 841–854 [DOI] [PubMed] [Google Scholar]
- Kaduce T.L., Figard P.H., Leifur R., Spector A.A. (1989) Formation of 9-hydroxyoctadecadienoic acid from linoleic acid in endothelial cells. J Biol Chem 264: 6823–6830 [PubMed] [Google Scholar]
- Kuhn H., Belkner J., Wiesner R., Schewe T., Lankin V.Z., Tikhaze A.K. (1992) Structure elucidation of oxygenated lipids in human atherosclerotic lesions. Eicosanoids 5: 17–22 [PubMed] [Google Scholar]
- Kuhn H., Belkner J., Zaiss S., Fahrenklemper T., Wohlfeil S. (1994) Involvement of 15-lipoxygen-ase in early stages of atherogenesis. J Exp Med 179: 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.J., Lee S., Lee K.S., Park J.H., Jang Y., Lee E.J., et al. (2006) PPARgamma activation induces CD36 expression and stimulates foam cell like changes in rVSMCs. Prostaglandins Other Lipid Mediat 80: 165–174 [DOI] [PubMed] [Google Scholar]
- Limor R., Sharon O., Knoll E., Many A., Weisinger G., Stern N. (2008) Lipoxygenase-derived metabolites are regulators of peroxisome proliferator-activated receptor gamma-2 expression in human vascular smooth muscle cells. Am J Hypertens 21: 219–223 [DOI] [PubMed] [Google Scholar]
- Mahipal S.V., Subhashini J., Reddy M.C., Reddy M.M., Anilkumar K., Roy K.R., et al. (2007) Effect of 15-lipoxygenase metabolites, 15-(S)-HPETE and 15-(S)-HETE on chronic myelogenous leukemia cell line K-562: reactive oxygen species (ROS) mediate caspase-dependent apoptosis. Biochem Pharmacol 74: 202–214 [DOI] [PubMed] [Google Scholar]
- Mani I., Iversen L., Ziboh V.A. (1998) Upregulation of nuclear PKC and MAP-kinase during hyperproliferation of guinea pig epidermis: modulation by 13-(S)-hydroxyoctadecadienoic acid (13-HODE). Cell Signal 10: 143–149 [DOI] [PubMed] [Google Scholar]
- Marx N., Bourcier T., Sukhova G.K., Libby P., Plutzky J. (1999) PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol 19: 546–551 [DOI] [PubMed] [Google Scholar]
- Miller C.C., Ziboh V.A. (1990) Induction of epidermal hyperproliferation by topical n-3 polyunsaturated fatty acids on guinea pig skin linked to decreased levels of 13-hydroxyoctadecadienoic acid (13-hode). J Invest Dermatol 94: 353–358 [DOI] [PubMed] [Google Scholar]
- Muellner M.K., Schreier S.M., Laggner H., Hermann M., Esterbauer H., Exner M., et al. (2009) Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J 420: 277–281 [DOI] [PubMed] [Google Scholar]
- Nagy L., Tontonoz P., Alvarez J.G., Chen H., Evans R.M. (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93: 229–240 [DOI] [PubMed] [Google Scholar]
- Obinata H., Izumi T., Obinata H., Izumi T. (2009) G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat 89: 66–72 [DOI] [PubMed] [Google Scholar]
- Parks B.W., Gambill G.P., Lusis A.J., Kabarowski J.H.S. (2005) Loss of G2A promotes macrophage accumulation in atherosclerotic lesions of low density lipoprotein receptor-deficient mice. J Lipid Res 46: 1405–1415 [DOI] [PubMed] [Google Scholar]
- Parks B.W., Srivastava R., Yu S., Kabarowski J.H., Parks B.W., Srivastava R., et al. (2009) ApoE-dependent modulation of HDL and atherosclerosis by G2A in LDL receptor-deficient mice independent of bone marrow-derived cells. Arterioscler Thromb Vasc Biol 29: 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboer I., Blin P., Lacape G., Daret D., Lamaziere J.M., Larrue J. (1992) Effects of monohydroxylated fatty acids on arterial smooth muscle cell properties. Kidney Int Suppl 37: S67–S72 [PubMed] [Google Scholar]
- Rikitake Y., Hirata K.-I., Yamashita T., Iwai K., Kobayashi S., Itoh H., et al. (2002) Expression of G2A, a receptor for lysophosphatidylcholine, by macrophages in murine, rabbit, and human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22: 2049–2053 [DOI] [PubMed] [Google Scholar]
- Rusinol A.E., Yang L., Thewke D., Panini S.R., Kramer M.F., Sinensky M.S. (2000) Isolation of a somatic cell mutant resistant to the induction of apoptosis by oxidized low density lipoprotein. J Biol Chem 275: 7296–7303 [DOI] [PubMed] [Google Scholar]
- Setty B.N., Berger M., Stuart M.J. (1987) 13-Hydroxyoctadecadienoic acid (13-HODE) stimulates prostacyclin production by endothelial cells. Biochem Biophys Res Commun 146: 502–509 [DOI] [PubMed] [Google Scholar]
- Stoll L.L., Morland M.R., Spector A.A. (1994) 13-HODE increases intracellular calcium in vascular smooth muscle cells. Am J Physiol 266: C990–C996 [DOI] [PubMed] [Google Scholar]
- Tloti M.A., Moon D.G., Weston L.K., Kaplan J.E. (1991) Effect of 13-hydroxyoctadeca-9,11-dienoic acid (13-HODE) on thrombin induced platelet adherence to endothelial cells in vitro. Thromb Res 62: 305–317 [DOI] [PubMed] [Google Scholar]
- Truitt A., McNeill G., Vanderhoek J.Y. (1999) Antiplatelet effects of conjugated linoleic acid isomers. Biochim Biophys Acta 1438: 239–246 [DOI] [PubMed] [Google Scholar]
- Upston J.M., Neuzil J., Witting P.K., Alleva R., Stocker R. (1997) Oxidation of free fatty acids in low density lipoprotein by 15-lipoxygenase stimulates nonenzymic, alpha-tocopherol-mediated peroxidation of cholesteryl esters. J Biol Chem 272: 30067–30074 [DOI] [PubMed] [Google Scholar]
- Waddington E., Sienuarine K., Puddey I., Croft K. (2001) Identification and quantitation of unique fatty acid oxidation products in human atherosclerotic plaque using high-performance liquid chromatography. Anal Biochem 292: 234–244 [DOI] [PubMed] [Google Scholar]
- Waddington E.I., Croft K.D., Sienuarine K., Latham B., Puddey I.B. (2003) Fatty acid oxidation products in human atherosclerotic plaque: an analysis of clinical and histopathological correlates. Atherosclerosis 167: 111–120 [DOI] [PubMed] [Google Scholar]
- Wang D., Strandgaard S., Iversen J., Wilcox C.S., Wang D., Strandgaard S., et al. (2009) Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol 296: R195–R200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E., Haas T.A., Muller T.H., Eisert W.G., Hirsh J., Richardson M., et al. (1990) Relationship between vessel wall 13-HODE synthesis and vessel wall thrombogenicity following injury: influence of salicylate and dipyridamole treatment. Thromb Res 57: 383–392 [DOI] [PubMed] [Google Scholar]
- Weng Z., Fluckiger A.C., Nisitani S., Wahl M.I., Le L.Q., Hunter C.A., et al. (1998) A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc Natl Acad Sci USA 95: 12334–12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst E.S., Jelk J., Rahner C., Asmis R. (2000) Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. Eur J Biochem 267: 6050–6059 [DOI] [PubMed] [Google Scholar]
- Wittwer J., Hersberger M. (2007) The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids 77: 67–77 [DOI] [PubMed] [Google Scholar]
- Xie S., Lee Y.F., Kim E., Chen L.M., Ni J., Fang L.Y., et al. (2009) TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci USA 106: 13353–13358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., et al. (2009) Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 284: 12328–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Kodai S., Takemura S., Minamiyama Y., Niki E. (2008) Simultaneous measurement of F2-isoprostane, hydroxyoctadecadienoic acid, hydroxyeicosatetraenoic acid, and hydroxycholesterols from physiological samples. Anal Biochem 379: 105–115 [DOI] [PubMed] [Google Scholar]
- Zhao L., Funk C.D., Zhao L., Funk C.D. (2004) Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med 14: 191–195 [DOI] [PubMed] [Google Scholar]