Abstract
In roots two distinct polar movements of auxin have been reported that may control different developmental and growth events. To test the hypothesis that auxin derived from the shoot and transported toward the root controls lateral root development, the two polarities of auxin transport were uncoupled in Arabidopsis. Local application of the auxin-transport inhibitor naphthylphthalamic acid (NPA) at the root-shoot junction decreased the number and density of lateral roots and reduced the free indoleacetic acid (IAA) levels in the root and [3H]IAA transport into the root. Application of NPA to the basal half of or at several positions along the root only reduced lateral root density in regions that were in contact with NPA or in regions apical to the site of application. Lateral root development was restored by application of IAA apical to NPA application. Lateral root development in Arabidopsis roots was also inhibited by excision of the shoot or dark growth and this inhibition was reversible by IAA. Together, these results are consistent with auxin transport from the shoot into the root controlling lateral root development.
Polar auxin transport in higher plants is a directional and regulated process. In stems auxin transport is from cell to cell and moves from the shoot apex toward the base (Lomax et al., 1995). Appropriate distribution of auxin has been shown to be necessary for a number of developmental processes. Normal embryogenesis, for example, requires auxin transport (Cooke et al., 1993), and the Arabidopsis mutants lop1, pin1-1, and monopterous (Okada et al., 1991; Carland and McHale, 1996; Przemeck et al., 1996), which may be altered in auxin transport, have altered vascular, floral, and pattern development, respectively.
In roots the movement of auxin is more complex. Analysis of the distribution of radiolabeled auxin applied to plants indicates that auxin is transported acropetally (from the base of the root toward the root tip) in the central cylinder of the root (Mitchell and Davies, 1975; Tsurumi and Ohwaki, 1978). However, there is also evidence for basipetal auxin transport near the root apex. In bean plants this transport appears to occur only between the root tip and the elongation zone (Mitchell and Davies, 1975), and microautoradiography suggests that auxin transport occurs in the root epidermis (Tsurumi and Ohwaki, 1978). Both polarities of auxin transport have been shown to be sensitive to inhibition by the auxin-transport inhibitor 2,3,5-triiodo-benzoic acid (Tsurumi and Ohwaki, 1978), suggesting that a similar mechanism may control both movements. The root apex may be capable of IAA biosynthesis (Feldman, 1980), but it is unknown whether auxin transported basipetally in the root originates in the shoot, the root apex, or both. Auxin transport is required for root elongation, gravity response, and lateral root development (Katekar and Geissler, 1980; Muday and Haworth, 1994). An important question is whether the two polarities of auxin movement in roots separately control these growth and developmental processes.
Lateral root development is highly dependent on auxin and auxin transport. Lateral roots originate in the root pericycle, in which individual quiescent cells are stimulated to dedifferentiate and proliferate to form the lateral root primordium (Blakely and Evans, 1979). Cells in the lateral root primordium differentiate and elongate, causing the lateral root to emerge through the primary root epidermis. Mature lateral roots structurally resemble the primary root and are themselves capable of producing new lateral roots, allowing for recursive branching and eventual development of a complex root system. Several lines of evidence indicate that auxin is necessary for the development of lateral roots. Application of IAA to growing plants stimulates lateral root development and lateral root elongation (Torrey, 1950; Blakely et al., 1982; Muday and Haworth, 1994). Conversely, growth of tomato roots on agar containing auxin-transport inhibitors, including NPA, decreases the number of lateral roots (Muday and Haworth, 1994). Natural variation in auxin transport may lead to differences in the development of lateral roots. Donaldson (1993) found a negative correlation between the degree of branching in root systems and the amount of NPA-binding activity present in roots in different species of plants (Lomax et al., 1995).
Genetic approaches have also established the connection between auxin and lateral root development. Mutants with reduced sensitivity to auxin exhibit reduction or loss of lateral roots. The tomato mutant diageotropica (dgt), isolated for its horizontal growth pattern, does not produce lateral roots (Zobel, 1974; Muday et al., 1995). This mutant appears to be reduced in auxin sensitivity in both the shoot (Kelly and Bradford, 1986) and the root (Muday et al., 1995). Like dgt, the dominant auxin-insensitive Arabidopsis mutant Dwf produces no lateral roots and displays no gravitropic response (Mirza et al., 1984). The Arabidopsis mutant aberrant lateral root formation-4 (alf-4) does not respond to exogenous auxin; this mutant also produces no lateral roots (Celenza et al., 1995). axr-1 and axr-2, which have reduced auxin sensitivities, produce fewer lateral roots than the wild type (Estelle and Somerville, 1987).
Conversely, increased lateral and adventitious rooting has been reported in plants with elevated auxin content. A transgenic tobacco plant transformed with a construct expressing bacterial auxin biosynthetic genes has a higher number of lateral roots (Sitbon et al., 1992). Extensive proliferation of adventitious and lateral roots in the mutant alf1-1, which is allelic to superroot and rooty, has been linked to elevated free IAA levels (Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995). Genetic approaches have also linked auxin transport to lateral root development. The tir3-1 mutant was isolated based on its reduced sensitivity to growth inhibition by auxin-transport inhibitors (Ruegger et al., 1997). This mutant has reduced polar auxin transport in floral inflorescences and fewer NPA-binding sites. This mutation also leads to a loss of lateral roots.
To fully understand how auxin controls lateral root development, knowledge of how auxin reaches its target tissues is necessary. An experiment in which radiolabeled IAA was applied to the shoot apical bud of pea seedlings found that radioactivity accumulates in lateral root primordia (Rowntree and Morris, 1979; Kerk and Feldman, 1995). In addition, removing the cotyledons and epicotyls from pea seedlings causes a decrease in the number of lateral root primordia, as well as in the percentage of primordia that develop into emergent lateral roots (Wightman et al., 1980). This excision-induced decrease in lateral root development can be partially rescued by applying IAA to the cut sites (McDavid et al., 1972; Hinchee and Rost, 1986). Localized application of auxin-transport inhibitors has been used to block one of the two polarities of auxin movement, but mixed results have been reported with this technique (McDavid et al., 1972; Hinchee and Rost, 1992). In experiments designed to prevent shoot-derived auxin from reaching the roots, auxin-transport inhibitors applied to pea plants at the base of the stem reduced lateral root development. However, these applications led to scorching and withering of the plant (McDavid et al., 1972), and with some auxin-transport inhibitors, the effect of inhibitor application was not position-specific (Hinchee and Rost, 1986).
To determine whether auxin from the shoot drives lateral root development in Arabidopsis wild-type plants, we prevented shoot-derived auxin from reaching the root through a variety of treatments in living Arabidopsis. These treatments were designed to block auxin transport with minimal damage to the plants. Lateral root inhibition by all of the treatments was reversible by localized application of IAA to the root-shoot junction.
MATERIALS AND METHODS
Chemicals and Radiochemicals
[2,3,4,5(n)-3H]NPA (58 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). NPA was from Chemical Services (West Chester, PA). ScintiVerse scintillation fluid, Triton X-100, and Suc were purchased from Fisher Scientific. Absolute ethanol was purchased from McCormick Distilling Co. (Weston, MO). All other chemicals were purchased from Sigma.
Seed Germination and Plant Growth
Wild-type Arabidopsis (ecotype Landsberg erecta) seeds were purchased from Lehle Seeds (Round Rock, TX). Seeds were soaked in distilled water for 30 min and surface sterilized with 95% ethanol for 5 min and 10% bleach with 0.01% Triton X-100 detergent for 5 min. After five washes in distilled water, seeds were germinated and grown on 9-cm Petri plates containing sterile control medium (0.8% agar [Sigma type M, plant tissue culture], 1× Murashige and Skoog salts, pH 6.0, 1.5% Suc, 1 μg mL−1 thiamine, 0.5 μg mL−1 pyridoxine HCl, 0.5 μg mL−1 nicotinic acid, and 50 μg mL−1 sterile filtered ampicillin). Seeds were grown in vertically oriented Petri dishes in continuous fluorescent light (94 μmol m−2 s−1) at room temperature (22°C) for 4 to 5 d, until cotyledons had emerged and roots were 1.0 to 1.5 cm long. For experiments in which plants were grown in the dark, Petri dishes were placed in light-tight boxes in the same room as light-grown plants to ensure consistent temperature.
Seeds were germinated on control agar and after growth for the indicated number of days, 10 seedlings were transferred to fresh plates containing control agar or agar-containing compounds at the indicated final concentrations. In experiments involving localized application of compounds, plants were grown on vertical control plates for an additional 3 to 6 d after application of compounds.
Preparation of Treatments in Agar
Control agar (0.8%), as described above, was supplemented with NPA, IAA, Lucifer Yellow, or Suc, and the agar was poured into Petri dishes or applied locally to plants under varying conditions. Compounds were added to cooled (50°C) molten control agar and poured into plates. NPA dissolved in DMSO was added to agar with a final DMSO concentration of 0.1%, and IAA dissolved in absolute ethanol was added to agar with a final ethanol concentration of 0.1%. Lucifer Yellow at 0.1% was dissolved in water and added to agar. Additional Suc was added to agar as a concentrated, sterile, filtered solution. In experiments using plates containing one-half control and one-half supplemented agar, sterile Petri plates divided into quadrants were used. Supplemented agar was poured into two adjacent quadrants, and control agar into the other two.
Treatments to Block Auxin Movements
In experiments involving localized application of compounds in agar, agar was allowed to harden in a sterile Pasteur pipette. The agar was dispensed directly from the pipette in 1- to 2-mm lines across the root-shoot junction. Lines of either control agar or agar containing NPA were applied to the root-shoot junction and 10 and 20 mm below the root-shoot junction. At the time of application, the roots were just over 20 mm long, so the lowest application of agar was at the root tip. The roots were then allowed to grow for the indicated period, and the density of lateral roots in each of the three zones was determined. These regions were designated zones 1, 2, and 3 for the areas between 0 and 10, 10 and 20, and 20 and 30 mm from the root-shoot junction, respectively.
In excision experiments the plants were cut with sterilized scissors and either the entire shoot at the root-shoot junction or the terminal 1 to 2 mm of the root tip were removed. After excision the plants were then grown on vertical plates, as indicated. In addition, the length of primary roots was measured using a ruler and dissecting microscope. The average number of lateral roots and the average length were calculated. The density of lateral roots along the primary root was calculated by dividing the number of lateral roots by the primary root length for each root. These paired values were then averaged and the se was calculated.
Diffusion Controls during Local Applications of Compounds
Diffusion of NPA from localized applications was measured by applying agar supplemented with 10 μm cold NPA and 5 nm [3H]NPA to the root-shoot junction of the seedlings. For each plate of 20 seedlings, the total volume of applied agar was approximately 2 mL. The plates were placed in a vertical position and the plants were allowed to grow for 3 d. The seedlings were removed from the plates and uniform samples of agar were taken, using a transfer pipette and gentle suction to cut and draw out cylindrical pieces of agar. Five samples were taken at the site of [3H]NPA agar application and every 1 cm down the plate. The radioactivity in each agar sample was determined by scintillation counting.
Uptake and diffusion of NPA into plants was measured by applying agar supplemented with 10 μm cold NPA and 8 nm [3H]NPA to the root-shoot junction of seedlings. The assay was performed using 20 seedlings per plate. The total volume of applied agar was approximately 2 mL. The plates were placed in a vertical position and the plants were allowed to grow for 2 or 8 d. Lateral root inhibition by localized NPA application was visible within 2 d. At 2 and 8 d, the seedlings were removed from the plates and any agar clinging to them was removed. The seedlings were cut into 1-cm sections, beginning at the root-shoot junction, with a razor blade. All of the sections that were at a constant distance from the root-shoot junction were combined and radioactivity was determined by scintillation counting.
Measurement of [3H]IAA Transport in Roots
To assess auxin-transport inhibition by NPA when applied locally to the root-shoot junction, 100 nm [3H]IAA was mixed with unlabeled IAA to reach a final IAA concentration of 10 μm. Unlabeled IAA was added, because IAA concentrations greater than 1 μm lead to significantly higher amounts of auxin transport (data not shown). Four-day-old seedlings were transferred to control plates, with 10 seedlings per plate. One percent agar blocks, which contained the [3H]IAA, were placed along the root-shoot junction of the intact plant, and agar blocks with or without 100 μm NPA were placed directly below them. The plates were oriented vertically, with the plants inverted, so the roots were above the shoots; thus, transport could be differentiated from diffusion or wicking along the surface in the direction of gravity. IAA transport was measured after 24 h by cutting the roots 2 mm below the NPA block and placing the roots into 2.5 mL of scintillation fluid (five roots per vial). Radioactivity in the samples was determined by scintillation counting with a LKB-Wallac counter (Wallac, Inc., Gaithersburg, MD) for 2 min.
Quantification of Endogenous Free IAA Levels
Endogenous free IAA levels in roots of Arabidopsis seedlings were determined after application of agar with or without 10 μm NPA to the root-shoot junction. All of the plants treated with NPA had qualitative reductions in numbers of lateral roots, compared with the controls. After treatment, roots were harvested from 60 to 120 plants, the fresh weight was determined, and the samples were frozen in liquid nitrogen and stored at −70°C.
Free IAA was purified and quantified using the procedure of Chen et al. (1988) in the laboratory of Dr. Jerry Cohen (USDA, Beltsville, MD). Between 50 and 100 mg of Arabidopsis root tissue was used for isolation of free IAA. Tissue was frozen in liquid nitrogen and ground with a mortar and pestle using IAA extraction buffer (65% isopropanol and 35% 0.2 m imidazole buffer, pH 7.0). [13C]IAA was used as an internal standard with either 25 ng/g fresh weight of tissue or with 20 ng total. [3H]IAA was used as a radiotracer using 50,000 dpm for each sample. Samples were extracted for 1 h at 4°C and the extract was centrifuged at 10,000g for 5 min. The supernatant fluid was then analyzed for free IAA. IAA was purified by an amino column (Prep Sep, Fisher Scientific) with several organic washes and eluted in methanol with 5% acetic acid. After concentration, the sample was purified by HPLC, methylated using ethereal diazomethane, and then analyzed by GC-SIM-MS. The GC-SIM-MS was used for selected ion measurements to quantify the free IAA concentrations in the root extracts relative to the [13C]IAA internal standard.
RESULTS AND DISCUSSION
Localized Application of NPA at the Root-Shoot Junction
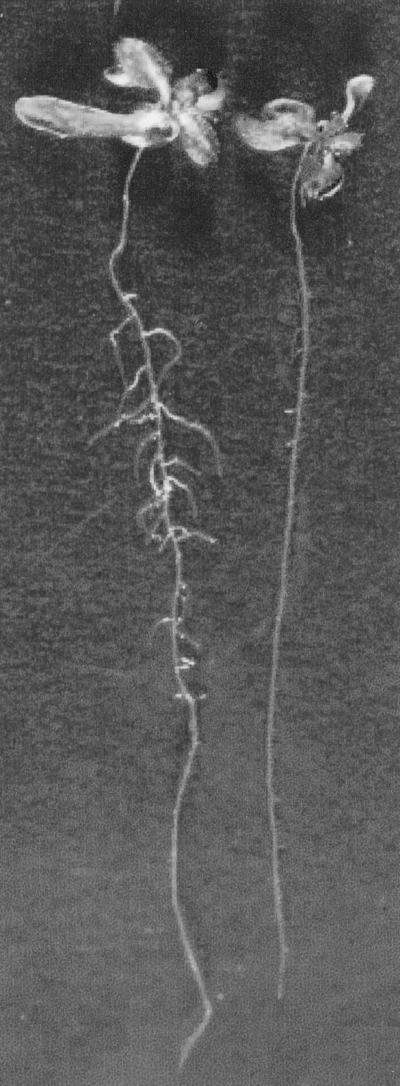
The effect of local application of NPA concentrations ranging from 5 to 100 μm on lateral root development is visible in Figure 1 and quantified in Table I. There was a dose-dependent decrease in the number of lateral roots with increasing concentration of NPA, with an almost complete inhibition at the highest NPA concentration. In contrast, growth was reduced less than 2-fold at the highest NPA concentration. The density of lateral roots was also calculated by dividing the number of lateral roots by the length to normalize for the effects of the treatment on length. Alone, the average number of lateral roots per plant may be misleading in some cases because the average primary root length varied according to treatments, allowing various lengths of root along which lateral roots can form. In this case, lateral root density decreased parallel to the decrease in the number of lateral roots.
Figure 1.
Comparison of lateral root development with localized application of control agar (left) or agar containing 100 μm NPA (right) to the root-shoot junction. Roots were grown for 4 d after application of agar.
Table I.
Effects of localized NPA application on lateral root development and root length
| [NPA] | Lateral Root No. | Root Length | Lateral Root Densitya |
|---|---|---|---|
| μm | mm | ||
| 0.0 | 15.9 ± 1.0 | 48.6 ± 1.4 | 0.33 ± 0.02 |
| 5.0 | 12.3 ± 0.8 | 47.2 ± 0.7 | 0.26 ± 0.02b |
| 10.0 | 10.4 ± 1.1 | 45.9 ± 1.1 | 0.23 ± 0.02b |
| 20.0 | 8.6 ± 0.7 | 39.0 ± 1.7 | 0.22 ± 0.20b |
| 50.0 | 6.4 ± 0.6 | 45.0 ± 0.9 | 0.14 ± 0.02b |
| 100.0 | 1.0 ± 0.3 | 38.7 ± 1.7 | 0.03 ± 0.00b |
Four-day-old seedlings were treated by application of agar containing NPA at the indicated concentrations to the root-shoot junction. After 6 additional d of growth, lateral roots were counted and the length of the primary roots was measured. The reported values are averages ± se of 10 plants.
The lateral root density was calculated by dividing the number of lateral roots by the primary root length for each plant, and is reported as the number of lateral roots per millimeter of primary root. The average ± se of the lateral root density calculated for each root is reported.
Lateral root density was significantly reduced by all concentrations of NPA (P ≤ 0.007).
To verify that NPA application at the root-shoot junction acted to reduce auxin movement into the roots, two approaches were used. First, endogenous levels of free IAA were measured after treatment with 10 μm NPA or control agar and compared with changes in lateral root number under similar conditions. The number of lateral roots and the levels of free IAA, as measured by GC-SIM-MS, were reduced in roots treated with NPA relative to those in control roots, as shown in Table II. Free IAA levels were 28% lower in samples treated with NPA. This reduction is statistically significant, as determined by a one-tailed Mann-Whitney U test (P = 0.04). This measurement reflects the differences in free IAA in the entire root, and there may be greater differences in cells and tissues of the root that form lateral roots not detectable by this analysis.
Table II.
Effects of localized NPA application at the root-shoot junction on free IAA concentration
| Treatment | Free IAA Concentrationa | Lateral Root No.b |
|---|---|---|
| ng/g fresh wt | ||
| Control agar | 25.8 ± 4.7 | 15.5 ± 0.8 |
| NPA agar | 17.6 ± 2.2 | 5.9 ± 0.5 |
Seven- or 8-d-old seedlings were treated by application of agar with or without NPA to the root-shoot junction. After 3 additional d of growth, roots were harvested and the free IAA concentration was determined by GC-SIM-MS or the number of lateral roots was determined.
The reduction in free IAA concentration was statistically significant using a Mann-Whitney U test (P = 0.04).
The reduction in lateral root number was statistically significant using Student's t test (P ≤ 0.001).
The second approach to demonstrate that application of NPA at the root-shoot junction reduced IAA movement was to measure [3H]IAA transport into the root from an application site at the root-shoot junction. The results from samples treated with or without NPA below the site of [3H]IAA application are shown in Table III. After 24 h there was 25% less [3H]IAA transport into roots treated with NPA, compared with controls. IAA-transport measurements with and without NPA show a statistically significant reduction in IAA movement, as determined by a Mann-Whitney U test (P = 0.04).
Table III.
Effects of localized NPA application on [3H]IAA movement
| Treatment | [3H]IAA Transported | |
|---|---|---|
| cpm | fmol | |
| Control agar | 1501 ± 175 | 61.4 ± 7.1 |
| NPA agar | 1124 ± 52 | 46.0 ± 2.1 |
Agar blocks containing [3H]IAA were applied to 4-d-old seedlings with and without NPA-containing blocks, and radioactivity in the roots was determined after 24 h. The reported values are averages ± se of four replicates, each containing five plants.
Using a Mann-Whitney U test, the differences in IAA are statistically significant (P ≤ 0.04).
To verify that NPA remained localized during these treatments, 10 μm NPA containing [3H]NPA was applied to seedlings in agar at the root-shoot junction. Samples of agar at the site of application and along the length of the plate were analyzed by liquid scintillation counting. Less than 10% of the tritiated NPA diffused into the agar at any point on the plate during a 3-d period (data not shown). Seedlings were also sectioned and analyzed by liquid scintillation counting after application of tritiated NPA to the root-shoot junction. Of the tritiated NPA that was taken up by the plants, 69% of the radioactivity was recovered in agar within 1 cm of the site of application 2 d after application and 47% remained within 1 cm of the site of application after 8 d. This indicates limited diffusion of NPA from the root-shoot junction to other areas of the plant. As another test of diffusion, the fluorescent dye Lucifer Yellow, used at 0.1%, was applied in agar at the root-shoot junction. Under fluorescence microscopy the dye could be visualized in plants no farther than 1 cm from the point of application. Substances applied in agar in this way appear to have limited rates of bulk transport and diffusion, and do not appear to wick along the surface of the roots. The effects of NPA applied at the root-shoot junction are therefore consistent with localized inhibition of auxin transport.
Effect of Position of NPA Application
Because elongating root tips tend to grow out from under a line of applied agar, two other approaches were used to examine the effect of local application of NPA to other positions on the root. In the first approach NPA was applied to either the apical or the basal halves of the root by growth in Petri dishes containing 10 μm NPA in one half and containing control agar in the other half. Although this approach led to a broader application of NPA, it did allow NPA to remain in continuous contact with the root tip and to be in contact with only part of the root. After transfer of plants to half-plates so that root tips were just below the middle line, the plates were placed in a vertical position in the light. When applied to the upper half of the root, NPA inhibited lateral root development in all parts of the root, relative to controls, as shown in Table IV. Almost no lateral roots formed on the lower half of these roots, although they were not in contact with the NPA. Conversely, application of NPA to the lower root half had no statistically significant effect on lateral root number or density in the upper half of the root, although it did significantly reduce lateral root number in the lower half. Thus, whereas NPA always has a local effect on the root tissue it contacts, NPA can only influence lateral root development at distant sites when it is in contact with the basal part of the root. This result is consistent with the direction of auxin movement controlling lateral root development moving from the shoot toward the root tip.
Table IV.
Effects of application of NPA to the upper and lower halves of seedling roots
| Agar | Lateral Root No. | Lateral Root Densitya | Change in Lengthb |
|---|---|---|---|
| mm | |||
| Controlc | 4.4 ± 0.6 | 0.46 ± 0.07 | |
| Controld | 5.3 ± 1.1 | 0.14 ± 0.03 | 30.2 ± 1.0 |
| NPAc | 1.7 ± 0.5 | 0.18 ± 0.05e | |
| Controld | 0.1 ± 0.1 | 0.00 ± 0.00e | 23.5 ± 1.0 |
| Controlc | 4.4 ± 0.5 | 0.49 ± 0.06 | |
| NPAd | 1.3 ± 0.6 | 0.05 ± 0.02e | 12.1 ± 0.7 |
Six-day-old seedlings were transferred to plates containing 10 μm NPA in the upper or lower half, as indicated. After 4 additional d of growth in the light, lateral roots in the upper and lower halves were counted and new primary root growth was measured. The reported values are averages ± se of 10 plants.
The lateral root density was calculated by dividing the number of lateral roots by the primary root length for each plant, and is reported as the number of lateral roots per millimeter of primary root.
The amount of elongation of the primary root after transfer to the indicated plates.
The compound in agar and the lateral root growth on the upper half of the plate is shown.
The compound in agar and the lateral root growth on the lower half of the plate is shown.
Lateral root density decreased significantly (P ≤ 0.002) as judged by Student's t test.
New root growth, measured as the change in primary root length after transfer to new plates, was also determined in these experiments. Plants treated with NPA in the upper half of the plate showed a modest 22% inhibition of elongation, whereas those treated with NPA in the lower half were inhibited by 60% (Table IV). Although both NPA treatments inhibited elongation, application of NPA at the root tip had a more profound inhibitory effect, suggesting a role for auxin originating near the root tip in root elongation. NPA application to the lower half of the root also reduced the gravitropic growth of the seedlings (data not shown). In contrast, application of NPA to the upper half of the root or to the root-shoot junction did not affect the gravitropic response.
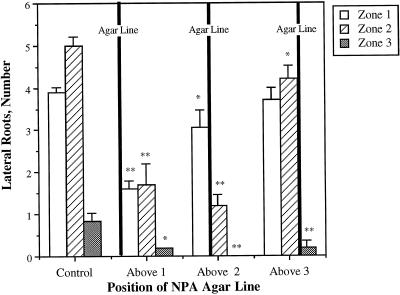
In the second approach, used to examine the effects of NPA applied to the root tip, lines of either control agar or agar containing 10 μm NPA were applied to the root-shoot junction and to positions 10 and 20 mm below the root-shoot junction. At the time of application, the roots were just over 20 mm long, so the most apical application of agar was at the root tip. The roots were then allowed to grow for 3 d and the density of lateral roots in each of three zones was determined. These regions were designated zones 1, 2, and 3 for the area between 0 and 10, 10 and 20, and 20 and 30 mm from the root-shoot junction, respectively. The results of this experiment are shown in Figure 2, and the site of application of NPA relative to the zone of measurement is indicated. In all cases there is a statistically significant reduction in lateral roots in regions apical to the site of application of NPA, with a range of 60% to 83% for lateral root inhibition. When the effect on lateral roots in regions basal to the site of application is examined, there are slight decreases in lateral root number, ranging from 5% to 22% inhibition. These data are consistent with auxin moving toward the tip controlling lateral root development.
Figure 2.
The effect of NPA application at several positions on the Arabidopsis primary root. Five-day-old Arabidopsis seedlings were treated with a line of agar containing 100 μm NPA at several positions along the root. The root was divided into 10-mm zones, with zone 1 beginning at the root-shoot junction. The lines of agar were placed above zone 1, 2, or 3, as indicated. The number and density of lateral roots were determined after 3 d of growth. The data were analyzed by Student's t test, and P values were determined for each sample compared with the controls and are indicated with asterisks (*P ≥ 0.015 and ≤ 0.038; **P ≤ 0.005).
Rescue of NPA Inhibition of Lateral Roots with Exogenous Auxin
Double lines of control agar and agar containing 100 μm NPA or 10 μm IAA were applied in different combinations to the root-shoot junction of seedlings. The number and density of lateral roots were determined, as shown in Table V. IAA application led to a greater than 2-fold, statistically significant (P < 0.0001) increase in lateral root density, whereas NPA application resulted in a statistically significant 10-fold decrease (P < 0.0001) in the lateral root density relative to control agar alone.
Table V.
Reversal of NPA-induced lateral root inhibition by localized IAA application
| Agara
|
Lateral Root No. | Lateral Root Density | |
|---|---|---|---|
| Upper | Lower | ||
| Control | Control | 6.7 ± 0.85 | 0.19 ± 0.02 |
| IAA | Control | 10.0 ± 0.56 | 0.43 ± 0.03 |
| Control | NPA | 0.33 ± 0.14 | 0.02 ± 0.01 |
| NPA | IAA | 4.25 ± 0.73 | 0.24 ± 0.04 |
| IAA | NPA | 1.72 ± 0.46 | 0.08 ± 0.02 |
After the indicated treatments, lateral root number and root length were determined after 4 d of additional growth in the light. The reported values are averages ± se of 10 plants.
Two, thin (1-mm), parallel lines of agar either with or without 10 μm IAA and 100 μm NPA were applied to the root-shoot junction of seedlings that were 4 d old. This technique allowed for the simultaneous application of two compounds to seedlings. Although both lines were over the root-shoot junction of the seedlings, one line was closer to the plant shoot (the Upper line) and the other was closer to the root (the Lower line).
When IAA and NPA were applied together in double lines to the root-shoot junction, the result depended on the placement of the two compounds. When IAA was applied apical to NPA, IAA reversed the inhibitory effect of NPA on lateral root development, as shown in Table V. These plants produced 14-fold more lateral roots than plants treated with NPA alone, and the increase in lateral root density was statistically significant (P < 0.0001). Therefore, plants apparently take up and transport the exogenously applied auxin below the site of local NPA inhibition, leading to a partial rescue of lateral root development. Conversely, when NPA was applied below IAA, the number and density of lateral roots were inhibited 6- and 5-fold, respectively, relative to plants treated with IAA alone (Table V). This was a statistically significant decrease in lateral root density (P = 0.0006). This NPA-mediated inhibition of lateral roots is not as complete when exogenous IAA is applied as when NPA is applied alone, presumably because NPA cannot completely block the movement of high levels of exogenous auxin.
The amount of IAA taken up by roots when the compound is applied at the root-shoot junction can be estimated by examination of the measurement of [3H]IAA transport in Table III. The average weight of an Arabidopsis root of the age in this analysis is approximately 0.6 mg (determined from the harvest of many plants for GC-SIM-MS analysis). Conversion of the femtomole values in Table III to picomoles per gram fresh weight suggests that radiolabeled IAA reaches a concentration of 3.5 ng/g fresh weight in the root after 24 h of application. Roots of this age had 26 ng/g fresh weight free IAA distributed throughout the entire root, so it is conceivable that several days of treatment with exogenous IAA led to physiologically significant changes in IAA levels in the pericycle cells in which lateral root initiation occurs.
Localized IAA Application
Table VI shows that when the basal half of the root was in contact with agar containing 0.1 μm IAA, there was a greater than 1.5-fold increase in number and density of lateral roots in both the upper and lower root halves, compared with plants on control agar. The increase in lateral root density was significant only in the lower half of these plants (P = 0.004). In contrast, exposure of the apical end of the root to IAA significantly increased the number and density of lateral roots 2-fold in the region of the root in contact with IAA (P ≤ 0.002 for root densities). The effect of IAA applied at the apical end of the root appeared to be local, in that lateral root number and density on the base of the root, which was in contact with control agar, did not differ significantly from the controls. Application of IAA using half-plates was less precise and presumably allowed loading of more IAA than the local application of IAA with lines of agar. The results are consistent with auxin transport from the shoot toward the root tip playing a controlling role in lateral root development and the opposite polarity of auxin movement not playing a role in regulation of this process.
Table VI.
Effects of application of IAA to the upper and lower halves of roots
| Agar | Lateral Root No. | Lateral Root Densitya | Change in Lengthb |
|---|---|---|---|
| mm | |||
| Controlc | 4.6 ± 0.4 | 0.62 ± 0.06 | |
| Controld | 5.3 ± 0.9 | 0.14 ± 0.02 | 43.7 ± 1.8 |
| IAAc | 7.2 ± 0.5 | 0.98 ± 0.27e | |
| Controld | 9.1 ± 1.8 | 0.33 ± 0.06f | 37.8 ± 1.8 |
| Controlc | 4.3 ± 0.4 | 0.47 ± 0.06e | |
| IAAd | 10.1 ± 0.9 | 0.30 ± 0.03f | 42.4 ± 2.5 |
Six-day-old seedlings were transferred to plates containing 0.1 μm IAA in the upper or lower half, as indicated. After 4 additional d of growth in the light, lateral roots in the upper and lower halves were counted and new primary root growth was measured. The reported values are averages ± se of 10 plants.
The lateral root density was calculated by dividing the number of lateral roots by the primary root length for each plant, and is reported as the number of lateral roots per millimeter of primary root.
The amount of elongation of the primary root after transfer to the indicated plates.
The compound in agar and the lateral root development on the upper half of the plate is shown.
The compound in agar and the lateral root development on the lower half of the plate is shown.
These values are not statistically different from the control values (P ≥ 0.06).
These values are statistically different from the control values (P ≤ 0.004).
Shoot and Root-Tip Excision
Table VII shows the effects of shoot and root-tip excision of seedlings on the number and density of lateral roots, measured 3 d after excision and growth in continuous light. Shoot excision caused a statistically significant (P < 0.0001) greater than 4-fold decrease in both the total number of lateral roots and the lateral root density, compared with intact plants (Table VII). Root-tip excision did not appear to disrupt the capacity of seedlings to form lateral roots. In fact, removing the root tip caused a statistically significant (P = 0.001) increase in the lateral root density. This is consistent with previous reports of lateral root stimulation by root-tip excision in pea seedlings (Wightman and Thimann, 1980). The effect of root-tip excision suggests that wounding responses from excision do not inhibit lateral root development, and that the root tip is not necessary for lateral root development. However, a substance coming from the shoot, presumably auxin, does appear to be necessary for lateral root development.
Table VII.
Effects of excision and localized IAA application on lateral root development
| Treatment | [IAA] | Lateral Root No. | Root Length | Lateral Root Densitya |
|---|---|---|---|---|
| μm | mm | |||
| Intact plantsb | 0.0 | 28.6 ± 2.3 | 51.9 ± 3.1 | 0.56 ± 0.04 |
| Shoot excisedb | 0.0 | 6.4 ± 0.4 | 45.7 ± 1.9 | 0.14 ± 0.01 |
| Root tip excisedb | 0.0 | 20.8 ± 0.8 | 27.7 ± 0.8 | 0.76 ± 0.03 |
| Shoot excisedc | 0.0 | 6.0 ± 1.0 | 42.4 ± 2.1 | 0.14 ± 0.02 |
| Shoot excised | 0.1 | 6.3 ± 0.9 | 46.7 ± 2.0 | 0.13 ± 0.02 |
| Shoot excised | 1.0 | 9.3 ± 1.2 | 43.5 ± 1.6 | 0.21 ± 0.03 |
| Shoot excised | 10.0 | 13.1 ± 1.3 | 36.7 ± 1.5 | 0.36 ± 0.03 |
Plants were treated by shoot or root-tip excision and application of agar containing IAA at the indicated concentrations to the root-shoot junction. After 3 additional d of growth, lateral roots were counted and the length of the primary roots was measured. The reported values are averages ± se of 10 plants.
The lateral root density was calculated by dividing the number of lateral roots by the primary root length for each plant, and is reported as the number of lateral roots per millimeter of primary root.
Five-day-old seedlings were treated and grown for 3 additional d in the light.
Eight-day-old seedlings were treated and grown for 3 additional d in the dark.
The apical 0.5 to 1.0 mm of the primary root, which included the root cap, root tip, and part or all of the elongation zone, was removed, leading to complete cessation of elongation of the remaining root. In contrast, both the control roots and the shoot-excised roots elongated throughout the growth period. The continued growth of roots from shoot-excised plants demonstrates that excision and any subsequent wounding response did not kill roots or prevent root growth. Removal of the shoot did slightly reduce elongation of the primary root, suggesting that either auxin or nutrients required for growth were depleted. Because Suc did not reverse this elongation inhibition by removal of the shoot (data not shown), the effect is not solely at the level of nutrient limitation.
To determine whether auxin may be the substance transported from the shoot that induces lateral roots, lateral root development in plants was inhibited by shoot excision. Then, agar containing IAA at concentrations from 0.1 to 10.0 μm was applied to the cut surfaces of shoot-excised plants. This experiment was performed in the dark to reduce light-induced IAA breakdown. IAA at the concentrations tested had a dose-dependent, stimulatory effect on the number of lateral roots formed by plants with the shoot excised (Table VII), although even at the highest concentration complete rescue was not possible. The increases in lateral roots were statistically significant at concentrations of 1 and 10 μm (P = 0.035 and P < 0.00001, respectively).
Dark Growth
Dark-grown seedlings formed fewer lateral roots than light-grown seedlings, as shown in Table VIII and as reported previously by Jensen et al. (1998). Auxin was applied to the root-shoot junction of whole seedlings at concentrations from 0.1 to 10 μm. IAA stimulated the development of lateral roots in dark-grown plants relative to plants treated with control agar. Table VIII shows a dose-dependent relationship between the concentration of IAA applied and the number of lateral roots formed. IAA at 1 μm, applied at the root-shoot junction, was sufficient to stimulate significant increases in lateral root development (P ≤ 0.0008), although greater number and density of lateral roots were obtained by application of 10 μm IAA. IAA application also increased the number and density of lateral roots in light-grown seedlings, but the magnitude of the effect was much less (Table V). This result is consistent with dark growth reducing the concentration of IAA that reaches the root, so that roots require exogenous IAA to form lateral roots.
Table VIII.
Effects of localized IAA application on lateral root development in dark-grown plants
| [IAA] | Lateral Root No. | Root Length | Lateral Root Densitya |
|---|---|---|---|
| μm | mm | ||
| 0.0 | 0.1 ± 0.1 | 33.5 ± 2.5 | 0.00 ± 0.00 |
| 0.1 | 0.7 ± 0.4 | 31.7 ± 1.4 | 0.02 ± 0.01 |
| 1.0 | 2.1 ± 0.4 | 27.6 ± 4.1 | 0.09 ± 0.02b |
| 5.0 | 6.0 ± 0.5 | 15.4 ± 0.8 | 0.40 ± 0.02b |
| 10.0 | 9.3 ± 0.7 | 13.0 ± 0.6 | 0.70 ± 0.07b |
Seven-day-old seedlings were treated by application of agar containing IAA at the indicated concentrations to the root-shoot junction. After 3 additional d of growth, lateral roots were counted and the length of the primary roots was measured. The reported values are averages ± se of 10 plants.
The lateral root density was calculated by dividing the number of lateral roots by the primary root length for each plant, and is reported as the number of lateral roots per millimeter of primary root.
The increase in lateral root density was statistically significant, as judged by Student's t test (P ≤ 0.0008).
Several lines of evidence suggest that the inhibition of lateral root development by dark growth is not solely at the level of carbon availability attributable to photosynthesis. Although Suc partially reverses the dark inhibition of lateral root development, auxin is more effective at reversing the effect. Plants on high-Suc agar plates (7.5% Suc, compared with control plates, which contained 1.5% Suc) produced an average of 4.5 ± 0.6 lateral roots after 5 d in the dark, compared with plants on control plates that formed no lateral roots (data not shown). When plants are grown in the dark on agar containing IAA and extra Suc, the effect of IAA seems to mask that of Suc. In the dark, plants grown on agar containing a high-Suc concentration (7.5% Suc) and IAA applied at the root-shoot junction have an average number of lateral roots that was not significantly different from that of plants treated with IAA alone (data not shown). It should be noted that this high level of Suc may cause additional alterations in plant growth through the action of sugars as signaling molecules (Jang and Sheen, 1997). High levels of Glc (up to 6%) alter the root growth and development of light-grown Arabidopsis plants (Jang et al., 1997), although Suc is much less potent in its effect than Glc (Jang and Sheen, 1997). Also, growth of Arabidopsis plants in the light on agar containing norflurazon, which inhibits photosynthesis, reduces but does not abolish lateral root development (M.-R. Cha and R. Hangarter, personal communication). This result suggests that dark growth prevents the movement of a substance from the shoot into the root, other than photosynthate, which mediates lateral root development. These results are consistent with the auxin signal being an important determinant of lateral root development, although carbon levels also play a role in this process.
CONCLUSIONS
This study examined whether lateral root development depends on specific, directional auxin transport in vivo. Disruption of polar auxin transport from the shoot through a variety of treatments resulted in inhibition of lateral roots. The effectiveness of the most potent and specific of these treatments, application of NPA to the root-shoot junction, was verified in two ways. Both free IAA levels and [3H]IAA transport into the root were reduced by local NPA application to the root-shoot junction. All of the treatments that reduced lateral root development were reversible by application of IAA to the root-shoot junction. In contrast, auxin movement from the root tip does not appear to be necessary for lateral root development. Two Arabidopsis mutants, agr1 and eir1, which are altered in root gravity response and may have reduced auxin transport specifically in the root, have normal lateral root development (Luschnig et al., 1998; R. Chen and P. Masson, personal communication). In contrast, the tir3 mutant has a defect in auxin transport in the inflorescence stem and does not form any lateral roots (Ruegger et al., 1997). These results are consistent with auxin transport from the shoot into the root being the sole source of the auxin required for lateral root development in Arabidopsis.
Why should the direction of auxin transport matter to the responding tissues? Perhaps the simplest explanation for this phenomenon is that acropetal and basipetal polar auxin transport occur in different root tissues. Whereas auxin moves basipetally in the epidermis, acropetal transport occurs in the central cylinder (Mitchell and Davies, 1975; Tsurumi and Ohwaki, 1978). Lateral roots originate in the pericycle, the ring of cells closest to the central cylinder (Schiefelbein and Benfey, 1994), and auxin moving acropetally is found in tissues with close proximity to the pericycle (Mitchell and Davies, 1975). Auxin moving through the central cylinder may have significantly better access to the pericycle than auxin moving through the epidermis, which is at least three cell layers removed from the pericycle in Arabidopsis (Schiefelbein and Benfey, 1994).
Polar auxin transport from the shoot into the root may be a means by which root and shoot developmental programs can be coordinated in response to environmental stimuli. The shoot is much more exposed than the root to variables such as light levels. Experimental evidence indicates that environmental variables, including light (Behringer and Davies, 1992) and production of ethylene (Suttle, 1988), can influence auxin movement in shoots. Therefore, regulation of transport of shoot-derived auxin into the root may be a mechanism by which environmental changes sensed by the shoot can be communicated to control root growth and development. These experiments clearly demonstrate that dark growth reduces lateral root development. Jensen et al. (1998) reported that NPA inhibits elongation of light-grown but not dark-grown hypocotyls, indicating changes in auxin transport or its regulation by NPA under different light conditions.
If auxin moving from the shoot into the root controls lateral root development, then what is the function of auxin moving from the tip toward the root base? Root elongation and gravity response have also been shown to be blocked by auxin-transport inhibitors (Katekar and Geissler, 1980; Muday and Haworth, 1994) and reduced in mutants proposed to be altered in auxin transport (Lushnig et al., 1998). Treatments parallel to those in this work indicate that inhibition of auxin moving from the tip toward the base will specifically block elongation and gravity response (S.R. Brady, R.C. Reed, and G. Muday, unpublished results). Therefore, these two distinct polarities of auxin movement may control different root growth and developmental processes.
ACKNOWLEDGMENTS
We appreciate Jerry Cohen's generosity in allowing us to conduct the GC-SIM-MS measurements of free IAA in his laboratory, through support from the U.S. Department of Energy (grant no. DE-AI02-94-ER20153). We thank Brian Tague for helpful advice and Roger Hangarter for discussion in preparation of the manuscript. We also appreciate Dave Anderson's assistance with the statistical analyses.
Abbreviations:
- GC-SIM-MS
gas chromatography-single ion monitoring-mass spectroscopy
- NPA
naphthylphthalamic acid
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAGW 4052 to G.K.M.) and a Sigma Xi Grant-in-Aid of Research to R.C.R.
LITERATURE CITED
- Behringer FJ, Davies PJ. Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark- and light-grown Pisum seedlings. Planta. 1992;188:85–92. doi: 10.1007/BF00198943. [DOI] [PubMed] [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Bot Gaz. 1982;143:341–352. [Google Scholar]
- Blakely LM, Evans TA. Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci Lett. 1979;14:79–83. [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Monatgu M, Inze D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, McHale NA. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development. 1996;122:1811–1819. doi: 10.1242/dev.122.6.1811. [DOI] [PubMed] [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Chen K-H, Miller AN, Patterson GW, Cohen JD. A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol. 1988;86:822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke TJ, Racusen RH, Cohen JD. The role of auxin in plant embryogenesis. Plant Cell. 1993;5:1494–1495. doi: 10.1105/tpc.5.11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AW (1993). Auxin transport and root development in different plant species: is there a correlation? MS thesis. Wake Forest University, Winston-Salem, NC
- Estelle M, Somerville C. Auxin-resistant mutants of Arabidopsisthaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Feldman LJ. Auxin biosynthesis and metabolism in isolated roots of Zeamays. Physiol Plant. 1980;49:145–150. [Google Scholar]
- Hinchee MAW, Rost TL. The control of lateral root development in cultured pea seedlings. I. The role of seedling organs and plant growth regulators. Bot Gaz. 1986;147:137–147. [Google Scholar]
- Hinchee MAW, Rost TL. The control of lateral root development in cultured pea seedlings. II. Root fasciation induced by auxin inhibitors. Bot Acta. 1992;105:121–126. [Google Scholar]
- Jang J-C, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in high plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;116:455–462. doi: 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar GF, Geissler AE. Auxin transport inhibitors. IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors, the phytotropins. Plant Physiol. 1980;66:1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MO, Bradford KJ. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol. 1986;82:713–717. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk NM, Feldman LJ. A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development. 1995;121:2825–2833. [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDavid CR, Sagar GR, Marshall C. The effect of auxin from the shoot on root development in Pisum sativum L. New Phytol. 1972;71:1027–1032. [Google Scholar]
- Mirza JI, Olsen GM, Iversen T-H, Maher EP. The growth and gravitropic responses of wild-type and auxin-resistant mutants of Arabidopsisthaliana. Physiol Plant. 1984;60:516–522. doi: 10.1111/j.1399-3054.1984.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Mitchell EK, Davies PJ. Evidence for three different systems of movement of indoleacetic acid in intact roots of Phaseolus coccineus. Physiol Plant. 1975;33:290–294. doi: 10.1007/BF00385573. [DOI] [PubMed] [Google Scholar]
- Muday GK, Haworth P. Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiol Biochem. 1994;32:193–203. [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- Rowntree RA, Morris DA. Accumulation of 14C from exogenous labelled auxin in lateral root primordia of intact pea seedlings (Pisum sativum L.) Planta. 1979;144:463–466. doi: 10.1007/BF00380123. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Benfey PN. Root development in Arabidopsis. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 335–353. [Google Scholar]
- Sitbon F, Hennion S, Sundberg B, Little CHA, Olsson O, Sandberg G. Transgenic tobacco plants coexpressing the Agrobacterium tumefaciens iaaM and iaaH genes display altered growth and indoleacetic acid metabolism. Plant Physiol. 1992;99:1062–1069. doi: 10.1104/pp.99.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC. Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 1988;88:795–799. doi: 10.1104/pp.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey JG. The induction of lateral roots by indoleacetic acid and root decapitation. Am J Bot. 1950;37:257–264. [Google Scholar]
- Tsurumi S, Ohwaki Y. Transport of 14C-labeled indoleacetic acid in Vicia root segments. Plant Cell Physiol. 1978;19:1195–1206. [Google Scholar]
- Wightman F, Thimann KV. Hormonal factors controlling the initiation and development of lateral roots. I. Sources of primordia-inducing substances in the primary root of pea seedlings. Physiol Plant. 1980;49:13–20. [Google Scholar]
- Zobel RW. Control of morphogenesis in ethylene-requiring tomato mutant, diageotropica. Can J Bot. 1974;52:735–741. [Google Scholar]