Abstract
The purpose of this study was to test the validity of the SenseWear Pro Armband (SWA) for the measurement of energy expenditure (EE) and step count against a criterion in persons with stroke. Twelve participants with chronic stroke (mean age 64.2 ± 10.4 years; mean gait speed 0.67 ± 0.25 m/sec) completed two trials of a six-minute walk test, while wearing a SenseWear Armband (SWA) on each arm and being continuously monitored using a portable metabolic cart. Agreement between estimates of energy expenditure from the SWA and the metabolic cart was fair for the armband on the hemiplegic arm (intraclass correlation cefficient (ICC) = 0.586) and good for the armband on the unaffected arm (ICC = 0.702). Agreement between the SWA estimate of step count, and step count as measured by the Step Activity Monitor was poor (ICC < 0.352), with significant underestimation by the SWA. Our results show that, for these moderately impaired persons with stroke, the SWA should be used with caution for the measurement of energy expenditure and should not be used to measure step count.
1. Introduction
Higher levels of physical activity energy expenditure (EE) are associated with reduced mortality and improvement in risks factors for chronic diseases such as heart disease or diabetes [1]. Maintenance of a 150
minutes/week of moderate intensity physical activity EE in older adults is associated with better functional outcomes [2]. In persons with stroke, exercise interventions show that coronary disease risk factors such as blood pressure and total cholesterol can be reduced with exercise training [3, 4], but that functional gains are lost without maintenance of activity [5]. Those with stroke are 2.6 times less likely to expend 1000 kcals per week than someone without a chronic condition [6]. Few meet activity guidelines, and time spent sedentary is greater than 80% of the day [7], which is significantly greater than sedentary time of their peers [8, 9]. Low levels of physical activity and fitness after discharge back to the community [10] have increased recognition of the need for community physical activity programming for persons with stroke [11, 12].
Accurate measuring devices for both physical activity EE and step count are important as a means to provide feedback about physical activity outcomes, before, during, and after a community based physical activity intervention. Additionally, persons with stroke expend more energy than their peers for everyday activities such as walking [13] and a device that can measure EE accurately may potentially be used to determine if exercise and activity programs lead to beneficial reductions in EE for functional activities. The SenseWear Pro ArmBand (SWA) (Body Media, Pittsburgh, PA, USA) uses multiple sensors including heat flux (heat dissipated from body), galvanic skin response (estimate of skin conductivity), skin temperature, and a two-axis accelerometer to provide estimates of EE and posture (lying or not lying) [14]. Information from the accelerometers about body position helps to predict user context (e.g., standing, walking, biking) which reportedly improves the accuracy of EE algorithms [15]. The use of information from multiple sensors makes the SWA a potentially more accurate measurement tool for assessing EE than accelerometry alone.
Energy expenditure studies testing the SWA show that they provide valid estimates of activity EE in comparison with doubly labeled water [16, 17]. A recent study with healthy individuals showed that agreement between the SenseWear Pro3 Armband and the SenseWear Mini with doubly labeled water was good (ICC = 0.80 and 0.85 resp.) [17]. The SWA appears to be sensitive to changes in EE [18, 19], but at higher EE the device generally underestimates EE [17, 18, 20]. The validity of the SWA has been tested with clinical populations including individuals with coronary heart disease, chronic obstructive pulmonary disease, cystic fibrosis, and arthritis [18, 19, 21, 22]. In two studies with individuals with chronic obstructive pulmonary disease, agreement between the SWA and the metabolic cart was found to be fair [19] or good [18] but use of a walker increased error variability and reduced agreement [19]. In cardiac patients, correlations between the metabolic cart EE and the SWA (version 2.2) ranged from 0.67 to 0.90 for arm and rowing ergometry, treadmill, and stepper [21]. Correlations between the metabolic cart EE estimates and the SWA improved with the use of cardiac-specific equations [21].
Few investigations have tested the validity of the measurement of step count by the SWA. At normal walking speeds, the SWA may provide a reasonably accurate estimation of step count [18, 19], though one research group found that the SWA underestimated steps at all speeds during treadmill walking [22]. However, measurement of step count was limited to three minutes [22], and it wasn't clear if participants achieved normal arm swing during treadmill walking, which may impact the accuracy of the SWA-derived step count. Studies with individuals with cystic fibrosis and chronic obstructive pulmonary disease show that step count from the SWA was significantly less than the criterion at slower speeds [18, 19, 22].
No study has reported the validity of the SWA for the measurement of EE or step count in individuals who have had a stroke. Thus, the primary purpose of this study was to test the validity of the SWA for the measurement of EE and steps against a criterion in a sample of individuals with stroke. We hypothesized that the SWA would underestimate EE and step count during walking in persons with stroke. Secondarily, we determined if EE and step counts were significantly different between the SWA worn on the nonhemiplegic and hemiplegic arm.
2. Materials and Methods
2.1. Study Participants and Recruitment
Participants with stroke were consecutively recruited from exercise and rehabilitation centres in Edmonton, AB, Canada. Inclusion criteria for recruitment were as follows: (1) able to ambulate with/without an assistive aid; (2) at least six months poststroke; (3) observable asymmetric gait; (4) medically stable; (5) ability to tolerate walking for at least a block or five minutes. The study was approved by the University of Alberta health research ethics board and participants signed written informed consent prior to participation.
2.2. Measurements Participant Characteristics
Baseline information including body weight, height, resting heart rate, gait speed, and blood pressure were measured. Impairment from stroke was measured by a physical therapist using the Chedoke-McMaster Stroke Assessment (CMSA) [23]. A score out of seven was determined for the hemiplegic arm, foot and leg. A score of one on the CMSA indicates no movement, a score of four indicates that the participant is able to complete some movements out of synergy, whereas seven is indicative of normal movement [23]. Because we hypothesized that active arm function may impact the accuracy of the SWA during walking, participants with scores of three or less on the CMSA arm scale were classified as having a nonfunctional hemiplegic arm. Balance was measured using the Berg Balance Scale, a 14 item observational test that provides information about standing balance and fall risk [24].
Instruments. The SWA (Body Media, Pittsburgh, PA, USA; software version 6.1) was used as the experimental method for estimating EE and step count. Utilizing proprietary equations developed by the manufacturer, EE is estimated by integrating acquired sensor data with participant's demographic characteristics including gender, age, smoking habit, handedness, height, and body weight [15]. Energy expenditure is estimated for each minute of data using complex pattern recognition algorithms that detect user context (i.e., walking, running) [17]. Each context or activity class has a linear regression model associated with it, estimating EE from the motion data and the physiological sensors [17]. Algorithms have been developed and refined through testing primarily young or middle aged adults and nondisabled reference groups, though more recent algorithms have incorporated data from clinical populations such as obesity and heart disease [14]. Step count is derived by the proprietary software from the raw accelerometer data.
The Oxycon Mobile metabolic cart (CareFusion Respiratory Care, Yorba Linda, CA, USA) was used to measure oxygen uptake continuously. Prior to testing, the metabolic cart was calibrated against known gases. The Oxycon Mobile provides valid estimates of oxygen uptake at a variety of different workloads [25]. It uses a breath by breath measurement method and continuously monitors the participants' respiratory rate, minute-by-minute oxygen uptake (VO2), carbon dioxide production, and respiratory exchange ratio (RER). Energy expenditure (kcal/min) was then determined by multiplying the caloric equivalent based on RER with oxygen uptake data (L/min) [26].
For step count, the StepWatch Activity Monitor (SAM) (Orthocare Innovations, Oklahoma City, OK, USA) was used as the criterion measure. This monitor is valid for the measurement of ambulatory activity (in steps per minute) in people with stroke when walking on level surfaces, uneven surfaces, outdoors, and on stairs, as long as the monitor is positioned on the nonparetic leg [27]. The SAM uses a uniaxial accelerometer and the software allows programming according to the participant's gait speed and gait quality (e.g., shuffling). The monitor is 98% accurate at a variety of gait speeds and across various surfaces with individuals with stroke [27].
2.3. Procedures
Data collection took place in one 90 minute laboratory visit. After signing informed consent, baseline information about participant characteristics was collected prior to the walking trials.
2.3.1. Participant Preparation
Participants were asked to abstain from coffee or food in the two hours prior to the test and not to exercise in the 24 hours prior to the test. The two SWA devices, worn posteriorly on each triceps, were set up by entering the participant's age, gender, height, weight, dominant handedness, and smoking history into the software. The guidelines defined by manufacturer state that the arm band should be worn on the right arm. Bilateral tests were conducted with two armbands (one on each arm) finding good repeatability of accelerometric and galvanic skin response sensor values (correlation coefficient >0.80) [15]. However, because of the asymmetrical placement of sensors (i.e., unilateral heat flux sensor), right arm placement is recommended. We placed one SWA on each of the hemiplegic and nonhemiplegic arms to allow investigation of potential differences in accuracy that may be related to hemiplegia. The monitors were worn for 15 minutes prior to data collection, as per the manufacturer's instructions. The SAM was programmed according to gait speed and then positioned over the lateral malleolus of the non-paretic leg.
Participants were oriented to the metabolic cart including the vest worn, as well as the face mask. The face mask was fitted over the participants' mouth and nose. Once the facemask was properly fitted (i.e., no leaks), the metabolic cart was started and participants rested quietly for 10 minutes in a seated position prior to starting the walking trials. Heart rate (HR) was derived from continuous monitoring with a pulse oximeter affixed to the participants' ear lobe. After application of all equipment, and the acclimation period, the walking trials were started.
2.3.2. Walking Trials
Each participant completed two six-minute walk tests over a 25 m course (walking in a straight line, and around pylons at each end). They used their own walking aid, as required, and a safety belt was worn during all walking testing, in the event that assistance with balance was required. The protocol from the American Thoracic Society [28] was used, and standard instructions were provided. Because there was no ability to time stamp the SAM to indicate the beginning and end of the walking trial, participants were transported to the start of the six-minute walk test in a wheelchair. They stood immediately prior to starting the walk test and began to walk once the investigators simultaneously started the metabolic cart and time-stamped the SWAs. After the participant walked for six-minutes, they were instructed to stop walking and stand. Simultaneously, investigators time-stamped the SWAs and the metabolic cart to indicate the end of the walk.
After the first six-minute walk test, the participant rested in the seated position for 15-minutes during which time their HR and blood pressure were recorded. During the rest period, the metabolic cart mask was removed for comfort. After the 15-minute rest break, the mask was reapplied, and the second six-minute walk test was completed. At the end of the second trial, the participant was monitored (e.g., HR and blood pressure) for 10 minutes. After the walking trials were complete, participants rested as needed prior to administering the Berg Balance Scale and the CMSA.
2.4. Statistical Analyses
All statistical analyses were performed using SPSS software (version 19.0; IBM, Markham, ON, Canada). The EE data from the metabolic cart and the SWA were grouped into one minute intervals and exported into a Microsoft Excel spreadsheet and synchronized for further analysis. Step count information in steps/min was downloaded from the SWA and the SAM at the same time as the EE download. The primary outcomes were mean EE in kcal/min and total steps and for both outcomes the full six-minutes of data from each walk test were used in the analysis. Means and standard deviations were calculated for physiological, energy expenditure, and walking data. Paired t-tests were used to determine if there were significant differences between outcomes on the two trials of the six-minute walk test.
For the two primary outcomes (mean EE and total steps) agreement between (1) the SWA on the hemiplegic and the nonhemiplegic arm and (2) the SWAs and the respective criterion measures (metabolic cart or SAM) was assessed using intraclass correlation (ICC) analyses (Model 2,1) [29]. The ICC analysis for EE was repeated: (1) after splitting the sample by nonfunctional and functional arm use and (2) using only the SWA information from the armband on the right arm. An ICC < 0.40 was considered to indicate poor agreement between outcomes for the devices, ICC between 0.41 and 0.60 fair agreement, ICC between 0.61 and 0.80 moderate agreement, and ICC > 0.80 substantial agreement [29]. Standard error of measurement, an estimate of the difference between observed values and the true value, was calculated using the formula SD , where SD is the standard deviation of the difference scores and r is the calculated ICC value [30]. Absolute percent differences were calculated as the difference between the criterion and the value from the SWA divided by the criterion, multiplied by 100.
For EE and steps, agreement between methods was presented graphically with the criterion on the x-axis and the difference between the criterion and the SWA values on the y-axis. Use of the criterion value instead of the mean of the two measurement methods on the x-axis is different than a traditional Bland Altman plot [31], but we deemed this to be more appropriate especially for step count where the difference between the methods was large. Finally, scatter plots were diagrammed to explore the associations between gait speed and error in EE and step measurement (i.e., percent difference between criterion and SWA). Data are expressed as mean ± standard deviation (SD) and significance was set at the P < 0.05 level.
3. Results
The characteristics of the twelve individuals with stroke who participated are displayed in Table 1. The majority had left hemiplegia (10 of 12) and eight of 12 had an average gait speed ≤ 0.8 m/sec indicating that they were not community ambulators [32]. One participant required stand-by assist to walk but all others walked independently. Most used a cane and an ankle foot orthosis to walk. Seven of 12 participants scored three or less on the Chedoke-McMaster Stroke Assessment (arm) indicating that they did not use their hemiplegic arm for functional activities.
Table 1.
Participant characteristics.
| Age (years) | 64.2 (10.4) |
| Gender, n male/n female | 7/5 |
| Body mass index | 29.4 (4.6) |
| Time after stroke (years) | 6.6 (4.3) |
| Gait speed (m/sec) | 0.67 (0.25) |
| Range | 0.29–1.10 |
| Berg balance scale (out of 56) | 40.7 (12.5) |
| CMSA Arm score (out of 7) | 3.5 (2.1) |
| Range | 1–7 |
| Number who scored 1–3 | 7 |
| CMSA Leg score (out of 7) | 4.4 (1.2) |
| Range | 2–6 |
| Number who scored 1–3 | 3 |
| Use of walking aid | |
| No aids, n | 2 |
| Cane, n | 10 |
| Use of lower extremity orthosis | |
| Yes, n | 8 |
| No, n | 4 |
Data are expressed at mean (SD); n: number; CMSA refers to Chedoke-McMaster Stroke Assessment. Baseline gait speed reported is from 10 m walk test.
Raw scores for the outcomes (physiological responses, EE, and steps) are presented in Table 2. Heart rate and oxygen uptake were slightly but significantly greater during the second six-minute walk test, as was the estimated EE from the metabolic cart. Estimated total steps during the six-minute walks were not different between the two walking trials for either device. Participants walked on average 10 m further on the 2nd six-minute walk but this difference was not statistically significant.
Table 2.
Physiological, energy expenditure, and walking data from six-minute walks.
| Six-minute walk one | Six-minute walk two | P | |
|---|---|---|---|
| Physiological data | |||
| Heart rate (beats/min) | 92.9 (13.3) | 95.1 (14.0) | 0.019 |
| Oxygen uptake (mL/kg/min) | 10.9 (2.0) | 11.4 (2.2) | 0.013 |
| Respiratory exchange ratio | 0.88 (0.08) | 0.86 (0.05) | 0.125 |
| Energy expenditure data | |||
| EE SWA (hemiplegic arm), kcal/min | 4.7 (1.4) | 4.7 (1.3) | 0.845 |
| EE SWA (nonhemiplegic arm), kcal/min | 4.8 (1.6) | 4.9 (1.3) | 0.432 |
| EE metabolic cart, kcal/min | 4.3 (1.0) | 4.5 (1.0) | 0.013 |
| Walking data | |||
| Steps from SWA (hemiplegic arm) | 296.9 (196.7) | 330.5 (197.6) | 0.062 |
| Steps from SWA (nonhemiplegic arm) | 335.3 (229.0) | 305.3 (231.3) | 0.21 |
| Steps from StepWatch Activity Monitor | 505.5 (98.0) | 515.8 (105.2) | 0.171 |
| Distance walked (m) | 215.2 (80.5) | 225.2 (86.4) | 0.084 |
| Walking speed (m/sec) | 0.60 (0.22) | 0.63 (0.24) | 0.084 |
All values are mean (SD). EE: energy expenditure; SWA: SenseWear ArmBand.
3.1. EE Agreement
Values from the two six-minute walks (for EE and steps) were combined for testing agreement, thus the sample size was 22 and 24 for tests utilizing the SWA on the hemiplegic arm and the nonhemiplegic arm respectively. For one participant, the SWA malfunctioned on the hemiplegic arm (i.e., no values were recorded). On average, the SWA reported greater EE during walking (Figure 1, Table 2), compared to the metabolic cart, and agreement was slightly better with the nonhemiplegic arm compared to the hemiplegic arm (Table 3). Agreement between the SWA on the hemiplegic arm versus the non-hemiplegic arm was moderate. Agreement between EE values from the SWA on the right arm (placement as per the manufacturer's instructions) and the MC was good and is similar to agreement between the EE values from SWA on the non-hemiplegic arm and the MC (ICC = 0.715, and 0.702 resp.). This should not be surprising as the right arm and the non-hemiplegic arm were one and the same for all but two individuals. Absolute percent difference in EE was 17.9% for the hemiplegic arm and 18.4% for the non-hemiplegic arm with only about one third of values falling within ±10% difference. Graphically (Figure 1), average overestimation by the SWA was 0.33 kcal/min. There does not appear to be better or worse agreement related to the average EE as measured by the criterion.
Figure 1.
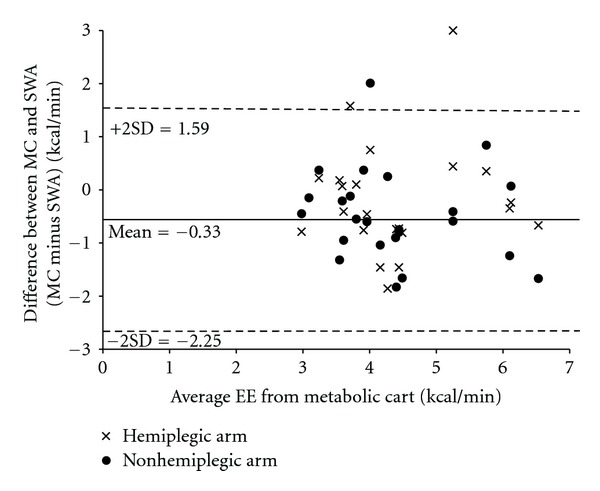
Graphical plot of average EE versus the difference between the two measurement methods.
Table 3.
Agreement, standard error of measurement, and absolute percent difference by condition.
| Variable, condition | ICC | SEM | Absolute percent difference |
|---|---|---|---|
| EE, SWA hemi versus SWA non-hemi | 0.620 | 0.73 | NC |
| EE, SWA hemi versus metabolic cart | 0.586 | 0.68 | 17.9 (15.3) |
| EE, SWA non-hemi versus metabolic cart | 0.702 | 0.48 | 18.4 (13.3) |
| Steps, SWA hemi versus SWA non-hemi | 0.682 | 97.92 | NC |
| Steps, SWA hemi versus SAM | 0.220 | 151.5 | 39.3 (40.4) |
| Steps, SWA non-hemi versus SAM | 0.352 | 132.34 | 41.7 (38.0) |
EE: energy expenditure; SWA: SenseWear Armband. SEM: standard error of measurement, units are kcal/min for EE, and steps for the steps variable. Values for absolute percent difference are mean (SD). NC: not calculated.
Agreement between the SWAs and the criterions was also tested by grouping values for those with (n = 5) and without (n = 7) functional use of their hemiplegic arm. Agreement between the SWA on the hemiplegic arm and the metabolic cart generally did not differ between the group with functional use of the arm (ICC = 0.577), as compared to those without functional arm use (ICC = 0.619). However, agreement between the SWA on the non-hemiplegic arm and the metabolic cart was better in the group that had functional arm use (ICC = 0.893), as compared to those without (ICC = 0.390).
3.2. Total Steps Agreement
Agreement between the two devices when measuring total steps was poor as evidenced by the ICCs and absolute percent differences between measures (Table 3). The SWA consistently underestimated steps by a mean of 193 steps over the six-minute walk (Figure 2). In spite of generally large underestimation, there were also two participants for whom the SWA overestimated steps (on both trials). Agreement between the devices does not appear to improve as the number of total steps increases. Limits of agreement were large (see Figure 2).
Figure 2.
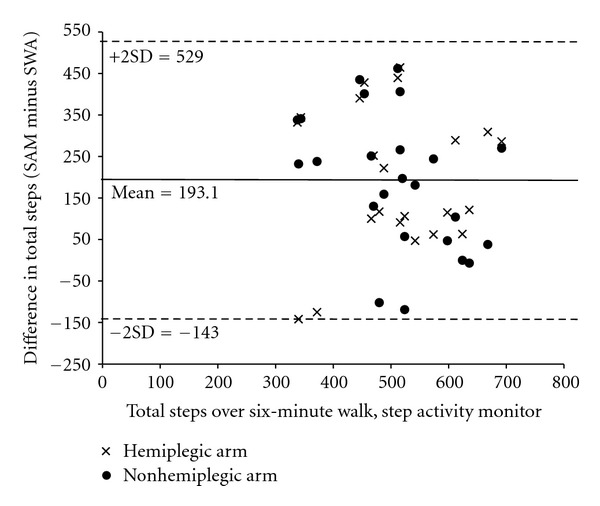
Graphical plot of average step count as measured by the Step Activity Monitor versus the difference between the two measurement methods.
3.3. Associations with Gait Speed
Association between gait speed and error in the measurement of EE or steps were explored. Figure 3 suggests that higher and lower gait speeds are associated with greater error in the measurement of EE. Slower gait speed appears to be associated with greater underestimation of step count by the SWA, but underestimation of step count occurs at all gait speeds (Figure 4).
Figure 3.
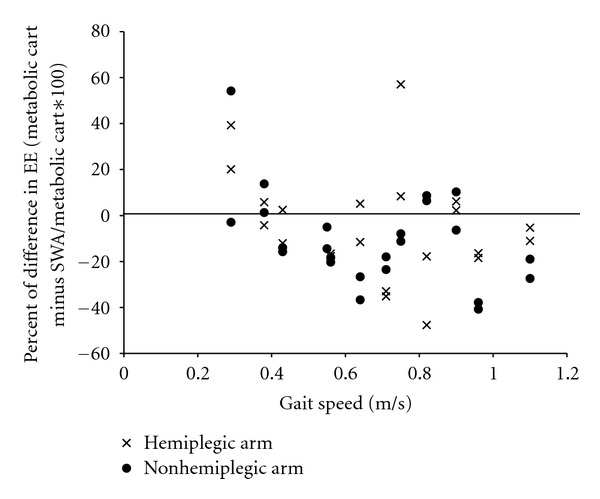
Scatterplot between gait speed and percent difference of energy expenditure (EE) for the SenseWear Armband (SWA) and metabolic cart. Percent differences less than zero indicate overestimation by the SWA. Each participant has four data points (one for each arm for each 6-minute walk test), viewed in a vertical line from the gait speed value. Note: there are only two data points for the participant for whom the SWA malfunctioned on the hemiplegic arm.
Figure 4.
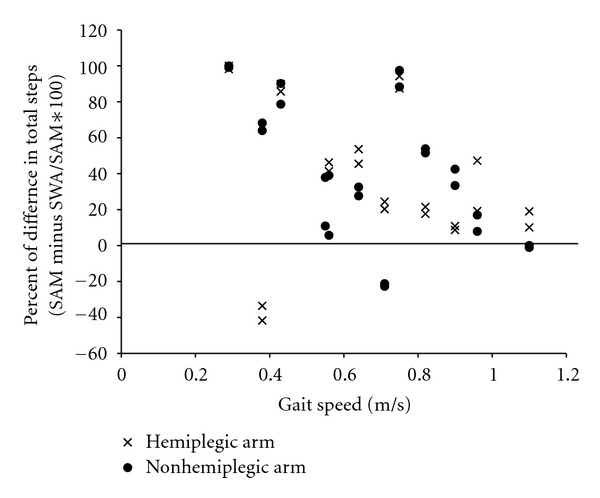
Scatterplot between gait speed and percent difference of step count as measured by the Step Activity Monitor (SAM) and the SenseWear Armband (SWA). Percent differences less than zero indicate overestimation by the SWA. Each participant has four data points (one for each arm for each 6-minute walk test), viewed in a vertical line from the gait speed value. Note: there are only two data points for the participant for whom the SWA malfunctioned on the hemiplegic arm.
4. Discussion
This study is the first to test the validity of the SWA for the measurement of EE and step count in individuals with stroke. We found that agreement between SWA EE estimates and the criterion was fair with the SWA on the hemiplegic arm and moderate with the SWA on the non-hemiplegic arm. This finding suggests that, similar to other activity monitors used for people with stroke [27], they work best when placed on the nonaffected side. And though the ICC values did reach the threshold for good agreement on the non-hemiplegic arm, the mean absolute percent difference was high (~18%), suggesting caution when using the SWA to estimate EE in individuals with stroke. For this group of individuals with stroke, who walked slowly, the SWA does not measure step count accurately.
Our results with respect to EE estimation and step count can be compared, in particular, to the findings from three clinical studies [18, 19, 22]. Hill and colleagues tested the measurement properties of the SWA in individuals with chronic obstructive pulmonary disease. They reported fair agreement, characterized by limits of agreement of 1.3 METS and an overall difference of 0.2 METS between estimates of EE by the SWA and EE as measured by indirect calorimetry [19]. Using our data, the difference between EE from the SWA on the hemiplegic arm and EE from the metabolic cart (0.4 kcal/min) roughly calculates to a 0.28 METS difference, similar to results from the Hill study. A study with individuals with cystic fibrosis reported strong correlations (r > 0.85) between EE as estimated with the SWA and indirect calorimetry [18]. Though not ICCs, these correlations are higher than our correlations and may be explained to some extent by the normal walking speed of participants with cystic fibrosis [18]. Our results related to step count are in line with other studies that found that slow walking or use of a rollator walker resulted in underestimation of step count by the SWA [19, 22]. A gait speed threshold of 50 m/min (0.83 m/sec) has been suggested (i.e., if a person walks more slowly than 50 m/min the SWA will not count steps accurately) [19, 33]. A threshold cannot be determined from our results; however, our average gait speed was well below the suggested threshold, and only three of our participants walked faster than 50 m/min.
Possible explanations of our findings may be related to step count information, difference between arms in terms of vascularity and arm swing, or gait speed. We put these explanations forward for discussion, but more work is required to fully understand the factors that may affect the accuracy of the SWA in the stroke population. The SWA, regardless of which arm it was worn on, did not provide a valid estimate of step count in this sample of stroke survivors. Estimates of step count are presumably derived exclusively from the raw accelerometer data. However, because the algorithms used by Body Media are proprietary, it was not possible to determine how much that incorrect information from the accelerometer during a walking activity contributed to inaccuracies in the EE estimates. Others have suggested that the accelerometer contributes relatively less to EE estimates [19, 34] than the other sensors and our findings support this view as in spite of lower accelerometer readings, the SWA generally overestimated EE.
Vascular differences from arm to arm may account for the difference observed in our stroke survivors. Many people with hemiplegia perceive their hemiplegic arm to be cold, and this sensation is associated with reduced arm and hand temperature as compared to the non-hemiplegic arm [35]. A small sample of people with stroke who perceived their arm to be cold had 35% less blood flow to the hemiplegic hand [35]. This compromised circulation could affect values for the temperature sensors and the sweat rate sensors of the SWA. However, we did not measure arm temperature or sweating so we can only speculate.
Arm swing may have also influenced our results. We found that when using the SWA on the non-hemiplegic arm, agreement between the SWA and the EE criterion was better generally, but especially in those with functional arm use, which suggests that more normal arm swing is associated with better accuracy. This result may be deceiving as those with arm function classified by the CMSA as functional, also had higher gait speed (0.82 m/sec versus 0.57 m/sec). We know from previous studies that slower gait speeds are associated with step count underestimation by the SWA [18, 19]. However, the effect of slow gait speed on EE estimation with the SWA is unknown. Our findings suggest that gait speed has little effect on EE measurement (Figure 3). Moreover, in spite of the classification of a functional hemiplegic arm, arm swing on the hemiplegic side often remained minimal during gait. Use of CMSA as a surrogate of arm swing is an imperfect measure and a better measure may have been calculations of arm swing amplitude from video recordings. Future studies may also consider standardizing arm swing (or lack thereof) by asking participants to wear a unilateral sling.
Though vascular changes and lack of arm swing are different in this group of stroke survivors, as compared to other samples, it would be expected that those differences might lead to underestimation of EE by the SWA. This was the case for a minority of participants (Figure 1) but overestimation by the SWA was more common and is challenging to explain. However, the galvanic skin response sensor does pick up on physiological stress [15], and it is possible that walking in a new environment (including wearing the metabolic cart vest) might increase readings to that sensor and help to explain overestimation. Vascular and arm swing differences between participants likely contributed to the large variability we observed in the EE measure but without knowing more about the algorithm used in the SWA software to calculate EE, and it is difficult to make sense of these findings. In future studies, measurement of vascular changes in the arms, as well as better measures of arm swing may help to explain the findings.
One of the challenges of testing a device like the SWA in people with mobility disability, and a limitation of this study, is the heterogeneity of samples, or representation of only part of a group of individuals with stroke. Our group of participants was small and at least moderately impaired with an average gait speed well below what would be expected in a similar age group without disability. A group of stroke survivors who walked faster, regardless of how much arm swing they had, would create more acceleration and perhaps the device would provide less variable and more accurate results for EE. Further studies with larger samples and more functionally varied groups are required to more fully understand the potential to use the SWA to measure EE in individuals with stroke. Also, because our sample almost exclusively had left hemiplegia, the SWA on the right arm (the arm on which the manufacturer recommends placement) was also the non-hemiplegic arm. More individuals with right hemiplegia should be tested.
5. Conclusions
Our results suggest caution when considering use of the SWA for measurement of EE in stroke survivors who walk slowly. Further studies are required to better understand the effects of hemiplegia on SWA accuracy. Use of the SWA to estimate step count in individuals with stroke who walk slowly is not recommended.
Acknowledgment
This study was funded by the Physiotherapy Foundation of Canada.
References
- 1.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Canadian Medical Association Journal. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding RA, Katula J, Miller ME, et al. Activity adherence and physical function in older adults with functional limitations. Medicine and Science in Sports and Exercise. 2007;39(11):1997–2004. doi: 10.1249/mss.0b013e318145348d. [DOI] [PubMed] [Google Scholar]
- 3.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41(1):129–135. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 4.Rimmer JH, Rauworth AE, Wang EC, Nicola TL, Hill B. A preliminary study to examine the effects of aerobic and therapeutic (nonaerobic) exercise on cardiorespiratory fitness and coronary risk reduction in stroke survivors. Archives of Physical Medicine and Rehabilitation. 2009;90(3):407–412. doi: 10.1016/j.apmr.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Mudge S, Barber PA, Stott NS. Circuit-based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2009;90(12):1989–1996. doi: 10.1016/j.apmr.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Sawatzky R, Liu-Ambrose T, Miller WC, Marra CA. Physical activity as a mediator of the impact of chronic conditions on quality of life in older adults. Health and Quality of Life Outcomes. 2007;5:p. 68. doi: 10.1186/1477-7525-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rand D, Eng JJ, Tang PF, Jeng JS, Hung C. How active are people with stroke?: use of accelerometers to assess physical activity. Stroke. 2009;40(1):163–168. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- 8.Hagströmer M, Oja P, Sjöström M. Physical activity and inactivity in an adult population assessed by accelerometry. Medicine and Science in Sports and Exercise. 2007;39(9):1502–1508. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- 9.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. American Journal of Epidemiology. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baert I, Daly D, Dejaeger E, Vanroy C, Vanlandewijck Y, Feys H. Evolution of cardiorespiratory fitness after stroke: a 1-year follow-up study. influence of prestroke patients' characteristics and stroke-related factors. Archives of Physical Medicine and Rehabilitation. 2012;93(4):669–676. doi: 10.1016/j.apmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Stuart M, Chard S, Roettger S. Exercise for chronic stroke survivors: a policy perspective. Journal of Rehabilitation Research and Development. 2008;45(2):329–336. doi: 10.1682/jrrd.2007.02.0027. [DOI] [PubMed] [Google Scholar]
- 12.Tang A, Closson V, Marzolini S, Oh P, McIlroy W, Brooks D. Cardiac rehabilitation after stroke-need and opportunity. Journal of Cardiopulmonary Rehabilitation and Prevention. 2009;29(2):97–104. doi: 10.1097/HCR.0b013e31819a00d4. [DOI] [PubMed] [Google Scholar]
- 13.Platts MM, Rafferty D, Paul L. Metabolic cost of overground gait in younger stroke patients and healthy controls. Medicine and Science in Sports and Exercise. 2006;38(6):1041–1046. doi: 10.1249/01.mss.0000222829.34111.9c. [DOI] [PubMed] [Google Scholar]
- 14.Andre D, Pelletier R, Farringdon J, et al. The development of the SenseWear ArmBand, A revolutionary energy assessment device to assess physical activity and lifestyle, http://www.bodymedia.com/Professionals/Whitepapers/, 2012.
- 15.Liden CB, Wolowicz M, Stivoric J, et al. Characterization and Implication of the Sensors Incorporated into the SenseWear ArmBand for Energy Expenditure and Activity Detection, http://www.bodymedia.com/Professionals/Whitepapers/, 2012.
- 16.Mackey DC, Manini TM, Schoeller DA, et al. Validation of an armband to measure daily energy expenditure in older adults. The Journals of Gerontology A. 2011;66(10):1108–1113. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Medicine and Science in Sports and Exercise. 2010;42(11):2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer TJ, Alison JA, McKeough ZJ, Elkins MR, Bye PTP. Evaluation of the SenseWear activity monitor during exercise in cystic fibrosis and in health. Respiratory Medicine. 2009;103(10):1511–1517. doi: 10.1016/j.rmed.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Hill K, Dolmage TE, Woon L, Goldstein R, Brooks D. Measurement properties of the SenseWear armband in adults with chronic obstructive pulmonary disease. Thorax. 2010;65(6):486–491. doi: 10.1136/thx.2009.128702. [DOI] [PubMed] [Google Scholar]
- 20.Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear pro armband™ to assess energy expenditure during exercise. Medicine and Science in Sports and Exercise. 2004;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 21.Cole PJ, Lemura LM, Klinger TA, Strohecker K, McConnell TR. Measuring energy expenditure in cardiac patients using the Body Media™ Armband versus indirect calorimetry: a validation study. Journal of Sports Medicine and Physical Fitness. 2004;44(3):262–271. [PubMed] [Google Scholar]
- 22.Furlanetto KC, Bisca GW, Oldemberg N, et al. Step counting and energy expenditure estimation in patients with chronic obstructive pulmonary disease and healthy elderly: accuracy of 2 motion sensors. Archives of Physical Medicine and Rehabilitation. 2010;91(2):261–267. doi: 10.1016/j.apmr.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Gowland CA. Staging motor impairment after stroke. Stroke. 1990;21(9, supplement 2):II19–II21. [PubMed] [Google Scholar]
- 24.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian Journal of Public Health. 1992;83(supplement 2):7–11. [PubMed] [Google Scholar]
- 25.Rosdahl H, Gullstrand L, Salier-Eriksson J, Johansson P, Schantz P. Evaluation of the Oxycon Mobile metabolic system against the Douglas bag method. European Journal of Applied Physiology. 2010;109(2):159–171. doi: 10.1007/s00421-009-1326-9. [DOI] [PubMed] [Google Scholar]
- 26.McArdle W, Katch F, Katch V. Exercise Physiology. Philadelphia, Pa, USA: Lea & Febiger; 1991. [Google Scholar]
- 27.Mudge S, Stott NS, Walt SE. Criterion validity of the stepwatch activity monitor as a measure of walking activity in patients after stroke. Archives of Physical Medicine and Rehabilitation. 2007;88(12):1710–1715. doi: 10.1016/j.apmr.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Society AT. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 29.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 30.Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. doi: 10.1519/JPT.0b013e318248e20d. Journal of Geriatric Physical Therapy. In press. [DOI] [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 32.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 33.Langer D, Gosselink R, Sena R, Burtin C, Decramer M, Troosters T. Validation of two activity monitors in patients with COPD. Thorax. 2009;64(7):641–642. doi: 10.1136/thx.2008.112102. [DOI] [PubMed] [Google Scholar]
- 34.Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients: a validation study. Journal of Chronic Obstructive Pulmonary Disease. 2007;4(2):107–112. doi: 10.1080/15412550701246658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanklyn P, Forster A, Young J, Mulley GP. Prevalence and associated features of the cold hemiplegic arm. Stroke. 1995;26(10):1867–1870. doi: 10.1161/01.str.26.10.1867. [DOI] [PubMed] [Google Scholar]


