Summary
Pheromones elicit innate sex-specific mating behaviors in many species. We demonstrate that in C. elegans, male-specific sexual attraction behavior is programmed in both sexes but repressed in hermaphrodites. Repression requires a single sensory neuron pair, the ASIs. To represses attraction in adults, the ASIs must be present, active, and capable of sensing the environment during development. The ASIs release TGF-β, and ASI function can be bypassed by experimental activation of TGF-β signaling. Sexual attraction in de-repressed hermaphrodites requires the same sensory neurons as in males. The sexual identity of both these sensory neurons and a distinct subset of interneurons must be male to relieve repression and release attraction. TGF-β may therefore act to change connections between sensory- and interneurons during development to engage repression. Thus, sensation in a single sensory neuron pair during development reprograms a common neural circuit from male to female behavior.
Introduction
In most species, males and females display sex-specific behavioral repertoires. Courtship and mating behaviors elicited by pheromones are among the most obvious sexually dimorphic repertoires because they are innate and stereotyped (Stowers and Logan, 2010). What are the neural differences that give rise to different behaviors in each sex? Behavioral differences could be due to differences in the ability of each sex to detect pheromone, or to differences in the processing of pheromone sensory information. For example, female mice with an impaired vomeronasal organ exhibit male mating behaviors, suggesting that the underlying neural circuity is the same in both sexes, but only active in males (Kimchi et al., 2007). It may be that females are capable of smelling pheromones that males cannot, and that smelling these compounds represses male mating. In this case, the difference is at the level of detection. Alternatively, male flies detect phero-mone identically to females (Kurtovic et al., 2007), but possess male-specific ganglia that initiate male courtship behavior (Clyne and Miesenböck, 2008; Kohatsu et al., 2011), even in an animal that is otherwise female (Kimura et al., 2008). Here, both sexes smell the same compound, cis-vaccenyl acetate, but male and female higher brain centers generate different responses (Kurtov-ic et al., 2007). Thus, in this case the difference is at the level of processing. The two mechanisms are not mutually exclusive. In Manduca sexta, transplanting the nascent male sensory apparatus (his antennae) to a female larva induces male development in the female brain, and the adult animal has male behaviors (Schneiderman et al., 1986). The reciprocal switch generates an animal that has female behaviors (Kalberer et al., 2010). In this case, a difference in detection induces sexually dimorphic wiring, resulting in a difference in processing. Behavior that depends only on differences in detection could be easily modulated, for example, by regulating chemore-ceptor expression. Behavior that is dependent on sex-specific brain structures must be hard-wired during development, and the different contributions of sex-determination pathways and the environment to this hard-wiring are not clear. Here, we address these fundamental questions in C. elegans, an animal with relatively few sex-specific neurons, but a rich sex-specific behavioral repertoire.
C. elegans reproduces both as a self-fertilizing hermaphrodite and by mating between hermaphrodites and males. C. elegans hermaphrodites are essentially females that make their own sperm for a short time during development, which they store to later fertilize their own eggs (White et al., 2007, Supplementary Figure). Hermaphrodites release pheromones that elicit behaviors in both sexes. Hermaphrodite pheromones fall into two broad classes: daf-22-dependent (Butcher et al., 2009; Pungaliya et al., 2009) and daf-22-independent (White et al., 2007). The daf-22 gene encodes a β-oxidase required for the synthesis of a family of small molecules whose distinguishing feature is an ascarylose sugar core (Butcher et al., 2009). The daf-22-dependent class of pheromones appear to act as density signals that mediate both development and behavior (Srinivasan et al., 2012). The daf-22 independent pheromones elicit robust male-specific attraction; males chemotax to a source of these pheromones and linger, but hermaphrodites do not (White et al., 2007). Behaviors elicited by the daf-22-dependent and daf-22-independent pheromone classes have different genetic and neural requirements (White et al., 2007; Srinivasan et al., 2008; Macosko et al., 2009; McGrath et al., 2011) and so appear to be distinct. Because daf-22-independent pheromones elicit behaviors in males reminiscent of copulation but in the absence of a mating partner, we refer to them as sex pheromones, and the behavior they elicit as sexual attraction (White et al., 2007). As in many species, both sexes are exposed to sex pheromones, but they compel sexual attraction only in males. The mechanism by which male-specific sexual attraction behavior is established in C. elegans is unknown.
Results
Hermaphrodites have latent male sexual attraction behavior, generated by a core neural circuit
We surveyed existing C. elegans mutants for those with altered sexual attraction, and found that daf-7 mutant hermaphrodites show sexual attraction behavior (Figure 1A). That is, daf-7 mutant hermaphrodites are attracted to sex pheromones, whereas wild-type hermaphrodites are not. In daf-7 males, sexual attraction is not detectably altered (Supplementary Figure). Thus, the absence of DAF-7/TGF-β reveals latent sexual attraction behavior in hermaphrodites.
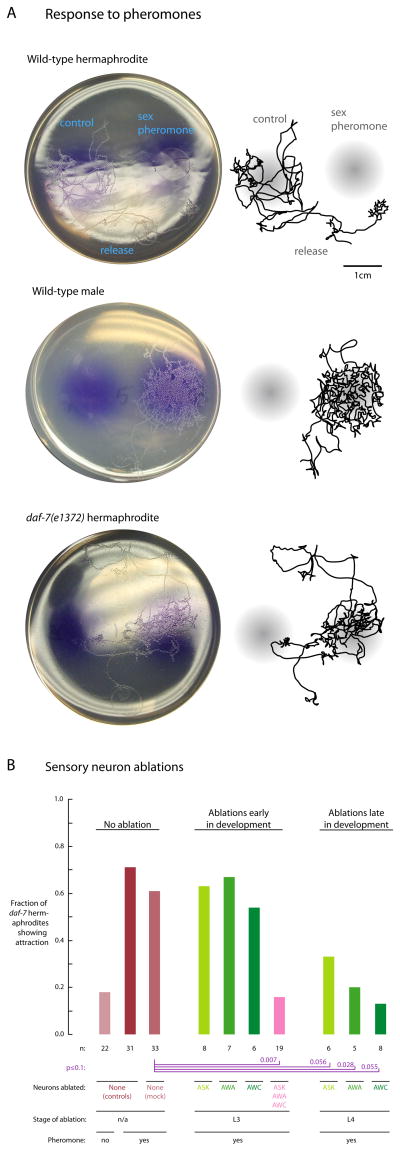
Figure 1. Sex pheromones elicit attraction behavior in wild-type males and daf-7 hermaphrodites; this behavior requires the same sensory neurons, and in both cases these neurons compensate for one another.
A. Sex pheromones elicit sexual attraction in wild-type males and daf-7 mutant hermaphrodites, but not wild-type hermaphrodites. Tracks on agar assay plates (single animal, 3 hrs elapsed) show that both wild-type males and daf-7 mutant hermaphrodites find a spot containing hermaphrodite sex pheromones and linger, but wild-type hermaphrodites do not. Photos of assay plates are on the left; hand tracings are on the right to assist in visualization. Quantitation is shown in Supplemental Figure 1.
B. Sexual attraction in daf-7 hermaphrodites requires the core sensory neurons AWA, AWC, and ASK, which compensate for one another. Simultaneous laser ablation in L3 larvae of the ASK, AWA, and AWC pairs of core sensory neurons (six pairs total, pink bar) reduces the attraction behavior of daf-7 hermaphrodites to a frequency comparable to background. Ablation of each pair singly in L3 larvae (each pair one at a time, green bars) does not appreciably affect attraction (compared to mock-ablated animals). In contrast, ablation of each pair singly in L4 larvae reduces the attraction behavior of daf-7 hermaphrodites to frequencies similar to the triple L3 ablation. The difference between L3 and L4 ablations is indicative of compensation, as observed for male sexual attraction.
Sexual attraction requires the same neurons in males and daf-7 hermaphrodites. Most of the C. elegans nervous system is the same in both sexes (Sulston et al., 1983): 294 neurons comprise this core nervous system (out of 302 total in the hermaphrodite). In males, sexual attraction requires the core sensory neurons AWA, AWC, ASK, and although male-specific sensory neurons normally contribute, these core sensory neurons are sufficient (White et al., 2007, Supplementary Figure). Furthermore, if any of these core neurons are surgically ablated in juvenile males, the remaining neurons compensate and the operated adults express full sexual attraction. As in males, in daf-7 hermaphrodites sexual attraction requires the core sensory neurons AWA, AWC, and ASK (Figure 1B). Furthermore, as in males, in daf-7 mutant hermaphrodites these sensory neurons compensate for one another (Figure 1B). If only one pair is removed late in development (L4 larval stage), the circuit is disrupted and behavior is compromised. However, if only one pair is removed early in development (L3 larval stage), the remaining pairs take over and behavior is not detectably affected, unless all three pairs are removed concurrently. Although other explanations for attraction in daf-7 hermaphrodites are formally possible, such as altered chemoreceptor expression (Nolan et al., 2002), it is striking that the same distinct set of sensory neurons are required and show the same property of compensation. Given these results, it is likely that the same neural circuit generates sexual attraction in both males and daf-7 hermaphrodites.
Sensation in the ASI neurons releases TGF-β to engage repression during development
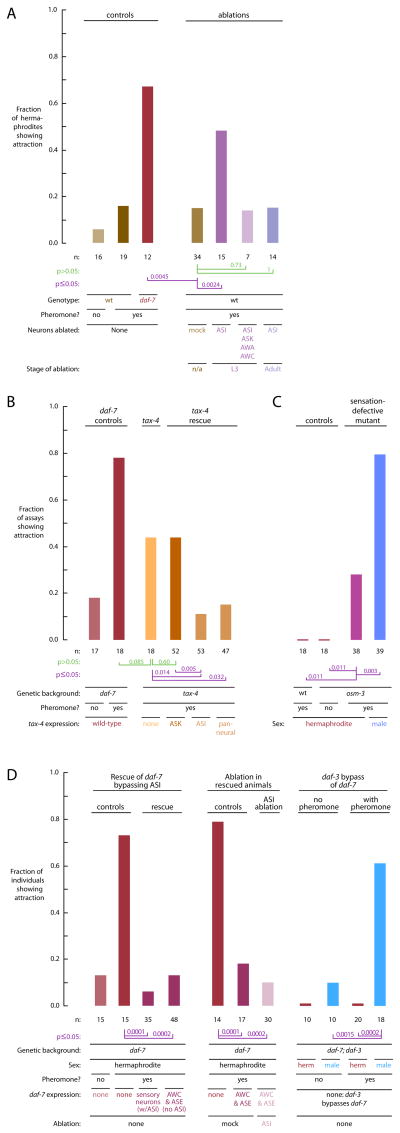
The sole source of DAF-7/TGF-β in C. elegans is the ASI sensory neuron pair (Ren et al., 1996; Schackwitz et al., 1996). Ablation of the ASI neurons reveals sexual attraction in hermaphrodites (Figure 2A). That is, ASI-ablated hermaphrodites are attracted to sex pheromones, whereas intact hermaphrodites are not. Sexual attraction in ASI-ablated hermaphrodites requires the ASK, AWA and AWC neurons (Figure 2A). The ASI neurons express a cGMP-gated channel containing the TAX-4 subunit (Komatsu et al., 1996; Coburn and Bargmann, 1996). This channel is required for ASI development and activity (Coburn and Bargmann, 1996; Peckol et al., 1999), but makes only a residual contribution to sexual attraction in males (White et al., 2007). Mutant tax-4 hermaphrodites show sexual attraction behavior (Figure 2B). Attraction in tax-4 hermaphrodites is not as consistent as in daf-7 hermaphrodites, suggesting that—as in males—TAX-4 may also function in cells that promote attraction. Expression of TAX-4 in ASI neurons completely restored wild-type behavior to tax-4 mutant hermaphrodites (Figure 2B; “wild-type behavior” means that attraction is repressed), but expression in other neurons, such as ASK, did not. Thus, TAX-4 function solely in the ASIs is sufficient to repress attraction. The ASIs are classified as sensory neurons in part because they have dendrites exposed to the external environment (White et al., 1986). Sensory dendrites in ASI require the OSM-3 kinesin to develop properly; osm-3 mutants have stunted sensory endings (Snow et al., 2004), but OSM-3 is not required in males for sexual attraction (White et al., 2007). osm-3 mutant hermaphrodites exhibit sexual attraction behavior (Figure 2C), most likely because their ASI neurons are not sensing the external environment and so do not engage repression. Thus, ASI neurons must be 1) present during development, 2) active, and 3) capable of sensing the external environment in order to repress sexual attraction in adult hermaphrodites.
Figure 2. ASI sensory activity is required to repress attraction in hermaphrodites, but the ASIs are bypassed by restoring DAF-7-signaling function elsewhere.
A. ASI is required for repression of attraction. Ablation of ASI neurons reveals sexual attraction behavior in wild-type hermaphrodites. When ASI neurons are ablated during the L3 larval stage, hermaphrodites exhibit attraction behavior comparable to daf 7 hermaphrodites, and significantly different from mock-ablated animals. Attraction in the absence of ASI requires the ASK, AWA, and AWC sensory neurons, because animals missing all eight neurons have significantly impaired sexual attraction behavior. The ASI neurons must be ablated during development to reveal sexual attraction behavior, because when the ASI neurons are ablated in adult animals, sexual attraction behavior remains repressed.
B. The TAX-4 cGMP-gated channel is required in ASI to repress sexual attraction. tax-4 mutant hermaphrodites show sexual attraction behavior comparable to daf-7 hermaphrodites. Expression of tax-4 in the ASI neurons, but not in ASK, restores full repression of attraction behavior.
C. Sensory cilia must be intact to repress sexual attraction in hermaphrodites. osm-3 mutants have impaired sensory cilia in a subset of sensory neurons that includes the ASIs (Snow et al., 2004; Signor et al., 1999); osm-3 mutant hermaphrodites have significant sexual attraction behavior compared to either wild-type hermaphrodites or to no-pheromone osm-3 controls.
D. Restoring DAF-7/TGF-β signaling function elsewhere bypasses ASI. Transgenic expression of DAF-7/TGF-β in the AWC and ASE neurons restores repression of sexual attraction in daf-7 mutant hermaphrodites. Restored repression is not ASI-dependent, because ablation of ASI during the L3 larval stage in transgenic animals does not significantly affect repression. Repression of attraction is restored genetically by the daf-3 mutation: the absence of DAF-3 activates DAF-7 signaling in target cells independent of DAF-7 (Thomas et al., 1993). Accordingly, daf-7; daf-3 double mutant hermaphrodites show significantly repressed attraction.
To repress sexual attraction, the ASI pair could act solely by releasing DAF-7/TGF-β or it could have additional roles. To separate the functions of DAF-7 from the ASI neurons, we experimentally activated TGF-β signaling independent of the ASIs in two ways. First, we expressed DAF-7/TGF-β specifically in the AWC and ASE sensory neurons, but not in ASI, in daf-7 mutant animals. As expected, DAF-7/TGF-β expression in the AWC and ASE neurons rescues three classic phenotypes of daf-7 mutants: 1) inappropriate induction of dauer larvae, 2) a dark intestine, and 3) aggregation. Importantly, DAF-7/TGF-β expression in the AWC and ASE neurons also rescues wild-type behavior in daf-7 mutant hermaphrodites: transgenic hermaphrodites are not attracted to sex pheromones (Figure 2D). Notably, ablation of the ASI neurons has no discernible effect on the attraction behavior of these transgenic hermaphrodites; sexual attraction is repressed regardless of whether ASI is present (Figure 2D). Second, we activated TGF-β signaling using genetics: in a daf-3 mutant, the absence of DAF-3 function activates the DAF-7 signaling pathway in target cells, independent of DAF-7 (Thomas et al., 1993). Accordingly, daf-7;daf-3 double-mutant hermaphrodites have repressed sexual attraction (Figure 2D). That is, daf-7;daf-3 hermaphrodites are no longer attracted to sex pheromones. Their brothers, daf-7;daf-3 double-mutant males, exhibit obvious sexual attraction behavior (Figure 2D) comparable to daf-3 single mutant males (not shown). Thus, although ASI activity normally modulates expression and release of DAF-7/TGF-β (Chang et al., 2006; Schackwitz et al., 1996), ASI activity may be bypassed either by forcing expression of DAF-7/TGF-β elsewhere, or by activating TGF-β signaling. Therefore, the sole role of ASI in repressing sexual attraction is to release DAF-7/TGF-β.
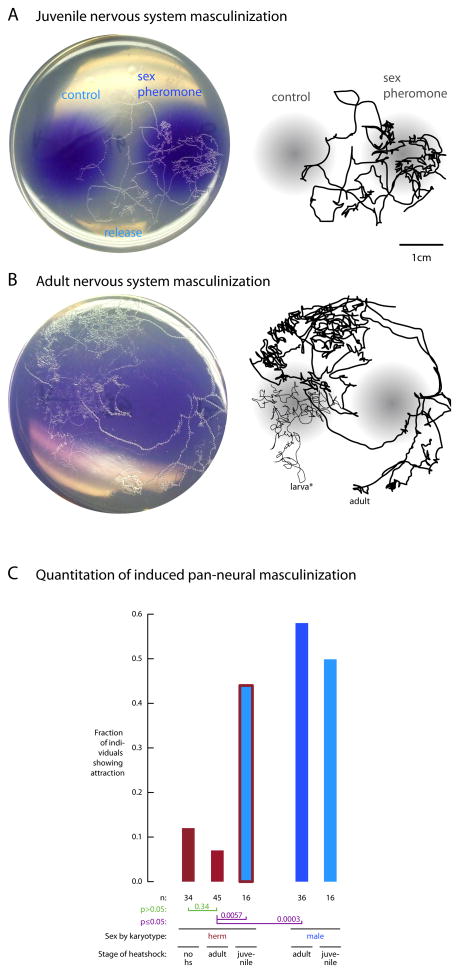
To establish sexually dimorphic behavior, DAF-7/TGF-β could alter either how the underlying neural circuit is built, how it is maintained, or how it is modulated. To address these possibilities, we determined when the nervous system must be sexualized to generate sexual attraction behavior. At different times during development we masculinized the hermaphrodite nervous system using a FLP-ON strategy (Davis et al., 2008). Sexual attraction behavior emerges in adults when the nervous system is switched during development (during the final, L4 larval stage or earlier), but not when switched in adults (Figure 3). Consistent with these results, sexual attraction is revealed in adult hermaphrodites only when the ASI neurons are ablated during development (prior to the L4 larval stage or earlier), not when ablated in adults (Figure 2A). The requirement for the ASI neurons during development coincides with the time when they express DAF-7/TGF-β (Ren et al., 1996; Schackwitz et al., 1996). Therefore, DAF-7/TGF-β most likely alters how the sexual attraction circuits are built.
Figure 3. The nervous system must be sexualized during development for sexual attraction behavior in adults.
A. Masculinization of the nervous system of XX animals (physically hermaphrodite) by expression of FEM-3 in the nervous system induced during development (“juvenile”) results in adult sexual attraction behavior comparable to control XO animals (physically male). Assay periods were three hours. Photos on left, hand-tracings of tracks on right.
B. Masculinization induced during adulthood and assayed a day later results in XX animals comparable to uninduced XX animals, and different from juvenile induction. An L1 larva was also present on the plate (*larva), but did not detectably interfere with the behavior of the adult.
C. Quantitation of masculinization of the core C. elegans nervous system induced in juveniles and adults.
Sexual attraction requires sex differences in both sensory- and interneurons
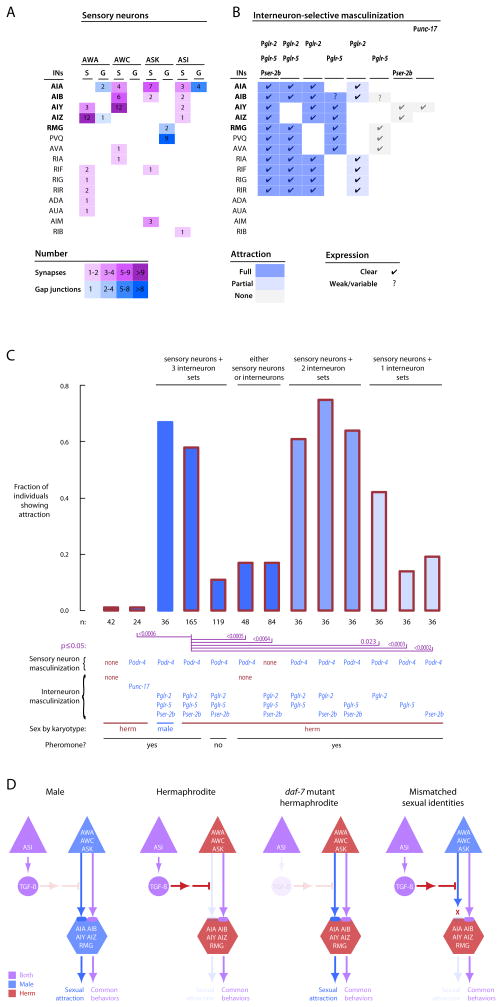
To localize neural sex differences required for sexual attraction behavior, we masculinized subsets of neurons in animals that were otherwise hermaphrodites. We focussed on the sensory neurons required for sexual attraction behavior (AWA, AWC, and ASK) and the interneurons that comprise their synaptic targets and gap-junction partners (Figure 4A). Because the male wiring diagram is incomplete, connectivity information is based on the hermaphrodite wiring diagram (White et al., 1986; Chen et al., 2006). Using cell-selective promotors, we masculinized sets of sensory neurons and interneurons in different combinations (Experimental Procedures; Figure 4B). Hermaphrodites with masculinized AWA, AWC, ASK and ASI sensory neurons exhibited no detectable sexual attraction (using Podr-4, Figure 4C). That is, attraction remained repressed in these animals. Likewise, hermaphrodites in which a broad set of interneurons were masculinized also exhibited no detectable sexual attraction (using a combination of Pglr-2, Pglr-5, and Pser-2b). In contrast, hermaphrodites in which both sensory neurons and interneurons were masculinized exhibited robust sexual attraction (Figure 4B and C, using a combination of Podr-4, Pglr-2, Pglr-5, and Pser-2b), indistinguishable from male controls (Figure 4C) and comparable to masculinization of the entire nervous system using Prab-3 (White et al., 2007). The particular set of interneurons is important, because masculinizing the Podr-4 sensory neurons together with Punc-17 interneurons and motor neurons did not enable expression of sexual attraction; the behavior remained repressed. Thus, both sensory and interneurons must be male for male-specific sexual attraction to emerge. Masculinization of only the Podr-4 sensory neuron set–which includes both ASI and the sensory neurons required for sexual attraction–is not sufficient. Likewise, masculinization of only an interneuron set–regardless of which–is not sufficient. If either set has a female sexual identity, DAF-7/TGF-β can act—either directly or indirectly—to repress sexual attraction in hermaphrodites.
Figure 4. Distinct but distributed sets of sensory neurons and interneurons must be sexualized for expression of sexual attraction behavior.
A. Direct sensory neuron-interneuron connectivity of the sensory neurons required for sexual attraction. The numbers of synapses (S) and gap junctions (G) are from the hermaphrodite wiring diagram (Chen et al., 2006; White et al., 1986); male connections may be different. Additional connections among sensory neurons and among interneurons are not shown.
B. Sensory neurons must be masculinized together with a distributed set of heavily-connected post-synaptic interneurons in order for transsexual hermaphrodites to exhibit sexual attraction behavior. The set of Podr-4 sensory neurons were masculinized together with interneurons sets defined by the Pglr-2, Pglr-5, Pser-2b, and Punc-17 promotors. Only the expression patterns of these promotors within the connected interneuron set are shown. Full expression patterns are given in Supplemental Tables 2, 3 and 4.
C. Masculinization of Podr-4 sensory neurons together with distinct subsets of interneurons imparts sexual attraction behavior on transexual animals. The graph shows the frequency of sexual attraction conferred by neural masculinization either with the Podr-4 promotor and none or more of the Pglr-2, Pglr-5, Pser-2b, and Punc-17 promotors, or the interneuron combination without Podr-4. Bars indicate the fraction of individuals exhibiting sexual attraction. Red outlined bars represent worms that are physically hermaphrodite but have been masculinized in the subset of neurons indicated.
D. Model. In males, synaptic connections that enable sexual attraction develop between the AWA, AWC, and ASK sensory neurons and the AIA, AIB, AIY and AIZ interneurons. In hermaphrodites, DAF-7/TGF-β signaling disables these connections, either by acting directly on the feminine nervous system, or indirectly through signals released by another tissue, such as the female gonad. In daf-7 hermaphrodites DAF-7/TGF-β signaling is absent, allowing male-type connections. In animals where the sexual identities of the sensory- and interneurons do not match, the feminine neurons respond to DAF-7/TGF-β signaling, repression is engaged, and male-type connections are disabled.
Interneuron sex differences required for sexual attraction are distributed
If pheromone sensory input converges on a single interneuron pair that functions, for example, as a modulatory hub (Macosko et al., 2009) or a site of integration (Shinkai et al., 2011), then these neurons might also be the site of the sex differences that account for sexual attraction. To address this, we masculinized a constant set of sensory neurons (using Podr-4) in combination with smaller subsets of interneurons. Based on the hermaphrodite wiring diagram (White et al., 1986; Chen et al., 2006), the most heavily connected interneurons that are directly post-synaptic to the AWA, AWC, and ASK sensory neurons are the AIA, AIB, AIY, and AIZ pairs (Figure 4A). Notably, no single interneuron class is post-synaptic to all three sensory neuron classes. In addition, the RMG interneuron pair modulates signaling from the ASKs via gap junctions (Macosko et al., 2009). A subset that should masculinize all of the AIA, AIB, AIY, and AIZ neurons but not the RMG interneurons (Pglr-2 + Pser-2b) fully expressed sexual attraction, comparable to broad masculinization (Pglr-2 + Pglr-5 + Pser-2b). That is, repression was not engaged. Subsets that should masculinize only some of the AIA, AIB, AIY, and AIZ neurons and include the RMG neurons also fully expressed sexual attraction (the Pglr-5 + Pglr-2 and Pglr-2 + Pser-2b combinations). In contrast, subsets that should masculinize only some of the AIA, AIB, AIY, and AIZ neurons but do not include RMG expressed sexual attraction less frequently (Pglr-2) or not at all (Pser-2b). Conversely, a subset that should masculinize RMG, but not AIA, AIY, and AIZ (and possibly not AIB; Pglr-5), did not exhibit sexual attraction. Within the framework provided by the hermaphrodite wiring diagram (White et al., 1986; Chen et al., 2006), a straightforward interpretation of these results is that sexual differences in AIA and AIB are most important for sexual attraction, with contributions from AIY and AIZ and possibly modulation by RMG. Independent of the hermaphrodite wiring, it appears unlikely that pheromone sensory input converges on a single interneuron class, but instead remains distributed.
Taken together, the neuron-selective masculinization experiments suggest that the AWA, AWC, and ASK sensory neurons and their interneuron partners—most likely the AIA, AIB, AIY, and AIZ neurons—must be male for the animal to display male behavior. A simple model based on these data is that a male-specific constellation of connections among these sensory- and inter-neurons forms during development to generate male-specific sexual attraction (Figure 4D). In this model, hermaphrodites are also capable of developing these connections, but repression either prevents them from being established or subsequently disables them.
Discussion
In general, sex-specific behaviors may generated by extra circuitry entirely present only in one sex, or by modification of circuitry present in both sexes (Stowers and Logan, 2010). In C. elegans, there are no additional male-specific neurons in the sex pheromone processing circuitry to account for male-specific sexual attraction, based on two facts. First, the nervous system is fully cataloged in males and hermaphrodites, establishing that there is core nervous system common to both sexes (White et al., 1986; Sulston, 1983; Sulston et al., 1980; Sulston and Horvitz, 1977). Second, this core nervous system is sufficient for male-specific sexual attraction behavior (White et al., 2007). Therefore, the essential differences that account for male-specific sexual attraction must reside not in the cellular composition, but rather in the properties of the circuit—for example, in connectivity, neural excitability, synaptic strengths, or receptor expression. Under most circumstances, these differences depend on sexual identity set by the somatic sex-determination pathway ( (White et al., 2007), Figures 3 and 4); however, it is unlikely that DAF-7/TGF-β alters sexual identity. Thus, daf-7 mutant hermaphrodites possess only neurons with a female sexual identity, yet express the essential differences for generating “male” behavior in the opposite sex.
Because the presence of DAF-7/TGF-β in wild-type hermaphrodites results in the absence of sexual attraction, DAF-7 functions to repress the behavior. However, because males also express DAF-7/TGF-β (Ren et al., 1996), and we have found no manipulation of DAF-7 expression in males that detectably alters sexual attraction, DAF-7 acts only on the feminine hermaphrodite core to repress attraction. That is, female sexual identity is permissive for repression. How might DAF-7/TGF-β repress sexual attraction? In general, DAF-7/TGF-β regulates diverse processes in C. elegans, from dauer development (Ren et al., 1996; Schackwitz et al., 1996) to fat metabolism and feeding behavior (Greer et al., 2008). Accordingly, DAF-7/TGF-β signaling culminates in the transcriptional regulation of a wide array of genes (Liu et al., 2004). Furthermore, DAF-7 receptors are widely expressed (Gunther et al., 2000), and their mutant phenotypes do not simply mimic the daf-7 mutant (Georgi et al., 1990; Estevez et al., 1993; Ren et al., 1996; Gunther et al., 2000). Based on the mechanisms of its other functions in C. elegans, DAF-7/TGF-β could act to repress sexual attraction in hermaphrodites either directly or indirectly (Figure 4D). In a direct model, similar to its broad action in dauer development, DAF-7/TGF-β acts on the neurons of the attraction circuit, possibly to disable synaptic connections during development. In an indirect model, similar to its role in feeding (Greer et al., 2008), DAF-7/TGF-β acts on a modulatory cell, which in turn alters the attraction circuit, plausibly via hormones, neuropeptides (Greer et al., 2008), or gap-junctions (Macosko et al., 2009). Regardless of the mechanism of repression, DAF-7/TGF-β signaling ultimately alters the attraction circuit, but only in hermaphrodites.
Unlike mice (Stowers et al., 2002; Kimchi et al., 2007), repression is set during development and does not have to be maintained by pheromone perception. That is, sexual attraction in wild-type C. elegans hermaphrodites cannot be revealed (de-repressed) in adults (Figure 2A). The developmental requirement for sensation in ASI to establish repression coincides with the period that the attraction circuit must be masculinized to establish attraction (Figure 3). Plausibly, masculinization during development renders the neurons of the attraction circuit unresponsive to repression. Furthermore, although only a limited subset of cells in the nervous system must be male to generate sexual attraction, both sensory and inter-neurons must be contemporaneously masculinized. A simple model based on these data is that sexual attraction requires male-type synaptic connections between sensory neurons (most likely AWA, AWC, and ASK) and inter-neurons (possibly AIA, AIB, AIY and/or AIZ), and that repression interferes with the establishment of these connections (Figure 4D). Thus, our data demonstrate that both sides of a particular constellation of synaptic connections must be functionally sexualized to generate a particular sex-specific behavior.
Although we have not found environmental conditions that lead to the display of sexual attraction in wild-type hermaphrodites, the requirements for properly formed sensory dendrites in ASI (Figure 2C) and for ASI activity suggest that sensation during development could modulate repression. The ASI neurons modulate behavior in other contexts (Coburn and Bargmann, 1996; Coburn et al., 1998; Peckol et al., 1999; Chang et al., 2006), so it may be that a general task of the ASIs is to integrate information about the environment (such as population density, food availability, p[CO2], or the presence of sex pheromone) and adjust either the function (Chang et al., 2006) or programming of neural circuits via DAF-7/TGF-β. Mechanisms linking environmental and genetic determinants of behaviors have implications for conceptually similar human conditions such as sexual preference and sexual identity.
Experimental Procedures
Behavior
Sexual attraction assays were as described (White et al., 2007), blind for strain and for pheromone vs. control and scored categorically based on track pattern (details in the Supplemental Experimental Procedures). Strains were cultivated at 20–22°C. At this temperature, daf-7 mutants frequently reach adulthood. The data are categorical (attraction or no attraction) and all data are shown. The number of assays for each condition are indicated in the Figures.
Statistics
Comparisons were made using Fisher‘s exact test at 90% confidence with the Bonfer-roni-Holm correction for multiple comparisons. For comparisons, α was taken at 0.05 unless otherwise indicated. Exact p values after correction are given in each Figure.
Laser ablations
Ablations were performed with a MicroPoint laser system as described (Bargmann and Avery, 1995; White et al., 2007) in L2-, L3-, or L4-stage larvae or young adults. Operated animals were assayed as one-day old adults, or after one day recovery for adult ablations. Ablations were verified post-assay anatomically or by checking for the absence of GFP, if appropriate. ASK and ASI were identified anatomically; other strains contained GFP markers to assist in neuron identification. Strains for ablations are described in detail in Supplementary Table 1.
Molecular biology
For neuron-specific expression of TAX-4 or DAF-7, a cDNA encoding either tax-4 or daf-7 was placed in an artificial operon also expressing either EGFP or mCherry under the control of a neuron-selective promotor and followed by a generic unc-54 3′UTR. Expression vectors were constructed from the following modules: a 4–1 Entry vector containing a cell-selective promotor, a 1–2 Entry vector containing a cDNA in an artificial operon, and a 2–3 Entry vector containing a generic 3 ′UTR from the unc-54 gene Promotors used were Pgpa-4 for ASI, Psrg-2 and Psrg-8 for ASK, Podr-4 for sensory neurons including ASI, Pceh-36 for AWC-ASE, and Prab-3 for the entire nervous system. Reported expression patterns and references are given in Supplemental Table 2.
Inducible neural masculinization
To inducibly masculinize the nervous system, we modified the published FLP-ON strategy (Davis et al., 2008). The masculinizing construct contained in order 5′ to 3′: the Prab-3 promotor, a let-858 stop-cassette marked with mCherry and flanked by FRT sites, EGFP in an artificial operon followed by a fem-3 cDNA (Mehra et al., 1999), and an unc-54 3′ UTR. FLP-recombinase was expressed in a separate construct under the control of the heatshock promotor Phsp16.41. In this strategy, heatshock (1 hr at 33°C) induces expression of FLP-recombinase, which excises the stop-cassette and mCherry, thus allowing expression of EGFP and fem-3. Animals for assays were selected prior to heatshock for no visible EGFP, and after heatshock for robust EGFP in the nervous system. Animals were assayed 24 hours after heatshock; EGFP was visible in the nervous system within 4 hours.
Masculinization of neural subsets
To masculinize subsets of the hermaphrodite nervous system, the Gateway system was used to fuse different neuron-selective promotors to a standard expression cassette containing fem-3 cDNA (Mehra et al., 1999) in an artificial operon with mCherry. Masculinized neurons therefore fluoresce red. For sensory neurons we used Podr-4. For interneurons we used Pglr-5, Pglr-2, Pser-2b, and Punc-17. Reported expression patterns and references are given in Supplemental Tables 2, 3, and 4.
Supplementary Material
Highlights.
Male sexual attraction is latent in the hermaphrodite brain, but normally repressed
Two sensory neurons, the ASIs, release TGF-β during development to engage repression
Males express TGF-β, but repression acts only on the hermaphrodite nervous system
Sexual attraction requires distributed sex differences in sensory- and interneurons
Acknowledgments
The authors wish to thank the Caenorhabditis Genetics Center for strains, Tom Nicholas, Eliott Davidson, Sarah Bodian, Bryan Benham and Nadja Schäfer for constructing reagents; Michael Ailion, Doug Portman, and Bill Mowrey for unpublished reagents and comments; Villu Maricq, Mike Shapiro, Randi Rawson, and Sean Merrill for comments; and Cori Bargmann, Kaveh Ash-rafi, Piali Sengupta, and Kyuhyung Kim for reagents and insight. This work was funded by a National Research Service Award and an American Cancer Society Fellowship to JQW, and NSF grants #0516815 and #0920069 to EMJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RA, Ragains JR, Li W, Ruvkun G, Clardy J, Mak HY. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci U S A. 2009;106:1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BL, Hall DH, Chklovskii DB. Wiring optimization can relate neuronal structure and function. Proc Natl Acad Sci U S A. 2006;103:4723–4728. doi: 10.1073/pnas.0506806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- Coburn CM, Mori I, Ohshima Y, Bargmann CI. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: a distinct pathway for maintenance of sensory axon structure. Development. 1998;125:249–258. doi: 10.1242/dev.125.2.249. [DOI] [PubMed] [Google Scholar]
- Davis MW, Morton JJ, Carroll D, Jorgensen EM. Gene activation using FLP recombinase in C. elegans. PLoS Genet. 2008;4:e1000028. doi: 10.1371/journal.pgen.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M, Attisano L, Wrana JL, Albert PS, Massagué J, Riddle DL. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Greer ER, Pérez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008;8:118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther CV, Georgi LL, Riddle DL. A Caenorhabditis elegans type I TGF-β receptor can function in the absence of type II kinase to promote larval development. Development. 2000;127:3337–3347. doi: 10.1242/dev.127.15.3337. [DOI] [PubMed] [Google Scholar]
- Kalberer NM, Reisenman CE, Hildebrand JG. Male moths bearing transplanted female antennae express characteristically female behaviour and central neural activity. J Exp Biol. 2010;213:1272–1280. doi: 10.1242/jeb.033167. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Liu T, Zimmerman KK, Patterson GI. Regulation of signaling genes by TGF-β during entry into dauer diapause in C. elegans. BMC Dev Biol. 2004;4:11. doi: 10.1186/1471-213X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra A, Gaudet J, Heck L, Kuwabara PE, Spence AM. Negative regulation of male development in Caenorhabditis elegans by a protein-protein interaction between TRA-2A and FEM-3. Genes Dev. 1999;13:1453–1463. doi: 10.1101/gad.13.11.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KM, Sarafi-Reinach TR, Horne JG, Saffer AM, Sengupta P. The DAF-7 TGF-β signaling pathway regulates chemosensory receptor gene expression in C. elegans. Genes Dev. 2002;16:3061–3073. doi: 10.1101/gad.1027702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckol EL, Zallen JA, Yarrow JC, Bargmann CI. Sensory activity affects sensory axon development in C. elegans. Development. 1999;126:1891–1902. doi: 10.1242/dev.126.9.1891. [DOI] [PubMed] [Google Scholar]
- Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Schneiderman AM, Hildebrand JG, Brennan MM, Tumlinson JH. Transsexually grafted antennae alter pheromone-directed behaviour in a moth. Nature. 1986;323:801–803. doi: 10.1038/323801a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Yamamoto Y, Fujiwara M, Tabata T, Murayama T, Hirotsu T, Ikeda DD, Tsunozaki M, Iino Y, Bargmann CI, Katsura I, Ishihara T. Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J Neurosci. 2011;31:3007–3015. doi: 10.1523/JNEUROSCI.4691-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Rose LS, Scholey JM. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol Biol Cell. 1999;10:345–360. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edi-son AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, Sengupta P, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O’Doherty OG, Edison AS, Sternberg PW, Schroeder FC. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Logan DW. Sexual dimorphism in olfactory signaling. Curr Opin Neurobiol. 2010;20:770–775. doi: 10.1016/j.conb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Sulston JE. Neuronal cell lineages in the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):443–452. doi: 10.1101/sqb.1983.048.01.049. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenor-habditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The sensory circuitry for sexual attraction in C. elegans males. Curr Biol. 2007;17:1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.