Abstract
Bacterial pneumonia remains associated with high morbidity and mortality. The gram-positive pathogen Streptococcus pneumoniae is the most common cause of community-acquired pneumonia. Lipoteichoic acid (LTA) is an important proinflammatory component of the gram-positive bacterial cell wall. R-roscovitine, a purine analog, is a potent cyclin-dependent kinase (CDK)-1, −2, −5 and −7 inhibitor that has the ability to inhibit the cell cycle and to induce polymorphonuclear cell (PMN) apoptosis. We sought to investigate the effect of R-roscovitine on LTA-induced activation of cell lines with relevance for lung inflammation in vitro and on lung inflammation elicited by either LTA or viable S. pneumoniae in vivo. In vitro R-roscovitine enhanced apoptosis in PMNs and reduced tumor necrosis factor (TNF)-α and keratinocyte chemoattractant (KC) production in MH-S (alveolar macrophage) and MLE-12/MLE-15 (respiratory epithelial) cell lines. In vivo R-roscovitine treatment reduced PMN numbers in bronchoalveolar lavage fluid during LTA-induced lung inflammation; this effect was reversed by inhibiting apoptosis. Postponed treatment with R-roscovitine (24 and 72 h) diminished PMN numbers in lung tissue during gram-positive pneumonia; this step was associated with a transient increase in pulmonary bacterial loads. R-roscovitine inhibits proinflammatory responses induced by the gram-positive stimuli LTA and S. pneumoniae. R-roscovitine reduces PMN numbers in lungs upon LTA administration by enhancing apoptosis. The reduction in PMN numbers caused by R-roscovitine during S. pneumoniae pneumonia may hamper antibacterial defense.
INTRODUCTION
Bacterial pneumonia is a common infection that, at present, remains associated with high morbidity and mortality (1,2). The gram-positive bacterium Streptococcus pneumoniae is the most common cause of community-acquired pneumonia (3,4). When antibiotics became available, mortality rates decreased from 77% to 28% (5). However, since then, mortality has not decreased dramatically, despite increasing medical aptitude in the following decades (6).
Much has been learned about gram-negative infections and the importance of lipopolysaccharide herein. However, less is known about the host response to gram-positive pathogens. Lipoteichoic acid (LTA) is a major constituent of the outer cell wall of gram-positive bacteria and the predominant mediator of inflammatory responses to these microorganisms (7–9).
A key element of the acute inflammatory response in the lung is the recruitment of polymorphonuclear cells (PMNs) to the bronchoalveolar space. The ability of PMNs to destroy invading microorganisms is potentially destructive for host tissue (10–12). PMNs contain and generate toxic substances that are harmful to the lung when they exocytose their granules and/or undergo uncontrolled necrosis. Therefore, successful resolution of infection entails removal of excess cellular infiltrate (11,13). Herein, apoptosis is a strong regulatory mechanism during lung inflammation (14): phagocytosis of apoptotic PMNs by macrophages reprograms macrophages to release antiinflammatory mediators, thus aiding resolution of (harmful) inflammation (15,16).
The purine analog R-roscovitine is a potent inhibitor of the cyclin-dependent kinase (CDK)-1, −2, −5 and −7, which have the ability to inhibit the cell cycle and to induce apoptosis, especially in PMNs (10,17–19). R-roscovitine’s apoptosis-inducing potential in PMNs has previously been established in several in vivo models of inflammation and infection, resulting in improved resolution of inflammation (20–22). Besides inducing beneficial PMN apoptosis, R-roscovitine possibly has direct antiinflammatory properties by inhibiting the transcription of proinflammatory cytokines in macrophages (19).
As proof of principle, we sought to investigate if the CDK inhibitor drug R-roscovitine reduces lung inflammation elicited by either LTA or viable S. pneumoniae in vivo. In the pneumonia setting, we combined R-roscovitine treatment with ceftriaxone, an antibiotic active against gram-positive and gram-negative bacteria, which was previously used in our laboratory in models of pneumococcal pneumonia (23,24). In the current study, we demonstrate that R-roscovitine has potent antiinflammatory effects in vitro on cells important for innate immune response of the pulmonary compartment. In vivo, R-roscovitine treatment reduced acute lung inflammation induced by purified LTA and S. pneumoniae.
MATERIALS AND METHODS
Experiments with Purified PMNs
Peripheral blood was drawn from healthy volunteers in tubes containing ethylenediaminetetraacetic acid (EDTA). Within 2 h of drawing blood, PMNs were isolated using Polymorphprep (Axis-Shield, Oslo, Norway). After isolation, 99% of PMNs were negative for trypane blue staining. There were 2 × 105 cells per well seeded in a 24-well plate. Cells were treated with 20 μmol/L R-roscovitine (LC Labs, Woburn, MA, USA) or vehicle (0.05% dimethyl sulfoxide [DMSO]) and incubated in RPMI 1640 (Gibco; Life Technologies Ltd, Paisley, UK) supplemented with 10% autologous serum. For apoptosis analysis, cells were incubated with 20 μmol/L R-roscovitine combined with 40 μmol/L zVAD-fmk (carboben-zoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone; Sigma-Aldrich, St. Louis, MO, USA), vehicle (0.05% DMSO) control or vehicle and 40 μmol/L zVAD-fmk. After 6 h, cells were lysed with lysis buffer (Cell Signaling Technology, Danvers, MA, USA), and 3 × Laemmli buffer was added before incubating the samples at 95°C for 5 min. Purity of the isolated cells was determined by assessing Giemsa-stained cytospin preparations (>90% PMNs). In a similar setup after 6 h of roscovitine or vehicle treatment, cells were stained using the Annexin V–FITC (fluorescein isothiocyanate) Apoptosis Detection Kit I (BD Pharmingen; BD, Franklin Lakes, NJ, USA) and analyzed on a FACSCalibur (BD Biosciences, San Jose, CA, USA).
Cell Line Experiments
The effects of R-roscovitine on cytokine responses of lung epithelium and resident macrophages were tested as follows: 1 × 105 MH-S (alveolar macrophage cell line; American Type Culture Collection, Rockville, MD, USA), MLE-12 and MLE-15 (mouse lung epithelial cell lines; provided by Jeffrey Whitsett, Division of Pulmonary Biology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center and the University of Cincinnati College of Medicine, Cincinnati, OH, USA) cells were seeded in a 24-well plate (Millipore, Billerica, MA, USA). MH-S cells were incubated with Iscove’s modified Dulbecco’s medium (IMDM; Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum (FCS) (Hycult, Uden, the Netherlands), 2 mmol/L l-glutamine (Sigma-Aldrich) and 0.1 units/mL penicillin-streptomycin (Sigma-Aldrich); MLE-12 and MLE-15 were maintained in RPMI 1640 supplemented with 2% FCS, 0.01 mg/mL insulin (Invitrogen; Life Technologies Ltd), 0.01 mg/mL transferrin (Invitrogen; Life Technologies Ltd), 30 nmol/L sodium selenite (Invitrogen; Life Technologies Ltd), 10 nmol/L hydro-cortisol (Sigma-Aldrich), 10 nmol/L β-estradiol (Sigma-Aldrich), 2 mmol/L l-glutamine and 0.04 units/mL penicillin-streptomycin in an atmosphere of 5% CO2.
After 24 h of culture, cells were stimulated with 10 μg/mL Staphylococcus aureus LTA (purified from S. aureus; endotoxin level: <1.25 endotoxin units (EU)/mg; InvivoGen, San Diego, CA, USA). Simultaneously, cells were treated with 20 μmol/L R-roscovitine or vehicle (0.05% DMSO). At 4 and 8 h, supernatant was harvested for enzyme-linked immunosorbent assay (ELISA). Cell viability was assessed by adding MTT ([3-(4,5-methylthiazol-2-yl)-2,5-dipheyl-tetrazolium bromide]; Sigma-Aldrich) reagent to all wells for 60 min. Supernatant was discarded, and the cells were lysed in acidic isopropanol (Merck, Darmstadt, Germany). Absorbance was measured at 570 nm.
Animals
For all in vivo experiments, female C57Bl6 mice (aged 10–12 wks) were purchased from Charles River (Maastricht, the Netherlands). The Animal Care and Use Committee of the University of Amsterdam approved all experiments.
Induction of Lung Inflammation and Pneumonia
Acute lung inflammation was induced by intranasal instillation of mice, anesthesized using isoflurane (Upjohn, Ede, the Netherlands) inhalation, with 100 μg LTA (InvivoGen) in 50 μL saline (8,9). Pneumonia was induced as previously described (25,26). In brief, S. pneumoniae serotype 3 (ATCC 6303) was grown to a mid-logarithmic phase at 37°C in Todd-Hewitt broth enriched with 0.5% yeast extract. Bacteria were harvested by centrifugation at 2,900g for 15 min and washed twice in sterile saline. For inoculation, bacteria were suspended in sterile saline at a concentration of 5 × 104 CFU (colony-forming units)/50 μL. Mice were anesthetized by isoflurane inhalation, and 5 × 104 CFUs were instilled intra nasally. At predefined endpoints, mice were anesthetized with Domitor (Pfizer Animal Health Care, Capelle aan den IJssel, the Netherlands: active ingredient medetomidine) and Nimatek (Eurovet Animal Health, Bladel, the Netherlands: active ingredient keta-mine) and sacrificed by cardiac puncture followed by cervical dislocation.
R-roscovitine and Ceftriaxone Administration
In the sterile lung inflammation experiments, 70 mg/kg R-roscovitine in 200 μL 10% DMSO/saline or 200 μL 10% DMSO/saline (vehicle) was administered intra -peritoneally at the start of the experiments. In a second set of experiments, mice were additionally treated with zVAD-fmk (5 mg/kg) or vehicle intraperitoneally (10% DMSO/saline) at the moment of LTA instillation. For the pneumonia experiments, 70 mg/kg R-roscovitine or vehicle was administered 24 h after infection, and a second dose was given at 72 h. A total of 20 mg/kg ceftriaxone (Fresenius Kabi, Bad Homburg, Germany) in 200 μL saline was administered intraperitoneally at 24 and 72 h after infection to all animals.
Bronchoalveolar Lavage
Through a midline incision, the trachea and lungs were exposed; the right lung was isolated from the airways via a suture. The trachea was cannulated with a 22-G Abbocath-T catheter (Abbott, Sligo, Ireland), and the left lung was instilled with two times 0.4 mL sterile phosphate-buffered saline. The fluid was retrieved and weighed and total cell counts were determined with a Coulter cell counter (Beckman Coulter, Fullerton, CA, USA). Differential cell counts were performed on Giemsa-stained cytospin preparations.
Determination of Bacterial Load
After sacrificing the animals as described above and taking blood samples, whole lungs and spleens were harvested and homogenized in four volumes of sterile saline with a tissue homogenizer (ProScience, Oxford, CT, USA). CFUs were determined from serial dilutions of the samples, plated on blood agar plates and incubated at 37°C for 16 h before colonies were counted.
Preparation of Homogenates
For cytokine measurements, right lungs were excised, weighed and diluted 1:4 in sterile saline. After homogenization, samples were diluted with one volume of lysis buffer containing 300 mmol/L NaCl, 30 mmol/L Tris, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 2% Triton X-100 and AEBSF [4-(2-aminoethyl)benzeensulfonyl fluoride, EDTA-Na2, pepstatin and leupeptin] (all 8 μg/mL, pH 7.4; Sigma-Aldrich) and incubated at 4°C for 30 min. Homogenates were centrifuged at 2,900g at 4°C for 15 min, and supernatants were stored at −20°C until assays were performed.
Assays
Annexin V binding in bronchoalveolar lavage (BAL) cells was assessed using an Annexin V PE Apoptosis Detection Kit PE (eBioscience, San Diego, CA, USA) in combination with anti-Ly-6G FITC (Miltenyi Biotec, Leiden, the Netherlands) and analyzed on an FACS Calibur (BD Biosciences). Interleukin (IL)-6, tumor necrosis factor (TNF)-α, cytokine-induced neutrophil keratinocyte chemoattractant (KC) and macrophage inflammatory protein (MIP)-2 were measured using ELISAs (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Total protein concentrations were measured using a DC protein assay (Bio-Rad Laboratories, Veenendaal, the Netherlands). Detection limits were as follows: 125 pg/mL for TNF-α and MIP-2; 31.25 pg/mL for IL-6 and KC.
Histological Examination
For histological examination, lungs were harvested at 48 and 96 h of bacterial infection or after 6 and 24 h after induction of sterile inflammation. These samples were fixed in formalin and embedded in paraffin. The 5-μm sections were made and stained with hematoxylin and eosin. The lungs were scored by a pathologist blinded for experimental groups for the following parameters, at a scale of 0 (absent) to 4 (very severe): interstitial damage, endothelialitis, peribronchitis, edema, thrombus formation and pleuritis. PMN staining was performed as described (26). In brief, slides were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched by a solution of 0.03% H2O2 (Merck). Slides were then digested by a solution of pepsin 0.25% (Sigma-Aldrich) in 0.01 mol/L HCl. After being rinsed, the sections were incubated in 10% normal goat serum (Dako, Glostrup, Denmark) and then exposed to FITC-labeled anti-mouse Ly-6G monoclonal antibody (Pharmingen, San Diego, CA, USA). After washes, slides were incubated with a rabbit anti-FITC antibody (Dako) followed by further incubation with a biotinylated swine anti-rabbit antibody (Dako), rinsed, incubated in a streptavidine-ABC solution (Dako) and developed using 1% H2O2 and 3.3-diaminobenzidin-tetra-hydrochoride (Sigma) in Tris-HCl. The sections were counterstained with methyl green and mounted in Pertex mounting medium (Leica Microsystems, Rijswijk, the Netherlands). The Ly-6G+ percentage of total lung surface was determined with ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij, 1997–2011).
Western Blotting
Samples for Western blotting were boiled at 95°C for 5 min in 3× Laemmli buffer and loaded onto sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels. After electrophoresis, the content of the gels was transferred onto Immobilon-PVDF (polyvinylidene fluoride) membranes (Millipore). The membranes were blocked in 5% bovine serum albumin (Roche, Basel, Switzerland) in Tris-buffered saline–Tween at room temperature for 60 min. Pan-CDK phosphorylated substrate and cleaved caspase-3 (Cell Signaling Technology) antibodies were diluted 1:500; β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted 1:4,000. The membranes were incubated overnight at 4°C. Next, the membranes were incubated for 60 min with anti-rabbit–horseradish peroxidase–conjugated secondary antibody (Cell Signaling Technology), and blots were imaged using LumiLight Plus ECL (Roche, Basel, Switzerland) on a LAS 4000 chemiluminescence imager (GE Healthcare Biosciences, Pittsburgh, PA, USA). Quantification was performed using ImageJ software.
Statistical Analysis
Data are expressed as means ± standard error of the mean (SEM). For in vivo data, two sample comparisons were performed by Mann-Whitney U tests by using GraphPad Prism, version 5.01 (GraphPad Software, San Diego, CA, USA). Comparisons between multiple groups were done using Kruskall-Wallis tests; if overall significant, individual groups were assessed by Mann-Whitney U tests. For in vitro analysis, Student t tests were applied. Multiple group analysis was performed by analysis of variance, with Bonferroni post hoc tests. P < 0.05 was considered statistically significant. Normality was determined by D’Agostino and Pearson omnibus normality test or Q-Q plots.
RESULTS
R-roscovitine Reduces CDK Activity and Increases Apoptosis in PMNs
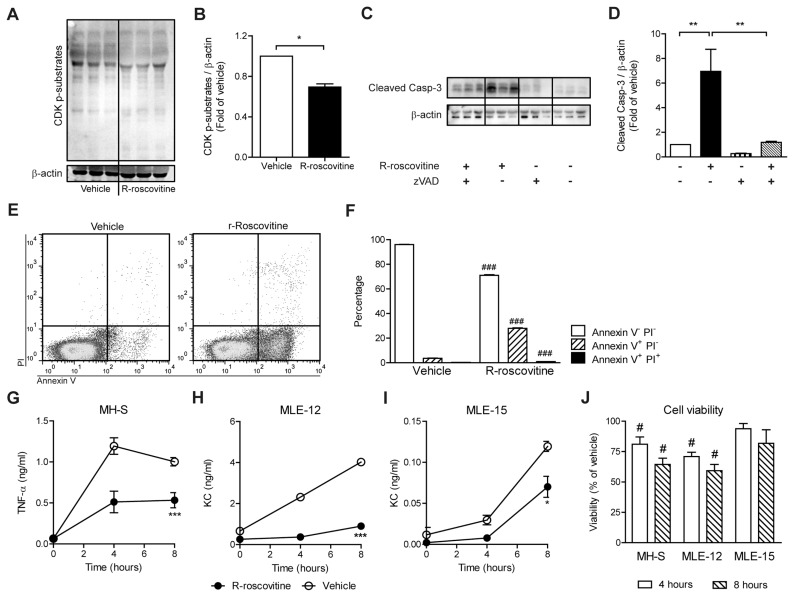
R-roscovitine is an inhibitor of CDK-1, −2, −5 and −7, as assessed with purified kinase assays (10,27). To test if R-roscovitine can reduce CDK activation in vitro, the phosphorylation state of CDK substrates in freshly isolated PMNs was assessed by Western blot. Compared to vehicle, a statistically significant (P < 0.05) decrease in phosphorylation of pan-CDK substrates was observed because of R-roscovitine treatment (Figures 1A, B). Recent studies demonstrated that R-roscovitine can induce apoptosis in PMNs (20,22). We confirmed that R-roscovitine–treated PMNs exhibit significantly enhanced levels of cleaved caspase-3 compared with vehicle after 6 h (Figures 1C, D; P < 0.01). Combined treatment with the caspase inhibitor zVAD-fmk reversed the amount of cleaved caspase-3 to near vehicle levels (P < 0.05), indicating that R-roscovitine induced caspase-dependent apoptosis. In accordance, incubation with R-roscovitine also resulted in enhanced annexin V+ PI−staining in PMNs, together with a slight increase in annexin V+ PI+ staining, when compared with vehicle (Figures 1E, F; P < 0.001).
Figure 1.
R-roscovitine inhibits CDKs, enhances caspase-3 cleavage and reduces inflammatory mediator production in vitro. Within 2 h of drawing, PMNs were isolated from peripheral blood and treated with 20 μmol/L R-roscovitine or vehicle (0.05% DMSO) for 6 h to determine pan-CDK substrate phosphorylation status (A, B). To determine the effects on caspase-3, PMNs were incubated with vehicle or 20 μmol/L R-roscovitine in the presence or absence of 40 μmol/L caspase inhibitor zVAD-fmk for 6 and 24 h (C, D). Annexin V binding was determined in vehicle- or R-roscovitine–treated cells. (E) PI + annexin V staining of vehicle and R-roscovitine treatment. (F) Bars show percentage positive PMNs for the staining indicated. Effects of R-roscovitine on TNF-α production were assessed in MH-S (alveolar macrophage cell line) (G) and KC production in MLE-12 (H) and MLE-15 (I) (mouse lung epithelial cell lines). Cells were stimulated with 10 μg/mL S. aureus LTA and treated with 20 μmol/L R-roscovitine (closed symbols) or vehicle (open symbols) for 4 and 8 h. Cell viability was determined by an MTT assay; the viability of vehicle-treated cells was set to 100% (J). The bar graphs (B, D) represent densitometric quantification of the relative amounts of pan-CDK phosphorylated substrates or cleaved caspase-3 normalized for β-actin as fold-change of vehicle. Data are expressed as means ± SEM; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05 and ###P < 0.001 versus vehicle control.
R-roscovitine Inhibits LTA-Induced Inflammatory Mediator Production In Vitro
Previously, R-roscovitine was shown to reduce COX-2, IL-6, IL-1β and TNF-α mRNA expression levels in LPS-stimulated RAW264.7 cells (murine macrophages) (19,28). We set out to investigate the effect of R-roscovitine on protein levels, rather than mRNA expression levels, of inflammatory mediators. In vitro, we tested three typical murine lung cell lines: MH-S (alveolar macrophages), MLE-12 and MLE-15 (alveolar epithelium cell lines). Cells were stimulated with 10 μg/mL LTA, and cytokine production was determined at 0, 4 and 8 h. R-roscovitine treatment strongly reduced TNF-α production in MH-S cells throughout 8 h of stimulation (Figure 1G; P < 0.01). Similar effects were observed in MLE-12 and MLE-15 cells, in which R-roscovitine treatment inhibited KC production (P < 0.001) (Figures 1H, I).
Viability was determined by MTT assay and was reduced by R-roscovitine in all cell lines after 4 and 8 h compared with vehicle-treated cells (Figure 1J). The effect of R-roscovitine treatment on viability was strongest on MLE-12 cells.
R-roscovitine Reduces LTA-Induced Acute Lung Inflammation
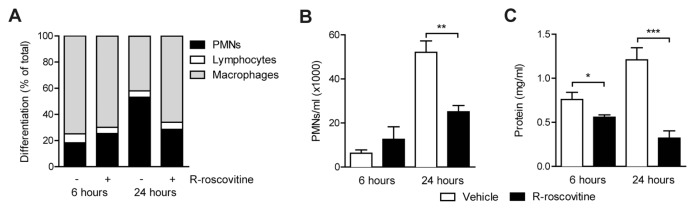
After demonstrating that R-roscovitine enhanced apoptosis in PMNs and reduced inflammatory mediator production in vitro, we applied R-roscovitine in vivo in LTA-induced lung inflammation. At t = 0 h, 100 μg LTA was administered intranasally and 70 mg/kg R-roscovitine or vehicle was injected intraperitoneally simultaneously. After 6 h of LTA inflammation, no differences in PMN counts were detected in BAL fluid of the two treatment groups. At 24 h, however, R-roscovitine treatment greatly reduced BAL fluid PMN counts (Figures 2A, B; P < 0.001 versus vehicle). Of note, no changes in cellular composition of blood collected from these mice were observed, ensuring that the observed differences did not result from R-roscovitine–induced leukocytopenia (data not shown).
Figure 2.
R-roscovitine reduces PMNs and total protein levels in LTA-induced lung inflammation. Lung inflammation was induced by intranasal instillation of 100 μg LTA. Simultaneously, 70 mg/kg R-roscovitine or vehicle (0.05% DMSO) was administered intraperitoneally. After 6 and 24 h, samples were harvested. Cell differentiations (A) and PMN numbers (B) were determined on Giemsa-stained cytospin preparations derived from BAL fluid. Total protein (C) levels were measured in BAL fluid. Data are expressed as mean ± SEM; n = 8. *P < 0.05; **P < 0.01, ***P < 0.001.
To measure vascular leak, total protein levels in BAL fluid were measured. After 6 h, treatment with R-roscovitine lowered protein levels by 26% (Figure 2C; P < 0.05 versus vehicle). At 24 h, this difference increased to 68% reduction (see Figure 2C; P < 0.001).
A strong effect of treatment was observed on cytokines and chemokine levels in the lung. Levels of TNF-α and chemokines KC and MIP-2 were reduced at both 6 and 24 h with R-roscovitine treatment (Table 1). IL-6 was reduced at the 24-h time point.
Table 1.
R-roscovitine inhibits cytokine and chemokine release in BAL fluid upon intrapulmonary delivery of LTA.
| TNF-α | IL-6 | KC | MIP-2 | ||
|---|---|---|---|---|---|
| 6 h | Vehicle | 0.61 ± 0.03 | 0.28 ± 0.02 | 0.96 ± 0.05 | 2.26 ± 0.11 |
| R-roscovitine | 0.48 ± 0.04a | 0.23 ± 0.02 | 1.05 ± 0.14 | 2.07 ± 0.10 | |
| 24 h | Vehicle | 15.96 ± 4.11 | 1.75 ± 0.24 | 1.31 ± 0.13 | 15.97 ± 1.67 |
| R-roscovitine | 3.68 ± 0.37a | 0.54 ± 0.04b | 0.57 ± 0.04b | 7.37 ± 0.42c |
Lung inflammation was induced by intranasal instillation of 100 μg LTA. Concurrently, 70 mg/kg R-roscovitine or vehicle (0.05% DMSO) was administered intraperitoneally. After 6 and 24 h, samples were harvested. Data are expressed as mean ± SEM in ng/mL. n = 8 per group at each time point;
P < 0.05;
P < 0.001;
P < 0.01 versus vehicle.
zVAD-fmk Reverts R-roscovitine–Induced Reduction in PMN Counts and Protein Levels in BAL Fluid
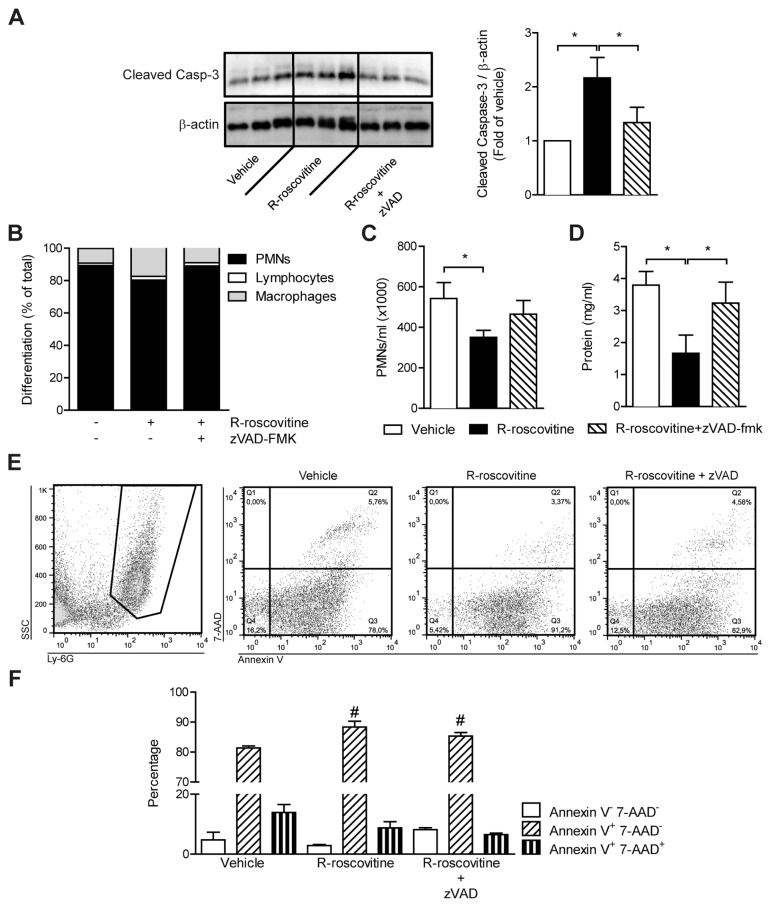
To assess if the observed effects of R-roscovitine solely depend on apoptosis induced by this compound, we combined R-roscovitine treatment with injection of the general caspase inhibitor zVAD-fmk (20). From the cells pelleted from BAL fluid after 24 h, cleaved caspase-3 levels were determined by Western blot (Figure 3A). As expected, R-roscovitine treatment enhanced cleaved caspase-3 levels in BAL fluid cells (P < 0.01). Combined treatment with zVAD-fmk reverted the cleaved caspase-3 levels to control levels. In line with the diminished cleaved caspase-3 levels, zVAD-fmk treatment prevented the R-roscovitine effects on PMN counts in BAL fluid (Figure 3B). Lung damage was assessed by measuring total protein in the BAL fluid. Total protein was reduced by R-roscovitine treatment (P < 0.05), and zVAD-fmk attenuated this effect (Figure 3C).
Figure 3.
zVAD-fmk reverts R-roscovitine–induced reduction in PMNs and protein content of BAL fluid. Lung inflammation was induced by intranasal instillation of 100 μg LTA. Simultaneously, 70 mg/kg R-roscovitine, 70 mg/kg R-roscovitine combined with 5 mg/kg zVAD-fmk or vehicle (10% DMSO) was administered intraperitoneally. After 24 h, animals were sacrificed and samples were taken. Cellular content obtained from pelleted BAL fluid was lysed and cleaved caspase-3 levels were determined by Western blot; representative Western blot is shown (A). The bar graph expresses data as fold-change of vehicle. Cell differentiations (B) and PMN counts (C) and total protein (D) were measured in BAL fluid. Annexin V binding was assessed in BAL-obtained cells. (E) The scatter plot of side scatter and Ly-6G staining (left) and scatter plots of 7-AAD + annexin V staining for vehicle, R-roscovitine and R-roscovitine + zVAD-fmk treatment (right graphs). (F) Bars show percentage-positive PMNs for the staining indicated. Data are expressed as mean ± SEM; n = 4–8. *P < 0.05, #P < 0.05 versus vehicle control.
Ly-6G–positive cells from the BAL fluid were assessed for annexin V and 7-aminoactinomycin D (7-AAD) binding by fluorescence-activated cell sorting. Annexin V+ 7-AAD− is indicative of early-stage apoptosis, whereas annexin V+ 7-AAD+–stained cells are either late apoptotic or necrotic (29). In line with the Western blot, R-roscovitine treatment enhanced annexin V+ 7-AAD− staining in Ly-6G–positive cells (Figures 3E, F; P < 0.05). The combination of R-roscovitine and zVAD-fmk resulted in less annexin V+ 7-AAD− staining, although staining was still significantly higher than in the vehicle treatment (Figures 3E, F; P < 0.05).
R-roscovitine Treatment Lowers PMN Counts in BAL Fluid During Gram-Positive Pneumonia
The experiments described above revealed that concurrent treatment with R-roscovitine attenuates acute lung inflammation induced by intrapulmonary delivery of LTA, as reflected by reductions in PMN counts, protein leak and cytokine/chemokine levels in BAL fluid. We were interested in determining the effect of R-roscovitine on an already ongoing and increasing inflammatory response in the lung elicited by a gram-positive stimulus. For this step, we chose our established model of gram-positive pneumonia in which mice are first challenged with a high dose of the clinically relevant respiratory pathogen S. pneumoniae and treated 24 h later with ceftriaxone (24,30). For the purpose of this study, ceftriaxone treatment was given 24 and 72 h after infection and combined with either R-roscovitine or vehicle.
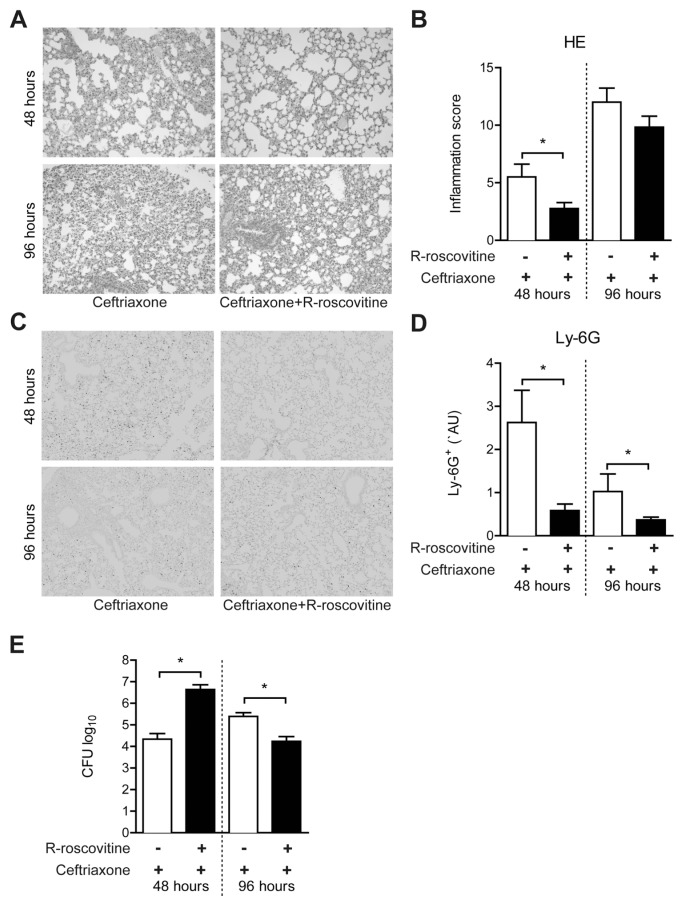
To evaluate the effect of R-roscovitine in this setting, histological slides were prepared from lungs 48 or 96 h after infection and stained with hematoxylin and eosin to assess histopathology. At 48 h, R-roscovitine–treated animals demonstrated a reduction in inflammation score; this difference had subsided at 96 h (Figures 4A, B). To determine the number of PMNs in lung tissue, slides were stained for Ly-6G and quantified (Figures 4C, D). In accordance with the effect of R-roscovitine during LTA- induced acute lung inflammation, treatment with R-roscovitine strongly reduced the number of Ly-6G+ cells in the lungs after 48 h of pneumonia. At 96 h, the number of Ly6+ cells was markedly lower in both groups, but still significantly lower in R-roscovitine–treated mice.
Figure 4.
R-roscovitine exerts antiinflammatory effects during gram-positive pneumonia. Gram-positive pneumonia was induced by intranasal inoculation of 5 × 104 CFU serotype 3 S. pneumoniae. Ceftriaxone (20 mg/kg) in combination with 70 mg/kg R-roscovitine or vehicle (10 % DMSO) was injected intraperitoneally after 24 and 72 h. Representative lung histology of ceftriaxone and R-roscovitine + ceftriaxone treatment (A) with corresponding inflammation scores (B) is shown. Representative images of Ly-6G staining on lung slides (C) and Ly-6G+ lung surface percentage (D) are shown. CFUs were determined in lung homogenates (E). Data are expressed as mean ± SEM; n = 8. *P < 0.05.
PMNs are an essential part of host defense against S. pneumoniae (31). We therefore wondered whether the effect of R-roscovitine on PMN numbers would affect bacterial growth and dissemination. Indeed, R-roscovitine treatment resulted in higher bacterial loads at 48 h after infection compared with vehicle (Figure 4E). Strikingly, the opposite effect was seen at 96 h; whereas bacterial burdens in the lungs of vehicle-treated mice had increased, R-roscovitine–administered animals displayed a reduction in the number of pneumococci recovered from the lungs, which were significantly lower than in vehicle controls (see Figure 4E). Bacterial loads in blood and spleen were influenced by R-roscovitine in the same (opposite) direction, although, due to a large individual variation, this is not statistically significant (data not shown).
R-roscovitine exerted variable effects on the levels of cytokines (TNF-α, IL-6) and chemokines (KC, MIP-2) in lung homogenates (Table 2). At 48 h, mediator levels were either similar or higher in R-roscovitine–treated mice (significantly for IL-6 and MIP-2). In contrast, at 96 h, mediator levels were either similar or lower in animals administered R-roscovitine (significantly for MIP-2).
Table 2.
Impact of R-roscovitine on cytokine and chemokine levels in whole lung homogenates during S. pneumoniae pneumonia.
| TNF-α | IL-6 | KC | MIP-2 | ||
|---|---|---|---|---|---|
| 48 h | Ceftriaxone | 3.21 ± 0.33 | 0.37 ± 0.12 | 1.29 ± 0.31 | 1.31 ± 0.10 |
| R-roscovitine + ceftriaxone | 4.78 ± 0.76 | 4.47 ± 2.52a | 1.41 ± 0.20 | 3.41 ± 0.33b | |
| 96 h | Ceftriaxone | 1.41 ± 0.13 | 3.65 ± 1.30 | 5.34 ± 2.69 | 7.31 ± 2.05 |
| R-roscovitine + ceftriaxone | 1.40 ± 0.22 | 3.26 ± 1.92 | 1.59 ± 0.55 | 1.30 ± 0.39b |
Pneumococcal pneumonia was induced by intranasal inoculation of 5 × 104 CFU serotype 3 S. pneumoniae (ATCC 6303). Ceftriaxone, 20 mg/kg, in combination with 70 mg/kg R-roscovitine or vehicle (10 % DMSO) was injected intraperitoneally after 24 and 72 h. TNF-α, IL-6, KC and MIP-2 were determined in lung homogenates. Data are expressed as mean ± SEM in ng/mL. n = 8 per group at each time point;
P < 0.05;
P < 0.01 versus vehicle.
DISCUSSION
Pneumonia is associated with a profound inflammatory response within the pulmonary compartment. Inflammation induced by gram-positive pathogens is predominantly elicited by bacterial cell wall components such as LTA and peptidoglycan (32). Pulmonary host defense against invading pathogens invariably involves activating macrophages and epithelial cells, releasing proinflammatory cytokines and chemokines and recruiting PMNs. These cells are to be removed for proper resolution of infection, since uncontrolled accumulation and/or activation may lead to severe lung damage (33,34).
We sought to determine the effect of the CDK inhibitor R-roscovitine on acute lung inflammation induced by intrapulmonary instillation of LTA and on already-established and progressing lung inflammation induced by S. pneumoniae. Although initially identified as key components of the cell cycle machinery, CDKs have played a role in cell differentiation, transcription (CDK-7 and −9), neural function and apoptosis, especially in PMNs (20,21). Our data indicate that R-roscovitine exerts profound antiinflammatory effects in lung inflammation elicited by either LTA or viable pneumococci.
We set out to assess the effects of R-roscovitine on freshly isolated PMNs. We used a novel approach to observe the effects of R-roscovitine on CDK activity: by using a pan-CDK phosphorylated substrate antibody, we found that PMN CDK activity was indeed reduced by R-roscovitine. Furthermore, we validated that R-roscovitine induces apoptosis in PMNs, by measuring increased levels of cleaved caspase-3 when comparing R-roscovitine–treated PMNs to vehicle controls (Figure 1). This result is in accordance with previous findings (20).
Because our interest is focused on the pulmonary compartment, we sought to investigate the effects of R-roscovitine on inflammatory responses in cells commonly present in the lung. R-roscovitine was shown to reduce TNF-α, IL-6 and IL-1β mRNA levels in the RAW 264.7 cell line (19). We expanded these findings with protein measurements of TNF-α and KC in three mouse cell lines relevant for lung inflammatory responses. In our experiments, these cell lines were less viable when treated with R-roscovitine (viability was reduced when compared with vehicle-treated cells). We believe the effects of R-roscovitine on inflammatory mediator production are not solely due to toxicity, since the difference in MTT levels is far less than the inhibition of cytokine/chemokine expression. How R-roscovitine influences cytokine and chemokine production is not well known. Several mechanisms have been proposed: myeloid cell leukemia sequence 1 (MCL-1) modulation of phosphatidylinositol 3-kinase and of mitogen-activated protein kinase signaling (35), as well as direct inhibition of IκB kinase by R-roscovitine (36). Moreover, inhibition of E2F transcription factor 1 (E2F1), linking nuclear factor κ–light chain enhancer of activated B cells (NF-κB) and cell cycle effects, has been implicated (19); however, R-roscovitine does not exert an effect on NF-κB in PMNs (37).
These strong in vitro effects prompted us to investigate effects of R-roscovitine during in vivo pulmonary inflammation. To this end, mice were intranasally instilled with LTA and treated with R-roscovitine or vehicle. R-roscovitine treatment especially attenuated inflammation at 24 hours after LTA administration. Whereas previous studies did not investigate R-roscovitine effects early after induction of inflammation in vivo, our investigations revealed only minor effects of this compound 6 h after LTA instillation. Our findings at 24 h concur with described reduced levels of IL-6, IFN-γ and MCP-1 after R-roscovitine treatment in carrageenan-induced pleurisy (20). Furthermore, the reduction of PMN influx into the lung is in accordance with previous studies showing a reduction of PMN counts in the pulmonary space in bleomycin-induced lung inflammation (20) and in cerebrospinal fluid in a mouse model for meningitis (22). Furthermore, we observed zVAD-fmk reversible cleaved caspase-3 levels in cells from the BAL fluid due to R-roscovitine treatment, indicating that it is the apoptosis-inducing effect of R-roscovitine that is responsible for the reduction in PMN counts. In vitro, direct antiinflammatory effects were observed because of R-roscovitine treatment, whereas in vivo, it seemed that induction of apoptosis is the primary effect of R-roscovitine treatment. In vitro, treatment was performed directly on the cell. It could well be that concentrations of R-roscovitine resulting in in vitro effects are not reached at epithelial and lung macrophage cells in vivo because of systemic treatment. However, in LTA inflammation at 6 h, there was no effect of R-roscovitine on PMN counts, whereas at this time, TNF-α levels were already reduced in the roscovitine-treated animals compared with the vehicle control. Further research is necessary to dissect the roles of apoptosis induction and antiinflammatory effects in the pulmonary compartment due to R-roscovitine.
LTA is known to contribute to the prolongation of the PMN lifespan (38). Increased lifespan is accompanied by increased probability of induction of damage to neighboring cells (34). Thus, induction of apoptosis in PMNs potentially reduces the chances of bystander damage. Moreover, phagocytosis of apoptotic PMNs by macrophages elicits release of antiinflammatory mediators, aiding resolution of (harmful) inflammation (15,16). The exact mechanism by which CDK inhibition contributes to apoptosis is elusive, although this property is attributed to destabilization of MCL-1, a prosurvival factor, which may play a role (21,37,39). It should be noted that CDK inhibition may also induce senescence (40). In the bloodstream, PMNs may be drawn to migrate into either tissues or senescence in the bone marrow before being phagocytosed (41). In the present study, we did not investigate this mechanism in PMNs. Therefore, we cannot exclude that the reduced numbers of PMNs in R-roscovitine–treated animals are due to senescence. R-roscovitine treatment per se did not alter systemic PMN counts (data not shown). As found in vitro, we observed enhanced cleaved caspase-3 levels in BAL fluid cells of R-roscovitine–treated mice, coinciding with reduced PMN counts (relative and absolute) and enhanced annexin V binding to PMNs. This result is indicative of the effect of R-roscovitine treatment on the arrival of PMNs in the lung compartment.
In vivo, zVAD-fmk cotreatment with R-roscovitine, as expected from a pan-caspase inhibitor, effectively inhibited cleaved caspase-3 formation; however, annexin V binding was not statistically altered compared with R-roscovitine. Whereas caspase-3 is indicative of end-stage apoptosis signaling, annexin V staining (that is, binding of flipped phosphatidylserine [PS] in the cell membrane) is an effect of apoptosis. Our observation suggests occurrence of non–caspase-dependent induction of apoptosis, which would be in agreement with the observed intermediate level of BAL PMNs in R-roscovitine + zVAD-fmk cotreatment. Few annexin V+ 7-AAD+ cells were measured, which could be because of either the experimental procedures or the phagocytosis (for example, by macrophages), since extracellular membrane PS targets cells for phagocytosis (13).
To expand our data on R-roscovitine effects on LTA-induced pulmonary inflammation, we determined the impact of R-roscovitine on a progressing inflammatory response in the lung elicited by the clinically relevant gram-positive pathogen S. pneumoniae. R-roscovitine treatment diminished PMN numbers in lung tissue at both 48 and 96 hours after infection; this result was associated with lower lung inflammation scores at 48 hours, but not at 96 hours. Interestingly, bacterial loads were 2.5 log10 higher at 48 h followed by a log10 reduction at the later time point due to R-roscovitine treatment. From our point of view, these data stress the importance of PMNs in the elimination of invading pathogens (48 hours) as well as PMN hindrance in resolution of infection/inflammation (96 hours) (11,42). In accordance, in a murine model of S. pneumoniae meningitis, R-roscovitine significantly improved the resolution of brain inflammation and accelerated recovery in the context of antibiotic therapy (22). R-roscovitine did not have a strong influence on cytokine and chemokine levels in lung homogenates harvested from infected mice, which likely is related to the fact that the extent of pulmonary mediator production strongly correlates with the bacterial burdens in this model of pneumococcal pneumonia (43,44).
R-roscovitine–treated animals exhibited reduced Ly-6G+ staining at both 48 and 96 hours. At 96 hours, the overall diminished Ly-6G+ staining suggests that PMN amounts had decreased in the pulmonary compartment compared with 48 hours after infection. Given the ongoing infection, it is not unexpected that the lung inflammation scores increase over time. Bacterial loads remain in excess of 104 CFUs throughout the time course and thus lung inflammation damage may accumulate. The decreasing PMN numbers in the presence of increasing total lung inflammation scores suggest that, in prolonged infection, neutrophils are not the sole cause of tissue inflammation and damage.
CONCLUSION
We show that R-roscovitine strongly reduces acute lung inflammation induced by LTA. Postponed administration of this compound after induction of gram-positive pneumonia similarly reduced PMN counts in lungs, which was accompanied by a primary increase and secondary decrease in lung bacterial loads. This step indicates that there is a possible role for CDK inhibition in infectious diseases: where antibiotics eradicate bacteria, R-roscovitine will likely diminish the severity of lung injury driven by microbial components as well as host inflammatory mediators. Despite being well tolerated by humans (45,46), clinical use of R-roscovitine to reduce lung injury is still far away. Further research is warranted to dissect the effects of R-roscovitine and CDK inhibition on pulmonary inflammation in the setting of infectious and noninfectious disease.
ACKNOWLEDGMENTS
This work was supported by a grant from the Netherlands Organization for Scientific Research to C W Wieland. We thank Joost Daalhuisen, Marieke ten Brink, Regina de Beer and Onno de Boer for expert technical assistance. We are indebted to Jeffrey Whitsett (Division of Pulmonary Biology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, and the University of Cincinnati College of Medicine, Cincinnati, OH, USA) for providing mouse respiratory epithelial cell lines.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Tsai KS, Grayson MH. Pulmonary defense mechanisms against pneumonia and sepsis. Curr Opin Pulm Med. 2008;14:260–5. doi: 10.1097/MCP.0b013e3282f76457. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RN, Smith BL. Deaths: leading causes for 2002. Natl Vital Stat Rep. 2005;53:1–89. [PubMed] [Google Scholar]
- 3.Niederman MS, et al. Guidelines for the Management of Adults with Community-acquired Pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–54. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 4.Feikin DR, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am. J. Public Health. 2000;90:223–9. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–96. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.File JTM. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–9. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- 8.Leemans JC, Vervoordeldonk MJBM, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med. 2002;165:1445–50. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- 9.Knapp S, et al. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–84. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 10.Leitch AE, Haslett C, Rossi AG. Cyclin-dependent kinase inhibitor drugs as potential novel anti-inflammatory and pro-resolution agents. Br J Pharmacol. 2009;158:1004–16. doi: 10.1111/j.1476-5381.2009.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 12.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–75. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi SM, Dockrell DH, Renshaw SA, Sabroe I, Whyte MKB. Granulocyte apoptosis in the pathogenesis and resolution of lung disease. Clin Sci. 2006;110:293–304. doi: 10.1042/CS20050178. [DOI] [PubMed] [Google Scholar]
- 14.Droemann D, et al. Decreased apoptosis and increased activation of alveolar neutrophils in bacterial pneumonia. Chest. 2000;117:1679–84. doi: 10.1378/chest.117.6.1679. [DOI] [PubMed] [Google Scholar]
- 15.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh M-LN, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach S, et al. Roscovitine targets, protein kinases and pyridoxal kinase. J Biol Chem. 2005;280:31208–19. doi: 10.1074/jbc.M500806200. [DOI] [PubMed] [Google Scholar]
- 18.Goodyear S, Sharma MC. Roscovitine regulates invasive breast cancer cell (MDA-MB231) proliferation and survival through cell cycle regulatory protein cdk5. Exp Mol Pathol. 2007;82:25–32. doi: 10.1016/j.yexmp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Jhou R-S, et al. Inhibition of cyclin-dependent kinases by olomoucine and roscovi-tine reduces lipopolysaccharide-induced inflammatory responses via down-regulation of nuclear factor kB. Cell Prolif. 2009;42:141–9. doi: 10.1111/j.1365-2184.2009.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi AG, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–64. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 21.Duffin R, et al. The CDK inhibitor, R-roscovitine, promotes eosinophil apoptosis by down-regulation of Mcl-1. FEBS Lett. 2009;583:2540–6. doi: 10.1016/j.febslet.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Koedel U, et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 2009;5:e1000461. doi: 10.1371/journal.ppat.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rijneveld AW, Florquin S, Hartung T, Speelman P, Poll T. Anti-tumor necrosis factor antibody impairs the therapeutic effect of ceftriaxone in murine pneumococcal pneumonia. J Infect Dis. 2003;188:282–5. doi: 10.1086/376454. [DOI] [PubMed] [Google Scholar]
- 24.Schouten M, et al. Therapeutic recombinant murine activated protein C attenuates pulmonary coagulopathy and improves survival in murine pneumococcal pneumonia. J Infect Dis. 2010;202:1600–7. doi: 10.1086/656787. [DOI] [PubMed] [Google Scholar]
- 25.Wieland CW, Stegenga ME, Florquin S, Fantuzzi G, van der Poll T. Leptin and host defense against gram-positive and gram-negative pneumonia in mice. Shock. 2006;25:414–9. doi: 10.1097/01.shk.0000209524.12873.da. [DOI] [PubMed] [Google Scholar]
- 26.Knapp S, et al. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–8. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 27.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci. 2002;23:417–25. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 28.Du J, et al. Inhibition of CDKs by roscovitine suppressed LPS-induced *NO production through inhibiting NF{kappa}B activation and BH4 biosynthesis in macrophages. Am J Physiol Cell Physiol. 2009;297:C742–9. doi: 10.1152/ajpcell.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Meth. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 30.Van Den Boogaard FE, et al. Recombinant human tissue factor pathway inhibitor exerts anticoagulant, anti-inflammatory and antimicrobial effects in murine pneumococcal pneumonia. J Thromb Haemost. 2011;9:122–32. doi: 10.1111/j.1538-7836.2010.04089.x. [DOI] [PubMed] [Google Scholar]
- 31.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–56. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Jeyaseelan S, et al. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–47. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 34.Brown KA, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–69. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 35.Cross A, Moots RJ, Edwards SW. The dual effects of TNF{alpha} on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood. 2008;111:878–84. doi: 10.1182/blood-2007-05-087833. [DOI] [PubMed] [Google Scholar]
- 36.Dey A, Wong ET, Cheok CF, Tergaonkar V, Lane DP. R-Roscovitine simultaneously targets both the p53 and NF-[kappa]B pathways and causes potentiation of apoptosis: implications in cancer therapy. Cell Death Differ. 2007;15:263–73. doi: 10.1038/sj.cdd.4402257. [DOI] [PubMed] [Google Scholar]
- 37.Leitch AE, et al. The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur J Immunol. 2010;40:1127–38. doi: 10.1002/eji.200939664. [DOI] [PubMed] [Google Scholar]
- 38.Lotz S, et al. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J Leukocyte Biol. 2004;75:467–77. doi: 10.1189/jlb.0803360. [DOI] [PubMed] [Google Scholar]
- 39.Farahi N, et al. Effects of the cyclin-dependent kinase inhibitor R-roscovitine on eosinophil survival and clearance. Clin Exp Allergy. 2011;41:673–87. doi: 10.1111/j.1365-2222.2010.03680.x. [DOI] [PubMed] [Google Scholar]
- 40.Alcorta DA, et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibro-blasts. Proc Natl Acad Sci U S A. 1996;93:13742–7. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lum JJ, Bren G, McClure R, Badley AD. Elimination of senescent neutrophils by TNF-related apoptosis-inducing ligand. J Immunol. 2005;175:1232–8. doi: 10.4049/jimmunol.175.2.1232. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 43.Bergeron Y, et al. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–22. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giebelen IA, Leendertse M, Florquin S, van der Poll T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur Respir J. 2009;33:375–81. doi: 10.1183/09031936.00103408. [DOI] [PubMed] [Google Scholar]
- 45.Benson C, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh W-S, et al. Pharmacodynamic effects of Seliciclib, an orally administered cell cycle modulator, in undifferentiated nasopharyngeal cancer. Clin Cancer Res. 2009;15:1435–42. doi: 10.1158/1078-0432.CCR-08-1748. [DOI] [PubMed] [Google Scholar]