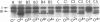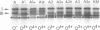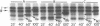Abstract
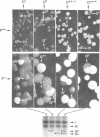
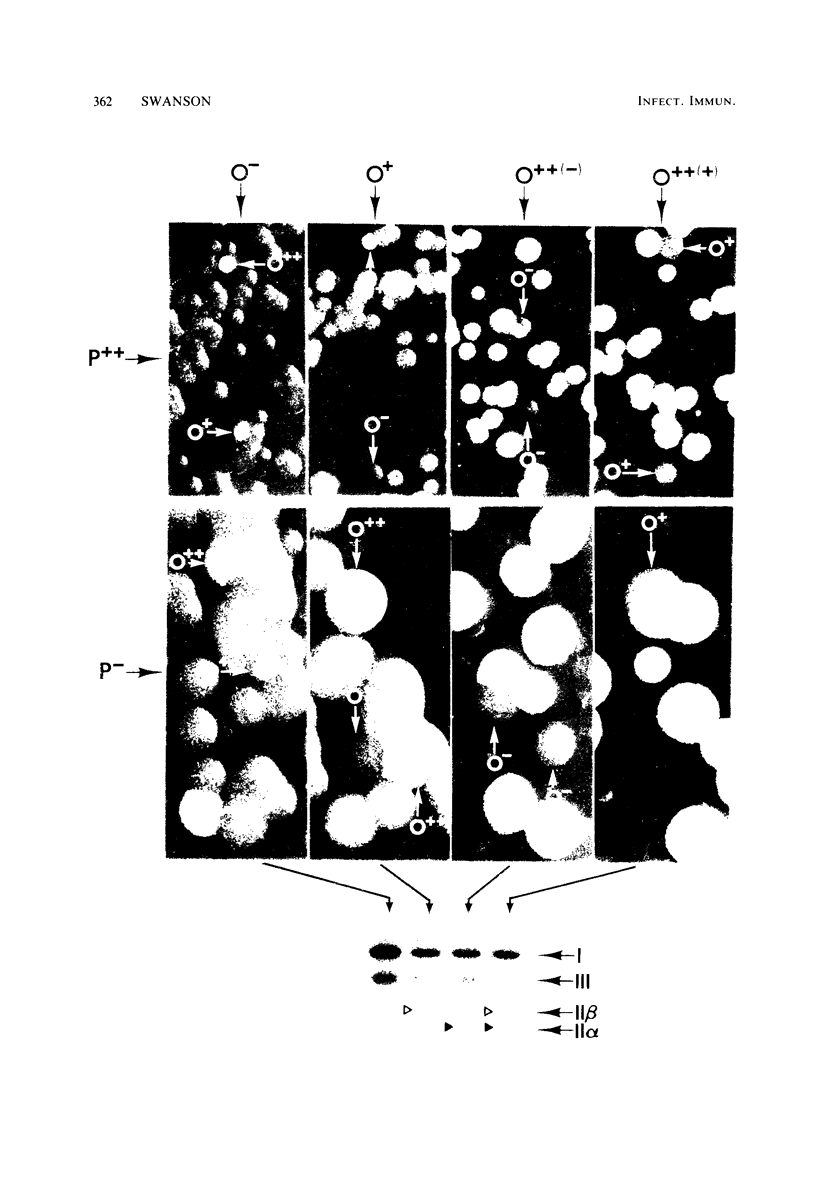
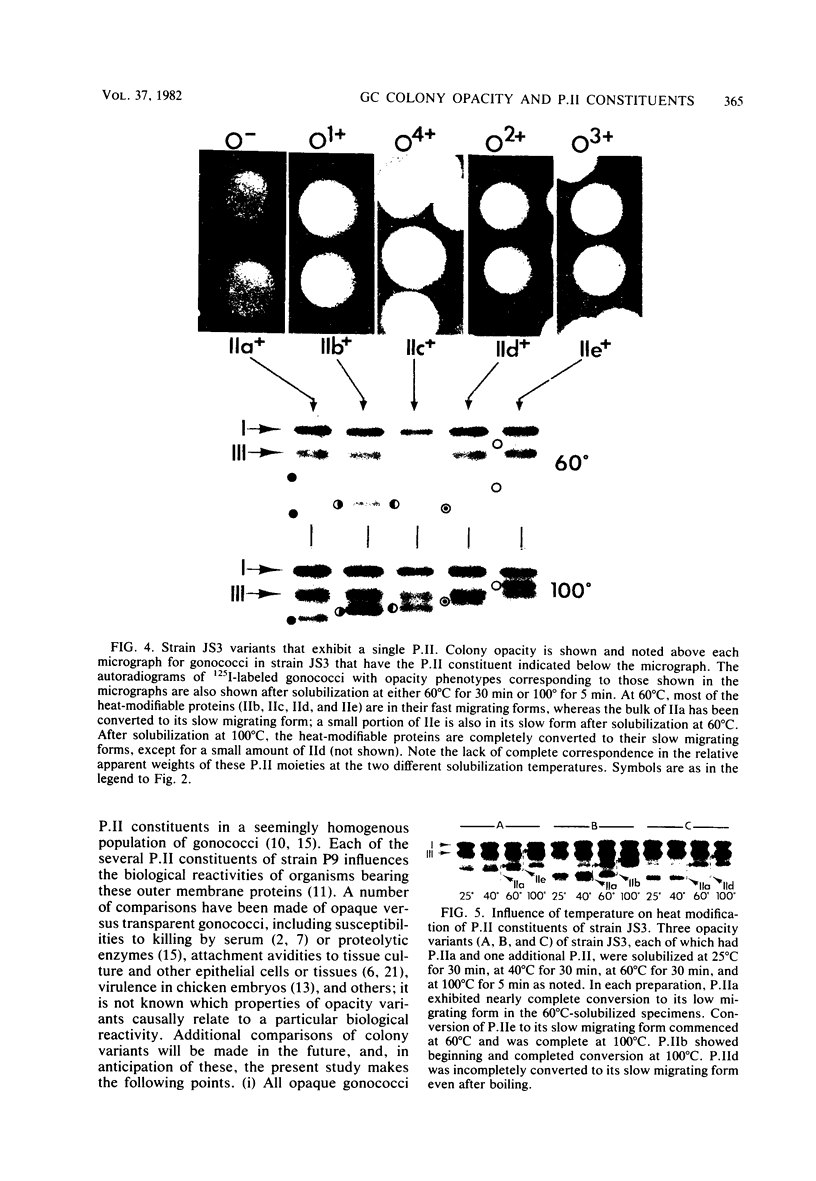
Changes in the presence of outer membrane protein II (P.II) constituents of gonococci were demonstrated by selecting opacity variants and defining the 125I-labeled bands of parental and variant organisms. In general, colony opacity phenotype was a convenient, reliable guide for obtaining variants that differed from their parents by the apparent single-step gain or loss of one P.II constituent. Within a given strain (three strains were studied), particular P.II species were associated with particular opacity phenotypes. This was well demonstrated in strain JS3, in which five different P.II constituents were identified and compared. Four of these five P.II moieties were consistently associated with a characteristic degree of colony opacity: presence of the fifth P.II (P.IIa) did not correlate with a discernible increase in opacity when present either alone or in combination with other opacity-associated P.II moieties. The electrophoretic migration characteristics for each of the five P.II constituents of this strain differed with regard to apparent molecular weight and the effects of temperature and 2-mercaptoethanol. The high prevalence of colony opacity variants indicates that gonococcal populations are capable of presenting a variety of surface components to their external environment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. L., James J. F., Brooks G. F., Sweet R. L. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect Immun. 1980 Mar;27(3):882–888. doi: 10.1128/iai.27.3.882-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):736–742. doi: 10.1128/jb.145.2.736-742.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Gonococcal color and opacity variants: virulence for chicken embryos. Infect Immun. 1978 Nov;22(2):359–364. doi: 10.1128/iai.22.2.359-364.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. 125I-labeled peptide mapping of some heat-modifiable proteins of the gonococcal outer membrane. Infect Immun. 1980 Apr;28(1):54–64. doi: 10.1128/iai.28.1.54-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981 Mar;31(3):965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]