Abstract
Behavioural findings have led to proposals that difficulties in attention and concentration in depression may have their roots in fundamental inhibitory impairments for irrelevant information. These impairments may be associated with reduced capacity to actively maintain relevant information to facilitate goal-directed behaviour. In light of mixed data from behavioural studies, the current study using direct neural measurement, examines whether dysphoric individuals show poor filtering of irrelevant information and reduced working memory (WM) capacity for relevant information. Consistent with previous research, a sustained event-related potential (ERP) asymmetry, termed contra-lateral delay activity (CDA), was observed to be sensitive to WM capacity and the efficient filtering of irrelevant information from visual WM. We found a strong positive correlation between the efficiency of filtering irrelevant items and visual WM capacity. Specifically, dysphoric participants were poor at filtering irrelevant information, and showed reduced WM capacity relative to high capacity non-dysphoric participants. Results support the hypothesis that impaired inhibition is a central feature of dysphoria and are discussed within the framework of cognitive and neurophysiological models of depression.
Keywords: attentional control, depression, inhibition, working memory capacity, contra-lateral delay activity, filtering efficiency
INTRODUCTION
Depression is recognized as a severe multifaceted disorder that includes affective, physiological, as well as cognitive symptoms. The cognitive symptoms observed in depression are typically deficits in attention and concentration (Mohanty and Heller, 2002). In a recent review (Levin et al., 2007), it was suggested that these cognitive symptoms may have their roots in fundamental executive function impairments. Generally, executive function describes a collection of top-down cognitive processes, mainly located in the prefrontal cortex, which control attention to produce goal-directed behaviour (Miller and Cohen, 2001). Hertel (1994) has proposed that depressed individuals have difficulty exercising attentional control in order to allocate available resources to task demands. It is thought that this is mainly reflected in inhibitory deficits, where an important function of inhibitory processes is to limit the disruptive influence of distractors on relevant information. One way inhibition may do this is by filtering out task-irrelevant information (Friedman and Miyake, 2004).
Interestingly, a wealth of cognitive neuropsychological research has investigated cognitive inhibition in depression using, among others, the n-back task (Harvey et al., 2005), oddball task (Kaiser et al., 2003), anti-saccade task (Sweeney et al., 1998), stroop task (Gohier et al., 2009) and rapid serial visual presentation tasks (Rokke et al., 2002). Although attention and concentration problems are considered important symptoms of depression, these studies have provided mixed support for the idea that depression is characterized by inhibitory deficits (Joormann et al., 2007). Often inhibitory performance was quite similar in depressed vs non-depressed individuals with mainly severely depressed individuals being characterized by marked impairments.
As studies have mainly used tasks relying on behavioural outcomes (i.e. reaction times, errors), one important limitation is that the absence of impairments does not imply that inhibitory functioning is as efficient in depressed as in non-depressed individuals. These behavioural outcomes do not generally speak to the ‘mechanisms’ underlying inhibition of task-irrelevant material. To this end, Dillon and Pizzagalli (2007) advocated a neuroscientific approach to study in a more direct fashion the brain mechanisms involved in inhibitory-related processes (see also Aron, 2007). For instance, in a recent fMRI study, activity within the prefrontal cortex and basal ganglia was found to precede filtering of irrelevant items in the posterior parietal cortex and this in turn predicted inter-individual differences in visual working memory capacity (WM capacity; McNab and Klingberg, 2008).
Inhibition has been proposed to facilitate efficient goal-directed behaviour by reducing the access and maintenance of irrelevant information in WM (Hasher et al., 1999). A crucial function of the WM system is to keep relevant information readily retrievable when the task context provides interfering information that would lead to an inappropriate response. The amount of relevant information that can remain active is the result of an ability to use attention to avoid distraction (Engle, 2002). In this view, inhibition modulates individual differences in WM capacity (Kane et al., 2001). As such, recent reviews and models of cognition in depression (Joormann et al. 2007; De Raedt and Koster, 2010) have highlighted inhibitory impairments as an important cognitive risk factor for depression. That is, within the context of emotion regulation, efficient inhibition of task irrelevant and/or negative information is crucial in regulating negative affect. Clear demonstration of the effects of inhibitory dysfunction on WM capacity in depression may then provide valuable insight into understanding why some people are more prone to cognitive risk factors, which increase the severity of depressive episodes such as depressive rumination (Nolen-Hoeksema et al., 2008), and help provide an index for cognitive therapies which target WM (De Raedt et al., 2010).
While cognitive symptoms of depression have mainly been described in terms of their verbal products such as the process of uncontrolled and persistent negative thoughts which characterizes depressive rumination (Nolen-Hoeksema et al., 2008), executive dysfunction may be expected to disrupt attentional control for processes of both the visual and verbal subsystems of WM due to their integrated structure (Repovs and Baddeley, 2006). Thus, if dysphoria is associated with impaired inhibition of irrelevant information a reduction in visual WM capacity should be expected.
There is recent ERP evidence to demonstrate that allocation of memory capacity to irrelevant information is significantly correlated with individual differences in overall WM capacity (Vogel et al., 2005). High WM capacity individuals tend to filter out irrelevant information and focus attention on the most relevant items within a cognitive task, whereas low WM capacity individuals tend to be less efficient and allocate attentional resources to irrelevant information. Vogel et al. (2005) measured WM capacity in a paradigm where on some trials participants were required to selectively remember a set of items (red rectangles) in the presence of task-irrelevant distractors (blue rectangles). WM capacity was then estimated from performance on the task with participants typically grouped based on a median split of their accuracy scores. To observe the association between the ability to efficiently filter irrelevant information and WM capacity each participant's brain activity was recorded during the task using Electroencephalography (EEG). Vogel and Machizawa (2004) previously observed a large negative voltage over posterior regions contra-lateral to the position of the to-be-remember items on the display (contra-lateral delay activity, CDA). CDA amplitudes have been found to be sensitive to the number of items remembered during each trial, increasing significantly between arrays of up to four items (McCollough et al., 2007).
In Vogel et al. (2005), the CDA was used as a direct neurophysiological measure of filtering efficiency (FE), ‘we used the CDA as a direct neurophysiological measure of whether or not the irrelevant distractor items unnecessarily consumed memory capacity. For example on the trials in which two red items were presented simultaneously with two blue items, if an individual was perfectly efficient at remembering only the red items and excluding the blue items from memory, then the CDA amplitude should be equivalent to that observed when two red items were presented alone. In contrast, if an individual was perfectly inefficient at excluding the blue items, all four of the items in the array (two red and two blue) would be stored in memory, resulting in an amplitude equal to that when four red items alone were presented’ (Vogel et al., 2005, p. 500). High WM capacity individuals were found to selectively filter out the irrelevant items. Low WM capacity individuals were found to have CDA amplitudes in the presence of distractors which were more similar to that of the four-item array, indicating they tended to inefficiently allocate attentional resources to irrelevant information.
The main aim of the current study was to examine the nature of impaired inhibition in depression, in relation to WM capacity using the visual WM task used by Vogel et al. (2005). We predicted that, relative to high capacity non-dysphoric individuals, dysphoric individuals will have reduced WM capacity for relevant information, and will have similar CDA amplitudes in the distractor condition and the four-item condition compared to the two-item condition, reflecting inefficient filtering of irrelevant information.
METHODS
Participants
The study was advertised online through the Birkbeck College and University College of London automated experiment management systems. Participants were not allowed to take part in the study if they suffered from migraine. Fifty one right-handed participants (27 males and 24 females) were selected for the study based on their initial scores on the Beck Depression Inventory, BDI-II (Beck et al., 1996). The inventory consists of 21 items assessing the severity of symptoms of depression. Each item has a four-point scale ranging from zero to three. The cut-off for presence of mild depressive symptoms is a score of 14 on the BDI (Beck et al., 1996). However, for the current study, participants were allocated to the dysphoric group only if their score was ≥20 as attention deficits have been shown to appear at a moderate level for the BDI-II within non-clinical university based samples (Rokke et al., 2002). Participants were allocated to the non-dysphoric group if their score was five or below. Accordingly, the dysphoric group had a mean score of M = 25.88 (s.d. = 7.04) and the non-dysphoric group a mean score of M = 2.18 (s.d. = 1.75). All participants were tested within 2 weeks of their first assessment. At testing, each participant provided demographic information by self report (Table 1) and was reassessed on the BDI-II. In the dysphoric group (n = 17) all scored above the cut-off for the presence of mild depressive symptoms (M = 24.94, s.d. = 7.08) and the non-dysphoric group (n = 34) scored below their cut-off (M = 2.03, s.d. = 2.12).
Table 1.
Demographic information for dysphoric and non-dysphoric groups
| Group | Time 1, Mean BDI | Time 2, Mean BDI | Gender |
Mean age | |
|---|---|---|---|---|---|
| Female | Male | ||||
| Dysphoric | 25.88 (7.04) | 24.94 (7.08) | 12 | 5 | 25.06 (10.13) |
| Non-dysphoric | 2.18 (1.75) | 2.03 (2.12) | 12 | 22 | 29.00 (10.09) |
| Non-dysphoric high capacity | 2.59 (1.82) | 2.17 (2.05) | 5 | 12 | 29.47 (12.70) |
| Non-dysphoric low capacity | 1.76 (1.64) | 1.88 (2.20) | 7 | 10 | 28.53 (6.92) |
Stimuli and procedure
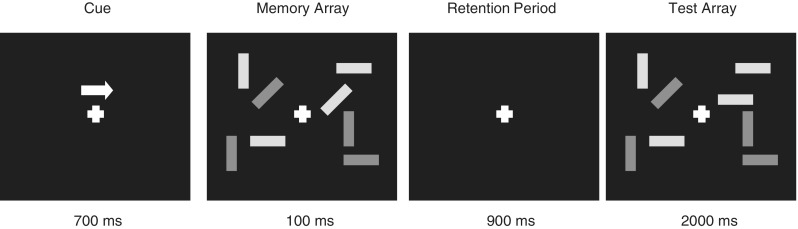
Stimuli were presented on a 17 inch LCD with a refresh rate of 16.6 ms. The experimental task was programmed and run using DMDX programming software (Forster and Forster, 2003) on a Dell Opitplex GX520. Stimulus design and procedure were adapted from those of Vogel et al. (2005). In the task, participants were presented with trials consisting of two stimulus arrays, a memory array and a test array. Participants were instructed to remember target items (red rectangles) from the memory array across a short retention period; accuracy for the target items was then assessed in the test array. The two stimulus arrays were each presented within 4° × 7.2° rectangular regions that were centred 3° from a white central fixation cross on a black background viewed at a distance of 60 cm. Within a trial each array (Figure 1) was presented either on the left or right side of the cross and consisted of two or four rectangles (0.64° × 1.21°). Participants were instructed which array to attend by a white arrow above the central fixation cross.
Fig. 1.
Example of a distractor condition in a change trial. Participants are instructed to remember the orientations of the red rectangles (light grey), and respond during the test array with one of two buttons to indicate whether a change was present or not.
The colour of each rectangle could be either red (target rectangle) or blue (distractor rectangle) depending on trial condition. Each rectangle was oriented randomly along one of four positions (vertical, horizontal, left 45°, right 45°). Rectangle positions were also random with the constraint that the distance between rectangles was at least 2°.
Each trial began with a central fixation (together with the white arrow), which remained on screen for 700 ms. After presentation of the cross and arrow, on both sides of the fixation, arrays of either two red rectangles (two-item condition), four red rectangles (four-item condition) or two red rectangles and two blue rectangles (distractor condition) were presented for 100 ms (memory array). All rectangles were then removed from the display for 900 ms (retention period). All rectangles were then redisplayed for 2000 ms (test array). Participants were instructed to maintain fixation during each trial and remember the orientations of the red rectangles on the side indicated by the arrow. During the test array, participants responded with one of two button presses to indicate whether the direction of one of the red rectangles changed or did not change. The inter-trial interval was varied randomly between 1500 and 2000 ms. Array size (conditions: two item, four item and distractor condition), arrow direction (left and right), change and no change trials were randomized and presented equally often across the experiment. Participants completed a short practice phase consisting of 24 trials (eight per condition) before the experimental blocks. The experiment was split into seven blocks of 84 trials (196 trials per condition), totalling 588 total trials across the experiment. Within each block, participants were given a short break after half of the trials were completed. Experimental session's lasted∼120 min. After the experiment participants were debriefed and paid £15 for their time.
Data preparation
WM capacity
Each participant's WM capacity was estimated from their performance on the task using a standard formula (Cowan, 2001). The formula is K = S (H–F), where K is WM capacity, S is the size of the array (i.e. four or two), H is the hit rate or proportion of correct responses when a change is present and F is the false alarm rate or the proportion of incorrect responses when no change is present. Memory capacity varies considerably within a population; as a result there are low and high WM capacity individuals. To account for variation in memory capacity, non-dysphoric participants were divided into high and low capacity groups using a median split on their K scores (cf. Vogel et al., 2005). The split led to the loss of one participant (who had a median score) resulting in 16 high capacity non-dysphoric participants and 17 low capacity non-dysphoric participants.
ERP recording
Participants were seated in an electrically isolated, sound proof room with dimmed lighting. Before recording each participant was given the instruction to avoid large movements during the task, to focus on the cross in the centre of the screen in order to avoid saccades during trials, and to try to time their blinks after button responses to stimuli within the inter-trial interval. The EEG was recorded using 32 Ag/AgCl sintered ring electrodes mounted on a fitted cap (EASYCAP) according to the international 10/20 system. The horizontal electrooculogram (EOG) was recorded from two electrodes placed 1 cm to the left and right of the external canthi to measure horizontal eye movements. Vertical EOG was recorded from a single electrode placed below the left eye to measure eye blinks. Electrode impedance was below 5 kΩ. EEG data were recorded referenced to the left mastoid, and re-referenced offline to the mean of the left and right mastoids (linked mastoids). EEG recordings were filtered with bandpass at 0.01–80 Hz and sampled at 250 Hz.
EEG processing
EEG data were processed in two stages using the MATLAB extension EEGLAB (Delorme and Makeig, 2004) and the EEGLAB plugin ERPLAB (Lopez-Calderon and Luck, 2010). EEG data were processed using both artefact correction and rejection. First independent component analysis (ICA) was conducted to identify ocular, muscle and noise components (Jung et al., 2001). Artefactual ICA components were then identified and removed from the data using standard methods (Jung et al., 2000a, b; Onton et al., 2006). Specifically, ICA was first applied to continuous EEG data to create time courses of temporally independent signals spatially filtered from the EEG data of each channel. Stereotypical artefactual wave shapes (e.g. blink activity: brief, large, deflections at frontal electrode sites and deflections of opposite polarity at the vertical EOG) were matched with that of simultaneous ICA time courses. Potential artefactual ICA components were then verified by plotting their scalp topography and removed if maps provided further evidence that the component was predominately artefactual activity (e.g. blink activity projects most strongly to far frontal sites). Artefact detection and rejection were then conducted on epoched uncorrected data files to identify and remove trials containing blinks and large eye movements at the time of stimulus presentation. Trials with ocular artefacts at stimulus presentation were removed from both behavioural and ICA corrected continuous data. The percentage of trials remaining after artefact rejection for each group was: 86% for the dysphorics, 85% for the non-dysphoric low capacity (NDLC) group and 86% for the non-dysphoric high capacity (NDHC) group. Across each group ERPs were based on an average of M = 169.23 (s.d. = 4.32) trials for the two-item condition, M = 168.63 (s.d. = 5.86) trials for the four-item condition and M = 171.28 (s.d. = 8.33) trials for the distractor condition. The groups did not differ on the number of artefact free trials per condition. Participants with rejection rates over 25% were removed from the analysis, which resulted in the removal of one participant from the low capacity non-dysphoric group leaving 16 participants in this group.
Contra-lateral delay activity
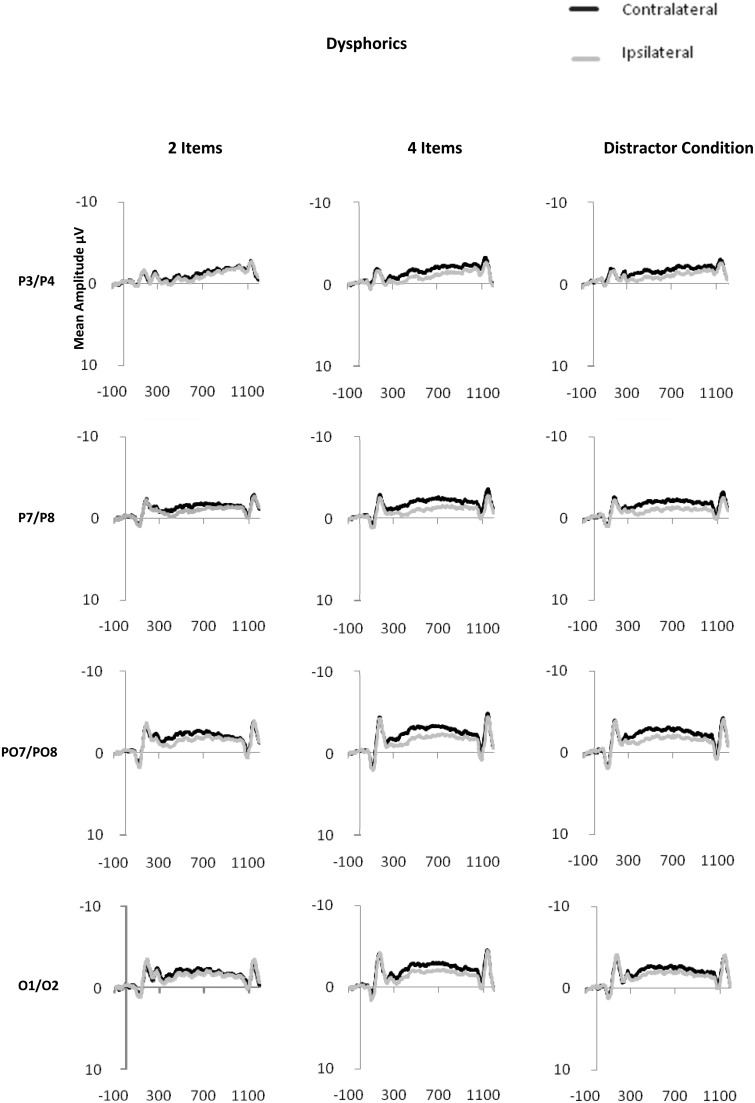
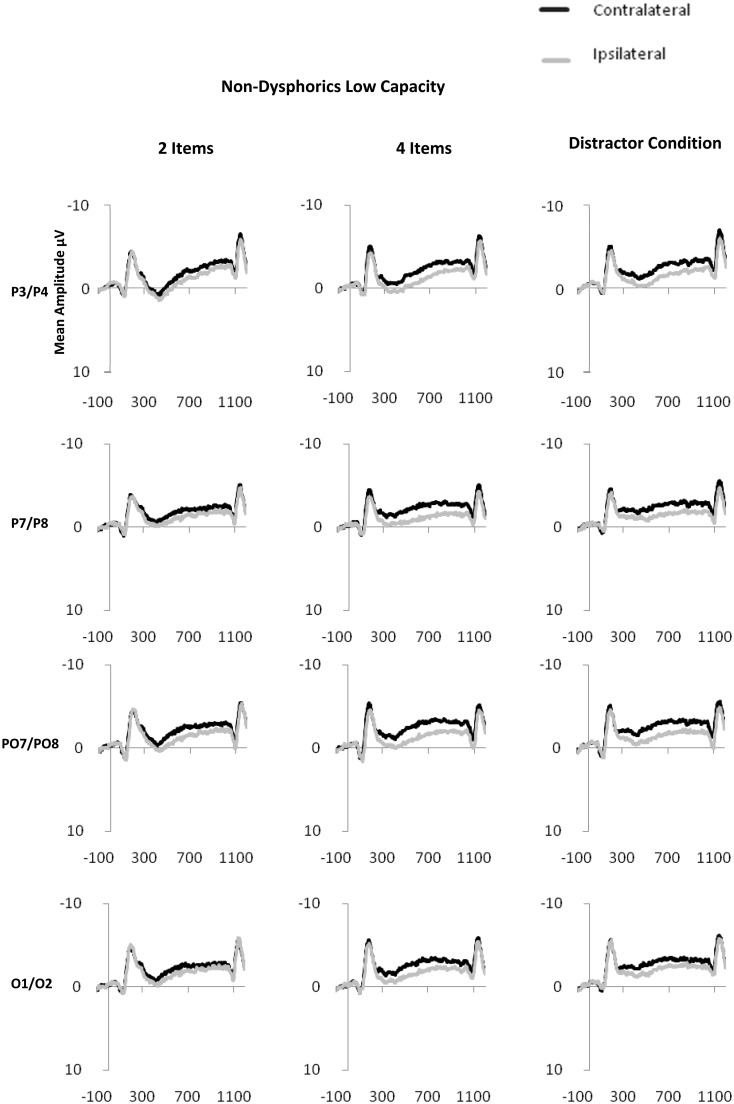
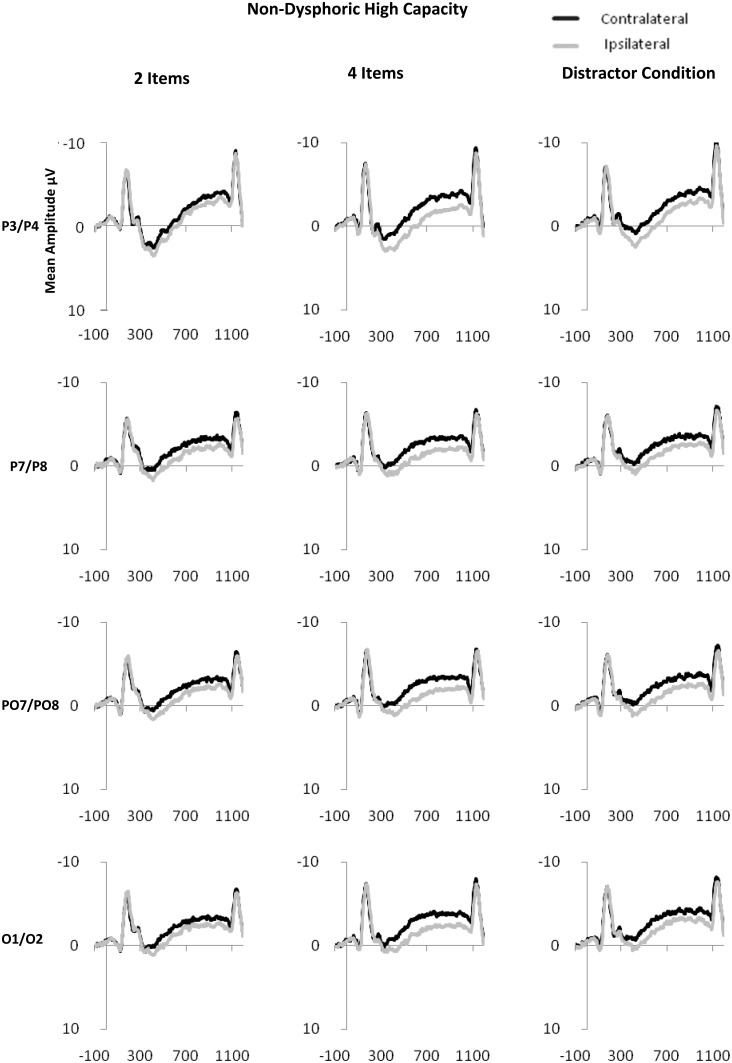
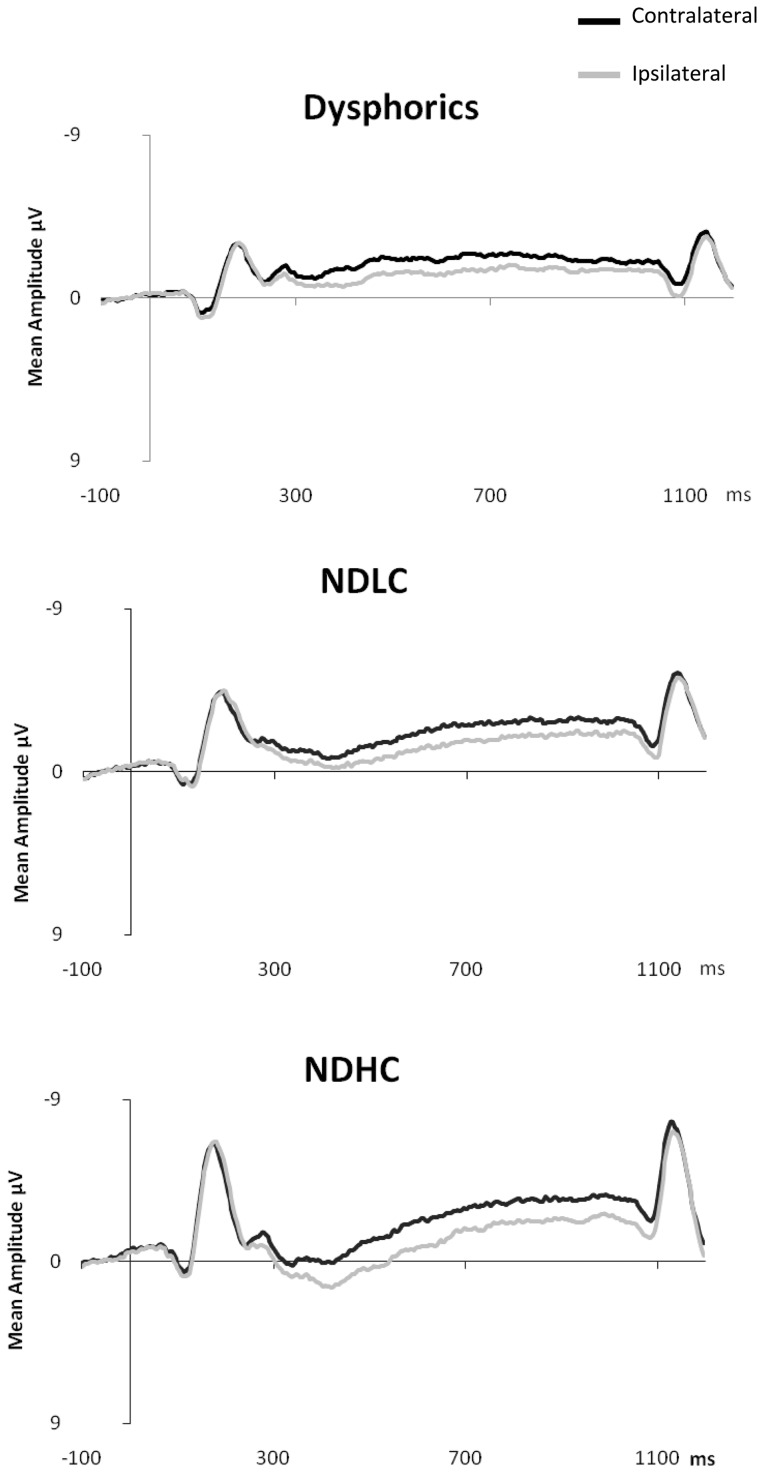
CDA is computed as the difference in mean amplitude between activity in hemispheres contra-lateral and ipsilateral to the memory array during the retention period. Activity from posterior electrode sites (P3/4, P7/8, PO3/4, PO7/8 and O1/2) within the time period of 300–900 ms after onset of the memory array was used in the calculation of CDA (see Figure 2 for display of contra-lateral and ipsilateral activity for each group by electrode sites). Contra-lateral waveforms were calculated by averaging activity recorded at right hemisphere electrode sites when participants were cued to remember items on the left side of the central fixation with activity recorded from the left hemisphere electrode sites when participants were cued to remember items on the right side of the central fixation. Conversely, ipsilateral waveforms were calculated by averaging the activity recorded at right hemisphere electrode sites when participants were cued to remember items on the right side of the central fixation with activity recorded from the left hemisphere electrode sites when participants were cued to remember items on the left side of the central fixation, see Figure 3 for contra-lateral and ipsilateral activity collapsed across posterior electrode sites.
Fig. 2.
Contra-lateral and ipsilateral activity time locked to the memory array by condition (two item, four item and distractor) at posterior electrode sites: P3/P4, P7/P8, PO7/PO8, O1/O2 for dysphoric (2a), NDHC (2b) and NDLC (2c) groups.
Fig. 3.
Grand averaged waveforms for activity contra-lateral and ipsilateral time locked to the memory array for dysphoric, NDHC and NDLC groups across posterior electrode sites.
ERP analysis: FE
CDA waveforms provide within-group representations of the number of items held in WM. The sensitivity of CDA makes it possible to use this measure to accurately determine the efficiency of inhibitory processes during the task. Analysis of CDA used the method of Vogel et al. (2005). This method uses a formula to determine each participant's ability to efficiently filter irrelevant information. The formula provides a quantitative measure of whether CDA amplitudes in the distractor condition are more similar to that in the four items or the two-items condition. Scores range from one (efficient: identical to two item) to zero (inefficient: identical to four item).1 The formula is FE = (F − D)/(F − T), where FE is filtering efficiency, F is the amplitude for four items, D is the amplitude in the distractor present condition and T is the amplitude in the two-items condition.
RESULTS
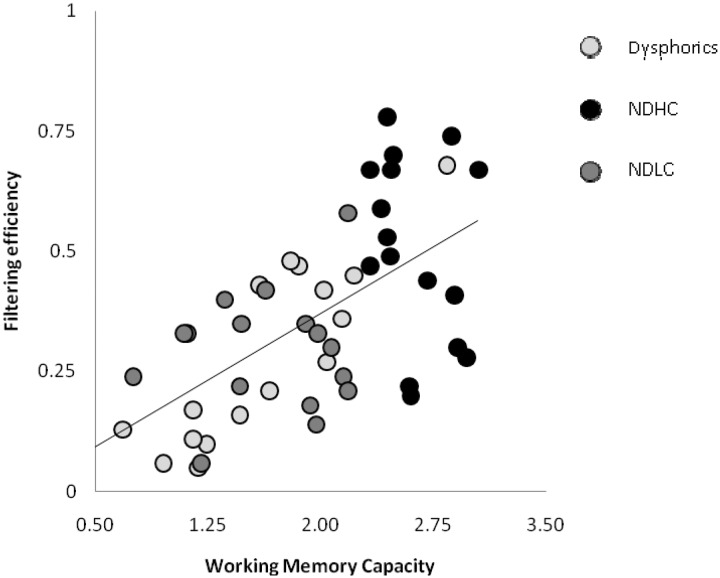
Previous research has shown that WM capacity and FE scores are strongly correlated (Vogel et al., 2005). Figure 4 shows the correlation between each participant's FE and WM capacity in the present study. In line with previous research, we found that these measures were strongly correlated across all participants (r = 0.63, n = 49, P < 0.001). This finding shows that low capacity individuals, in the current sample, have low FE scores and high capacity individuals have high FE scores.2
Fig. 4.
Correlation between memory capacity and the efficiency of excluding distractors from storage in visual WM for dysphoric, NDHC and NDLC groups.
To determine if dysphoria was associated with reduced memory WM capacity and impaired FE, performance of the dysphoric group was compared to that of each of the non-dysphoric subgroups.3 A multivariate ANOVA was conducted with group (dysphoric, NDHC and NDLC) as between-subject factor and WM capacity (K scores) and FE as dependent variables.
WM capacity
For WM, there was a main effect of group, F(2,46) = 24.941, P < 0.001.4 The NDHC group showed higher K scores (M = 2.62, s.d. = 0.24), than the dysphoric (M = 1.53, s.d. = 0.63), and the NDLC group (M = 1.65, s.d. = 0.45). Using a Bonferroni adjustment, these differences were found to be significant in post hoc comparisons between the NDHC and NDLC groups, P < 0.001, and for the NDHC group and dysphoric individuals, P < 0.001. There were no group differences between the dysphoric and the NDLC groups, P = 1.
CDA analysis
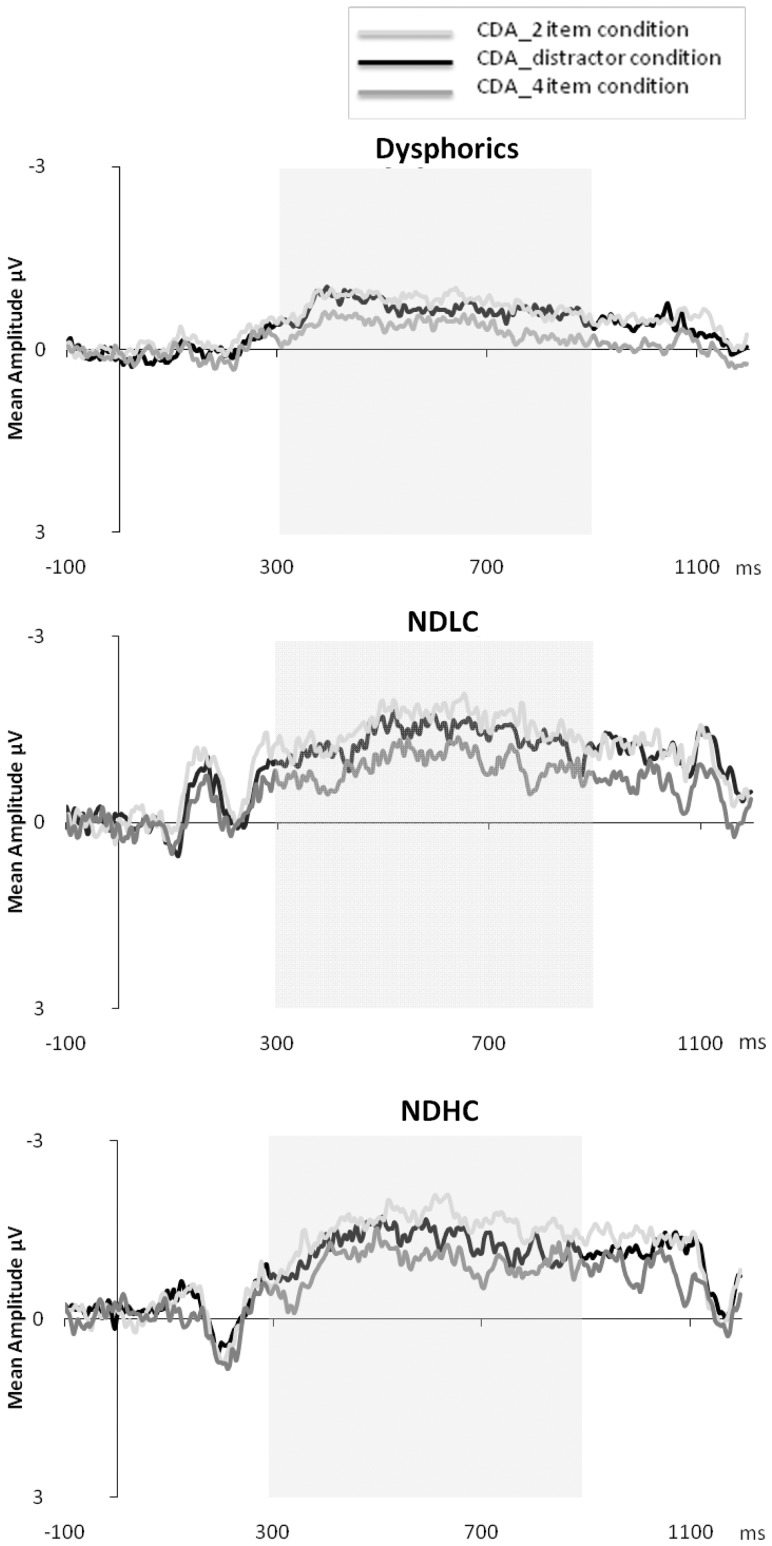
Figure 5 shows grand mean CDA waveforms as a function of condition for the dysphoric group and non-dysphoric subgroups averaged across posterior electrode sites. For all groups, it appears that within the 300–900 ms time window CDA amplitudes were highest for the four-item array followed by the distractor condition and two-item conditions. A mixed ANOVA with Group (dysphoric, NDLC and NDHC) as the between-subject factor and Condition (two item, distractor and four item) as the within-subject factor yielded a main effect of Condition, Wilks λ = 0.30, F(2,45) = 51.11, P < 0.001 showing that CDA amplitudes were significantly different between all conditions (P's < 0.001, Bonferroni corrected) (see Table 2 for descriptive statistics). A significant difference between the four- and two-item conditions shows CDA amplitudes are sensitive to the number of representations held in memory (McCollough et al., 2007). CDA amplitudes were also significantly different for these two conditions in comparison to the distractor condition, indicating all participants did not completely filter the distractors and stored at least some irrelevant information in visual WM.
Fig. 5.
Grand averaged CDA waveforms (contra-lateral–ipsilateral activity) for dysphoric, NDHC and NDLC. Each graph shows waveforms by trial condition; two item (CDA_2 item condition), distractor (CDA_distractor condition) and four item (CDA_4 item condition). Highlighted region shows analysis window (300–900 ms).
Table 2.
Mean CDA amplitudes (standard deviations in brackets) for dysphorics, NDLC, NDHC and Condition; two item, four item and distractor
| Dysphoric | NDLC | NDHC | Condition mean | |
|---|---|---|---|---|
| Two item | −0.48 (0.42) | −0.74 (0.54) | −0.92 (0.49) | −0.71 (0.59) |
| Four item | −0.93 (0.49) | −1.26 (0.76) | −1.55 (0.67) | −1.24 (0.70) |
| Distractor | −0.82 (0.48) | −1.09 (0.66) | −1.24 (0.58) | −1.04 (0.51) |
Analysis also revealed a significant Group × Condition interaction, Wilks λ = 0.73, F(4,90) = 3.76, P < 0.008. This interaction showed that for dysphorics and the NDLC group the differences in CDA amplitudes between the distractor and four-item conditions was lower than that of the NDHC group [Mean differences of 0.11, 0.16 and 0.31, respectively, F(2,46) = 6.02, P < 0.006]. This suggested that the NDHC group held fewer irrelevant items in WM relative to the NDLC and the D groups. In contrast the differences in CDA amplitudes between the four- and the two-item condition were not different across groups, F < 1. To investigate the relationship between CDA amplitudes reflecting the ability to filter irrelevant items from WM and the number of items held in WM we conducted the FE analyses below.
FE
FE was calculated using the formula: FE = (F −D)/(F − T), where F is the amplitude for four items, D is the amplitude in the distractor present condition and T is the amplitude in the two-items condition. Scores range from one (efficient: identical to two item) to zero (inefficient: identical to four item). FE scores ranged from 0.05 to 0.78. The dysphorics had a mean FE score of 0.27 (s.d. = 0.18). The NDLC group had a mean FE score of 0.29 (s.d. = 0.12) and for the NDHC group the mean FE score was 0.51 (s.d. = 0.18). Analysis revealed a main effect of Group, F(2,46) = 9.674, P < 0.001.5 The NDHC group were significantly more efficient at filtering irrelevant information from storage in visual WM than dysphoric and NDLC individuals. Using Bonferroni adjustments, these differences were found to be significant in post hoc comparisons between NDHC and NDLC groups, P = 0.002, and NDHC and dysphoric groups, P = 0.001. There were no group differences between dysphoric and NDLC groups, P = 1.
Additional analysis
We conducted additional analyses to rule out the possibility that the poor levels of performance in the NDLC and dysphoric groups was simply due to an inability to voluntarily allocate visual attention to the task (the relevant side of the memory array as indicated by the cue). To assess voluntary attention to the task, the difference in mean amplitude was measured between contra-lateral and ipsilateral activity from 75–175 ms after onset of the memory array at posterior electrode sites (P3/4, P7/8, PO3/4, PO7/8 and O1/2) (cf. Fukuda and Vogel, 2009). This time range encompasses early visual sensory responses (P1/N1) reflecting spatial attention to the task. Mean amplitudes were compared between groups (NDHC, NDLC and dysphoric) and within condition (two item, four item and distractor) using a mixed ANOVA. We found that P1/N1 amplitudes did not differ by group, F(2, 46) < 1 ruling out the possibility that dysphorics and the NDLC differed from the NDHC in their ability to voluntarily orient spatial attention to the task.
DISCUSSION
In the present study, we examined the nature of impaired inhibition of irrelevant information in depression in relation to WM capacity. For this purpose, we administered a well-investigated task (Vogel et al., 2001, 2005), which provides a specific neural marker of filtering of irrelevant information and WM capacity. The results of the study are that (i) FE and WM capacity are positively related and (ii) inhibitory functioning in dysphoric individuals is significantly lower than high capacity non-dysphoric individuals and similar to functioning of non-dysphoric individuals low in WM capacity. These findings are discussed below.
We calculated WM and FE according to methods developed by Vogel and colleagues. The present findings replicated the results of Vogel et al. (2005); specifically, across all participants, FE and WM capacity were positively correlated with high capacity individuals showing high FE and low capacity individuals showing low FE scores. Thus, the current study provided further evidence that misallocation of attentional resources to irrelevant information may drive individual differences in overall WM capacity (Vogel et al., 2005). In this regard, the results of the present study are also in line with theoretical proposals and recent neural evidence which have linked inhibition and WM (Hasher et al., 1999; McNab and Klingberg, 2008).
Based on previous work, we hypothesized that due to impaired inhibition (Joormann et al., 2007), dysphoric individuals would have lower WM capacity relative to high capacity non-dysphoric individuals. Our hypothesis was supported by the results: dysphoric individuals were characterized by reduced filtering of irrelevant information and WM capacity relative to high capacity non-dysphoric individuals. No differences were observed for WM capacity or FE when the dysphoric group were compared to low capacity non-dysphoric group. These findings emerged at the neural and behavioural level, respectively. Importantly, it was found that the performance of the dysphoric group was not associated with poor voluntary attention to task and was related specifically to inefficient filtering of irrelevant information so these results provide an important validation for the use of this methodology in cognitive research in depression.
Previous behavioural paradigms so far have provided mixed results for the existence of inhibitory impairments in depression. Specifically, our findings showed that it is important to separate the (control) non-dysphoric group by their WM capacity scores as it was through this division that the similarity between non-dysphoric (low WM capacity) and dysphoric individuals could be observed. In the absence of such a division, the differences between dysphoric and non-dysphoric groups may be masked by their differences in WM capacity. Our findings further highlight the benefit of combining behavioural and electrophysiological measures in examining the inhibitory processes linked with depression. The current approach also highlights that with a more specific measurement it can be observed that dysphoric individuals are less efficient in filtering irrelevant information.
The results of the present study imply that impaired inhibition is a central feature of dysphoria, and are thus important for cognitive theory and research in depression. It has been proposed that impaired inhibition may act as a cognitive vulnerability factor for depression as it could explain some of the typical cognitive symptoms of depression (e.g. lack of concentration and memory deficits) as well as being associated with a reduced capacity to engage in emotion regulation (cf. Joormann et al., 2007). The further elucidation of the nature of inhibitory deficits in depression is also of importance from a translation research perspective. That is, a more precise understanding of the cognitive impairments in depression will allow examination of its direct role in the pathophysiology of depression and can illuminate potential ways to remediate such problems (De Raedt et al., 2010). There are several interventions, such as repetitive transcranial magnetic stimulation (Leyman et al., 2011) or cognitive training regimes (Siegle et al., 2007; MacLeod et al., 2009) that could strengthen inhibitory control and may alleviate depressive symptoms.
There are some restrictions to the present study. The use of a dysphoric sample hampers generalization to clinically depressed populations. However, if anything, our findings provide an overestimation of inhibitory functioning in depression as a largely high-functioning student population was tested who had slightly lower depression scores than observed in clinical samples. Also, as a result of the use of neutral stimuli, the current study cannot inform whether inhibition is impaired and WM capacity is reduced in the context of emotional material. There is growing evidence of inhibitory impairment for emotional material in depression (Joormann et al., 2007; Derakshan et al., 2009). However, it is interesting that in the current study basic impairments were observed. As these impairments may be even more pronounced for emotional material, future research will need to be conducted to extend findings of the current study to emotional processing in depression. Finally, the current study did not consider the influence of anxiety and given the comorbidity between anxiety and depression, it is important for future research to examine the independent and interactive effects of anxiety and depression in relation to FE. Still, it should be noted that impaired inhibitory functions have been more strongly associated with depression (Joormann et al., 2007) than anxiety.
In conclusion, the present study provides clear evidence of fundamental attentional control impairments in depression. Results indicate that dysphoric individuals have trouble filtering distracting irrelevant information from the focus of attention while engaged in goal-directed behaviour. This disruption of information processing may have severe cognitive and emotional consequences. The results of the present study collectively show utilizing direct neural and behavioural measures offers a promising way forward for exploring attentional control impairment in depression.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Taherah L. Ansari and Marie Smith for advice with programming and data analysis. Thanks also to Gilles Pourtois for helpful comments on an early draft of the article. This work was supported by a PhD studentship (awarded to M.O. and carried out under the supervision of Nazanin Derakshan at Birkbeck University of London); and a joint international Royal Society grant [grant number 2007/R1-IJP to N.D. and E.H.W.K., in part].
Footnotes
1Calculation of FE can produce outliers if mean CDA amplitudes for the two-item condition are, for example, greater than the four-item condition (i.e. negative FE). However, all participants in the current study had FE scores within the range of zero to one so were included in the analysis.
2A significant positive correlation was found for both the full non-dysphoric group r = 0.44, n = 32, P = 0.011, and the dysphoric group r = 0.83, n = 17, P < 0.001.
3It must be noted that firstly a two-group analysis comparing the dysphoric and the full non-dysphoric group was conducted to examine if the two groups differed overall in terms of Overall K and FE. The analysis showed that the dysphoric group had significantly lower levels of K and FE than the non-dysphoric group.
4The full non-dysphoric group had significantly higher WM capacity (M = 2.13, s.d. = 0.60) than the dysphoric group (M = 1.53, s.d. = 0.63), F(1,47) = 10.279, P = 0.002.
5The full non-dysphoric group had significantly greater FE (M = 0.40, s.d. = 0.19) than the dysphoric group (M = 0.27, s.d. = 0.18), F(1,47) = 4.98, P < 0.05.
REFERENCES
- Aron A. The neural basis of inhibition in cognitive control. The Neuroscientist. 2007;13:214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective, and Behavioral Neuroscience. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW, Joorman J. Attentional control in depression: a translational affective neuroscience approach. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:1–7. doi: 10.3758/CABN.10.1.1. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Salt M, Koster E. Attentional control in dysphoria: an investigation using the antisaccade task. Biological Psychology. 2009;80:251–5. doi: 10.1016/j.biopsycho.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Dillon D, Pizzagalli D. Inhibition of action, thought, and emotion: a selective neurobiological review. Applied and Preventive Psychology. 2007;12:99–114. doi: 10.1016/j.appsy.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle R. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. [Google Scholar]
- Forster KI, Forster JC. DMDX: a Windows Display Program with millisecond accuracy. Behavior Research Methods, Instruments & Computers. 2003;35:116–24. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Friedman N, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. Journal of Experimental Psychology-General. 2004;133:101–35. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel E. Human variation in overriding attentional caputure. The Journal of Neuroscience. 2009;29:8726–33. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohier B, Ferracci L, Surguladze SA, et al. Cognitive inhibition and working memory in unipolar depression. Journal of Affective Disorder. 2009;116:100–5. doi: 10.1016/j.jad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Foassati P, Pochon J-B, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage. 2005;26:860–9. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R, May C. Inhibitory control, circadian arousal, and age. Attention and Performance. 1999;17:653–75. [Google Scholar]
- Hertel P. Depression and memory – are impairments remediable through attentional control? Current Directions in Psychological Science. 1994;3:190–3. [Google Scholar]
- Joormann J, Yoon K, Zetsche U. Cognitive inhibition in depression. Applied & Preventive Psychology. 2007;12:128–39. [Google Scholar]
- Jung T, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000a;37:163–78. [PubMed] [Google Scholar]
- Jung T, Makeig S, McKeown M, Bell A, Lee T, Sejnowski T. Imaging brain dynamics using independent component analysis. Proceedings of the IEEE. 2001;89:1107–22. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski T. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000b;80:1745–58. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, Weisbrod M. Executive control deficit in depression: event related potentials in a go/nogo task. Psychiatry Research. 2003;122:169–84. doi: 10.1016/s0925-4927(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Kane M, Bleckley M, Conway A, Engle R. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology-General. 2001;130:169–83. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Levin R, Heller W, Mohanty A, Herrington J, Miller G. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;30:211–33. [Google Scholar]
- Leyman L, De Raedt R, Vanderhasselt MA, Baeken C. Effects of repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex on the attentional processing of emotional information in major depression: a pilot study. Psychiatry Research. 2011;185:102–7. doi: 10.1016/j.psychres.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB (version 1.0.0.33a) (Computer Software). UC-Davis Center for Mind & Brain. 2010 http://erpinfo.org/erplab/erplab-download (7 October 2010, date last accessed) [Google Scholar]
- MacLeod C, Koster EHW, Fox E. Whither cognitive bias modification research: a commentary on the special section. Journal of Abnormal Psychology. 2009;118:89–99. doi: 10.1037/a0014878. [DOI] [PubMed] [Google Scholar]
- McCollough A, Machizawa M, Vogel E. Electrophysiological measures of maintaining representations in visual working memory. Cortex. 2007;43:77–94. doi: 10.1016/s0010-9452(08)70447-7. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal Cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–7. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Heller W. The neuropsychology of depression: affect, cognition, and neural circuitry. In: D'haenen H, den Boer JA, Westenberg H, Willner P, editors. Textbook of Biological Psychiatry. Chichester, West Sussex: Wiley; 2002. pp. 791–802. [Google Scholar]
- Nolen-Hoeksema S, Wisco B, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–24. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neuroscience and Biobehavioral Reviews. 2006;30:808–22. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Rokke P, Arnell K, Koch M, Andrews J. Dual-task attention deficits in dysphoric mood. Journal of Abnormal Psychology. 2002;111:370–9. [PubMed] [Google Scholar]
- Siegle G, Ghinassi F, Thase M. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research. 2007;31:235–62. [Google Scholar]
- Sweeney J, Strojwas M, Mann J, Thase M. Prefrontal and cerebellar abnormalities in major depression: evidence from oculomotor studies. Biological Psychiatry. 1998;43:584–94. doi: 10.1016/s0006-3223(97)00485-x. [DOI] [PubMed] [Google Scholar]
- Vogel E, Machizawa M. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–51. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel E, McCollough A, Machizawa M. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–3. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Vogel E, Woodman G, Luck S. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology-Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]