Abstract
Imitation is an important component of human social learning throughout life. Theoretical models and empirical data from anthropology and psychology suggest that people tend to imitate self-similar individuals, and that such imitation biases increase the adaptive value (e.g., self-relevance) of learned information. It is unclear, however, what neural mechanisms underlie people's tendency to imitate those similar to themselves. We focused on the own-gender imitation bias, a pervasive bias thought to be important for gender identity development. While undergoing fMRI, participants imitated own- and other-gender actors performing novel, meaningless hand signs; as control conditions, they also simply observed such actions and viewed still portraits of the same actors. Only the ventral and dorsal striatum, orbitofrontal cortex and amygdala were more active when imitating own- compared to other-gender individuals. A Bayesian analysis of the BrainMap neuroimaging database demonstrated that the striatal region preferentially activated by own-gender imitation is selectively activated by classical reward tasks in the literature. Taken together, these findings reveal a neurobiological mechanism associated with the own-gender imitation bias and demonstrate a novel role of reward-processing neural structures in social behavior.
Keywords: imitation, neuroimaging, reward, gender, cultural learning
INTRODUCTION
Imitation is widespread in humans, emerges early in development and is the means by which many critical skills are learned throughout life (Tomasello et al., 1993). Theoretical models and behavioral data from psychology and anthropology indicate that people tend to imitate certain individuals, including those who are self-similar (Bandura, 1977; Henrich and McElreath, 2003). These ‘similarity biases’ are thought to increase the adaptive value of learned information by increasing its self-relevance.
One of the best-documented similarity biases is the own-gender bias, which is thought to play a critical role in the acquisition of gender roles and continues to guide learning in adulthood (Bussey and Bandura, 1984). In a series of foundational studies, Bandura and colleagues found that a diverse set of behaviors, ranging from aggression to color preference, were more readily transmitted via imitation of own-gender than other-gender models (Bandura et al., 1961; Bussey and Bandura, 1984). These studies also demonstrated that a preference for own-gender imitation is present in children before gender identity is fully formed, suggesting that own-gender imitation is not only an effect, but also a cause of gender identity development (Bussey and Bandura, 1984).
Following Bandura's findings, preference for own-gender models has been documented for models such as parents (Basow and Howe, 1980), teachers (Gilbert et al., 1983), peers (Slaby and Frey, 1975; Perloff, 1982) and even strangers, like musicians (Killian, 1990) and celebrities (Mesoudi, 2009). Own-gender imitation biases are thus pervasive, yet neuroimaging studies of human imitation have provided little insight into the neural underpinnings of such model-based imitative learning biases, as they have typically utilized stimuli depicting an isolated, gender-neutral effector (e.g. a hand or foot) performing simple actions (e.g. Iacoboni et al., 1999; Buccino et al., 2004; Frey and Gerry, 2006).
Here, we used fMRI to investigate the neural circuitry underlying the own-gender imitation bias. We addressed two main questions. First, which neural systems encode gender during imitation? Second, are these neural mechanisms imitation-specific and thus more likely related to the own-gender imitative bias? We have previously proposed that neural systems related to imitation, mental state attribution and reinforcement learning might underlie human cultural imitative learning (Losin et al., 2009). Given that imitative biases such as the own-gender bias are a key component of cultural learning, we predicted that one or more of these neural systems would differentially encode own- and other-gender individuals during imitation and do so to a greater degree or exclusively during imitation.
METHODS
Participants
Participants were 20 (10 males), right-handed, European American individuals, 18 to 26 years old (mean = 22.92, s.d. = 2.09). Seventeen participants reported being heterosexual, and three participants reported being homosexual (two males, one female). Participants were recruited through the volunteers section on Craigslist (8/20 were students). Participants had no history of medication or drug use other than oral contraceptives, no heavy use of alcohol and no prior or concurrent diagnosis of any neurological, psychiatric, or developmental disorders according to self-report. The study was approved by the UCLA Institutional Review Board. Written informed consent was obtained from all participants.
fMRI task
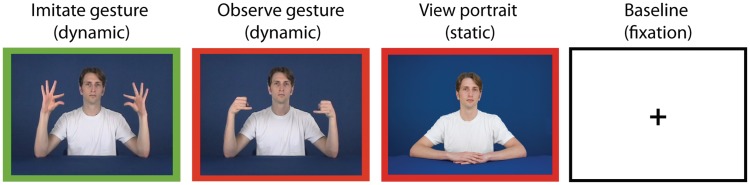
Stimuli were color, waist-up videos of 12 actors (six males), of three different ethnicities (European American, African American and Chinese American), performing 16 bimanual, symmetrical hand signs derived from New Zealand Sign Language and described as meaningless to both actors and participants. Actor and stimulus appearance was standardized (e.g. neutral expression, white t-shirt, consistent lighting, position and background). Stimuli were either outlined with a red border, indicating that the participant should observe passively (observe gesture condition), or a green border, indicating that the participant should imitate the signs during the video presentation (imitate gesture condition). Two control conditions were utilized: (i) portraits of each actor to control for viewing vs imitating actors (view portrait condition, also outlined with a red border) and (ii) a fixation cross (baseline) (Figure 1).
Fig. 1.
Example stimuli from four experimental conditions (Imitate gesture stimuli have a green border = participants imitate and observe gesture and view portrait stimuli have red borders = participants observe).
Four stimuli from the same condition and portraying the same actor were presented in a block. For example, during a block of the imitate gesture condition, a participant would imitate the same actor performing four different hand signs. Each stimulus within a block was presented for 2.5 s and separated from the next stimulus by a 0.5-s fixation cross. All blocks were preceded by an instruction screen that was either green with the word, ‘imitate’ or red with the word, ‘observe’. Stimulus blocks were divided into four balanced runs such that each actor, each hand sign and each condition were seen an equal number of times in each run. The order of blocks was pseudorandomized within a run, ensuring less than two of same gender in a row, no two of same ethnicity in a row, and no two of same hand sign in a row. Five 22.5-s rest blocks were evenly spaced throughout each run. This run configuration resulted in one block of each condition (imitate gesture, observe gesture and view portrait) per actor, per run for a total run time of 13:45. Over the course of the experiment, gestures were each seen an equal number of times as each gesture was performed by each actor once in each condition. Also over the course of the experiment, each participant saw 96 stimuli (24 blocks) portraying own-gender actors and 96 stimuli (24 blocks) portraying other-gender actors in each of the conditions. The stimulus order for each participant was unique. These functional data were acquired over a total of 55 min of scan time. The fMRI task was created and presented in the scanner using Presentation® software (Neurobehavioral Systems, Inc., Albany, CA, USA) and viewed in the scanner on magnet-compatible goggles (Resonance Technologies, Inc.).
Prior to scanning, each subject completed two training tasks: a hand-sign familiarization task during which participants imitated each sign in slow motion and then at full speed, and a task structure familiarization during which participants performed one block of each task condition (task structure familiarization hand signs and actor were not later seen in scanner). During training tasks, participants performed the imitation condition with their hands in their laps and under a table to mimic scanner conditions.
fMRI data acquisition
Data were collected using a 3 Tesla Siemens Trio whole-body MRI scanner at the UCLA Ahmanson–Lovelace Brain Mapping Center. The following scans were performed on each participant: (i) four functional echo-planar imaging (EPI) scans (3 × 3 × 4 mm voxels, TR: 2250 ms, TE: 28 ms, slices: 34, flip angle: 90°, FoV read: 192 mm, echo spacing: 47 ms, bandwidth: 2442 Hz/Px, time: 13:45); (ii) co-planar high-resolution T2-weighted structural scan (1.5 × 1.5 × 4 mm voxels, TR: 5000 ms, TE: 34 ms, slices: 34, flip angle: 90°, FOV Read: 192 mm, echo spacing: 0.89 ms, bandwidth: 1302 Hz/Px, time: 1:30); (iii) high-resolution T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) structural scan (1 × 1 × 1 mm voxels, TR: 1900 ms, TE: 2.26 ms, Flip angle: 90°, T1: 900 ms, FoV Read: 250 mm, echo spacing 6.9 ms, bandwidth: 200 hz/px, time: 6:50).
Behavioral measures
To quantify participants’ task compliance and imitation accuracy, participants were visually monitored during scanning to ensure that no movement occurred during observation-only blocks. Additionally, for 16 of 19 participants, hand-sign imitation accuracy was assessed by watching participants’ hands through the control-room window. Each sign was assigned a rating of 2 (performed sign correctly); 1 (performed sign but with errors); or 0 (did not perform sign). Imitation accuracy was high with an average of 94.8% (subject range = 82.6%–99.7%) of signs receiving the highest accuracy rating, suggesting participants were able to perform the hand-sign imitation task accurately.
fMRI data analysis
One male participant was not included in the analysis due to a failure of the stimulus randomization script. Additionally, the fourth run was dropped from two participants due to failure of the stimulus presentation computer, and two runs were dropped from each of two participants due to head motion. This resulted in a total of 19 (10 females) participants and 70 runs being utilized in the statistical analyses. Head motion in the remaining data was low, with an average mean relative head motion per run of 0.077 mm, s.d. = 0.004 mm and a an average maximum relative head motion per run of .648 mm, s.d. = 0.074 mm.
Structural and functional MRI data analyses were performed using FSL (FMRIB's Software Library:http://www.fmrib.ox.ac.uk/fsl/), AFNI (Cox, 1996) and ART (Ardekani et al., 1995). Preprocessing included skull-stripping (AFNI), realignment (mean image, FSL), highpass filtering (100 ms, FSL) and spatial smoothing (6 mm, FSL). Functional data were registered to the in-plane high-resolution scan (3-parameter affine) and in turn to the T1 MPRAGE (7-parameter affine). Finally, registration of the MPRAGE to MNI space (FSL's MNI Avg152, T1 2x2x2mm) was carried out with FSL (12-parameter affine) and refined using ART (non-linear transformation). First-level analyses included voxel pre-whitening, double-gamma hemodynamic response function (HRF) convolution, temporal filtering, and temporal derivative inclusion.
The following contrasts were entered in the first-level analysis: (own gender > other gender), (other gender > own gender), (own gender > baseline), (other gender > baseline), for each of the imitate gesture, observe gesture and view portrait conditions. Interaction contrasts were also entered, subtracting the above contrasts for the observe gesture or view portrait condition from the equivalent contrasts in the imitate gesture condition {e.g., [(imitate gesture own gender > other gender) > (observe gesture own gender > other gender)]}. Interaction contrasts were intended to reveal whether results found in the imitate gesture condition reflected processes unique to imitation. For each participant, the four runs were averaged using a fixed-effects analysis. A mixed-effects analysis (i.e. random and fixed effects) was then used to average across all participants (FLAME 1 + 2). All data were thresholded at Z > 2.3 and whole-brain cluster corrected for multiple comparisons (P < 0.05).
Additional analyses were conducted to explore the robustness of the significant effect observed in the (imitate gesture own gender > other gender) contrast. The consistency of this effect across participants was evaluated by examining whether significant activity for this contrast was present in each individual (fixed effect 4-run average, P < 0.05, uncorrected) within the region where significant activity was observed at the group level. An additional group analysis excluding the three homosexual participants was also conducted. Finally, in order to further test whether differential activity for own- and other-gender models was specific to the imitate gesture condition, parameter estimates of activity were extracted for all conditions from an anatomical region of interest (ROI) of the bilateral nucleus accumbens from the Harvard–Oxford probabilistic atlas (Desikan et al., 2006), thresholded at P = 0.25 (at least 25% of people have nucleus accumbens tissue in every voxel) and entered into a two gender (own and other) × three condition (imitate gesture, observe gesture and view portrait) repeated measures ANOVA in SPSS.
Bayesian analysis of the BrainMap database
To assess how selectively the region more active for own- than other-gender imitation is activated by reward tasks in the literature, we performed a Bayesian analysis of the BrainMap neuroimaging database, following the methods outlined by Poldrack (2006). We used a 10-mm cuboid ROI around the peak voxel of the cluster more active for own- than other-gender imitation [(14, 14, −8), converted from MNI to Talairach using the BrainMap search tool]. This ROI was fully contained within the active cluster. We searched for all experiments containing activity within this region that did and did not employ reward tasks (denoted by the Paradigm Class code in the database) and also for all experiments without activity in this region that did and did not employ reward tasks (Table 1). We used these frequencies along with a neutral prior estimate of a reward task being used (P = 0.5) to calculate a posterior probability and corresponding Bayes factor (P/1 − P) for the likelihood a reward task was employed based on the presence of activity within our nucleus accumbens-centered functional ROI. We also calculated the conditional probability of a reward task being used given the activity within the ROI (Table 1, first row).
Table 1.
Frequency table for searches conducted in BrainMap database reflecting number of experimental comparisons found for each search
| Reward task | Not reward task | |
|---|---|---|
| Activated | 47 | 37 |
| Not activated | 460 | 8666 |
Note. Search location was (14, 14, −8) MNI converted to Talairach (12, 13, −3) using icbm2tal through the BrainMap search tool Sleuth v1.2, extending 5 mm in each direction.
RESULTS
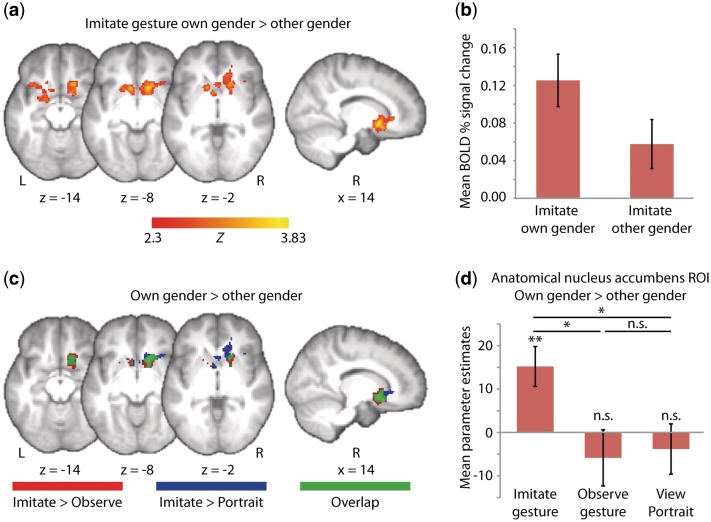
Comparing imitation of one's own gender to the other gender (imitate gesture own gender > other gender) produced a single cluster of significant activity, centered in bilateral nucleus accumbens and extending into the dorsal striatum, orbitofrontal cortex (OFC) and left amygdala (Table 2 and Figure 2a-b). This result held when the three homosexual participants were removed from the analysis and in both males and females when the sexes were analyzed separately. Additionally, 16 of 19 study participants had significant activity within this cluster when imitating own- compared to other-gender individuals, confirming the robustness of the own-gender effect even at the single subject level (two-tailed sign test, P = .004). No significant activity was found for the reverse contrast (imitate gesture other gender > own gender). Furthermore, no significant differences were found for the (own gender > other gender) contrast in either the observe gesture or view portrait conditions.
Table 2.
Peaks of activity for own > other gender during imitation and the interaction between imitation and the other conditions
| Anatomical region | X | y | z | Z |
|---|---|---|---|---|
| Imitate gesture own gender > other gender | ||||
| R putamen/nucleus accumbens | 14 | 14 | −8 | 3.83 |
| R orbitofrontal cortex/putamen | 18 | 18 | −12 | 3.76 |
| L nucleus accumbens | −8 | 14 | −8 | 3.43 |
| R caudate | 18 | 18 | 2 | 3.38 |
| R putamen | 20 | 18 | −2 | 3.38 |
| (Imitate gesture own gender > other gender) > (observe gesture own gender > other gender) | ||||
| R putamen/nucleus accumbens | 16 | 14 | −10 | 3.96 |
| R orbitofrontal cortex/putamen | 18 | 18 | −12 | 3.78 |
| R caudate | 20 | 16 | 6 | 3.26 |
| R pallidum/putamen | 16 | 4 | −16 | 3.19 |
| (Imitate gesture own gender > other gender) > (view portrait own gender > other gender) | ||||
| R putamen/caudate | 18 | 18 | −10 | 3.61 |
| R orbitofrontal cortex/insula | 28 | 20 | −8 | 3.59 |
| R insula/orbitofrontal cortex | 32 | 20 | −6 | 3.55 |
| R nucleus accumbens/putamen | 14 | 16 | −10 | 3.54 |
Note. All clusters present at statistical threshold of Z > 2.3, whole-brain corrected for multiple comparions (P < 0.05) (n = 19). Anatomical regions of peak voxel within cluster assigned using Harvard–Oxford Cortical and Subcortical Probabilistic Structural Atlases. First cluster within each anatomical region listed. Interaction contrasts masked by significant clusters in imitate condition. L and R refer to left and right hemispheres; x, y and z refer to the MNI coordinates corresponding to the left–right, anterior–posterior and inferior–superior axes, respectively; Z refers to the highest Z score within a cluster.
Fig. 2.
Results. Functional activity is thresholded at Z > 2.3 with whole-brain correction for multiple comparisons at the cluster level (P < .05), and overlaid on a group average (n = 19) T1-weighted structural scan. (a-b) Whole-brain analysis. (a) During imitation of own gender compared to other gender models, significantly more activity was seen in reward-related neural regions, including the bilateral ventral striatum [MNI coordinates: R (14,14,−8), L (−8, 14, −8)], dorsal striatum and left amygdala. Cluster is 1148 voxels, cluster P = 0.0023, peak Z = 3.83. (b) Percent fMRI signal change from baseline averaged across entire cluster of significant activity shown in (a). Error bars are s.e. (c-d) Gender x condition interaction. (c) Whole-brain interaction effects in regions exhibiting greater activity for own- than other-gender in the imitate gesture condition as compared to the observe gesture condition (red) and the view portrait condition (blue). The overlap between these interaction effects (green) can be interpreted as activity that is unique to imitation. All contrasts are post-threshold masked by significant activity within the (imitate gesture own gender > other gender) contrast; (d) mean differences for (own gender - other gender) parameter estimates averaged across a bilateral nucleus accumbens anatomical ROI. Results displayed are from two gender (own and other) × three condition (imitate gesture, observe gesture and view portrait) repeated measures ANOVA. *P < 0.05, **P < 0.01.
To determine whether own-gender enhanced activity within reward-related regions was unique to imitation, we next compared the (own gender > other gender) contrast in the imitate gesture condition to both the observe gesture and view portrait conditions [(imitate gesture own gender > other gender) > (observe gesture own gender > other gender)] and [(imitate gesture own gender > other gender) > (view portrait own gender > other gender)]. Since the purpose of these analyses was to determine what activity from the (imitate gesture own gender > other gender) contrast was unique to imitation, interaction contrasts were post-threshold masked by the result of the (imitate gesture own gender > other gender) contrast. There was reliably higher activity in the nucleus accumbens, dorsal striatum, OFC and amygdala for imitating own- compared to other-gender actors, even after activity associated with passively observing the gestures (Figure 2c, red activity) or portraits (Figure 2c, blue activity) of own- compared to other-gender actors was removed. Furthermore, there was considerable overlap between these two analyses (Figure 2c, green activity), suggesting that much of the enhanced activity in response to own- compared to other-gender individuals during imitation was unique to imitation.
We also conducted a more targeted inquiry into whether the own-gender effect seen in the imitate condition was also seen during gesture observation or portrait viewing, by restricting our search to an anatomical nucleus accumbens ROI. We extracted parameter estimates of activity from this cluster for all six conditions (as compared to baseline) and entered these into a two-gender (own and other) × three-condition (imitate gesture, observe gesture and view portrait) repeated measures ANOVA. We found a significant gender × condition interaction [F (2,17) = 4.26, P = 0.02], whereby significantly greater activity for own than other gender was observed in the imitate gesture condition [t (18) = 3.3, P = 0.004] but not in the observe gesture [t (18) = −0.9, P = 0.378] or view portrait [t (18) = −0.7, P = 0.518] condition. Furthermore, the (own gender > other gender) contrast in the imitate gesture condition was significantly different from both the observe gesture [F(1,18) = 7.26, P = 0.015] and view portrait condition [F(1,18) = 6.69, P = 0.019], but the non-imitative conditions did not differ from one another (Figure 2d). This ROI analysis suggests that the increased activity in the nucleus accumbens when participants imitated individuals of their own gender is indeed specific to the process of imitation, as no effect was found during gesture observation or portrait viewing, even when the search was restricted to an anatomical region where this difference was seen during imitation.
To assess the likelihood that the enhanced activity in the nucleus accumbens and other regions indicated the engagement of reward-related cognitive processes, as opposed to other cognitive functions related to these structures, we conducted a Bayesian analysis of the BrainMap neuroimaging database (Laird et al., 2005). We estimated the degree to which the region found to be more active for own- compared to other-gender imitation is selectively activated by reward tasks within the BrainMap database (Poldrack, 2006). We found that activity within this nucleus accumbens region was associated with a substantial increase in the probability that a reward task was used (i.e. from a neutral prior probability of 0.5–0.96, corresponding to a Bayes factor of 21.8). A Bayes factor of >10 is thought to reflect strong increases in confidence over the prior probability (Jeffreys, 1998). We also calculated the conditional probability of a reward task being used, given the presence of activity within this nucleus accumbens region. This measure provides a different metric of the likelihood that activity within this region is related to reward processing, which unlike the Bayesian calculation does not depend on a specific comparison set. We found that 56% of contrasts elliciting activity within this region involved reward tasks, indicating that activity within this area is more likely to be related to reward than to any other cognitive function. Notably, this metric likely underestimates the conditional probability because a number of contrasts among the 44% not explicitly labeled as reward tasks involved comparison between conditions that may differ in their reward value (e.g. amphetamine > saline; erotic pictures > neutral pictures). Taken together, these analyses suggest that own-gender imitation is most likely associated with cognitive processes similar to those associated with more traditional reward tasks, such as reward and reinforcement.
DISCUSSION
While preferential imitation of own-gender models has been well documented behaviorally, until now no proximate neural mechanism underlying own-gender imitation was known. Consistent with our prediction, we found that reward-related structures, including the ventral striatum, OFC, dorsal striatum and left amygdala, were more active during own- compared to other-gender imitation. The specificity of the own-gender effect to the imitation condition suggests that this effect is not merely due to low-level perceptual features of the stimuli (e.g. simply looking at own- compared to other-gender individuals) but rather is related to the process of imitation of own-gender models in particular. Furthermore, using a Bayesian analysis, we demonstrated that the region more active for own- than other-gender imitation is most often activated by reward tasks in the BrainMap database. Taken together, these findings provide a plausible neural mechanism for the pervasive gender similarity bias in imitative learning.
The neural regions observed to be preferentially active during own-gender imitation are part of a dopaminergic neural system that has been associated with processing reward and reinforcement learning in both humans and animals (Haber and Knutson, 2010). Specifically, the OFC, ventral striatum and amygdala have each been implicated in coding the value of current and future rewards. The ventral striatum and amygdala are also sensitive to reward salience. These structures are thought to work in concert with the dorsal striatum (caudate and putamen; also structures preferentially active for own-gender imitation) to guide subsequent action selection (O'Doherty, 2004; Haber and Knutson, 2010). Thus, activity in reward-related circuitry during own-gender imitation may be providing a reinforcement signal that facilitates learning from own-gender models. It has previously been demonstrated that activation of reward-related neural structures including the ventral striatum may be contingent on the interaction between anticipated reward and the need to perform an instrumental response (Bjork and Hommer, 2007). Accordingly, the reinforcement signal in the present study may result from the act of own-gender imitation itself or the interaction between neural activity directly related to imitation with activity related to the salience and self-similarity of own-gender models.
Importantly, these data reveal a similarity between the neural underpinnings of own-gender imitation and those of classical reward tasks, which would not have been apparent from behavior alone. Bayesian analysis of the BrainMap database using the peak of the region found to be more active for own- vs other-gender imitation, located in the nucleus accumbens, confirmed that this region is most often activated by reward tasks in the literature. Ariely and Berns (2010) found a similar level of selective activation of the nucleus accumbens by reward tasks in the BrainMap database using an anatomical nucleus accumbens ROI. Such Bayesian calculations are heavily influenced by the nature of the comparison set, but even when we only considered studies that had activation within the nucleus accumbens, reward tasks still constitute the majority. Thus, although inferring cognition from brain activity in many areas of the brain may be complicated due to the large number of functions these brain areas may perform, the ventral striatum appears to be most often active during reward-related tasks, thereby increasing our confidence that own-gender imitation is an intrinsically rewarding process.
Previous theoretical and empirical work on gender identity development suggests why own-gender imitation may be rewarding. Social learning theory proposes that acting like own-gender individuals is encouraged by parents, teachers and peers from an early age, thus facilitating gender identity formation (Bandura, 1977). Social-cognitive theory posits that once gender identity has begun to form, own-gender imitation is perpetuated by the confidence derived from perceiving one's self to be similar to a group of same-sex individuals (Kohlberg, 1966). Indeed, both the reception of praise from others (Izuma et al., 2008) and the act of conforming to a group (Klucharev et al., 2009) have been associated with increases in activity within reward-related neural circuitry including the ventral striatum. Future studies are needed to determine how the positive relationship found between own-gender imitation and reward in the present study is related to gender identity development.
The present study represents the first step toward elucidating the neural mechanisms of model-observer similarity biases during imitation, a key element of real-world imitative learning. Our findings provide strong support for the hypothesis that imitation of own-gender models is indeed accompanied by intrinsic reinforcement and may thus facilitate the acquisition of gender norms and gender role behaviors. This finding is in keeping with our recently proposed model of the neural architecture of cultural imitative learning, which posits that reward-related responses play a critical role in reinforcing the learning of culturally transmitted behavior (Losin et al., 2009). Future developmental and cross-cultural studies may help determine the extent to which increased responsiveness in the reward system when imitating individuals of one's own gender may be driven by experiential vs biologically determined factors.
Conflict of Interest
None declared.
Acknowledgments
The first author was supported by a National Science Foundation Graduate Research fellowship. The research was supported by a research grant from the FPR-UCLA Center for Culture Brain and Development.
We thank Kathleen Quach, Drew Morton and Kambria Nguyen for their helpful assistance in data collection and analysis and Clark Barrett, Naomi Eisenberger and Neil Losin for their helpful discussions and comments on the manuscript.
REFERENCES
- Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image resistration algorythm. Journal of computer assisted tomography. 1995;19:615–23. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- Ariely D, Berns GS. Neuromarketing: the hope and hype of neuroimaging in business. Nature Reviews Neuroscience. 2010;11:283–292. doi: 10.1038/nrn2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Social Learning Theory. New Jersey: Prentice-Hall; 1977. [Google Scholar]
- Bandura A, Ross D, Ross SA. Transmission of aggression through imitation of aggressive models. Journal of Abnormal and Social Psychology. 1961;63:575–82. doi: 10.1037/h0045925. [DOI] [PubMed] [Google Scholar]
- Basow SA, Howe KG. Role-model influence: effects of sex and sex-role attitude in college students. Psychology of Women Quarterly. 1980;4:558–72. [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behavioural Brain Research. 2007;177:165–70. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, et al. Neural circuits underlying imitation learning of hand actions an event-related fMRI atudy. Neuron. 2004;42:323–34. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Bussey K, Bandura A. Influence of gender constancy and social power on sex-linked modeling. Journal of Personality and Social Psychology. 1984;47:1292–302. doi: 10.1037//0022-3514.47.6.1292. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Frey SH, Gerry VE. Modulation of neural activity during observational learning of actions and their sequential orders. Journal of Neuroscience. 2006;26:13194–201. doi: 10.1523/JNEUROSCI.3914-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Gallessich JM, Evans SL. Sex of faculty role model and students' self-perceptions of competency. Sex Roles. 1983;9:597–607. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, McElreath R. The evolution of cultural evolution. Evolutionary Anthropology. 2003;12:123–35. [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–8. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jeffreys SH. Theory of Probability. USA: Oxford University Press; 1998. [Google Scholar]
- Killian JN. Effect of model characteristics on musical preference of junior high students. Journal of Research in Music Education. 1990;38:115–23. [Google Scholar]
- Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–51. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Kohlberg L. A cognitive-developmental analysis of children's sex-role concepts and attitudes. In: Maccoby EE, editor. The Development of Sex Differences. Stanford, CA: Stanford University Press; 1966. pp. 82–173. [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. The social evolution of a human brain mapping database. Neuroinformatics. 2005;3:65–77. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Losin EA, Dapretto M, Iacoboni M. Culture in the mind's mirror: how anthropology and neuroscience can inform a model of the neural substrate for cultural imitative learning. Progress Brain Research. 2009;178:175–90. doi: 10.1016/S0079-6123(09)17812-3. [DOI] [PubMed] [Google Scholar]
- Mesoudi A. The cultural dynamics of copycat suicide. PLoS ONE. 2009;4:e7252. doi: 10.1371/journal.pone.0007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiologia. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Perloff RM. Gender constancy and same-sex imitation: a developmental study. Journal of Psychology. 1982;111:81–6. [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Science. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Slaby RG, Frey KS. Development of gender constancy and selective attention to same-sex models. Child Development. 1975;46:849–56. [PubMed] [Google Scholar]
- Tomasello M, Kruger AC, Ratner HH. Cultural Learning. Behavioural Brain Science. 1993;16:495–511. [Google Scholar]