Abstract
The influence of personality on the neural correlates of emotional processing is still not well characterized. We investigated the relationship between extraversion and neuroticism and emotional perception using functional magnetic resonance imaging (fMRI) in a group of 23 young, healthy women. Using a parametric modulation approach, we examined how the blood oxygenation level dependent (BOLD) signal varied with the participants’ ratings of arousal and valence, and whether levels of extraversion and neuroticism were related to these modulations. In particular, we wished to test Eysenck's biological theory of personality, which links high extraversion to lower levels of reticulothalamic–cortical arousal, and neuroticism to increased reactivity of the limbic system and stronger reactions to emotional arousal. Individuals high in neuroticism demonstrated reduced sustained activation in the orbitofrontal cortex (OFC) and attenuated valence processing in the right temporal lobe while viewing emotional images, but an increased BOLD response to emotional arousal in the right medial prefrontal cortex (mPFC). These results support Eysenck's theory, as well as our hypothesis that high levels of neuroticism are associated with attenuated reward processing. Extraversion was inversely related to arousal processing in the right cerebellum, but positively associated with arousal processing in the right insula, indicating that the relationship between extraversion and arousal is not as simple as that proposed by Eysenck.
Keywords: arousal, extraversion, fMRI, neuroticism, valence
INTRODUCTION
Extraversion and neuroticism are two of the most widely studied personality traits (Canli et al., 2001; Hamann and Canli, 2004) and they are common to several dominant theories of personality including Costa and McCrae's Big Five model (2003) and Eysenck's biological approach (1967, 1994). Extraversion and neuroticism have been found to correlate robustly with positive and negative emotionality, respectively (Costa and McCrae, 1980; Rusting and Larsen, 1997), a relationship which seems to remain stable across the lifespan (Wilson and Gullone, 1999); and differences in these traits are known to influence emotional and cognitive processing (Bradley and Mogg, 1994; Amin et al., 2004; Canli, 2004; Kumari et al., 2004, 2007).
Although neuroticism describes differences in a personality dimension rather than a clinical disorder, it can be of high clinical relevance as it is a risk factor for developing anxiety and depression disorders (Derryberry and Reed, 1994; Kendler et al., 2004). Several neuroimaging studies have found that this trait is most notably associated with biases towards negative emotional processing (Canli et al., 2001; Haas et al., 2007, 2008; Chan et al., 2008, 2009; Cremers et al., 2010). However, there has been little focus to date on the relationship between neuroticism and positive emotional perception. There is evidence that depressed patients demonstrate reduced neural responding to positive emotional stimuli (Shestyuk et al., 2005), and given that neuroticism is a risk factor for depression, we were interested in investigating whether high levels of this trait are associated with an attenuated neural response to positive valence.
According to Eysenck’s biological theory of personality (Eysenck and Eysenck, 1991; Eysenck, 1994), the neuroticism dimension is also posited to affect how individuals respond to emotional arousal. Whereas extraversion is considered to be linked to differences in the functioning of the reticulothalamic–cortical arousal system, high levels of neuroticism are theorized to reflect increased reactivity of the limbic system, which predisposes individuals high in neuroticism to react strongly to emotionally arousing experiences and take longer to return to pre-arousal states. In spite of these predictions about the relationship between personality and reactivity to emotional arousal, the neural bases of these relationships have never been investigated using functional magnetic resonance imaging (fMRI). Therefore, we were interested in examining whether differences in this trait are related to how emotional arousal is processed in the brain.
Eysenck’s biological theory of extraversion (1967, 1994) proposes that differences in this personality dimension are reflective of differences in a reticulothalamic–cortical arousal system, with extraverts experiencing both lower baseline levels of cortical arousal, as well as low arousability of the cortex, i.e. they show a smaller change in cortical activity in response to arousing stimuli than introverts (Eysenck, 1967; Eysenck and Eysenck; 1991; Hagemann et al., 2009). It is this chronic intrinsic under-arousal which is thought to drive highly extraverted people to engage in typically extraverted behaviours in order to enhance their low arousal states (Eysenck, 1994). Furthermore, this under-arousability enables extraverts to tolerate much higher levels of arousal than introverts, who withdraw to avoid further increases in arousal which they find difficult to withstand (Eysenck, 1967, 1994).
Several neuroimaging studies have supported this theory of extraversion (Kumari et al., 2004; O'Gorman et al., 2006; Hagemann et al., 2009); for example, Hagemann and colleagues (2009) found that alpha activity as measured by resting-state electroencephalography (EEG), which is indicative of lower arousal states (Barry et al., 2011; De Cesarei and Codispoti, 2011) was positively associated with extraversion levels in left and right frontal sites, indicating that extraverted individuals had lower baseline levels of cortical arousal; while Kumari et al. (2004) found that levels of extraversion were negatively associated with resting fMRI signals in the thalamus and in Broca's area extending to Wernicke's area. Given the predictions of Eysenck's arousal hypothesis, one might expect that in the context of emotional processing, extraversion levels would have a pervasive influence on the perception of emotional arousal; however, the relationship between extraversion and the neural substrates of emotional arousal processing are unknown. Therefore, we sought to investigate this question using fMRI.
In the present study, we used fMRI to examine how levels of extraversion and neuroticism are related to the neural substrates of emotional arousal and valence processing in a group of 23 young healthy women. We utilized an amplitude modulation approach to dissociate the effects of arousal and valence on the blood oxygenation level-dependent (BOLD) signal during an emotional image viewing task. We examined single trial dynamics, whereby the subject's valence and arousal ratings for each stimulus image were included as a parametric weight in our model. We then examined how these effects varied as a function of neuroticism and extraversion levels.
We used the Eysenck personality questionnaire to measure levels of neuroticism and extraversion (Eysenck and Eysenck, 1991). The primary reason we used Eysenck's scale and theoretical framework is that this personality theory has a strong biological component and causal explanation, with individual differences in personality thought to arise from differences in brain function. Specifically, Eysenck proposed that differences in the extraversion dimension are reflective of differences in the functioning of the reticular arousal system, while neuroticism reflects differences in the limbic system's emotional responses (Eysenck, 1967, 1994). Gray's (1982, 1990, 1997) theory proposes that there are two basic motivational systems in the brain which drive behavioural responses, an approach and an inhibition system, which differ between individuals, representing differences in sensitivity to environmental cues of reward and punishment. This theory also has a strong biological component, however, due to the focus on behavioural inhibition in the avoidance of punishment cues, we choose Eysenck's model as having potentially more predictive power in the context of our experimental questions, namely concerning the processing of rewarding stimuli, and arousal in particular. Finally, the Big Five model of Costa and McCrae (McCrae and Costa, 2003), although very popular in the field of personality has been criticized for being more of a descriptive model, with less scope for explanation as it contains no specific predictions about the biological basis of personality (Block, 2010).
In particular, we were interested in testing the relationship between extraversion and the perception of emotional arousal, and predicted that individuals high in this trait would experience less brain activation in response to increasing levels of arousal, in accord with the predictions of Eysenck's arousal hypothesis (Eysenck, 1994). Second, we wished to examine the relationship between neuroticism and positive valence and arousal processing. We predicted that high levels of neuroticism would be associated with reduced neural activation in response to positive valence, but an increased response to emotional arousal.
MATERIALS AND METHODS
Participants
Twenty-three young (mean age = 23.04 ± 3.46 years, age range = 19–29 years), healthy, right-handed women took part in the study. Only women were included as there have been considerable differences found between genders in emotional reactivity (Bradley et al., 2001b) in the neural representation of emotion (Beck et al., 1996; Cahill et al., 2001; Wager et al., 2003; Wrase et al., 2003) and in levels of neuroticism (Lynn and Martin, 1997). They had no history of psychiatric or neurological illness and were not depressed [Beck Depression Inventory scores: 4.83 ± 3.38; cut-off score for mild depression = 14 (Beck et al., 1996)]. The study had full ethical approval from the St James Hospital and the Adelaide and Meath Hospital, incorporating the National Children's Hospital Research Ethics Committee. All of the participants gave written informed consent and were paid €40 for taking part.
Personality measurement
The participants completed the Eysenck personality questionnaire, revised edition short scale (EPQ-R, Eysenck and Eysenck, 1991), a self-report questionnaire which measures levels of extraversion, neuroticism and psychoticism on scales ranging from 0 to 12, with 0 indicating the lowest level of the trait and 12 the highest level of the trait. We focused our analyses on neuroticism and extraversion.
Stimuli
The stimuli consisted of 190 coloured photographs. They were a combination of 98 images from the International Affective Picture System (IAPS, Lang et al., 2007) and 92 images gathered by the experimenter from various sources. They were either positive or neutral in valence and they varied in arousal levels. The stimuli were limited to neutral and positive valence only for several reasons. First, although arousal and valence tend to be highly correlated (Bradley et al., 1992; Ribeiro et al., 2005), we included images which were positive or neutral in valence and which varied in arousal level, allowing us to match the stimuli along one dimension while varying along the other and so investigate their effect on the BOLD signal separately. However, the co-variation of arousal with valence is particularly strong in the case of negative images, which tend to be rated as more arousing than positive images (Lang et al., 2008). Therefore, it was not possible to have enough negative low arousal exemplars. Second, we were particularly interested in examining the relationship between neuroticism and positive valence. Third, we wanted to be sure that any linear associations with valence in the imaging paradigm could be interpreted clearly as being associated with increasing valence from neutral to positive, and not confounded by the inclusion of negative stimuli.
The IAPS was supplemented to increase the number of positive, low arousal and neutral, higher arousal images. None of the images we used were of a sexual nature. The new images were chosen to be as similar as possible to the IAPS images and so, included very similar content and a mixture of objects, scenes, animals and people as does the IAPS.
As far as was possible the images of different valence and arousal levels were chosen to contain the same number of people, animals, scenes and objects. Before the experiment, all of the images were rated by 15 age-matched controls, of which 8 were women, along the dimensions of valence, arousal and dominance. These ratings were collected to both devise approximate ratings for the new images and also to assess the level of agreement between the IAPS standard ratings, which are devised from a US sample, and the ratings of a group of young Irish adults. Further details of the ratings of the new images and the IAPS images, as well as examples of images from our stimulus set can be found in the Supplementary Data. The stimuli were delivered using Presentation v. 13.0 (Neurobehavioral Systems, Albany, CA, USA).
Experimental design
Functional MRI paradigm
The participants viewed 190 coloured images as they underwent fMRI. To avoid long-lasting mood states, the images were pseudo-randomized so that no more than three images of the same valence or arousal type were presented in a row. The task consisted of two experimental runs, each containing 95 trials and lasting ∼20 min. In each trial, an image was presented in the centre of a white background for 3000 ms, and after a delay of 1000–3000 ms (pseudo-random jitter), a prompt appeared on screen for 2000 ms asking the participants to classify the image they had just seen as ‘Living’ or ‘Non-living’. The participants were instructed to make their response by pressing either the left or right button on a MR-compatible button response box held in their right hand, to correspond with the left/right position of the ‘Living/Non-living’ word on the screen. This shallow encoding task was intended to maintain the participants' focus for the duration of the task, without explicitly drawing their attention to the emotional content of the stimuli (Kensinger et al., 2007). The onset of both the images and the prompts were jittered to ensure optimal sampling of the haemodynamic response (Josephs and Henson, 1999).
Post-scanning image rating
The participants did not rate the images while in the scanner as emotional evaluation has been found to result in attenuation of the neural response to emotional stimuli, thought to be due to the top–down influences of cognitive re-evaluation and judgement (Hariri et al., 2000; Taylor et al., 2003). Instead, they returned 2–3 days later and rated all of the images they had seen during the scanning session, as well as 48 negative images from the IAPS along the dimensions of valence, arousal and dominance. (The 2–3 days gap was to facilitate the collection of memory recall information that is not part of this report). The negative images were included in the rating task to provide contrast to the others, to ensure that the participants understood the full remit of the valence dimension. A computerized version of the Self-Assessment Manikin (SAM, Lang et al., 2008) was used to operationalize valence, arousal and dominance.
MRI scanning protocol
Imaging data were acquired using a Philips Intera Achieva 3.0 T MR system (Best, The Netherlands). The BOLD signal changes were measured using a T2*-weighted echo-planar imaging sequence with TR = 2000 ms and TE = 30 ms. Each volume of data covered the entire brain with 39 slices, and the slices were acquired in interleaved sequence from inferior to superior direction. In total 598 volumes were acquired during each of the two runs, with voxel dimensions of 3.5 × 3.5 × 3.85 mm and a 0.35 mm gap between the slices. A T1W/IR sequence was used to collect a 3D high-resolution anatomical image with voxel dimensions equal to 0.9 × 0.9 × 0.9 mm for structural localization.
Analysis
Behavioural data
The experimental log files were parsed using python scripts (version 2.6.2, http://www.python.org/) to extract performance and rating information. These data were then used to create individually tailored regressors for each participant based on their subjective ratings of the images. Statistical analyses of the behavioural results were conducted using Minitab (version 15, Coventry, UK).
MRI data analysis
The MRI data were analysed using Analysis of Functional NeuroImaging (AFNI) (Cox, 1996) (http://afni.nimh.nih.gov/afni/) and FSL (FMRIB Software Library- http://www.fmrib.ox.ac.uk/fsl/). The first four dynamics were obtained to correct for T1 equilibration effects and were subsequently discarded. The data were motion-corrected by realignment to the first volume of the first run, concatenated into a single run, global mean adjusted by proportional scaling and smoothed with a 6-mm full-width-at-half-maximum Gaussian kernel.
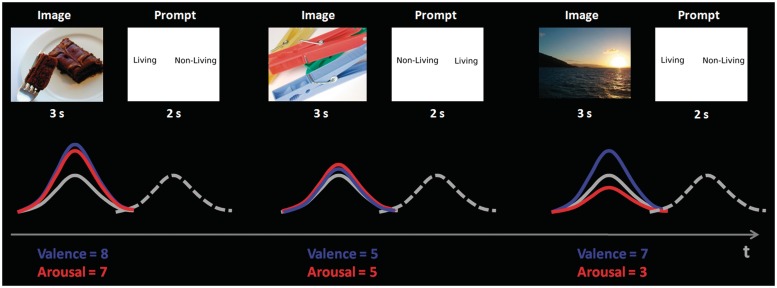
A general linear model (GLM) analysis was conducted in AFNI. Figure 1 shows a schema of the experimental design. Two regressors of interest were included to model the variance due to the image and prompt trials—these two regressors modelled the mean BOLD signal change from baseline across all trials. To model the additional effects of arousal and valence on the BOLD response during the image trials, subjective ratings of arousal and valence for each image were included as single trial parametric weights. This resulted in three image regressors: a constant unmodulated regressor describing the mean BOLD response during the image presentations, regardless of the arousal or valence of the image (this will be referred to in this manuscript as the constant BOLD response), BOLD activation during the image trials modulated by arousal, and BOLD activation during the image trials modulated by valence. A separate regressor was included for all of the images rated as negative, so that these trials were not included in the amplitude modulation analysis. Several regressors of no interest were also included in the GLM to model the following sources of variance: (i) six motion parameters, (ii) eight regressors to model low frequency noise and (iii) two regressors to model the mean differences between the two runs.
Fig. 1.
Illustration showing a schema of the fMRI analysis and parametric modelling of the haemodynamic response function. Each trial consisted of an image presented for 3000 ms, a pseudo-random jitter of 1000–3000 ms before the onset of the prompt for 2000 ms, followed by an inter-trial interval of between 5000 ms and 8000 ms. The valence and arousal ratings were included in the GLM as amplitude modulators and are shown in blue and red, respectively, whereas the constant or average BOLD response is represented by the solid grey response. The BOLD response to the prompts is shown as a dashed grey line.
All statistical analyses were calculated in the participants’ native space, then the regressor coefficients maps were normalized into standard stereotactic space by warping them to the MNI brain template (Montreal Neurological Institute/International Consortium for Brain Mapping 152 standard atlas as provided in the FSL software package) using FSL's linear registration tool, FLIRT. The transformation matrix (12 parameter affine) from native space to MNI space was calculated using the high resolution structural images from each subject.
The group statistical analyses were based on a random-effects model, and activation maps of the constant and modulated responses were calculated with a series of independent t-tests. The relationship between personality and brain activation was examined with a series of whole brain correlations using AFNI's tool 3dRegAna. The participants’ levels of extraversion and neuroticism were separately regressed against the constant BOLD response, the arousal-modulated response and the positive valence-modulated response. In order to control for the near inverse correlation between extraversion and neuroticism, when examining the relationship between extraversion and the BOLD signal, neuroticism was included as a covariate of no interest in the model, while extraversion was included as a covariate of no interest when examining the effects of neuroticism on brain activation.
Significant voxels passed a voxel-wise statistical threshold of P ≤ 0.01. To correct for multiple comparisons across the brain, each cluster had to have a minimum size of 708 µl of contiguous statistically significant voxels to be considered statistically significant. This minimum cluster size was calculated using a Monte Carlo simulation (in AFNI) to obtain a (familywise error) corrected P < 0.05 statistical significance in the t-tests. The SPM anatomy toolbox (V1.7b, Eicknoff et al., 2005) was used to localize activation clusters; however, where there were no probabilistic cytoarchitectonic labels available, a Brodmann area (BA) is given in the results table instead.
RESULTS
Behavioural results
Image ratings
The participants' individual arousal and valence ratings of the stimuli were used to create regressors which were unique for each subject. Average ratings were calculated to give an idea of the distribution of the scores; however, they are included here for guidance only. On average, 53.87 (±26.91) images were rated as positive (i.e. having a valence of 7, 8 or 9), 113.04 (±37.19) as neutral (i.e. having a valence of 4, 5 or 6), and 18.43 (±13.96) as negative (i.e. having a valence of 1, 2 or 3). The participants rated 99.57 (±13.76) images as having an arousal level of between 1 and 5, while 67.35 were rated as having an arousal level of 6 or more. All negative images were excluded from the parametric modulation analysis and were included in the GLM as a regressor of no interest.
EPQ scores
The mean and standard deviation of the neuroticism and extraversion scores were 4.8 ± 2.6 and 8.0 ± 3.6, respectively. These scores were not correlated, although there was a trend towards an inverse correlation (Pearson's r = −0.397, P = 0.061). In order to investigate whether there was a linear relationship between the emotional ratings and levels of neuroticism and extraversion, each subject's neuroticism and extraversion scores were correlated against the number of images they rated as positive and negative, and high and low in arousal. In all cases, the results were not statistically significant (P ≥ 0.38 in all cases), indicating that personality scores were not associated with the ratings of the images.
fMRI results
The constant and modulated BOLD responses independent of personality variables
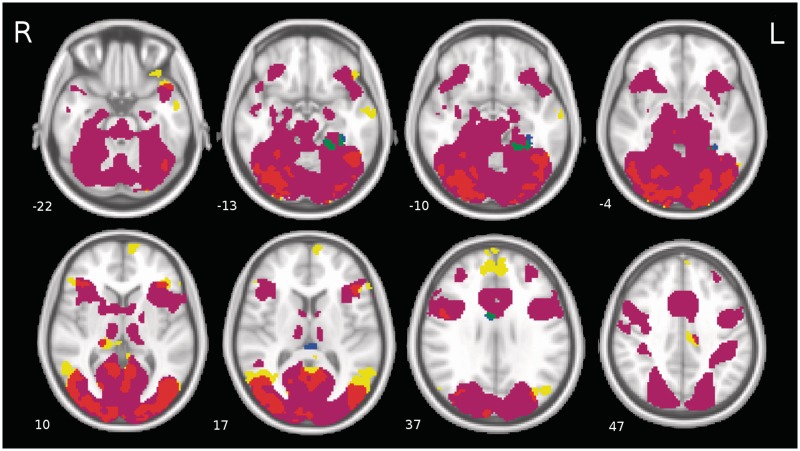
The constant BOLD response during image viewing, as well as the modulations due to arousal and positive valence were first examined independent of neuroticism and extraversion levels. A conjunction analysis was performed to examine which regions of the brain were activated in the constant response only, which regions were modulated only by increasing levels of arousal and positive valence and whether there were regions which were active in both the constant response and which were also modulated by the arousal and valence of the stimuli. The results of this conjunction analysis are shown in Figure 2, and the interested reader can find a table summarizing the significant activation clusters in Supplementary Table S3 in the Supplementary Data.
Fig. 2.
The group level results independent of the personality traits. A conjunction map was created to show the brain regions with a statistically significant constant BOLD signal not modulated by the emotional content of images, and brain regions where the BOLD signal was modulated by the arousal and valence characteristics of the stimuli. The colours represent the following: Purple = Constant BOLD response; yellow = regions modulated by arousal; red = regions activated in both the constant response and the arousal-dependent response; blue = regions modulated by valence; green = regions activated in both the constant response and the valence-dependent response. The numbers of the slices indicate the direction along the inferior–superior axis in millimetres in standard MNI space (−LPI).
Regions which were significantly active compared with baseline in the constant response only, and which were not modulated by the arousal or valence level of the stimuli are shown in purple in Figure 2. They include a very large cluster spanning widespread areas of the occipital and temporal lobes and which included the hippocampus, and clusters in the right superior temporal gyrus, the left middle frontal gyrus, the left and right cerebellum and the right middle occipital gyrus.
Overlap between the constant and arousal-dependent BOLD responses
There was a large degree of overlap between regions which were activated in the constant BOLD response and also modulated by increasing levels of emotional arousal, especially in the occipital and temporal visual processing areas. These regions are shown in red in Figure 2 and include a large cluster that spans the fusiform gyrus and primary and secondary visual cortex (BA 17 and 18) bilaterally, and further clusters in the left temporal pole, the right superior temporal gyrus, the right and left inferior frontal gyrus, the right thalamus and the right precentral gyrus.
The arousal-dependent BOLD response
Several brain regions in the temporal and frontal lobes were not activated in the constant BOLD response but only preferentially responded to increasing levels of arousal. These are shown in yellow in Figure 2, and included clusters in the middle temporal gyri bilaterally, the left inferior temporal gyrus, the right superior temporal gyrus, the left superior medial frontal gyrus, the left and right inferior frontal gyrus, the precuneus, the left middle cingulate cortex, the right thalamus and the left middle occipital gyrus.
The valence-dependent BOLD response
The valence-dependent BOLD response was much less extensive than the arousal-dependent response. There was a single cluster located in the left inferior temporal gyrus which was not activated in the constant BOLD response but whose BOLD signal was significantly modulated by increasing levels of positive valence. This cluster is shown in blue in Figure 2. There was also a cluster in the left fusiform gyrus that was both activated in the constant BOLD response and which also showed a positive modulation of the BOLD signal in response to increasingly positive valence. This is shown in green in Figure 2.
The influence of personality on emotional processing
The relationship between neuroticism and the BOLD signal
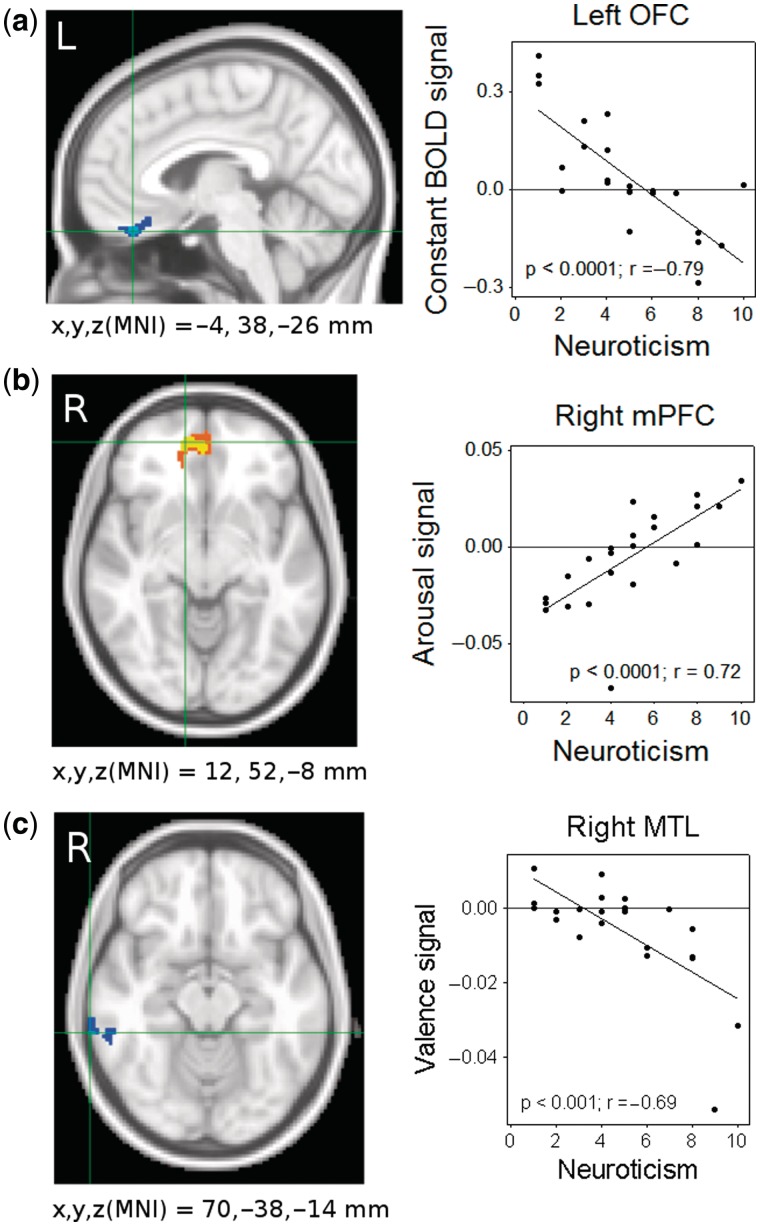
There was a strong negative linear association between neuroticism and the constant BOLD response in the middle orbitofrontal cortex (OFC). There was a negative linear association between neuroticism and the valence-dependent BOLD modulation in the right middle temporal gyrus and the right rolandic operculum. There was a positive linear association between neuroticism and the degree of arousal-dependent BOLD modulation in the right medial prefrontal cortex (mPFC; Table 1 and Figure 3).
Table 1.
Linear associations between neuroticism levels and the BOLD signal during image viewing
| Voxels | t-valuea | MNI co-ordinates | Area: prob or BA | |
|---|---|---|---|---|
| Constant BOLD response | ||||
| Frontal lobe | ||||
| Left rectal gyrus | 161 | −4.96 | −4, 38, −26 | BA 11 |
| Left rectal gyrus | −3.62 | −6, 30, −22 | BA 11 | |
| Arousal modulation | ||||
| Frontal Lobe | ||||
| Right middle orbital gyrus | 211 | 5.65 | 12, 52, −8 | BA 11 |
| Right middle orbital gyrus | 4.72 | 2, 48, −6 | BA 10 | |
| Right middle orbital gyrus | 3.47 | 2, 58, −14 | BA 11 | |
| Valence modulation | ||||
| Temporal Lobe | ||||
| Right middle temporal gyrus | 102 | −4.43 | 70, −38, −14 | BA 21 |
| Right middle temporal gyrus | −4.3 | 70, −34, −16 | BA 21 | |
| Right inferior temporal gyrus | −4.16 | 64, −26, −18 | BA 21 | |
| Right rolandic operculum | 98 | −3.92 | 54, −18, 18 | OP 1: 50%; OP 4: 30% |
at ≥ 2.82, P < 0.01; t ≥ 3.79, P < 0.001, corrected. OP = opercular cortex.
Labels were generated using SPM's anatomy toolbox; however, where there was no cytoarchitectonic information available, a Brodmann area is given. MNI co-ordinates are—LPI (i.e. negative indicates left, posterior and inferior directions).
Fig. 3.
Neuroticism levels were negatively associated with the constant BOLD signal in the (a) orbitofrontal cortex (OFC), positively associated with the arousal-dependent BOLD signal in the (b) medial prefrontal cortex (mPFC) and negatively associated with the valence-dependent BOLD signal in the (c) right middle temporal gyrus.
The relationship between extraversion and the BOLD signal
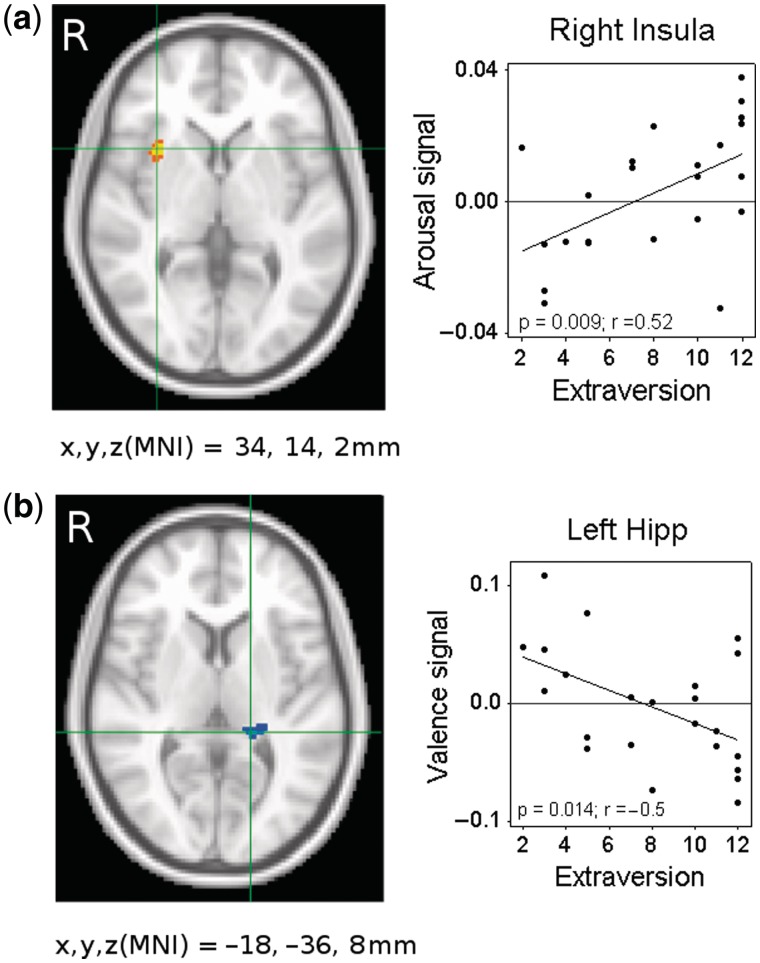
Extraversion was negatively associated with the constant BOLD response in a region of BA 6 spanning the middle cingulate cortex and supplemental motor area (SMA). There was a negative linear relationship between extraversion and the arousal-dependent BOLD response in the right cerebellum and a positive linear association between extraversion and the arousal-dependent BOLD response in the right insula. Extraversion was negatively associated with the valence-dependent BOLD signal in the right superior parietal lobule, the right postcentral gyrus and the left posterior hippocampus (Table 2 and Figure 4).
Table 2.
Linear associations between extraversion levels and the BOLD signal during image viewing.
| Voxels | t-valuea | MNI Co-ordinates | Area: prob or BA | |
|---|---|---|---|---|
| Constant BOLD response | ||||
| Frontal Lobe | ||||
| Left middle cingulate cortex | 189 | −4.01 | −6, −0, 40 | Area 6: 10% |
| Right SMA | −3.79 | 10, 0, 46 | Area 6: 40% | |
| Arousal modulation | ||||
| Cerebellum | ||||
| Right cerebellum | 96 | −4.17 | 24, −68, −26 | Lobule VI: 75% |
| Right cerebellum | −3.85 | 26, −72, −38 | Lobule VIIa Crus I: 57% | |
| Right cerebellum | 91 | −3.85 | 30, −84, −42 | Lobule VIIa Crus II: 98% |
| Right cerebellum | −3.84 | 22, −86, −38 | Lobule VIIa Crus II: 93% | |
| Frontal Lobe | ||||
| Right insula Lobe | 130 | 5.46 | 34, 14, 2 | BA 13 |
| Right insula Lobe | 4.23 | 32, 10, 14 | BA 13 | |
| Valence modulation | ||||
| Parietal Lobe | ||||
| Right superior parietal lobule | 102 | −4.72 | 24, −58, 72 | SPL (7A): 50% |
| Right postcentral gyrus | −3.99 | 30, −48, 74 | BA 2 | |
| Right superior parietal lobule | −3.68 | 14, −56, 68 | SPL (5L): 60% | |
| Right postcentral gyrus | 132 | −3.2 | 30, −32, 42 | Area 3a: 40% |
| Temporal Lobe | ||||
| Left hippocampus | 109 | −4.47 | −18, −36, 8 | Hipp (CA): 50% |
| Left hippocampus | −4.19 | −24, −34, 2 | Hipp (CA): 20% |
at ≥ 2.82, P < 0.01; t ≥ 3.79, P < 0.001, corrected. SPL = superior parietal lobule. Refer to Table 1 legend.
Fig. 4.
Extraversion levels were positively associated with the arousal-dependent BOLD signal in the (a) right insula lobe and negatively associated with the valence-dependent BOLD signal in the (b) left hippocampus.
DISCUSSION
The results of the present study demonstrate several relationships between personality and emotional processing that are novel findings, in particular the relationships between neuroticism and three different elements of emotional processing. First, neuroticism levels were negatively associated with the constant BOLD signal in the OFC, regardless of the valence or arousal level of the stimuli. Although this relationship was not valence specific as the images included both neutral and positive exemplars, it is of particular interest given that the OFC is known to play a pivotal role in the processing of emotion and reward (Schultz et al., 2000; O'Doherty, 2004; Rolls, 2004; Kringelbach, 2005), and in also in the successful down-regulation of negative emotional states (Ochsner et al., 2004; Eippert et al., 2007).
Second, we found that in accordance with our prediction, neuroticism was negatively associated with valence processing, in two regions of the right temporal lobe. It has previously been reported that patients with major depression show reduced brain activation in response to positive emotional words on an emotional working memory task (Shestyuk et al., 2005). Given that higher levels of neuroticism are associated with an increased risk of developing affective disorders such as depression and anxiety (Kendler et al., 2004), we hypothesized that a similar pattern may be evident in the case of high neuroticism individuals. Indeed, we found a negative relationship between neuroticism and the neural response to positive valence in the right middle temporal gyrus and the right rolandic operculum. The right middle temporal gyrus has been found to be activated in both emotional processing and encoding tasks (Critchley et al., 2000; Dolcos et al., 2004; Olson et al., 2007), and the group results from the current study also indicate widespread activation in the temporal lobes associated with emotional image viewing. Previous neuroimaging studies have identified this trait to be strongly associated with negative, rather than positive emotional processing (Canli et al., 2001; Haas et al., 2007, 2008; Chan et al., 2008, 2009; Cremers et al., 2010). We found in the current study that the converse is also apparent—neuroticism is associated with a tendency for several regions in the brain to be less responsive to positive emotional stimuli.
The attenuated neural response to the stimuli in the OFC and reduced activation to positive emotional valence in the right middle temporal gyrus might tentatively be interpreted as a predisposition in cases of high neuroticism for under activation in reward-processing or emotional regulation. We believe that this is the first demonstration of such a finding in a non-clinical population, and it may represent a biological vulnerability marker or putative phenotypic characteristic at a neural network level (Lesch et al., 1996; Sen et al., 2003; Strobel et al., 2003), which may help to further explain the relationship between this trait and the increased risk of developing depression and anxiety disorders. However, further elucidation of these types of functional phenotypes will be needed to understand the complex relationship between personality, emotional processing and the risk for affective disorders.
Last, we found that neuroticism was associated with an increased BOLD response to emotional arousal in the left mPFC. This is the first time, to our knowledge, that a link has been identified between neuroticism and increased reactivity to emotional arousal, rather than valence, in the brain. Eysenck (1967, 1994) proposed in his biological theory of personality that high neuroticism is associated with increased reactivity of the limbic system, which predisposes individuals high in neuroticism to react strongly to emotionally arousing experiences and take longer to return to pre-arousal states. This suggests that neuroticism influences emotional processing by enhancing neural sensitivity to high levels of emotional arousal, making high neuroticism individuals predisposed or primed to react strongly to arousing experiences (Eysenck, 1967). Indeed this trait is associated with greater sensitivity to negative mood inductions (Larsen and Ketelaar, 1991); and it has been found to correlate with larger electrodermal responses (EDR) to both arousing negative and positive emotional images, as well as with a longer period of recovery in the EDR signal (Norris et al., 2007). The results of the present study support Eysenck’s theory, with individuals high in neuroticism showing an enhanced response to emotional arousal in the left mPFC. This cortical region has previously been implicated to play a central role in self-referential processing and emotional attribution and appraisal (Eysenck, 1967; Fossati et al., 2003; Mitchell et al., 2005, 2006; Ochsner et al., 2005). Furthermore, constant activation in the mPFC in response to sad faces has been found to correlate with neuroticism (Haas et al., 2008). Thus, the enhancement of activation in this key emotional appraisal area in high neuroticism individuals suggests a heightened response to high levels of emotional arousal which may go some way to explaining why arousing experiences might have a more intense and lasting effect.
The relationship we identified between extraversion and the neural response to emotional arousal in the right cerebellum is consistent with Eysenck's arousal hypothesis of extraversion (1967, 1994), which proposes that extraverts demonstrate both lower baseline levels of cortical arousal and under-arousability of the cortex, and can tolerate higher levels of arousal better than introverts (Eysenck, 1967; Kumari et al., 2004; O'Gorman et al., 2006; Hagemann et al., 2009). This is postulated to explain why extraverts typically engage in more risk-taking and impulsive behaviours (Costa and McCrae, 1980; Eysenck, 1994), as they endeavour to enhance their intrinsic low arousal levels. Our results provide some supporting evidence for the under-arousability component of this hypothesis, with individuals high in extraversion showing less activation in response to increasing levels of arousal in areas Crus I and Crus II in the right cerebellum.
Although once considered a purely motor region, it is now known that outputs from the cerebellum influence more widespread regions of the cerebral cortex than previously recognized, and there is converging evidence from primate connectivity studies and human resting-state fMRI data that there are several anatomical circuits between the cerebellum and PFC (Kelly and Strick, 2003; Habas et al., 2009; O'Reilly et al., 2010). For example, there are reciprocal connections between Crus II and BA 46 (Strick et al., 2009), supporting the idea that the cerebellum may play an important role in attention and working memory among other diverse cognitive and emotional functions (Dolan, 1998; Rapoport et al., 2000; Stoodley and Schamahmann, 2009; Leiner, 2010). Therefore, it is not entirely surprising to find a relationship between personality and arousal processing in these regions, and in a review of neuroimaging studies which have reported cerebellar activations, Stoodley and Schamahmann (2009) localized the topography of emotion-related activation to Crus I in particular. In the context of our study, the fact that highly extraverted individuals showed less arousal modulation in Crus I and II may indicate under-sensitivity to the effects of emotional arousal.
However, we also found there to be a strong positive linear association between extraversion and arousal processing in the right insula lobe, which contradicts Eysenck's predictions about this trait. The insula is thought to play a central role in the perception of bodily sensations and in particular the perception of pain (Bornhovd et al., 2002); and there is also evidence that this structure has a role in the maintenance of drug addiction (Naqvi and Bechara, 2009; Garavan, 2010), as well as more general evidence for its involvement in a myriad different tasks involving emotional processing (Lamm and Singer, 2010) and attention (Nelson et al., 2010). The fact that highly extraverted individuals in our study displayed increased insula activity to high levels of arousal does not support the notion that extraverts are less sensitive to the effects of arousal. However, Kumari and colleagues (2004) found that high extraversion was associated with increased activation in the dorsolateral PFC and anterior cingulate in response to increasing levels of cognitive demand on an n-back working memory task, indicating that extraverts were more sensitive to the effects of cognitive arousal. It may well be the case that the relationship between extraversion and arousal is a more complex story than that originally proposed by Eysenck, with further research required to elucidate if and how arousal processing differences are a central feature of this personality trait, and whether there are effects related to emotional arousal, cognitive arousal or both.
The negative relationship we identified between extraversion and activation due to positive valence was somewhat unexpected, as previous studies have found this trait to be associated with increased activation in response to positive emotional stimuli (Canli et al., 2001; Haas et al., 2006). It may be, however, that our results contrast with previous neuroimaging studies of personality (e.g. Canli et al., 2001; Haas et al., 2006; Chan et al., 2008) due to the slightly different design of our study. For example, we used only neutral and positive stimuli, whereas the other studies also employed negative stimuli (Canli et al., 2001; Canli, 2004), which have been found to activate the key emotional processing centres such as the amygdala more substantially than either neutral or positive stimuli (For a review, see Carretie et al., 2009). The contrast between positive versus negative image viewing may also reveal greater differences in brain activation than the contrast between positive and neutral image viewing. Further, our positive stimuli had only moderately high arousal levels (few were rated as 8 or 9 on a 9-point scale) as we did not include any erotic stimuli. This may indicate that high extraversion is associated with greater neural sensitivity to highly arousing, highly positive stimuli rather than all positive stimuli. The amplitude modulation analysis and use of subjective rather than group ratings may also have contributed to these different results as previous neuroimaging studies have tended to rely on standard ratings and compare across image categories rather than using a parametric modulation approach.
A general observation of our results is that, whereas the linear associations between neuroticism and the neural response to the emotional stimuli were found in areas which have previously been linked to emotional processing, e.g. the OFC and the mPFC (Phan et al., 2002; O'Doherty, 2004), the relationships we found between extraversion and the fMRI results were in regions which would not be considered purely ‘emotional’, e.g. the SMA, cerebellum and parietal cortex. The evidence for a link between extraversion levels and emotional processing is not as strong as the body of literature supporting the influence of neuroticism on emotional processing at a behavioural (Bolger and Schilling, 1991; Rusting and Larsen, 1997; Kendler et al., 2004; Denissen and Penke, 2008) and neural level (Canli, 2004; Haas et al., 2007, 2008; Kumari et al., 2007; Cremers et al., 2010). It may well be the case that extraversion does not play a large modulatory role in affective processing, which is why we do not see strong evidence for variation in this trait being related to neural activation in emotional processing regions. Given that the associations we observed were mostly in motor and parietal regions, it seems likely that extraversion may not play a prominent mediating role in the experience of emotion, but rather may affect other elements of the task performance captured by these differences in the BOLD signal. The negative association between extraversion and the mean BOLD signal for example may indicate that those low in this trait were more susceptible to motor priming effects in readiness to press the button to respond to the prompt following the image. It is known, for example, that highly emotional stimuli capture attention faster than neutral stimuli (Bradley, 2009); therefore, it is possible that the associations with arousal and/or valence are due to attentional differences, e.g. high extraversion may be linked to increased susceptibly to the effects of attention. Obviously, there is no way using the current data to disentangle the effects of emotion and other factors such as attention, however, it would be interesting in future studies to investigate whether extraversion levels influence cognitive rather than affective aspects of emotional perception.
The amplitude modulation analysis we employed in this study has been used in other neuroimaging studies to examine single trial dynamics, such as in motor learning (Friston et al., 1992) and stimulus–response pairings (Buchel et al., 1998); however, it has only been used to examine how emotional judgements modulate the BOLD signal in a few studies (Phan et al., 2004; Heinzel et al., 2005; Anders et al., 2008; Northoff et al., 2009), and has not been applied in the study of personality and emotional processing. It proved highly effective at disentangling the effects of emotional arousal and valence on the BOLD signal, and the results are broadly similar to studies which have used different analysis methods, such as contrasting the activation maps associated with viewing emotional versus neutral images (Lang et al., 1998; Bradley et al., 2001a, Mourao-Miranda et al., 2003; Anders et al., 2004). The extensive modulations in the occipital and temporal lobes associated with increasing arousal corroborate many neuroimaging studies which have found increases in neural activation associated with increasing emotional arousal in the occipital cortex and inferotemporal regions of the ventral visual pathway (Bradley et al., 2003; Sabatinelli et al., 2005; Junghofer et al., 2006; Sabatinelli et al., 2007). Furthermore, the arousal-associated increases in the BOLD signal that we observed in the prefrontal gyrus have been found in others’ studies of emotional processing and evaluation (Grimm et al., 2006). The amplitude modulation due to valence during image viewing revealed a less extensive and distinct different pattern of neural activation, including significant clusters in the visual processing regions noticeably concentrated around the fusiform gyrus. Mourao-Miranda et al. (2003) also reported increased activation in the occipitotemporal visual processing areas when they compared responses with positive versus neutral images with the same arousal level. Lang et al. (1998) found that only positive images, not neutral or negative valences, increased the BOLD signal in the left fusiform gyrus. These patterns of activation are very similar to the modulation due to valence in the current study, and suggest that arousal and valence are represented by quite distinct neural networks.
The analysis of the fMRI results with respect to the subject's individual ratings is also a relatively novel approach, having been used only rarely (Anders et al., 2008) and we are confident that this is preferable to using standard or group average ratings as actual emotional ratings are quite variable. We believe that this design approach allowed us the opportunity to explore the interaction of personality with emotional processing in ways that have not yet been examined. Although beyond the scope of the current study, it may be interesting in future studies to compare this approach with the standard approach of using average emotional ratings, in order to see what effect this has on the fMRI results. It would also be interesting to investigate whether there are any interactions between mood, personality and emotional perception, with the administration of a mood scale such as the PANAS (Positive and Negative Affective Scales; Watson et al., 1988) or POMS (Profile of Mood States; McNair et al., 1971).
There has been some discussion and controversy in the past few years about the reliability of studies in social and personality neuroscience which correlate brain activations as measured by fMRI with measures of personality (Vul et al., 2009; Yarkoni, 2009). Some authors have argued that the results of such studies may be inflated due to lack of statistical power in small sample sizes (Vul et al., 2009; Yarkoni, 2009), while others have criticized the methods they claim are used by certain experimenters, whereby, region of interest analyses are carried out on the basis of whole-brain correlations, giving exaggeratedly high correlation coefficients (Vul et al., 2009). We are confident, however, that the results of the current study are reflective of real differences in brain function related to personality, and are not spurious results or inflated correlations. First, we employed a whole-brain correlation approach which has been validated as a reliable, independent measure to detect brain–behaviour relationships (Lieberman et al., 2009; Poldrack and Mumford, 2009). Second, we employed a robust multiple comparison procedure which protects against the possibility of false positives. Furthermore, given the anatomical specificity of our results, for example, reduced activation in the OFC associated with neuroticism, we are confident that our results are not simply spurious correlations in random brain regions. Finally, by including only women in the current study, we greatly reduced the heterogeneity of our sample given that there have been considerable differences found between genders in emotional reactivity (Bradley et al., 2001b), in the neural representation of emotion (Beck et al., 1996; Cahill et al., 2001; Wager et al., 2003; Wrase et al., 2003) and in levels of neuroticism (Lynn and Martin, 1997).
In summary, our findings offer a unique insight into how extraversion and neuroticism interact with the neural representation of emotional arousal and valence, two critical components of emotional processing. We identified several novel relationships between personality and the emotional processing, notably reduced sustained activation in the OFC and attenuated valence processing associated with high levels of neuroticism. These results provide further evidence for the important role that this trait plays in individual responses to affective stimuli, and they also suggest similarities between emotional processing in individuals with high levels of neuroticism and in depressed patients which may help to elucidate the role that this trait plays in the development of depression and other affective disorders. Furthering our understanding of individual differences in neural reactivity to emotional stimuli is pivotal to increasing our understanding of the role that personality plays in emotional processing, and may help to elucidate how personality influences the development of affective disorders.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
E.G.K. is supported by a PhD grant from the Health Research Board (HRB). J.H.B. is supported by a postdoctoral fellowship from the Irish Research Council for Science Engineering and Technology (IRCSET). A.L.W.B is supported by the Stokes program from Science Foundation Ireland (SFI).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We would like to thank Mr Sojo Josephs for his assistance in acquiring the neuroimaging data and the Trinity Centre for High Performance Computing for making computer resources available for the study.
REFERENCES
- Amin ZR, Constable T, Canli T. Attentional bias for valenced stimuli as a function of personality in the dot-probe task. Journal of Research in Personality. 2004;38:15–23. [Google Scholar]
- Anders S, Eippert F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Social Cognitive and Affective Neuroscience. 2008;3:233–43. doi: 10.1093/scan/nsn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping. 2004;23:200–9. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. Caffeine and opening the eyes have additive effects on resting arousal measures. Clinical Neurophysiology. 2011;122:2010–5. doi: 10.1016/j.clinph.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Second Edition Manual. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Block J. The five-factor framing of personality and beyond: some ruminations. Psychological Inquiry. 2010;21:2–25. [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59:355–86. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–36. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K. Mood and personality in recall of positive and negative information. Behaviour Research and Therapy. 1994;32:137–41. doi: 10.1016/0005-7967(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001a;1:276–98. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001b;1:300–19. [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. Journal of Experimental Psychology. Learning, Memory and Cognition. 1992;18:379–90. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–80. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Buchel AP, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions unsing nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–8. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotional processing. Journal of Personality. 2004;72:1105–32. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carretie L, Albert J, Lopez-Martin S, Tapia M. Negative brain: an integrative review on the neural processes activated by unpleasant stimuli. International Journal of Psychophysiology. 2009;71:57–63. doi: 10.1016/j.ijpsycho.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Chan SWY, Harmer CJ, Goodwin GM, Norbury R. Risk for depression is associated with neural biases in emotional categorisation. Neuropsychologia. 2008;46:2896–903. doi: 10.1016/j.neuropsychologia.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Chan SWY, Norbury R, Goodwin GM, Harmer CJ. Risk for depression and neural responses to fearful facial expressions of emotion. The British Journal of Psychiatry. 2009;194:139–45. doi: 10.1192/bjp.bp.107.047993. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Influence of extraversion and neuroticism on subjective well-being: happy and unhappy people. Journal of Personality and Social Psychology. 1980;38:668–78. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, et al. Neuroticism modulates amygdala–prefrontal connectivity in response to negative emotional facial expressions. NeuroImage. 2010;49:963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Affective modulation of the LPP and alpha-ERD during picture viewing. Psychophysiology. 2011;48:1397–404. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- Denissen JJA, Penke L. Neuroticism predicts reactions to cues of social inclusion. European Journal of Personality. 2008;22:497–517. [Google Scholar]
- Derryberry D, Reed MA. Temperament and attention: orientating towards and away from positive and negative signals. Journal of Social and Personality Psychology. 1994;66:1128–39. doi: 10.1037//0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. A cognitive affective role for the cerebellum. Brain. 1998;121:545–6. doi: 10.1093/brain/121.4.545. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–63. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Eicknoff S, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H. The Biological Basis of Personality. Springfield, IL: Thomas; 1967. [Google Scholar]
- Eysenck HJ. Personality: biological foundations. In: Vernon PA, editor. The Neuropsychology of Individual Differences. London: Academic Press; 1994. [Google Scholar]
- Eysenck HJE, Eysenck SBG. Manual of the Eysenck Personality Scales (EPS Adult) London: Hodder and Stoughton; 1991. [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Passingham RE, Liddle PF, Frackowiak RS. Motor practice and neurophysiological adaptation in the cerebellum: a positron tomography study. Proceedings of the Royal Society B: Biological Science. 1992;248:223–8. doi: 10.1098/rspb.1992.0065. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Structure and Function. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Gray JA. The Neuropsychology of Anxiety: an Enquiry into the Functions of the Septal-hippocampal System. New York: Oxford University Press; 1982. [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition and Emotion. 1990;4:269–88. [Google Scholar]
- Gray JA. The Psychology of Fear and Stress. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, et al. Segregrated neural representation of distinct emotion dimensions in the prefrontal cortex- an fMRI study. Neuroimage. 2006;30:325–40. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Haas BW, Constable T, Canli T. Stop the sadness: neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. NeuroImage. 2008;42:385–92. doi: 10.1016/j.neuroimage.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Amin ZR, Constable T, Canli T. Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Social Neuroscience. 2006;1:16–24. doi: 10.1080/17470910600650753. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience. 2007;121:249–56. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, et al. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of Neuroscience. 2009;29:8586–94. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Walter C, Schankin A, Danner D, Naumann E. Positive evidence for Eysenck's arousal hypothesis: a combined EEG and MRI study with multiple measurement occasions. Personality and Individual Differences. 2009;47:717–21. [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14:233–8. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziota JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Bermpohl F, Niese R, et al. How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. Cognitive Brain Research. 2005;25:348–58. doi: 10.1016/j.cogbrainres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philosophical Transactions of the Royal Society of London B: Biological Science. 1999;354:1215–28. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghofer M, Sabatinelli D, Bradley MM, Schupp HT, Elbert TR, Lang PJ. Fleeting images: rapid affect discrimination in the visual cortex. Neuroreport. 2006;17:225–9. doi: 10.1097/01.wnr.0000198437.59883.bb. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience. 2003;23:8432–44. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. American Journal of Psychiatry. 2004;161:631–6. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience. 2007;19:1872–87. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Das M, Wilson GD, Goswami S, Sharma T. Neuroticism and brain responses to anticipatory fear. Behavioral Neuroscience. 2007;121:643–52. doi: 10.1037/0735-7044.121.4.643. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Williams SCR, Gray JA. Personality predicts brain responses to cognitive demands. Journal of Neuroscience. 2004;24:10636–41. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of the anterior insular cortex in social emotions. Brain Structure and Function. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley BP, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. Journal of Personaliy and Social Psychology. 1991;61:132–40. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Leiner HC. Solving the mystery of the human cerebellum. Neuropsychol Review. 2010;20:229–35. doi: 10.1007/s11065-010-9140-z. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;29:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Berkman ET, Wager TD. Correlations in social neuroscience aren't voodoo. Commentary on Vul et al. (2009) Perspectives on Psychological Science. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn R, Martin T. Gender differences in extraversion, neuroticism, and psychoticism in 37 nations. Journal of Social Pscyhology. 1997;137:369–73. doi: 10.1080/00224549709595447. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Personality in Adulthood: a Five-factor Theory Perspective. New York/London: Guilford; 2003. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontla cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgements of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Volchan E, Moll J, et al. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20:1955–63. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohe AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Structure and Function. 2010;214:669–80. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, Cacioppo JT. Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology. 2007;44:823–6. doi: 10.1111/j.1469-8986.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, Rotte M, et al. Differential parametric modulation of self-relatedness and emotions in different brain regions. Human Brain Mapping. 2009;30:369–82. doi: 10.1002/hbm.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Kumari V, Williams SCR, et al. Personality factors correlate with regional cerebral perfusion. Neuroimage. 2006;31:489–95. doi: 10.1016/j.neuroimage.2005.12.048. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral Cortex. 2010;20:953–65. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected slef-knowledge. NeuroImage. 2005;21:1484–96. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage. 2004;21:768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience. 2009;4:208–13. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, van Reekum R, Mayberg H. The role of the cerebellum in cognition and behavior. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12:193–8. doi: 10.1176/jnp.12.2.193. [DOI] [PubMed] [Google Scholar]
- Ribeiro RL, Pompeia S, Bueno OF. Comparison of Brazilian and American norms for the International Affective Picture System (IAPS) Rev Bras Psiquiatr. 2005;27:208–15. doi: 10.1590/s1516-44462005000300009. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rusting CL, Larsen RJ. Extraversion, neuroticism, and susceptibility to positive and negative affect: a test of two theoretical models. Personality and Individual Differences. 1997;22:607–12. [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–70. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of Neurophysiology. 2007;98:1374–9. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–83. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, et al. A BDNF coding variant is associated with the neo personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Shestyuk YA, Deldin PJ, Brand JE, Deveney CM. Reduced sustained brain activity during processing of positive emotional stimuli in major depression. Biological Pscyhiatry. 2005;57:1089–96. doi: 10.1016/j.biopsych.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schamahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. The Annual Review of Neuroscience. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, et al. Allelic variation in 5-HT 1A receptor expression is associated with anxiety- and depression-related personality traits. Journal of Neural Transmission. 2003;110:1445–53. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–90. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personailty and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilson K, Gullone E. The relationship between personality and affect over the lifespan. Personality and Individual Differences. 1999;27:1141–56. [Google Scholar]
- Wrase J, Klein S, Gruesser SM, et al. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neuroscience Letters. 2003;348:41–5. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. Big correlations in little studies. Perspectives on Psychological Science. 2009;4:294–8. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.