Abstract
Asymmetrical patterns of frontal cortical activity have been implicated in the development and expression of aggressive behavior. Along with individual motivational tendencies, the ability to restrain one's impulses might be a factor in aggressive behavior. Recently, a role for the inhibitory cortical beta rhythm was suggested. The present study investigated whether individual differences in resting state asymmetries in the beta frequency band were associated with trait aggression and behavioral inhibition. In addition, the selective contributions of the prefrontal and motor cortex areas to these associations were examined. Results showed that relative dominant right frontal beta frequency activity was associated with both heightened trait aggression, especially hostility, and reduced response inhibition. Moreover, asymmetries over the anterior electrode locations proved to be related most closely to trait aggression, while asymmetries over the central electrode locations were associated with response inhibition. Together these findings show that right-dominant frontal beta activity is positively associated with aggressive tendencies and reduced behavioral inhibition.
Keywords: aggression, behavioral inhibition, beta oscillations, electroencephalography, frontal cortex, hostility, motor cortex
INTRODUCTION
Over the past decades, scientific interest in human socio-emotive behavior has increased dramatically. Theorizing on the neural correlates of emotion and motivation has evolved into a rich branch of neuroscientific research which has increasingly gained the attention of scientists and the general public alike (Illes et al., 2010). Owing to its impact on society as well as individuals especially behaviors expressing aggressive intention defensively have become a topic of extensive investigation. Although several patterns of structural and functional connectivity (Nelson and Trainor, 2007; Hofman and Schutter, 2009; Hoptman et al., 2010) and frontal cortical activity (Harmon-Jones, 2003; Harmon-Jones et al., 2010) have been implicated in the development and expression of aggressive behavior, sources of individual differences that contribute to aggressive behavior however still remain relatively unknown.
A line of investigation particularly concerned with dispositional individual differences has been focusing on left and right frontal electrophysiological (EEG) activity and their respective relation with emotional processing (Davidson, 1988, 1992). The main tenet in this field has advanced from right hemispheric processing of negative emotion vs left hemispheric processing of positive emotion to a model of motivational direction in which the right cerebral hemisphere is associated with avoidance related incentive and the left cerebral hemisphere with approach related incentive (Harmon-Jones, 2003; Harmon-Jones et al., 2010). Recent empirical advances show asymmetrical frontal EEG activity to be directive in motivational incentives, and interestingly, have strongly linked anger and aggressive behaviors to approach motivation (Harmon-Jones and Allen, 1998; Harmon-Jones, 2007). The vast majority of the studies investigating frontal asymmetry in motivation and emotion have made use of the possibility to measure surface EEG activity from the scalp noninvasively (Harmon-Jones, 2003; Davidson, 2004). Manipulations of frontal EEG activity patterns strengthened the correlational claim of lateralized cerebral involvement in emotional processing (Peterson et al., 2008). Studies employing repetitive transcranial magnetic stimulation (rTMS), a method capable of changing local cortical activity transiently (Hallett, 2007), showed altered performance on emotionally laden behavioral tasks after changing anterior cortical excitability (d'Alfonso et al., 2000; van Honk and Schutter, 2006). Consequently, activity patterns in the frontal cortices can be regarded to be crucially involved in the processing of emotional stimuli.
The vast majority of the literature on asymmetrical frontal activity patterns has focused on the alpha (8–12 Hz) frequency band of the EEG spectrum. Prefrontal alpha EEG asymmetry has proved to be a consistent marker of motivational tendencies, in resting state as well as manipulation studies (for a contemporary review see Harmon-Jones et al., 2010). As an assumed index of cortical idling, inverse alpha band activity has served a successful predictor of especially approach-related behaviors. Early studies employing alpha band activity have shown left hemispheric dominance to be predictive of approach motivation as measured by the Behavioral Approach (or behavioral activation system, BAS) and Behavioral Inhibition system (BIS) (Harmon-Jones and Allen, 1997), and were able to discern motivational direction and affective valence (Sutton and Davidson, 1997). Consistent with the latter notion, it was shown that anger, a negatively valenced emotion with clear approach motivational inclination, could also be linked to left-hemispheric dominance (Harmon-Jones and Allen, 1998) as described in terms of inverse alpha power. Conversely, manipulations of patterns of prefrontal activity in the alpha band, for instance by contralateral hand contraction, have also been shown to relate to increases in approach motivation as well as anger (Harmon-Jones, 2006; Peterson et al., 2008).
A recent study, however, suggested a role for activity in the beta (12–30 Hz) frequency band in motivation-related incentives (Schutter et al., 2008). In this study, the relationship between asymmetrical beta frequency range activity and the behavioral asymmetry in approach (behavioral activation, BAS) and avoidance (behavioral inhibition, BIS) motivation as indexed by the BIS/BAS questionnaire (Carver and White, 1994) was examined. The focus on beta band activity originates from earlier reports on its inhibitory function as illustrated by, for instance, its relationship with the inhibitory neurotransmitter γ-aminobutyric acid (GABA) activity (Jensen et al., 2005). Results indicated a positive relationship between relative dominant right-hemispheric beta power and dominant BAS motivation, and were interpreted in terms of a left hemispheric functional dominance over the right cerebral cortex due to dominant right hemispheric inhibitory activity. These findings suggest that rather than focusing on local activity in one of the cerebral hemispheres, the balance of either excitatory or inhibitory activity between the cortices is of pivotal importance when considering functional dominance in terms of motivational tendencies. In a stimulus-dependent design, prestimulus beta activity measured directly from the cortex in a go-no-go task has been shown to predict successful inhibition of response (Swann et al., 2009b). Together these findings call for investigation of the relationships between beta frequency activity, motivational tendencies and behavioral inhibition.
Arguably, the ability to exert behavioral control over one's actions plays an important role in restraining aggressive tendencies. Studies on aggressive behavior in substance abuse have suggested a role for impaired self-control in aggressive and in particular hostile behavior (Dawe et al., 2009; Lapworth et al., 2009). Following from these studies, scrutinizing a possible role for impaired behavioral inhibition in the expression of aggressive behaviors might provide for additional insights in the relationship between frontal cortical activity and aggressive behaviors. Indeed, several studies have implicated impaired inhibition of behavioral responses in psychopathologies such as antisocial personality disorder (Kiehl et al., 2000; Swann et al., 2009a). Integrating these findings into a model of functional dominance on the level of the cerebral cortex therefore would petition the incorporation of the primary motor cortex (M1) into measures of cortical activity. As an important effector of human behavior, M1 is highly connected to the anterior parts of the cortex, and indeed, some of the physiological mechanisms by which M1 operates are highly similar to those of the prefrontal cortex (Kahkonen et al., 2005; Daskalakis et al., 2008). It therefore is not surprising that recent studies employing asymmetrical measures of cortical activity in motivation research have also either incorporated (Schutter et al., 2008; Hofman and Schutter, 2009) or made use of (Harmon-Jones, 2006; Peterson and Harmon-Jones, 2008; Peterson et al., 2008) the connectivity between the prefrontal and more central parts of the human frontal cortex.
The main objective of the present study was to establish whether individual differences in asymmetrical resting state cortical activity in the beta frequency band were predictive of trait aggression and behavioral inhibition. Conform the inhibitory property of beta frequency activity and the asymmetrical involvement of the frontal cortices in approach and withdrawal-related motivational tendencies, we hypothesized relative dominant right-hemispheric beta activity to be related to higher trait aggression, and that activity recorded from anterior sites would be particularly predictive of trait aggression. Similarly, reduced behavioral inhibition is hypothesized to be related to relative right-dominant beta activity. In addition, we hypothesized activity recorded from the central electrodes would be closest associated with behavioral inhibition scores.
METHODS
Participants
Thirty healthy nonsmoking right-handed male volunteers mean ± s.d. age, 23.3 ± 2.0 years were recruited among the student population of Utrecht University, the Netherlands. None of the participants had a history of psychiatric or neurological conditions. Written informed consent was obtained and volunteers received course credit for participation. All volunteers were naïve to the aim of the study. The study was in accordance with the standards set by the Declaration of Helsinki (Edinburgh Amendments).
EEG recordings
Resting state EEG was collected during a 4 min eyes opened—eyes closed—eyes opened—eyes closed recording session. Recordings were made using the BiosemiActiveTwo system (Biosemi, Amsterdam, The Netherlands) at a 256 Hz sampling rate from 32 Ag/AgCl pin electrodes placed over the scalp according to the International 10/20 EEG system. The ground consisted of the active common mode sense and passive driven right leg electrode.
Buss-Perry aggression questionnaire
Aggressive personality style was assessed with the Dutch translation of the 29 item Buss-Perry aggression questionnaire (AQ) (Buss and Perry, 1992; Meesters, et al., 1996). The ordinal response scale ranged from ‘1- extremely like me’ to ‘5- extremely unlike me’. The total AQ score provides a general index of trait aggression that can be further subdivided into four categories: Physical aggression (nine items), verbal aggression (five items), anger (seven items) and hostility (eight items).
Go-no-Go task
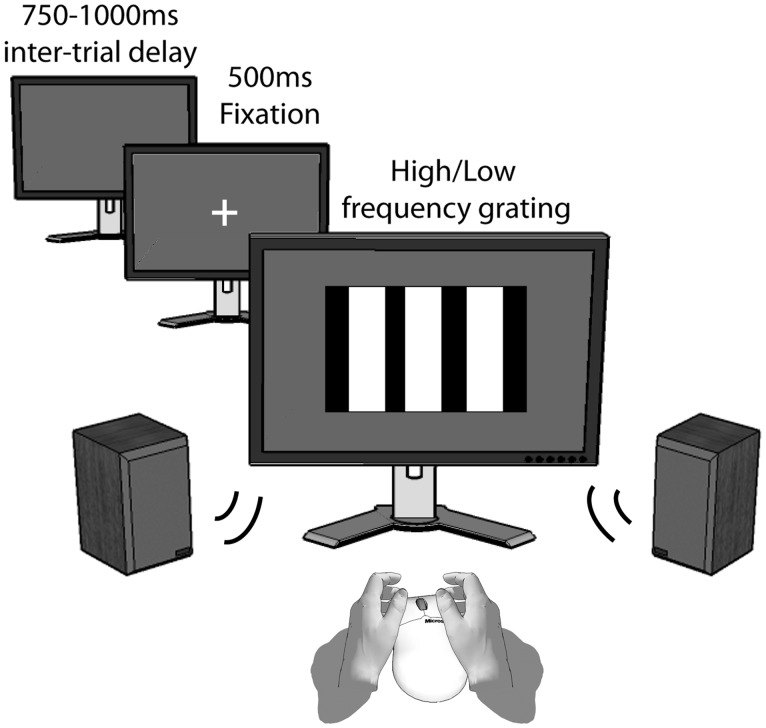
Behavioral inhibition was assessed in a Go-no-Go paradigm. The Go-no-Go (GnG) task consisted of three practice trials and 200 experimental trials. Participants were instructed to respond as quickly as possible, but accurately, to low- and high-frequency gratings by pressing the left or right mouse button, respectively. This response had to be inhibited if the grating presentation was accompanied by a 400 ms, 1000 Hz tone, presented at 50 dB (20% of total number of trials, 40) as opposed to the trials accompanied by a low volume and frequency tone. Individual trials were delivered as follows: fixation cross against black background (500 ms), grating presentation (1000 ms) and a delay randomly varying from 750 to 1000 ms. Gratings were sized 800 × 800 pixels and presented on a 24″ LCD monitor at ∼100 cm viewing distance. Figure 1 depicts a single trial of the Go-no-Go task.
Fig. 1.
Single trial of the Go-no-Go task. Trials were separated by a time-varying intertrial delay, after which a fixation cross was presented. Participants were instructed to respond as quickly as possible to the high or low frequency grating, except for the trials that were accompanied by a loud high frequency tone.
Procedure
Upon arrival at the laboratory participants received oral and written information on the experiment after which written informed consent was obtained. Next, participants were subjected to a short semi-structured interview to rule out history of psychiatric or neurological illness. The experiment continued with the resting state EEG recording session. Next, participants completed the AQ. The experiment continued with administration of the Go-No-Go task, and ended with debriefing.
Data reduction and statistical analyses
Go-no-Go data
Failed inhibition of response (i.e. response if response was to be inhibited) rates were calculated as the percentage of failed inhibitions of trials in which responses were to be inhibited 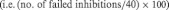 .
.
Resting state EEG data
Raw EEG traces were re-referenced offline to the average activity of all electrode locations. Electro-oculogram recorded from electrodes placed on the suborbit and supraorbit of the right eye and on the external canthi of both eyes was used to correct for eye movements (Gratton et al., 1983). EEG was 1–30 Hz band pass filtered with a 24 dB roll-off per octave. Data were subsequently divided in segments of 1 min length, and the two remaining segments containing the eyes-closed data were segmented further in 2 s epochs. Next, artifacts >±50 mV were rejected before further analysis by removal of the containing epoch for all channels. Spectral power (μV2) in the alpha (8–12 Hz) and beta (12.5–30 Hz) frequency bandwidth was estimated by a fast Fourier transform (Hamming window: length 10%). Regions of interest were identical to the regions reported in earlier reports from our laboratory (left FC: C3, Fc1, Fc5, F3; right FC: C4, Fc2, Fc6, F4) and mean spectral power for the left and right frontal cortex was calculated by averaging the four corresponding electrodes. Asymmetrical power distribution was computed using the following equation: 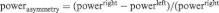
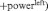 (Schutter et al., 2008), in order to control for individual differences in nonneurogenic variables including skull-to-cortex distance, skull thickness and orientation of underlying cortical tissue (Herbsman et al., 2009).
(Schutter et al., 2008), in order to control for individual differences in nonneurogenic variables including skull-to-cortex distance, skull thickness and orientation of underlying cortical tissue (Herbsman et al., 2009).
Statistical analyses
Pearson product-moment correlation analyses were run to assess the relations between asymmetrical frontal activity, AQ and behavioral inhibition scores. Exploratory follow-up Pearson product-moment analyses were performed to assess the relationship between asymmetrical frontal activity and the four factors of the AQ.
To test our hypotheses regarding the contributions of the anterior and more centrally located electrode pairs, separate step-wise linear regressions (method: probability of F to enter <0.05; criteria probability of F to remove >0.1) using F3/F4, Fc1/Fc2, Fc5/Fc6 and C3/C4 asymmetries as predictors were run for both AQ and behavioral inhibition scores if the Pearson correlations yielded a significant relation.
RESULTS
Data exclusion
Due to technical failure, data of one participant were lost and one participant did not comply with instructions in the Go-no-Go task. Exploratory analyses indicated that data of two participants should be omitted from the analyses due to outlier values (>2 s.d.) on the βasymmetry variable. In Table 1, the group means and standard deviations of asymmetrical frontal activity, AQ scores and percentages failed inhibition of response are depicted. All results reported in the following section are based on the analysis of the data from the remaining 26 participants.
Table 1.
Means and standard deviations (s.d.'s) of the αasymmetry, βasymmetry, percentages failed inhibition of response scores and AQ total and individual factor scores
| Measure | Mean score (s.d.) |
|---|---|
| αasymmetry | 0.0067 (0.0759) |
| βasymmetry | 0.0142 (0.0893) |
| Failed inhibition (no-go trials) (%) | 8.31 (6.583) |
| Responses (go trials) (%) | 99.78 (0.56) |
| AQ total score | 66.19 (7.156) |
| AQ_factor physical aggression | 20.92 (3.752) |
| AQ factor hostility | 17.19 (4.079) |
| AQ factor verbal aggression | 13.15 (2.866) |
| AQ factor anger | 14.92 (3.698) |
Relations between αasymmetry, βasymmetry, trait aggressionand behavioral inhibition.
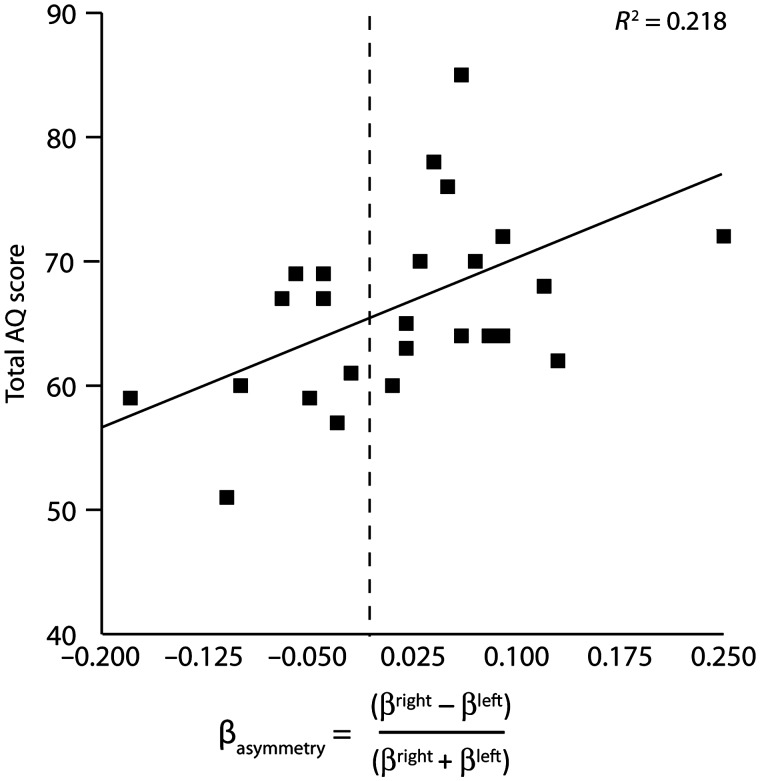
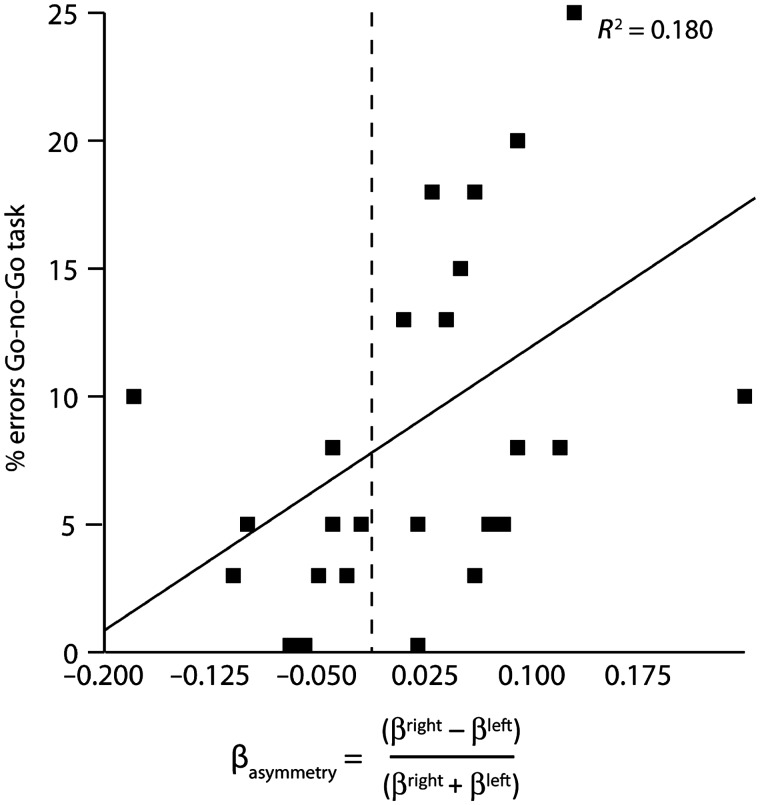
First, Pearson product-moment correlational analysis showed that βasymmetry significantly predicted AQ scores, r = 0.467, P = 0.016 (Figure 2). Next, the Pearson correlational analysis of the relationship between βasymmetry and percentage failed inhibition also resulted in a significant association, r = 0.424, P = 0.031 (Figure 3).
Fig. 2.
Dominant right hemispheric beta frequency activity is positively correlated to AQ scores.
Fig. 3.
Dominant right hemispheric beta frequency activity is positively correlated to error percentages in the Go-no-Go task.
Exploratory follow-up analyses of the relationship between βasymmetry and the four factors of the AQ (i.e. anger, hostility, physical aggression and verbal aggression) revealed that βasymmetry was significantly related to the AQ factor hostility solely, r = 0.513, P = 0.007, all other P > 0.172.
To examine contributions of individual electrode pairs in predicting trait aggression and behavioral inhibition, exploratory step-wise regression analyses in which F3/F4, Fc1/Fc2, Fc5/Fc6 and C3/C4 asymmetries were entered as predictors were run for AQ and behavioral inhibition scores separately. In line with our expectations, the step-wise linear regression in which F3/F4, Fc1/Fc2, Fc5/Fc6 and C3/C4 asymmetries were entered to predict AQ scores demonstrated that the F3/F4asymmetry was the only significant predictor, F(4, 25) = 5.829, P = 0.024 (R2 = 0.195).
The step-wise linear regression in which F3/F4, Fc1/Fc2, Fc5/Fc6 and C3/C4 asymmetries served to model behavioral inhibition scores yielded two significant models. Model I consisted only of the C3/C4asymmetry, F(4, 25) = 4.873, P = 0.037 (R2 = 0.169). In model II, however, adding the lateral fronto-central pair Fc5/Fc6 resulted in a significantly better fit, F(4, 25) = 6.462, P = 0.018 (R2 change = 0.182). In Tables 2 and 3 the constants, betas, standard errors and the standardized betas can be found for the respective models.
Table 2.
AQ scores
| Model | b | SE b | β |
|---|---|---|---|
| Step 1 βasymmetry | |||
| Constant | 66.003 | 1.287 | |
| F3/F4 | 22.889 | 9.481 | 0.442* |
| Step 1 αasymmetry | |||
| Constant | 65.818 | 1.288 | |
| F3/F4 | 39.669 | 16.085 | 0.450* |
βasymmetry R2 = 0.195 (P < 0.05). *P < 0.05.
αasymmetry R2 = 0.202 (P < 0.05). *P < 0.05.
Table 3.
Go-no-Go scores
| Model | B | SE b | β |
|---|---|---|---|
| Step 1 | |||
| Constant | 0.077 | 0.012 | |
| C3/C4 | 0.191 | 0.086 | 0.411* |
| Step 2 | |||
| Constant | 0.077 | 0.011 | |
| C3/C4 | 0.210 | 0.078 | 0.452* |
| Fc4/Fc6 | 0.210 | 0.083 | 0.429* |
R2 = 0.169 for step 1, ΔR2 = 0.182 for step 2 (P < 0.05). *P < 0.05.
To examine how the present data relate to the larger body of research on asymmetrical frontal activity, the statistical analyses were repeated for the asymmetries in the alpha band. In agreement with prior findings, Pearson product-moment correlational analysis showed that αasymmetry significantly predicted AQ scores, r = 0.391, P = 0.048. However, the Pearson correlational analysis of the relationship between αasymmetry and behavioral inhibition scores was not significant, r = 0.275, P = 0.173. The step-wise linear regression analysis in which F3/F4, Fc1/Fc2, Fc5/Fc6 and C3/C4 alpha asymmetries were entered to model AQ-scores demonstrated that the F3/F4asymmetry was the only significant predictor, F(4, 25) = 6.082, P = 0.021 (R2 = 0.202). In Table 2, the constant, beta, standard error and the standardized beta can be found for the model.
DISCUSSION
The present study aimed to explore the interrelations between frontal asymmetrical beta activity, trait aggression and behavioral inhibition. Additionally, we investigated whether activity recorded from anterior areas of the frontal cortex was especially predictive of trait aggression scores, whereas activity recorded from more central locations was hypothesized to be related closest to inhibition scores. We found asymmetrical frontal activity in the beta frequency range a significant predictor of both trait aggression as measured with the self-report AQ and behavioral inhibition as measured with a Go-no-Go task. Past research reporting interrelations between alpha asymmetries and trait aggression was replicated. However, whereas a significant relationship was found between frontal asymmetry and behavioral inhibition for the beta band, this relation was not found for the alpha band.
Exploratory analyses of the relationship between AQ and beta asymmetrical activity indicated that the relationship originated from the strong association of the beta asymmetry with the AQ factor hostility. Further scrutinizing the contributions of separate electrode pairs yielded the frontal electrode pair F3/F4 as the most important predictor of trait aggression scores. For the behavioral inhibition scores, in a model which also included the lateral locations Fc5/Fc6, the most important predictor was the C3/C4 pair.
These findings confirm and extend earlier reports on the relationship between asymmetrical frontal activity and approach and withdrawal motivated behaviors, in particular the possibility of left hemispheric dominance on a functional level due to increased levels of inhibitory activity in the right hemisphere. This study corroborates previous results showing that dominant right hemispheric beta activity is paralleled by increased BAS motivation and relative left hemispheric cortical excitability (Schutter et al., 2008), and links this pattern of functional dominance to aggressive behavior, a relation that has already become apparent from studies employing alpha band EEG asymmetries(for reviews see Harmon-Jones, 2003; Harmon-Jones et al., 2010).
Beta frequency band activity is proposed to be indicative of active cortical inhibition (Jensen et al., 2005) and therefore may well play a role in the physiology underling hemispheric asymmetries. The beta inhibitory function is illustrated by its relationship with the inhibitory neurotransmitter γ-aminobutyric acid (GABA) activity (Jensen et al., 2005), and reports on beta frequency predicting successful inhibition of behavioral responses (Ruiz et al., 2010). Conversely, prestimulus alpha activity has recently been shown to, contrary to beta frequency activity, predict failure to inhibit motor response in a Go-no-Go task (Mazaheri et al., 2009). These findings suggest an organizing role for beta rather than alpha frequency activity in successfully regulating behavioral responses and inhibition.
A possible explanation for the higher predictive value of beta band activity may lie in the observation that specific brain regions have preferential natural rhythms, which tend to increase from posterior to anterior locations (Rosanova et al., 2009). In an interleaved TMS–EEG design, Rosanova and collegues (2009) disturbed ongoing activity over occipital, parietal and premotor areas and observed the rate of subsequent oscillations (Rosanova et al., 2009). Applying this approach, it was demonstrated that the frequency of the observed natural rhythms following single-pulse TMS increased from around 10 Hz over occipital areas to around 20 Hz over parietal areas and around 30 Hz over premotor areas. Therefore, if the more frontal areas of the human brain tend to oscillate at frequencies of 20 Hz and higher, this might partly explain increased sensitivity of beta band activity over motor areas in predicting behavioral performance.
Moreover, M1 is considered to be a major output target of inhibitory control in the stopping network. Studies employing TMS while participants engaged in Go-no-Go tasks have shown rises in GABA mediated intracortical inhibition in no-go trials, suggesting that volitional inhibition of behavior is exerted at the M1 level, and is related to local GABA-ergic activity (Sohn et al., 2002; Coxon et al., 2006). Since beta activity has been suggested to reflect GABA-ergic activity (Jensen et al., 2005), this might also contribute to the observed increased sensitivity of beta activity over M1 in predicting response inhibition.
Involvement of the right frontal cortex in the regulation of affect is underlined by clinical studies involving patients suffering from selective damage to the frontal and anterior temporal lobes (Mychack et al., 2001). Frontotemporal dementia is a clinical syndrome marked by the selective degeneration of the frontal and anterior parts of the temporal lobes. Patients suffering from damage to the right frontal lobe are characterized by poorly modulated affect, poor impulse control and become highly critical of others (Mychack et al., 2001). These clinical observations fit the results presented here as relative hypofunctioning of the right frontal cortex was marked by higher levels of impulsivity, trait aggression and hostility in particular. Hostility can be described as a negative evaluation of people and objects (Ramirez and Andreu, 2006), and is proposed as a driving force behind angry and aggressive tendencies (Eckhardt et al., 1997).
In the present study, we show that the electrode pairs incorporated in the compound measure of asymmetrical beta power besides their ability to predict trait aggression and behavioral inhibition as a global measure of asymmetrical frontal cortical activity have selective explanatory power. Following our hypotheses, F3/F4 asymmetrical activity proved to be related to the measure of trait aggression in a selective manner. First, this finding is in line with the extensive body of work on asymmetrical frontal EEG activity (Harmon-Jones et al., 2010). Second, since participants were asked to judge the appropriateness of the statements in regard to their own personality and history, the questionnaire may partly implicitly probe successful nonaggressive coping in situations where participants had the opportunity to exert aggressive behavior. Therefore, since the prefrontal areas are implicated in emotion control (Davidson et al., 2000; Wager et al., 2008), activity as measured over these locations may be indicative for successful regulation of these behaviors over the lifespan. Conversely, the pair overlying the M1 (C3/C4) proved the strongest predictor for behavioral inhibition scores. This result suggests that in addition to the prefrontal locations, beta oscillations recorded over M1 are predictive of response inhibition. This finding is in line with earlier reports on beta band activity over the M1 and its involvement in successful stopping behavior (Swann et al., 2009b), as well as reports on M1 involvement in approach and withdrawal behaviors (Schutter et al., 2008; Hofman and Schutter, 2009) and studies employing the M1 as a proxy to induce prefrontal EEG asymmetries (Harmon-Jones, 2006; Peterson and Harmon-Jones, 2008; Peterson et al., 2008).
Some limitations on the generalizability and specificity of the present results must however be noted. First, it must be noted that the entire sample consisted of male university students without any history of psychiatric illness. In this sample, the relation between beta asymmetrical activity and hostility proved particularly strong. It would be conceivable that in more aggressive populations, other factors of the AQ would also become significantly related to asymmetrical beta activity.
Also, considering the characteristics of EEG regarding the localization of sources of cortical activity we cannot claim the activity as recorded over the locations reported originates from the cortical tissue beneath the electrodes.
Furthermore, the association between asymmetrical beta activity and aggressive behaviors and behavioral inhibition in particular may also be dependent on other personality characteristics which were not studied in the present study (Knyazev et al., 2008). For example, in a study employing a stop signal paradigm it was shown that synchronization of cortical electrophysiological activity postresponse was modulated by trait anxiety (Savostyanov et al., 2009).
Finally, the correlational nature of the findings presented should be considered as a limitation of the present study. Testing causality of the relationship between asymmetrical beta activity and aggressive behaviors and behavioral inhibition by manipulation of frontal activity patterns in the beta range, for instance by using transcranial alternating current stimulation (tACS), might be able to decide on the specificity and origin of the relation between asymmetrical beta activity patterns (Kanai et al., 2008; but see Schutter and Hortensius, 2010).
In conclusion, we here present data showing that resting state asymmetrical frontal beta activity is associated with trait aggression and response inhibition.
Conflict of Interest
None declared.
Acknowledgments
This study was supported by an Innovational Research Grant (VIDI 452-07-012) from the Netherlands Organization for Scientific research (NWO) to D.J.L.G. Schutter. The authors would like to thank Richard Bethlehem, Carlos Del Rio and Joris van Oosterhout for assistance in data collection.
REFERENCES
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–33. [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology. 2006;95:3371–83. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- d'Alfonso AA, van Honk J, Hermans E, Postma A, de Haan EH. Laterality effects in selective attention to threat after repetitive transcranial magnetic stimulation at the prefrontal cortex in female subjects. Neuroscience Letters. 2000;280:195–8. doi: 10.1016/s0304-3940(00)00781-3. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology. 2008;33:2860–9. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. EEG measures of cerebral asymmetry: conceptual and methodological issues. International Journal of Neuroscience. 1988;39:71–89. doi: 10.3109/00207458808985694. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–51. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67:219–33. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dawe S, Davis P, Lapworth K, McKetin R. Mechanisms underlying aggressive and hostile behavior in amphetamine users. Current Opinion in Psychiatry. 2009;22:269–73. doi: 10.1097/YCO.0b013e32832a1dd4. [DOI] [PubMed] [Google Scholar]
- Eckhardt CI, Barbour KA, Stuart GL. Anger and hostility in maritally violent men: conceptual distinctions, measurement issues, and literature review. Clinical Psychological Review. 1997;17:333–58. doi: 10.1016/s0272-7358(96)00003-7. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Early career award. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–48. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology. 2006;43:598–603. doi: 10.1111/j.1469-8986.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Trait anger predicts relative left frontal cortical activation to anger-inducing stimuli. International Journal of Psychophysiology. 2007;66:154–60. doi: 10.1016/j.ijpsycho.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–63. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–6. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84:451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Herbsman T, Forster L, Molnar C, et al. Motor threshold in transcranial magnetic stimulation: the impact of white matter fiber orientation and skull-to-cortex distance. Human Brain Mapping. 2009;30:2044–55. doi: 10.1002/hbm.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman D, Schutter DJ. Inside the wire: aggression and functional interhemispheric connectivity in the human brain. Psychophysiology. 2009;46:1054–8. doi: 10.1111/j.1469-8986.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophrenia Bulletin. 2010;36:1020–8. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Moser MA, McCormick JB, et al. Neurotalk: improving the communication of neuroscience research. Nature Reviews Neuroscience. 2010;11:61–9. doi: 10.1038/nrn2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–55. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage. 2005;24:955–60. doi: 10.1016/j.neuroimage.2004.09.048. [DOI] [PubMed] [Google Scholar]
- Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Current Biology. 2008;18:1839–43. doi: 10.1016/j.cub.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biological Psychiatry. 2000;48:210–21. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Bocharov AV, Levin EA, Savostyanov AN, Slobodskoj-Plusnin JY. Anxiety and oscillatory responses to emotional facial expressions. Brain Research. 2008;1227:174–88. doi: 10.1016/j.brainres.2008.06.108. [DOI] [PubMed] [Google Scholar]
- Lapworth K, Dawe S, Davis P, Kavanagh D, Young R, Saunders J. Impulsivity and positive psychotic symptoms influence hostility in methamphetamine users. Addictive Behaviors. 2009;34:380–5. doi: 10.1016/j.addbeh.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis IL, van Dijk H, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Human Brain Mapping. 2009;30:1791–800. doi: 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meesters C, Muris P, Bosma H, Schouten E, Beuving S. Psychometric evaluation of the Dutch version of the Aggression Questionnaire. Behaviour Research and Therapy. 1996;34:839–43. doi: 10.1016/0005-7967(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Mychack P, Kramer JH, Boone KB, Miller BL. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56:S11–5. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Reviews Neuroscience. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Peterson CK, Harmon-Jones E. Proneness to hypomania predicts EEG coherence between left motor cortex and left prefrontal cortex. Biological Psychology. 2008;78:216–9. doi: 10.1016/j.biopsycho.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Peterson CK, Shackman AJ, Harmon-Jones E. The role of asymmetrical frontal cortical activity in aggression. Psychophysiology. 2008;45:86–92. doi: 10.1111/j.1469-8986.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Andreu JM. Aggression, and some related psychological constructs (anger, hostility, and impulsivity); some comments from a research project. Neuroscience and Biobehavioral Reviews. 2006;30:276–91. doi: 10.1016/j.neubiorev.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. Journal of Neuroscience. 2009;29:7679–85. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MH, Strubing F, Jabusch HC, Altenmuller E. EEG oscillatory patterns are associated with error prediction during music performance and are altered in musician's dystonia. Neuroimage. 2011;55:1791–803. doi: 10.1016/j.neuroimage.2010.12.050. [DOI] [PubMed] [Google Scholar]
- Savostyanov AN, Tsai AC, Liou M, et al. EEG-correlates of trait anxiety in the stop-signal paradigm. Neuroscience Letters. 2009;449:112–6. doi: 10.1016/j.neulet.2008.10.084. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, Hortensius R. Retinal origin of phosphenes to transcranial alternating current stimulation. Clinical Neurophysiology. 2010;121:1080–4. doi: 10.1016/j.clinph.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, de Weijer AD, Meuwese JD, Morgan B, van Honk J. Interrelations between motivational stance, cortical excitability, and the frontal electroencephalogram asymmetry of emotion: a transcranial magnetic stimulation study. Human Brain Mapping. 2008;29:574–80. doi: 10.1002/hbm.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. Journal of Neurophysiology. 2002;88:333–8. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–10. [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Trait impulsivity and response inhibition in antisocial personality disorder. Journal of Psychiatric Research. 2009a;43:1057–63. doi: 10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. Journal of Neuroscience. 2009b;29:12675–85. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ. From affective valence to motivational direction: the frontal asymmetry of emotion revised. Psychological Science. 2006;17:963–5. doi: 10.1111/j.1467-9280.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]