Abstract
Most choices are complex and can be considered from a number of different perspectives. For example, someone choosing a snack may have taste, health, cost or any number of factors at the forefront of their mind. Although previous research has examined neural systems related to value and choice, very little is known about how mindset influences these systems. In the current study, participants were primed with Health or Taste while they made decisions about snack foods. Some neural regions showed consistent associations with value and choice across Health or Taste mindsets. Regardless of mindset, medial orbitofrontal cortex (MOFC) tracked value in terms of taste, regions in left lateral prefrontal cortex (LPFC) tracked value in terms of health, and MOFC and dorsal anterior cingulate were associated with choice. However, activity in other neural regions was modulated by the mindset manipulation. When primed with Taste, rostral anterior cingulate tracked value in terms of taste whereas left amygdala and left putamen were associated with choice. When primed with Health, right LPFC and posterior MOFC tracked value in terms of health. The findings contribute to the neural research on decision-making by demonstrating that changing perspectives can modulate value- and choice-related neural activity.
Keywords: preference, evaluation, fMRI, ventromedial, prefrontal
INTRODUCTION
People's mindsets can influence the way they evaluate options and make choices. For example, people choosing a snack may be focused on finding something tasty until they happen to walk by a gym, which additionally places the health implications of food choices at the forefront of their minds. Mindset indicates the factor(s) that may be at the forefront of the mind when evaluating and choosing between options. Although mindset can influence the types of value people are likely to consider and the choices people make, previous neural research has paid little attention to whether mindset affects the neural representations of value and choice. In the food example above, it may be that neural representations of a food's value in terms of taste or health are consistent regardless of mindset. On the other hand, mindset may affect neural representations of value or choice by bolstering or refining the computations that are important given our mindset. For example, a brain region might represent the healthiness of a food, but only when one's mindset is focused on health. The current study examines whether mindset influences the neural representations of value and choice when people make real-world decisions about snack foods.
Although the influence of mindset on the neural representation of value and choice has not been well-studied, many studies have examined neural representations of value and a few studies have examined neural regions that distinguish choices. Value in a number of domains (e.g. food, consumer product and monetary decisions) has been associated with activity in ventromedial prefrontal cortex [VMPFC, including medial orbitofrontal cortex (MOFC) and rostral anterior cingulate cortex (RACC)], amygdala, striatum, dorsal anterior cingulate (DACC) and lateral prefrontal cortex (LPFC) (LaBar et al., 2001; Erk et al., 2002; Arana et al., 2003; Gottfried et al., 2003; Killgore et al., 2003; Hinton et al., 2004; Blair et al., 2006; O’Doherty et al., 2006; Porubska et al., 2006; Kable and Glimcher, 2007; Knutson et al., 2007; Plassmann et al., 2007; Fuhrer et al., 2008; Chib et al., 2009; Hare et al., 2009; Lebreton et al., 2009). Although many studies have examined neural representations of value, only a few studies have additionally examined neural representations of choice. Measures of value are often related to people's choices, but measures of value and choice are not wholly redundant. For example, a health mindset does not rule out the possibility that other factors will be weighed when choosing a snack (e.g. How tasty is the food? How filling is the food? How expensive is the food?). In other words, people in a health mindset should evaluate whether foods are valuable in terms of their health implications but may not choose the healthiest option because they are ultimately willing to sacrifice health for taste or some other factor. The few studies that have examined neural activity related to choice have shown that some regions associated with value are also associated with choice. Ventral striatum and VMPFC increase activity when viewing options that are chosen compared to options that are not chosen (Kim et al., 2007; Knutson et al., 2007; Hare et al., 2009; Lebreton et al., 2009). Some research suggests that temporal aspects of decisions may modulate the neural associations of value and choice. For example, some studies show differences depending on whether a decision has immediate or long-term consequences (McClure et al., 2007; Ersner-Hershfield et al., 2009; Mitchell et al., 2011). However, these studies examine the influence of delayed consequences rather than how focusing on different properties of an option may influence neural representations of value and choice. Therefore, previous studies have identified neural regions that we might expect to represent value and a subset of these regions may represent choice, but no previous studies have examined whether these representations are affected by property-based mindsets.
Previous neural research raises the possibility that property-based mindsets affect neural representations of value and choice. Neural representations of a stimulus value will differ depending on whether alternatives are higher or lower in value (Tremblay and Schultz, 1999; Elliott et al., 2008). Additionally, individual differences in core values, reward sensitivity and dieting behavior modulate neural activity related to value (Beaver et al., 2006; Hare et al., 2009; Brosch et al., 2011). Individual differences may imply the type of mindset that a person is likely to adopt when making a decision. For example, successful dieters may be especially likely to have health at the forefront of their minds when choosing foods. Dieters who make healthy choices show a parametric relation between VMPFC and a food's value in terms of taste and health. But dieters who make unhealthy choices only show VMPFC modulation in relation to a food's value in terms of taste (Hare et al., 2009). These differences may arise because only successful dieters take a health mindset when evaluating and choosing food. Or it may be that all dieters take a health mindset yet choices are only consistent with this mindset when health value is neurally represented in VMPFC. An important complement to studies that examine individual differences will be studies that experimentally examine the influence of mindset on neural representations of both value and choice in more general populations (e.g. nondieters).
The current study is the first to experimentally manipulate how mindsets (i.e. focusing on different properties) influence neural representations of value and choice. Following from previous decision-making research that has contrasted the consideration of taste compared to health properties in food decisions (Shiv and Fedorikhin, 1999, 2002; Hare et al., 2009), participants evaluated and chose between snack foods while they were primed with a taste or health mindset. If neural representations of value are insensitive to mindset, then previously established parametric relations between value and VMPFC, amygdala, striatum, DACC and LPFC should exist regardless of whether taste or health properties of foods are primed. However, if mindset does influence neural representations of value, then some of the previously established neural regions may only be modulated by value in terms of taste or health when those particular properties are at the forefront of participants’ minds. As mentioned above, although value may contribute to choice, it is not redundant. People may evaluate health in a health mindset but ultimately choose based on other factors. Therefore, we conduct a parallel set of analyses to examine how mindset affects neural representations of choice.
MATERIALS AND METHODS
Participants
The results from 24 female participants (right-handed; age 18–29 years, mean age = 20.7, s.d. = 2.9) are reported. Data from one additional participant were excluded from analysis because the participant failed to stay awake and data from three additional participants were excluded due to excessive head movement (>4 mm over the scanning session). All participants provided informed consent and the study approved by the institutional review board of the University of California at Davis. Pre-screening ensured that participants were not on any kind of restrictive diet. Body mass index (BMI: weight in kilograms divided by squared height in meters) indicated that, according to National Heart, Lung, and Blood Institute obesity guidelines (NHLBI, no date), no participants were in the obese (BMI > 30) or underweight (BMI < 18.5) range (mean BMI = 22.3, s.d. = 3.0). Participants were instructed not to eat for 3 h preceding their session to ensure that they were hungry during the experiment. All participants verified that they followed this instruction.
Stimuli
Participants chose from pairs of snack food pictures. The snack food stimuli set consisted of 100 pictures that lent themselves to evaluations of taste and health. In order to measure whether participants choose options that were consistent with the mindset manipulation, 50 stimuli were characteristic of healthy foods (Healthy category) and 50 stimuli were characteristic of unhealthy foods (Unhealthy category). All were common snacks that people typically consume and were therefore rated at least somewhat favorably on Taste Value. Healthy category items were judged as highest on Health Value (e.g. almonds compared to a cupcake).
Behavioral paradigm
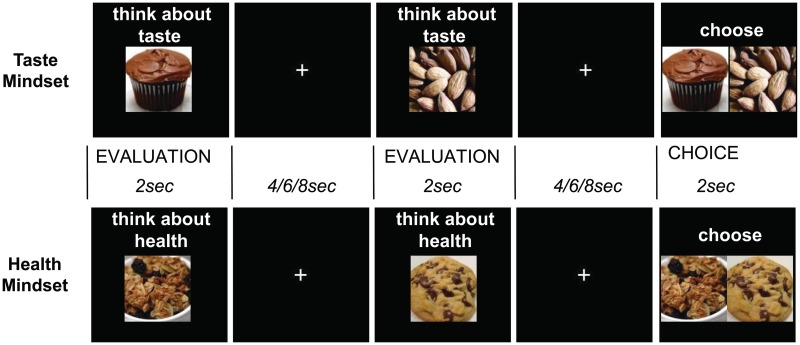
Participants performed a food decision task while undergoing functional magnetic resonance imaging (fMRI) (Figure 1). Mindset was manipulated while participants evaluated two snack foods in succession (Evaluation) and then indicated their final choice (Choice) in each trial of the task. A Mindset cue (4 s) indicated whether participants should adopt a mindset of Health (think about healthiness of the food) or Taste (think about tastiness of the food). After the Mindset cue, participants completed five decision trials. Each trial began with an Evaluation phase in which participants were sequentially presented with two food pictures (one Healthy: 2 s, one Unhealthy: 2 s; order counterbalanced). In the Choice part of each trial, the foods were simultaneously presented (2 s) and participants pressed a button to indicate which item they wanted to eat, regardless of the Mindset condition. As mentioned above, the Mindset manipulation was intended to affect the property of food that was at the forefront of participants’ minds but it was not meant to cue which food to choose. Instead, we wanted to examine spontaneous choices in the different Mindset conditions. Therefore, to ensure that participants actually made their selection based on what they wanted to eat at the moment rather than according to the Taste or Health Mindset condition, we gave participants two key instructions. First, we made it clear that although we wanted them to focus on health or taste as instructed during Evaluation, we wanted to know their actual preference for the snack foods during the Choice phase. Additionally, participants were motivated to express their actual preferences because they were aware that they would receive their food choice from a random trial at the end of the experiment (McClure et al., 2004).
Fig. 1.
Decision-making task. Participants evaluate one food item, view a fixation cross, evaluate a second food item, view a fixation cross and then indicate their choice between the two items. Depending on the condition, participants were either primed to think about taste properties (Taste Mindset) or health properties (Health Mindset) while evaluating single items. Participants were instructed to always choose the item that they wanted, regardless of the Mindset condition. The two items were always from different categories: Healthy (e.g. almonds, granola) or Unhealthy (e.g. cupcake, cookie) in a balanced pseudorandom order. The same set of items was used in each condition but items were paired differently.
The experiment consisted of four functional runs that included 25 trials and lasted 10 min, 14 s each. For each trial, the two Evaluation screens and Choice screen were randomly jittered with variable length fixation screens (50% 4 s, 25% 6 s and 25% 8 s) in order to permit independent estimates of neural activity (Donaldson et al., 2001). Within a functional run, the Mindset condition alternated every five trials and the order of the alternation was counterbalanced across participants. All 100 stimuli were presented in both the Taste and Health Mindset condition. Occurrences of specific item pairs in the Mindset conditions were counterbalanced across subjects. After leaving the scanner, participants rated the Taste and Health Values for all stimuli using 5-point scales with endpoints labeled ‘not at all’ and ‘extremely’ for three questions: ‘How tasty is this food?’; ‘How motivated are you towards this food?’; ‘How healthy is this food?’ Consistent with previous research (Glanz et al., 1998), subjective Taste Value was operationalized as an average of taste and motivation ratings. Motivation ratings were significantly positively correlated with taste ratings (r = 0.77), and significantly more highly correlated with taste ratings than health ratings (motivation and health r = −0.23; z = 4.06, P < 0.05). After completing food ratings, participants received one food item that they chose during the task.
MR data acquisition
All images were collected on a 1.5-T GE Signa scanner at the University of California, Davis, Imaging Research Center. Functional images were acquired with a gradient echo echo-planar imaging (EPI) sequence (time to repeat = 2000 ms, echo time = 40 ms, field of view = 220, 64 × 64 matrix, 24 slices tilted ∼15° from the anterior commissure-posterior commissure line, voxel size, 3.44 × 3.44 × 5 mm, 307 volumes per scan). These parameters optimized VMPFC coverage while preserving whole-brain coverage. Functional volume acquisitions were time-locked to the onset of each trial. The first five volumes of each scan were discarded to allow for T1-equilibration effects. Coplanar and high-resolution T1-weighted anatomical images were also collected.
fMRI data analysis
Data were preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, London). Images were corrected for differences in timing of slice acquisition, followed by rigid body motion correction. Structural and functional volumes were respectively normalized to T1 and EPI templates based on the Montreal Neurological Institute (MNI) atlas space. Functional volumes were resampled to 2-mm cubic voxels and spatially smoothed with an 8-mm FWHM isotropic Gaussian kernel.
Statistical analyses were performed on individual participants’ data using the general linear model (GLM) in SPM2. The fMRI time series data were high-pass filtered (128-s cut-off period) and modeled by regressors convolved with a canonical hemodynamic response function. For single participants, the least-squared parameter estimates for each regressor were used to create contrast images. At the group level, participants were treated as a random effect and group statistical maps were computed for each contrast by calculating single sample t-tests on participants’ contrast images (threshold at P < 0.005, minimum cluster volume of 270 mm3 = 34 contiguous 2 mm3 voxels, which is equivalent in volume to 10 3 mm3 voxels). The P-value and cluster volume threshold was selected to balance Types I and II error rates (Lieberman and Cunningham, 2009). Value and choice-related parameter estimates were extracted (Brett et al., 2002) to identify interaction patterns and visualize effects in clusters within LPFC, MOFC, RACC, DACC, striatum and amygdala based on previous research reporting neural regions specifically associated with food value and choice (LaBar et al., 2001; O’Doherty et al., 2002; Tzourio-Mazoyer et al., 2002; Arana et al., 2003; Gottfried et al., 2003; O’Doherty et al., 2006; Porubska et al., 2006; Plassmann et al., 2007; Chib et al., 2009; Hare et al., 2009).
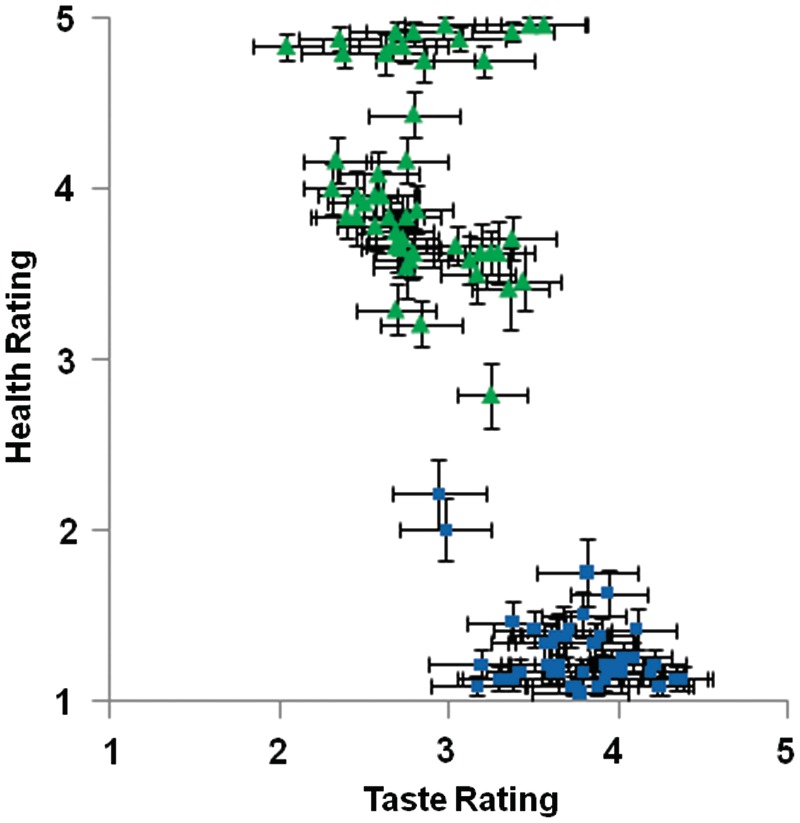
Analyses of activation during the evaluation of each option addressed several research questions. Our first analytic goal was to identify neural regions that parametrically tracked Taste or Health Value and examine how these relations were insensitive or sensitive to the Mindset manipulation. We conducted parametric analyses modeled after previous research on neural representations of food value (Hare et al., 2009). Subjective Taste and Health Value ratings were used to predict trial-by-trial neural activity during the evaluation of each item. Consistent with previous research showing that people tend to have preconceived attitudes that healthy foods are less tasty (Raghunathan et al., 2006), subjective Taste and Health Value ratings of stimuli were moderately negatively correlated (mean r across participants = −0.32, Figure 2). However, we wanted to examine taste and health value independently. Therefore, parametric modulation analyses relating Taste Value to neural activity are based on residual Taste Value ratings after controlling for Health Value ratings. Likewise, parametric modulation analyses relating Health Value to neural activity are based on residual Health Value ratings after controlling for Taste Value ratings.
Fig. 2.
Taste and health ratings for each food. Each point in the chart represents the average taste and health rating for a food. Green triangles represent Healthy category foods and blue squares represent Unhealthy category foods. Error bars represent standard error.
Separate GLMs examined activity related to Taste Value and Health Value. Each analysis focused on two parametric regressors: (i) subjective Taste Value (or Health Value) for each item in the Taste Mindset condition and (ii) subjective Taste Value (or Health Value) for each item in Health Mindset condition. Each GLM also included regressors modeling experimental events: Health Mindset Evaluation, Taste Mindset Evaluation, Taste Mindset Choice, Health Mindset Choice, Instruction screens and missed-trials. Neural activation was tested for significant parametric relations to Taste or Health value in relation to null baseline.
We next examined whether parametric relations with value existed regardless of the Mindset condition. Therefore, we conducted a conjunction analysis using the Minimum Statistic compared to the conjunction null (Nichols et al., 2005) to identify common neural regions showing parametric relations to Taste Value or Health Value in both Taste and Health Mindset conditions. We also examined whether the Mindset condition modulated neural associations with value. We investigated whether there were significant differences in neural regions’ parametric relation to Taste Value in the Taste Mindset condition compared to their parametric relation to Taste Value in the Health Mindset condition. Similarly, we contrasted neural regions’ parametric relation to Health Value in the Health Mindset condition with their parametric relation to Health Value in the Taste Mindset condition.
A second analytic goal was to examine whether neural regions associated with choice were modulated by Taste compared to Health Mindset. An additional GLM estimated neural responses during evaluation of subsequently chosen compared to nonchosen items in the Taste and Health Mindset conditions. Regressors of noninterest were the same as in the GLMs described above. Conjunction analysis identified regions related to choice regardless of Mindset: [(Taste Mindset Chosen vs Taste Mindset Non-chosen) conjoined with (Health Mindset Chosen vs Health Mindset Non-chosen)] (Minimum Statistic compared to conjunction null, Nichols et al., 2005). Next, we examined neural activity related to choice that was modulated by the Mindset condition. A contrast examined the interaction of Choice and Mindset [(Taste Mindset Chosen vs Taste Mindset Non-chosen)] vs [(Health Mindset Chosen vs Health Mindset Non-chosen)]. The interaction pattern of interest was in neural activity that increased for chosen items compared to nonchosen items, but only in one of the Taste or Health Mindset conditions.
Finally, we examined neural activity modulated by the mindset condition without respect to value or choice. All Taste Mindset evaluation events were compared to all Health Mindset evaluation events [(Taste Mindset Chosen + Taste Mindset Non-chosen) vs (Health Mindset Chosen + Health Mindset Non-chosen)] and all Health Mindset evaluation events were compared to all Taste Mindset evaluation events [(Health Mindset Chosen + Health Mindset Non-chosen) vs (Taste Mindset Chosen + Taste Mindset Non-chosen)]. These analyses were conducted both with and without the inclusion of individual response times for each choice as a covariate of noninterest. The inclusion of reaction times in the GLM addressed whether differences in neural activation were merely attributable to differences in response times. Table 3 reports findings from the analyses that include the response time covariate because it was largely similar to the analysis that did not include the response time covariate (one exception is noted in Table 3).
Table 3.
Neural activity related to main effect of Mindset
| Region of activation (right/left) | Brodmann | MNI co-ordinates |
t-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Greater activity in Taste Mindset than Health Mindset during evaluation | |||||
| Superior parietal (R) | 7 | 30 | −54 | 50 | 5.42 |
| Occipital (L) | 18/19 | −18 | −74 | 34 | 4.31 |
| Occipital (R) | 19 | 44 | −84 | 10 | 3.75 |
| Occipital (L) | 18/19 | −34 | −90 | 14 | 3.17 |
| Greater activity in Health Mindset than Taste Mindset during evaluation | |||||
| LPFC (L) | 44 | −36 | 16 | 28 | 3.92 |
| Inferior temporal (L) | 20 | −38 | 0 | −42 | 4.26 |
| Middle temporal (L) | 21 | −64 | −48 | 6 | 3.27 |
| Middle temporal (R) | 21 | 52 | −54 | 16 | 3.84 |
| Precuneus | 7 | −6 | −60 | 36 | 3.96 |
| Angular gyrus (L) | 39 | −54 | −66 | 20 | 4.49 |
MNI co-ordinates indicate location of peak, P < 0.005, >34 contiguous voxels. Results based on GLM that includes individual response times for each choice as a covariate of noninterest. When the individual response time covariate is omitted from the GLM, an additional cluster in left inferior parietal cortex (BA 40, peak at −40, −40, 46, t = 3.58) shows significantly greater activity in the Taste Mindset than the Health Mindset. Approximate Brodmann's areas are shown.
RESULTS
Behavioral results
Participants were most likely to choose snack foods based on taste overall but this was less likely in the Health Mindset condition. Across the whole experiment, Healthy food items were chosen less frequently than Unhealthy items [Healthy items mean frequency = 36%, s.d. = 25%; remainder of choices were Unhealthy; t(23) = 2.76, P < 0.05, d = 0.56]. Although Healthy food items were chosen less frequently overall, they were chosen significantly more often in the Health Mindset condition (M = 44%, s.d. = 33%) compared to the Taste Mindset condition [M = 28%, s.d. = 24%; t(23) = 2.50, P < 0.05, d = 0.52]. The Health Mindset condition tended to slow down response times (M = 1001 ms, s.d. = 153 ms) compared to Taste Mindset trials [M = 940 ms, s.d. = 142 ms; t(23) = 4.30, P < 0.05, d = 0.87]. Finally, healthy foods were not chosen significantly more or less at the end of a block compared to the beginning of a block in either the Health [t(23) = 0.42, P > 0.05, d = 0.09] or Taste Mindset condition [t(23) = 1.37, P > 0.05, d = 0.27]. In this nondieting sample, taste was a dominant factor in choices but when health information was made relatively salient, choices were made more slowly and were pushed in the direction of the healthier food option.
Imaging results
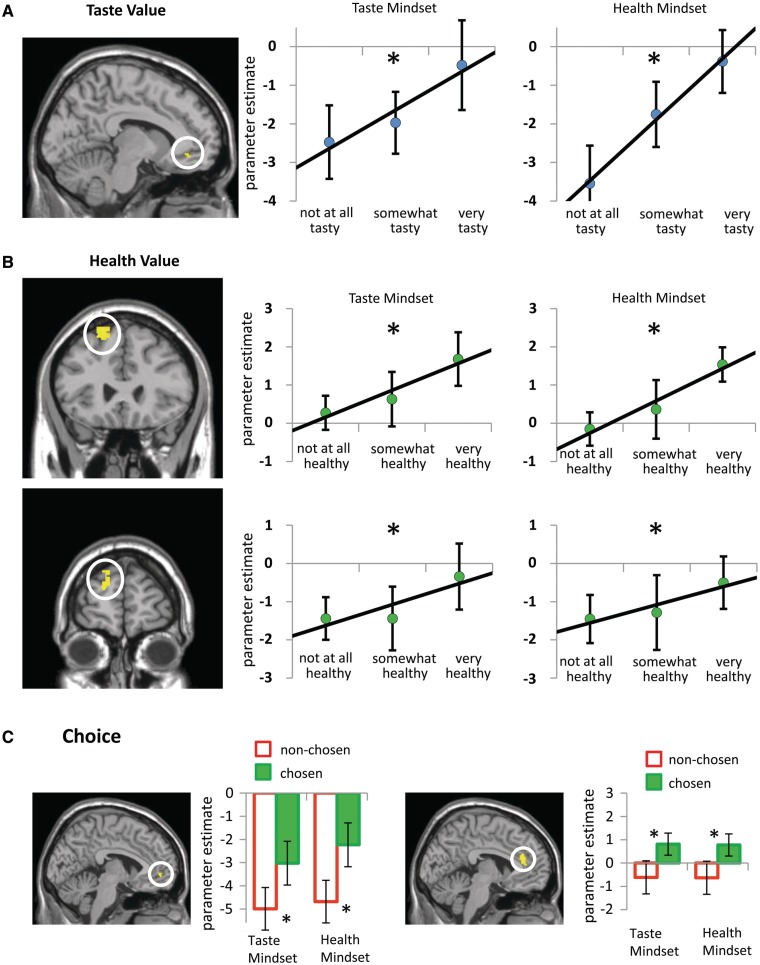
MOFC relates to Taste Value regardless of Taste or Health Mindset
MOFC activity was parametrically related to Taste Value in both the Health Mindset and the Taste Mindset conditions (BA 11, Table 1 and Figure 3). The parametric relation to Taste Value in MOFC did not differ significantly across Taste and Health Mindset conditions [t(23) = 0.31, n.s.]. In other words, MOFC tracked taste value of foods regardless of whether taste or health information was emphasized.
Table 1.
Neural associations with value and choice that persist across Mindsets
| Region of activation (right/left) | Brodmann | MNI co-ordinates |
t-value |
|||
|---|---|---|---|---|---|---|
| x | y | z | Taste Mindset | Health Mindset | ||
| Consistent parametric relation to Taste Value across Taste and Health Mindsets | ||||||
| MOFC (L) | 11 | −8 | 40 | −8 | 3.78 | 3.52 |
| Insula (R) | 36 | −8 | 4 | 3.90 | 3.62 | |
| Midbrain (L/R) | 0 | −28 | −12 | 3.73 | 4.03 | |
| Precuneus (L) | 30 | −8 | −54 | 12 | 3.69 | 4.89 |
| Consistent parametric relation to Health Value across Taste and Health Mindsets | ||||||
| LPFC (L) | 9 | −12 | 60 | 34 | 3.61 | 3.83 |
| LPFC (L) | 8 | −18 | 26 | 60 | 4.16 | 4.94 |
| Occipital (L) | 19 | −44 | −68 | 38 | 4.73 | 4.70 |
| Occipital (L) | 18 | −20 | −80 | −16 | 5.08 | 5.11 |
| Occipital (L) | 17/18 | −14 | −102 | 2 | 5.05 | 7.48 |
| Consistent relation to choice (chosen > nonchosen) across Taste and Health Mindsets | ||||||
| MOFC (L) | 11 | −6 | 48 | −4 | 4.02 | 3.27 |
| DACC (L) | 24 | −4 | 36 | 18 | 3.51 | 4.57 |
Clusters parametrically related to Taste Value, Health Value or choice across Mindsets were identified by conjunction analyses, P < 0.005, >34 contiguous voxels. Peak t-values with each conjunction cluster are given for each Mindset condition. MNI co-ordinates indicate center of mass of cluster, regions ordered from anterior to posterior. Approximate Brodmann’s areas are shown. Regions listed in bold are depicted in Figure 3.
Fig. 3.
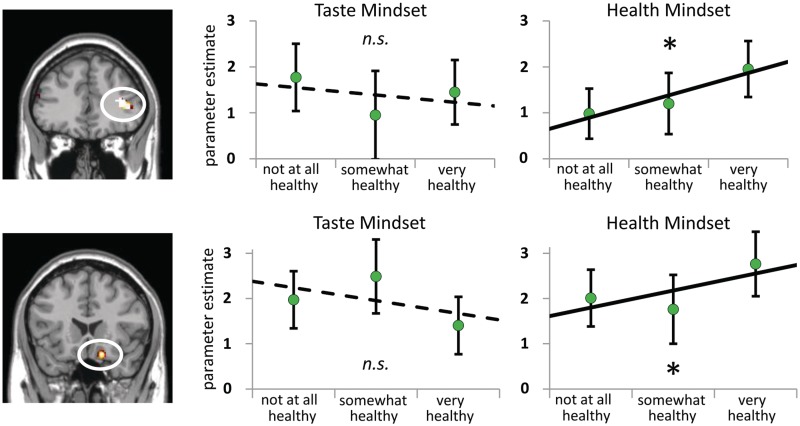
Neural regions related to (A) Taste Value, (B) Health Value and (C) Choice that are insensitive to Mindset. (A) MOFC parametrically relates to Taste Value across Mindset conditions (Taste Mindset Taste Value and Health Mindset Taste Value conjunction, P < 0.005, 34 contiguous voxels). Charts on the right illustrate parametric relations with value in terms of taste by showing activation parameter estimates for foods rated low (rated two or lower), middle (rated three) and high (rated four or higher) on tastiness. (B) LPFC regions parametrically relate to value in terms of health across Mindset conditions (top: BA 8; bottom: BA 9; Taste Mindset Health Value and Health Mindset Health Value conjunction, P < 0.005, >34 contiguous voxels). Charts to the right of each brain image illustrate parametric relations to value in terms of health by showing activation parameter estimates for foods rated low (rated two or lower), middle (rated three) and high (rated four or higher) on healthiness. A ‘asterisks’ and solid line indicates that the parametric relationship is significant based on the criterion for the parametric modulation analysis (P < 0.005, >34 contiguous voxels). Ratings were categorized as low, middle or high to visualize the parametric relations, but no statistical tests were conducted based on the categorical estimates. (C) MOFC and DACC activity is greater for chosen than nonchosen foods across Taste and Health Mindset conditions. Maps show neural association with choice conjunction analysis (Taste Mindset Chosen vs Taste Mindset Non-chosen conjoined with Health Mindset Chosen vs Health Mindset Non-chosen, P < 0.005, >34 contiguous voxels). Bar charts to the right of each brain image show activation parameter estimates for chosen and nonchosen foods in each condition. ‘asterisks’ indicates a significant difference between parameter estimates for chosen and nonchosen foods. Error bars represent standard error.
Left LPFC region relates to Health Value regardless of Health or Taste Mindset
Left LPFC BA 8 and BA 9 activity was parametrically related to Health Value in both the Health Mindset and the Taste Mindset conditions (Table 1 and Figure 3). The parametric relation to Health Value in these regions of left LPFC did not differ significantly across Health and Taste Mindset conditions [BA 8 t(23) = 1.02, BA 9 t(23) = 1.15, n.s.]. In other words, regions of left LPFC tracked health value of foods regardless of whether health or taste information was emphasized.
MOFC and DACC relate to behavioral choice regardless of Taste or Health Mindset
MOFC and DACC increased activity for chosen foods relative to nonchosen foods in both Taste and Health Mindset conditions (Table 1 and Figure 3). The MOFC cluster associated with choice in both Mindset conditions did not overlap with the MOFC cluster parametrically related to Taste Value in both conditions. MOFC and DACC differentiate between chosen and nonchosen foods, and this relation exists when either health or taste information is emphasized.
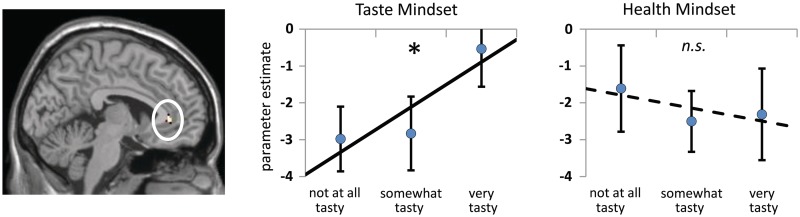
Taste Mindset modulates RACC relation to Taste Value
The parametric relation between RACC and Taste Value was significantly greater in the Taste Mindset condition than in the in the Health Mindset condition (Table 2 and Figure 4). Furthermore, parameter estimates from the RACC cluster show that the parametric relation to Taste Value was significantly greater than zero in the Taste Mindset [t(23) = 2.43, P < 0.05] but not significantly different from zero in the Health Mindset condition [t(23) = −0.53, n.s.]. In other words, RACC activity tracked the taste value of foods only when taste information was emphasized.
Table 2.
Neural associations with value and choice that are modulated by Mindset
| Region of activation (right/left) | Brodmann | MNI co-ordinates |
t-value |
||||
|---|---|---|---|---|---|---|---|
| x | y | z | Mindset difference | Taste Mindset | Health Mindset | ||
| Greater parametric relation to Taste Value in Taste Mindset than Health Mindset | |||||||
| RACC (L) | 25/11 | −2 | 34 | 2 | 4.34 | 3.01 | −1.40 |
| Greater parametric relation to Health Value in Health Mindset than Taste Mindset | |||||||
| Anterior prefrontal (L) | 10 | −6 | 66 | 12 | 4.89 | −.86 | 3.91 |
| LPFC (R) | 45 | 38 | 38 | 8 | 4.04 | −1.33 | 3.83 |
| MOFC (R) | 11 | 16 | 22 | −20 | 4.37 | −1.64 | 3.52 |
| Precentral (L) | 6 | −26 | −6 | 54 | 3.66 | −1.9 | 3.44 |
| Inferior parietal (L) | 40 | −38 | −48 | 50 | 4.10 | −1.02 | 3.98 |
| Inferior parietal (R) | 40 | 44 | −50 | 52 | 4.89 | −1.64 | 4.38 |
| Occipital (L) | 17/18 | −10 | −100 | −8 | 3.61 | 1.48 | 5.23 |
| Greater relation to choice (chosen > nonchosen) in Taste Mindset than Health Mindset | |||||||
| Putamen (L) | −24 | 6 | 10 | 4.41 | 3.66 | −1.53 | |
| Amygdala (L) | −24 | −2 | −20 | 3.84 | 3.36 | −1.48 | |
| Insula/Operculum (R) | 44 | −26 | 22 | 4.85 | 3.68 | −2.84 | |
| Inferior parietal (L) | 40 | −52 | −32 | 44 | 4.42 | 4.33 | −1.63 |
| Inferior parietal (L) | 40 | −50 | −50 | 54 | 3.27 | 2.98 | −1.03 |
| Inferior temporal (L) | 37 | −42 | −64 | −10 | 4.20 | 2.90 | −1.24 |
| Superior parietal (L) | 7 | −26 | −72 | 50 | 5.04 | 4.22 | −.43 |
| Occipital (L) | 19 | −26 | −74 | 26 | 3.68 | 3.36 | −1.25 |
| Occipital (R) | 19 | 26 | −78 | 38 | 3.23 | 3.26 | −.29 |
The ‘Mindset difference’ column indicates peak t-value for the effect of Mindset on neural associations with value and choice; MNI co-ordinates indicate location of peak, P < 0.005, >34 contiguous voxels. Columns for Taste Mindset and Health Mindset indicate the t-value for the association with value or choice within each Mindset condition in the listed voxel. Approximate Brodmann's areas are shown. Regions listed in bold are depicted in Figures 4–6.
Fig. 4.
Taste compared to Health Mindset modulates the parametric relation between neural activity and Taste Value. An RACC cluster shows a significant parametric relation to value in terms of taste only in the Taste Mindset condition (Taste Mindset Taste Value > Health Mindset Taste Value contrast, P < 0.005, >34 contiguous voxels). Charts illustrate parametric relations with value in terms of taste by showing activation parameter estimates for foods rated low (rated two or lower), middle (rated three) and high (rated four or higher) on tastiness. A ‘Asterisks’ and solid line indicates that the parametric relationship is significantly greater than zero (P < 0.05), ‘n.s.’ and dotted line indicate that the parametric relationship is not significantly different from zero (P > 0.05). Ratings were categorized as low, middle or high to visualize the parametric relations, but no statistical tests were conducted based on the categorical estimates. Error bars represent standard error.
Health Mindset modulates the relation of LPFC and MOFC to Health Value
The parametric relation between neural activity and Health Value in right LPFC and right posterior MOFC was significantly greater in the Health Mindset condition than in the in the Taste Mindset condition (Table 2 and Figure 5). Furthermore, parameter estimates from the clusters show that the parametric relation to Health Value was significantly greater than zero in the Health Mindset condition [right LPFC BA 45 t(23) = 4.25; right posterior MOFC BA 11 t(23) = 3.10; P < 0.05] but not significantly different from zero in the Taste Mindset condition [right LPFC BA 45 t(23) = −1.22; right posterior MOFC BA 11 t(23) = −1.65]. The right posterior MOFC cluster associated with Health Value in the Health Mindset condition did not overlap with the MOFC clusters related to Taste Value or Choice across conditions. In summary, activity in regions of right LPFC and right posterior MOFC tracked the health value of foods only when health information was emphasized.
Fig. 5.
Health compared to Taste Mindset modulates the parametric relation between neural activity and Health Value. In the left column, clusters show a significant parametric relation to value in terms of health only in the Health Mindset condition (Health Mindset Health Value > Taste Mindset Health Value contrast, P < 0.005, >34 contiguous voxels). In the right column, neural parametric relations to value in terms of health are illustrated by showing activation parameter estimates for foods rated low (rated two or lower), middle (rated three) and high (rated four or higher) on healthiness. ‘Asterisks’ and solid line indicates that the parametric relationship is significantly greater than zero (P < 0.05), ‘n.s.’ and dotted line indicate that the parametric relationship is not significantly different from zero (P > 0.05). Ratings were categorized as low, middle or high to visualize the parametric relations, but no statistical tests were conducted based on the categorical estimates. Error bars represent standard error.
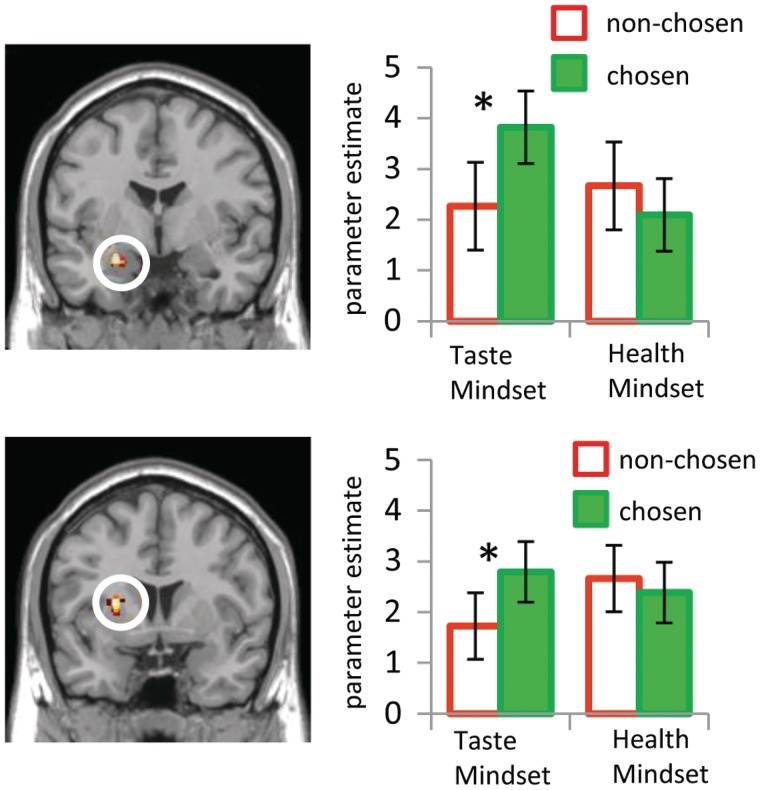
Taste compared to Health Mindset modulates the relation of amygdala and putamen to choice
Left amygdala and left putamen activity for chosen compared to nonchosen foods was modulated by the Mindset condition (Table 2 and Figure 6). Activity in left amygdala and left putamen regions defined by this contrast significantly increased for chosen compared to nonchosen foods only in the Taste Mindset condition [Taste Mindset Chosen vs Non-chosen: left amygdala t(23) = 3.30, left putamen t(23) = 3.58, P < 0.05; Health Mindset Chosen vs Non-chosen: left amygdala t(23) = −1.39; left putamen t(23) = −1.30, n.s.]. In summary, left amygdala and left putamen increased activity for foods that were chosen compared to nonchosen foods, but only when taste information was emphasized.
Fig. 6.
Taste compared to Health Mindset modulates brain regions associated with choice. Left amygdala and left putamen show an interaction of Choice and Mindset such that activity increases for chosen compared to nonchosen foods only in the Taste Mindset condition [(Taste Mindset Chosen vs Taste Mindset Non-chosen) vs (Health Mindset Chosen vs Health Mindset Non-chosen), P < 0.005, >34 contiguous voxels]. Bar charts to the right of each brain image show activation parameter estimates for chosen and nonchosen foods in each condition. ‘Asterisks’ indicates a significant difference between parameter estimates for chosen and nonchosen foods. Error bars represent standard error.
Main effect of Mindset on neural activity
The main effect of Mindset on neural activity during evaluation was examined by contrasts of all Taste Mindset evaluation events with all Health Mindset events and all Health Mindset evaluation events with all Taste Mindset events (regardless of food value or choice; individual response times included as a covariate of noninterest in the GLM). Superior parietal and occipital regions showed increased activity when participants adopted a Taste Mindset. Left LPFC (BA 44), inferior temporal, precuneus and angular gyrus regions showed increased activity when participants adopted a Health Mindset (Table 3). The LPFC (BA 44) region did not overlap with any of the LPFC regions that were parametrically related to Health Value.
DISCUSSION
The current study builds on neural models of decision-making by more precisely characterizing neural representations of value and choice. Do neural regions relate to value and choice regardless of the decision context, or are these relations modulated by the mindsets we adopt when evaluating options? Previous research on food choice has frequently contrasted food decisions driven by taste with decisions driven by health considerations (Shiv and Fedorikhin, 1999, 2002; Hare et al., 2009). The present study built on this research by examining how health and taste mindsets influence previously established neural representations of value and choice. When in a health mindset, people were slower to make choices and were more likely to favor healthy options. The fMRI results build on previous neural research by showing that some neural regions previously associated with value and choice are consistent across mindsets and others are modulated by mindset. Regardless of mindset, MOFC activity parametrically relates to value in terms of taste, left LPFC activity parametrically relates to value in terms of health and MOFC and DACC activity relates to choice. In a taste mindset, RACC parametrically relates to value in terms of taste, and left amygdala and left putamen relate to choice. In a health mindset, right LPFC and posterior MOFC activity parametrically relates to value in terms of health. These findings are the first experimental demonstration that mindsets focused on different properties influence the neural regions that relate to value and choice.
A subset of neural representations of value and choice persist across distinct mindsets
The present findings partially support previously established neural associations with value and choice by showing that some associations persist regardless of mindset. As in previous research, MOFC and LPFC activity was related to value and MOFC activity was related to choice (Erk et al., 2002; Arana et al., 2003; Gottfried et al., 2003; Hinton et al., 2004; Blair et al., 2006; Kable and Glimcher, 2007; Knutson et al., 2007; Plassmann et al., 2007; Fuhrer et al., 2008; Chib et al., 2009; Hare et al., 2009; Lebreton et al., 2009). The current study more specifically showed that mindset did not significantly affect the parametric relation between MOFC activity and taste value or the parametric relation between left LPFC (BA 8, 9) activity and health value. Similarly, mindset did not significantly affect the association of MOFC activity with chosen compared to nonchosen foods.
One important future direction will be to understand the similarity between neural regions associated with value and choice across mindsets. In the current study, the MOFC region related to choice in the Taste and Health Mindset conditions did not overlap with the MOFC cluster related to taste value across conditions. The distance between peak voxels in each cluster was 9 mm and future research will be beneficial for understanding whether distinct clusters are replicated or whether the same region of MOFC can relate to taste value as well as choice.
The MOFC, LPFC and DACC associations with value and choice across mindsets raise the question of what function is served by neural representations of value and choice across different mindsets. For example, why would MOFC track value in terms of taste when a person is in a health mindset? While mindset may ensure that certain values are computed, it is unlikely that people fail to notice other ways that options are valuable. In fact, it may be that some computations of value occur automatically. MOFC activation relates to value even when participants perform a distracting task (Kim et al., 2007; Lebreton et al., 2009). Previous research has shown that taste is a prepotent concern when evaluating food (Glanz et al., 1998; Shiv and Fedorikhin, 1999, 2002). In the current study, MOFC activity may have automatically computed foods’ value in terms of taste even when mindset increased attention to other types of value (e.g. health mindset). Furthermore, mindset may not always completely account for decisions. For example, people in a health mindset may be especially likely to evaluate foods in terms of health but they may be ultimately unwilling to sacrifice taste for health in their choice. Indeed, although participants in the current study made healthier choices in a health mindset compared to a taste mindset, they still favored the unhealthy food more than half of the time. Similarly, merely conceiving of one's self as a dieter does not ensure that food choices are never affected by taste. One reason that mindset may not perfectly predict decisions is that values that are not emphasized by the mindset are computed and integrated into choice. Neural representations of value that persist across different mindsets may allow choices to stray away from a given mindset. This possibility suggests that further research should explore whether MOFC taste value-related activity may be linked to innate preferences for sweet foods with high caloric content (Hill and Peters, 1998; Birch, 1999). Additionally, future research might examine whether the MOFC and DACC regions associated with choice across mindsets reflect the integration of different components of value.
Mindset influences how the brain evaluates options and makes a choice
The current study extends research on the neural basis of decision-making by showing that mindset affects previously reported neural representations of value and choice. Neural regions show not only increased activity for one mindset compared to another, neural representations of decision components such as taste value, health value and choice are also modulated by the mindset we adopt. Only the Taste Mindset condition showed (i) a significant parametric relation between taste value and rACC activation and (ii) a significant relation between choice and activity in left amygdala and left putamen. Only the Health Mindset condition showed a significant parametric relation between health value and activity in right LPFC (BA 45) and posterior MOFC. Furthermore, a nonoverlapping region of left LPFC (BA 44) was generally engaged by a Health Mindset. Finally, it is important to note that the influence of mindset on neural activity during evaluation is not attributable to differences in response time. Although response times are longer in the Health Mindset compared to Taste Mindset, the response time difference is related to the behavioral choices rather than the evaluation of each option that precedes the choices. Taken together, the findings show that mindset is an important moderator of what have previously been considered robust neural representations of value and choice.
Future research is needed to understand the psychological function of mindset-specific neural representations of value and choice. With regard to value representations, one possibility is that specific regions represent value when one's mindset makes fine-tuned distinctions of value more important. For example, if health is at the forefront of people's minds, they may make finer distinctions between foods based on their health properties than in times when health is not emphasized. With regard to choice representations, one possibility is that neural activity represents choices that are consistent rather than inconsistent with one's mindset (e.g. choosing a tasty food when one's mindset is focused on taste properties) rather than a more general distinction between chosen and nonchosen options. Another possibility is that mindset-specific neural representations reflect characteristics of the mindset such as effort or self-control. For example, previous research has sometimes suggested that health considerations may require more effort or self-control than taste considerations (Shiv and Fedorikhin, 1999, 2002; Hare et al., 2009). Therefore, it is possible that mindsets that require effort or self-control may moderate neural representations of value and choice in a manner similar to the Health mindset condition in the current study. Future research that addresses fine-tuned vs coarse distinctions in value, the consistency between mindset and choice, and characteristics of mindsets will further our understanding of why mindset affects neural representations of value and choice.
Furthermore, future research should explore how experience with a mindset affects neural representation of value and choice. Experience with a task can automatize performance and influence the brain regions recruited for the task (Cohen et al., 1990; Weissman et al., 2002). Does this extend to neural representations of value and choice? Taken together, the current study and a previous study (Hare et al., 2009) suggest that successful dieters represent health value in a manner that is different than nondieters and unsuccessful dieters. Successful dieters are likely to have more experience considering health properties of foods. Therefore, successful dieters’ computations of health value may become more automatic or specialized than the health value computations of unsuccessful dieters and nondieters. In addition to experimental manipulations, future research should consider experience with particular mindsets.
Individual components of value are represented in distinct neural regions
The current findings add to a growing literature demonstrating that neural activity can be related to a single component of value or the integration of multiple components of value. MOFC and RACC regions were related to taste value but not health value, while LPFC regions were related to health value but not taste value. Other regions were related to choices, which may reflect the integration of taste value, health value and other components of value that affect preference. These findings build on previous research that has demonstrated that neural activity may represent single components of value such as reward magnitude, reward probability, effort required for reward and delay to reward (Ballard and Knutson, 2009; Kennerley et al., 2009).
CONCLUSION
Although previous neural research on decision-making provides convergent evidence for neural regions associated with value and choice, very little is known about how these associations are modulated when the mind is focused on different properties. In the current study, focusing participants’ mindsets on the taste or health properties of snack foods affected neural representations of value and choice. Furthermore, regions associated with value are not completely redundant with neural regions associated with choice. Neural models of decision-making will benefit from taking mindset into account and more precisely understanding the neural processes related to the computation of value and choice (Fellows, 2004; Ernst and Paulus, 2005; Trepel et al., 2005). More research is needed to understand the specific functions that are served by neural representations of value and choice in different mindsets. With a better understanding of these neural relations to value and choice we can begin to address how regions related to value may interact with regions related to choice, and how this interaction may be influenced by the mindsets we adopt.
Conflict of Interest
None declared.
Acknowledgments
This research was supported by an NIMH Predoctoral Training Fellowship in Affective Science to Jamil Palacios Bhanji, grant T32 MH2006-10.
REFERENCES
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–8. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. NeuroImage. 2009;45:143–50. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL. Development of food preferences. Annual Reviews Nutrition. 1999;19:41–62. doi: 10.1146/annurev.nutr.19.1.41. [DOI] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, et al. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. Journal of Neuroscience. 2006;26:11379–86. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan: 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Brosch T, Coppin G, Scherer KR, Schwartz S, Sander D. Generating value(s): psychological value hierarchies reflect context-dependent sensitivity of the reward system. Social Neuroscience. 2011;6:198–208. doi: 10.1080/17470919.2010.506754. [DOI] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O’Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. Journal of Neuroscience. 2009;29:12315–20. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–61. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. NeuroImage. 2001;13:129–42. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Medial orbitofrontal cortex codes relative rather than absolute value of financial rewards in humans. European Journal of Neuroscience. 2008;27:2213–8. doi: 10.1111/j.1460-9568.2008.06202.x. [DOI] [PubMed] [Google Scholar]
- Erk S, Spitzer M, Wunderlich AP, Galley L, Walter H. Cultural objects modulate reward circuitry. Neuroreport. 2002;13:2499–503. doi: 10.1097/00001756-200212200-00024. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ersner-Hershfield H, Wimmer GE, Knutson B. Saving for the future self: neural measures of future self-continuity predict temporal discounting. Social Cognitive and Affective Neuroscience. 2009;4:85–92. doi: 10.1093/scan/nsn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK. The cognitive neuroscience of human decision making: a review and conceptual framework. Behavioral and Cognitive Neuroscience Reviews. 2004;3:159–72. doi: 10.1177/1534582304273251. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity. 2008;16:945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. Journal of the American Dietetic Association. 1998;98:1118–26. doi: 10.1016/S0002-8223(98)00260-0. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–90. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. European Journal of Neuroscience. 2004;20:1411–8. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. Journal of Cognitive Neuroscience. 2009;21:1162–78. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage. 2003;19:1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kim H, Adolphs R, O’Doherty JP, Shimojo S. Temporal isolation of neural processes underlying face preference decisions. Proceedings at National Academy of Sciences USA. 2007;104:18253–8. doi: 10.1073/pnas.0703101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–56. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioural Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64:431–9. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. Journal of Neuroscience. 2007;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Schirmer J, Ames DL, Gilbert DT. Medial prefrontal cortex predicts intertemporal choice. Journal of Cognitive Neuroscience. 2011;23:857–66. doi: 10.1162/jocn.2010.21479. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. Calculate your BMI. http://www.nhlbisupport.com/bmi/ (10 December 2009, date last accessed) [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–66. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27:9984. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32:1273–80. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- Raghunathan R, Naylor RW, Hoyer WD. The unhealthy = tasty intuition and its effects on taste inferences, enjoyment, and choice of food products. Journal of Marketing. 2006;70:170–84. [Google Scholar]
- Shiv B, Fedorikhin A. Heart and mind in conflict: the interplay of affect and cognition in consumer decision making. The Journal of Consumer Research. 1999;26:278–92. [Google Scholar]
- Shiv B, Fedorikhin A. Spontaneous versus controlled influences of stimulus-based affect on choice behavior. Organ. Behav. Hum. Dec. 2002;87:342–70. [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Brain Research Cognitive Brain Research. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Woldorff MG, Hazlett CJ, Mangun GR. Effects of practice on executive control investigated with fMRI. Brain Research Cognitive Brain Research. 2002;15:47–60. doi: 10.1016/s0926-6410(02)00215-x. [DOI] [PubMed] [Google Scholar]