Abstract
In emotional learning tasks, sex differences, stress effects and an interaction of these two moderators have often been observed. The sex hormones estradiol (E2) and progesterone (P4) vary over the menstrual cycle. We tested groups with different sex hormone status: 39 men, 30 women in the luteal phase (LU, high E2+P4) and 29 women taking oral contraceptives (OC, low E2+P4). They received either 30 mg cortisol or placebo prior to instructed differential fear conditioning consisting of neutral conditioned stimuli (CS) and an electrical stimulation (unconditioned stimulus; UCS). One figure (CS+) was paired with the UCS, the other figure (CS−) never. During extinction, no electrical stimulation was administered. Regarding fear acquisition, results showed higher skin conductance and higher brain responses to the CS+ compared to the CS− in several structures that were not modulated by cortisol or sex hormones. However, OC women exhibited higher CS+/CS− differentiations than men and LU women in the amygdala, thalamus, anterior cingulate and ventromedial prefrontal cortex during extinction. The suppression of endogenous sex hormones by OC seems to alter neuronal correlates of extinction. The observation that extinction is influenced by the current sex hormone availability is relevant for future studies and might also be clinically important.
Keywords: amygdala, fMRI, instructed fear conditioning, menstrual cycle, oral contraceptives
INTRODUCTION
Anxiety disorders are the most common mental diseases with a lifetime prevalence of ∼28.8%. Of note, women are more prone to develop an anxiety disorder than men (Kessler et al., 2005). The neurobiological understanding of this phenomenon is still incomplete. Sex hormones such as estradiol (E2), progesterone (P4) and testosterone (T) are considered as potential mediators of the observed sex differences in susceptibility (Toufexis et al., 2006; Solomon and Herman, 2009). Altered fear acquisition and extinction during classical fear conditioning are well-established models for the etiology and the maintenance of anxiety disorders (Mineka and Zinbarg, 2006; Mineka and Oehlberg, 2008). In the present study, we investigated activational effects of sex hormones on fear extinction that could potentially contribute to the sex differences in the prevalence of anxiety disorders.
Differential fear conditioning paradigms typically consist of a stimulus, which is paired with an aversive event (unconditioned stimulus; UCS) and becomes a conditioned stimulus (CS+), whereas another stimulus is never paired (CS−). Higher responses toward the CS+ compared to the CS− indicate successful fear acquisition. On the neuronal level, activations of the amygdala, the anterior cingulate cortex (ACC), the hippocampus, the insula, the orbitofrontal cortex (OFC) and the thalamus have been identified as correlates of fear acquisition and fear expression (Rolls, 1999; LeDoux, 2000; Knight et al., 2004a, 2004b; Sehlmeyer et al., 2009).
In extinction learning, the CS+ is no longer paired with the UCS, so conditioned behavior diminishes. This mechanism comprises a new learning process including neuronal activation of the amygdala, the hippocampus and the ventromedial prefrontal cortex (vmPFC; Myers and Davis, 2002; Quirk and Mueller, 2008; Sotres-Bayon and Quirk, 2010). Hence, the amygdala is involved in the acquisition as well as in the extinction of fear, with fear extinction probably being modulated by the inhibitory activation of the vmPFC leading to reduced conditioned responses (CRs; Knight et al., 2004b; Phelps et al., 2004; Delgado et al., 2006). In rodents, sex differences in extinction have been frequently reported (Dalla and Shors, 2009). In detail, E2 and P4 administration facilitated extinction, whereas blockade of their receptors led to decreased extinction (Milad et al., 2009). Besides, it has been shown that higher E2 levels enhanced fear extinction (Markus and Zecevic, 1997) by involving the estrogen receptor β (Chang et al., 2009). In humans, low levels of E2 have been associated with heightened fear recall (Milad et al., 2006, 2010). All in all, sex hormones (in particular E2) seem to impact extinction processes. Especially in women, this fact is highly relevant because of fluctuating E2 levels over the course of the menstrual cycle and the frequent use of oral contraceptives (OC) suppressing endogenous E2 concentrations.
In addition to sex hormones, several studies have found cortisol application facilitating extinction, which could support psychotherapy (e.g. Bentz et al., 2010; de Quervain et al., 2011). But it has also been shown that stress, with its accompanying release of cortisol, might impair extinction processes by shifting the activation from the vmPFC to the amygdala (Izquierdo et al., 2006; Miracle et al., 2006; Akirav and Maroun, 2007). Stress types and exact protocols with timing of stress seem to be crucial (Sandi and Pinelo-Nava, 2007; Rodrigues et al., 2009; Wolf, 2009; Schwabe et al., 2010).
A possible interaction between stress and sex has also been proposed in extinction learning (Jackson et al., 2006; Baran et al., 2009) with stress reducing extinction in males, but enhancing extinction in females. The relevance of differing E2 levels on extinction learning in humans when stress hormones are heightened has been neglected so far. To address this issue in more detail, we investigated men and women characterized by different endogenous sex hormone levels. Women in the luteal phase of the menstrual cycle (LU; high endogenous E2 and P4) and women taking OC (low endogenous E2 and P4) were included. Half of the participants received an oral dose of cortisol to explore possible effects of this stress hormone on CRs.
A differential fear conditioning paradigm was employed with an instructed fear acquisition phase to ensure that all participants were aware of the contingencies between the CS and UCS. The acquisition phase was immediately followed by an extinction phase. Contingency awareness has a considerable impact on fear acquisition (Tabbert et al., 2006, 2011; Klucken et al., 2009). In our previous fear acquisition studies, we observed an interaction of cortisol and sex in a mixed sample comprising participants who learned the contingencies during the experiment (learned aware) and participants who did not notice the CS–UCS contingencies (unaware; Stark et al., 2006). The same interaction effect was found in a sample of unaware participants (Merz et al., 2010). Enhancing effects of cortisol in learned aware OC women have also recently been reported in fear acquisition and extinction (Tabbert et al., 2010). In the present independent sample, we extend these observations to instructed fear conditioning, which probably more closely reflects fear expression rather than fear learning, because fear is experienced already at the first presentation of the CS+. To the best of our knowledge, this is the first fMRI study exploring extinction learning with respect to a possible interaction between stress (cortisol) and sex (E2, P4, T) hormones. Congruent with previous studies on the impact of E2 on fear conditioning, it was hypothesized that LU women would display facilitated extinction processes because of heightened E2 concentrations. In OC women, low endogenous E2 levels were expected to interfere with extinction learning. In line with the stress literature mentioned above, cortisol was hypothesized to enhance extinction in women but impair it in men.
MATERIALS AND METHODS
Subjects
In total, 99 participants completed the study, 92 were undergraduate students and 7 had already graduated. None of them was taking regular medication except OCs or had a history of neurological or psychiatric treatment. Exclusion criteria were somatic diseases, in particular endocrine diseases, which can influence hormone concentrations. Inclusion criteria were an age between 18 and 35 years and a body mass index (BMI) between 18 and 28 kg/m2. All participants had normal or corrected vision and were right handed as assessed by the Edinburgh Inventory of Handedness (Oldfield, 1971). To assess different sex hormone statuses in women, we invited free-cycling women and OC taking women. Free-cycling women were required to report having a regular menstrual cycle and were invited in the luteal phase of their individual menstrual cycle (3rd to 9th day before the onset of their next menses; Buffet et al., 1998). OC women were required to have been taking their birth control pill (only monophasic preparations with an ethinylestradiol component) for at least the last 3 months and were tested during the pill intake phase. All subjects were instructed to refrain from smoking, food intake and drinking anything but water for at least 2 h before the experiment.
At the beginning, participants received a detailed explanation of the procedure in general. The cover story was the investigation of the impact of cortisol and several distractors on memory performance. All participants were informed about the relationship between CS and UCS in advance of the experiment, but received no details about the absence of the UCS in extinction. One OC woman fell asleep during extinction; her data were removed from all analyses. Thus, the final sample consisted of 98 participants subdivided into six groups according to sex hormone status and treatment: 20 men, 15 LU and 14 OC women in the placebo group; 19 men, 15 LU and 15 OC women in the cortisol group. The mean age for the six groups ranged from 22.4 to 25.3 years and the mean BMI from 21.5 to 23.2 kg/m2. All participants gave written informed consent and received 25 Euros for their attendance. The study was approved by the ethics committee of the German Psychological Society.
An analysis of a subsample of the present data (n = 49 from the placebo group combined with additional 68 participants) has been published previously (Tabbert et al., 2011). But this prior study was concerned with the differential impact of contingency awareness on fear acquisition. The remaining 50 participants receiving cortisol have not been analyzed separately or are part of any other study. In the present report, we concentrate on the effects of cortisol and sex hormone status on fear extinction in instructed aware participants only. As a side analysis, we were able to extend previous effects of stress and sex hormones in learned and unaware persons (Stark et al., 2006; Merz et al., 2010; Tabbert et al., 2010) to instructed fear conditioning.
Conditioned visual stimuli, UCS and experimental procedure
Three pictures of geometric figures (rhomb, square and triangle) served as CS+, CS− and as distractor stimulus (non-CS; always the triangle). All figures were gray in color, had identical luminance and were presented with a duration of 8 s against a black background. Stimuli were projected onto a screen at the end of the scanner (visual field = 18°) using an LCD projector (EPSON EMP-7250) and were viewed through a mirror mounted on the head coil. A custom-made impulse-generator (833 Hz) provided transcutaneous electrical stimulation (UCS) for 100 ms through two Ag/AgCl electrodes (1 mm2 surface each). Electrodes were fixed to the middle of the left shin and stimulus intensity was set individually using a gradually increasing procedure to achieve an ‘unpleasant but not painful’ level of sensation. The onset of the UCS presentation started 7.9 s after CS+ onset (delay conditioning; 100% reinforcement). Non-UCS was defined as the UCS omission 7.9 s after the CS− onset. The CS− and the non-CS were never paired with the UCS. No electrical stimulation was given in the extinction.
The conditioning experiment consisted of an acquisition phase, an extinction phase and an implemented two-back task (cf. Merz et al., 2010 for further details). The conditioning procedure was adapted from prior studies in our laboratory (Tabbert et al., 2005, 2006; Stark et al., 2006) with an additional extinction phase as described previously (Merz et al., 2010; Tabbert et al., 2010, 2011). In short, 20 trials of CS+ as well as CS− and ten trials of non-CS were presented in the acquisition phase (total duration: ∼20 min; starting 45 min after tablet administration). During extinction, 11 trials of CS+ and CS−and 5 trials of non-CS were presented (total duration: ∼11 min; starting 70 min after tablet intake). Inter-trial intervals (ITIs) between the numbers and the geometrical figures lasted 5 s and were randomly jittered between 0 and 2.5 s (i.e. ITI of 5–7.5 s). For each participant, pseudo-randomized stimulus orders were used (cf. Merz et al., 2010).
Participants were told precisely which geometrical figure will precede the electrical stimulation (i.e. instructed fear conditioning; see Tabbert et al., 2011). Immediately after the acquisition, participants had to rate the contingencies between the UCS and CS+, CS− and non-CS, which were presented in random order. Next to the picture of the respective CS, the question was always: ‘Please estimate how often the electrical stimulation succeeded the following geometrical figure’ with the possible answers: ‘I do not know’, ‘never’, ‘sometimes’ and ‘always’. Contingency awareness was confirmed in all participants by indication that the CS+ ‘always’ and the CS− ‘never’ preceded the UCS.
Treatment, hormone analyses and skin conductance responses
This study was conducted as a double-blind, randomized and placebo-controlled experiment. Forty-nine participants (see ‘Subjects’ section for further allocation to sex hormone status group) received three 10 mg tablets of cortisol (30 mg hydrocortisone; Hoechst) 45 min before the start of the fear conditioning protocol. Visually identical placebos (tablettose and magnesium) were given to the other 49 participants. Each experiment started between 2 and 5 p.m. to guarantee low and relatively stable endogenous cortisol concentrations.
Saliva samples for the analyses of free cortisol, E2, P4 and T were collected from the participants by use of glass tubes. Samples were taken directly before as well as 25 min (immediately before the fMRI run) and 90 min after tablet intake (immediately after the fMRI run). Directly after sampling, the saliva was stored at −20°C until assayed. All hormones were analyzed within one lot and in duplicates by use of commercial enzyme immunoassays (IBL International, Hamburg, Germany). Inter-assay coefficients of variations (CVs) for all analyses were below 8% with an inter-assay CV below 11%.
Skin conductance responses (SCRs) were sampled with an in-house built optical fiber SCR coupler especially designed for measuring SCRs concurrently to fMRI. Ag/AgCl electrodes were used filled with isotonic (0.05 M NaCl) electrolyte medium placed hypothenar at the non-dominant hand. Raw SCR data were low pass filtered with a cutoff frequency of 10 Hz. SCRs were defined in three analysis windows (cf. Prokasy and Ebel, 1967): the maximum amplitude within a window of 1–5 s after the CS onset was counted as the first interval response (FIR), within the time window of 5–8.5 s as the second interval response (SIR), and within the time window of 8.5–13 s as the unconditioned response (UCR). The baseline was the skin conductance level immediately preceding the inflexion point. Electrodermal data of seven participants (for extinction: 11) had to be discarded because of several problems (fallen off electrodes, malfunction of the SCR coupler, or random noise in the dataset). Data were transformed with the natural logarithm in order to attain a normal distribution.
All statistical analyses were conducted in PASW for Windows 18.0 via analyses of variance (ANOVA) with the between-subjects factors sex hormone status (men vs LU women vs OC women) and treatment (placebo vs cortisol). Greenhouse–Geisser correction was applied when the sphericity assumption was not met and statistical significance was set at P < 0.05. Statistical analyses of cortisol included the repeated measurement factor time (first vs second vs third sample). E2, P4 and T were analyzed without the repeated measurement factor time. Sex hormones were only determined in the first and the third saliva sample and their concentrations were averaged to check for expected differences between men, LU and OC women. Statistical comparisons of SCRs were performed separately for the acquisition and the extinction with the within-subject factors stimulus type (CS+ and CS− for the FIR and SIR) and trial (20 for fear acquisition; 10 for fear extinction). Results of the UCR can be found in the Supplementary Data. Only main effects or interactions with the factor stimulus type will be reported to emphasize fear learning-related modulations.
Image acquisition and analyses
Brain images were acquired using a 1.5 T whole-body tomograph (Siemens Symphony with a quantum gradient system) with a standard head coil. Data were analyzed using Statistical Parametric Mapping (SPM5, Wellcome Department of Cognitive Neurology, London, UK, 2005) implemented in MatLab R2007b (Mathworks Inc., Sherborn, MA). Standard preprocessing steps were used as described before (cf. Merz et al., 2010; Tabbert et al., 2010, 2011, also for details concerning structural and functional image acquisition).
Fear acquisition and extinction were integrated as separate sessions in one model in SPM5 including the following experimental conditions: CS+, CS−, non-CS, UCS, non-UCS, targets and non-targets (excluding UCS and non-UCS for extinction). The linear temporal trend of the CS+, CS−, non-CS, UCS and non-UCS were added as regressors in the statistical design to account for possible habituation or sensitization effects. An additional regressor was introduced containing the first two numbers and the first two geometrical figures of the extinction, because learning could not have yet occurred (Phelps et al., 2004). All regressors were modeled by a stick function convolved with the canonical hemodynamic response function in the general linear model, without specifically modeling the durations of the different events. Six regressors counting information about motion correction were introduced as covariates in the model separately for the acquisition and the extinction. The high pass filter was set at 128 s.
The individual contrasts were analyzed in random effects group analyses in SPM8 (Wellcome Department of Cognitive Neurology, London, UK, 2009) and focused on the contrasts CS+ minus CS− and CS+ by time minus CS− by time during acquisition and extinction. Results of the contrast UCS minus non-UCS during acquisition can be found in the Supplementary Data. ANOVA was conducted with the group factors sex hormone status and treatment in the full factorial model implemented in SPM8. F-contrasts will be reported for main effects and interactions with subsequent post hoc t-tests for significant results.
For statistical analyses, we used region of interest (ROI) analyses: ACC, amygdala, insula and thalamus were included as ROI for the UCS and CS analyses. In addition, for CS analyses in the acquisition, the hippocampus and the OFC were included. For CS analyses in the extinction, the hippocampus and the vmPFC were additionally tested. The structural masks were designed using the software-program MARINA (Walter, 2002). The significance threshold was set to α = 0.05 on voxel level, corrected for multiple testing (family-wise error (FWE) correction using the small volume correction options of SPM8) with Pcorr. < 0.05. Trends in the amygdala will also be reported at a more liberal level (Pcorr. < 0.10) due to its prominent role in fear learning.
RESULTS
Endocrinological data and SCRs
A small number of participants (six men, one LU and one OC woman) showed extremely high cortisol concentrations (>1000 nmol/l) 25 min after hydrocortisone intake. These subjects were excluded from cortisol analyses, because these high levels most likely reflect micro hydrocortisone residues of the uncoated tablet in the mouth of the participants. ANOVA revealed a significant main effect of time [F(1.1, 91.9) = 32.1; P < 0.001], treatment [F(1, 84) = 58.5; P < 0.001] and a time × treatment interaction [F(1.1, 91.9) = 33.3; P < 0.001]. In the cortisol compared to the placebo group, cortisol levels were elevated in the second and third sample (both Ps < 0.001; first sample: P = 0.55; Table 1) pointing to a successful treatment. No other main effects or interactions occurred. Further, no differences in baseline cortisol concentrations (first sample) with respect to treatment, sex hormone status, or an interaction of these factors were observed. A cortisol response resulting from the paradigm was tested in the placebo group only, because cortisol levels were too high in the group receiving 30 mg hydrocortisone to detect slight changes due to the experimental procedure. Comparing cortisol levels of the second and third sample (which might reflect a cortisol response due to the paradigm most adequately), no significant effect of time was found.
Table 1.
Mean (s.e.) cortisol concentrations (in nmol/l) before, 25 min after and 90 min after the administration of 30 mg hydrocortisone or placebo
| Cortisol (nmol/l) | Before treatment | 25 min after treatment | 90 min after treatment |
|---|---|---|---|
| Cortisol | 7.12 (0.85) | 224.17 (37.02)a | 100.96 (10.31)a |
| Placebo | 6.51 (0.59) | 4.47 (0.44) | 5.25 (0.45) |
Unrealistically high hormone concentrations (>1000 nmol/l) were excluded from the analyses and the descriptives of this table.
aSignificant increase compared to the placebo group (P < 0.001).
Analyses of sex hormones revealed implausibly high levels for some participants (larger than 3 s.d. from the mean of the respective sex hormone status group), which could be a sign of sample contamination. These subjects (E2: one man, one LU and one OC woman; P4 and T: one OC woman) were excluded from sex hormone analyses. There was a significant main effect of sex hormone status for E2 [F(2, 89) = 6.6; P = 0.002], P4 [F(2, 91) = 56.0; P < 0.001] and T [F(2, 91) = 29.6; P < 0.001; Table 2]. LU women had higher E2 and P4 concentrations than OC women and men (all Ps ≤ 0.005). Concerning T, men had higher levels than LU and OC women (both Ps < 0.001); LU women had higher concentrations than OC women (P = 0.01). No other main effects or interactions emerged. Recruiting of the present sample was successful, because sex hormone levels of the three sex hormone status groups were in the expected range.
Table 2.
Mean (s.e.) estradiol, progesterone and testosterone concentrations (in pmol/l) for men, LU and OC women
| Sex hormone (pmol/l) | Estradiol | Progesterone | Testosterone |
|---|---|---|---|
| Men | 7.45 (0.82) | 175.06 (16.24) | 322.13 (36.23)a |
| LU women | 11.93 (0.82)a,b | 616.97 (59.93)a,b | 99.65 (12.02)a,b |
| OC women | 7.71 (0.90) | 141.80 (12.56) | 61.13 (8.00) |
Unrealistically high hormone concentrations (>3s.d. from the mean of the respective sex hormone status group) were excluded from the analyses and the descriptives of this table.
aSignificant difference compared to OC women (largest P ≤ 0.01).
bSignificant difference compared to men (P ≤ 0.002).
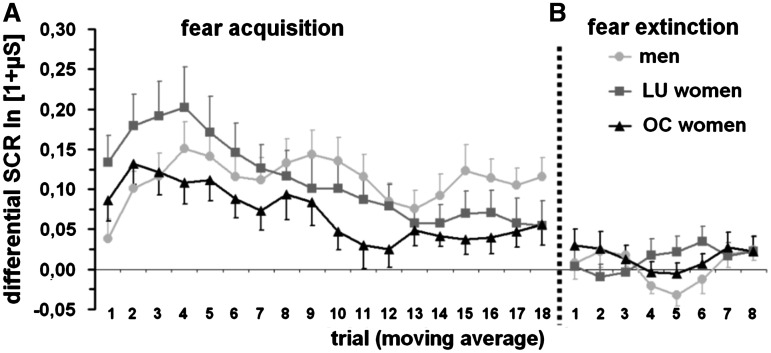
Concerning SCRs in the acquisition, ANOVA demonstrated a main effect of stimulus type for the FIR [F(1, 85) = 98.2; P < 0.001] and the SIR [F(1, 85) = 59.1; P < 0.001]. These effects were based on higher SCRs toward the CS+ than toward the CS−. Moreover, a significant interaction between stimulus type and trial could be detected for the FIR [F(11.1, 945.8) = 5.3; P < 0.001] and the SIR [F(11.3, 957.3) = 4.4; P < 0.001] indicating that stimulus processing differed over time. Further, ANOVA revealed a stimulus type × trial × sex hormone status interaction in the SIR [F(22.5, 957.3) = 1.6; P = 0.048]. Post hoc tests could not detect differences between the sex hormone status groups. Presumably, this interaction effect is due to differences in single trials; for an illustration of these results, time courses of the differential SCRs in the SIR are depicted in Figure 1A. It appears that LU women had initially higher differential SCRs, which habituated more rapidly compared to those of men and OC women. In the extinction, no main or interaction effects with stimulus type were found. In particular, sex hormone status did not influence extinction learning (see Figure 1B). Additional tests in the six groups separated for the acquisition and the extinction phase can be found in the Supplementary Data (see Supplementary Table S1).
Fig. 1.
Mean differential SCRs (CS+ minus CS− in the SIR) in (A) the fear acquisition and (B) the fear extinction phase separated for men, LU and OC women. A moving average over three trials is illustrated to enhance temporal smoothness. Error bars are standard errors of the mean.
Hemodynamic responses
In all analyses of the acquisition and the extinction, the factor treatment (placebo vs cortisol) did not result in significant main or interaction effects.
Fear acquisition
In the contrast CS+ by time minus CS− by time, we found significant neuronal activation in the right ACC (x = 6, y = 48, z = 27, Fmax = 16.42 and Pcorr. = 0.023) and the right insula (x = 33, y = 9, z = 6, Fmax = 15.92 and Pcorr. = 0.038). These differentiations were based on linear increases toward the CS− and linear decreases toward the CS+.
In the contrast CS+ minus CS−, ANOVA revealed significant neuronal activations in all ROI (all Ps ≤ 0.008) with higher responses to the CS+ than to the CS−.
Thus, in fear acquisition, reliable CRs could be found without a modulation by cortisol or sex hormone status.
Fear extinction
In the contrast CS+ by time minus CS− by time, we found a significant linear CS+/CS−differentiation in the right amygdala (x = 21, y = 3, z = −21, Fmax = 16.34 and Pcorr. = 0.005) revealing a linear increase toward the CS− and a linear decrease toward the CS+ in all participants.
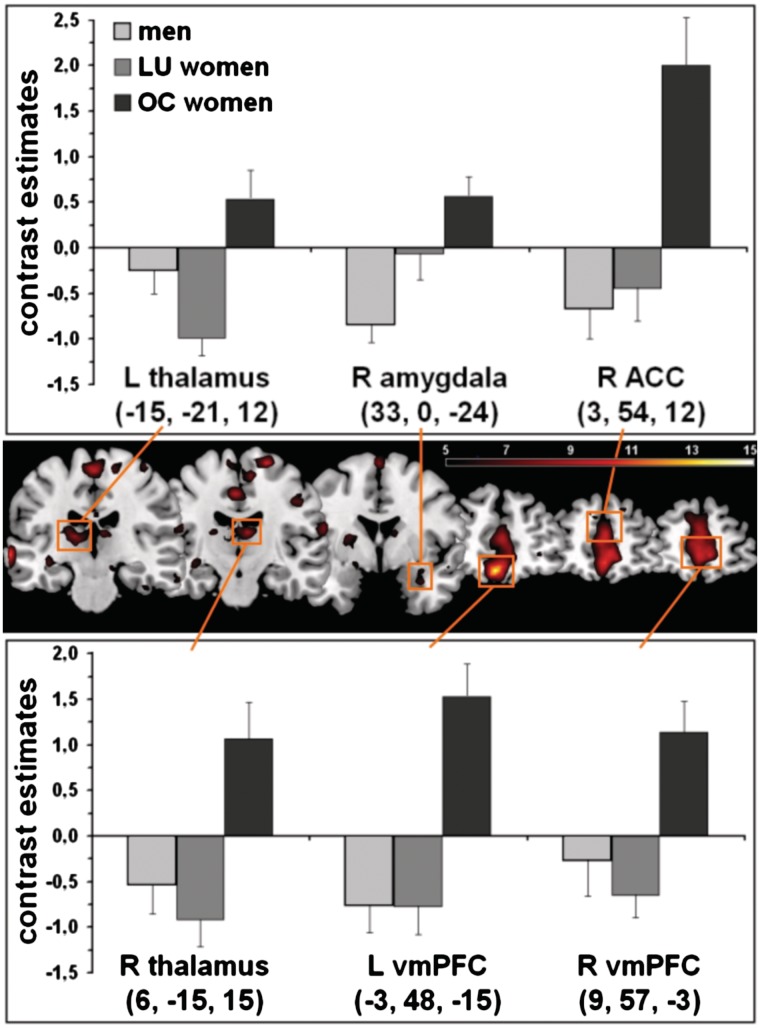
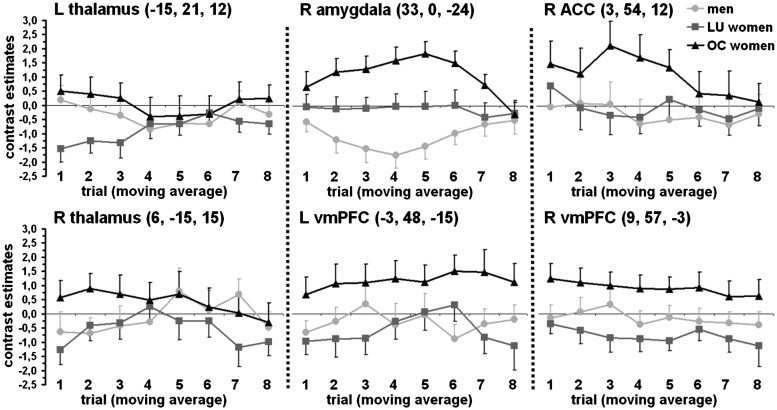
In the F-test for the main effect sex hormone status in the contrast CS+ minus CS−, ANOVA revealed significant CS+/CS− differentiations in the right ACC, the right amygdala, bilaterally in the thalamus as well as in the vmPFC (Table 3 and Figure 2). Subsequent t-tests in these structures revealed that OC women had significantly higher CS+/CS− differentiation than men and LU women in all regions (except the left thalamus for the comparison OC women minus men; Table 3). As shown in Figure 2, this pattern resulted from higher responses to the CS− compared to the CS+ in men and LU women, whereas OC women had higher neuronal activation to the CS+ in comparison to the CS−. Additionally, plots of the time courses of differential neuronal activation in the peak voxels showed rather steady CRs in the left and the right vmPFC in OC women (Figure 3). In the right amygdala and the right ACC, OC women showed increasing CRs at first; these decreased over time leading to a low level comparable to men and LU women. Time courses of the thalamus activation seem to be quite unspecific.
Table 3.
Localization, cluster size (k) and statistics of the peak voxels within the respective ROI resulting from the contrast CS+ minus CS− in the extinction
| Group | Brain region | x | y | z | k | Fmax | Pcorr. |
|---|---|---|---|---|---|---|---|
| All participants | R ACC | 3 | 54 | 12 | 166 | 10.42 | 0.021 |
| R amygdala | 33 | 0 | −24 | 65 | 6.72 | 0.060 | |
| L thalamus | −15 | −21 | 12 | 147 | 8.84 | 0.032 | |
| R thalamus | 6 | −15 | 15 | 124 | 9.13 | 0.026 | |
| L vmPFC | −3 | 48 | −15 | 192 | 14.16 | 0.001 | |
| R vmPFC | 9 | 57 | −3 | 220 | 10.68 | 0.014 |
| Group | Brain region | x | y | z | k | Tmax | Pcorr. |
|---|---|---|---|---|---|---|---|
| OC women minus men | R ACC | 6 | 45 | 6 | 285 | 4.12 | 0.012 |
| R amygdala | 30 | −3 | −21 | 122 | 4.20 | 0.002 | |
| R thalamus | 21 | −27 | 3 | 172 | 3.54 | 0.029 | |
| L vmPFC | −6 | 48 | −15 | 269 | 4.91 | 0.001 | |
| R vmPFC | 9 | 51 | −12 | 305 | 3.99 | 0.014 | |
| Men minus OC women | No significant activations | ||||||
| OC women minus LU women | R ACC | 3 | 54 | 12 | 191 | 3.94 | 0.023 |
| R amygdala | 33 | 0 | −15 | 76 | 2.77 | 0.082 | |
| L thalamus | −15 | −21 | 12 | 254 | 4.31 | 0.005 | |
| R thalamus | 9 | −9 | 15 | 182 | 3.97 | 0.011 | |
| L vmPFC | −3 | 48 | −15 | 227 | 4.65 | 0.002 | |
| R vmPFC | 3 | 51 | −9 | 264 | 4.41 | 0.005 | |
| LU women minus OC women | No significant activations | ||||||
| Men minus LU women | No significant activations | ||||||
| LU women minus men | No significant activations |
Here, the undirected F-tests revealed a main effect of sex hormone status. Further, results for the post hoc directed t-tests between the sex hormone status groups (men vs LU women versus OC women) are shown.
The significance threshold was Pcorr. < 0.05 (FWE-corrected according to SPM8; small volume correction); for neuronal activation in the amygdala Pcorr. < 0.10 was chosen. All coordinates (x, y, z) are given in MNI space. L = left and R = right.
Fig. 2.
Neuronal activation in the extinction for the main effect of sex hormone status on conditioned responses (CS+ minus CS−). Data are illustrated with F ≥ 5.0 (see color bar for exact F-values). The depicted slices were selected according to the reported activations in the left (y = −21) and right thalamus (y = −15), the right amygdala (y = 0), the right anterior cingulate cortex (ACC; y = 54), as well as in the left (y = 48) and right ventromedial prefrontal cortex (vmPFC; y = 57). Additionally, mean contrast estimates as well as the respective standard errors of the mean to CS+ minus CS− for men, LU and OC women in the respective peak voxels are displayed in the bar graphs. L = left, R = right.
Fig. 3.
Extinction learning curves for the contrast CS+ minus CS− for the three sex hormone status groups (men, LU women and OC women) in the peak voxels of the group statistics (cf. Table 3 and Figure 2). A moving average over three trials is illustrated to enhance temporal smoothness. Error bars are standard errors of the mean. L = left, R = right.
To gain further insights into the potential association between electrodermal activity (showing no sex hormone status effect) and neuronal activation in fear extinction learning, we correlated differential SCRs with the contrast CS+ minus CS− in all significant ROI (cf. Table 3). The mean differential SCR (separately for the FIR and the SIR) was included as regressor in simple regression models for all participants as well as separately for men, LU and OC women. Significant t-values identify brain activation significantly correlating with differential SCRs. Results in the SIR revealed no associations. However in the FIR, positive correlations were found in the right ACC, the right amygdala, the right thalamus and the right vmPFC in OC women only (see Table 4).
DISCUSSION
This fMRI study with a large sample size investigated how sex and stress hormones influence instructed fear acquisition (probably more closely reflecting fear expression rather than fear learning) and in particular fear extinction. Cortisol alone as well as in the interaction with sex hormone status did not influence instructed fear acquisition or extinction. However, sex hormone status influenced neuronal correlates of extinction: OC women showed altered extinction learning compared to men and free-cycling women in the luteal phase. Additionally, an association of neuronal activation with differential SCRs during extinction was observed in OC women only.
In general in extinction, amygdala activation linearly increased toward the CS− and decreased to the CS+. As the amygdala is involved in the detection of salience (Davis and Whalen, 2001; Phan et al., 2002), this finding could be interpreted as a shift in salience from the CS+ toward the CS− due to the (for the participants initially surprising) absence of the UCS in extinction. After having noticed that the CS+ does not predict the UCS anymore, attention might have been directed to the CS− as a possible new cue for the UCS. It has to be mentioned that analysis of the contrast CS+ by time minus CS− by time in the present study reflects linear changes in neuronal activation over time. Different time courses of CS+/CS− differentiation might also be presumed in extinction, e.g. quadratic or cubic trends. However, the exact time course of the learning process, which is required for the set up of an adequate statistical model, is still unclear, in particular concerning different brain structures.
Most interestingly, we observed differences in extinction learning in our three sex hormone status groups. Neuronal activation in the contrast CS+ minus CS− was higher in OC women compared to men and LU women in the ACC, the amygdala, the thalamus and the vmPFC. These brain structures have been associated with fear extinction processes (Myers and Davis, 2002; Quirk and Mueller, 2008; Sotres-Bayon and Quirk, 2010) as well as more generally with emotion processing (Bush et al., 2000; Phan et al., 2002, 2004). Furthermore, the amygdala and parts of the ACC seem to be overactive in patients with specific phobia or post-traumatic stress disorder (PTSD; Etkin and Wager, 2007; Shin and Liberzon, 2010). An increased fear acquisition (Grillon and Morgan, 1999; Orr et al., 2000) and a prolonged fear extinction (Orr et al., 2000; Peri et al., 2000; Blechert et al., 2007) have already been observed in patients with PTSD. Women are much more prone to develop an anxiety disorder (Breslau et al., 1997; Kessler et al., 2005) with a possible maintenance mechanism being e.g. a failure to adequately diminish fear reactions. In the present study, the opposite neuronal activation pattern in OC women compared to men and LU women suggests at least altered extinction learning (van Haaren et al., 1990).
As far as the amygdala is concerned, a previous study observed higher responses to the CS− compared to the CS+ in extinction learning (Phelps et al., 2004; but see LaBar et al., 1998), which were correlated with diminishing differential SCRs. However, in the present study, a statistical significant effect of sex hormone status could not be found in SCRs during extinction. Thus, at the electrodermal level, we cannot confirm the results of the fMRI analyses at first glance. But several important facts have to be taken into consideration. Potentially, SCRs are not sensitive enough to detect slight changes due to extinction learning, especially with a 100% reinforcement schedule in the acquisition phase. BOLD responses might be considered as a more sensitive measure, although they do not necessarily represent the same measure of the fear conditioning process as SCRs. In this line, a dissociation of the electrodermal and the neuronal level in fear conditioning has already been reported (e.g. Tabbert et al., 2006, 2011; Kalisch et al., 2009). Further, to attain a comparable extinction pattern in SCRs (i.e. no significant differentiation between CS+ and CS−) between the sex hormone status groups, a different underlying neuronal activation was necessary. Descriptively, at the beginning of the extinction phase, high CS+/CS− differentiations were found in the amygdala and the ACC in OC women. Both structures have been related to fear expression (LeDoux, 2000; Phelps et al., 2004; Shin and Liberzon, 2010). Thus, it could be probably suggested that this initial higher activation reflects fear responses, which are still present in the first part of the extinction in OC women but not in men or LU women. Later, neuronal activation in the amygdala and the ACC diminishes resulting in a similar activation pattern in all sex hormone status groups. The assumedly inhibitory vmPFC activation sustained over time in OC women. The vmPFC has been associated with fear extinction and emotion regulation (Quirk and Mueller, 2008; Sotres-Bayon and Quirk, 2010; Hartley and Phelps, 2010); in the present study, this critical structure seems to be recruited steadily in OC women only. Thus, sustained differential vmPFC activation might be necessary to inhibit the initial fear responses still elicited by the amygdala and the ACC in OC women, but not in men or LU women.
Table 4.
Localization and statistics of the peak voxels for the correlation analyses between differential SCRs in the FIR and neuronal activation in the contrast CS+ minus CS− in the extinction phase
| Group | Brain region | x | y | z | Tmax | Pcorr. | r |
|---|---|---|---|---|---|---|---|
| All participants | No significant correlation | ||||||
| Men | No significant correlation | ||||||
| LU women | No significant correlation | ||||||
| OC women | R ACC | 12 | 18 | 27 | 4.00 | 0.055 | 0.641 |
| R amygdala | 24 | 3 | −24 | 3.99 | 0.013 | 0.640 | |
| R thalamus | 15 | −18 | −3 | 3.75 | 0.044 | 0.616 | |
| R vmPFC | 21 | 18 | −12 | 4.20 | 0.030 | 0.659 |
Results are listed for all participants as well as separately for men, LU and OC women within the respective ROI. In order to gain a quantitative measure for the magnitude of these correlations, we calculated the correlation coefficients r for the resulting brain structures using the t-values at the peak voxels of the respective analysis (cf. Rosenthal, 1994).
The significance threshold was Pcorr < 0.05 (FWE-corrected according to SPM8; small volume correction); trends up to Pcorr < 0.10 are shown. All coordinates (x, y, z) are given in MNI space. L = left and R = right.
Despite the lacking effect of sex hormone status on SCRs in the extinction phase, we could demonstrate in correlation analyses that higher CS+/CS− responses at the electrodermal level were positively associated with higher differential activation in the ACC, the amygdala, the thalamus and the vmPFC. This might also be interpreted in terms of fear responses still being present in OC women. However, a negative correlation between vmPFC activation and SCRs would have been expected regarding the literature (Quirk and Mueller, 2008; Hartley and Phelps, 2010; Sotres-Bayon and Quirk, 2010). Nevertheless, the present results indicate that extinction learning is at least altered in OC women as compared to men and LU women.
In addition, the present study suggests that the interpretation of previous conditioning experiments including women without specifying OC usage or the time of measurement in the menstrual cycle might be compromised. Differing sex hormone levels in women could explain divergent results reported in the literature. Future conditioning studies should consider sex hormones as a relevant source of variation. It would be interesting to investigate women in the early follicular phase. They have low E2 and P4 levels comparable to OC women, but resulting from endogenous release instead of exogenous treatment. With this approach, direct effects of OC intake could be tested (cf. Kuhlmann and Wolf, 2005).
LU women have high E2 concentrations, whereas men and OC women have comparable low E2 levels. If sex hormones, in particular E2, were involved in the modulation of fear extinction, men and OC women should have displayed comparable CS+/CS− differentiations. At first sight, this seems not to be the case, because men and LU women showed the same neuronal activation pattern. Yet, high T concentrations in men might intervene either directly or indirectly after aromatization into E2 leading to higher E2 availability in men (cf. Milad et al., 2010). In conditioned taste aversion, it has been observed that female rats extinguish CRs more rapidly than male rats, with an application of E2 accelerating extinction (Chambers, 1976; Yuan and Chambers, 1999a, 1999b). Our present results are also in line with studies associating high E2 levels with facilitated extinction (Markus and Zecevic, 1997; Milad et al., 2006, 2009, 2010; Chang et al., 2009) and OC treatment in rats with anxiety-like behavior (Follesa et al., 2002). Besides fear extinction, E2 is related to the modulation of learning and memory processes in general (Korol, 2004). Our findings appear to suggest that the current sex hormone status has a stronger influence on emotional learning processes than biological sex (cf. Andreano and Cahill, 2010).
Long-term effects of sex hormones on neuronal morphology and physiology during development are termed organizational effects. In contrast, activational effects refer to the impact of current availability of sex hormones on morphological and physiological changes throughout the whole lifespan (McCarthy and Konkle, 2005; Gillies and McArthur, 2010). In this line, the present study proposes that sex hormones exert their influence on fear extinction rather via activational and not via organizational actions; otherwise, LU and OC women should have displayed the same response pattern. Experiments using E2 and P4 administration or blockade of their receptors (e.g. Bowman et al., 2002; Milad et al., 2009; Walf and Frye, 2009) might be able to disentangle activational from organizational effects.
In the contrast CS+ minus CS− in the instructed fear acquisition, we observed significant activations in all preselected ROI, i.e. in the amygdala, the ACC, the hippocampus, the insula, the OFC and the thalamus. This supports the assumption that the fear conditioning protocol was successful in eliciting CRs in fear-related structures, which is also mirrored in conditioned SCRs in the FIR and SIR. Importantly, prior instruction about the CS–UCS contingencies probably leads to a measure of fear expression (rather than genuine fear learning) in the acquisition phase when comparing CS+ and CS−. A more detailed discussion of the results of the acquisition phase is provided in the Supplementary Data.
Extinction learning was not modulated by cortisol, which is in contrast to several studies in rodents (e.g. Yang et al., 2006, 2007) and humans (Jackson et al., 2006; Zorawski et al., 2006; see Akirav and Maroun, 2007 for a review). Some of these reports used stress induction also leading to an activation of the autonomous nervous system besides a cortisol release. Further, the specific dose of cortisol (30 mg) can be responsible for the divergent results. An inverted U-shaped curve as well as linear associations have been proposed for cortisol effects to occur (de Kloet et al., 1999; Lupien et al., 2007; Sandi and Pinelo-Nava, 2007). Hence, we cannot exclude that different results would have been obtained with a lower dosage of hydrocortisone or with an induction of psychosocial stress. In this line, lowered cortisol levels, that have been proposed in PTSD (de Kloet et al., 2006; Yehuda, 2009), might also be interesting. Differences in response to cortisol increases in women compared to men should also be considered (cf. Paris et al., 2010). Moreover, cortisol exerts its influence on learning and memory processes via rapid non-genomic effects as well as via a slower genomic pathway (Joëls et al., 2006). Thereby, a gradual shift between these two effects has been suggested (de Kloet et al., 2005). In our design, the acquisition phase took place between 45 and 65 min and the extinction phase between 70 and 81 min after the intake of the tablets. So, a clear distinction between non-genomic and genomic effects of cortisol cannot be made (cf. Henckens et al., 2010).
The exact conditioning protocol might also be responsible for the discrepant findings. A recent study in learned aware OC women with the identical experimental design revealed that placebo compared to cortisol was associated with higher differential neuronal responses during extinction learning (Tabbert et al., 2010). One possible reason for these discrepancies is that in the present sample, we investigated instructed fear learning. So, differences in contingency awareness during fear acquisition (cf. Tabbert et al., 2011) might explain the lacking cortisol effects in the present study. One could speculate that the type of fear learning (instructed vs learned) alters cortisol effects on subsequent extinction learning. Presumably, learned aware participants needed more time to detect the correct CS–UCS contingencies during fear acquisition and extinction. But extinction learning seems to occur faster with a prior instruction about the relationship between CS and UCS in fear acquisition. Potentially, cortisol cannot influence this rapid process or its impact is detectable in the first trials only. Speculatively, contingency awareness seems to be relevant not only for fear acquisition (Klucken et al., 2009; Tabbert et al., 2011), but also for fear extinction and its modulation by cortisol. This clearly has to be tested in future studies.
As a limitation of sex hormone status effects, several methodologies suggest that prefrontal functioning, e.g. attention, is altered at particular stages of the menstrual cycle (e.g. McCourt et al., 1997; Solis-Ortiz et al., 2004; Holländer et al., 2005; Solis-Ortiz and Corsi-Cabrera, 2008). Further, conscious and, more importantly, subconscious attention levels might influence fear conditioning processes. Since we did not measure objective attention or other dimensions of prefrontal activation, it remains unclear if these confounding variables might have influenced the present results.
In conclusion, we observed that sex hormone status modulates fear extinction learning in several brain structures. In contrast, instructed fear acquisition was comparable between the groups. Therefore, a carry-over effect of different fear acquisition prior to extinction can be excluded. No interaction of cortisol and sex hormone status occurred in fear acquisition and extinction. During extinction, OC women exhibited higher CS+/CS− differentiation in ACC, amygdala, thalamus and vmPFC compared to men and LU women. Moreover, differential SCRs were positively correlated with neuronal activation in these fear-related brain regions, but only in OC women. Descriptively, different time courses of the activation of the fear network could also be observed in the sex hormone status groups. Taken together, it is suggested that extinction learning is altered during times of low sex hormone availability. However, direct effects of exogenous sex steroids in OC cannot be excluded. Of note, biological sex is not a major factor explaining the variance in our paradigm, but rather the current presence or absence of sex hormones. These results should be taken into account in future conditioning studies. Moreover, the altered fear extinction of OC women might be relevant for the treatment of patients with anxiety disorders (Anderson and Insel, 2006).
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Dr Dr Jürgen Hennig, Dr Yvonne Küpper and Cornelia Meineke (Justus Liebig University Giessen) for assistance with the hormone analyses as well as Dr Carlo Blecker (Bender Institute of Neuroimaging) for technical assistance and Dr Bertram Walter (Bender Institute of Neuroimaging) for statistical support. Further, we thank Lisa Bulganin, Kristina Haase, Klio Hilber, Adriane Icenhour, Agnes Kroczek and Lisa Koob for subject recruitment and data collection. Finally, we would like to thank the three anonymous reviewers for their helpful comments. This research was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation: STA 475/7-1 to R.S., WO 733/8-1 to O.T.W.).
REFERENCES
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007 doi: 10.1155/2007/30873. doi:10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biological Psychiatry. 2006;60:319–21. doi: 10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53:1286–93. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory. 2009;91:323–32. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, de Quervain DJ-F, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. Journal of Anxiety Disorders. 2010;24:223–30. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour research and therapy. 2007;45:2019–33. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–10. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:1044–8. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Buffet NC, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Frontiers in Neuroendocrinology. 1998;19:151–86. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chambers KC. Hormonal influences on sexual dimorphism in rate of extinction of a conditioned taste-aversion in rats. Journal of Comparative and Physiological Psychology. 1976;90:851–6. doi: 10.1037/h0077270. [DOI] [PubMed] [Google Scholar]
- Chang Y-J, Yang C-H, Liang Y-C, Yeh C-M, Huang C-C, Hsu K-S. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19:1142–50. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiology & Behavior. 2009;97:229–38. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HGM. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research. 2006;40:550–67. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–6. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biological Psychology. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences. 2011;108:6621–25. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Porcu P, Sogliano C, Cinus M, Biggio F, Mancuso L, et al. Changes in GABA(A) receptor gamma2 subunit gene expression induced by long-term administration of oral contraceptives in rats. Neuropharmacology. 2002;42:325–36. doi: 10.1016/s0028-3908(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological Reviews. 2010;62:155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in gulf war veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, Fernandez G. Time-dependent effects of corticosteroids on human amygdala processing. The Journal of Neuroscience. 2010;30:12725–32. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländer A, Hausmann M, Hamm JP, Corballis MC. Sex hormonal modulation of hemispheric asymmetries in the attentional blink. Journal of the International Neuropsychological Society. 2005;11:263–72. doi: 10.1017/S1355617705050319. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. The Journal of Neuroscience. 2006;26:5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–22. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends in Cognitive Sciences. 2006;10:152–8. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Holt B, Petrovic P, Martino B, de Klöppel S, Büchel C, et al. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cerebral Cortex. 2009;19:187–96. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klucken T, Tabbert K, Schweckendiek J, Merz CJ, Kagerer S, Vaitl D, et al. Contingency learning in human fear conditioning involves the ventral striatum. Human Brain Mapping. 2009;30:3636–44. doi: 10.1002/hbm.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of Neuroscience. 2004a;24:218–28. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective & Behavioral Neuroscience. 2004b;4:317–25. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of learning and memory. 2004;82:309–23. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: influence of menstrual cycle and oral contraceptives. Psychopharmacology. 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu FS, Tu MT, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain and cognition. 2007;65:209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Zecevic M. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. 1997;25:246–52. [Google Scholar]
- McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Frontiers in neuroendocrinology. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Mark VW, Radonovich KJ, Willison SK, Freeman P. The effects of gender, menstrual phase and practice on the perceived location of the midsagittal plane. Neuropsychologia. 1997;35:717–24. doi: 10.1016/s0028-3932(96)00115-7. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal homones influence conditioned fear extinction. Neuroscience. 2009;164:887–95. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders - It’s not what you thought it was. American psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–80. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of learning and memory. 2006;85:213–8. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–84. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of abnormal psychology. 2000;109:290–8. [PubMed] [Google Scholar]
- Paris JJ, Franco C, Sodano R, Freidenberg B, Gordis E, Anderson DA, et al. Sex differences in salivary cortisol in response to acute stressors among healthy participants, in recreational or pathological gamblers, and in those with posttraumatic stress disorder. Hormones and Behavior. 2010;57:35–45. doi: 10.1016/j.yhbeh.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47:512–9. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrums. 2004;9:258–66. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Prokasy WF, Ebel HC. Three components of the classically conditioned GSR in human subjects. Journal of Experimental Psychology. 1967;73:247–56. [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annual Review of Neuroscience. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. New York: Oxford University Press; 1999. [Google Scholar]
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 231–44. [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007 doi: 10.1155/2007/78970. doi:10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: quantity and quality. Neuroscience and Biobehavioral Reviews. 2010;34:584–91. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Ortiz S, Corsi-Cabrera M. Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology. 2008;33:989–98. doi: 10.1016/j.psyneuen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Solis-Ortiz S, Guevara MA, Corsi-Cabrera M. Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: an electroencephalographic study. Psychoneuroendocrinology. 2004;29:1047–57. doi: 10.1016/j.psyneuen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiology & Behavior. 2009;97:250–8. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:1–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, et al. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32:1290–8. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, et al. Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiology of Learning and Memory. 2010;94:392–401. doi: 10.1016/j.nlm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, et al. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Social Cognitive and Affective Neuroscience. 2011;6:495–506. doi: 10.1093/scan/nsq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. International Journal of Psychophysiology. 2005;57:15–23. doi: 10.1016/j.ijpsycho.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage. 2006;32:761–70. doi: 10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Hormones and Behavior. 2006;50:539–49. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Hest A, Heinsbroek RPW. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neuroscience and Biobehavioral Reviews. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiology & Behavior. 2009;99:169–74. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B. MARINA: an easy to use tool for the creation of masks for region of interest analyses. Proceedings of the 9th International Conference on Functional Mapping of the Human Brain. 2002 available on CD-Rom in NeuroImage, 19(2) [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Research. 2009;1293:142–54. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Yang Y-L, Chao P-K, Lu K-T. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropharmacology. 2006;31:912–24. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yang Y-L, Chao P-K, Ro L-S, Wo Y-YP, Lu K-T. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropharmacology. 2007;32:1042–51. doi: 10.1038/sj.npp.1301215. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Hormones and Behavior. 1999a;36:1–16. doi: 10.1006/hbeh.1999.1520. [DOI] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. Estradiol accelerates extinction of lithium chloride-induced conditioned taste aversions through its illness-associated properties. Hormones and Behavior. 1999b;36:287–98. doi: 10.1006/hbeh.1999.1551. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Blanding NQ, Kuhn CM, LaBar KS. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learning & Memory. 2006;13:441–50. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.