Abstract
Many cystic fibrosis transmembrane conductance regulator (CFTR)-expressing epithelia secrete bicarbonate (HCO3−)-containing fluids. Recent evidence suggests that defects in epithelial bicarbonate secretion are directly involved in the pathogenesis of cystic fibrosis, in particular by building up hyperviscous mucus in the ductal structures of the lung and pancreas. Pancreatic juice is one of the representative fluids that contain a very high concentration of bicarbonate among bodily fluids that are secreted from CFTR-expressing epithelia. We introduce up-to-date knowledge on the basic principles of transepithelial bicarbonate transport by showing the mechanisms involved in pancreatic bicarbonate secretion. The model of pancreatic bicarbonate secretion described herein may also apply to other exocrine epithelia. As a central regulator of bicarbonate transport at the apical membrane, CFTR plays an essential role in both direct and indirect bicarbonate secretion. The major role of CFTR in bicarbonate secretion would be variable depending on the tissue and cell type. For example, in epithelial cells that produce a low concentration of bicarbonate-containing fluid (up to 80 mm), either CFTR-dependent Cl−/HCO3− exchange or CFTR anion channel with low bicarbonate permeability would be sufficient to generate such fluid. However, in cells that secrete high-bicarbonate-containing fluids, a highly selective CFTR bicarbonate channel activity is required. Therefore, understanding the molecular mechanism of transepithelial bicarbonate transport and the role of CFTR in each specific epithelium will provide therapeutic strategies to recover from epithelial defects induced by hyposecretion of bicarbonate in cystic fibrosis.
Bicarbonate secretion is coordinated by ion transporters (e.g., CFTR). This process can be effectively studied in pancreatic duct epithelial cells, because pancreatic juice contains a very high concentration of bicarbonate.
Epithelial cells in respiratory, gastrointestinal, and genitourinary systems secrete bicarbonate (HCO3−)-containing fluids, which include saliva, pancreatic juice, intestinal fluids, airway surface fluid, and fluids secreted by reproductive organs. Bicarbonate is an essential ingredient in these fluids and plays critical roles. For example, bicarbonate is the biological pH buffer that guards against toxic intracellular and extracellular fluctuations in pH (Roos and Boron 1981). Bicarbonate in pancreatic juice and duodenal fluids neutralizes gastric acid and provides an optimal pH environment for digestive enzymes to function properly in the duodenum (Lee and Muallem 2008). In addition, as a moderate chaotropic ion, bicarbonate facilitates the solubilization of macromolecules such as mucins (Hatefi and Hanstein 1969). Recent studies suggest that the abnormal bicarbonate secretion observed in cystic fibrosis (CF) leads to altered mucin hydration and solubilization (Quinton 2010), resulting in hyperviscous mucus that blocks ductal structures of the lung and pancreas (Quinton 2001, 2008).
Pancreatic juice is one of the representative fluids that contain a very high concentration of bicarbonate among bodily fluids secreted from exocrine epithelia. At pH 7.4 and 5% CO2, the bicarbonate equilibrium concentration is ∼25 mm according to the Henderson–Hasselbalch equation. In humans and several other species, such as dogs, cats, pigs, and guinea pigs, the pancreas is capable of generating ∼140 mm HCO3−-containing pancreatic fluid upon stimulation, which is at least a fivefold higher concentration than the plasma. (Domschke et al. 1977; Lee and Muallem 2008). Therefore, pancreatic bicarbonate secretion has attracted attention as a typical model to gain insight into the bicarbonate transport mechanism in diverse epithelial cells. How exocrine glands secrete copious amounts of fluid and bicarbonate has long been a puzzle. The discovery of acidic pancreatic juice from patients with CF was an important advance in understanding the physiological mechanisms of pancreatic bicarbonate secretion (Johansen et al. 1968). In addition, significant progress has been made during the last 25 years with the identification of the molecular nature of many epithelial ion transporters and channels including the cystic fibrosis transmembrane conductance regulator (CFTR), which is mutated in patients with CF (Kerem et al. 1989). We introduce the basic principles of transepithelial bicarbonate transport, in particular in pancreatic duct cells, and the role of CFTR in this process. Additional information on pancreatic bicarbonate secretion can be found in Lee and Muallem (2008) and Lee et al. (2012).
TRANSPORTERS INVOLVED IN TRANSEPITHELIAL BICARBONATE TRANSPORT
Overview
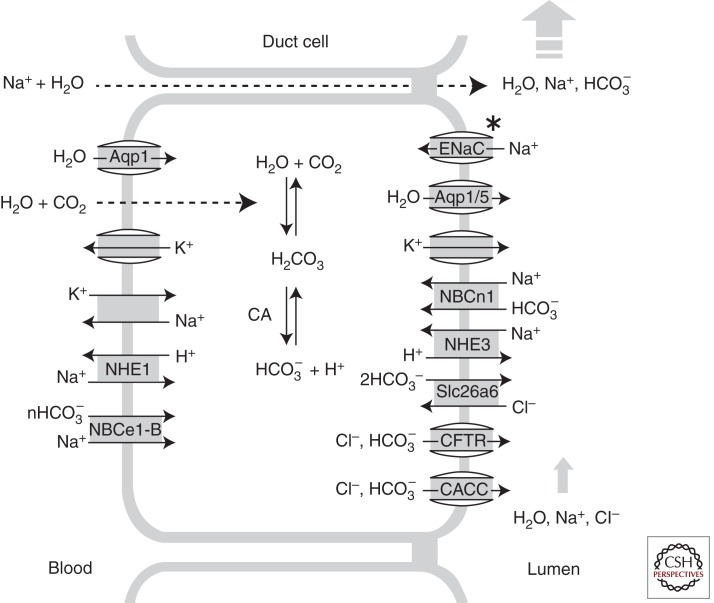
Transepithelial bicarbonate secretion is mediated by a coordinated function of transporters in epithelial cells, whereby transporters in the basolateral membrane absorb bicarbonate from the blood and those in the apical membrane secrete bicarbonate to the luminal space of hollow viscus or exocrine ducts. Recent progress in molecular and physiological techniques revealed the molecular identity and function of epithelial ion transporters at the basolateral and apical membranes (Lee et al. 2012). Major discoveries include the identification of anion channels and transporters in the apical membrane, such as CFTR and Cl−/HCO3− exchangers belonging to the SLC26 family (Ko et al. 2004; Dorwart et al. 2008), in combination with the basolateral bicarbonate uptake mechanisms, such as the Na+-HCO3− cotransporter (NBC) (Zhao et al. 1994; Lee et al. 2000). This article describes major ion transporters expressed at the basolateral and apical membranes in bicarbonate-secreting epithelial cells and their roles in transepithelial bicarbonate secretion. The basic characteristics of basolateral and apical transporters are illustrated in Figure 1.
Figure 1.
Ion transporters involved in transepithelial bicarbonate transport. Major transporters in the basolateral and luminal membranes of bicarbonate-secreting epithelial cells are illustrated. Bicarbonate uptake through the basolateral membrane is achieved by the NBCe1-B and the combinatorial function of NHE1 and CAs. Apical HCO3− secretion is mostly mediated by the CFTR anion channel and the Cl−/HCO3− exchanger Slc26a6 in pancreatic duct cells. Some epithelial cells also express (1) bicarbonate-reabsorbing mechanisms such as NHE3 and NBCn1-A; (2) ENaC, which mediates electrogenic Na+ absorption; and (3) K+ channels that secrete K+ to the luminal fluids in the apical membrane. Overall fluid secretion is driven by HCO3− secretion, and Na+ and water follow via a paracellular route. Water can also travel through a transcellular route via aquaporins.
Transporters in the Basolateral Membrane
Na+/K+ ATPase Pump
The Na+/K+ ATPase pump is expressed in the basolateral membrane of the epithelial cells that actively secrete fluid and electrolytes such as pancreatic duct cells. The Na+/K+ ATPase exchanges three Nai+ for two Ko+ with energy generated by ATP hydrolysis (Morth et al. 2011). The Na+/K+ ATPase pump in conjunction with the basolateral K+ channels converts the chemical energy of ATP into osmotic energy in the form of the Na+ and K+ gradients and into electrical energy of a negative membrane potential. These osmotic and electrical energies fuel the fluid and electrolyte transport in epithelial monolayers. The Na+ gradient is used for cytosolic bicarbonate accumulation by Na+-HCO3− cotransporters (NBCs) and Na+/H+ exchangers (NHEs) in the basolateral membrane, and the negative membrane potential facilitates bicarbonate efflux via the electrogenic anion channels and transporters in the apical membrane.
Na+/H+ Exchanger (NHE), V-Type H+ Pump, and H+/K+ ATPase Pump
Transepithelial bicarbonate secretion requires bicarbonate entry through the basolateral membrane to maintain adequate bicarbonate concentration in the cytoplasm. This can be achieved by the function of H+ extrusion mechanisms in the basolateral membrane in conjunction with cytosolic carbonic anhydrases (CAs) that eventually produce bicarbonate from membrane-diffused CO2 and water molecules (Fig. 1). The NHEs are electroneutral 1 Na+/1 H+ exchangers and exchange Nao+ for Hi+ in physiological ion gradients. The mammalian NHE gene family (SLC9A) is composed of three gene clusters: (1) five plasma membrane-type Na+-selective NHEs (NHE1–NHE5); (2) four organellar cation nonselective NHEs (NHE6–NHE9); and (3) two distantly related NHE-like genes, termed Na+/H+ antiporter 1 (NHA1) and NHA2 (Lee et al. 2012). The ubiquitous housekeeping NHE1 is essential for pHi homeostasis, and it is localized at the basolateral membrane in epithelial cells. Bicarbonate secretion through apical transporters will decrease the bicarbonate concentrations and induce intracellular acidification. In acidic pHi, NHE1 is activated, and it extrudes Hi+ in exchange for Nao+. This, in turn, facilitates the production and accumulation of bicarbonate inside epithelial cells. Similarly, H+ extrusion through a vesicular V-type H+-ATPase pump or H+/K+ ATPase pumps at the basolateral membrane can accumulate bicarbonate in epithelial cells. It has been shown that the V-type H+-ATPase pump is expressed in the basolateral membrane of pig pancreatic duct cells (Villanger et al. 1995) and that the gastric and nongastric types of H+/K+ ATPase pumps are expressed in the basolateral membrane of rat pancreatic duct cells (Novak et al. 2011).
However, the ability of these basolateral H+ extrusion mechanisms to accumulate bicarbonate seems to be limited and does not fully meet the required bicarbonate uptake through the basolateral membrane in cells that secrete high bicarbonate-containing fluids, such as human and guinea pig pancreatic duct cells. For example, NHE1 is activated below a pHi of 7.0, indicating that it can retain intracellular bicarbonate concentrations only ∼10 mm at 5% CO2. In this case, the maximum bicarbonate concentration in pancreatic juice would be only 100 mm even when the hypothetical bicarbonate channel in the apical membrane is maximally activated (membrane potential at −60 mV). Several mathematical models and experimental data suggest that pancreatic duct cells maintain a 20 mm intracellular bicarbonate concentration (pHi 7.3) that is suitable to generate >140 mm bicarbonate-containing fluid (Ishiguro et al. 1996a; Sohma et al. 2000; Whitcomb and Ermentrout 2004). In addition, inhibitors of NHE1 and the V-type H+-ATPase pump produced no or variable effects in reducing pancreatic bicarbonate secretion (Lee and Muallem 2008). Therefore, a more direct bicarbonate uptake mechanism is required in the basolateral membrane of pancreatic duct cells.
Na+-HCO3− Cotransporter (NBC)
Evidence suggests that NBC activity at the basolateral membrane is the major route for the basolateral bicarbonate uptake in the pancreatic duct cells (Ishiguro et al. 1996a). The basolateral NBC isoform was cloned from the pancreas and named pNBC1 (Abuladze et al. 1998). Subsequently, pNBC1 was renamed NBCe1-B, which is an electrogenic transporter with 1 Na+:2 HCO3− stoichiometry in pancreatic duct cells. The stoichiometry of electrogenic NBC1 (NBCe1) is an important parameter that determines the direction of bicarbonate movement. The stoichiometry of NBCe1 appears to be dependent on the cell type and PKA-dependent phosphorylation status (Gross et al. 2003). For example, the kidney type NBC1 (kNBC1, NBCe1-A), another variant transcribed from the same gene, seems to have 1 Na+:3 HCO3− stoichiometry in the basolateral membrane of proximal tubule, where it mediates transepithelial NaHCO3 absorption (hence, outward movement of bicarbonate at the basolateral membrane). In this case, the electrorepulsive force of 3 HCO3− overcomes the inward movement of 1 Na+ molecule. The pancreatic electrogenic NBCe1-B uses the Na+ gradient more efficiently to accumulate cytosolic bicarbonate than the electroneutral NHE1, because NBCe1-B transports two bicarbonate molecules into the cells using the electrochemical energy of one Na+ molecule. In fact, it has been shown in guinea pig pancreatic duct cells, that NBCe1-B mediates the bulk of basolateral bicarbonate entry during stimulated secretion (Ishiguro et al. 1996a,b). Recent studies suggested that IRBIT (inositol-1,4,5-triphosphate [IP3] receptor-binding protein released with IP3) is an important regulator of NBCe1-B in pancreas (Shirakabe et al. 2006).
K+ Channel
The negative membrane potential generated by K+ channels in the basolateral membrane of epithelial cells provides the driving force for Cl− and bicarbonate to exit through the apical membrane, which is a key step that precedes fluid and electrolyte secretion in all secretory epithelial cells. In general, secretory epithelial cells express two important K+ channels in the basolateral membrane, a Ca2+- and voltage-activated K+ channel of a large conductance (Maruyama et al. 1983) and a time- and voltage-independent K+ channel of intermediate conductance (Hayashi et al. 1996; Nehrke et al. 2003). The molecular identity of the channels was subsequently determined as the MaxiK channels encoded by KCNMA1 (Nehrke et al. 2003; Romanenko et al. 2006) and the IK1 channels encoded by KCNN4 (Begenisich et al. 2004; Hayashi et al. 2004), respectively. In the pancreatic duct, MaxiK seems to be the major channel maintaining a negative membrane potential during stimulated bicarbonate secretion (Gray et al. 1990). However, MaxiK channels do not appear to contribute to resting membrane potential, possibly because they have a very low open probability during the unstimulated state. The IK1 channel is a potential basolateral K+ channel responsible for the resting K+ permeability (Novak and Greger 1988).
Na+/K+/2 Cl− Cotransporter (NKCC) and Cl−/HCO3− Exchanger (Anion Exchanger, AE)
Epithelial cells that secrete Cl−-rich fluids, such as acinar cells in the exocrine pancreas and salivary glands, express the Na+/K+/2 Cl− cotransporter NKCC1 in the basolateral membrane. Owing to the electroneutrality of its transport process, NKCC1 maintains intracellular Cl− concentrations above the electrochemical equilibrium. This high intracellular Cl− concentration together with the negative membrane potential generated by the basolateral K+ channel provides the driving force for fluid and electrolyte secretion to the luminal space when the apical Cl− channel is opened by physiological stimuli. However, as we discuss below, a high intracellular Cl− concentration is unfavorable to secrete a high concentration of bicarbonate via CFTR or Cl−/HCO3− exchangers at the apical membrane. Therefore, basolateral NKCC is absent in epithelial cells that produce fluids containing an extremely high concentration of bicarbonate, such as human and guinea pig pancreatic duct cells.
The Cl−/HCO3− exchanger AE2 is found in the basolateral membrane of almost all epithelial cells. AE2 is activated by alkaline pHi to extrude excessive cytosolic bases (Olsnes et al. 1986). In physiological ion gradients, the basolateral AE accumulates Cl− inside the cells and dissipates accumulated intracellular bicarbonate. Therefore, although basolateral AE2 is required for a housekeeping function of cells preventing overt intracellular alkalinization, its activation would inhibit apical bicarbonate secretion in epithelial cells. In fact, mathematical models suggest that inhibition of basolateral AE activity is required for the high bicarbonate secretion in pancreatic duct cells (Sohma et al. 1996, 2000; Whitcomb and Ermentrout 2004).
Transporters in the Apical Membrane
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
CFTR (ABCC7) belongs to the “C” branch of the ATP-binding cassette (ABC) transporter superfamily. Most ABC transporters function as membrane pumps, which transport their substrates against the electrochemical gradient using energy generated from ATP hydrolysis (Deeley et al. 2006). Unlike other ABC transporters, CFTR is an anion channel that permits diffusion of substrate ion molecules down to the preexisting electrochemical gradient. In expression cloning, CFTR functions as a cAMP-activated Cl− channel that has a small conductance (5–10 pS) and a linear current–voltage relationship (Tabcharani et al. 1991).
CFTR is expressed in the apical membrane of secretory epithelial cells. CFTR Cl− channels have a limited permeability to bicarbonate in typical physiologic conditions. At normal intracellular (>20 mm) and extracellular (>100 mm) Cl− concentrations, the PHCO3/PCl of CFTR is 0.2–0.5 (Poulsen et al. 1994; Linsdell et al. 1997; Shcheynikov et al. 2004). Importantly, CFTR bicarbonate permeability is dynamically regulated by intracellular Cl− concentration-sensitive kinases (Park et al. 2010). Recently, two related kinase families—with-no-lysine (WNK) kinases and sterile 20 (STE20)-like kinases—have emerged as osmotic sensors that modulate diverse ion transporters (Anselmo et al. 2006; Richardson and Alessi 2008). In general, osmotic stress such as a decrease in the intracellular Cl− concentration, [Cl−]i, activates WNK kinases, including WNK1, which subsequently phosphorylate and activate downstream STE20-like kinases, especially oxidative stress-responsive kinase 1 (OSR1) and STE20/SPS1-related proline/alanine-rich kinase (SPAK) (Richardson and Alessi 2008). In pancreatic duct cells, CFTR activation greatly reduces [Cl−]i, which triggers the activation of WNK1-SPAK/OSR1 kinase cascade. Activation of the WNK1-SPAK/OSR1 pathway results in a dramatic increase in CFTR bicarbonate permeability, making CFTR primarily a bicarbonate-selective channel (Park et al. 2010). A bicarbonate channel forms an electrodiffusive bicarbonate efflux pathway that is essential for the generation of pancreatic juice containing bicarbonate at concentrations exceeding 140 mm.
In addition to Cl− and bicarbonate channel activity, CFTR has been suggested to be a central regulator of fluid and electrolyte secretion in many epithelia by regulating other membrane transporters (Lee et al. 2012). CFTR exists in a macromolecular complex at the apical membrane of secretory epithelia. The carboxyl terminus of CFTR forms a PDZ ligand that binds to PDZ domain-containing scaffolds (Short et al. 1998; Wang et al. 1998). In addition, CFTR interacts with SNARE proteins, AKAPs, kinases, and phosphatases (Guggino 2004). In these complexes, CFTR directly or indirectly regulates the activity of several transporters. Functional interactions with CFTR were reported for epithelial Na+ channels (ENaCs), outwardly rectifying Cl− channels, Ca2+-activated Cl− channels, ROMK2 and KvLQT1 K+ channels, SLC26 transporters, NHE3, NBCn1-A (NBC3), and aquaporins (AQPs) (Kunzelmann 2001; Lee and Muallem 2008).
Cl−/HCO3− Exchanger (Anion Exchanger, AE)
Many exocrine glands are composed of two types of cells: the acinar cells initially secreting Cl−-rich fluids, and the duct cells modifying the ionic composition of the fluids. Pancreatic duct cells absorb luminal Cl− and secrete the bulk of bicarbonate and fluids. Although the CFTR anion conductive pathway could directly produce bicarbonate-containing fluids, thermodynamically, an apical Cl−/HCO3− exchanger can much more efficiently secrete bicarbonate into the lumen when the luminal space contains Cl−. In fact, it has been proposed that the Cl−/HCO3− exchanger in the apical membrane mediates bicarbonate secretion in cooperation with an apical Cl− channel in pancreatic ducts (Steward et al. 2005; Lee and Muallem 2008). In this case, apical Cl− channels facilitate bicarbonate secretion by recycling Cl− to the lumen, which maintains the luminal Cl− concentration for continuous apical Cl−/HCO3− exchange.
The first family of Cl−/HCO3− exchangers to be considered is members of the solute-linked carrier 4 (SLC4) family (Lee et al. 2012). The pancreatic duct cells express the SLC4 transporter AE2 (SLC4A2), but it is localized on the basolateral membrane (Roussa et al. 1999, 2001). The second type of Cl−/HCO3− exchangers comprise transporters belonging to the SLC26 transporter family, which consists of 10 members and transports diverse anions, such as chloride, bicarbonate, oxalate, and sulfate (Ohana et al. 2009). Among the members, SLC26A3, SLC26A4, and SLC26A6 function as Cl−/HCO3−/I− exchangers (Ko et al. 2002; Xie et al. 2002). Slc26a6 appears to be the major Cl−/HCO3− exchanger in the apical membrane of rat pancreatic duct cells and has electrogenic 1 Cl−/2 HCO3− exchange activity (Wang et al. 2006; Shcheynikov et al. 2008; Stewart et al. 2009). As discussed below, this electrogenicity of SLC26A6 further contributes to the outward transport of bicarbonate at physiological negative membrane potential. An important feature of the SLC26 transporters and CFTR is their mutual regulation. Thus, the STAS domain of SLC26 transporters interacts with the R domain of CFTR. In addition, the two transporters are connected by PDZ-based adaptors. These interactions are required for activation of both the SLC26 transporters and CFTR (Ko et al. 2004).
Ca2+-Activated Cl− Channel (CaCC)
CaCC activity is present in the apical membrane of almost all secretory epithelial cells (Gray et al. 1989, 1994; Zeng et al. 1997; Venglovecz et al. 2008). The biophysical property of the channel has been characterized to be a voltage- and Ca2+-activated, time-dependent outwardly rectifying channel (Melvin et al. 2005; Kunzelmann et al. 2009). Recently, members of the anoctamin (ANO; also known as TMEM16) family, in particular ANO1/TMEM16A and ANO2/TMEM16B, were shown to function as CaCCs in several epithelial and neuronal tissues (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008; Stephan et al. 2009; Romanenko et al. 2010). ANO1 is expressed at high levels in the apical membranes of salivary glands (Schroeder et al. 2008; Yang et al. 2008) and pancreatic acinar cells (Huang et al. 2009). However, the molecular identity of CaCC in the pancreatic duct cell is still unknown. The discovery of the ANO/TMEM16 family as the CaCC in several epithelial cells suggests that the ductal CaCC is likely a member of this family. Whether the other ANO/TMEM16 isoforms mediate CaCC activity in pancreatic duct cells awaits further studies (Lee et al. 2012). An interesting possibility is that CaCCs may replace the anion channel function of CFTR in bicarbonate secretion (Zsembery et al. 2000). The PHCO3/PCl of heterologously expressed ANO1 is 0.1–0.5, indicating that CaCC has a limited ability to produce bicarbonate secretion at typical physiological conditions. However, the PHCO3/PCl of ANO1 seems to be dynamically regulated by [Ca2+]i (MG Lee, unpubl.), suggesting that CaCC may play a role in epithelial bicarbonate secretion under certain specific conditions.
K+ Channel
Some secretory epithelial cells secrete K+ into the lumen. For example, the salivary gland duct cells absorb Na+ and secrete K+ into salivary fluid. Deletion of Kcnma1, which encodes MaxiK, impairs salivary K+ secretion, suggesting MaxiK to be the channel responsible for K+ efflux in salivary glands (Nakamoto et al. 2008). A recent study showed that MaxiK channels are also expressed at the apical membrane of pancreatic duct cells in guinea pigs (Venglovecz et al. 2011), which may contribute to the potentiation of secretin and CCK (or other Ca2+ agonists such as cholinergic) response in pancreatic secretion (Gray et al. 1990). However, the physiological role of the apical K+ channel in pancreatic duct cells is not fully understood at present, because unlike saliva, the major cation in pancreatic juice is Na+ and pancreatic duct cells do not secrete the bulk of K+.
Epithelial Na+ Channel (ENaC)
Some surface epithelial cells of respiratory and digestive tracts and duct cells of salivary glands express ENaC at the apical membrane, which mediates electrogenic Na+ uptake in these cells (Cook et al. 2002; Catalan et al. 2010). However, pancreatic duct cells do not express ENaC, because they do not absorb Na+. Simple absorption of Na+ without secreting K+ is unfavorable for bicarbonate and fluid secretion because it will evoke a lumen-negative transepithelial potential and net fluid absorption. In the lungs, CFTR is proposed to inhibit ENaC channel function, and deletion of CFTR increases ENaC activity and fluid absorption, which causes a contraction in airway surface fluids (Berdiev et al. 2009). However, in salivary glands, deletion of CFTR inhibited Na+ absorption by ENaC and greatly diminished the expression of α-ENaC (Catalan et al. 2010). Similarly, ENaC activity is markedly reduced in the sweat ducts of CF patients (Reddy et al. 1999). It is not known whether these tissue-specific regulations of ENaC by CFTR affect transepithelial bicarbonate secretion in ENaC-expressing epithelial cells.
NHE and NBC
Many Na+- and fluid-absorbing epithelial cells express NHE in the apical membrane, which mediates electroneutral Na+ uptake (Cook et al. 2002; Catalan et al. 2010). NHE2 and NHE3 are the major NHE isotypes expressed in the apical membrane of epithelial cells. NHE activity in the apical membrane will nullify bicarbonate secretion by donating protons to the lumen. Interestingly, NHEs are expressed in the apical membrane of cells in the distal parts of large-sized salivary and pancreatic ducts, and they may reabsorb bicarbonate when bicarbonate secretion is not needed in these cells (Marteau et al. 1995; Park et al. 1999; Lee et al. 2000; Luo et al. 2001; Bobulescu et al. 2005). At resting state or low flow rates, pancreatic juice is acidic and contains a high amount of CO2, indicating an active H+ secretion by the apical transporters (Gerolami et al. 1989; Marteau et al. 1993). This seems to be mainly mediated by NHE3 in the large-sized ducts (Ahn et al. 2001). The salivary and pancreatic ducts also express the electroneutral NBCn1-A (NBC3) in the apical membrane (Park et al. 2002), which absorbs Na+ and bicarbonate from luminal fluids. Similar to the case of NHE3, NBCn1-A appears to have a bicarbonate-salvaging function in the resting state (Park et al. 2002).
Other Factors
Aquaporin (AQP)
Transcellular ion secretion results in osmotic water flow. Although the membrane lipid bilayer and paracellular junctions are partially permeable to water, transepithelial water flow is facilitated by the water channel AQP. The AQP family consists of 13 members, all of which can function as water channels, but some can transport additional molecules such as glycerol, urea, ions, and CO2 (Verkman 2008). In epithelia, the AQPs show highly restricted and cell-specific expression patterns. For example, AQP1 is expressed in both the apical and basolateral domains (Burghardt et al. 2003), whereas AQP5 is expressed in the apical membrane of salivary gland and pancreatic duct cells (Delporte and Steinfeld 2006).
Carbonic Anydrase (CA)
CAs comprise a class of proteins essential for all bicarbonate-related transport functions. The global CA inhibitor acetazolamide significantly inhibits pancreatic fluid and bicarbonate secretion (Pak et al. 1966; Dyck et al. 1972). Further studies revealed that CAs are present at the bicarbonate transporting complex to facilitate bicarbonate transport via membrane transporters, such as NBCs and SLC26 transporters (McMurtrie et al. 2004).
PDZ-Based Adaptor
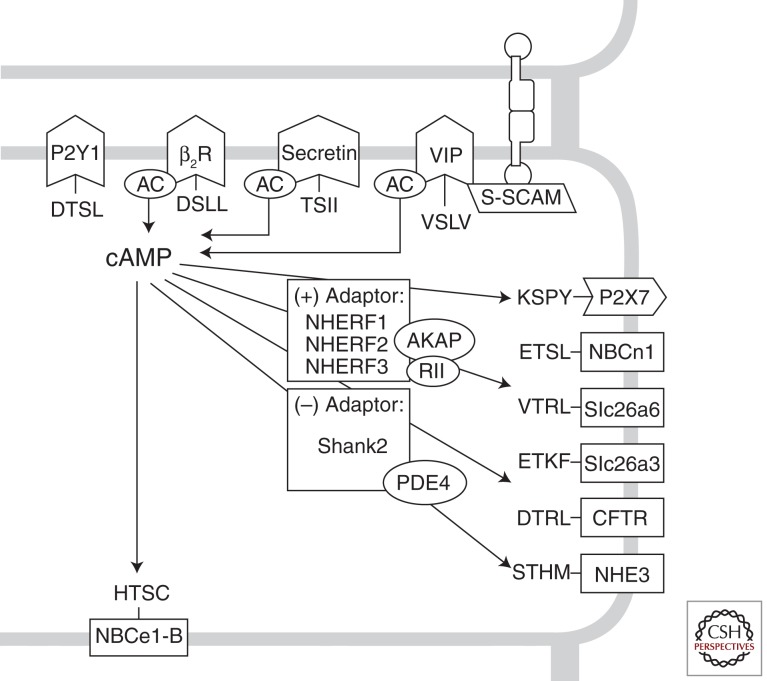
Transepithelial bicarbonate transport is achieved by the cooperative operation of several membrane proteins in the basolateral and apical membranes. A mechanism that enhances the efficiency of this close cooperation is protein complex formation by adaptor proteins containing PDZ domains (Fig. 2). The PDZ domain was identified as a conserved domain in three proteins: PSD-95, Discs-large, and ZO-1. The PDZ domain is a protein–protein interaction module consisting of 80–90 amino acids that typically binds to target proteins harboring specific carboxy-terminal sequences called PDZ-binding motifs. Although they were initially identified in neuronal tissues, subsequent studies revealed that PDZ-based adaptor proteins are also expressed in diverse epithelia and play critical roles in transepithelial fluid and electrolyte transport (Gee et al. 2009).
Figure 2.
PDZ-based protein–protein interaction in bicarbonate-transporting epithelia. Many membrane receptors and transporters participating in the bicarbonate homeostasis in epithelial cells have a PDZ-binding motif (-X-T/S-X-hydrophobic amino acid) on their carboxyl terminus. The carboxy-terminal sequences are based on the human clones. Excluding purinergic receptors, most of the proteins are associated with cAMP-dependent processes. AC, Adenylyl cyclase; AKAP, cAMP-dependent protein kinase-anchoring protein; P2, purinergic receptor; RII, regulatory subunit of protein kinase A type II. (Other abbreviations are the same as described in the text.)
The most well-known PDZ-based adaptors in epithelia are the Na+/H+ exchanger regulatory factor (NHERF) proteins. NHERF1 (EBP50), NHERF2 (E3KARP), and NHERF3 (CAP70, PDZK1) facilitate the PKA-dependent phosphorylation and membrane trafficking of CFTR and NHE3 in epithelial cells of respiratory and digestive systems, including pancreatic duct cells. These adaptors assemble a large protein complex in the apical membrane of secretory epithelia, where CFTR functions as a central regulator. For example, the mutual regulation of CFTR and SLC26 transporters is enhanced by protein–protein interactions through PDZ-based scaffolds (Ko et al. 2002). In addition, the apical NHE3 and NBCn1-A are associated with CFTR via PDZ-based adaptors, such as NHERF1 and NHERF2, and their activity is regulated by CFTR in the protein complex (Ahn et al. 2001; Park et al. 2002). Assembling a large protein complex greatly enhances signaling efficiency in confined regions of the apical membrane. Upon stimulation with cAMP, bicarbonate-secreting transporters, such as CFTR and apical Cl−/HCO3− exchangers, are activated, and at the same time, bicarbonate-absorbing transporters, such as NHE3 and NBCn1-A, are inhibited to efficiently mediate bicarbonate and fluid secretion (Lee et al. 2012).
In addition to the NHERFs, several other scaffolds with PDZ domains, such as Shank2, S-SCAM, SAP97, and PSD-95, are expressed in various epithelial cells and participate in the regulation of transepithelial fluid and ion transport (Kim et al. 2004; Gee et al. 2009). For example, Shank2 is expressed at the apical region of transporting epithelial cells and modulates the activity of CFTR and NHE3 (Kim et al. 2004; Han et al. 2006). In contrast with NHERF proteins that deliver cAMP/PKA signals, Shank2 mediates an inhibitory effect on the cAMP/PKA pathway. Shank2 associates with phosphodiesterase 4D, which hydrolyzes cAMP, hence lowering local cAMP concentrations in the apical microdomains (Lee et al. 2007). Therefore, the activity of CFTR is dynamically regulated by a competitive balance between CFTR-activating (NHERFs) and CFTR-inactivating (Shank2) PDZ-domain interactions (Lee et al. 2012).
Many G-protein-coupled receptors (GPCRs) in epithelial cells also have PDZ ligands at their carboxyl termini (Fig. 2). This recruits the GPCRs to the transporting complex, resulting in polarized GPCR expression and delivery of the second messengers to the specific intracellular microdomains. A good example for such an arrangement is the vasoactive intestinal polypeptide (VIP) receptor VPAC1, which binds to the PDZ-based scaffold S-SCAM (Gee et al. 2009). S-SCAM associates with E-cadherin, a key protein at the adherens junction, and recruits VPAC1 to the junctional area near the apical pole. Confined localization of VPAC1 at the junctional area generates a localized cAMP signal close to the apical effectors such as CFTR. This, in turn, enables efficient bicarbonate and fluid secretion in epithelial cells in response to VIP with minimal effects on the cell interior (Gee et al. 2009; Lee et al. 2012).
MODEL FOR PANCREATIC BICARBONATE SECRETION
Studies on the mechanism and regulation of epithelial bicarbonate secretion have been concentrated in the pancreatic ducts, because the pancreatic duct epithelial cells secrete copious amounts of bicarbonate. Argent and Case (1994) proposed the first model to explain pancreatic bicarbonate secretion. In this model, basolateral NHE1 in cooperation with cytosolic CAs provides a bicarbonate entry mechanism. Bicarbonate is then secreted by Cl−/HCO3− exchange at the apical membrane. During this process, the apical Cl−/HCO3− exchange absorbs the Cl−, which is then recycled by exiting through CFTR (Argent and Case 1994; Steward et al. 2005). Subsequently, this model was revised by several major findings. For example, the major basolateral bicarbonate influx mechanism was identified as NBCe1-B, which functions as a 1 Na+/2 HCO3− cotransporter (Zhao et al. 1994; Ishiguro et al. 1996a; Abuladze et al. 1998). In addition, studies in perfused guinea pig pancreatic ducts and mathematical modeling suggested that a bicarbonate channel activity is required to set the final bicarbonate concentration in the pancreatic juice to 140 mm (Sohma et al. 2000; Whitcomb and Ermentrout 2004).
The pancreatic duct absorbs Cl− and secretes bicarbonate to generate pancreatic juice containing ∼20 mm Cl− and 140 mm HCO3−. The loss of pancreatic bicarbonate secretion in patients with CF (Johansen et al. 1968) indicates that CFTR plays a critical role in bicarbonate secretion. Interestingly, the activity of the apical Cl−/HCO3− exchanger is dependent on the expression of CFTR (Lee et al. 1999a,b). These results strengthened the idea that Cl−/HCO3− exchange mediates pancreatic bicarbonate secretion. However, a limitation of this model has been realized by many people that a classical 1:1 electroneutral Cl−/HCO3− exchanger is able to secrete only a maximum of 80 mm HCO3− when intracellular concentrations of Cl− and bicarbonate are estimated to be equal. In comparison to these electroneutral transporters, electrogenic bicarbonate transporters are capable of secreting higher concentrations of bicarbonate when the electrorepulsive force generated by the negative membrane potential is coupled to the efflux of bicarbonate. Interestingly, recent studies have suggested that the apical Cl−/HCO3− exchangers may be electrogenic with distinct Cl−:HCO3− stoichiometry (Ko et al. 2004). In case of an electrogenic 1 Cl−/2 HCO3−, it can accumulate greater amounts of bicarbonate in pancreatic juice compared with an electroneutral exchanger (Steward et al. 2005). Nevertheless, this transporter cannot fully account for the bicarbonate-driven fluid secretion in pancreatic duct cells because a Cl−/HCO3− exchanger with 1:2 stoichiometry is still insufficient to attain 140 mm HCO3− in the lumen (Steward et al. 2005). More importantly, the driving force for bicarbonate secretion by the Cl−/HCO3− exchanger are greatly weakened when the luminal Cl− concentration decreases in the subsequent step of pancreatic secretion, because luminal Cl− must be required for the Cl−/HCO3− exchanger-mediated bicarbonate secretion. However, it has been shown that a significant fraction of pancreatic bicarbonate secretion is retained even in the absence of luminal Cl− (Ishiguro et al. 1998, 2009). This implies that an unknown mechanism is likely to be responsible for the ductal bicarbonate secretion, especially at the subsequent stage when the bicarbonate concentration mediated by Cl−/HCO3− exchange approaches equilibrium.
Bicarbonate channels are strong candidates for the transporter responsible for high-concentration bicarbonate secretion (Steward et al. 2005; Lee and Muallem 2008). Having a bicarbonate-selective channel in the apical membrane of a duct cell makes it theoretically possible to secrete up to 200 mm HCO3− if cells maintain a membrane potential of −60 mV. It has been previously suggested that CFTR functions as a bicarbonate channel in pancreatic duct cells under some specific conditions (O’Reilly et al. 2000; Reddy and Quinton 2003; Shcheynikov et al. 2004; Whitcomb and Ermentrout 2004; Ishiguro et al. 2009). However, the PHCO3/PCl of CFTR was reported to be ∼0.2–0.5 (O’Reilly et al. 2000; Sohma et al. 2000). With this permeability ratio, the CFTR anion channel would secrete Cl− much faster than it would secrete bicarbonate. Thus, CFTR is unable to secrete sufficient amounts of bicarbonate when cells retain a significant intracellular concentration of Cl−. In one of the previous models, the basolateral membrane was set to be absolutely impermeable to Cl− but allowed to uptake bicarbonate continuously via basolateral pNBC (Whitcomb and Ermentrout 2004). This caused an extreme reduction in [Cl−]i close to 0 mm by CFTR activation, which secretes Cl− to the lumen. Under these circumstances, the CFTR anion channel secretes isotonic bicarbonate, because of the absence of Cl− inside the cells. As exemplified in this model, [Cl−]i is a critical parameter that determines the anion composition in fluids secreted by epithelial cells, and a decrease in [Cl−]i can greatly increase bicarbonate secretion via apical anion channels and transporters. In fact, experimental data in guinea pig ducts and human pancreatic duct cells revealed that [Cl−]i in duct cells is ∼20 mm at the resting state, and this level is reduced when CFTR is stimulated with cAMP (Ishiguro et al. 2002; Park et al. 2010). However, the minimum [Cl−]i that can be induced by a maximum cAMP stimulation is 5 mm in a practical situation, perhaps because of a small Cl− leak via basolateral Cl−/HCO3− exchangers or Cl− channels. At this [Cl−]i, known anion transporters in the apical membrane are still unable to accumulate bicarbonate at concentrations exceeding 140 mm in pancreatic juice. At 20 mm [HCO3−]i and 5 mm [Cl−]i, the maximum bicarbonate equilibrium concentration in pancreatic juice that can be attained by the 1Cl−/2HCO3− exchanger is 128 mm (Steward et al. 2005), and that attained by the CFTR anion channel with a PHCO3/PCl of 0.2 is 100 mm (Whitcomb and Ermentrout 2004).
Recently, these limitations were complemented by a unique mode of CFTR regulation whereby the PHCO3/PCl of CFTR is dynamically modulated by the [Cl−]i-sensitive WNK1-SPAK/OSR1 kinase pathway (Park et al. 2010). In pancreatic duct cells, CFTR activation reduces [Cl−]i, which subsequently activates the WNK1-SPAK/OSR1 kinase cascade. Activation of the WNK1-SPAK/OSR1 pathway significantly increases the bicarbonate permeability of CFTR, making CFTR into a bicarbonate-selective channel with a PHCO3/PCl of ∼1.5. This CFTR-derived bicarbonate channel would form an electrodiffusive bicarbonate efflux pathway, which can effectively generate pancreatic juice containing >140 mm bicarbonate. Interestingly, SPAK and OSR1 activation simultaneously inhibits the CFTR-dependent Cl−/HCO3− exchange activity. It is conceivable that continuous activation of apical 1 Cl−/2 HCO3− exchange would actually absorb bicarbonate from the lumen by its reversed activity when the luminal bicarbonate concentration becomes >128 mm. Therefore, inhibition of the apical Cl−/HCO3− exchange is required to prevent the reverse mode of Cl−/HCO3− exchange activity and ultimately achieve high-bicarbonate-containing pancreatic juice. In fact, the requirement of inhibiting the apical Cl−/HCO3− exchange during stimulated secretion had been previously assumed by the mathematical models on high pancreatic bicarbonate secretion (Sohma et al. 2000; Steward et al. 2005).
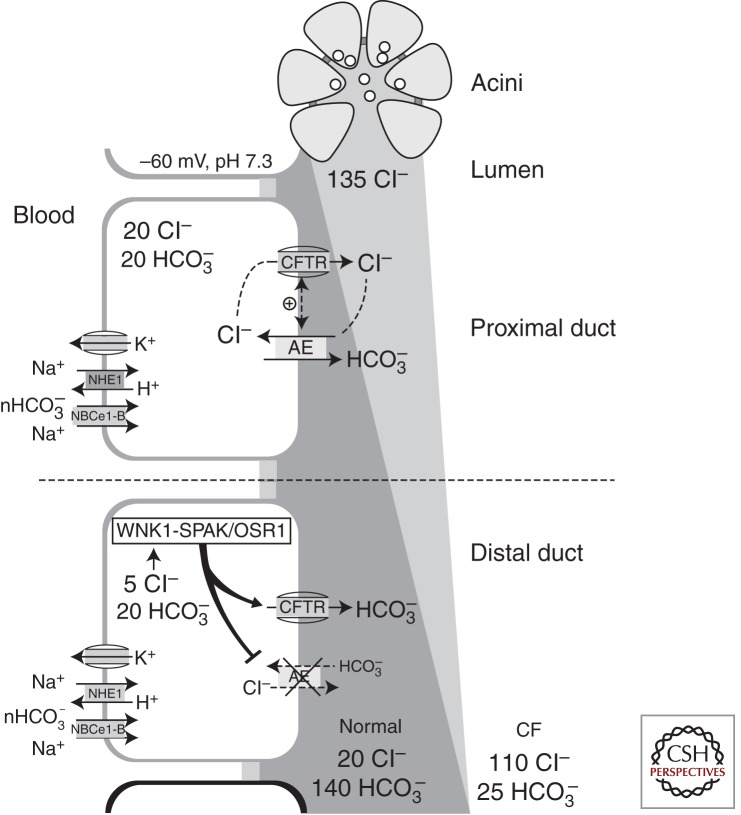
An integrated model for pancreatic bicarbonate secretion based on the aforementioned findings is illustrated in Figure 3. In response to a meal, secretin hormone and VIP released from the vagal nerve endings stimulate pancreatic duct cells and generate cAMP signals (Lee and Muallem 2008). This initially activates bicarbonate secretion mediated by CFTR-dependent Cl−/HCO3− exchange. The continuous increase in luminal bicarbonate and consequent reduction in luminal Cl− causes a decrease in [Cl−]i to close to an electrical equilibrium (10-fold lower than luminal [Cl−] at a membrane potential of −60 mV) via apical CFTR Cl− channel activity. The low [Cl−]i, in turn, activates WNK1 and the downstream STE20-like kinases, such as SPAK and OSR1. Activation of WNK1-SPAK/OSR1 exerts two critical effects on the apical ion-transporting proteins. First, activation of WNK1-SPAK/OSR1 generates an electrogenic pathway for bicarbonate secretion by increasing the bicarbonate permeability of CFTR, which is essential for the secretion of pancreatic juice containing bicarbonate at a concentration exceeding 140 mm. Second, WNK1-SPAK/OSR1 activation inhibits apical Cl−/HCO3− exchange activity that may reabsorb bicarbonate from the high-bicarbonate-containing pancreatic juice.
Figure 3.
A model of pancreatic fluid and bicarbonate secretion. In the proximal pancreatic duct, cAMP signals activate the CFTR-dependent Cl−/HCO3− exchange at the apical membrane, which enables the duct to absorb part of the Cl− and secrete as much as 80–100 mm HCO3− along with a large volume of fluid into the pancreatic juice. As the fluid arrives at the more distal portions of the duct, the reduced luminal Cl− and activated CFTR lower the intracellular Cl− concentration [Cl−]i to <10 mm. The low [Cl-]i activates WNK1, which phosphorylates SPAK/OSR1, which, in turn, acts on CFTR by converting it into a bicarbonate-selective channel. In contrast, the WNK1-SPAK/OSR1 pathway concurrently inhibits the function of apical Cl−/HCO3− exchange to prevent bicarbonate reabsorption.
CONCLUSION
The model in Figure 3 describes the mechanisms underlying fluid and bicarbonate secretion in the pancreatic duct, which may also apply to other exocrine epithelia. Mechanisms of basolateral bicarbonate uptake are now relatively well established. The H+ extrusion mechanisms including NHE1 expressed in the basolateral membrane of almost all epithelial cells contribute to the cellular accumulation of bicarbonate. In some cells specialized for bicarbonate secretion, such as pancreatic duct cells, NBC mediates the bulk of basolateral bicarbonate uptake. In contrast, the bicarbonate exit pathways in the apical membrane are diverse and depend on the specific condition of each type of epithelia. In epithelial cells that secrete a low concentration of bicarbonate-containing fluids (up to 80 mm), either Cl−/HCO3− exchange or CFTR anion channel with low bicarbonate permeability is sufficient to perform this task. Expression patterns of ion transporters at the apical membrane and the presence of Cl− in the lumen will determine the route of apical bicarbonate exit. In cells that secrete high-bicarbonate-containing fluids, a CFTR bicarbonate channel activity that is activated by low [Cl−]i and WNK1-SPAK/OSR1 kinases is required. It is important to mention that many pathological conditions in the respiratory tract and intestinal lumen can induce hypo- or hyperosmotic stress, which also activates the WNK1-SPAK/OSR1 kinases. Therefore, CFTR bicarbonate channel activity may play a role in epithelial defense against various noxious stimuli in these organs. For example, bicarbonate is an important ingredient to maintain appropriate viscosity and proper function of mucin molecules, which play a critical role in mucosal immunity and the epithelial defense system (Lee and Muallem 2008; Quinton 2008). The fact that CFTR functions not only as a Cl− channel but also a bicarbonate channel will greatly influence the arena of CFTR research and drug development toward CF patients. In addition, models of epithelial bicarbonate secretion will continue to progress as our knowledge on established pathways expands and novel mechanisms are further elucidated.
ACKNOWLEDGMENTS
We thank Dong-Su Jang for editorial assistance. M.G.L. is supported by grant 2012-0005644 from the National Research Foundation; the Ministry of Education, Science, and Technology, Korea; and grant A111218-11-PG03 from the National Project for Personalized Genomic Medicine, Korea Health 21 R&D Project, Ministry of Health & Welfare, Korea.
Footnotes
Editors: John R. Riordan, Richard C. Boucher, and Paul M. Quinton
Additional Perspectives on Cystic Fibrosis available at www.perspectivesinmedicine.org
REFERENCES
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I 1998. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273: 17689–17695 [DOI] [PubMed] [Google Scholar]
- Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG 2001. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3− salvage mechanisms in model systems and the mouse pancreatic duct. J Biol Chem 276: 17236–17243 [DOI] [PubMed] [Google Scholar]
- Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH 2006. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci 103: 10883–10888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent B, Case R 1994. Pancreatic ducts. Cellular mechanism and control of bicarbonate secretion. In Physiology of the gastrointestinal tract (ed. Johnson LR), pp. 1473–1497 Raven, New York [Google Scholar]
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE 2004. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem 279: 47681–47687 [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Qadri YJ, Benos DJ 2009. Assessment of the CFTR and ENaC association. Mol Biosyst 5: 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobulescu IA, Di Sole F, Moe OW 2005. Na+/H+ exchangers: Physiology and link to hypertension and organ ischemia. Curr Opin Nephrol Hypertens 14: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S 2003. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut 52: 1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ 2008. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594 [DOI] [PubMed] [Google Scholar]
- Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE 2010. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol 588: 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DI, Dinudom A, Komwatana P, Kumar S, Young JA 2002. Patch-clamp studies on epithelial sodium channels in salivary duct cells. Cell Biochem Biophys 36: 105–113 [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP 2006. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86: 849–899 [DOI] [PubMed] [Google Scholar]
- Delporte C, Steinfeld S 2006. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta 1758: 1061–1070 [DOI] [PubMed] [Google Scholar]
- Domschke S, Domschke W, Rosch W, Konturek SJ, Sprugel W, Mitznegg P, Wunsch E, Demling L 1977. Inhibition by somatostatin of secretin-stimulated pancreatic secretion in man: A study with pure pancreatic juice. Scand J Gastroenterol 12: 59–63 [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S 2008. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 23: 104–114 [DOI] [PubMed] [Google Scholar]
- Dyck WP, Hightower NC, Janowitz HD 1972. Effect of acetazolamide on human pancreatic secretion. Gastroenterology 62: 547–552 [PubMed] [Google Scholar]
- Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, Kim KS, Kim H, Baba A, Yang J, et al. 2009. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology 137: 607–617 [DOI] [PubMed] [Google Scholar]
- Gerolami A, Marteau C, Matteo A, Sahel J, Portugal H, Pauli AM, Pastor J, Sarles H 1989. Calcium carbonate saturation in human pancreatic juice: Possible role of ductal H+ secretion. Gastroenterology 96: 881–884 [PubMed] [Google Scholar]
- Gray MA, Harris A, Coleman L, Greenwell JR, Argent BE 1989. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol 257: C240–C251 [DOI] [PubMed] [Google Scholar]
- Gray MA, Greenwell JR, Garton AJ, Argent BE 1990. Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J Membr Biol 115: 203–215 [DOI] [PubMed] [Google Scholar]
- Gray MA, Winpenny JP, Porteous DJ, Dorin JR, Argent BE 1994. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol 266: C213–C221 [DOI] [PubMed] [Google Scholar]
- Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I 2003. Phosphorylation-induced modulation of pNBC1 function: Distinct roles for the amino- and carboxy-termini. J Physiol 549: 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino WB 2004. The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins. Proc Am Thorac Soc 1: 28–32 [DOI] [PubMed] [Google Scholar]
- Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, Moe OW, Lee J, Kim E, Lee MG 2006. Shank2 associates with and regulates Na+/H+ exchanger 3. J Biol Chem 281: 1461–1469 [DOI] [PubMed] [Google Scholar]
- Hatefi Y, Hanstein WG 1969. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci 62: 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Young JA, Cook DI 1996. The Ach-evoked Ca2+-activated K+ current in mouse mandibular secretory cells. Single channel studies. J Membr Biol 151: 19–27 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kunii C, Takahata T, Ishikawa T 2004. ATP-dependent regulation of SK4/IK1-like currents in rat submandibular acinar cells: Possible role of cAMP-dependent protein kinase. Am J Physiol Cell Physiol 286: C635–C646 [DOI] [PubMed] [Google Scholar]
- Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY 2009. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci 106: 21413–21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay AR, Case RM 1996a. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Wilson RW, Case RM 1996b. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol 495: 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM 1998. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol 511: 407–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Kitagawa M, Mabuchi T, Kondo T, Hayakawa T, Case RM, Steward MC 2002. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol 539: 175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A 2009. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 133: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen PG, Anderson CM, Hadorn B 1968. Cystic fibrosis of the pancreas. A generalised disturbance of water and electrolyte movement in exocrine tissues. Lancet 1: 455–460 [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC 1989. Identification of the cystic fibrosis gene: Genetic analysis. Science 245: 1073–1080 [DOI] [PubMed] [Google Scholar]
- Kim JY, Han W, Namkung W, Lee JH, Kim KH, Shin H, Kim E, Lee MG 2004. Inhibitory regulation of cystic fibrosis transmembrane conductance regulator anion-transporting activities by Shank2. J Biol Chem 279: 10389–10396 [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, et al. 2002. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J 21: 5662–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al. 2004. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K 2001. CFTR: Interacting with everything? News Physiol Sci 16: 167–170 [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R 2009. Bestrophin and TMEM16-Ca2+ activated Cl− channels with different functions. Cell Calcium 46: 233–241 [DOI] [PubMed] [Google Scholar]
- Lee MG, Muallem S 2008. Physiology of duct cell secretion. In Pancreas: An integrated textbook of basic science, medicine, and surgery (ed. Beger H, et al. ), pp. 78–90 Blackwell, Oxford [Google Scholar]
- Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S 1999a. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3− exchange in mouse submandibular and pancreatic ducts. J Biol Chem 274: 14670–14677 [DOI] [PubMed] [Google Scholar]
- Lee MG, Wigley WC, Zeng W, Noel LE, Marino CR, Thomas PJ, Muallem S 1999b. Regulation of Cl−/ HCO3− exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 3T3 and HEK 293 cells. J Biol Chem 274: 3414–3421 [DOI] [PubMed] [Google Scholar]
- Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S 2000. Na+-dependent transporters mediate HCO3− salvage across the luminal membrane of the main pancreatic duct. J Clin Invest 105: 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Richter W, Namkung W, Kim KH, Kim E, Conti M, Lee MG 2007. Dynamic regulation of cystic fibrosis transmembrane conductance regulator by competitive interactions of molecular adaptors. J Biol Chem 282: 10414–10422 [DOI] [PubMed] [Google Scholar]
- Lee MG, Ohana E, Park HW, Yang D, Muallem S 2012. Molecular mechanism of pancreatic and salivary gland fluid and HCO3− secretion. Physiol Rev 92: 39–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW 1997. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol 110: 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, Lee MG, Muallem S 2001. HCO3− salvage mechanisms in the submandibular gland acinar and duct cells. J Biol Chem 276: 9808–9816 [DOI] [PubMed] [Google Scholar]
- Marteau C, Blanc G, Devaux MA, Portugal H, Gerolami A 1993. Influence of pancreatic ducts on saturation of juice with calcium carbonate in dogs. Dig Dis Sci 38: 2090–2097 [DOI] [PubMed] [Google Scholar]
- Marteau C, Silviani V, Ducroc R, Crotte C, Gerolami A 1995. Evidence for apical Na+/H+ exchanger in bovine main pancreatic duct. Dig Dis Sci 40: 2336–2340 [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Petersen OH, Flanagan P, Pearson GT 1983. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature 305: 228–232 [DOI] [PubMed] [Google Scholar]
- McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR 2004. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem 19: 231–236 [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T 2005. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469 [DOI] [PubMed] [Google Scholar]
- Morth JP, Pedersen BP, Buch-Pedersen MJ, Andersen JP, Vilsen B, Palmgren MG, Nissen P 2011. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol 12: 60–70 [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE 2008. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol 294: C810–C819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrke K, Quinn CC, Begenisich T 2003. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol 284: C535–C546 [DOI] [PubMed] [Google Scholar]
- Novak I, Greger R 1988. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: Properties of the basolateral membrane. Pflugers Arch 411: 58–68 [DOI] [PubMed] [Google Scholar]
- Novak I, Wang J, Henriksen KL, Haanes KA, Krabbe S, Nitschke R, Hede SE 2011. Pancreatic bicarbonate secretion involves two proton pumps. J Biol Chem 286: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Yang D, Shcheynikov N, Muallem S 2009. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587: 2179–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S, Tonnessen TI, Sandvig K 1986. pH-regulated anion antiport in nucleated mammalian cells. J Cell Biol 102: 967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly CM, Winpenny JP, Argent BE, Gray MA 2000. Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: Inhibition by bicarbonate ions. Gastroenterology 118: 1187–1196 [DOI] [PubMed] [Google Scholar]
- Pak BH, Hong SS, Pak HK, Hong SK 1966. Effects of acetazolamide and acid–base changes on biliary and pancreatic secretion. Am J Physiol 210: 624–628 [DOI] [PubMed] [Google Scholar]
- Park K, Olschowka JA, Richardson LA, Bookstein C, Chang EB, Melvin JE 1999. Expression of multiple Na+/H+ exchanger isoforms in rat parotid acinar and ductal cells. Am J Physiol 276: G470–G478 [DOI] [PubMed] [Google Scholar]
- Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, et al. 2002. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem 277: 50503–50509 [DOI] [PubMed] [Google Scholar]
- Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, et al. 2010. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139: 620–631 [DOI] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE 1994. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci 91: 5340–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM 2001. The neglected ion: HCO3−. Nat Med 7: 292–293 [DOI] [PubMed] [Google Scholar]
- Quinton PM 2008. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417 [DOI] [PubMed] [Google Scholar]
- Quinton PM 2010. Role of epithelial HCO3 transport in mucin secretion: Lessons from cystic fibrosis. Am J Physiol Cell Physiol 299: C1222–C1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 2003. Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature 423: 756–760 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM 1999. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature 402: 301–304 [DOI] [PubMed] [Google Scholar]
- Richardson C, Alessi DR 2008. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293–3304 [DOI] [PubMed] [Google Scholar]
- Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T 2006. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem 281: 27964–27972 [DOI] [PubMed] [Google Scholar]
- Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE 2010. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285: 12990–13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Boron WF 1981. Intracellular pH. Physiol Rev 61: 296–434 [DOI] [PubMed] [Google Scholar]
- Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F 1999. Immunolocalization of anion exchanger AE2 and Na+-HCO3− cotransporter in rat parotid and submandibular glands. Am J Physiol 277: G1288–G1296 [DOI] [PubMed] [Google Scholar]
- Roussa E, Alper SL, Thevenod F 2001. Immunolocalization of anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J Histochem Cytochem 49: 463–474 [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY 2008. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Kim KH, Kim KM, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S 2004. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J Biol Chem 279: 21857–21865 [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S 2008. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: Role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol 586: 3813–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K 2006. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci 103: 9542–9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL 1998. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem 273: 19797–19801 [DOI] [PubMed] [Google Scholar]
- Sohma Y, Gray MA, Imai Y, Argent BE 1996. A mathematical model of the pancreatic ductal epithelium. J Membr Biol 154: 53–67 [DOI] [PubMed] [Google Scholar]
- Sohma Y, Gray MA, Imai Y, Argent BE 2000. HCO3− transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol 176: 77–100 [DOI] [PubMed] [Google Scholar]
- Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H 2009. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci 106: 11776–11781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM 2005. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67: 377–409 [DOI] [PubMed] [Google Scholar]
- Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H 2009. Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol 296: G1307–G1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW 1991. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352: 628–631 [DOI] [PubMed] [Google Scholar]
- Venglovecz V, Rakonczay Z Jr, Ozsvari B, Takacs T, Lonovics J, Varro A, Gray MA, Argent BE, Hegyi P 2008. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut 57: 1102–1112 [DOI] [PubMed] [Google Scholar]
- Venglovecz V, Hegyi P, Rakonczay Z Jr, Tiszlavicz L, Nardi A, Grunnet M, Gray MA 2011. Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut 60: 361–369 [DOI] [PubMed] [Google Scholar]
- Verkman AS 2008. Mammalian aquaporins: Diverse physiological roles and potential clinical significance. Expert Rev Mol Med 10: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanger O, Veel T, Raeder MG 1995. Secretin causes H+/HCO3− secretion from pig pancreatic ductules by vacuolar-type H+-adenosine triphosphatase. Gastroenterology 108: 850–859 [DOI] [PubMed] [Google Scholar]
- Wang S, Raab RW, Schatz PJ, Guggino WB, Li M 1998. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR). FEBS Lett 427: 103–108 [DOI] [PubMed] [Google Scholar]
- Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S 2006. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: Relevance to cystic fibrosis. EMBO J 25: 5049–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC, Ermentrout GB 2004. A mathematical model of the pancreatic duct cell generating high bicarbonate concentrations in pancreatic juice. Pancreas 29: e30–e40 [DOI] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB 2002. Molecular characterization of the murine Slc26a6 anion exchanger: Functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838 [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, et al. 2008. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215 [DOI] [PubMed] [Google Scholar]
- Zeng W, Lee MG, Muallem S 1997. Membrane-specific regulation of Cl− channels by purinergic receptors in rat submandibular gland acinar and duct cells. J Biol Chem 272: 32956–32965 [DOI] [PubMed] [Google Scholar]
- Zhao H, Star RA, Muallem S 1994. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol 104: 57–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsembery A, Strazzabosco M, Graf J 2000. Ca2+-activated Cl− channels can substitute for CFTR in stimulation of pancreatic duct bicarbonate secretion. FASEB J 14: 2345–2356 [DOI] [PubMed] [Google Scholar]