Abstract
In leaves of Egeria densa Planchon, N-ethylmaleimide (NEM) and other sulfhydryl-binding reagents induce a temporary increase in nonmitochondrial respiration (ΔQO2) that is inhibited by diphenylene iodonium and quinacrine, two known inhibitors of the plasma membrane NADPH oxidase, and are associated with a relevant increase in electrolyte leakage (M. Bellando, S. Sacco, F. Albergoni, P. Rocco, M.T. Marré [1997] Bot Acta 110: 388–394). In this paper we report data indicating further analogies between the oxidative burst induced by sulfhydryl blockers in E. densa and that induced by pathogen-derived elicitors in animal and plant cells: (a) NEM- and Ag+-induced ΔQO2 was associated with H2O2 production and both effects depended on the presence of external Ca2+; (b) Ca2+ influx was markedly increased by treatment with NEM; (c) the Ca2+ channel blocker LaCl3 inhibited ΔQO2, electrolyte release, and membrane depolarization induced by the sulfhydryl reagents; and (d) LaCl3 also inhibited electrolyte leakage induced by the direct infiltration of the leaves with H2O2. These results suggest a model in which the interaction of sulfhydryl blockers with sulfhydryl groups of cell components would primarily induce an increase in the Ca2+ cytosolic concentration, followed by membrane depolarization and activation of a plasma membrane NADPH oxidase. This latter effect, producing active oxygen species, might further influence plasma membrane permeability, leading to the massive release of electrolytes from the tissue.
The ability of NEM and a number of other sulfhydryl group blockers to induce ΔQO2 in Egeria densa Planchon leaves has been reported previously (Albergoni et al., 1996; Bellando et al., 1997; Marrè and Albergoni, 1998). ΔQO2 induced by these reagents is completely insensitive to mitochondrial inhibitors such as cyanide, salicylhydroxamic acid, and propyl gallate and to the uncoupler carbonylcyanide-m-chlorphenylhydrazone, whereas it is suppressed by quinacrine and diphenylene iodonium, two inhibitors of plasma membrane NADPH oxidase, an enzyme responsible for the oxidative burst induced by pathogenic factors in granulocytes and in plants (Babior, 1992; Doussière and Vignais, 1992; Auh and Murphy, 1995; Desikan et al., 1996; Murphy and Auh, 1996; Van Gestelen et al., 1997). The respiratory burst induced by sulfhydryl blockers is also associated with an early depolarization of the transmembrane electrical potential difference (Em), with a rapid increase in electrolyte leakage (mainly K+, but also HPO42− and Cl−), and with an increase in H+ influx (Bellando et al., 1997; Marrè and Albergoni, 1998). All of these features are similar to the effects of pathogenic elicitors in higher plants in which the hypersensitive reaction has been investigated. This suggests that a common general mechanism is involved in the two orders of phenomena.
In addition to its intrinsic value, the elucidation of the mode of action of sulfhydryl reagents on O2 uptake and plasma membrane permeability might contribute to the understanding of the mechanism of the pathogen-induced oxidative burst and the increase in membrane permeability that are characteristic of the hypersensitive response (Apostol et al., 1989; Conrath et al., 1991; Goodman and Novacky, 1994). Two important observations concerning the pathogen-derived, elicitor-induced oxidative burst are: (a) the production of active oxygen species, mainly O2− and H2O2 (Doke, 1983; Sutherland, 1991; Levine et al., 1994; Mehdy, 1994; Doke and Miura, 1995; Lamb and Dixon, 1997; Xing et al., 1997), which are potentially responsible for changes at the plasma membrane; and (b) the demonstration of a strict Ca2+ dependence of the effects of treatments with a variety of pathogenic elicitors, leading to the conclusion that an increase in the cytosolic Ca2+ level is a very early step in the sequence of events involved in the phenomenon (Bach et al., 1993; Tavernier et al., 1995; Levine et al., 1996; Gelli et al., 1997; Jabs et al., 1997; Pugin et al., 1997).
The present investigation was undertaken to determine whether the effects of sulfhydryl blockers in E. densa are associated with the production of active oxygen species, and whether in this case, as well as in the induction of the respiratory burst, membrane depolarization and electrolyte leakage depend on the presence of Ca2+ in the medium and on its influx into the tissue. We also investigated the possibility that the production of H2O2, as a product of the oxidative burst, could contribute to the changes in membrane permeability associated with electrolyte leakage.
MATERIALS AND METHODS
Plant Material
Egeria densa Planchon plants were grown in flowing tap water in a greenhouse. Young, fully expanded leaves were cut about 2 to 4 cm from the branch apex, randomized, and then grouped into samples. Before the experiments, the samples were washed twice for 15 min with 20 mL of 0.5 mm CaSO4 and preincubated for 30 min in 20 mL of 0.5 mm CaSO4 and 5 μm DCMU, with continuous shaking at 20°C in the dark. DCMU, a specific inhibitor of photosynthetic O2 evolution and C fixation, was added to prevent the eventual effects of photosynthesis caused by occasional exposure to light (Marrè et al., 1989; Albergoni et al., 1996).
QO2 Measurements
QO2 was measured on four leaves (about 30 mg fresh weight) in 2 mL of medium using a Clark-type O2 electrode (unit KW2, control box CB1, Hansatech, Norfolk, UK). Measurements were carried out continuously, as described by Marrè and Albergoni (1998), and medium was renewed every 8 min to restore maximal concentration of dissolved O2. The basal medium contained 5 μm DCMU, 10 mm Mes buffer (pH 6.0), with BTP. CaSO4 and other reagents were added at the concentrations specified in the legends to the figures.
H2O2 Production
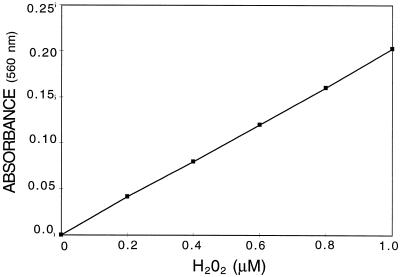
The low values of H2O2 accumulation in the samples revealed that there was a problem with the measurement procedure. The widely used luminol method presented the disadvantages of a relatively low sensitivity, together with a large variability. After several trials using this method (as described by Viard et al., 1994), we decided to follow the method described by Jiang et al. (1990) using the peroxide-mediated oxidation of Fe2+, followed by the reaction of Fe3+ with xylenol orange. This method produced reproducible results in the 0.1 to 1 μm H2O2 concentration range (Fig. 1).
Figure 1.
Standard curve of the H2O2 concentration determined by the xylenol method in the range of 0.1 to 1 μm. H2O2 was measured with 100 μm xylenol orange, 250 μm Fe2+, and 100 μm sorbitol in 25 mm H2SO4. A560 was read after 45 min at room temperature. Data are the means of triplicate determinations. sd did not exceed ±3%.
The possibility of interference by other chemicals present in the various assay media used in our experiments was excluded by a series of tests, and the specificity for H2O2 was tested by determining the effects of H2O2 elimination by catalase. For the determination of H2O2 with this method 500-μL aliquots of the incubation medium, drawn from the O2 electrode chamber every 8 min, were added to 500 μL of the reaction mixture containing 500 μm ammonium ferrous sulfate, 50 mm sulfuric acid, 200 μm xylenol orange, and 200 mm sorbitol. After 45 min at room temperature the changes in A560 were evaluated with a Biochrom Ultrospec 2 spectrophotometer (LKB, Bromma, Sweden). The addition of catalase (1600 units) to the sample aliquots for 5 min decreased the absorbance changes to less than 10%. The H2O2 values reported in Figures 1 and 6 are those left after subtraction of the small values found after treatment with catalase.
Figure 6.
Effects of the presence of 0.25 mm La3+ (added at time 0) on the rate of H2O2 production induced by 0.2 mm NEM and 5 μm Ag+. Experimental conditions were as in Figure 2. Data are from one representative experiment out of three. FW, Fresh weight.
Electrophysiological Measurements
A single leaf held in a 5-mL plexiglass chamber was irrigated with a continuously flowing (approximately 15 mL min−1) aerated medium thermoregulated at 20°C. The basal medium was the same as that described above, except that 0.5 mm CaSO4 was added to the solution. Other reagents were added at the concentrations reported in the legends to the figures. Micropipettes (tip resistance, 10 MΩ) filled with 1 m KCl were used as microsalt bridges of Ag/AgCl electrodes and were inserted vertically into the tissue by means of a micromanipulator (Leitz, Wetzlar, Germany). Em was measured with a high-impedance electrometer amplifier (model WPI K5–700, World Precision Instruments, New Haven, CT) connected to a chart recorder. The data presented represent one of at least three experiments for each set of experimental conditions.
Measurement of Electrolyte Leakage
After pretreatment, samples of 10 leaves (approximately 100 mg fresh weight) were incubated in 10 mL of a stirred solution thermoregulated at 20°C. Basal medium contained 5 μm DCMU and 10 mm Mes buffer (pH 6.0), with BTP. Other reagents were added at the concentrations specified in the legends to the figures. The conductivity of the medium was measured continuously by a conductivity meter (model MO101, Analytical Control, Milan, Italy) connected to a chart recorder for the required time. The data represent the changes in medium conductivity during the treatments, calculated per gram fresh weight, taking 0 as the value of conductivity measured at the end of the equilibration period before starting the treatment.
Ca2+ Uptake and Net Flux Measurements
Ca2+ influx was measured as 45Ca2+ uptake, and the radioactivity was provided as 45CaCl2 (1.48 GBq mol−1). After pretreatment, samples of leaves (100 mg fresh weight) were incubated in 20 mL of basal medium containing 0.5 mm CaSO4, 10 mm Mes-BTP (pH 6.0), and 5 μm DCMU, with or without 0.2 mm NEM. To remove by exchange the 45Ca2+ contained in the free space and in the cell wall, after 30 and 90 min of treatment the samples were briefly rinsed twice in 20 mL of an unlabeled ice-cold solution containing 10 mm CaSO4, 10 mm Mes-BTP (pH 6.0), and 5 μm DCMU. The leaves were then transferred twice (for 2.5 and 18 min, respectively) in 20 mL of the same unlabeled solution and finally collected, blotted on filter paper, and placed into scintillation vials. Digestion and bleaching of the tissue were performed as described by Romani and Beffagna (1991). The radioactivity was measured by liquid-scintillation counting in a TriCarb counter (Packard Instruments, Meriden, CT) after addition of 10 mL of scintillation liquid (Hionic Fluor, Packard Instruments).
Net Ca2+ fluxes were measured in samples of leaves incubated in the same experimental conditions described above, without 45Ca2+. The changes in Ca2+ concentrations in the assay media were measured after 90 min of incubation using a Ca2+-selective electrode (MI-600, Microelectrodes, Bedford, NH).
SOD Assay
E. densa leaves (130 mg/mL) were homogenized in 50 mm potassium phosphate (pH 7.8) containing 0.1 mm EDTA and 0.3% (w/v) Triton X-100 at 4°C. The homogenate was filtered through four layers of cheesecloth and promptly used for the SOD assay. SOD activity was determined spectrophotometrically as the inhibition of xanthine-xanthine oxidase-mediated reduction of Cyt c (McCord and Fridovich, 1969). The assay was performed at 25°C in a 3-mL cuvette containing 50 mm potassium phosphate (pH 7.8), 0.1 mm EDTA, 50 μm Cyt c, and 1 mm xanthine. The reduction of Cyt c was followed by a double-beam spectrophotometer (ZWS II, Sigma). The data are means of three determinations on two different leaf extracts.
RESULTS
H2O2 Production in NEM and the Ag+-Induced Oxidative Burst
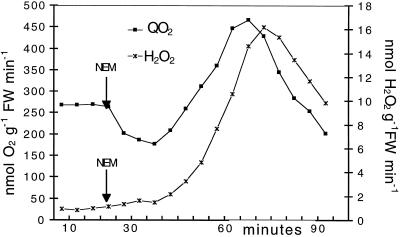
Previously, it was shown that the ΔQO2 induced by NEM or by Ag+ was insensitive to cyanide and salicylhydroxamic acid but completely inhibited by diphenylene iodonium and quinacrine. This suggested that it was mediated by a type of NADPH oxidase operating in the oxidative burst induced by pathogen-derived elicitors, both in granulocytes and in plants, and responsible for the production of O2− and (through SOD) H2O2 (Mehdy, 1994; Doke and Miura, 1995; Desikan et al., 1996). To confirm this conclusion we investigated whether H2O2 was the product of the oxidative reaction, as in the case of treatment with the sulfhydryl blockers. Figure 2 shows that NEM-induced ΔQO2 was indeed closely associated with and parallel to the production of H2O2. Similar results were obtained on leaves in which the oxidative burst had been induced by treatment with 5 μm Ag+. Attempts to demonstrate the appearance of O2− in the incubation medium failed to give significant and reproducible results. This might be a consequence of SOD rapidly metabolizing the products of NADPH oxidase activity. Measurements of SOD activity in E. densa leaves gave a very high value of this enzyme (1.3 ± 0.15 μmol min−1 g−1 fresh weight; see Methods).
Figure 2.
Effects of 0.2 mm NEM on the rate of O2 uptake and of H2O2 production by E. densa leaves. The incubation medium contained 10 mm Mes buffer (pH 6.0) with BTP, 5 μm DCMU, and 0.5 mm CaSO4. Aliquots for ΔQO2 measurements and H2O2 determination came from the same incubation medium. Data are from one representative experiment out of five. FW, Fresh weight.
Ca2+ Dependence of Sulfhydryl Reagent-Induced ΔQO2, Membrane Depolarization, and Electrolyte Leakage
Effects of Ca2+ Concentration in the Medium
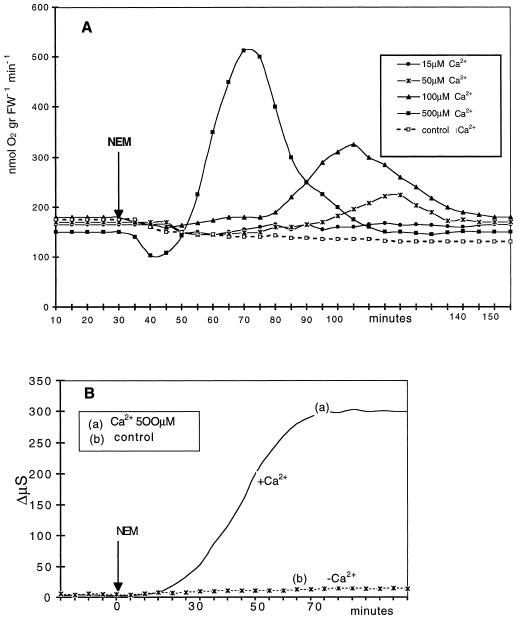
In our previous studies on the oxidative burst induced by the sulfhydryl blockers in aquatic plants, 0.5 mm Ca2+ was always present in the medium (Albergoni et al., 1996; Bellando et al., 1997; Marrè and Albergoni, 1998). In this study the relevance of extracellular Ca2+ concentration on the NEM- and Ag+-induced increase in O2 consumption was investigated at Ca2+ concentrations ranging from 0 to 0.5 mm. The results shown in Figure 3A indicate that the ability of NEM to induce the respiratory burst was completely abolished by the absence of extracellular Ca2+ (control) or by its presence at 15 μm, whereas it was barely appreciable at 50 μm, and steadily increased from 0.1 to 0.5 mm Ca2+. Figure 3B shows that the absence of Ca2+ in the medium completely suppressed the NEM-induced electrolyte leakage, an aspect of sulfhydryl-blocker action similar to that reported for the pathogenic elicitor-induced respiratory burst (Atkinson at al., 1990; Bach et al., 1993; Goodman and Novacky, 1994; Jabs et al., 1997).
Figure 3.
Effects of increasing concentrations of Ca2+ on 0.2 mm NEM-induced respiratory burst (A) and Ca2+ dependence of NEM-induced electrolyte leakage (B). Experimental conditions were as in Figure 2. ΔQO2 data (A) are from one representative experiment out of three for each Ca2+ concentration tested. Conductivity change data (B) are the means of three experiments. se did not exceed ±8%. gr FW-1, Per gram fresh weight.
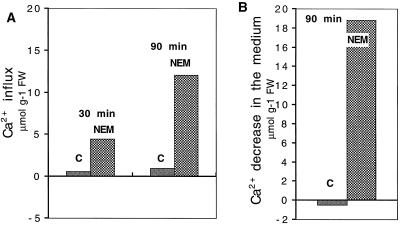
The effect of Ca2+ in conditioning the NEM-induced oxidative burst and electrolyte leakage might have depended on an increase of its influx in the cells. To test this hypothesis, we measured the effects of NEM on Ca2+ influx in a medium containing 45Ca2+ (Fig. 4) after 30 and 90 min of incubation. The results shown in Figure 4A demonstrate a very large increase of Ca2+ influx in the NEM-treated leaves. This finding was confirmed by measurements of Ca2+ concentration changes in the incubation medium, which showed a decrease of 20 μmol g−1 fresh weight (Fig. 4B). Although changes in the Ca2+ content in the cell walls contributed somewhat to these results, these data are interpreted as indicating a marked stimulation of Ca2+ influx in the NEM-treated leaves.
Figure 4.
45Ca2+ influx in E. densa leaves (A) and changes in external Ca2+ concentration (B) induced by 0.2 mm NEM. The 45Ca2+ uptake was determined after 30 and 90 min of incubation, as described in Methods. Changes in external Ca2+ concentration were measured in the medium with a Ca2+-selective electrode after 90 min of incubation. Samples were incubated under the same conditions described for the influx, except for the absence of 45Ca2+. The data represent the means of two experiments run in triplicate. se did not exceed ±10%. FW, Fresh weight.
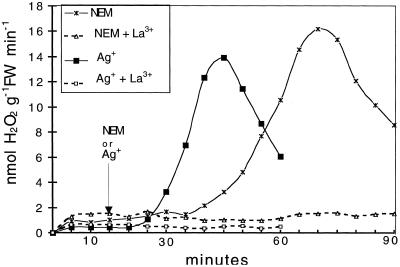
Effects of La3+ on ΔQO2 and H2O2 Production
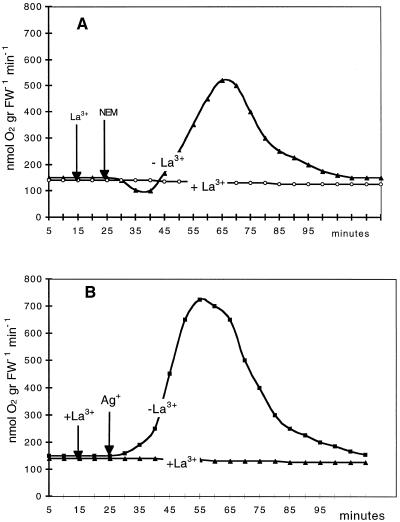
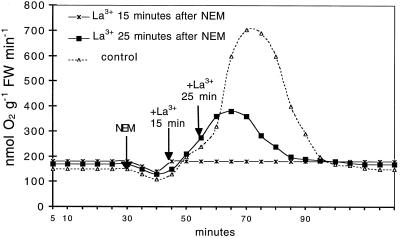
In several plant systems the ability to respond to a stress stimulus (i.e. temperature shock, drought, salinity, or pathogen-derived elicitors) requires the presence of extracellular Ca2+. The inhibition by Ca2+-channel blockers such as La3+ suggests that Ca2+ influx increases the cytosolic Ca2+ concentration (Atkinson et al., 1990; Bach et al., 1993; Price et al., 1994; Tavernier et al., 1995; Gelli et al., 1997; Pugin et al., 1997; Takahashi et al., 1997). The results shown in Figures 5 and 6 indicate that in our system the induction of ΔQO2 and the production of H2O2 by NEM or Ag+ were completely inhibited by the presence of 0.25 mm La3+. After treatment with NEM, the onset of the oxidative burst was seen after a lag of about 20 min, and the addition of La3+ was still able to counteract the appearance of ΔQO2. As shown in Figure 7, the development of the oxidative burst was completely prevented when La3+ was added 15 min after NEM, and was interrupted almost immediately when La3+ was added at the ΔQO2 already initiated.
Figure 5.
Effects of 0.25 mm La3+ on 0.2 mm NEM-induced (A) or 5 μm Ag+-induced (B) respiratory burst. Experimental conditions were as in Figure 2. Data are from one representative experiment out of three. gr FW-1, Per gram fresh weight.
Figure 7.
Effects of the addition of La3+ 15 and 25 min after the addition of NEM on the NEM-induced respiratory burst. Experimental conditions were as in Figure 2. Data are from one representative experiment out of three. FW, Fresh weight.
Effect of La3+ on the Em and on Electrolyte Leakage
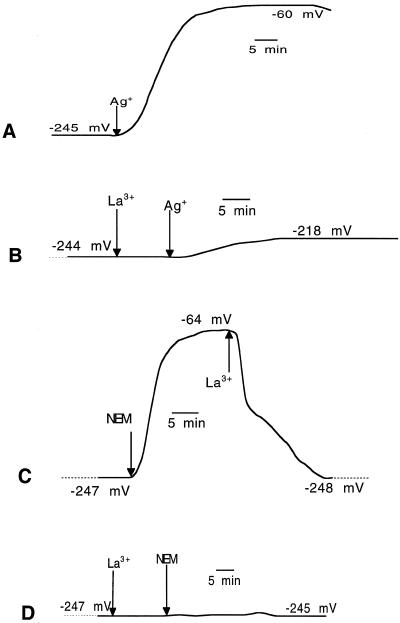
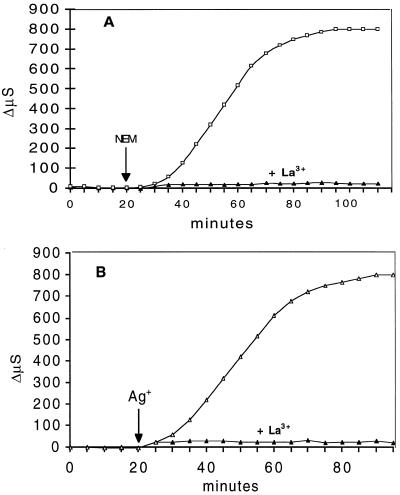
Previous experiments have shown that sulfhydryl blockers induced a respiratory burst and an increase in electrolyte leakage that were preceded by an early, strong depolarization of the plasma membrane (Bellando et al., 1997; Marrè and Albergoni, 1998). As shown in Figure 8, the addition of 0.25 mm LaCl3 to the flowing solution before the addition of either NEM or Ag+ completely suppressed the depolarizing effect of the two drugs (Fig. 8B). In other experiments La3+ was added 20 min after NEM, i.e. toward the end of the lag period between the addition of NEM and the onset of the respiratory burst, but when the depolarization of NEM had fully developed and electrolyte leakage had already started. In this case La3+ completely reversed the NEM-induced depolarization (Fig. 8C) (for a similar effect of La3+ on elicitor-induced depolarization in tobacco cells, compare Pugin et al., 1997). La3+ alone did not significantly change the basal Em value of E. densa cells, indicating a weak ability of this cation to enter the cells. The La3+-induced inhibition of the membrane depolarization caused by NEM or Ag+ was associated with an equally complete suppression of the increase in electrolyte leakage (Fig. 9).
Figure 8.
Effects of the presence of 0.25 mm La3+ on 5 μm Ag+-induced (A and B) and 0.2 mm NEM-induced (C and D) membrane depolarization. Note that in C, La3+ was added after NEM addition, so that NEM-induced depolarization had already reached its maximum (about 15 min) but ΔQO2 had not yet started (compare Fig. 7). Data are from one representative experiment out of three. A, Depolarization by 5 μm Ag+; B, its inhibition by La3+; C, depolarization by 0.2 mm NEM and its reversal by La3+; and D, inhibition by the presence of La3+ of the NEM-induced depolarization.
Figure 9.
Effects of 0.25 mm La3+ on 0.2 mm NEM-induced (A) and 5 μm Ag+-induced (B) electrolyte leakage. Basal medium was as in Figure 2 with 0.5 mm CaSO4 always present; 0.25 mm La3+ was added at time 0. Data are the means of three experiments. se did not exceed ±10%. ΔμS, Change in medium conductivity.
Effect of La3+ on H2O2-Induced Electrolyte Leakage
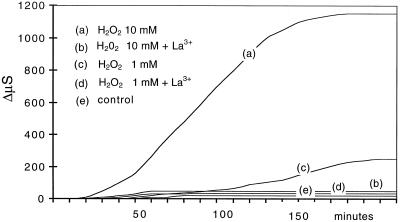
A role has been proposed for H2O2 in the changes in the plasma membrane leading to electrolyte leakage in the oxidative burst induced by pathogen-derived elicitors (Levine et al., 1994; Mehdy, 1994; Lamb and Dixon, 1997). This also appears to be true in the case of sulfhydryl-blocker-induced H2O2 production in E. densa. Figure 10 shows that infiltration of E. densa leaves with 1 and 10 mm H2O2 induced a rapid increase in electrolyte leakage. H2O2 concentrations less than 0.5 mm did not induce any detectable response.
Figure 10.
Effects of the presence of 1 and 10 mm H2O2 in the incubation medium on electrolyte leakage in the presence and absence of 0.25 mm La3+. To facilitate H2O2 entry, before the experiment was started leaves were rapidly infiltrated with the same medium used in the treatment. Experimental conditions were as in Figure 2. Data are from one representative experiment out of four. ΔμS, Change in medium conductivity.
The ability of H2O2 to induce electrolyte leakage might depend on the activation of Ca2+ influx (Price et al., 1994; Levine et al., 1996). Thus, one might expect an effect of La3+ on the effect of H2O2 on membrane permeability and electrolyte leakage. In our experiments the presence of La3+ completely inhibited the H2O2 effect on electrolyte leakage, suggesting the involvement of some step influenced by Ca2+ extracellular concentration (for a similar conclusion in soybean cells, see Levine et al., 1996).
DISCUSSION
The results presented in this paper integrate the previously reported evidence concerning the effects of sulfhydryl reagents on respiration and ion transport in E. densa leaves. These new data demonstrate that the respiratory burst induced by sulfhydryl blockers (NEM or Ag+) in E. densa leaves was associated with a parallel production of H2O2. The presence of Ca2+ in the incubation medium was strictly required for the effects of NEM and Ag+ on transmembrane potential, electrolyte leakage, O2 uptake, and H2O2 production, and Ca2+ influx was markedly stimulated by NEM. With 0.5 mm Ca2+ present, the addition of the Ca2+-channel blocker La3+ completely inhibited all of these effects of NEM or Ag+, suggesting that Ca2+ action depends on its influx. The addition of H2O2 to the medium induced an electrolyte leakage of the same order as that induced by NEM or Ag+, and this effect was completely inhibited by La3+.
The integration of these results with those of previous studies allows a coherent interpretation of the mechanism of action of the sulfhydryl blockers on respiration and membrane activities in E. densa leaves. According to a model covering the available evidence to date, the primary interaction of the sulfhydryl blockers with the sulfhydryl groups of some cell component would lead to a rapid increase in Ca2+ influx. The consequent increase in cytosolic Ca2+ concentration would influence ion-transport systems at the plasma membrane (for a similar interpretation of the effects of Ca2+, see Schroeder and Hagiwara, 1989; Price et al., 1994; Tavernier et al., 1995; Pugin et al., 1997), and would therefore explain the observed rapid membrane depolarization and increased release of electrolytes. According to our previous data, the main effects on ionic fluxes induced by sulfhydryl reagents are the effluxes of K+, Cl−, and H2PO4− and the influx of H+ and Ca2+ (according to the present results). In guard cells an increase in cytosolic Ca2+ concentration has been reported to favor the opening of anion (Cl− and H2PO4−) channels and the closure of inwardly rectified K+ channels (Schroeder and Hagiwara, 1989), which would explain the changes in anion fluxes in our system. Membrane depolarization would depend at least in part on anion efflux and might be responsible for the opening of outward K+ channels and for K+ efflux. Thus, one might propose that all changes in the fluxes of these ions and in the Em are consequences of Ca2+ entry into the cell, whereas the increase of H+ net uptake (this is potentially important because of its influence on cytosolic pH and the membrane potential) would depend on a Ca2+-induced increase in passive membrane permeability to H+ and perhaps also on the inhibition of the H+-ATPase by the sulfhydryl blockers (Brooker and Slayman, 1982).
The identification of H2O2 production during the QO2 burst confirms the previous conclusion that an O2−-producing NADPH oxidase of the type speculated to be involved in the hypersensitive reaction mediates the oxidative burst induced by the sulfhydryl blockers (Doussière and Vignais, 1992; Felix et al., 1994; Auh and Murphy, 1995; Van Gestelen et al., 1997; Xing et al., 1997), a conclusion previously drawn from the inhibition of the ΔQO2 effect by diphenylene iodonium and quinacrine (Bellando et al., 1997). The activation mechanism of the NADPH oxidase by the sulfhydryl blockers remains open to investigation and probably involves various steps, as proposed for NADPH oxidase activation by pathogen-derived elicitors (Legendre et al., 1992; Morrè et al., 1993; Mehdy, 1994; Vera-Estrella et al., 1994). The conclusion that the responses of membrane potential and electrolyte leakage are largely independent of the activation of the NADPH oxidase is suggested by the findings that: (a) after treatment with NEM, the two responses are clearly separated in time; (b) NADPH oxidase inhibitors completely suppress the oxidative burst, whereas they inhibit electrolyte leakage only partially (Bellando et al., 1997; Marrè and Albergoni, 1998); and (c) the addition of La3+ 15 to 20 min after that of NEM completely prevents the oxidative burst, although membrane depolarization has reached its maximum and electrolyte leakage has already started. The findings that the addition of La3+ completely suppresses NEM-induced ΔQO2 and H2O2 production and that it reverses the previous effects of NEM on membrane potential suggests that all of the leaf responses to the sulfhydryl blockers require the continuous influx of Ca2+ into the cells.
These experiments demonstrate that Ca2+ influx is strictly required for sulfhydryl reagent-induced changes in both ion transport and oxidative metabolism. However, an unresolved question is whether the influx of Ca2+ by itself is sufficient to induce the oxidative burst. The necessary involvement of ion fluxes other than that of Ca2+ in the induction of the oxidative burst has been suggested by Jabs et al. (1997). Further data are requested to define the situation in our system.
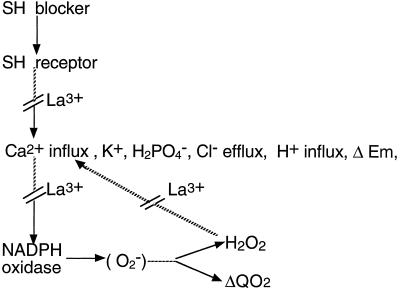
The model shown in Figure 11 summarizes the main lines of our interpretation of the action of the sulfhydryl blockers in E. densa leaves. A strict similarity is evident between this model and that proposed for the action of pathogen-derived elicitors in the induction of the oxidative burst (for review, see Lamb and Dixon, 1997). Our model is similar to that presented by Pugin et al. (1997) in a recent and very complete investigation regarding the action of cryptogein in tobacco cells, in which the effects of this elicitor on membrane depolarization, ion transport, and oxidative metabolism appeared to be very similar to those induced by the sulfhydryl blockers in E. densa leaves. According to our model, the H2O2 produced by NADPH oxidase might converge with the primary effect of the sulfhydryl reagent increasing the passive permeability of the plasma membrane to ions. Such an effect of active oxygen species produced by pathogen-derived elicitors in various plant materials has been proposed by various authors (Keppler and Novacky, 1987; Levine et al., 1994; Mehdy, 1994; Lamb and Dixon, 1997). In our case an interesting feature of the activation of electrolyte leakage by H2O2 was its inhibition by La3+, suggesting that Ca2+ influx was involved. A stimulation of Ca2+ influx by exogenous H2O2 has been reported by Price et al. (1994) in tobacco cells, and a stimulating effect of Ca2+ on anion efflux (and thus plasma membrane depolarization) has been reported by Schroeder and Hagiwara (1989). Thus, it seems possible that the effect of H2O2 depends on its nature as a sulfhydryl-oxidizing agent, endowed with a mode of action on Ca2+ influx similar to that of Ag+ or NEM, rather than on its direct interaction with the plasma membrane.
Figure 11.
Proposed model for the action of the sulfhydryl (SH) blockers and La3+ on membrane potential, electrolyte leakage, H+ influx, O2 uptake, and H2O2 production burst in E. densa leaves. According to this scheme, Ca2+ influx would influence the effects on NADPH oxidase independently of its action on ion transport and transmembrane potential. However, the present evidence is compatible with the alternative interpretation, that the activation of NADPH oxidase is a consequence of the changes at the ion-transport level (see Jabs et al., 1997).
The concentrations of H2O2 in the medium required to induce a consistent electrolyte leakage are much higher than those that accumulated in the medium after treatment with NEM. This might be attributable to the fact that in the first case, H2O2 reaches the cell from the outside, whereas in the second case, it is produced inside of the cell. A high H2O2 concentration has been used in studies of the possible relevance of H2O2 in pathogen-derived effects on ion transport (Apostol et al., 1989; Levine et al., 1994, 1996; Price et al., 1994). However, the very high H2O2 concentration required to induce leakage raises some doubt about its possible role in NEM action.
Taken together, these new results emphasize the similarity between the effect of the sulfhydryl blockers in E. densa and the effects of pathogen-derived elicitors on plasma membrane activities and oxidative metabolism in a number of plant materials. This underscores the need for a comparative analysis of the possibly common steps in the mechanism of action of the sulfhydryl reagents and the pathogen-derived elicitors. Useful information in this regard should come from the investigation of the effects of nonspecific pathogen-derived elicitors in the E. densa system, an approach that we plan to develop in the near future. Progress in the study of the responses to the sulfhydryl blockers is of interest not only for the understanding of the mode of action of the sulfhydryl reagents, the nature of plasmalemma redox systems, and the role of Ca2+ in the regulation of other ion fluxes, but also for the elucidation of the mechanism of defense reactions in plants.
Abbreviations:
- BTP
1,3-bis[tris(hydroxymethyl)methylamino] propane
- Em
transmembrane electric potential difference
- NEM
N-ethylmaleimide
- QO2
oxygen uptake
- ΔQO2
increase in nonmitochondrial respiration
- SOD
superoxide dismutase
Footnotes
This work was supported in part by the Ministero Italiano dell' Università e della Ricerca Scientifica e Tecnologica.
LITERATURE CITED
- Albergoni F, Rocco P, Bellando M, Sacco S, Marrè MT. serie IX. 1996;7:171–177. [Google Scholar]
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MM, Keppler LD, Orlandi EW, Baker CJ, Mischke CF. Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ and hypersensitive responses in tobacco. Plant Physiol. 1990;92:215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auh CK, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase. Adv Enzymol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- Bach M, Schnitzler J-P, Seitz HU. Elicitor-induced changes in Ca2+ influx, K+ efflux, and 4-hydroxybenzoic acid synthesis in protoplasts of Daucus carota L. Plant Physiol. 1993;103:407–412. doi: 10.1104/pp.103.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellando M, Sacco S, Albergoni F, Rocco P, Marrè MT. Transient stimulation of oxygen uptake induced by sulfhydryl reagents in Egeria densa and Potamogeton crispus leaves. Bot Acta. 1997;110:388–394. [Google Scholar]
- Brooker RJ, Slayman CW. Inhibition of the plasma membrane H+-ATPase of Neurospora crassa by N-ethylmaleimide. J Biol Chem. 1982;257:12051–12055. [PubMed] [Google Scholar]
- Conrath U, Jeblick W, Kauss H. The protein kinase inhibitor, K-252a, decreases elicitor-induced Ca2+ uptake and K+ release, and increases coumarin synthesis in parsley cells. FEBS Lett. 1991;279:141–144. doi: 10.1016/0014-5793(91)80269-9. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Coffey MJ, Neill SJ. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 1996;382:213–217. doi: 10.1016/0014-5793(96)00177-9. [DOI] [PubMed] [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to hyphal wall components. Physiol Plant Pathol. 1983;23:345–357. [Google Scholar]
- Doke N, Miura Y. In vitro activation of NADPH-dependent O2− generating system in a plasma membrane-rich fraction of potato tuber tissues by treatment with an elicitor from Phytophthora infestans or with digitonin. Physiol Mol Plant Pathol. 1995;46:17–28. [Google Scholar]
- Doussière J, Vignais PV. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Eur J Biochem. 1992;208:61–71. doi: 10.1111/j.1432-1033.1992.tb17159.x. [DOI] [PubMed] [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelli A, Higgins VJ, Blumwald E. Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol. 1997;113:269–279. doi: 10.1104/pp.113.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RN, Novacky AJ (1994) The Hypersensitive Reaction in Plants to Pathogens. American Phytopathological Society, St. Paul, MN
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defence gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZJ, Woollard ACS, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- Keppler LD, Novacky A. The initiation of membrane lipid peroxidation during bacteria-induced hypersensitive reaction. Physiol Mol Plant Pathol. 1987;30:233–245. [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Legendre L, Heinstei PF, Low PS. Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cell. J Biol Chem. 1992;267:20140–20147. [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Marrè MT, Albergoni F. Fusicoccin counteracts the NEM- and Ag+-induced stimulation of oxygen uptake in Egeria densa leaves. Plant Physiol. 1998;116:681–686. doi: 10.1104/pp.116.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrè MT, Albergoni FG, Moroni A, Marrè E. Light-induced activation of electrogenic H+ extrusion and K+ uptake in Elodea densa depends on photosynthesis and is mediated by the plasma membrane H+ ATPase. J Exp Bot. 1989;40:343–352. [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrè DJ, Brightman AO, Barr R, Davidson M, Crane FL. NADH oxidase activity of plasma membrane of soybean hypocotyls is activated by guanine nucleotides. Plant Physiol. 1993;102:595–602. doi: 10.1104/pp.102.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TM, Auh CK. The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 1996;110:621–629. doi: 10.1104/pp.110.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AH, Taylor A, Scott JR, Griffiths, Trewavas AJ, Knight MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell. 1994;6:1301–1310. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani G, Beffagna N. Effect of some triazole fungicides on intracellular pH and on cell membrane permeability in leaves of Elodea densa (Planch.) Casp. New Phytol. 1991;117:431–437. doi: 10.1111/j.1469-8137.1991.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–93. [Google Scholar]
- Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Hypoosmotic shock induces increase in cytosolic Ca2+ in tobacco suspension-culture cells. Plant Physiol. 1997;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier E, Wendehenne D, Blein JP, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 1995;109:1025–1033. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestelen P, Asard H, Caubergs RJ. Solubilization and separation of plant plasma membrane NADPH-O2− synthase from other NAD(P)H oxidoreductases. Plant Physiol. 1997;115:543–550. doi: 10.1104/pp.115.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Higgins VJ, Blumwald E. Plant defense response to fungal pathogens. II. G-protein-mediated changes in host plasma membrane redox reactions. Plant Physiol. 1994;106:97–102. doi: 10.1104/pp.106.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Ricci P, Blein JP. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Higgins J, Blumwald E. Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell. 1997;9:249–259. doi: 10.1105/tpc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]