Abstract
Neurovascular dysfunction is an integral part of Alzheimer disease (AD). Changes in the brain vascular system may contribute in a significant way to the onset and progression of cognitive decline and the development of a chronic neurodegenerative process associated with accumulation of amyloid β-peptide (Aβ) in brain and cerebral vessels in AD individuals and AD animal models. Here, we review the role of the neurovascular unit and molecular mechanisms in cerebral vascular cells behind the pathogenesis of AD. In particular, we focus on blood–brain barrier (BBB) dysfunction, decreased cerebral blood flow, and impaired vascular clearance of Aβ from brain. The data reviewed here support an essential role of the neurovascular and BBB mechanisms in AD pathogenesis.
Changes in the brain vascular system (e.g., blood–brain barrier dysfunction) significantly contribute to amyloid-β accumulation and Alzheimer disease pathogenesis.
Alzheimer disease (AD) is a neurodegenerative disorder associated with neurovascular dysfunction (Zlokovic 2005, 2010; de la Torre 2010; Marchesi 2011), cognitive decline (Cummings 2004), and accumulation in brain of amyloid β peptide (Aβ; Querfurth and LaFerla 2010) and tau-related lesions in neurons termed neurofibrillary tangles (Ballatore et al. 2007; Ittner and Gotz 2011). Multiple epidemiological studies have demonstrated a remarkable overlap among risk factors for cerebrovascular disorder and sporadic, late-onset AD (de la Torre 2010; Jellinger 2010; Kalaria 2010). For example, mid-life diabetes (Luchsinger et al. 2007; Knopman and Roberts 2010), hypertension (Iadecola and Davisson 2008), and obesity (Whitmer et al. 2008) have all been shown to increase the risk for both AD and vascular dementia. It is now generally acknowledged that most AD cases have mixed vascular pathology and small-vessel disease (Jellinger 2010; Marchesi 2011). Moreover, reduced brain blood perfusion (Ruitenberg et al. 2005), silent infarcts (Vermeer et al. 2003), and the presence of one or more infarctions (Snowdon et al. 1997) all increase the risk of AD.
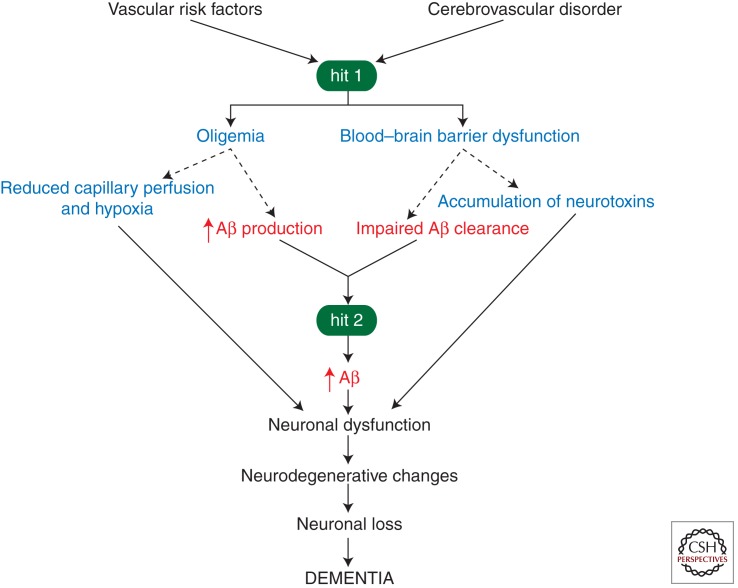
The amyloid hypothesis states that Aβ initiates a cascade of events leading to neuronal injury and loss (Hardy and Selkoe 2002) associated with cognitive decline (Cummings 2004). According to an alternative two-hit vascular hypothesis of AD (Zlokovic 2005, 2010; de la Torre 2010; Marchesi 2011), Aβ accumulation in the brain is a second insult (hit 2) that is initiated by vascular damage (hit 1; Fig. 1). Although the molecular and cellular events for each step in the disease process and for each risk factor are not absolutely clear, all vascular factors might share a common final disease pathway, involving brain microvascular dysfunction and/or degeneration, as well as Aβ and tau pathology (Zlokovic 2011), as discussed below. The vascular hypothesis maintains that reduced cerebral blood flow (CBF) and hypoxia, from one end, and blood–brain barrier (BBB) dysfunction associated with accumulation of different vasculotoxic and neurotoxic macromolecules in the brain, from the other, can initiate neuronal dysfunction and neurodegnerative changes independently and/or prior to Aβ deposition (Zlokovic 2005, 2010; Bell et al. 2010; de la Torre 2010; Marchesi 2011). Moreover, several studies have suggested that cerebrovascular dysfunction and injury lead to faulty Aβ clearance from brain (Deane et al. 2004; Zlokovic 2005), increased influx of peripheral Aβ across the BBB (Deane et al. 2003; Eisele et al. 2010), and/or elevated expression of β-amyloid precursor protein (APP; Atwood et al. 2002; Kumar-Singh et al. 2005; Cullen et al. 2006; Weller et al. 2008), resulting in Aβ accumulation in the brain and around cerebral blood vessels. Elevated levels of Aβ in brain may in turn accelerate neurovascular (Deane et al. 2003; Bell et al. 2009) and neuronal (Yan et al. 1996; Walsh et al. 2002; Takuma et al. 2009) dysfunction and promote self-propagation (Meyer-Luehmann et al. 2006, 2008; Eisele et al. 2010), as in prion diseases (Prusiner 1996), leading to cerebral β-amyloidosis (Zlokovic 2008).
Figure 1.
The vascular hypothesis of Alzheimer disease. Vascular risk factors (e.g., hypertension, diabetes, obesity, cardiac disease) and/or an initial vascular damage mediated by a cerebrovascular disorder (e.g., ischemia, stroke) lead to brain hypoperfusion (oligemia) and/or blood–brain barrier (BBB) dysfunction (hit 1), which is associated with a diminished brain capillary flow/hypoxia and accumulation of multiple neurotoxins in brain, respectively, that can impact neuronal function contributing to the development of neurodegenerative changes and cognitive decline (solid lines). In a parallel pathway, BBB dysfunction and hypoperfusion/hypoxia can reduce amyloid β peptide (Aβ) vascular clearance across the BBB and increase Aβ production from Aβ-precursor protein (APP), respectively, causing Aβ accumulation in brain (hit 2; dashed lines). Elevated Aβ levels lead to formation of neurotoxic Aβ oligomers, causing neuronal dysfunction, on the one hand, and self-aggregation, on the other, which leads to self-propagation of Aβ-mediated brain disorder and the development of cerebral β-amyloidosis. According to the vascular hypothesis, a pathogenic tau phosphorylation in neurons and the development of tau-related pathology including neurofibrillary tangles (not shown in the diagram) may be triggered independently or simultaneously by a hypoperfusion/hypoxia insult and/or direct Aβ neurotoxicity.
Here we will review the role of the neurovascular unit and molecular mechanisms within cerebral vascular cells behind the pathogenesis of AD. In particular, we will focus on BBB dysfunction, decreased CBF, and impaired vascular clearance of Aβ from brain.
NEUROVASCULAR UNIT
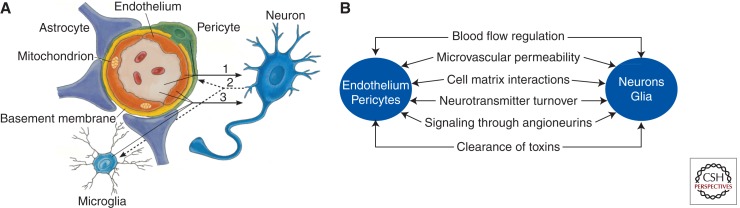
The neurovascular unit (NVU) consists of different cell types, including (1) vascular cells such as brain endothelial cells, a site of the anatomical BBB in vivo, pericytes, and vascular smooth muscle cells (VSMCs), (2) glial cells such as astrocytes, microglia and oliogodendroglia, and (3) neurons (Fig. 2A; Zlokovic 2008; Guo and Lo 2009; Moskowitz et al. 2010). The close proximity of nonneuronal neighboring cells with each other and with neurons allows for effective cell-to-cell cross-communications that are critical for normal functions in the healthy central nervous system (CNS) and are increasingly recognized as important in the disease process in multiple neurological disorders (Boillee et al. 2006; Zhong et al. 2008; Zlokovic 2008).
Figure 2.
The neurovascular unit and neurovascular functions. (A) A schematic illustration of the neurovascular unit at the level of brain capillary consisting of brain endothelial cells, pericytes, astrocytes, microglia, and neurons. Endothelial cells and pericytes share a common basement membrane and form direct “peg and socket” contacts. Astrocyte end-feet processes ensheath the capillary wall made up of pericytes and endothelial cells. (B) Vascular cells (endothelium and pericytes), glia (e.g., astrocytes), and neurons regulate multiple neurovascular functions. (Modified from Zlokovic 2008.)
The NVU functions in the healthy brain include control of neurovascular coupling and BBB permeability, matrix interactions, inactivation of neurotransmitters, signaling through angioneurins (i.e., growth factors that have both the neurotrophic and vasculotrophic functions) (Zacchigna et al. 2008), and clearance of toxins from brain (Fig. 2B).
The BBB is a highly specialized, continuous endothelial cell membrane that normally prevents the entry of plasma components, red blood cells, and leukocytes into the brain. In addition, through specific transporters in brain endothelium, the BBB regulates delivery of energy metabolites and essential nutrients that are required for proper neuronal and synaptic functions. The BBB is responsible for maintaining the constant “chemical” composition of brain interstitial fluid required for optimal brain function. Under physiological conditions, the BBB and pericytes control entry from blood and promote clearance from brain of various potentially neurotoxic and vasculotoxic macromolecules (Zlokovic 2008).
Alzheimer disease is associated with microvascular dysfunction, defective BBB, and vascular factors (Bailey et al. 2004; Wu et al. 2005; Zlokovic 2005, 2008; Paul et al. 2007; Zipser et al. 2007; Kalaria 2010; Knopman and Roberts 2010; Miyazaki et al. 2011; Neuwelt et al. 2011). Microvascular degeneration diminishes CBF, resulting in shortages in oxygen supply, energy substrates, and nutrients to the brain. On the other hand, microvascular defects compromise clearance of neurotoxic molecules from brain, resulting in accumulation of pathological deposits in brain interstitial fluid, nonneuronal cells, and neurons. According to the recent evidence, microvascular injury ultimately leads to neuronal dysfunction and development of neurodegenerative changes. Vascular damage may also contribute to the development of cerebral β-amyloidosis and cerebral amyloid angiopathy (CAA) caused by accumulation of Aβ in brain and the vessel wall, respectively (Zlokovic 2008).
CEREBRAL BLOOD FLOW DYSREGULATION AND REDUCTIONS
An adequate blood supply is ensured by a tight coupling between local tissue metabolic demands and blood perfusion of the active neuronal site. The link between regional synaptic activity and a CBF increase is known as functional hyperemia. Neurovascular coupling requires intact and effectively innervated pial and intracerebral arteries and normal responsiveness of brain endothelium, VSMCs, and pericytes to vasoactive stimuli (Iadecola 2004; Peppiatt et al. 2006; Bell et al. 2010). In addition to VSMCs, recent studies have shown that pericytes control brain capillary diameter by constricting the vessel wall (Peppiatt et al. 2006), which under ischemic conditions can completely obstructs capillary flow (Yemisci et al. 2009). Astrocytes have also been shown to regulate CBF responses by influencing contractile properties of small penetrating intracerebral arteries (Takano et al. 2007; Kuchibhotla et al. 2009).
Functional hyperemia is the basis for functional magnetic resonance imaging that has revolutionized our understanding of human brain in health and disease (Girouard and Iadecola 2006). Functional neuroimaging studies in AD individuals have shown that neurovascular uncoupling or diminished CBF responses to brain activation may occur prior to neurodegenerative changes (Smith et al. 1999; Bookheimer et al. 2000; Ruitenberg et al. 2005; Knopman and Roberts 2010). In addition, it has been reported that cognitively normal individuals bearing the apolipoprotein E (APOE) ε4 allele, which is known to be the major genetic risk factor for late-onset AD (Bertram et al. 2007; Kim et al. 2009; Verghese et al. 2011), have reduced functional hyperemia response in the absence of brain atrophy or Aβ/amyloid accumulation (Sheline et al. 2010). Diminished resting CBF has been also shown in elderly individuals at risk to develop AD (Iadecola 2004; Knopman and Roberts 2010).
Studies in animal models have indicated that CBF reductions can induce and/or amplify neuronal dysfunction and/or neuropathological changes resembling AD pathology. For example, it has been shown that Aβ constricts cerebral arteries (Thomas et al. 1996), and that endothelium-dependent regulation of cortical microcirculation is diminished in a mouse model of AD before Aβ accumulation (Iadecola et al. 1999). Moreover, in AD mice, mild hypoperfusion increases neuronal Aβ levels and tau phosphophorylation at an epitotope associated with AD-type paired helical filaments (Koike et al. 2010). Brain ischemia in rodents leads to accumulation of hyperphosphorylated tau in neurons and filament formation similar to that present in human AD tauopathy (Gordon-Krajcer et al. 2007). Arterial carotid occlusion in rats leads to memory impairment, neuronal dysfunction, synaptic changes and accumulation of neurotoxic Aβ oligomers (Wang et al. 2010). Mice expressing APP and transforming growth factor β develop neurovascular uncoupling, cholinergic denervation, accelerated Aβ deposition and age-dependent cognitive decline (Ongali et al. 2010).
It has been demonstrated that moderate CBF reductions, comparable to those as seen in the aging brain, are associated with diminished cerebral protein synthesis (Hossmann 1994; Iadecola 2004). CBF reductions >50% impair ATP synthesis and decrease the ability of neurons to fire action potentials. In addition, focal CBF reductions comparable to those as in chronic neurodegenerative disorders such as AD lead to shifts in intracellular pH, water and electrolytes that are attributed to a loss of activity of multiple energy-dependent ion pumps such as sodium/hydrogen exchanger and ATP-dependent sodium pump at the BBB (Zlokovic 2008). CBF reductions may also lead to accumulation of different toxins and glutamate in brain owing to a loss of activity at the BBB of ATP-binding cassette (ABC) efflux transporters (Dutheil et al. 2010; Elali and Hermann 2011) and Na-dependent transporters for the excitatory amino acids (O’Kane et al. 1999; Hardingham 2009), respectively. Severe reductions in CBF (>80%), similar to those found after an ischemic stroke lead to neuronal death. It is of note that changes in the NVU including degeneration of brain capillaries and/or reductions in the resting CBF may be the first sign of the disease process prior to neuronal changes and neurodegeneration.
MICROVASCULAR DEGENERATION
Alzheimer disease individuals and other dementia patients frequently have focal degenerative changes in brain microcirculation including atrophy and reductions in capillary network, a rise in endothelial vacuolization and loss of mitochondria, accumulation of collagen and perlecans in the basement capillary membrane, loss of BBB tight junction proteins (Farkas and Luiten 2001; Bailey et al. 2004; Iadecola 2004; Wu et al. 2005; Zlokovic 2005; Kalaria 2010), and leakage of blood-derived molecules (Paul et al. 2007; Zipser et al. 2007; Kalaria 2010). Aβ accumulation and amyloid deposition in pial and intracerebral arteries lead to CAA, which according to some studies is present in >80% of AD patients (Jellinger 2010; Viswanathan and Greenberg 2011). AD patients with CAA frequently develop atrophy in the VSMC layer of small arteries, causing a rupture of the vessel wall and intracerebral bleeding in about 30% of patients, which in turn aggravates dementia (Ghiso and Frangione 2002; Cordonnier 2011). Patients with hereditary cerebral β-amyloidosis with CAA in leptomeningeal and intracerebral arteries of the Dutch, Iowa, Arctic, Flemish, Italian, and Piedmont L34V type develop massive hemorrhagic strokes and dementia owing to VSMC degeneration in the vessel wall (Fossati et al. 2010). Similar, duplication of the APP gene results in AD dementia with CAA and intracerebral hemorrhage (Rovelet-Lecrux et al. 2006).
BBB DYSFUNCTION IN AD
Changes in the expression of several BBB transporters mediating nutrient transport, ion pumps, ABC transporters, and/or receptors mediating transport of peptides and proteins, including blood-to-brain and brain-to-blood exchanges of Aβ, have been described in AD and AD models (see below). Here we will focus on (1) glucose transporter 1 (GLUT1), which is a BBB-specific transporter that is of special importance because glucose is a key energy source for brain, (2) the receptor for advanced glycation end products (RAGE) that mediates Aβ reentry into the brain from circulation and the neurovascular inflammatory response, and (3) lipoprotein receptor-related protein 1 (LRP), which is a major Aβ clearance receptor at the BBB mediating Aβ efflux from brain and its systemic clearance.
GLUT1
GLUT1 expression at the BBB is diminished in AD individuals (Mooradian et al. 1997), suggesting a shortage in glucose supply to the brain. Positron emission tomography (PET) studies with 18F-2-fluoro-2-deoxy-d-glucose (FDG), have demonstrated diminished glucose uptake by the brain in individuals at increased risk for dementia (Hunt et al. 2007; Herholz 2010). Several studies have suggested that reduced glucose uptake across the BBB as seen by FDG-PET can precede brain atrophy (Mosconi et al. 2006; Hunt et al. 2007; Samuraki et al. 2007; Mosconi et al. 2008; Herholz 2010) and may be used as a potential biomarker for AD (Miller 2009; Perrin et al. 2009).
RAGE
RAGE is a multiligand receptor of the immunoglobulin superfamily (Neeper et al. 1992). RAGE binds distinct classes of ligands including AGE proteins, S100/calgranulins, Aβ, amphoterin, and the family of crossed β-sheet macromolecules (Yan et al. 2010). RAGE interaction with ligands activates signal transduction pathways, leading to sustained cellular stress as shown in chronic diseases such as diabetes, inflammation, and AD (Bucciarelli et al. 2002; Bierhaus et al. 2005; Schmidt et al. 2009). The extracellular domain of RAGE contains one V-type and two C-type immunoglobulin domains (Yan et al. 2010). Most ligands bind to RAGE’s V-domain. A single, transmembrane spanning domain is followed by a short, charged cytoplasmic domain-mediating signal transduction after ligand binding to RAGE (Yan et al. 2010). A recent crystal structure analysis of RAGE revealed a versatile structure, which explains the ability of RAGE to bind multiple, structurally distinct ligands (Koch et al. 2010; Park et al. 2010).
As a cell surface receptor for Aβ (Yan et al. 1996), RAGE binds monomeric and oligomeric Aβ via its V-domain and aggregated Aβ via its C1 domain (Sturchler et al. 2008; Yan et al. 2010). RAGE mediates Aβ-induced neurotoxicity directly by causing oxidant stress and indirectly by activating microglia (Yan et al. 1996). In addition, intraneuronal Aβ transport via RAGE leads to mitochondrial dysfunction (Takuma et al. 2009). Targeted expression of RAGE in neurons in APP-transgenic mice accelerates cognitive decline and Aβ-induced neuronal perturbation (Arancio et al. 2004).
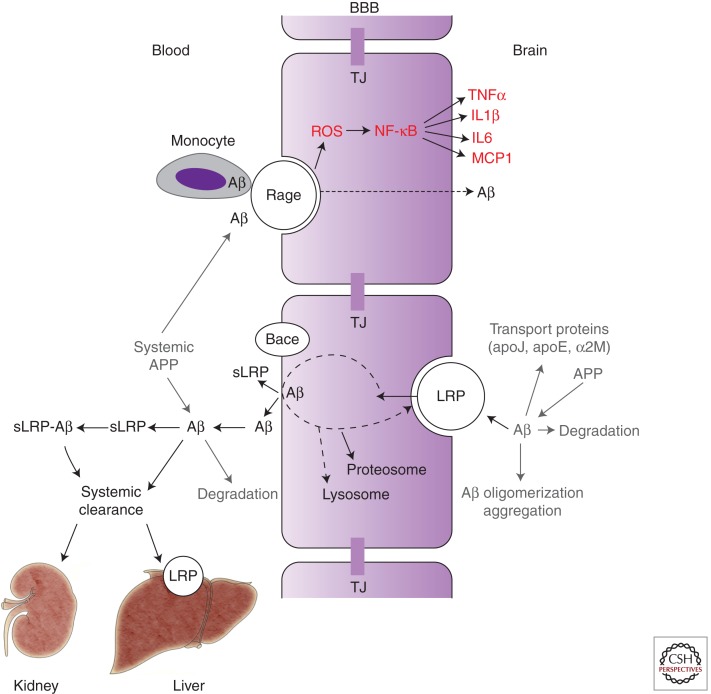
Expression of RAGE is increased in cerebrovascular endothelial cells under pathological conditions, including those seen in AD models and AD (Yan et al. 1995, 1996; Deane et al. 2003). At the BBB RAGE mediates (1) transport of circulating Aβ into the brain (Mackic et al. 1998; Deane et al. 2003), (2) NF-κB-dependent endothelial cell activation resulting in neuroinflammatory response, and (3) generation of endothelin-1 suppressing the CBF (Fig. 3; Deane et al. 2003). In addition, expression of RAGE in brain endothelium initiates cellular signaling, leading to monocyte trafficking across the BBB (Giri et al. 2000). It is of note that RAGE expression is increased in both neurons and endothelium in an Aβ-rich or AGE-rich environment as in AD (Yan et al. 1995), which amplifies Aβ-mediated pathogenic responses.
Figure 3.
The role of blood–brain barrier (BBB) transport in homeostasis of brain Aβ. Influx pathway: RAGE, the receptor for advanced glycation end products, mediates influx and reentry of circulating Aβ across the BBB. RAGE-mediated Aβ influx is accompanied by generation of reactive oxygen species (ROS) and activation of nuclear factor-κB (NF-κB)-mediated inflammatory response in endothelium, that is, increased production of cytokines including tumor necrosis factor α (TNFα), interleukin (IL) 1β and 6, and monocyte chemotactic protein-1 (MCP1), as well as increased expression of several leukocyte adhesion molecules (not shown). RAGE also mediates transport of Aβ-laden monocytes across the BBB. Efflux pathway: LRP, the low-density lipoprotein receptor-related protein-1, mediates Aβ clearance from brain via transport of free Aβ and Aβ-bound to apoE2 and apoE3, but not apoE4, across the BBB. Other Aβ transport proteins in brain interstitial fluid such as apoJ and α-2 macroglobulin (α2M) influence Aβ clearance from brain. Aβ enzymatic clearance, oligomerization, and aggregation also control Aβ levels in brain. Soluble form of LRP (sLRP) in plasma is a major binding protein of plasma Aβ. sLRP is produced by the proteolytic cleavage from LRP mediated by β-secretase (BACE). Liver and kidneys mediate systemic clearance of free Aβ and of sLRP–Aβ complexes. APP, Aβ-precurosr protein. TJ, tight junctions. (Modified from Zlokovic 2008.)
The cellular events triggered by RAGE at the BBB, neurons, microglia, and VSMCs may be implicated in the onset and progression of disease in AD models and possibly in AD. Therefore, RAGE is a potential therapeutic target in AD and blocking RAGE might contribute to control of Aβ-mediated brain disorder.
LRP
LRP, a member of the LDL receptor family, has a dual role as a rapid cargo endocytotic cellular transporter and a transmembrane cell signaling receptor (Zlokovic et al. 2010). LRP regulates transport and metabolism of apoE-associated cholesterol (Herz 2001; Herz and Strickland 2001; Herz et al. 2009). Its extracellular heavy α-chain (515 kDa) is noncovalently linked to a transmembrane and cytoplasmic light β-chain domain (85 kDa). The α-chain has four ligand-binding domains (clusters I–IV; Obermoeller-McCormick et al. 2001; Meijer et al. 2007). Domains II and IV bind more than 40 structurally diverse ligands including, to name a few, apoE, α2-macroglobulin (α2M), tissue plasminogen activator (tPA), proteinase-inhibitors, blood coagulation factor VIII, receptor-associated protein (RAP), Aβ, prion protein, and aprotinin (Hussain et al. 1999; Neels et al. 1999; Herz 2001; Herz and Strickland 2001; Croy et al. 2003; Deane et al. 2004; Meijer et al. 2007; Demeule et al. 2008; Lillis et al. 2008; Parkyn et al. 2008; Herz et al. 2009; Zlokovic et al. 2010). LRP’s cytoplasmic tail comprises two NPXY motifs, and one YXXL motif and two di-leucine motifs that both are required for rapid endocytosis of LRP ligands (Li et al. 2001; Deane et al. 2004, 2008). The cytoplasmic tail phosphorylated on serine and/or tyrosine residues (Bu et al. 1998; van der Geer 2002) interacts with different adaptor proteins associated with cell signaling such as disabled-1, FE65, and postsynaptic density protein 95 (Trommsdorff et al. 1998; Gotthardt et al. 2000; Herz et al. 2009).
Within the NVU, LRP is expressed in brain endothelium, VSMCs, pericytes, astrocytes, and neurons (Herz and Bock 2002; Polavarapu et al. 2007). LRP internalizes its ligands and directs them to lysosomes for proteolytic degradation. Recent studies have demonstrated that LRP also transports its ligands transcellularly across the BBB including Aβ (Shibata et al. 2000; Deane et al. 2004), RAP (Pan et al. 2004), tPA (Benchenane et al. 2005), lipid-free and lipidated apoE2 and apoE3, including their respective complexes with Aβ (Deane et al. 2008), and a family of Kunitz domain-derived peptides (Demeule et al. 2008).
Initial studies have suggested that LRP is linked genetically to AD and CAA (Kang et al. 1997; Lambert et al. 1998; Wavrant-DeVrieze et al. 1999; Christoforidis et al. 2005; Ballatore et al. 2007), but this has not been confirmed by later studies (Harold et al. 2009; Lambert et al. 2009). LRP and many of its ligands are normally deposited in senile plaques (Rebeck et al. 1995; Arelin et al. 2002). It has been shown that LRP interacts with APP, which influences Aβ generation (Pietrzik et al. 2004; Waldron et al. 2008). LRP also mediates Aβ neuronal uptake via α2M and apoE (Narita et al. 1997; Qiu et al. 1999; DeMattos et al. 2004; Zerbinatti et al. 2004; Zerbinatti and Bu 2005; Deane et al. 2008). The exact implication of these findings for the development of Aβ pathology remain, however, unclear. On another note, LRP interacts with γ-secretase, an APP processing enzyme, which results in inhibition of the inflammatory response, suggesting a potential for modulating inflammation (Zurhove et al. 2008).
As illustrated in Figure 3, several studies have demonstrated that LRP has a key role in a three-step serial clearance mechanism mediating Aβ elimination from brain and body (Zlokovic et al. 2010). In multiple animal models, binding of Aβ to LRP at the abluminal side of the BBB results in its rapid clearance into the blood (Shibata et al. 2000; Deane et al. 2004, 2008; Shiiki et al. 2004; Cirrito et al. 2005; Ito et al. 2006; Bell et al. 2007; Jaeger et al. 2009; Shinohara et al. 2010). A decreased expression of LRP in the choroid plexus epithelium (Johanson et al. 2006) leads to Aβ accumulation in the choroid plexus (Behl et al. 2009, 2010). Because RAP blocks apoE-dependent uptake of Aβ by astrocytes, it has been suggested that LRP and/or another member of the LDL receptor family are involved in astrocyte-mediated clearance of Aβ (Koistinaho et al. 2004). Studies using in vitro BBB models (Nazer et al. 2008; Yamada et al. 2008) have confirmed the role of LRP in Aβ endothelial cellular uptake and endocytosis, respectively, resulting in clearance of Aβ.
Reduced LRP levels in brain microvessels correlate with endogenous Aβ deposition in a chronic hydrocephalus model in rats (Klinge et al. 2006) and Aβ cerebrovascular and brain accumulation in AD patients (Shibata et al. 2000; Donahue et al. 2006). Several studies have indicated that LRP expression in brain endothelium decreases with normal aging in rodents, nonhuman primates, and humans, as well as in AD models and AD patients (Kang et al. 2000; Shibata et al. 2000; Bading et al. 2002; Deane et al. 2004; Donahue et al. 2006; Bell and Zlokovic 2009). LRP reductions have been reported in cerebral VSMCs associated with Aβ accumulation in the wall of small pial and intracerebral arteries (Bell et al. 2009). Therefore, LRP down-regulation at the BBB and in vascular cells may contribute to cerebrovascular and focal parenchymal Aβ accumulations.
In blood, the circulating form of LRP (i.e., soluble LRP, sLRP) provides a key endogenous peripheral “sink” activity for Aβ, as shown in a mouse model of AD (Sagare et al. 2007). In neurologically healthy humans and mice, sLRP binds >70% of circulating Aβ, preventing free Aβ access to the brain (Fig. 3; Sagare et al. 2007). In AD patients and AD transgenic mice, Aβ binding to sLRP is compromised by oxidation, resulting in increased levels of oxidized sLRP, which does not bind Aβ (Sagare et al. 2007). This is associated with elevated levels of free Aβ40 and Aβ42 in plasma that can reenter the brain via RAGE-mediated transport across the BBB (Deane et al. 2003; Ujiie et al. 2003; Donahue et al. 2006; Sagare et al. 2007). Moreover, in the human hippocampus, an increased RAGE expression in brain endothelium of the BBB has been shown in advanced AD compared with early stage AD and/or individuals with mild cognitive impairment (MCI; Miller et al. 2008). This might further contribute to Aβ accumulation in brain via accelerated Aβ influx from blood. In one study, a diminished sLRP-Aβ peripheral binding has been shown to precede an increase in the tau/Aβ42 CSF ratio and a drop in global cognitive decline in individuals with MCI converting into AD (Sagare et al. 2011a). Importantly, recombinant LRP fragments can effectively replace oxidized sLRP and sequester free Aβ in plasma in AD patients and AD transgenic mice, ultimately reducing Aβ-related pathology in brain (Sagare et al. 2007). Consistent with these findings, it has been suggested that sLRP and anti-RAGE antibodies that are present in Baxter’s intravenous immunoglobulin preparation Gammagard Liquid may contribute to the observed beneficial effects of Gammagard Liquid in AD patients (Relkin et al. 2009; Weber et al. 2009) by improving the peripheral Aβ sequestration and preventing entry of free Aβ into the brain (Dodel et al. 2010).
LRP in the liver mediates rapid peripheral clearance of Aβ (Tamaki et al. 2006, 2007). It is of note that reduced hepatic LRP levels have been shown to be associated with decreased peripheral Aβ clearance in the aged rats (Tamaki et al. 2006, 2007). Regulation of Aβ brain levels by the liver has been recently demonstrated in an independent study (Sagare et al. 2011b; Sutcliffe et al. 2011).
VASCULAR-SPECIFIC GENES
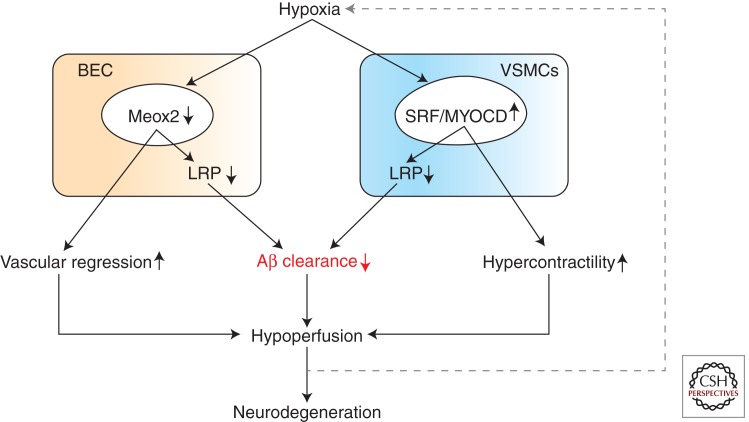
Recent findings suggest that unsuccessful vascular regeneration may lead to degeneration of brain endothelium in AD and AD models. It has been shown that brain endothelial cells in AD express extremely low levels of the mesenchyme homeobox gene 2 (MEOX-2; Wu et al. 2005), a transcription factor that regulates vascular cell differentiation and remodeling, and whose expression in the adult brain is restricted to the vascular system (Gorski and Walsh 2003). Low levels of MEOX-2 expression in AD brain endothelium have been shown to mediate an aberrant angiogenic response to vascular endothelial growth factor (Wu et al. 2005), ultimately resulting in vessel regression associated with reductions in the resting CBF (Fig. 4). Low levels of MEOX-2 also promote proteasomal degradation of LRP in brain endothelium (Wu et al. 2005) that diminishes Aβ clearance at the BBB. On the other hand, accumulation of Aβ on the outer membrane of the blood vessels is anti-angiogenic per se (Paris et al. 2004a,b). Therefore, Aβ may act in concert with low expression of MEOX2 at the BBB to focally reduce brain capillary density in AD models and AD. Importantly, MEOX2 expression is diminished by hypoxia, suggesting that hypoxia may be upstream of MEOX2 depletion seen in AD brain endothelium (Wu et al. 2005).
Figure 4.
Alterations in vascular-specific gene expression mediating neurovascular dysfunction in AD. (Left) Hypoxia down-regulates mesenchyme homeobox gene-2 (MEOX2) in brain endothelial cells (BEC). Reduced levels of MEOX2 lead to unsuccessful vascular remodeling and vascular regression, resulting in a primary endothelial hypoplasia and brain hypoperfusion. On the other hand, reduced levels of MEOX2 stimulate proteosomal degradation of LRP, a major Aβ clearance receptor, leading to a loss of LRP from BEC and reduced Aβ clearance from brain. (Right) Hypoxia increases expression of myocardin (MYOCD) in vascular smooth muscle cells (VSMCs) resulting in elevated levels of MYOCD and serum response factor (SRF). Elevated SRF/MYOCD levels lead to increased expression of several contractile proteins and calcium-regulated channels in VSMCs, resulting in a hypercontractile phenotype of small cerebral arteries and brain hypoperfusion. On the other hand, increased SRF/MYOCD activity stimulates directed expression of the sterol binding protein-2, which is a major transcriptional suppressor of LRP. Loss of LRP from VSMCs diminishes Aβ clearance from small cerebral arteries, leading to deposition of Aβ and amyloid in the arterial wall known as CAA, cerebral amyloid angiopathy. It is of note that changes in the expression of vascular-restricted genes MEOX2 and MYCD can trigger both an Aβ-independent brain hypoperfusion and Aβ accumulation, mediating neuronal dysfunction. Interestingly, hypoxia seems to be upstream to both a diminished MEOX2 expression in BEC and an increased MYOCD expression in VSMCs.
Interestingly, mice with a single allele of Meox2 develop a primary cerebral endothelial hypoplasia with an intact BBB, but a significant brain perfusion deficit (Wu et al. 2005), which has been shown to lead to secondary neurodegenerative changes prior to Aβ accumulation (Bell et al. 2010). Neurodegenerative changes in Meox2+/− mice were, however, significantly less pronounced than in pericyte-deficient mice, which have a comparable brain hypoperfusion to Meox2+/− mice but also a compromised BBB (Bell et al. 2010). These data indicate that chronic hypoperfusion alone can cause neuronal injury, but not to the same extent as when combined with BBB breakdown.
Recent studies have also shown that AD patients as well as mouse models with high cerebrovascular levels of serum response factor (SRF) and myocardin (MYOCD), the two transcription factors that control VSMC differentiation, develop a hypercontractile cerebral arterial phenotype, resulting in brain hypoperfusion, diminished functional hyperemia, and CAA (Chow et al. 2007; Bell et al. 2009). MYOCD, a SAF-A/B, Acinus, and PIAS domain family nuclear protein, is a VCMC-specific transcriptional co-activator that binds SRF to induce gene expression (Wang et al. 2001). SRF is a ubiquitously expressed transcription factor that binds to a ten-base pair cis element called a CArG box, which is located in the regulatory region of numerous target genes (Sun et al. 2006). MYOCD and SRF constitute a molecular switch for the VSMC differentiation program (Chen et al. 2002; Li et al. 2003). In addition, it has been shown that increased levels of MYOCD and SRF in AD VSMCs may suppress Aβ clearance and thus exacerbate CAA (Bell et al. 2009). Namely, high levels of MYOCD and SRF in VSMCs lead to directed expression of sterol response element binding protein 2 (SREBP2), which is a major LRP transcriptional suppressor ultimately resulting in LRP depletion, which diminishes LRP-mediated Aβ clearance from the vessel wall (Fig. 4). Hypoxia increases MYOCD levels in VSMCs (Reynolds et al. 2004; Chow et al. 2007; Bell et al. 2009), and it has been shown that it is also upstream of elevated MYOCD/SRF expression in cerebral arterial VSMCs (Bell et al. 2009). More studies are needed, however, to establish the exact role of vascular-specific genes MEOX2 and MYOCD in the development of Alzheimer neurovascular dysfunction.
CONCLUDING REMARKS
Recent clinical observations provide strong evidence for the link between cerebrovascular disease and AD and the role of vascular risk factors in AD. In this chapter, we have briefly reviewed literature on dysregulated and diminished CBF, BBB dysfunction, and impaired vascular clearance of Aβ from brain, supporting an essential role of the neurovascular and BBB mechanisms in AD pathogenesis. Several studies in animal models of AD and more recently in AD patients (Mawuenyega et al. 2010) have demonstrated a diminished Aβ clearance from brain. The recognition of Aβ clearance pathways opens exciting new therapeutic opportunities for AD. It is now established that faulty clearance from brain and across the BBB leads to elevated Aβ levels in brain that in turn have been shown to contribute to the formation of neurotoxic Aβ oligomers (Walsh et al. 2002) and the development of Aβ-mediated brain storage disorder and cerebral β-amyloidosis (Zlokovic 2008).
The activation of neurovascular pathogenic pathways has been shown to compromise synaptic and neuronal functions prior to and/or in parallel with Aβ accumulation and development of intraneuronal tangles, neuronal loss, and dementia. Some early molecular targets within the neurovascular pathway include receptors RAGE and LRP at the BBB and possibly vascular-specific genes MEOX2 and MYOCD.
Focusing on comorbidity, vascular risk factors associated with AD such as hypoperfusion, hypertension, ministrokes, and/or diabetes might generate useful models of human dementia. The proposed neurovascular model of AD raises a set of new important questions that require further study, as recently discussed (Zlokovic 2011). For example, the molecular basis of the neurovascular link with neurodegenerative disorders is still poorly understood as well as the molecular cues underlying the cross talks between different cell types of the NVU, including vascular and glia cells, and how these cellular interactions influence neuronal activity. Addressing these questions will lead to better understanding of the neurovascular link with neurodegeneration process, which will lead to the development of novel neurovascular-based approaches for AD (Zlokovic 2011).
ACKNOWLEDGMENTS
B.V.Z. thanks the United States National Institutes of Health grants R37 AG023084 and R37 NS34467 and the Zilkha family for supporting his research.
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, et al. 2004. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J 23: 4096–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT 2002. LRP and senile plaques in Alzheimer’s disease: Colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res 104: 38–46 [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bishop GM, Perry G, Smith MA 2002. Amyloid-β: A vascular sealant that protects against hemorrhage? J Neurosci Res 70: 356. [DOI] [PubMed] [Google Scholar]
- Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, Ghiso J, Frangione B, Zlokovic BV 2002. Brain clearance of Alzheimer’s amyloid-β40 in the squirrel monkey: A SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target 10: 359–368 [DOI] [PubMed] [Google Scholar]
- Bailey TL, Rivara CB, Rocher AB, Hof PR 2004. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res 26: 573–578 [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ 2007. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8: 663–672 [DOI] [PubMed] [Google Scholar]
- Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W 2009. Increased β-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol Appl Pharmacol 240: 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Zhang Y, Shi Y, Cheng J, Du Y, Zheng W 2010. Lead-induced accumulation of β-amyloid in the choroid plexus: Role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology 31: 524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV 2009. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118: 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV 2007. Transport pathways for clearance of human Alzheimer’s amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, et al. 2009. SRF and myocardin regulate LRP-mediated amyloid-β clearance in brain vascular cells. Nat Cell Biol 11: 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV 2010. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68: 409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, et al. 2005. Tissue-type plasminogen activator crosses the intact blood–brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation 111: 2241–2249 [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE 2007. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet 39: 17–23 [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP 2005. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 83: 876–886 [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW 2006. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312: 1389–1392 [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW 2000. Patterns of brain activation in people at risk for Alzheimer’s disease. New Engl J Med 343: 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Sun Y, Schwartz AL, Holtzman DM 1998. Nerve growth factor induces rapid increases in functional cell surface low density lipoprotein receptor-related protein. J Biol Chem 273: 13359–13365 [DOI] [PubMed] [Google Scholar]
- Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, et al. 2002. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation 106: 2827–2835 [DOI] [PubMed] [Google Scholar]
- Chen J, Kitchen CM, Streb JW, Miano JM 2002. Myocardin: A component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356 [DOI] [PubMed] [Google Scholar]
- Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV 2007. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci 104: 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis M, Schober R, Krohn K 2005. Genetic-morphologic association study: Association between the low density lipoprotein-receptor related protein (LRP) and cerebral amyloid angiopathy. Neuropathol Appl Neurosci 31: 11–19 [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. 2005. P-glycoprotein deficiency at the blood–brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest 115: 3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C 2011. Brain microbleeds: More evidence, but still a clinical dilemma. Curr Opin Neurol 24: 69–74 [DOI] [PubMed] [Google Scholar]
- Croy JE, Shin WD, Knauer MF, Knauer DJ, Komives EA 2003. All three LDL receptor homology regions of the LDL receptor-related protein bind multiple ligands. Biochemistry 42: 13049–13057 [DOI] [PubMed] [Google Scholar]
- Cullen KM, Kocsi Z, Stone J 2006. Microvascular pathology in the aging human brain: Evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging 27: 1786–1796 [DOI] [PubMed] [Google Scholar]
- Cummings JL 2004. Alzheimer’s disease. New Engl J Med 351: 56–67 [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al. 2003. RAGE mediates amyloid-β peptide transport across the blood–brain barrier and accumulation in brain. Nat Med 9: 907–913 [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. 2004. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43: 333–344 [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV 2008. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J Clin Invest 118: 4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC 2010. Vascular risk factor detection and control may prevent Alzheimer’s disease. Aging Res Rev 9: 218–225 [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, et al. 2004. ApoE and clusterin cooperatively suppress Aβ levels and deposition: Evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron 41: 193–202 [DOI] [PubMed] [Google Scholar]
- Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, Gabathuler R, Castaigne JP, Beliveau R 2008. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem 106: 1534–1544 [DOI] [PubMed] [Google Scholar]
- Dodel R, Neff F, Noelker C, Pul R, Du Y, Bacher M, Oertel W 2010. Intravenous immunoglobulins as a treatment for Alzheimer’s disease: Rationale and current evidence. Drugs 70: 513–528 [DOI] [PubMed] [Google Scholar]
- Donahue J, Flaherty S, Johanson C, Duncan J, Silverberg G, Miller M, Tavares R, Yang W, Wu Q, Sabo E, et al. 2006. RAGE, LRP-1, and amyloid-β protein in Alzheimer’s disease. Acta Neuropathol 112: 405–415 [DOI] [PubMed] [Google Scholar]
- Dutheil F, Jacob A, Dauchy S, Beaune P, Scherrmann JM, Decleves X, Loriot MA 2010. ABC transporters and cytochromes P450 in the human central nervous system: Influence on brain pharmacokinetics and contribution to neurodegenerative disorders. Expert Opin Drug Metab Toxicol 6: 1161–1174 [DOI] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M 2010. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science 330: 980–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elali A, Hermann DM 2011. ATP-binding cassette transporters and their roles in protecting the brain. Neuroscientist 17: 423–436 [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG 2001. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64: 575–611 [DOI] [PubMed] [Google Scholar]
- Fossati S, Cam J, Meyerson J, Mezhericher E, Romero IA, Couraud PO, Weksler BB, Ghiso J, Frangione B 2002. Amyloidosis and Alzheimer’s disease. Adv Drug Deliv Rev 54: 1539–1551 [DOI] [PubMed] [Google Scholar]
- Ghiso J, Rostagno A 2010. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-β variants in cells composing the cerebral vessel walls. FASEB 24: 229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK 2000. β-Amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol 279: C1772–C1781 [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C 2006. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335 [DOI] [PubMed] [Google Scholar]
- Gordon-Krajcer W, Kozniewska E, Lazarewicz JW, Ksiezak-Reding H 2007. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem Res 32: 729–737 [DOI] [PubMed] [Google Scholar]
- Gorski DH, Walsh K 2003. Control of vascular cell differentiation by homeobox transcription factors. Trends Cardiovasc Med 13: 213–220 [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J 2000. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem 275: 25616–25624 [DOI] [PubMed] [Google Scholar]
- Guo S, Lo EH 2009. Dysfunctional cell–cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke 40: S4–S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE 2009. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans 37: 1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: 353–356 [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, et al. 2009. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41: 1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K 2010. Cerebral glucose metabolism in preclinical and prodromal Alzheimer’s disease. Expert Rev Neurother 10: 1667–1673 [DOI] [PubMed] [Google Scholar]
- Herz J 2001. The LDL receptor gene family: (Un)expected signal transducers in the brain. Neuron 29: 571–581 [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH 2002. Lipoprotein receptors in the nervous system. Annu Rev Biochem 71: 405–434 [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK 2001. LRP: A multifunctional scavenger and signaling receptor. J Clin Invest 108: 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Chen Y, Masiulis I, Zhou L 2009. Expanding functions of lipoprotein receptors. J Lip Res 50: S287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA 1994. Viability thresholds and the penumbra of focal ischemia. Ann Neurol 36: 557–565 [DOI] [PubMed] [Google Scholar]
- Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J 2007. Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiat Res 155: 147–154 [DOI] [PubMed] [Google Scholar]
- Hussain MM, Strickland DK, Bakillah A 1999. The mammalian low-density lipoprotein receptor family. Annu Rev Nutr 19: 141–172 [DOI] [PubMed] [Google Scholar]
- Iadecola C 2004. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5: 347–360 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL 2008. Hypertension and cerebrovascular dysfunction. Cell Metab 7: 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA 1999. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2: 157–161 [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Terasaki T 2006. Functional characterization of the brain-to-blood efflux clearance of human amyloid-β peptide (1–40) across the rat blood–brain barrier. Neurosci Res 56: 246–252 [DOI] [PubMed] [Google Scholar]
- Ittner LM, Gotz J 2011. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12: 65–72 [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, et al. 2009. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood–brain barrier clearance, increases brain levels of amyloid-β protein, and impairs cognition. J Alzheimer’s Dis 17: 553–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA 2010. Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegen Dis 7: 112–115 [DOI] [PubMed] [Google Scholar]
- Johanson C, Flaherty S, Messier A, Duncan J III, Silverberg G 2006. Expression of the β-amyloid transporter, LRP1, in aging choroid plexus: Implications for the CSF–brain systemin NPH and Alzheimer’s disease. Cerebrospinal Fluid Res 3: S29 [Google Scholar]
- Kalaria RN 2010. Vascular basis for brain degeneration: Faltering controls and risk factors for dementia. Nutr Rev 68: S74–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R 1997. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology 49: 56–61 [DOI] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, et al. 2000. Modulation of amyloid β-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest 106: 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM 2009. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63: 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge PM, Samii A, Niescken S, Brinker T, Silverberg GD 2006. Brain amyloid accumulates in aged rats with kaolin-induced hydrocephalus. Neuroreport 17: 657–660 [DOI] [PubMed] [Google Scholar]
- Knopman DS, Roberts R 2010. Vascular risk factors: Imaging and neuropathologic correlates. J Alzheimers Dis 20: 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Chitayat S, Dattilo BM, Schiefner A, Diez J, Chazin WJ, Fritz G 2010. Structural basis for ligand recognition and activation of RAGE. Structure 18: 1342–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike MA, Green KN, Blurton-Jones M, Laferla FM 2010. Oligemic hypoperfusion differentially affects tau and amyloid-β. Am J Pathol 177: 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, et al. 2004. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat Med 10: 719–726 [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ 2009. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323: 1211–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C 2005. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167: 527–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Wavrant-De Vrieze F, Amouyel P, Chartier-Harlin MC 1998. Association at LRP gene locus with sporadic late-onset Alzheimer’s disease. Lancet 351: 1787–1788 [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, et al. 2009. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41: 1094–1099 [DOI] [PubMed] [Google Scholar]
- Li Y, Lu W, Marzolo MP, Bu G 2001. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J Biol Chem 276: 18000–18006 [DOI] [PubMed] [Google Scholar]
- Li S, Wang DZ, Wang Z, Richardson JA, Olson EN 2003. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci 100: 9366–9370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK 2008. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 88: 887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R 2007. Relation of diabetes to mild cognitive impairment. Arch Neurol 64: 570–575 [DOI] [PubMed] [Google Scholar]
- Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, et al. 1998. Human blood–brain barrier receptors for Alzheimer’s amyloid-β 1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest 102: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi VT 2011. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB J 25: 5–13 [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ 2010. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AB, Rohlena J, van der Zwaan C, van Zonneveld AJ, Boertjes RC, Lenting PJ, Mertens K 2007. Functional duplication of ligand-binding domains within low-density lipoprotein receptor-related protein for interaction with receptor associated protein, α2-macroglobulin, factor IXa and factor VIII. Biochim Biophys Acta 1774: 714–722 [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. 2006. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313: 1781–1784 [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT 2008. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature 451: 720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G 2009. Alzheimer’s biomarker initiative hits its stride. Science 326: 386–389 [DOI] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG 2008. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res 1230: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K 2011. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res 89: 718–728 [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Chung HC, Shah GN 1997. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging 18: 469–474 [DOI] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, Tsui W, Ginestroni A, Bessi V, Fayyazz M, et al. 2006. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 47: 1778–1786 [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ 2008. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging 29: 676–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C 2010. The science of stroke: Mechanisms in search of treatments. Neuron 67: 181–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Holtzman DM, Schwartz AL, Bu G 1997. α2-Macroglobulin complexes with and mediates the endocytosis of β-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem 69: 1904–1911 [DOI] [PubMed] [Google Scholar]
- Nazer B, Hong S, Selkoe DJ 2008. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-β peptide in a blood–brain barrier in vitro model. Neurobiol Dis 30: 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neels JG, van Den Berg BM, Lookene A, Olivecrona G, Pannekoek H, van Zonneveld AJ 1999. The second and fourth cluster of class A cysteine-rich repeats of the low density lipoprotein receptor-related protein share ligand-binding properties. J Biol Chem 274: 31305–31311 [DOI] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A 1992. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 267: 14998–15004 [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O’Donnell ME, Povlishock JT, et al. 2011. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12: 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G 2001. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci 114: 899–908 [DOI] [PubMed] [Google Scholar]
- O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA 1999. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood–brain barrier. A mechanism for glutamate removal. J Biol Chem 274: 31891–31895 [DOI] [PubMed] [Google Scholar]
- Ongali B, Nicolakakis N, Lecrux C, Aboulkassim T, Rosa-Neto P, Papadopoulos P, Tong XK, Hamel E 2010. Transgenic mice overexpressing APP and transforming growth factor-β1 feature cognitive and vascular hallmarks of Alzheimer’s disease. Am J Pathol 177: 3071–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Zankel TC, van Kerkhof P, Terasaki T, Bu G 2004. Efficient transfer of receptor-associated protein (RAP) across the blood–brain barrier. J Cell Sci 117: 5071–5078 [DOI] [PubMed] [Google Scholar]
- Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M 2004a. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett 366: 80–85 [DOI] [PubMed] [Google Scholar]
- Paris D, Townsend K, Quadros A, Humphrey J, Sun J, Brem S, Wotoczek-Obadia M, DelleDonne A, Patel N, Obregon DF, et al. 2004b. Inhibition of angiogenesis by Aβ peptides. Angiogenesis 7: 75–85 [DOI] [PubMed] [Google Scholar]
- Park H, Adsit FG, Boyington JC 2010. The 1.5 A crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem 285: 40762–40770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkyn CJ, Vermeulen EG, Mootoosamy RC, Sunyach C, Jacobsen C, Oxvig C, Moestrup S, Liu Q, Bu G, Jen A, et al. 2008. LRP1 controls biosynthetic and endocytic trafficking of neuronal prion protein. J Cell Sci 121: 773–783 [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP 2007. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med 204: 1999–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D 2006. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Fagan AM, Holtzman DM 2009. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 461: 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH 2004. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci 24: 4259–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M 2007. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood 109: 3270–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB 1996. Molecular biology and genetics of prion diseases. Cold Spring Harb Sym 61: 473–493 [PubMed] [Google Scholar]
- Qiu Z, Strickland DK, Hyman BT, Rebeck GW 1999. Alpha2-macroglobulin enhances the clearance of endogenous soluble β-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J Neurochem 73: 1393–1398 [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM 2010. Alzheimer’s disease. New Engl J Med 362: 329–344 [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Harr SD, Strickland DK, Hyman BT 1995. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the α 2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol 37: 211–217 [DOI] [PubMed] [Google Scholar]
- Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, Younkin S, Younkin L, Schiff R, Weksler ME 2009. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging 30: 1728–1736 [DOI] [PubMed] [Google Scholar]
- Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA 2004. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 279: 37124–37132 [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. 2006. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38: 24–26 [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM 2005. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam Study. Ann Neurol 57: 789–794 [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, et al. 2007. Clearance of amyloid-β by circulating lipoprotein receptors. Nat Med 13: 1029–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Deane R, Zetterberg H, Wallin A, Blennow K, Zlokovic BV 2011a. Impaired lipoprotein receptor-mediated peripheral binding of plasma amyloid-β is an early biomarker for mild cognitive impairment preceding Alzheimer’s disease. J Alzheimer’s Dis 24: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Winkler EA, Bell RD, Deane R, Zlokovic BV 2011b. From the liver to the blood–brain barrier: An interconnected system regulating brain amyloid-β levels. J Neurosci Res 89: 967–968 [DOI] [PubMed] [Google Scholar]
- Samuraki M, Matsunari I, Chen WP, Yajima K, Yanase D, Fujikawa A, Takeda N, Nishimura S, Matsuda H, Yamada M 2007. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer’s disease. Euro J Nucl Med Mol Imag 34: 1658–1669 [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM 2009. The role of RAGE in amyloid-β peptide-mediated pathology in Alzheimer’s disease. Curr Opin Investig Drugs 10: 672–680 [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, et al. 2010. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 30: 17035–17040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. 2000. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J Clin Invest 106: 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiki T, Ohtsuki S, Kurihara A, Naganuma H, Nishimura K, Tachikawa M, Hosoya K, Terasaki T 2004. Brain insulin impairs amyloid-β(1–40) clearance from the brain. J Neurosci 24: 9632–9637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Sato N, Kurinami H, Takeuchi D, Takeda S, Shimamura M, Yamashita T, Uchiyama Y, Rakugi H, Morishita R 2010. Reduction of brain β-amyloid (Aβ) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Aβ clearance. J Biol Chem 285: 22091–22102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ 1999. Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology 53: 1391–1396 [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR 1997. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277: 813–817 [PubMed] [Google Scholar]
- Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW 2008. Site-specific blockade of RAGE-Vd prevents amyloid-β oligomer neurotoxicity. J Neurosci 28: 5149–5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen G, Streb JW, Long X, Yang Y, Stoeckert CJ Jr, Miano JM 2006. Defining the mammalian CArGome. Genome Res 16: 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, Hedlund PB, Thomas EA, Bloom FE, Hilbush BS 2011. Peripheral reduction of β-amyloid is sufficient to reduce brain β-amyloid: Implications for Alzheimer’s disease. J Neurosci Res 89: 808–814 [DOI] [PubMed] [Google Scholar]
- Takano T, Han X, Deane R, Zlokovic B, Nedergaard M 2007. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann NY Acad Sci 1097: 40–50 [DOI] [PubMed] [Google Scholar]
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, et al. 2009. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-β and neuronal dysfunction. Proc Natl Acad Sci 106: 20021–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T 2006. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid β-peptide by the liver. Pharm Res 23: 1407–1416 [DOI] [PubMed] [Google Scholar]
- Tamaki C, Ohtsuki S, Terasaki T 2007. Insulin facilitates the hepatic clearance of plasma amyloid β-peptide (1–40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharm 72: 850–855 [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M 1996. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380: 168–171 [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J 1998. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem 273: 33556–33560 [DOI] [PubMed] [Google Scholar]
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA 2003. Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 10: 463–470 [DOI] [PubMed] [Google Scholar]
- van der Geer P 2002. Phosphorylation of LRP1: Regulation of transport and signal transduction. Trends Cardiovasc Med 12: 160–165 [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM 2011. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 10: 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM 2003. Silent brain infarcts and the risk of dementia and cognitive decline. New Engl J Med 348: 1215–1222 [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Greenberg SM 2011. Cerebral amyloid angiopathy (CAA) in the elderly. Ann Neurol 10.1002/ana.22516 [DOI] [PMC free article] [PubMed]
- Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU 2008. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol Dis 31: 188–197 [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ 2002. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539 [DOI] [PubMed] [Google Scholar]
- Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN 2001. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862 [DOI] [PubMed] [Google Scholar]
- Wang X, Xing A, Xu C, Cai Q, Liu H, Li L 2010. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-β oligomerization in rats. J Alzheimers Dis 21: 813–822 [DOI] [PubMed] [Google Scholar]
- Wavrant-DeVrieze F, Lambert JC, Stas L, Crook R, Cottel D, Pasquier F, Frigard B, Lambrechts M, Thiry E, Amouyel P, et al. 1999. Association between coding variability in the LRP gene and the risk of late-onset Alzheimer’s disease. Hum Genet 104: 432–434 [DOI] [PubMed] [Google Scholar]
- Weber A, Engelmaier A, Teschner W, Ehrlich HJ, Schwarz HP 2009. Intravenous immunoglobulin (IVIg) Gammagard Liquid contains anti-RAGE IgG and sLRP. Alzheimer’s Dement 5: P416 [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO 2008. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 18: 253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K 2008. Central obesity and increased risk of dementia more than three decades later. Neurology 71: 1057–1064 [DOI] [PubMed] [Google Scholar]
- Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, et al. 2005. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 11: 959–965 [DOI] [PubMed] [Google Scholar]
- Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, et al. 2008. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid β peptides in an in vitro model of the blood–brain barrier cells. J Biol Chem 283: 34554–34562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, et al. 1995. Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid β-peptide. Nat Med 1: 693–699 [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. 1996. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature 382: 685–691 [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM 2010. The RAGE axis: A fundamental mechanism signaling danger to the vulnerable vasculature. Circul Res 106: 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T 2009. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 15: 1031–1037 [DOI] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P 2008. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci 9:169–81 [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Bu G 2005. LRP and Alzheimer’s disease. Rev Neurosci 16: 123–135 [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, Bales KR, Zhuo M, Paul SM, Holtzman DM, Bu G 2004. Increased soluble amyloid-β peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci 101: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, et al. 2008. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11: 420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG 2007. Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease. Neurobiol Aging 28: 977–986 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV 2005. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 28: 202–208 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV 2008. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV 2010. Neurodegeneration and the neurovascular unit. Nat Med 16: 1370–1371 [DOI] [PubMed] [Google Scholar]
- Zlokovic BZ 2011. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci (in press) [DOI] [PMC free article] [PubMed]
- Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA 2010. Low-density lipoprotein receptor-related protein-1: A serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem 115: 1077–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurhove K, Nakajima C, Herz J, Bock HH, May P 2008. γ-Secretase limits the inflammatory response through the processing of LRP1. Sci Signal 1: ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]