Abstract
Whether thymocytes adopt an αβ or a γδ T cell fate in the thymus is determined at a checkpoint (β-selection) by the relatively weak or strong signals that are delivered by either the pre-T cell receptor (preTCR) or the γδ TCR, respectively. However, how these signals are initiated, and how different signal strengths are generated, remains unclear. Although binding of thymic agonist ligand would predict strong signaling, the preTCR and TCRγδ appear to be capable of ligand-independent signaling. Some reports have suggested that receptor oligomerization, which is thought to be mediated by either the immunoglobulin (Ig)-like domain of the preTCR α-chain (pTα) or the variable domain of TCRδ, is a unifying mechanism that initiates signaling in early CD4− CD8− double negative (DN) thymocyte progenitors. Here, we demonstrate that the extracellular regions of pTα and TCRd that were implicated in mediating receptor oligomerization were not required for signal initiation from the preTCR or TCRγδ. Indeed, a truncated TCRγδ that lacked all of its extracellular Ig-like domains still formed a signaling-competent TCR that drove cells through the β-selection checkpoint. These observations suggest that signal initiation in DN thymocytes is simply a consequence of the surface-pairing of TCR chains, with signal strength being a function of the abundances of surface TCR. Thus, processes that regulate the surface abundances of TCR complexes in DN cells, such as oligomerization-induced endocytosis, would be predicted to have a major influence in determining whether cells adopt an αβ versus γδ T cell fate.
Introduction
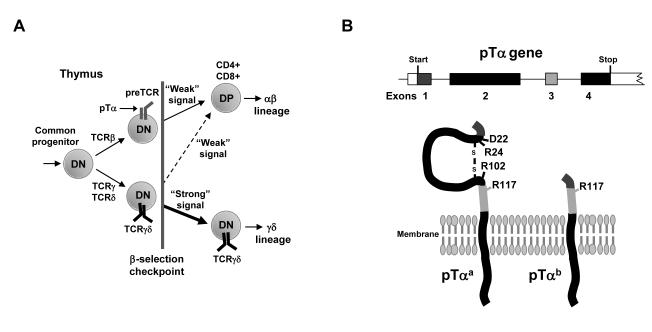
T cells defined by the presence of either an αβ or a γδ T cell receptor (TCR) on the cell surface develop in the thymus from a common hematopoietic progenitor (1, 2). Commitment to either the αβ or γδ lineage fate occurs in immature double-negative (DN) thymocytes (so called because of their lack of both CD4 and CD8) shortly after rearrangement of the genes that encode the TCRβ, TCRγ, and TCRδ chains at a stage of development referred to as the β-selection checkpoint (Fig. 1A) (3). To progress beyond this checkpoint, thymocytes must express at the cell surface either TCRγδ or the preTCR [which consists of a TCRβ chain paired with the invariant preTCRα (pTα) chain] (4). Nonetheless, lineage fate is not simply instructed by the type of rearranged TCR complex that is found on the surface of a given cell. Instead, the “strength” of the signal that is delivered by the TCR complex is critical (5, 6). Thus, TCRγδ, which appears to signal relatively strongly, largely directs cells towards the γδ lineage, whereas the preTCR, which generates a weaker signal than does TCRγδ, promotes the development of αβ lineage cells (Fig. 1A). However, despite being a major factor in αβ versus γδ cell fate determination, the mechanism that mediates TCR signal initiation and subsequent modulation of TCR signal strength in DN thymocytes has not been resolved.
Fig. 1.
Schematic representations of early thymocyte development and the pTα chains used in this study. (A) Schematic of αβ versus γδ T cell lineage fate during early thymocyte development. Cells that do not express TCR complexes are blocked at the β-selection checkpoint. Weak signaling from the preTCR or TCRγδ drives commitment to the αβ T cell lineage, whereas strong signaling from the TCRγδ drives the development of γδ cells. DN, CD4−CD8− cells; DP, CD4+CD8+ cells. (B) Schematic showing the four exons of the gene that encodes pTα and the proteins that are generated from the two known alternative splice products: pTαa and pTαb. The amino acid residues Asp22 (D22), Arg24 (R24), Arg102 (R102), and Arg117 (R117) have been implicated in preTCR oligomerization.
The consensus view is that signal initiation at the β-selection checkpoint is not, for the most part, a consequence of ligand engagement of the receptor. Instead, it is thought to reflect both a low signaling threshold in DN thymocytes and intrinsic properties of the signaling receptors themselves (7-10). Ligand-independent signaling from preTCR is suggested to result from oligomerization of preTCR complexes (9, 11). Yamasaki and co-workers implicated four charged amino acid residues in the extracellular domain of pTα, mutation of any one of which abolished pTα oligomerization in vitro, and prevented preTCR-driven developmental progression at the β-selection checkpoint in bone marrow chimeras in vivo (9). By contrast, Pang and colleagues used data from the crystal structure of the preTCR to propose that the extracellular immunoglobulin (Ig)-like domain of pTα mediated oligomerization of the preTCR and subsequent signal initiation (11).
Further weight was added to this idea of oligomerization-induced signal initiation from work on TCRγδ (10). From experiments using a T22 tetrameric FACS staining reagent to identify and characterize a subset of γδ T cells (~1% of all γδ T cells in normal animals) that recognizes the only identified murine TCRγδ ligand, T10/T22 (12), it was extrapolated that most γδ cells had not interacted with TCR ligands during thymic development. Moreover, the authors of this study demonstrated that the variable region of the TCRδ chain (Vδ) formed homodimers in vitro, and therefore they proposed that TCRγδ signaling was as a result of TCR oligomerization at the surface of DN thymocytes, rather than of ligand binding (10). Thus, the intrinsic ability of both the preTCR and TCRγδ to oligomerize at the cell surface of DN thymocytes, presumably also clustering molecules that initiate phosphorylation of signaling cascades, has emerged as an underlying mechanism by which these receptors are proposed to promote ligand-independent development beyond the β-selection checkpoint.
We have focused on the function of pTαb, which arises from an alternatively spliced isoform of the pTα-encoding gene that lacks exon 2 (13). Similar to full-length pTα (which we shall refer to as pTαa), pTαb rescues the block in thymocyte development in pTα-deficient mice (14), implying that it forms a signaling-competent preTCR. However, pTαb lacks the entire extracellular Ig-like domain of pTα, which was proposed to be necessary for preTCR oligomerization and signal initiation by Pang and co-workers, and which contains three of the four charged amino acid residues that were implicated in preTCR oligomerization by Yamasaki and colleagues (13). This prompted us to reassess the mechanism of TCR signal initiation (of both the preTCR and the TCRγδ) at the β-selection checkpoint. Here, we demonstrate that regions of pTα and TCRδ previously implicated in receptor oligomerization were not required for signal initiation in DN thymocytes. Moreover, TCRγδ receptors that lacked all of their extracellular Ig-like domains retained the ability to signal, suggesting that DN thymocytes are particularly responsive to the formation and surface expression of TCR-CD3 complexes. Collectively, these results provide insight into the mechanism of TCR signal initiation at the β-selection checkpoint, suggesting that processes that control the surface expression of appropriately paired TCR chains in DN thymocyte progenitors will directly affect αβ versus γδ cell fate determination.
Results
Extracellular regions of pTα implicated in receptor oligomerization are not required for signal initiation by the preTCR
We built on previous reports that pTαb is capable of forming a signaling-competent preTCR (14) by using a green fluorescent protein (GFP)-expressing retroviral vector (pLZ) to transduce embryonic day-14 (E14) thymocytes from pTα-deficient mice with either pTαb, full-length pTα (pTαa), or an empty vector control. Transduced cells were then cultured for up to 15 days in fetal thymic organ cultures (FTOCs) or on OP9-DL1 cells, which are both well-characterised culture systems that enable T cell development to be monitored and manipulated in vitro (15, 16).
PreTCR signaling drives the differentiation and expansion of CD4+CD8+ double-positive (DP) cells from a common precursor that also generates γδ T cells (Fig. 1A). However, TCRγδ signaling can also generate a low, but variable, number of DP cells, which are particularly evident in pTα-deficient animals (17). Thus, to assess the efficiency of preTCR signaling, we performed a combination of functional read-outs, including measurements of the increase in total thymocyte and DP cell numbers, the decrease in the percentage of γδ cells, and the increase in the ratio of DP cells to γδ cells. This combined approach was also made necessary because variable retroviral transduction efficiencies between experiments (in the range of 25 to 75%) precluded assessment by simply measuring the absolute number of GFP+ cells in any one subset alone.
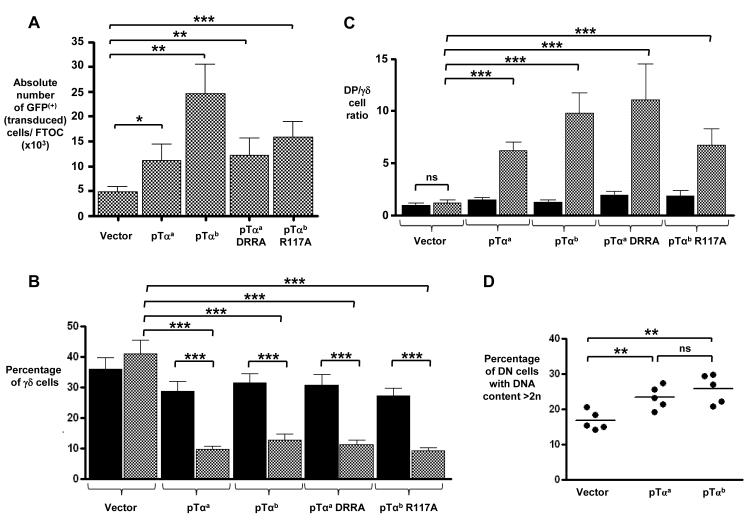
FTOCs of pTα-deficient thymocytes transduced with control vector yielded ~5 × 103 GFP+ cells (Fig. 2A), of which ~40% were γδ cells (Fig. 2B and fig. S1A), and displayed a DP:γδ cell ratio of just over one (Fig. 2C). As expected, pTα-deficient cells transduced with full-length pTαa formed a functional preTCR that increased the yields of total and DP cells (Fig. 2A and fig. S1B), decreased the representation of γδ cells to ~10% (Fig. 2B), and increased the DP:γδ cell ratio to ~6 (Fig. 2C). Cultures of pTα-deficient thymocytes transduced with pTαb also yielded substantially more total and DP cells than did cells transduced with the control vector, with the proportion of γδ cells (~12%), and the increase in the DP:γδ cell ratio (to ~10) being comparable to that observed with cells transduced with vector expressing pTαa (Fig. 2, A to C and fig. S1). We also observed an increase in the rate of DN cell proliferation in pTαa- and pTαb-expressing cultures (Fig. 2D). Together, these data confirm that pTαb formed a signaling-competent preTCR even in the absence of the extracellular Ig-like domain that contains three of four charged amino acids (Asp22, Arg24, and Arg102) implicated in signal initiation from the preTCR in previous studies (9, 11). Indeed, a full-length pTαa protein in which these three amino acids were mutated to alanines [pTαaDRRA] also formed a signaling-competent preTCR that could compete with TCRγδ to a similar degree to preTCRs containing either pTαa or pTαb (Fig. 2, A to C and fig. S1).
Fig. 2.
pTα chains that lack regions implicated in preTCR oligomerization are able to initiate signaling that complements pTα deficiency. Bar charts showing (A) the absolute number of GFP+ cells after 10 days in FTOC (n = 8 experiments), (B) the percentage of γδ cells after 12 days in FTOC (n = 7 experiments), and (C) the ratio of DP cells to γδ cells after 12 days in FTOC (n = 7 experiments) for embryonic (E14) pTα−/− thymocytes transduced with a GFP-expressing control vector or with GFP-expressing retroviral vectors encoding pTαa, pTαb, pTαaDRRA (a mutant pTαa containing alanines substituted at positions 22, 24, and 102), or pTαbR117A (a mutant pTαb with an alanine at position 117). Black bars represent GFP-negative (that is, untransduced) cells, whereas white bars represent GFP+ cells. (D) Graph showing the percentage of GFP+ pTα−/− CD4− CD8−TCRδ− DN cells transduced with vector only, vector expressing pTαa, or vector expressing pTαb after 8 days in FTOC, with >2n DNA content as judged by FAC-staining for cellular DNA with the intercalating fluorescent dye 7-Aminoactinomycin D (7-AAD), (n = 5 experiments). ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05; ns = not significant.
Of the four charged amino acid residues implicated in pTα-mediated oligomerization, the equivalent residue to Arg117 in pTαa is still present in pTαb (Fig. 1B) (9), which might be sufficient, in the absence of the Ig-like domain, to promote receptor oligomerization and signaling of pTαb-containing preTCR complexes. We therefore mutated this arginine to alanine to generate pTαbR117A. In transduced pTα-deficient thymocytes, pTαbR117A formed a signaling-competent preTCR, as determined by the resulting increased yield of total and DP cells, the reduction in the proportion of γδ cells, and the increase in the DP:γδ cell ratio, which were all comparable to those observed in pTα-deficient thymocytes that expressed pTαa or pTαb (Fig. 2, A to C and fig. S1). Collectively, these data suggest that the extracellular Ig-like domain of pTα and the residues Asp22, Arg24, Arg102, and Arg117, which have all previously been implicated in pTα oligomerization and signal transduction, were not required for signal initiation from the preTCR complex.
The variable region of TCRδ is not required for TCRγδ signal initiation in DN thymocytes
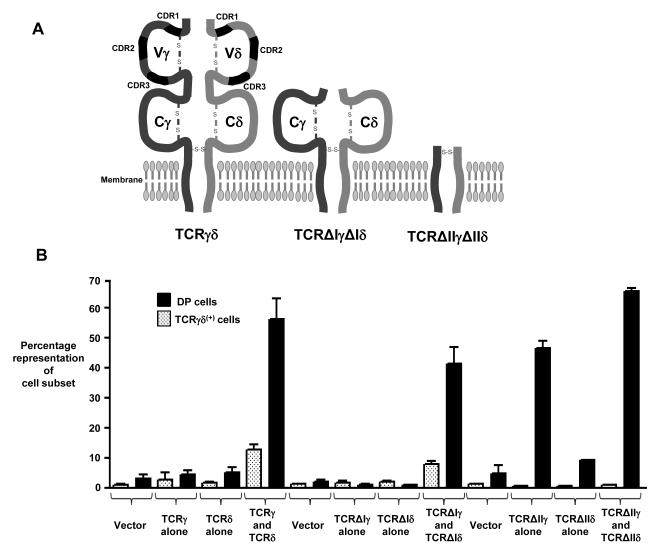
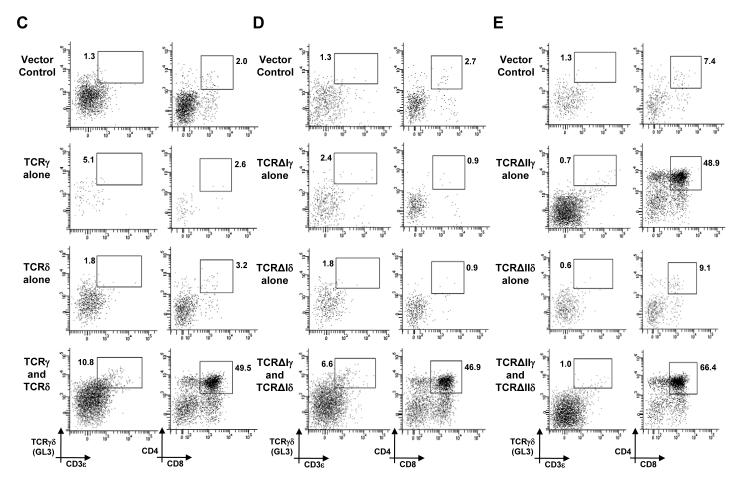
Oligomerization of surface-expressed TCRγδ complexes in DN cells, which is mediated by the variable region of TCRδ (Vδ), is proposed as a mechanism of ligand-independent signaling that promotes the thymic development of γδ cells (10). However, our data demonstrating that regions previously implicated in pTα oligomerization were not required for signal initiation by the preTCR prompted us to reassess whether oligomerization of the TCRδ chain was required to generate signaling-competent TCRγδ complexes. Thus, we transduced RAG-2-deficient E14 thymocytes with retroviral vectors expressing TCRγ and TCRδ chains that lacked Vγ and Vδ, respectively. This truncated TCRγδ, termed TCRΔγIΔδI (Fig. 3A), not only lacked the entire Vδ region that was implicated in receptor oligomerization, but also lacked complementarity determining region 1 (CDR1), CDR2, and CDR3 regions from TCRγ and TCRδ that, by analogy to TCRαβ, are implicated in ligand binding. RAG-2-deficient thymocytes are unable to rearrange the genes encoding their endogenous TCR chains, and so they are completely blocked at the β-selection checkpoint. Indeed, a RAG-2-deficient DN cell must receive a CD3-mediated signal to develop further; for example to increase the surface expression of CD4 and CD8 to become a DP cell (Fig. 1A). Simultaneous transduction of RAG-2-deficient thymocytes with vectors encoding full-length TCRγ and TCRδ resulted in the presence of TCRγδ+ cells in 7-day FTOCs, as detected by the binding of the GL3 antibody to the constant region of TCRδ (Fig. 3, B and C, left column). Nonetheless, simply detecting the surface expression of TCRγδ on DN thymocytes does not imply that the TCR has initiated signal transduction (6). Instead, a clear indication of signal initiation from the TCRγδ was the appearance of DP thymocytes (Fig. 3, B and C, right column). TCRγδ-dependent DP cells are observed in normal mice, and the generation of these cells absolutely requires signaling from a TCR complex at the DN stage (17-19).
Fig. 3.
Truncated TCRγδ receptors that lack Vδ or regions implicated in ligand binding initiate signaling in DN cells. (A) Schematic showing the TCRγ and TCRδ chains and receptors used in this study. CDRs, Ig-like domains (Vγ, Vδ, Cγ, and Cδ), and disulfide bonds are indicated. (B) Bar chart showing the percentage representation of γδ cells (white bars) or DP cells (black bars) and (C to E) representative flow cytometry plots from 8-day FTOC of E14 RAG-2−/− thymocytes transduced with GFP-expressing retroviral vector alone (vector control) or with GFP-expressing retroviral vectors encoding TCRγ, TCRδ, TCRΔIγ, TCRΔIδ, TCRΔIIγ, TCRΔIIδ, or the indicated combinations thereof. Percentages of gated cells are indicated. Data in (B) are from at least n = 3 experiments, while figures (C to E) are representative of data summarized in (B).
If Vδ is indispensible for TCRδ oligomerization and ligand-independent signaling from TCRγδ, a truncated form of TCRγδ, TCRΔγIΔδI, which lacks both Vγ and Vδ, should not enable RAG-2-deficient cells to progress beyond the block at the DN stage. Consistent with this, transduction of RAG-2-deficient thymocytes with vectors expressing either TCRΔγI alone or TCRΔδI alone did not result in the appearance of GL3+ cells and did not enable the cells to progress to the DP stage (Fig. 3, B and D). However, when RAG-2-deficient thymocytes were simultaneously transduced with vectors encoding TCRΔγI and TCRΔδI, DP cells and GL3+ thymocytes were readily detected in 7-day cultures (Fig. 3, B and D). Furthermore, these GL3+, TCRΔγIΔδI-expressing cells displayed unambiguous characteristics of genuine γδ thymocytes (rather than of DN thymocytes), as judged by multiplex polymerase chain reaction (PCR) analysis for the expression of signature genes of γδ cells, such as those encoding interferon-γ (IFN-γ) and the transcription factor T-bet (fig. S2). Thus, the variable regions of both TCRγ and TCRδ were not required for either the surface expression of a TCR complex or for the initiation of physiologically relevant signaling that can drive DN cells past the β-selection checkpoint. Moreover, signal initiation from TCRΔγIΔδI was not mediated by Vδ-domain-dependent oligomerization of TCR complexes or ligand binding to the CDR regions of the Vγ or Vδ domains. Indeed, these data suggest that initiation of ligand-independent TCRγδ signaling in DN cells proceeds by an alternative mechanism.
A TCRγδ complex lacking all extracellular Ig-like domains initiates signaling in DN thymocytes
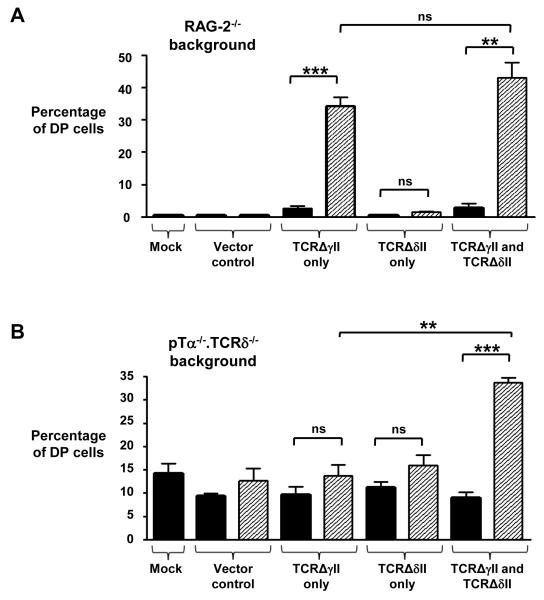
Although TCRΔγIΔδI lacks Vγ and Vδ, it still contains Ig-like domains encoded by the Cγ and Cδ portions of the TCRγ and TCRδ genes, respectively (Fig. 3A). Thus, it is possible that these Ig-like domains could initiate receptor signaling by promoting receptor oligomerization or even by binding to a ligand. To address this, we transduced RAG-2-deficient E14 thymocytes with vectors that encoded TCRγ and TCRδ chains that lacked the extracellular Ig-like domains of both the variable and constant regions. This truncated form of TCRγδ, TCRΔγIIΔδII, retained only the membrane-proximal connecting peptides of both TCRγ and TCRδ, which included cysteine residues required to form an interchain disulfide bond (Fig. 3A). After simultaneous transduction of RAG-2-deficient thymocytes with vectors encoding TCRΔγII and TCRΔδII and culture for 7 days, we could detect only a marginal increase in the number of cells bound to by an antibody against CD3ε (CD3ε+) (Fig. 3, B and E, left column). Note that the GL3 antibody could not be used to detect TCRΔγIIΔδII because the GL3-specific epitope is not present in TCRΔδII. Nonetheless, the joint expression of TCRΔγII and TCRΔδII promoted developmental progression of RAG-2-deficient DN thymocytes to the DP stage (Fig. 3, B and E, right column), implying that TCRΔγIIΔδII was capable of initiating signal transduction. We also observed substantial developmental progression to the DP stage when TCRΔγII alone was expressed in RAG-2-deficient thymocytes (Fig. 3, B and E). Together, these data suggest that the extracellular Ig-like domains of TCRγ and TCRδ are not required for the initiation of signaling in DN thymocytes, and that ligand-binding to the variable or constant Ig-like domains of TCRγδ, and Vδ-mediated TCRδ oligomerization of TCRγδ complexes are not essential mechanisms for TCRγδ signal initiation in DN thymocytes.
A TCRγ chain lacking both extracellular Ig-like domains can pair with pTα to initiate signaling in DN thymocytes
That a TCRγ chain lacking both the variable and constant extracellular Ig-like domains (TCRΔγII) could drive RAG-2-deficient DN thymocytes to the DP stage (Fig. 3, B and E) implied that TCRΔγII, but not full-length TCRγ or TCRΔγI, could initiate signaling in DN cells in the absence of any form of the TCRδ chain. It is possible that TCRΔγII might either signal alone or homodimerize. Alternatively, TCRΔγII might form a TCR complex with any pTα that is still present in RAG-2-deficient thymocytes despite the absence of all rearrangement-dependent TCR chains (TCRα, TCRβ, TCRγ, and TCRδ). To investigate this, we used our retroviral vector to express TCRΔγII in [pTα−/− × TCRδ−/−] E14 thymocytes and cultured these cells for 7 days on OP9-DL1 cells. Consistent with our observations from the FTOC system, RAG-2-deficient thymocytes transduced with either the control vector or the vector encoding TCRΔδII did not induce the development of DP cells (Fig. 4A and fig. S3A). By contrast, TCRΔγII alone, or TCRΔγII together with TCRΔδII, led to the generation of ~30 to 40% DP cells, which was consistent with the data from our FTOC system. Untransduced E14 thymocytes from [pTα−/− x TCRδ−/−] mice generated ~10 to 15% DP cells in vitro and in vivo because of rare, precocious TCRα gene rearrangements that formed TCRαβ complexes with TCRβ (17) (Fig. 4B and fig. S3B). This proportion of DP cells increased to ~35% when both TCRΔγII and TCRΔδII were present together in transduced cells, confirming that TCRγ and TCRδ chains that lack both Ig-like extracellular domains are capable of initiating signaling in DN thymocytes (Fig. 4B). However, unlike the situation observed in RAG-2-deficent thymocytes, the introduction of TCRΔγII alone did not result in an increase in the number of DP cells, but instead resulted in cells that behaved in a similar manner to that of cells expressing the vector control and the TCRΔδII chain alone (Fig. 4B and fig. S3B). These data suggest that a TCRγ chain lacking both extracellular Ig-like domains is capable of forming a TCR complex with pTα that initiates signaling in DN cells. Thus, we suggest that signal initiation in DN thymocytes appears to require pairing of appropriately matched TCR chains, rather than being a consequence of receptor oligomerization or ligand binding.
Fig. 4.
A truncated TCRγ chain lacking both extracellular Ig-like domains can pair with pTα to initiate signaling. Graphical representation of the percentages of CD4+CD8+ DP cells generated when (A) E14 RAG-2−/− (n = 3 experiments) or (B) [pTα−/−.TCRδ−/−] thymocytes (n = 3 experiments) were transduced with retroviral vector expressing GFP alone (vector control) or with retroviral vectors expressing GFP and TCRΔIIγ, TCRΔIIδ, or both, as indicated, and cultured on OP9-DL1 stromal cells for 7 days. Mock cells received no virus. Black bars represent GFP− cells from the culture, whereas white bars represent GFP+ cells. **, P ≤ 0.002; ***, P ≤ 0.0005; ns = not significant.
Discussion
The strength of signal delivered by TCR complexes expressed on the surface of DN thymocyte progenitors at the β-selection checkpoint is now accepted as a major factor in determining αβ versus γδ T cell fate (5, 6, 20). Weak signaling from the preTCR promotes the development of αβ T cells, whereas TCRγδ, which generally delivers a stronger signal than that of the preTCR, drives the development of γδ T cells. However, the critical aspects of how TCR complexes initiate signaling in DN thymocytes and how the subsequent TCR signal strength is modulated are still unclear.
Initial study of the preTCR revealed that it signalled at the cell surface in a ligand-independent manner (21, 22). Such signaling was suggested to initiate from constitutive targeting of the preTCR to kinase-rich lipid rafts through the palmitoylation of an intracellular cysteine in the pTα chain (23); however, it was subsequently shown that pTα chains lacking this cysteine signal efficiently (7, 14). Two independent reports proposed that ligand-independent preTCR signaling was a consequence of pTα oligomerization, mediated by the extracellular Ig-like domain (11) or by four essential charged amino acid residues on the outward face of the extracellular region of pTα (9). Indeed, in the latter study, mutation of any one of these amino acid residues to alanine was sufficient to abolish pTα oligomerization in vitro, and preTCR function in mixed bone marrow chimeras in vivo. TCR oligomerization as a mechanism for signal initiation in DN thymocytes was further supported by the demonstration that the variable domain of TCRδ (Vδ) promoted TCRγδ oligomerization in vitro, a process implicated by the authors of these studies in ligand-independent signaling from the TCRγδ in DN cells (10, 24). Thus, taken together with studies on pTα, oligomerization of the extracellular domains of TCR complexes (both preTCR and TCRγδ) had emerged as a unifying model for ligand-independent signal initiation at the β-selection checkpoint.
Our report challenges this model by demonstrating that signal initiation from TCR complexes at the DN stage of thymocyte development is independent of extracellular domains previously implicated in TCR oligomerization. Our observations originated from studies on a second spliced isoform of pTα, pTαb, which can signal in DN thymocytes as part of an alternative preTCR (preTCRb) (13, 14). The pTαb chain lacks the extracellular Ig-like domain of pTα that, as described earlier, is implicated in preTCR oligomerization and signal initiation (9, 11); however, both pTαb and a pTαa mutant that has alanine residues in place of Asp22, Arg24, and Arg102 [pTα aDRRA] still initiated signaling in DN cells in a manner comparable to that of pTαa. Furthermore, a mutant form of pTαb in which the remaining “oligomerization residue” (Arg117 in pTαa) was replaced by alanine [pTαbR117A] also formed a signaling-competent preTCR. We additionally demonstrated that a truncated TCRγδ that lacked both Vγ and Vδ [the latter of which is implicated in TCRδ oligomerization (10)], or that lacked all extracellular Ig-like domains (that is, Vγ, Cγ, Vδ, and Cδ) both initiated signaling in DN cells. These truncated TCRγδ receptors also lacked all six CDRs that are implicated, by analogy to TCRαβ, in ligand binding. Thus, in DN thymocytes, neither Vδ-mediated oligomerization of TCRδ chains nor ligand binding were absolutely required for the initiation of TCRγδ signaling.
Collectively, these data suggest that signal initiation from TCR complexes in DN thymocytes is simply a consequence of the surface expression of successfully paired TCR chains. This is consistent with previous observations that retrovirally expressed TCRα forms a signaling-competent TCR complex with TCRβ that complements pTα deficiency in the absence of MHC (7), and that a TCR complex consisting of TCRα and TCRγ (which contains Cγ4) can drive DN cells to the DP stage (25). This view is further supported by our observation that a TCRγ chain that lacks both extracellular Ig-like domains (that is both the Vγ and Cγ Ig-like loops) paired with pTα to initiate signaling in DN cells. The absence of comparable signaling when full-length TCRγ was given the opportunity to pair with pTα suggests that the extracellular Ig-like domains of TCRγ have a previously unappreciated role in specifying appropriate TCRγ pairing with TCRδ. Other than in DN thymocytes, there is no evidence that simple pairing of TCR chains at the cell surface results in the initiation of TCR signal transduction. DN thymocytes display a lower signaling threshold than that of mature thymocytes (7, 9) and they contain a higher concentration of lipid rafts (7). Lipid rafts were initially proposed to facilitate signaling from TCR complexes because of the increased concentration of downstream signaling elements located within them (26); however, later evidence suggested that lipid raft localisation was not required for signal initiation in DN thymocytes (9, 14).
A possible explanation for the increased signaling sensitivity in DN cells may be the absence of the CD4 and CD8 coreceptors. The cytoplasmic tails of CD4 and CD8α bind to the protein tyrosine kinase Lck (27, 28), which is critical for signal initiation from all TCRs (29). CD4 and CD8α effectively remove free Lck from the cell membrane, restricting its accessibility to TCR complexes. This is thought to confer MHC restriction during TCRαβ–mediated selection of CD4+CD8+ thymocytes, because only TCRs that bind to MHC can draw CD4 or CD8α into the TCR complex, and hence only these TCRs will have access to Lck (30). The absence of Lck sequestration by CD4 and CD8α in DN thymocytes means that free Lck is accessible to successfully formed TCR pairings, possibly through interaction with the intracellular domain of CD3ε (31). Thus, we propose that the initiation of signal transduction in DN thymocytes simply reflects the association of two CD3ε-containing signaling modules (that is, CD3εγ or CD3εδ), mediated by the pairing of two compatible TCR chains in the presence of readily accessible, free Lck. Indeed, this would explain why the cross linking of CD3ε on RAG-deficient DN thymocytes by monoclonal antibodies (32) or the dimerization of a human CD8-CD3ε fusion protein in RAG-deficient thymocytes (9) efficiently promote progression past the β-selection checkpoint.
Although we showed that regions implicated in receptor oligomerization were not required for TCR signal initiation in DN thymocytes, they may substantially affect the surface abundance of TCRs by inducing increased endocytosis and degradation of the receptors (33). This mechanism may be important for maintaining cell-surface concentrations of TCR complexes in “operating windows” that, according to the strength-of-signal hypothesis (5, 6, 20), can ensure optimal development of both αβ and γδ T cells. The regulation of the cell-surface abundance of TCR-CD3 complexes by tonic ubiquitination in more mature CD4+CD8+ DP thymocytes is implicated in setting appropriate thresholds for the positive and negative selection of T cells (34).
In summary, our study demonstrates that signal initiation by the preTCR and TCRγδ at the DN stage of thymocyte development does not require regions of the extracellular domains of pTα and TCRδ that were previously implicated in receptor oligomerization. Instead, we suggest that the simple pairing of appropriately matched TCR chains at the cell surface is sufficient for signal transduction. These conclusions suggest a revised view of TCR signaling in early thymocytes. Moreover, they suggest that processes that actively modulate the cell-surface amounts of preTCR and TCRγδ in DN thymocytes will directly affect signal strength, which in turn will determine αβ versus γδ T cell lineage commitment at the β-selection checkpoint.
Materials and Methods
Mice
C57BL/6 mice were from Harlan Laboratories. pTα−/− (4), TCRδ−/− (35), and RAG-2−/− (19) mice have been previously described and were obtained from Jackson Laboratories. [pTα−/− x TCRδ−/−] mice were obtained by crossing pTα−/− mice with TCRδ−/− mice. Embryos were obtained by the setting up of timed pregnancies. Mice were bred and maintained in the specific pathogen-free animal facilities at Queen Mary University of London. All experiments involving animals were performed in compliance with relevant laws and institutional guidelines and were approved by a local ethics committee.
Cell culture
The Phoenix ecotropic packaging cell line was kindly provided by G. Nolan (Stanford University). Phoenix cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with Glutamax (Invitrogen), 10% heat-inactivated fetal calf serum (FCS, Invitrogen), and 1% penicillin and streptomycin (Invitrogen) at 37°C and 5% CO2. Phoenix cells were transfected with the Fugene 6 transfection reagent (Roche) and then incubated for 48 hours at 37°C and 5% CO2. Transfectants were selected in puromycin (2 μg/ml, Sigma). The OP9-DL1 cell line was kindly provided by J. C. Zuniga-Pflucker (University of Toronto) (36). OP9-DL1 cells were maintained in OP9-DL1 medium [DMEM with Glutamax, 10% heat-inactivated FCS, 1% penicillin and streptomycin, β-mercaptoethanol (50 μM), and 1% non-essential amino acids] at 37°C and 5% CO2. OP9-DL1 cells were passaged 1:4 or 1:5 every two days, and were maintained at 75% confluency.
Plasmid constructs
Complementary DNAs (cDNAs) encoding pTαa and pTαb were cloned from adult C57BL/6 DN thymocytes with Phusion polymerase (Finnzymes) and the following primers: pTαForward1: 5′-TAGCCCACACCTCAGAGCTGCAG-3′; pTαReverse1: 5′-GCTATCCTATCAGAGACTGGGCTCT-3′. The PCR products were cloned into the pCRBlunt plasmid (Invitrogen) and then into the pLZRS-IRES-eGFP vector by digestion with Bam HI and Xho I (NEB). pLZRS-IRES-eGFP and pLZRS-IRES-HuNGFR were kindly provided by the G. Nolan. Site-directed mutagenesis was performed with PfuTurbo polymerase (Stratagene). To generate pTαaDRRA, a first mutagenesis step introduced the D22A and R24A mutations with the following primers: DRRAForward1: 5′-TCACACTGCTGGTAGCTGGAGCGCAGCACATGCTG-3′; DRRAReverse1: 5′-AGCATGTGCTGCGCTCCAGCTACCAGCAGTGTGA-3′. The second mutagenesis step then introduced the R102A mutation with the following primers: DRRAForward2: 5′-TGGGGGACAGAACGCGAGCACACACC-3′; DRRAReverse2: 5′-GGTGTGTGCTCGCGTTCTGTCCCCCA-3′. pTαbR117A was generated with the following primers: R117AForward1: 5′-TCTTCGACAGCCGCGAGCTGCTTTCCG-3′; R117AReverse1: 5′-CGGAAAGCAGCTCGCGGCTGTCGAAGA-3′. PCR products were digested with Dpn I (NEB) before they were used in the transformation of TOP10 competent cells (Invitrogen) for nick repair. Mutant constructs were then subcloned into pLZRS-IRES-eGFP vectors. The cDNA encoding full-length TCRγ (Vγ7Jγ1Cγ1) was cloned from adult C57BL/6 splenocytes with the following primers: Vγ7Forward: 5′-CACAAGGCATGCTGTGGGCTCTGG-3′; Vγ7Reverse: 5′-GCTGACTTGCTGTACCACCACTCG-3′. The Vγ7Jγ1Cγ1 cDNA was then cloned into the pLZRS-IRES-eGFP and pLZRS-IRES-HuNGFR vectors. TCRγ chain truncations were generated by two rounds of PCR with full length TCRγ as a template. Primers for the first round amplification were as follows: ΔI/IIγ-Forward1: 5′-GTAGGATCCACTAGTAACGGCCG-3′; ΔI γ-Reverse1: 5′-GGGCTTGGGGGAAATTTCCAAGTTGGAGGATGTTTGTCTGC-3′; ΔIγ-Forward2: 5′-ATTTCCCCCAAGCCCACTATTTTCC-3′; ΔI/IIγ-Reverse2: 5′-TATCTCGAGAATTCAGGCTTGCTGTACCACC-3′; ΔIIγ-Reverse1: 5′-ACTCACAGCAACTTTTTCCAAGTTGGAGGATGTTTGTCTGC-3′; ΔIIγ-Forward2: 5′-AAAGTTGCTGTGAGTACCAAGCCTAC-3′. These PCR products were then used as templates for PCR reactions with ΔI/IIγ-Forward1 and ΔI/IIγ-Reverse2, before being cloned into pLZRS-IRES-eGFP or pLZRS-IRES-HuNGFR. The cDNA encoding full-length TCRδ (Vδ5Dδ2Jδ1Cδ) was cloned from adult C57BL/6 thymocytes with the following primers: Vδ5Forward: 5′-CGACTGGAAGGATGATTGTTGC-3′; Vδ5Reverse: 5′-CTTAAAAGAATAACTTAACAGTCAAG-3′. The Vδ5Dδ2Jδ1Cδ cDNA was then cloned into the pLZRS-IRES-eGFP vector. TCRδ chain truncations were generated by two rounds of PCR with full-length TCRδ as a template. Primers for the first round amplification were as follows: ΔI/IIδ-Forward1: 5′-CGAGCTCGGATCCACTAGTAACGGC-3′; ΔIδ-Reverse1: 5′-AACAGATGGTTTGGCAGTGGAGCTCTGGGTCAGCGTGATG-3′; ΔIδ-Forward2: 5′-GCCAAACCATCTGTTTTCATCATG-3′; ΔI/IIδ-Reverse2: 5′-CTCTAGATGCATGCTCGAGCG-3′; ΔIIδ-Reverse1: 5′-TTGTGTGTCATTTTCAGTGGAGCTCTGGGTCAGCGTGATG-3′; ΔIIδ-Forward2: 5′-GAAAATGACACACAAATTTCAGAGCC-3′. These PCR products were then used as templates for PCR reactions with ΔI/IIδ-Forward1 and ΔI/IIδ-Reverse2, before being cloned into pLZRS-IRES-eGFP.
Retroviral harvest and transduction of primary thymocytes
Retroviral supernatants were collected from phoenix cell cultures cultured in DMEM, 20% Hi-FCS at 32°C for 16 hours after transfection with the appropriate vectors described earlier. The supernatants were centrifuged at 13,000g for 45 min at 4°C, concentrated eight-fold, and frozen. For transduction of thymocytes, 1 ml of concentrated retroviral supernatant was mixed with 1 ml of an E14 thymocyte suspension in FTOC-media [RPMI with 10% FCS, 1% penicillin and streptomycin, 2 mM L-glutamine, and β-mercaptoethanol (50 μM)] and cultured for 5 hours at 37°C and 5% CO2 in 3.5-cm2 plates coated with retronectin (12 μg/ml, Takara Bio Inc).
FTOCs
E15 thymic lobes from C57/BL6 mice were cultured on nucleopore membrane filter discs (Whatman) in FTOC medium containing 2-deoxyguanosine (1.35 mM, Sigma) for 5 days. After resting for 12 to 24 hours in FTOC medium alone, the depleted lobes were suspended in hanging drop cultures and seeded with retrovirally-transduced E14 thymocytes for 48 hours at 37°C, 5% CO2. The repopulated lobes were then cultured on nucleopore membrane filter discs for 5 to 15 days in FTOC medium.
Culture of transduced thymocytes on OP9-DL1 cells
Transduced thymocytes were seeded onto a semi-confluent monolayer of OP9-DL1 cells cultured in 6-well plates with 4 ml of OP9-DL1 media supplemented with Flt3 ligand (5 ng/ml) and IL-7 (1 ng/ml, Miltenyi Biotec). Cells were incubated for 7 days at 37°C and 5 % CO2.
Flow cytometry
All flow cytometric analysis was performed with BD-LSR-II, BD-CANTO-II, or BD-Aria flow cytometers (BD Biosciences). Single-cell suspensions were prepared from FTOCs by straining through 40-μm strainers (BD Biosciences) in FACS buffer [phosphate-buffered saline (PBS) containing 2% HI-FCS]. Thymocytes in FACS buffer were incubated on ice with conjugated antibodies specific for surface molecules. Thymocytes were washed before analysis. The following antibodies were used: Alexa Fluor 450–, phycoerythrin (PE)-Cy7–, or allophycocyanin (APC)-conjugated antibody against CD4 (GK1.5) (eBioscience); Alexa fluor 780– or PerCP-Cy5.5–conjugated antibody against CD8α (53-6.7) (BD Pharmingen); APC-conjugated antibody against TCRδ (GL3, eBioscience); PerCP-Cy5.5–conjugated antibody against TCRβ (H57-597) (eBioscience); PE-Cy5–conjugated antibody against CD3ε (145-2C11) (BD Pharmingen), PE-conjugated antibody against CD271 (NGF, BD Pharmingen). Data were analyzed with FACS DIVA software. For cell cycle analysis, cells were stained for 1 hour at 37°C with 30μl of 7-Aminoactinomycin D (7-AAD) (BD Pharmingen) in permeabilisation buffer (eBioscience).
Multiplex PCR analysis
RNA equivalent to 200 to 400 FACS-sorted cells was extracted with TRIzol reagent (Invitrogen) and treated with RNase-free RQ1 DNase (Promega). cDNA was generated with Superscript III according to the manufacturer’s protocol (Invitrogen). First round multiplex PCR used all forward and reverse primers: Ifng-F 5′-TTTGCAGCTCTTCCTCATGG-3′; Ifng-R 5′-GCCTTGCTGTTGCTGAAGAA-3′; Rorc-F 5′-AGCGCACCAACCTCTTTTCA-3′; Rorc-R 5′-TGCACATTCTGACTAGGACG-3′; Tbx21-F 5′-GGGAACCGCTTATATGTCCA-3′; Tbx21-R 5′-CTCTGGCTCTCCATCATTCA-3′; Ef1a-F 5′-TGGAATCGACAAGCGAACCA-3′; Ef1a-R 5′-CTGGGATGTGCCTGTAATCA-3′. From this mixture, a 2-μl aliquot was used as a template for each one of four individual nested PCRs. Ef1a was used as a house-keeping control. Nested primers were as follows: Ifng-Nest-R 5′-GCTGATGGCCTGATTGTCTT-3′; Rorc-Nest-R 5′-TGGCAAACTCCACCACATAC-3′; Tbx21-Nest-F 5′-CGCCAGGAAGTTTCATTTGG-3′: Ef1a-Nest-R 5′-CACGCTCAGCTTTCAGTTTG-3′.
Supplementary Material
Footnotes
Supplementary Materials Fig. S1. Fig. S2. Fig. S3.
References and Notes
- 1.Pennington DJ, Silva-Santos B, Hayday AC. Gammadelta T cell development--having the strength to get there. Curr Opin Immunol. 2005;17:108–115. doi: 10.1016/j.coi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nat Immunol. 2007;8:137–144. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- 3.Mallick CA, Dudley EC, Viney JL, Owen MJ, Hayday AC. Rearrangement and diversity of T cell receptor beta chain genes in thymocytes: a critical role for the beta chain in development. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 5.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SM, Li L, Love PE. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Haks MC, Belkowski SM, Ciofani M, Rhodes M, Lefebvre JM, Trop S, Hugo P, Zuniga-Pflucker JC, Wiest DL. Low activation threshold as a mechanism for ligand-independent signaling in pre-T cells. J Immunol. 2003;170:2853–2861. doi: 10.4049/jimmunol.170.6.2853. [DOI] [PubMed] [Google Scholar]
- 8.Borowski C, Li X, Aifantis I, Gounari F, von Boehmer H. Pre-TCRalpha and TCRalpha are not interchangeable partners of TCRbeta during T lymphocyte development. J Exp Med. 2004;199:607–615. doi: 10.1084/jem.20031973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamasaki S, Ishikawa E, Sakuma M, Ogata K, Sakata-Sogawa K, Hiroshima M, Wiest DL, Tokunaga M, Saito T. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 10.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang SS, Berry R, Chen Z, Kjer-Nielsen L, Perugini MA, King GF, Wang C, Chew SH, La Gruta NL, Williams NK, Beddoe T, Tiganis T, Cowieson NP, Godfrey DI, Purcell AW, Wilce MC, McCluskey J, Rossjohn J. The structural basis for autonomous dimerization of the pre-T-cell antigen receptor. Nature. 2010;467:844–848. doi: 10.1038/nature09448. [DOI] [PubMed] [Google Scholar]
- 12.Kreslavsky T, von Boehmer H. gammadeltaTCR ligands and lineage commitment. Semin Immunol. 2010;22:214–221. doi: 10.1016/j.smim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber DF, Passoni L, Wen L, Geng L, Hayday AC. The expression in vivo of a second isoform of pT alpha: implications for the mechanism of pT alpha action. J Immunol. 1998;161:11–16. [PubMed] [Google Scholar]
- 14.Gibbons D, Douglas NC, Barber DF, Liu Q, Sullo R, Geng L, Fehling HJ, von Boehmer H, Hayday AC. The biological activity of natural and mutant pTalpha alleles. J Exp Med. 2001;194:695–703. doi: 10.1084/jem.194.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson EJ, Anderson G, Owen JJ. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992;176:845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Pooter R, Zuniga-Pflucker JC. T-cell potential and development in vitro: the OP9-DL1 approach. Curr Opin Immunol. 2007;19:163–168. doi: 10.1016/j.coi.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Buer J, Aifantis I, DiSanto JP, Fehling HJ, von Boehmer H. Role of different T cell receptors in the development of pre-T cells. J Exp Med. 1997;185:1541–1547. doi: 10.1084/jem.185.9.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 19.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 20.Hayes SM, Laird RM, Love PE. Beyond alphabeta/gammadelta lineage commitment: TCR signal strength regulates gammadelta T cell maturation and effector fate. Semin Immunol. 2010;22:247–251. doi: 10.1016/j.smim.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Shea CC, Thornell AP, Rosewell IR, Hayes B, Owen MJ. Exit of the pre-TCR from the ER/cis-Golgi is necessary for signaling differentiation, proliferation, and allelic exclusion in immature thymocytes. Immunity. 1997;7:591–599. doi: 10.1016/s1074-7613(00)80380-5. [DOI] [PubMed] [Google Scholar]
- 22.Irving BA, Alt FW, Killeen N. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science. 1998;280:905–908. doi: 10.1126/science.280.5365.905. [DOI] [PubMed] [Google Scholar]
- 23.Aifantis I, Borowski C, Gounari F, Lacorazza HD, Nikolich-Zugich J, von Boehmer H. A critical role for the cytoplasmic tail of pTalpha in T lymphocyte development. Nat Immunol. 2002;3:483–488. doi: 10.1038/ni779. [DOI] [PubMed] [Google Scholar]
- 24.Jensen KD, Chien YH. Thymic maturation determines gammadelta T cell function, but not their antigen specificities. Curr Opin Immunol. 2009;21:140–145. doi: 10.1016/j.coi.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erman B, Feigenbaum L, Coligan JE, Singer A. Early TCRalpha expression generates TCRalphagamma complexes that signal the DN-to-DP transition and impair development. Nat Immunol. 2002;3:564–569. doi: 10.1038/ni800. [DOI] [PubMed] [Google Scholar]
- 26.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 27.Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 28.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 29.Veillette A, Horak ID, Horak EM, Bookman MA, Bolen JB. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988;8:4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Laethem F, Sarafova SD, Park JH, Tai X, Pobezinsky L, Guinter TI, Adoro S, Adams A, Sharrow SO, Feigenbaum L, Singer A. Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity. 2007;27:735–750. doi: 10.1016/j.immuni.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Brodeur JF, Li S, Martins Mda S, Larose L, Dave VP. Critical and multiple roles for the CD3epsilon intracytoplasmic tail in double negative to double positive thymocyte differentiation. J Immunol. 2009;182:4844–4853. doi: 10.4049/jimmunol.0803679. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs H, Vandeputte D, Tolkamp L, de Vries E, Borst J, Berns A. CD3 components at the surface of pro-T cells can mediate pre-T cell development in vivo. Eur J Immunol. 1994;24:934–939. doi: 10.1002/eji.1830240423. [DOI] [PubMed] [Google Scholar]
- 33.Panigada M, Porcellini S, Barbier E, Hoeflinger S, Cazenave PA, Gu H, Band H, von Boehmer H, Grassi F. Constitutive endocytosis and degradation of the pre-T cell receptor. J Exp Med. 2002;195:1585–1597. doi: 10.1084/jem.20020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Holst J, Woo SR, Guy C, Bettini M, Wang Y, Shafer A, Naramura M, Mingueneau M, Dragone LL, Hayes SM, Malissen B, Band H, Vignali DA. Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development. EMBO J. 2010;29:1285–1298. doi: 10.1038/emboj.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.