Abstract
In response to injury or stress, the adult heart undergoes maladaptive changes, collectively defined as pathological cardiac remodeling. Here, we focus on the role of A-kinase anchoring proteins (AKAPs) in 3 main areas associated with cardiac remodeling and the progression of heart failure: excitation–contraction coupling, sarcomeric regulation, and induction of pathological hypertrophy. AKAPs are a diverse family of scaffold proteins that form multi-protein complexes, integrating cAMP signaling with protein kinases, phosphatases, and other effector proteins. Many AKAPs have been characterized in the heart, where they play a critical role in modulating cardiac function.
Keywords: A-kinase anchoring protein (AKAP), signal transduction, cardiac remodeling, heart failure, excitation-contraction coupling, sarcomeric regulation, pathological hypertrophy, cAMP, protein kinase, protein phosphatase, protein phosphorylation
INTRODUCTION
The adult heart responds to injury or stress by activating a variety of intracellular signaling pathways that promote reexpression of an embryonic gene program, myocyte hypertrophy, and remodeling of the extracellular matrix.1–3 Collectively, these changes, defined as pathological cardiac remodeling, are associated with the progression of heart failure, leading to a decline in ejection fraction and dilated cardiomyopathy. It is estimated that about 1 in 3 cases of congestive heart failure is due to dilated cardiomyopathy, which can be viewed as the final phenotype of a number of primary diseases, including coronary artery disease, hyper-tension, diabetes, and valvular disease.4,5
Typically, cardiac stress occurs through injury to the ventricles due to acute myocardial infarction, promoting myocardial necrosis and left ventricular remodeling, associated with the progression of heart failure. The initial remodeling phase after a myocardial infarction results in repair of the necrotic area and myocardial scarring. This may be considered beneficial to some extent, because there is an improvement in or maintenance of left ventricle function and cardiac output. However, over time, ventricular remodeling is a maladaptive process leading to a progressive decline in left ventricle performance.1–5
The concept of cardiac remodeling was initially developed to describe cardiac changes after myocardial infarction. It has also been extended to cardiomyopathies of nonischemic origin, such as idiopathic dilated cardiomyopathy or chronic myocarditis, suggesting common mechanisms for the progression of cardiac dysfunction. Factors causing increased pressure or volume overload, including chronic hypertension, congenital heart disease, and valvular heart disease, will induce cardiac remodeling.
The cardiac myocyte is the major cell involved in remodeling and will be the main focus of this review. Fibroblasts, collagen, the interstitium, and the coronary vessels also play a role in cardiac remodeling.5
A-KINASE ANCHORING PROTEINS
A-kinase anchoring proteins (AKAPs) are a diverse family of scaffold proteins that regulate the spatiotemporal signaling within a cell by providing a framework for the formation of multienzyme signaling complexes. Dysregulation of these signaling complexes may cause a breakdown in the organization of signal transduction underlying pathophysio-logical conditions leading to the development of disease.6,7
All members of the AKAP family possess a conserved protein kinase A (PKA) anchoring domain and binding sites for additional signaling components. AKAPs act to directly couple PKA to upstream activators (ie, adenylyl cyclases), feedback inhibitors (ie, phosphodiesterases and protein phosphatases), and other signal transduction pathways (ie, small molecular weight GTPases).8 Importantly, these scaffold proteins target signaling complexes to distinct subcellular locations, thereby generating substrate specificity.9 Many AKAPs have been characterized in the heart, where they play a critical role in modulating phosphorylation of numerous PKA-dependent substrates that regulate cardiac function.10–12 AKAPs also modulate signal transduction through additional protein kinases, phosphatases, and effector proteins. Thus, the altered expression of AKAPs may result in drastic consequences, through dysregulation of localized signal transduction events.
CARDIAC PROTEIN KINASE A SIGNALING AND HEART FAILURE
PKA is important in mediating the effects of adrenergic stimulation on the heart. In response to sympathetically released catecholamines, PKA regulates contractility in cardiac myocytes through the phosphorylation of numerous substrates, including the L-type Ca2+-channel, the ryanodine receptor (RyR), phospholamban (PLB), and cardiac troponin I (cTnI).13 Although acute stimulation of PKA has beneficial effects on heart function, chronic heart failure is associated with elevated catecholamines and is characterized by dysregulation in β-adrenergic receptor (AR) function and downstream PKA signaling.14,15 Overexpression of β1-ARs in transgenic mice initially increases contractile function and responsiveness to the β-AR agonist isoproterenol. However, chronic over-stimulation of β-ARs leads to progressive deterioration of cardiac performance, cardiac hypertrophy, and heart failure.16 Transgenic overexpression of the catalytic subunit of PKA results in hypertrophy and fibrosis, suggesting that prolonged signaling through this protein kinase causes some of the detrimental consequences associated with chronically elevated β-adrenergic signaling.17
CARDIAC FIBROBLASTS AND CARDIAC REMODELING
Cardiac fibroblasts are interspersed between cardiac myocytes and comprise the majority of nonmyocytic cells in all species.18 Crosstalk between fibroblasts and myocytes is important for both cardiac development and in response to tissue injury during remodeling. Cardiac fibroblasts act mainly as a structural support during ventricular wall thickening from embryogenesis until adulthood, but they also serve as a source of mitogens, extracellular matrix proteins, cytokines, and growth factors. Intracellular communication between cardiac fibroblasts and myocytes occurs through paracrine signals (cytokines and growth factors), direct interactions (via connexins and cadherins), and indirectly by collagen and fibronectin secretion from fibroblasts to influence extracellular matrix composition, or by activating matrix metalloproteinases to promote integrin signaling through the extracellular matrix, thereby mediating hypertrophy.18,19
Fibrosis is one of the largest groups of diseases for which there is no therapy. It is believed to occur because of a persistent tissue repair program. During connective tissue repair, “activated” fibroblasts migrate into the wound area, where they synthesize and remodel newly created extracellular matrix.20 Currently, very little is known regarding the specific expression and roles of AKAPs in cardiac fibroblasts and whether they play a role in fibrosis. This is one area of research that might benefit from insight into AKAP-mediated signal transduction. Interestingly, a role for PKA in fibroblast cell migration has been demonstrated, including a defect in migration when anchored PKA is disrupted. Using mouse embryonic fibroblasts and T cells, Lim et al21 report that α4 integrins are type I PKA AKAPs. PKA–RI interacts with the integrin α4β1 and the isolated α4 cytoplasmic domain and is required for the localized phosphorylation of α4 and other substrates at the leading edge. Integrin signaling is a compelling therapeutic target for autoimmune disease and inflammation and may also be of use in the treatment of fibrosis.
CARDIAC MYOCYTES AND CARDIAC REMODELING
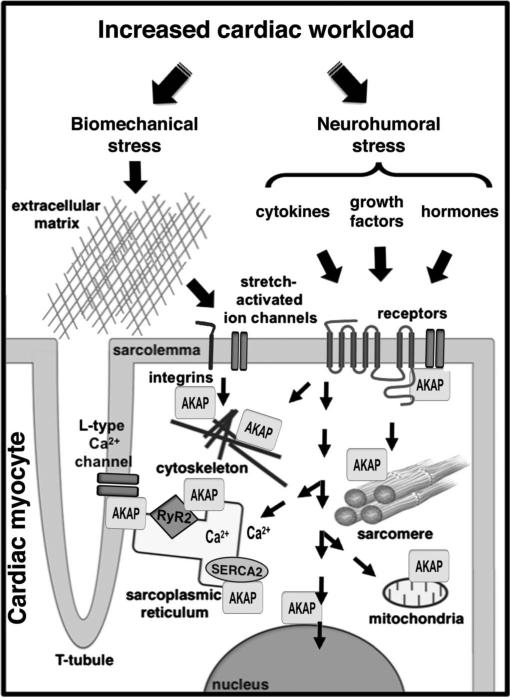
Diverse disease pathways have been identified in cardiac myocytes, implicating perturbations in force generation, force transmission, intracellular calcium homeostasis, myocardial energetics, and cardiac metabolism.1–4 AKAPs function in many of these processes, as indicated in Figure 1. This review will focus on the role of these anchoring proteins in 3 main areas: excitation–contraction coupling, sarcomeric regulation, and induction of pathological hypertrophy.
FIGURE 1.
AKAPs coordinate signaling complexes in cardiac myocytes that function in cardiac remodeling. The adult heart responds to injury or stress by activating a variety of intracellular signaling pathways that may affect calcium handling, the cytoskeleton, and sarcomeric and mitochondrial function. Different AKAPs in cardiac myocytes play a critical role in coordinating these signaling events to promote reexpression of an embryonic gene program, myocyte hypertrophy, and extracellular matrix remodeling. Refer to the text for specific details.
MODULATION OF EXCITATION– CONTRACTION COUPLING
Abnormal calcium handling and dysregulation in calcium homeostasis is a prominent feature in the transition from compensatory hypertrophy to heart failure. Several AKAPs play a role in the regulation of calcium homeostasis, including isoforms of AKAP15/18 (which target PKA to the L-type Ca2+ channel22 and the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA),23 muscle-specific A-kinase anchoring protein (mAKAP; which targets PKA in the proximity of the RyR and integrates calcineurin signaling).24
AKAP15/18 (AKAP7)
Cardiac AKAP15/18 isoforms modulate the cardiac L-type Ca2+ channel and SERCA2 activity22,23; however, it is not known exactly how these complexes are affected during cardiac remodeling and the progression of heart failure.
Alternative splicing of the AKAP7 gene yields several isoforms that possess distinct subcellular localizations. Shorter isoforms α and β are expressed in the heart and are located at the plasma membrane due to an N-terminal lipid-anchoring domain. Lipid modification of 3 residues in the N-terminus of the protein is required for association with the plasma membrane, whereas a leucine zipper–like motif in the C-terminus facilitates interaction of the cytoplasmic domain with the L-type Ca2+ channel.25,26 Thus, AKAP15/18 provides an anchored pool of PKA that is readily available to phosphorylate and regulate the channel. AKAP15/18 expression potentiates cAMP-responsive Ca2+ currents, whereas expression of a peptide to disrupt PKA–AKAP interaction results in the inhibition of L-type Ca2+ channel activity.27
The active transport of Ca2+ back into the lumen of the SR initiates myocyte relaxation. The SERCA2 pump and PLB control this process. PLB inhibits SERCA2 activity; however, β-adrenergic stimulation enhances PKA phosphorylation of PLB, which promotes its oligomerization and the derepression of SERCA2 activity.28–31
The interaction of the long splice variant of AKAP15/18 with PLB is necessary for PKA-mediated phosphorylation of PLB at Ser16, leading to the effects of adrenergic stimulation on calcium reuptake. Disruption of AKAP15/18δ-anchored PKA, interferes with PLB phosphorylation, preventing the release of PLB from SERCA2, thereby attenuating Ca2+ reuptake and myocyte relaxation.23 Reversible control of the cAMP response may be provided by an anchored pool of type 4 PDE, which is believed to associate with AKAP15/18δ.32,33 Decreased SERCA expression/activity and PLB hypophosphorylation are intricately involved in heart failure; thus, AKAP15/18δ plays a crucial role in maintaining cardiac function. Previous studies have demonstrated that expression of a pseudophosphorylated PLB34 or enhanced expression/activity of SERCA35 is sufficient not only to halt the progression of cardiomyopathy but can also restore cardiac function. Because AKAP15/18δ mediates PLB phosporylation and a subsequent increase in SERCA activity, the modulation of AKAP15/18δ may represent a novel pharmacologic target for restoring heart function.
AKAP79/150 (AKAP5)
AKAP79/150 also modulates the entry of extracellular Ca2+ through L-type calcium channels in arterial myocytes. In addition to PKA, AKAP79/150 targets the protein phosphatase calcinuerin (PP2B) and protein kinase C (PKC) to the plasma membrane.36 AKAP79/150-associated pools of PKCα participate in the induction of persistent Ca2+ sparklets.37 These sparklets are local Ca2+ signals produced by recurrent openings of L-type Ca2+ channels in arterial myocytes that enhance arterial tone.
AKAP79/150 may also be involved in the regulation of gene expression in the vascular smooth muscle during the development of hypertension. In these cells, activated PKCα stimulates persistent Ca2+ sparklets and an increase in local Ca2+ influx that acivates AKAP150-targeted PP2B. Upon activation, PP2B dephosphorylates NFATc3 to allow for translocation into the nucleus of arterial myocytes where the transcription factor can modulate gene expression. In cases of hypertension, there is an upregulation of PKCα activity that increases persistent Ca2+ sparklet activity, leading to elevated PP2B activity and NFATc3-mediated transcription.38AKAP5–/– mice were found to lack persistent Ca2+ sparklets and have lower arterial wall intracellular calcium and decreased myogenic tone. These mice were hypotensive and did not develop angiotensin II-induced hypertension.37 Cardiomyocytes from these mice exhibit disrupted β-adrenergic regulation of Ca2+ transients and phosphorylation of proteins involved in calcium handling.39 Thus, AKAP79/150 is likely to play a role in cardiac remodeling.
MUSCLE-SPECIFIC A-KINASE ANCHORING PROTEIN (AKAP6)
The mAKAP is highly expressed in cardiac myocytes where it is localized to the perinuclear membrane and junctional SR.40,41 By serving as a scaffold for a wide range of molecules including PKA, PDE4D3, ERK5, and Epac1, this anchoring protein assembles a highly regulated signaling network that can act upon nearby substrates.42 For example, mAKAP interacts with the RyR, which is regulated in response to phosphorylation by PKA.13,40 The FK506-binding protein FKBP12.6, PP2A, and PP1 form a complex with RyR2, and PKA phosphorylation of the RyR2 promotes the dissociation of FKBP12.6 from the channel, resulting in an increased probability of channel opening.43,44 Increasing the open probability of the channel enhances EC coupling gain, resulting in increased cardiac muscle contraction. Thus, localization of PKA to the RyR2 by mAKAP is critical in modulating RyR2 activity and cardiac Ca2+ transients.
Another important regulatory mechanism of cardiac contractility is mediated through phosphodiesterases that are able to rapidly hydrolyze cAMP and restore PKA to an inactive state. By scaffolding phosphodiesterases, mAKAP functions to regulate localized PKA signaling thereby maintaining efficient heart function. Recently, it has been shown that type 4 phosphodiesterases (PDE4) are important for the spatiotemporal control of cAMP after β-AR receptor stimulation.45 The alternatively spliced PDE4 isoform D3 (PDE4D3) binds to mAKAP and regulates the levels of cAMP in the vicinity of the mAKAP complex. Together, cAMP, PKA, and PDE4D3 constitute a negative feedback loop that modulates both local cAMP levels and PKA activity. Under resting conditions, the PDE maintains low cAMP levels and keeps PKA inactive. However, in response to elevated cAMP levels, mAKAP-bound PKA phosphorylates PDE4D3 on serine residues 13 and 54, resulting in increased catalytic activity of the PDE and accelerated degradation of local cAMP levels.46 In addition to its phosphodiesterase activity, mAKAP-bound PDE4D3 also acts as an adapter protein by recruiting the mitogen-activated protein kinase ERK5 and its upstream activator MEK5, and the cAMP-dependent Rap1-guanine nucleotide exchange factor Epac1 to mAKAP complexes. Activation of ERK5 leads to phosphorylation of PDE4D3 on serine residue 579, resulting in phosphodiesterase inhibition, promotion of cAMP accumulation and PKA activation. Elevated cAMP levels will also activate mAKAP-associated Epac1. Through Rap1, Epac1 suppresses ERK5 activity and prevents PDE4D3 inhibition. Thus, Epac1, ERK5, and PDE4D3 compose a second negative feedback loop through which the mAKAP signaling complex controls local cAMP levels.42 Importantly, both of these negative feedback loops depend upon regulation of PDE4D3 activity by mAKAP-bound PP2A, which catalyzes dephosphorylation of PDE4D3 Ser-54 and inhibits phosphodiesterase activity.47 In addition to regulating calcium handling, mAKAP also functions in the induction of pathological hypertrophy (Fig. 2).
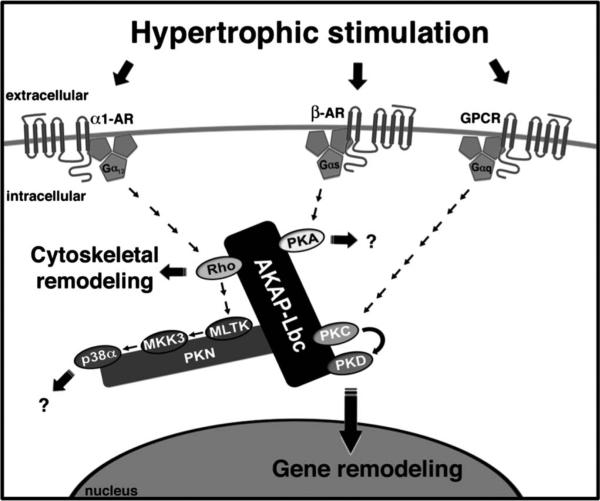
FIGURE 2.
AKAP-Lbc coordinates hypertrophic signaling to promote cytoskeletal and gene remodeling. AKAP-Lbc is present in the cytoplasm, displaying a cytoskeletal and perinuclear localization. This anchoring protein serves as a scaffold for PKA, PKD, and its upstream activating kinase PKC. By bringing PKC and PKD into close proximity, AKAP-Lbc facilitates the phosphorylation and subsequent activation of PKD by PKC. Upon activation, PKD translocates to the nucleus promoting hypertrophic gene expression through phosphorylation of a histone deacetylase (HDAC5), leading to HDAC5 nuclear export and derepression of Mef2 transcription.AKAP-Lbc is a GEF for Rho. The Rho-GEF activity of AKAP-Lbc is stimulated via the Gα12 family of heterotrimeric G-proteins in response to α1-adrenergic receptor (AR) activation. GEF activity can be inactivated by an AKAP-Lbc anchored PKA-dependent mechanism. AKAP-Lbc also coordinates a p38α MAPK complex, downstream of Rho, composed of PKNα, MLTK, MKK3, and p38α. Presently, the downstream targets and functional consequences of this AKAP-Lbc-associated signaling cascade are unknown.
PATHOLOGICAL HYPERTROPHY
Adult myocytes respond to an increase in workload through an increase in cell size (hypertrophy), rather than proliferation. Concentric hypertrophy occurs in response to pressure overload, increasing myocyte thickness, whereas eccentric hypertrophy is due to volume overload, causing the myocytes to elongate.2
Hypertrophy is initially a beneficial compensatory process as it decreases wall stress and increases cardiac output and stroke volume. However, prolonged cardiac hypertrophy is maladaptive, transitioning to decompensation and cardiac failure, where there is downregulation of β-ARs and dysregulation of PKA signaling events.14 A decreased phosphorylation of the type II regulatory subunit of PKA is observed, and as a result, there may be a change in the localization of PKA due to a difference in the affinity of AKAPs for phosphorylated and unphosphorylated RII.48
Many major pathways for pathological hypertrophy converge on a set of transcriptional regulators. mAKAP and AKAP121 have been implicated in the induction of hypertrophy through the activation of nuclear factor of activated T cells (NFAT)-mediated transcription.49,50 AKAP-Lbc promotes the induction of hypertrophy through the modulation of myocyte enhancer factor 2 (MEF2)-mediated gene remodeling.51
mAKAP (AKAP6)
Activation of the cytokine gp130 receptor by leukemia inhibitory factor through the ERK5 signaling pathway induces in cardiac hypertrophy.52 The hypertrophic effects of leukemia inhibitory factor are ablated by the activation of Epac1, suggesting the importance of the cAMP-mediated inhibition of mAKAP-bound ERK5.53 It is not entirely clear as to how the mAKAP complex is involved in the hypertrophic ERK5 signal transmission. A proposed mechanism is that ERK5-induced inhibition of PDE4D3 results in PKA potentiation of RyR-mediated release and activation of the calcineurin/NFAT pathway.54 Thus, mAKAP functions in the hypertrophic response through the coordination of multiple feedback mechanisms as depicted in Figure 3.
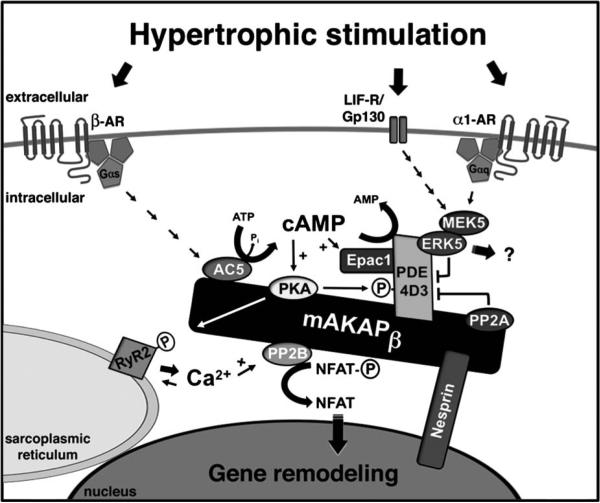
FIGURE 3.
mAKAPβ coordinates hypertrophic signaling leading to cardiac gene remodeling. mAKAPβ is predominantly localized at the cardiomyocyte outer nuclear membrane through interaction with nesprin-1α. β-AR stimulation will result in the production of cAMP through Gs-coupled activation of mAKAPβ-bound adenylyl cyclase (AC5), likely present on transverse tubules adjacent to the nucleus. cAMP synthesis promotes mAKAP-bound PKA activation, leading to the phosphorylation of multiple substrates, including the RyR2, acting to potentiate Ca2+-induced RyR2 Ca2+ release. Local Ca2+ may activate mAKAP-associated PP2B (calcineurin Aβ), which will dephosphorylate the transcription factor NFATc, resulting in NFATc nuclear translocation and hypertrophic gene expression. cAMP metabolism is tightly regulated by the mAKAP signaling complex. PKA phosphorylates both AC5 and PDE4D3, inhibiting local cAMP production and increasing cAMP degradation, respectively. PKA also phosphorylates and stimulates PP2A, opposing PKA phosphorylation of PDE4D3, leading to greater, longer lasting cAMP signals. ERK5 will also phosphorylate and inhibit PDE4D3, thereby promoting PKA activity. α1-Adrenergic and gp130/leukemia inhibitory factor receptor (LIF-R) stimulation will result in MEK5 and ERK5 activation through a JAK/STAT/Ras/Raf pathway. Other substrates for mAKAPβ-associated ERK5 have not yet been identified. For simplicity, only a single mAKAP molecule is depicted.
AKAP121 (AKAP1)
In contrast to mAKAP and AKAP-Lbc, AKAP121, has recently been identified as a negative regulator of cardiomyocyte hypertrophy. Knockdown of AKAP121 induces hypertrophy, whereas overexpression reduces cell size and inhibits the effect of the hypertrophic adrenergic agonist, isoproterenol. The effect of AKAP121 knockdown is mediated by the calcineurin/NFAT pathway, illustrated by an increase in NFATc3 nuclear localization, which can be blocked through the inhibition of calcineurin by cyclosporine A.50 The NFAT pathway is a potent inducer of cardiac hypertrophy, and its activity is tightly regulated. It is possible that calcineurin may be maintained in an inactive state due to spatial sequestration and inhibition of phosphatase activity in the AKAP121 complex. Thus, the loss of AKAP121 results in the release of an active pool of calcineurin in the cytosol. A critical target of calcineurin is the NFAT transcription factor family, which includes NFATc3. Phosphorylated NFAT is largely restricted to the cytosol. Upon dephosphorylation, NFAT translocates to the nucleus where it promotes hypertrophic gene expression.2
AKAP121 may also facilitate PKA phosphorylation of a downstream target in the NFAT/calcineurin pathway resulting in the formation of a transcriptional complex with NFAT to activate hypertrophic gene expression. Inhibition of either calcineurin or PKA activity would therefore prevent the formation of the transcriptional complex and efficient gene expression.1 Abrenica et al speculate that the identity of this putative PKA target is the transcription factor GATA4, which stimulates hypertrophic gene expression and directly interacts with NFAT.1,6–8,50
Interestingly, AKAP121 is generally thought to be located at the mitochondria; however, the relevance of this in the process of cardiac hypertrophy and remodeling was not addressed in this study. Additionally, an important question remains as to whether the loss of AKAP121 is critical for the induction of cardiac hypertrophy in vivo. Currently, it is unclear whether downregulation of AKAP121 expression is a significant mechanism in physiological or pathological hypertrophy.
AKAP-LBC (AKAP13)
AKAP-Lbc plays central role in coordinating multiple pathways involved in the induction of pathological cardiac hypertrophy (Fig. 2). AKAP-Lbc expression is upregulated in hypertrophic cardiac myocytes, and siRNA-mediated silencing of AKAP-Lbc expression in primary rat neonatal ventricular myocytes leads to a reduction in phenylephrine-stimulated hypertrophy.51,55 AKAP-Lbc serves as a scaffold for PKA, PKC, protein kinase D (PKD),54 and also contains a DH (Dbl-homology)–PH (pleckstrin-homology) domain that acts as a guanine nucleotide exchange factor (GEF) for the low molecular weight GTPase Rho,56 which is a known mediator of cardiac hypertrophy. Rho promotes the activation of mitogen-activated protein kinase (MAPK) pathways downstream of α1-adrenergic receptors (α1-ARs).57,58 Interestingly, AKAP-Lbc Rho-GEF activity is critical for α1-AR activation of Rho, through a Gα12-coupled receptor signaling pathway.55,56 Recently, it has been reported that in HEK-293 cells, AKAP-Lbc organizes a p38 MAPK complex composed of the RhoA effector PKNα and the MAPKs: MLTK, MKK3, and p38a. Thus, AKAP-Lbc assembles a signaling complex, which specifically promotes Rho-dependent activation of p38 in response to α1-AR stimulation.59 The p38 MAPK family has been implicated in several signaling pathways including promotion of proliferation, growth, inflammation, and contraction, through cytokine and G protein–coupled receptors. Specifically, α1-AR activation of p38α can regulate smooth muscle cell contractility and promote cardiomyocyte sarcomere remodeling during cardiac hypertrophy.59,60 Inactivation of AKAP-Lbc Rho-GEF activity occurs via AKAP-Lbc-bound PKA phosphorylation, which induces oligomerization and recruitment of 14-3-3 proteins.61,62 This promotes dissociation of the p38α activation module and prevents AKAP-Lbc from activating Rho.56 Together, these data indicate that AKAP-Lbc integrates signaling pathways that can regulate either activation or inhibition of p38α. It would be interesting to determine if these molecular mechanisms controlling signaling specificity occur in the heart in vivo.
In addition to Rho-mediated myocardial hypertrophy, AKAP-Lbc also promotes the initiation of a developmental gene reprogramming paradigm (often termed the fetal gene response). These “fetal” cardiac genes encode proteins involved in contraction, calcium handling, and metabolism, and their activation accompanies cardiac hypertrophy.1–5 AKAP-Lbc facilitates the activation of PKD in response to hypertrophic stimuli (phenylephrine and endothelin), through recruitment of its upstream activator, PKC.63 Activated PKD can then phosphorylate class II histone deacetylases to promote their nuclear export, leading to the derepression of the transcription factor MEF2, resulting in cardiac hypertrophy and tissue remodeling through MEF2-dependent transcription of hypertrophic genes.51,64 Although most of the study was conducted in rat neonatal ventricular myocytes, analysis of human heart tissue samples provides some further support for this mechanism. Samples obtained from individuals who exhibited cardiac hypertrophy showed that AKAP-Lbc mRNA increased 2 ± 0.5-fold over normal age-matched patient control samples.51 Thus, further research is required to determine if AKAP-Lbc may possibly be a valid biomarker for cardiac hypertrophy.
AKAP-Lbc also acts to promote stress fiber formation downstream of Rho. This was demonstrated in NIH3T3 fibroblasts,56 and the significance of this in cardiac cytoskeletal remodeling in response to stress is currently unknown. Other AKAPs have been identified that target PKA to the sarcomere for regulation of muscle fiber proteins.
SARCOMERIC REGULATION
Regulation of myofilament proteins by PKA is necessary for optimal contractile function. Phosphorylation of both cTnI or myosin-binding protein C (MyBP-C) by PKA is essential in shortening the heart beat cycle time in response to an increased demand in cardiac output.13 PKA-mediated phosphorylation is also associated with cardioprotection65 and confers resistance to calpain-mediated proteolysis of cTnI and MyBP-C.65–67 Furthermore, studies of human and experimental heart failure models show a significant decrease in the phosphorylation levels of myofilament proteins, which may account for a large part of the contractile dysfunction of the failing myocardium.68,69 Collectively, these data suggest that sarcomeric PKA anchoring is critical for contraction and that altered PKA anchoring at the myofilaments may play a role in the progression of heart failure. Studies demonstrate that both PKA types I and II are located at the cardiac sarcomere; however, the identity, composition, and function of PKA complexes at the sarcomere remains poorly understood.
SYNEMIN
Synemin colocalizes in the heart at the Z line with RII and desmin. By localizing PKA near intermediate filaments, synemin may enhance PKA phosphorylation of the substrates at the Z line such as desmin, vimentin, tubulin, and αB-crystallin. This anchoring protein may also serve to localize PKA near myofibril filaments for potential phosphorylation of substrates such as cTnI or MyBP-C. Localization of synemin at the Z line and the plasma membrane would make it ideally suited to be involved in cardiac remodeling or cardiomyopathies, where altered phosphorylation of PKA substrates is observed.70 In addition, Synemin is implicated in myofibrillar myopathy and other muscle diseases, suggesting an important pathological role.71
MYOSPRYN
Myospryn is another striated muscle-specific AKAP. It interacts with α-actinin and is located at the Z-discs. This AKAP has been studied in the context of muscle disease and in the dystrophin-deficient mdx mouse, a model of Duchenne muscular dystrophy (DMD), myospryn and its anchored PKA is mislocalized. It is thought that this is because myospryn is unable to interact with dystrophin.72 Mislocalization and dysregulation of PKA activity in muscle tissue of DMD patients may play a role in cardiac pathology, leading to cardiac remodeling. Disrupted Ca2+ homeostasis, metabolism, vulnerability to mechanical stress, and cardiac hypertrophy are observed in the mdx mice, with a concomitant decrease in expression of mAKAP, RyR, and SERCA2A.73 Interestingly, >95% of patients with DMD exhibit dilated cardiomyopathy, due to a cardiac dystrophin integrity defect, which is generally genetic, but can also be acquired.74 In addition, the expression of myospryn is upregulated by MEF2A, under hypertrophic conditions, suggesting that it is likely to play a role in cardiac remodeling.75
CARDIAC TROPONIN T
Cardiac troponin T (cTnT) has recently been reported as a novel dual-specificity sarcomeric AKAP, associating with thin filaments in isolated rat myofibrils. Sumandea et al identified cTnT as a PKA–RII interacting protein through a yeast 2-hybrid screen. Immunoprecipitation and mapping studies demonstrate that cTnT can bind both type I and II PKA through a characteristic amphipathic helix domain in cTnT. The displacement of sarcomeric PKA–cTnT complexes leads to reduced myofibrilar PKA activity.76 Currently, there are no data relating to the role of this signaling complex in the progression of heart failure.
SUMMARY/FUTURE DIRECTIONS
The regulation and localization of PKA activity is very important for proper cardiac function, and perturbation of this can lead to heart failure. We are now just beginning to understand the spatiotemporal aspects of AKAP-mediated signaling in the heart. Therapeutically, although the global inhibition of PKA activity may not be efficacious, there may be great utility in specifically inhibiting localized PKA activity that contributes to cardiac dysfunction, for example, in the modulation of calcium-handling proteins, such as the L-type Ca2+ channel, SERCA pump, and RyR. Additionally, targeting PKA for regulation of sarcomeric proteins may be important for the modulation of contractile function in heart failure and muscle diseases.
It is likely that more cardiac AKAPs will be identified in the regulation of cardiac (patho)physiology. One area that has not been explored is cardiac metabolism, where a continuous production of ATP by oxidative phosphorylation is required to sustain the contractile cycle. PKA plays a key role in regulating glycolysis and oxidative phosphorylation and is also located at the mitochondria. Changes in metabolism are observed in cardiac remodeling, which may be modulated through AKAP signaling complexes at the mitochondria, possibly in response to oxidative stress.
The progression of heart failure is a multifactorial process; therefore, we need to understand how multiple signals are integrated leading to a disease outcome. Currently, we do not know if and how AKAP signaling complexes are altered during heart disease progression and how this could affect cardiac remodeling. For example, is AKAP complex stoichiometry modified, and are enzymes regulated or targeted differently under pathological conditions? Clearly, more work is required to determine precisely how AKAP signaling complexes function in the progression of heart failure.
Footnotes
The authors declare no conflicts of interest.
(See Editorial, A-Kinase Anchoring Proteins: Temporal and Spatial Regulation of Intracellular Signal Transduction in the Cardiovascular System by Michael S. Kapiloff and Kshama D. Chandrasekhar, Journal of Cardiovascular Pharmacology 2011;58:337-338)
REFERENCES
- 1.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 2.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. NatRev MolCellBiol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 3.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 5.Barry SP, Townsend PA. What causes a broken heart—molecular insights into heart failure. Int Rev Cell Mol Biol. 2010;284:113–179. doi: 10.1016/S1937-6448(10)84003-1. [DOI] [PubMed] [Google Scholar]
- 6.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they're apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61:394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 9.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nature Rev Mol Cell Biol. 2004;5:959–971. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 10.Diviani D. Modulation of cardiac function by A-kinase anchoring proteins. Curr Opin Pharmacol. 2008;8:166–173. doi: 10.1016/j.coph.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pidoux G, Tasken K. Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J Mol Endocrinol. 2010;44:271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- 13.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 14.Movsesian MA, Bristow MR. Alterations in cAMP-mediated signaling and their role in the pathophysiology of dilated cardiomyopathy. Curr Top Dev Biol. 2005;68:25–48. doi: 10.1016/S0070-2153(05)68002-7. [DOI] [PubMed] [Google Scholar]
- 15.Bristow MR. Myocardial beta-adrenergic receptor downregulation in heart failure. Int J Cardiol. 1984;5:648–652. doi: 10.1016/0167-5273(84)90179-7. [DOI] [PubMed] [Google Scholar]
- 16.Bisognano JD, Weinberger HD, Bohlmeyer TJ, et al. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 17.Antos CL, Frey N, Marx SO, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 18.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottaviano FG, Yee KO. Communication signals between cardiac fibroblasts and cardiac myocytes. J Cardiovasc Pharmacol. 2011;57:513–521. doi: 10.1097/FJC.0b013e31821209ee. [DOI] [PubMed] [Google Scholar]
- 20.van den Borne SW, Diez J, Blankesteijn WM, et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 21.Lim CJ, Han J, Yousefi N, et al. Alpha4 integrins are type I cAMP-dependent protein kinase-anchoring proteins. Nat Cell Biol. 2007;9:415–421. doi: 10.1038/ncb1561. [DOI] [PubMed] [Google Scholar]
- 22.Gray PC, Tibbs VC, Catterall WA, et al. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 23.Lygren B, Carlson CR, Santamaria K, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauman AL, Michel JJ, Henson E, et al. The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life. 2007;59:163–169. doi: 10.1080/15216540701358593. [DOI] [PubMed] [Google Scholar]
- 25.Fraser ID, Tavalin SJ, Lester LB, et al. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulme JT, Lin TW, Westenbroek RE, et al. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray PC, Scott JD, Catterall WA. Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr Opin Neurobiol. 1998;8:330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 29.Katz AM. Discovery of phospholamban. A personal history. Ann N Y Acad Sci. 1998;853:9–19. doi: 10.1111/j.1749-6632.1998.tb08252.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt JP, Kamisago M, Asahi M, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 31.Kelly EM, Hou Z, Bossuyt J, et al. Phospholamban oligomerization, quaternary structure, and sarco(endo)plasmic reticulum calcium ATPase binding measured by fluorescence resonance energy transfer in living cells. J Biol Chem. 2008;283:12202–12211. doi: 10.1074/jbc.M707590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefan E, Wiesner B, Baillie GS, et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 33.Lygren B, Tasken K. Compartmentalized cAMP signalling is important in the regulation of Ca(2+) cycling in the heart. Biochem Soc Trans. 2006;34(pt 4):489–491. doi: 10.1042/BST0340489. [DOI] [PubMed] [Google Scholar]
- 34.Hoshijima M, Ikeda Y, Iwanaga Y, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto MI, del Monte F, Schmidt U, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klauck TM, Faux MC, Labudda K, et al. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 37.Navedo MF, Nieves-Cintron M, Amberg GC, et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 38.Nieves-Cintron M, Amberg GC, Navedo MF, et al. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols CB, Rossow CF, Navedo MF, et al. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001;114(pt 17):3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Drazba JA, Ferguson DG, et al. A-kinase anchoring protein 100 (AKAP100) is localized in multiple subcellular compartments in the adult rat heart. J Cell Biol. 1998;142:511–522. doi: 10.1083/jcb.142.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodge-Kafka KL, Soughayer J, Pare GC, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marx SO, Reiken S, Hisamatsu Y, et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 45.Fischmeister R, Castro LR, Abi-Gerges A, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 46.Dodge KL, Khouangsathiene S, Kapiloff MS, et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dodge-Kafka KL, Bauman A, Mayer N, et al. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem. 2010;285:11078–11086. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakhary DR, Fink MA, Ruehr ML, et al. Selectivity and regulation of A-kinase anchoring proteins in the heart. The role of autophosphorylation of the type II regulatory subunit of cAMP-dependent protein kinase. J Biol Chem. 2000;275:41389–41395. doi: 10.1074/jbc.M004212200. [DOI] [PubMed] [Google Scholar]
- 49.Pare GC, Bauman AL, McHenry M, et al. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118(pt 23):5637–5646. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 50.Abrenica B, AlShaaban M, Czubryt MP. The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2009;46:674–681. doi: 10.1016/j.yjmcc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Carnegie GK, Soughayer J, Smith FD, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicol RL, Frey N, Pearson G, et al. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodge-Kafka KL, Kapiloff MS. The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways. Eur J Cell Biol. 2006;85:593–602. doi: 10.1016/j.ejcb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q, Hofmann PA. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H2204–H2212. doi: 10.1152/ajpheart.01050.2003. [DOI] [PubMed] [Google Scholar]
- 55.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, et al. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:10140–10145. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 57.Maruyama Y, Nishida M, Sugimoto Y, et al. Galpha(12/13) mediates alpha(1)-adrenergic receptor-induced cardiac hypertrophy. Circ Res. 2002;91:961–969. doi: 10.1161/01.res.0000043282.39776.7c. [DOI] [PubMed] [Google Scholar]
- 58.Charron F, Tsimiklis G, Arcand M, et al. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 2001;15:2702–2719. doi: 10.1101/gad.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cariolato L, Cavin S, Diviani D. A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in alpha1-adrenergic receptor-induced p38 activation. J Biol Chem. 2011;286:7925–7937. doi: 10.1074/jbc.M110.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srinivasan R, Forman S, Quinlan RA, et al. Regulation of contractility by Hsp27 and Hic-5 in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2008;294:H961–H969. doi: 10.1152/ajpheart.00939.2007. [DOI] [PubMed] [Google Scholar]
- 61.Diviani D, Abuin L, Cotecchia S, et al. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin J, Smith FD, Stark C, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 63.Carnegie GK, Smith FD, McConnachie G, et al. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Vega RB, Harrison BC, Meadows E, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadayappan S, Osinska H, Klevitsky R, et al. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci U S A. 2006;103:169. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 67.Nagayama T, Takimoto E, Sadayappan S, et al. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation. 2007;116:2399–2408. doi: 10.1161/CIRCULATIONAHA.107.706523. [DOI] [PubMed] [Google Scholar]
- 68.Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovasc Res. 2008;77:616–618. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- 69.Hamdani N, de Waard M, Messer AE, et al. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 70.Russell MA, Lund LM, Haber R, et al. The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys. 2006;456:204–215. doi: 10.1016/j.abb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Paulin D, Huet A, Khanamyrian L, et al. Desminopathies in muscle disease. J Pathol. 2004;204:418–427. doi: 10.1002/path.1639. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds JG, McCalmon SA, Donaghey JA, et al. Deregulated PKA signaling andmyosprynexpression inmusculardystrophy. J Biol Chem. 2008;283:8070–8074. doi: 10.1074/jbc.C700221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rohman MS, Emoto N, Takeshima Y, et al. Decreased mAKAP, ryanodine receptor, and SERCA2a gene expression in mdx hearts. Biochem Biophys Res Commun. 2003;310:228–235. doi: 10.1016/j.bbrc.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- 75.Durham JT, Brand OM, Arnold M, et al. Myospryn is a direct transcriptional target for MEF2A that encodes a striated muscle, alpha-actinin-interacting, costamere-localized protein. J Biol Chem. 2006;281:6841–6849. doi: 10.1074/jbc.M510499200. [DOI] [PubMed] [Google Scholar]
- 76.Sumandea CA, Garcia-Cazarin ML, Bozio CH, et al. CTnT, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J Biol Chem. 286:530–541. doi: 10.1074/jbc.M110.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]