Abstract
Human and animal model studies have linked brain-derived neurotrophic factor (BDNF) with the etiology of anxiety disorders. This pleiotropic neurotrophin and its receptor, TrkB, promote neuronal survival, differentiation and synaptic plasticity. Here we interrogated the role of BDNF in serotonergic neurotransmission in the basolateral amygdala (BLA), a limbic brain region associated with the neurobiology of anxiety. We found that both GABAergic and pyramidal projection neurons in the wild-type BLA contained TrkB receptors. Examination of BDNF2L/2LCk-cre mutant mice with brain-selective depletion of BDNF revealed mild decreases in serotonin content in the BLA. Notably, whole cell recordings in BLA pyramidal cells uncovered significant alterations in 5-HT2-mediated regulation of GABAergic and glutamatergic transmission in BDNF2L/2LCk-Cre mutant mice that result in a hyperexcitable circuit. These changes were associated with decreased expression of 5-HT2 receptors. Collectively, the results indicate a required role of BDNF in serotonin transmission in the BLA. Furthermore, they suggest a mechanism underlying the reported increase in anxiety-like behavior elicited by perturbed BDNF signaling.
Keywords: BDNF, serotonin, amygdala, anxiety, pyramidal, GABA, excitability
Brain-derived neurotrophic factor (BDNF) is a multifunctional growth factor active in the developing and mature brain that signals through the TrkB receptor to promote neuronal survival, differentiation and synaptic plasticity. Perturbed BDNF signaling has been implicated in the pathophysiology of anxiety disorders but the underlying mechanisms remain poorly defined. For example, human and rodent studies indicate that the single nucleotide Val66Met polymorphism in the Bdnf gene (BDNFMet), which impedes regulated secretion and signaling of BDNF, confers susceptibility to anxiety (Chen et al. 2006; Frielingsdorf et al. 2010; Lang et al., 2005; Bath et al., 2012). Previous investigations in our laboratory demonstrated that mice with central deletion of Bdnf (BDNF2L/2LCk-Cre) exhibit increased anxiety-like behavior in the light/dark exploration test (Rios et al, 2001). Furthermore, mice with TrkB overexpression display reduced anxiety in the elevated plus maze (Koponen et al., 2004).
One possible mechanism underlying the effects of BDNF is regulation of serotonergic neurotransmission, which is universally recognized to play critical roles in the control of affective and anxiety-related behaviors (Stutzmann and LeDoux, 1999; Inoue et al., 2004). BDNF and TrkB are co-expressed in serotonergic neurons within the dorsal raphe and median raphe (Madhav et al., 2001), where they promote expression of serotonergic markers, including tryptophan hydroxylase, the rate-limiting enzyme in serotonin synthesis (Siuciak et al., 1998). Alterations in serotonin systems were reported in BDNF+/− mice previously (Lyons et al, 1999). Moreover, we showed that albeit having a largely intact presynaptic serotonergic system, BDNF2L/2LCk-Cre mutant mice exhibit severe deficits in 5-HT2A-mediated responses to serotonin in the medial prefrontal cortex (Rios et al. 2006). Similar alterations in other limbic areas associated with the neurobiology of anxiety are possible. Of particular interest is the basolateral amygdala (BLA), which contains both BDNF and TrkB (Conner et al. 1997; Rattiner et al. 2004) and is densely innervated by ascending serotonergic fibers from the raphe nucleus (Muller et al. 2007; Sadikot and Parent 1990).
The BLA contains two major classes of cells, projection glutamatergic pyramidal (McDonald et al. 1989) and GABAergic local-circuit neurons (McDonald 1985), both of which express serotonin receptors (Cornea-Hebert et al. 1999; McDonald and Mascagni 2007; Stein et al. 2000). Serotonin negatively regulates activity of pyramidal neurons in the BLA via direct and indirect mechanisms. Direct modulation involves activation of Gi-coupled 5-HT1A receptors in projection neurons with a hyperpolarizing effect (Rainnie 1999). Indirect modulation, the predominant mechanism, entails stimulation of Gq-coupled 5-HT2A and 5-HT2C receptors in GABAergic interneurons and a consequent increase in GABA release onto projection neurons (Jiang et al. 2009; Rainnie 1999). Alterations in serotonin signaling that disinhibit the BLA might contribute to increases in anxiety-like behavior. Indeed, manipulations that increase the excitability of output neurons in the BLA, including focal administration of the GABAA receptor antagonist bicuculline, have reported anxiogenic-like effects (Sanders and Shekhar, 1995).
Here, we tested the hypothesis that deficient BDNF signaling interferes with serotonergic regulation of BLA pyramidal neurons. Using electrophysiological, biochemical and molecular approaches, we show that 5-HT2 receptor activation modulates GABAergic and AMPA receptor-mediated glutamatergic transmission in the BLA and that these processes are significantly altered in BDNF2L/2LCk-Cre mice, possibly contributing to the anxiety-related phenotypes that they exhibit.
Experimental Procedures
Animals
Male BDNF2L/2LCk-Cre mice were generated as previously described and were in a hybrid background with C57BL/6 and 129 strain contributions (Rios et al. 2001). Briefly, for the generation of mice with floxed Bdnf alleles, loxP sites were inserted around exon 9, the single coding exon of Bdnf. Thus, cre-mediated recombination of floxed Bdnf results in a null Bdnf allele. BDNF2L/2LCk-Cre conditional mutants were generated by crossing mice carrying floxed Bdnf alleles with transgenic mice (CamKII-cre93) in which expression of cre recombinase was driven by the α-calcium/calmodulin protein kinase II (CamKII). BDNF2L/2LCk-Cre mutant mice are depleted of BDNF stores everywhere in the brain except the cerebellum starting during the first post natal week, maximal depletion being completed by post natal day 21 (Rios et al., 2001). Age-matched littermate wild-type controls were used in every experiment to reduce genetic background differences. Electrophysiological studies were optimally performed in mice 4 to 6 weeks of age while biochemical and molecular studies were performed in tissues obtained from mice aged 10 to 16 weeks. Preliminary studies did not reveal differences in neurochemistry between the ages, and younger ages provide more viable slices for electrophysiological recordings. All of the procedures were approved by the Institutional Animal Care and Use Committee at Tufts University and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the animal suffering and to reduce the number of animals used.
In situ hybridization analysis
To measure BDNF mRNA levels in the amygdala and dorsal raphe nucleus, brains from wild- type and BDNF2L/2LCk-Cre mice (males, 10–12 weeks of age) were collected and immediately frozen on dry ice. Twelve-micrometer-thick sections containing BLA from each animal were cut on a cryostat. Sections were hybridized for 16 h at 60°C with a 35S-labeled, antisense riboprobe representing bases 507–833 of the BDNF cDNA (Genebank Accession No X55573). After the hybridization step, sections were stringently washed and placed on x-ray film for 12 days.
Immunohistochemistry
Wild-type mice (males, 10–12 weeks of age) were transcardially perfused with 20 ml of cold saline followed by 50 ml of 4% paraformaldehyde (PFA). Brains were immediately removed, postfixed in 4% PFA for 2 hours at 4°C, cryoprotected in a 30% sucrose solution, and frozen in mounting media (Tissue-Tek, Torrance, CA) until further use. Thirty-micrometer-thick cryostat-cut coronal brain sections containing BLA were immunolabeled with anti-TrkB, anti-CamKII and anti-GAD antibodies using standard methods. In brief, free floating brain sections were pre-incubated in a 5% normal donkey serum, 0.1% Triton X-100, 1X PBS solution for 1 hr at room temperature. Sections were then incubated for 24 hr at 4°C with anti-TrkB (1:1000, Santa Cruz Biotechnology, CA) and anti-CamKII (1:100; Millipore, Billerica, MA) or anti-GAD (1:1000, Millipore). After washes in 1XPBS, the sections were incubated with the appropriate fluorescence-conjugated secondary antibodies (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 hr at room temperature and visualized using a Zeiss Axioplan upright microscope with epifluorescence.
High Pressure Liquid Chromatography (HPLC)
Serotonin and its metabolite 5-HIAA were separated and quantified using high performance liquid chromatography (HPLC). Male wild-type and BDNF2L/2LCk-cre mice (12–16 weeks of age) were anesthetized with isoflurane and then decapitated. Their brains were rapidly removed, chilled in ice-cold PBS, and 500 μm coronal slices (Bregma: −1.2 mm to −1.7 mm) cut using a vibratome (VT 1000S, Leica Microsystems). Coronal sections were laid rostral side up and the BLA outlined laterally by the white matter tract of the external capsule (corpus callosum) and medially by the white matter tract of the longitudinal association bundle was dissected using a scalpel blade under a dissecting microscope. Tissues were immediately frozen on dry ice. HPLC for biogenic amines was performed by the Neurochemistry Core Facility at Vanderbilt University.
Electrophysiology
2.5.1 Slice Preparation
Male wild-type and BDNF2L/2LCk-cre mice (4–6 weeks old) were anesthetized and decapitated. Brains were rapidly removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) composed of (in mM) NaCl 124; KCl 3.75; KH2PO4 1.25; MgCl2 6; CaCl2 1; NaHCO3 26; Glucose 10 and kynurenic acid 10, bubbled with 95% O2/5% CO2. The aCSF was adjusted to minimize the excitotoxicity associated with glutamate release during tissue slicing, and involved the addition of the glutamate antagonist, kynurenic acid, decreased calcium levels to minimize neurotransmitter release, and increased magnesium levels. A block containing the amygdala region was prepared by rostral and caudal coronal cuts, and coronal slices, 320 μm thick, were cut using a vibratome (VT 1000S, Leica Microsystems). Following slicing, tissues were hemisected and kept in a holding chamber containing oxygenated aCSF at 32°C for ~1 hr. Slices were then transferred to oxygenated aCSF maintained at room temperature for at least 30 min before recording and experiments started ≥1 hr after slice preparation.
Whole Cell Recordings
Slices were transferred to a submersion-type recording chamber where they were continuously perfused with oxygenated aCSF containing (in mM) NaCl 124; KCl 3.75; KH2PO4 1.25; MgCl2 1.3; CaCl2 2.5; NaHCO3 26; and Glucose 10 at a rate of 2–3 ml/min. All experiments were carried out at 32°C. Tight-seal (>1G Ω) whole-cell recordings were obtained from the cell body of neurons in the BLA region. Patch electrodes were fabricated from borosilicate glass and had a resistance of 5 – 8MΩ when filled with a solution containing (in mM) K-gluconate 135; KCl 2; HEPES 10; MgCl2 3; phosphocreatinine 5; K-ATP 2; and Na-GTP 0.2; adjusted to pH 7.3 with KOH and having an osmolarity of 280–290 mOsm. To record spontaneous GABAergic synaptic events, we used a high Cl-based intracellular solution. The above patch solution was modified to contain (in mM) K-gluconate 70 and KCl 62. Under these conditions, the reversal potential for GABA is 20 mV and all events appear as negatively going events. Individual neurons were visualized with a Nikon E600FN microscope (Nikon, USA) using differential interference contrast (DIC) optics and infrared (IR) illumination with an IR-sensitive CCD camera (Dage-MTI, Michigan City, IN). Neurons with a pyramidal appearance were selected for recordings, and were voltage clamped using a Multiclamp 700A amplifier (Axon Instruments Inc., Foster City, CA, USA). Access resistance (8–26 MΩ ) was regularly monitored during recordings, and cells were rejected if it changed by more than 15% during the experiment. The signals were filtered at 2 kHz, digitized (Digidata 1322A, Axon Instruments Inc.), and stored on a computer using Clampex software of pCLAMP9 (Axon Instruments Inc.). Traces were analyzed offline with Clampfit software of pClamp 9.2 (Axon Instruments). Postsynaptic current (PSC) frequency and amplitude were analyzed with Mini Analysis software (Synaptosoft, Decatur, GA).
Drugs
Drugs were applied directly to the aCSF using a continuous gravity-fed bath application. Serotonin-mediated (glutamatergic or GABAergic) currents were studied by recording for a 5 min baseline period, followed by recordings following addition of 10 ml serotonin (50 μM) to the bath perfusion at a flow rate of 3ml/min. At this perfusion rate, it took between 1 to 2.5 min for the response to develop and this was followed by recording during a 10 min washout period. Other drugs were also added to the bath in specific experiments and included: D-(-)-2-Amino-5-phosphonopentanoic acid (D-AP-5, 50 μM); 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline -7-sulfonamide (NBQX, 10 μM); bicuculline (20 μM), Cinanserine (20 μM), Serotonin (5-HT; 50 μM); and MDL 100907 (30 nM, Advanced Biochemical Compounds, Germany). The compounds were acquired from Tocris (Ballwin, MO, USA) or Sigma (St Louis, MO, USA) unless indicated otherwise and were stored in stock solutions at −20°C before being diluted and applied to the slice in oxygenated aCSF.
Quantitative Reverse Transcription-PCR Analysis
For determination of levels of 5HT2A and 5-HT2C total RNA was extracted from the BLA of wild-type and BDNF2L/2LCk-Cre mice. Briefly, mice (12–14 weeks of age) were anesthetized with isoflurane, and then decapitated. The brains were rapidly removed from the skulls, 320 μm coronal slices cut on the vibratome and the BLA dissected out as described above. Tissue was immediately frozen on dry ice and RNA extracted using Tri Reagent (Molecular Research Center). RNA samples were treated with DNase and tested for genomic DNA contamination in PCRs. Reverse transcription to generate cDNA was conducted with 1 μg of RNA and using 200 units of Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and 150 ng of random primers (Invitrogen, Carlsbad, CA) in a 20μl reaction. cDNA (2 μl) from the RT reaction was used as the template for quantitative real-time PCR reaction with a final PCR reaction volume of 20 μl, with the 5′ and 3′ gene-specific PCR primer concentrations at 500 nM each. Real time PCR amplification was performed using a MX-3000P Stratagene cycler and SYBR green PCR master mix (Qiagen, Valencia, CA). For each primer set, the specificity of the product amplification was confirmed by dissociation curve analysis and agarose gel electrophoresis. Furthermore, curves were created using serial dilutions and the efficiencies for each primer set were calculated. The amplification efficiency for all the primers used in this study was >90%. For each target primer set, a validation experiment was performed to demonstrate that the PCR efficiencies were approximately equal to those of the reference gene. A two-step protocol was used: 95°C for 10 min and 45 cycles with 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec. GAPDH and cyclophilin were used as normalizers. The data shown represent experiments were cyclophilin was used as a normalizer. The following primers were used: 5-HT2A: forward (F), 5- ATAGCCGCTTCAACTCCAGA-3′; reverse (R), 5′-TCATCCTGTAGCCCGAAGAC-3′; 5-HT2C: F, 5′-TAATGGTGAACCTGGGCACTGCGG -3′; R, 5′-TAAAAGTGTCAGTTACTATAGCTGC -3′; GAPDH: F, 5′-CGTCCCGTAGACAAAATGGT- 3′; R, 5′-TCAATGAAGGGGTCGTTGAT-3′ Cyclophilin: F, 5′-CACCGTGTTCTTCGACATCA-3′; R, 5′-CAGTGCTCAGAGCTCGAAAG-3′. All samples were analyzed in triplicates, and non-template controls were included to ascertain any level of contamination. Data obtained were analyzed using the comparative Ct method.
Statistical analysis
All data sets were first tested for normality using the Kolmogorov-Smirnov’s normality test. Amygdala serotonin and serotonin receptor levels in the HPLC and quantitative PCR studies were analyzed using unpaired, two-tailed Student’s t-tests. Statistical comparisons of electrophysiological data were performed using the Student’s paired t test for within-group cell comparisons and the Student’s unpaired t test for between-group comparisons. The one-way repeated measures ANOVA was used for multiple comparisons followed by post hoc analysis using Bonferroni correction to determine differences between multiple groups. All data are expressed as mean ± standard error of the mean. Probability values <0.05 were considered statistically significant for all comparisons.
Results
TrkB is expressed in pyramidal and GABAergic neurons in the BLA
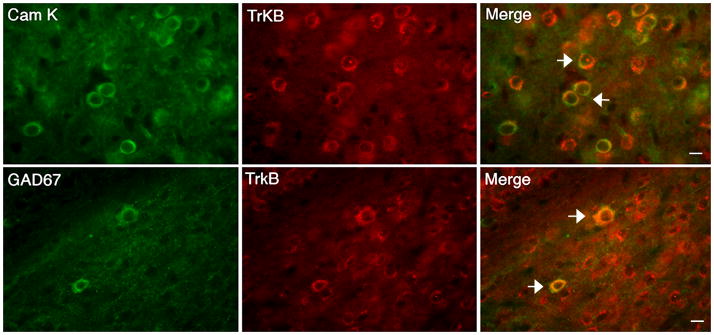
As a first step towards ascertaining the role of BDNF in the BLA, we asked whether glutamatergic pyramidal projection and GABAergic neurons within this brain region are able to respond to this neurotrophin. Double immunolabeling studies showed that TrkB co-localizes with Cam kinase (marks pyramidal neurons) and GAD (marks GABAergic neurons) in the wild-type BLA (Fig. 1). Whereas 70.1 ± 5.8% of pyramidal neurons contained TrkB receptors, 82.4 ± 3.3% of GABAergic neurons expressed TrkB (n = 3 wild-type mice). These data indicate that BDNF might act on both of these cell populations to regulate activity of the BLA.
Figure 1. TrkB receptors are expressed in BLA Projection and GABAergic neurons.
Representative images illustrating co-localization of TrkB receptor with CamK and GAD67 in the wild-type BLA (arrows). CamK marks pyramidal neurons and GAD denotes GABAergic interneurons. Scale bars = 10 μm
BDNF2L/2LCk-Cre mutant mice exhibit a mild decrease in serotonin content in the BLA
The BLA receives a dense serotonergic innervation from the raphe nucleus (Muller et al. 2007) that appears to play a critical role in the regulation of mood and anxiety. Therefore, we investigated whether perturbed BDNF signaling affected serotonin synthesis and turnover in the BLA of adult BDNF2L/2LCk-Cre mutant mice. In these mice, BDNF is depleted throughout the brain, excluding the cerebellum, during the early post natal period (Rios et al. 2001). In situ hybridization analysis confirmed that BDNF mRNA was vastly depleted throughout the brain, including the BLA (Fig 2A and C) and the raphe nucleus of these mutants (Fig. 2B and D).
Figure 2. Depletion of BDNF mRNA in the BLA and raphe nucleus of BDNF2L/2LCk-Cre mutant mice.

In situ hybridization analysis of representative coronal brain sections obtained from wild-type (A and B) and BDNF2L/2LCk-Cre mutant mice (C and D) showing extensive depletion of BDNF mRNA in the BLA (A and C) and dorsal raphe nucleus (B and D) (arrows) of BDNF2L/2LCk-Cre mice compared to wild-types.
HPLC analysis of BLA tissue punches obtained from wild-type and BDNF2L/2LCk-Cre mice showed a modest but statistically significant decrease in serotonin content in the mutants (wild-types: 15.3 ± 0.6; BDNF2L/2LCk-Cre mutants: 13.3 ± 0.5 ng/mg protein, p = 0.03, n = 8) (Fig. 3A). However, levels of 5-HIAA (wild-types: 2.3 ± 0.2; BDNF2L/2LCk-Cre mutants: 2.1 ± 0.1 ng/mg protein), the main metabolite of serotonin and the 5-HT/5-HIAA ratio (wild types: 0.15 ± 0.01; BDNF2L/2LCk-Cre mutants: 0.16 ± 0.007 ng/mg protein), a common index of serotonin turnover, were normal in BDNF2L/2LCk-Cre mice (Fig. 3B and C). Because the HPLC analysis measures total levels of serotonin in the BLA, changes in serotonin release within the BLA may be masked. Nonetheless, these data show that central depletion of BDNF results in a mild reduction in serotonin content in the BLA.
Figure 3. Serotonin content in the BLA of wild-type and BDNF2L/2LCk-Cre mutant mice.
Levels of (A) serotonin (B), 5-HIAA and (C) 5HIAA/5-HT ratio in the BLA of wild-type (WT) and BDNF2L/2LCk-Cre (CM) mice. A significant decrease in 5-HT content was observed in BDNF2L/2LCk-Cre mice whereas levels of 5-HIAA and the 5-HT/5-HIAA ratio were normal. *, p = 0.03; n = 8 animals.
Basal electrophysiological properties of BLA pyramidal neurons in BDNF2L/2LCk-Cre mutants are normal
We sought to ascertain whether BDNF influenced BLA output by regulating the activity of pyramidal projection neurons. Projection neurons in acute brain slices obtained from wild-type and BDNF2L/2LCk-Cre mice were identified by their distinct pyramidal shape and electrophysiological properties, including lack of spontaneous discharges at rest, generation of spike trains exhibiting varying degrees of frequency adaptation during membrane depolarization and low input resistance (<100 MOhm) (Rainnie et al. 1993). Whole cell recordings indicated that basic electrophysiological properties of BDNF2L/2LCk-Cre pyramidal cells, including resting membrane potential, input resistance, membrane time constant, spike threshold, spike amplitude, spike half-width and spike rise time were normal compared to wild-type controls (Table 1).
Table 1.
Physiological Properties of BLA Projection neurons
| Parameter | WT | BDNF2L/2LCKcre |
|---|---|---|
| Vm (mV) | −61 ± 1.2 | −63 ± 1.1 |
| Rm (mΩ) | 127 ± 12 | 110 ± 10 |
| Tau (msec) | 25 ± 2.3 | 23 ± 1.8 |
| Spike Threshold (msec) | −41 ± 0.6 | −40 ± 2.8 |
| Spike Amp (mV) | 77 ± 3.1 | 77 ± 2.5 |
| Half-spike width (msec) | 0.95 ± 0.04 | 1.02 ± 0.05 |
| 10–90% Rise time (msec) | 0.34 ± 0.01 | 0.37 ± 0.02 |
Vm, resting membrane potential; Rm, membrane input resistance
No significant difference was observed in the physiological properties of BLA projection neurons from controls and mutants (p > 0.05 for all categories).
Next, we measured spontaneous inhibitory (IPSC) and excitatory (EPSC) post synaptic currents under baseline conditions. IPSCs were recorded at a holding potential of −70 mV and in the presence of D-AP5 (50 μM) and NBQX (10 μM) to block NMDA and AMPA/kainate receptors, respectively. The frequencies (wild-types: 4.0 ± 0.7 Hz; BDNF2L/2LCk-Cre mutants: 4.7 ± 1.6 Hz) and amplitudes (wild-types: 9.9 ± 1.7 pA; BDNF2L/2LCk-Cre mutants: 9.9 ± 1.4 pA) of IPSCs in wild-type and BDNF2L/2LCk-Cre mutant cells were similar at baseline (n = 7 - 9; p = n/s). EPSCs were studied in the presence of bicuculline (20 μM) to block inhibitory events. Basal level of glutamatergic transmission in BLA neurons of wild type and mutants in untreated slices was relatively low with some neurons receiving no detectable currents. No significant differences in amplitude (wild-types: 10.3 ± 1.1 pA; BDNF2L/2LCk-Cre mutants: 12.1 ± 1.3 pA; n = 22–24 cells; p = n/s) or frequency (wild-types: 3.8 ± 0.4 Hz; BDNF2L/2LCk-Cre mutants: 4.6 ± 0.4 Hz; p = n/s; n = 22–24 cells) of EPSCs were observed in BDNF2L/2LCk-Cre pyramidal cells compared to wild-type controls.
5-HT2-mediated inhibitory responses of BLA pyramidal neurons to serotonin are severely impaired in BDNF2L/2LCk-Cre mutant mice
Anxiety disorders have been linked to alterations in the GABAergic system in the BLA (Davies et al. 2010). Stimulation of 5-HT2 receptors on rat GABAergic interneurons by serotonin induces GABA release onto projection neurons and concomitant reductions in BLA output (Jiang et al. 2009; Rainnie 1999). To determine whether serotonergic facilitation of the BLA GABAergic system is dependent on BDNF, we measured spontaneous IPSCs in wild-type and BDNF2L/2LCk-Cre mutant pyramidal cells following bath application of serotonin. As previously demonstrated in rats (Jiang et al. 2009; Rainnie 1999), serotonin induced a significant increase in frequency (206.0 ± 32.7% of baseline values, p = 0.009, n = 6) of IPSCs in pyramidal neurons of wild-type mice compared to aCSF treatment (Fig. 4A and C). There was also a trend towards a significant increase in amplitude of the wild type response to serotonin (217.0 ± 34.7% of baseline values, p = 0.06, n = 6). Bath application of bicuculline completely eliminated IPSCs (Fig. 4A and C), indicating that they were mediated by GABAA receptors. In the presence of the 5-HT2 receptor antagonist cinanserine (20 μM), serotonin no longer induced changes in the frequency or amplitude of IPSCs in 5 of the 6 wild-type cells tested (Fig. 4A and C), indicating that 5-HT2A/2C receptors were responsible for the facilitative effect of serotonin on GABAergic drive. Additionally, 5-HT2A receptor blockade with MDL 100,907 (30 nM; n = 4) (Fig. 4C), abrogated the effects of serotonin, further defining 5-HT receptors involved in the negative regulation of BLA pyramidal neurons.
Figure 4. 5-HT2A-mediated inhibitory responses in BLA pyramidal neurons are reduced in BDNF2L/2LCk-Cre mutant mice.
Traces in voltage clamp from wild-type (A) and BDNF2L/2LCk-Cre (B) mice show inhibitory postsynaptic currents (IPSCs) under basal conditions, in the presence of serotonin (5-HT; 50 μM, 1 min), 5-HT and bicuculline (Bic., 20 μM) or 5-HT and cinanserine (Cin., 20 μM). Serotonin elicits a significant increase in spontaneous IPSC frequency in neurons from wild-type (p = 0.03) but not mutant (p = n/s) mice. C, Bar graph summarizes IPSC frequency data (mean ± SE) for wild-type (WT) and BDNF2L/2LCk-Cre mice (CM) following 5-HT application and in the presence of bicuculline (Bic), cinanserine (Cin.) and MDL 100,907 (MDL). *, p = 0.01; n = 6 (wild types) and 7 (BDNF2L/2LCk-Cre) cells.
In far contrast to its effects on wild-type cells, serotonin failed to induce significant increases in IPSC frequency in BLA pyramidal cells in BDNF2L/2LCk-Cre mice compared to BDNF2L/2LCk-Cre mutant cells treated with aCSF (114 ± 20% of baseline values, p =n/s; n = 7) (Fig. 4B and C). Accordingly, this corresponds to a significant reduction in serotonin-induced IPSCs in BDNF2L/2LCk-Cre cells compared to wild-type controls (wild-types: 9.4 ± 1.8 Hz; BDNF2L/2LCk-Cre mutants: 5.2 ± 1.5 Hz; p = 0.01, n = 6–7 cells). However, as it was the case with wild-type cells, BDNF2L/2LCk-Cre mutant principal neurons in the BLA exhibited a trend towards a significant increase in the amplitude of serotonin-induced IPSCs (BDNF2L/2LCk-Cre mutants: 197 ± 55% of baseline values; p = 0.06, n = 7). Furthermore, the mean amplitude of mutant IPSCs following serotonin application was not significantly different from that observed in wild-type cells (wild-types: 18.7 ± 4.4 pA; BDNF2L/2LCk-Cre mutants: 18.4 ± 4.5 pA; p = n/s, n = 6–7 cells).
Serotonin predominantly inhibits BLA pyramidal neurons through indirect mechanisms as described above (Jiang et al. 2009; Rainnie 1999). However, it can also activate Gi-coupled 5-HT1A receptors expressed in these cells with a hyperpolarizing effect (Rainnie 1999). Because BDNF was linked previously to 5-HT1A receptor function in the hippocampus (Hensler et al., 2003; Burke et al., 2012), we investigated whether the hyperpolarizing effect of serotonin on BLA pyramidal neurons was altered in BDNF2L/2LCk-Cre mutants. Whole cell recordings indicated that bath application of serotonin hyperpolarized projection neurons from both wild type and BDNF2L/2LCk-Cre mice to a similar degree (wild-types: 3.1 ± 0.5 mV; BDNF2L/2LCk-Cre mutants: 5.2 ± 0.3 mV; p > 0.05, n = 5), suggesting that 5-HT1A-mediated responses are preserved in BDNF2L/2LCk-Cre cells. The cumulative data indicate that BDNF is required for the facilitative effects of serotonin on GABAergic transmission in BLA pyramidal cells.
BDNF depletion results in exaggerated excitatory responses of BLA pyramidal neurons to serotonin
Serotonin is known to influence glutamatergic transmission in several areas of the brain, including cortical pyramidal neurons (Aghajanian and Marek 1997; Lambe et al. 2000; Zhou and Hablitz 1999). We asked whether it also influences glutamatergic drive onto BLA projection neurons and/or postsynaptic glutamate-mediated events. To test this, we recorded EPSCs from BLA pyramidal neurons following serotonin application and blockade of GABAA receptor- mediated signaling with bicuculline. The holding potential was at 70 mV, favoring the activation of AMPA rather than NMDA receptors. In agreement with this, synaptic currents were completely blocked in the presence of the AMPA receptor blocker, NBQX (n = 6, p = 0.03) (Fig 5B). Cells exhibiting 25% or higher changes in EPSC frequencies or amplitude in response to serotonin were defined as responding cells.
Figure 5. BLA pyramidal neurons in BDNF2L/2LCk-Cre mutants show exaggerated 5-HT2-mediated excitatory responses.
A, Representative traces in voltage clamp from wild-type and BDNF2L/2LCk-Cre conditional mutant mice show excitatory postsynaptic currents (EPSCs) under basal conditions and in the presence of serotonin (5-HT, 50 μM, 1 min). B, Bar graph summarizes EPSC frequency data (mean ± SE) for wild-type (WT) and BDNF2L/2LCk-Cre mutant (CM) mice following 5-HT application. This effect was blocked in the presence of NBQX (5-HT and NBQX) or cinanserine (5-HT and Cin.). The data are based on recordings from wild-type (n = 16) and BDNF2L/2LCk-Cre mutant (n = 21) responding pyramidal cells. While serotonin elicits a significant increase in spontaneous EPSC frequency in BLA pyramidal neurons from both BDNF2L/2LCk-Cre (p < 0.0001) and wild-type (p = 0.01) mice, the excitatory effect was more pronounced in mutant neurons and blocked by NBQX and cinanserine. *, p = 0.01; **, p = 0.03; ***, p = 0.004.
Serotonin increased glutamatergic transmission in a subpopulation of BLA pyramidal neurons. In wild type slices, 42% of cells examined (6/14 cells) responded to serotonin with a significant increase in EPSC frequency (143.8 ± 17.5% of baseline values, p = 0.02) compared to aCSF treatment (Fig 5A and B). Interestingly, the facilitative effect of serotonin on glutamate transmission in the BLA was enhanced in BDNF2L/2LCk-Cre mice (Fig. 5A and B). Indeed, 91% of BDNF2L/2LCk-Cre mutant pyramidal neurons (19/21 cells) exhibited increased EPSC frequency following serotonin bath application. The mean frequency of EPSCs in BDNF2L/2LCk-Cre responders was 201.4 ± 16.6% of baseline values following serotonin treatment (p < 0.0001) (Fig. 5B). Because this analysis was conducted in the presence of bicuculline to study excitatory events in isolation, the observed increase in EPSC’s cannot be attributed to the previously identified disinhibition of mutant projection neurons (Fig. 4B and C). Enhanced glutamatergic transmission in BDNF2L/2LCk-Cre mutant cells was abolished by the 5-HT2 receptor antagonist cinanserine (p = 0.004, n = 5/6) and the AMPA receptor blocker, NBQX (n = 6, p = 0.03), indicating the involvement of 5-HT2 and AMPA receptors (Fig. 5B). Importantly, the frequency of serotonin-induced EPSCs was significantly increased in BDNF2L/2LCk-Cre mutants compared to wild-type responders (wild-types: 5.3 ± 0.9 Hz; BDNF2L/2LCk-Cre mutants: 8.3 ± 0.9 Hz; p = 0.01). However, similar amplitudes of serotonin-mediated EPSCs were observed in responding BDNF2L/2LCk-Cre pyramidal neurons compared to wild-type controls, (BDNF2L/2LCk-Cre mutants: 159.5 ± 14.7% of baseline values, p = 0.009; wild-type; 160 ± 11.9% of baseline values, p = 0.001). The mean amplitude of BDNF2L/2LCk-Cre mutant EPSCs following serotonin application was not significantly different from that observed in wild-type cells (wild-types: 11.69 ± 1.3 pA; BDNF2L/2LCk-Cre mutants: 12.7 ± 1.7 pA; wild-type versus BDNF2L/2LCk-Cre mutants; p = n/s).
To determine whether the influence of serotonin on membrane firing properties of BLA neurons is altered by BDNF, the effects of serotonin were examined under current-clamp. Action-potential firing patterns evoked by depolarizing current steps (100 pA, 150 ms) were monitored before and during bath application of serotonin. The action potential firing patterns remained unchanged in wild-type BLA projection neurons (data not shown). However, although not significant, a sub population of BDNF mutant BLA neurons fired more spikes in response to serotonin (BDNF2L/2LCk-Cre mutants: 197 ± 60% of baseline firing; n= 3/5, p = ns). Nonetheless, the spike threshold of neurons remained unaffected by the application of serotonin. The mean spike threshold before and during the application of serotonin was similar to that observed in normal aCSF (wild-type −40 ± 2mV n = 3; BDNF2L/2LCk-Cre mutants: −41 ± 0.4mV, n = 5), suggesting that serotonin did not significantly affect the electrophysiological properties of BLA cell membranes.
In summary, our data indicate that the BLA contains a heterogeneous population of pyramidal cells, a subset of which exhibit excitatory responses to serotonin. Furthermore, they show that the facilitative effects of serotonin on excitatory transmission in principal cells are enhanced by diminished BDNF signaling in the BLA.
The BLA of BDNF2L/2LCk-Cre mutant mice exhibits reduced content of 5-HT2A and 2C receptor mRNA
We asked whether altered responses to serotonin evident in BDNF2L/2LCk-Cre pyramidal neurons could be explained by changes in levels of serotonin receptors in the BLA. We measured mRNA levels of 5HT2A and 2C receptors in BLA tissue punches using quantitative RT-PCR analysis. As illustrated in Figure 6B, BDNF2L/2LCk-Cre mice exhibited a 32% decrease in 5-HT2C transcripts in the BLA (n = 6, p = 0.001). There was also a trend towards a significant 46% decrease in 5-HT2A mRNA content in the BDNF2L/2LCk-Cre mutant BLA (n = 6, p = 0.06) (Fig. 6A). These results indicate that BDNF is required for normal expression of 5-HT2 receptors in the BLA.
Figure 6. Expression of 5-HT2A and 5-HT2C receptor mRNA in the BLA.

Content of 5-HT2A (A) and 5-HT2C (B) receptor mRNA in BLA tissue punches obtained from wild-type (WT) and BDNF2L/2LCk-Cre (CM) mice (n = 6). Data are expressed as expression of 5-HT receptor mRNA in BDNF2L/2LCk-Cre mice relative to wild-type controls. Diminished BDNF signaling resulted in decreased 5-HT2A and 5-HT2C receptor mRNA levels in the BLA. *, p < 0.01
Discussion
BDNF and its facilitation of synaptic plasticity have been implicated in the regulation of emotional behaviors. Its actions in the hippocampus and mesolimbic dopamine pathway have been linked to the regulation of depressive-like behaviors (Berton et al., 2006; Gourley et al., 2008; Taliaz et., 2010). BDNF also plays an important role in the regulation of anxiety-related behavior, but less is known regarding the nature of this function. Here we show that BDNF facilitates the modulatory effects of serotonin on GABAergic and glutamatergic systems in the BLA, a brain region linked to the etiology of anxiety. Specifically, we found that deficient BDNF signaling in the brain triggers aberrant responses of BLA pyramidal neurons to serotonin, ultimately enhancing excitatory events. These data extend our previous findings of abnormal serotonin-mediated synaptic transmission in the dorsal raphe nucleus and PFC of BDNF2L/2LCk-Cre mice (Rios et al. 2006).
Serotonin-mediated increases in GABA release appeared to be reduced in the BLA of BDNF2L/2LCk-Cre mice. In wild-type mice, administration of serotonin to BLA slices enhanced the frequency and amplitude of spontaneous IPSCs in pyramidal neurons. The frequency but not amplitude of serotonin-induced IPSCs was significantly reduced in pyramidal neurons in BDNF2L/2LCk-Cre mice, suggesting a required presynaptic function of BDNF. Consistent with this idea, our quantitative RT-PCR studies showed decreased expression of 5-HT2A and 5-HT2C mRNA in the BLA of BDNF2L/2LCk-Cre mice. Stimulation of 5-HT2 receptors located in BLA interneurons, which as shown here also express TrkB receptors, was demonstrated previously to mediate GABA release onto pyramidal neurons (Jiang et al. 2009). Therefore, decreased density of 5-HT2 receptors in GABA neurons is likely to underlie the diminished inhibitory drive onto BLA pyramidal neurons observed in BDNF2L/2LCk-Cre mutants. This deficit is more likely to be related to reduced 5-HT2A signaling because we showed that selective 5-HT2A receptor blockade prevented serotonin-induced increases in IPSCs. Furthermore, it was reported previously that application of the selective 5-HT2C receptor agonist WAY629 in rat slices did not influence GABA release in the BLA (Jiang et al. 2009). It is also worth noting that Jiang et al. (2009) reported that chronic stress reduces both 5-HT2A receptor content and frequency of pyramidal IPSC’s induced by α-methyl-5-HT in the BLA. Because chronic stress was shown previously to reduce BDNF expression in the amygdala (Pizarro et al. 2004), it is reasonable to speculate that perturbed BDNF signaling might contribute to the impaired serotonergic facilitation of GABA release in the BLA elicited by this challenge. However, it is important to recognize that BDNF depletion during the early post natal period in BDNF2L/2LCk-Cre mutants might exert developmental effects that are distinct from those induced by altered BDNF levels in the mature brain.
Projection neurons in the BLA of BDNF2L/2LCk-Cre mice also exhibited enhanced excitation in response to serotonin. This was measured as an increase in the serotonin-mediated glutamatergic (AMPA) EPSCs in the presence of bicuculline. Whereas only 42% of BLA pyramidal cells in wild-types responded with excitation to serotonin, 91% of BDNF2L/2LCk-Cre cells were responders. Moreover, the frequency but not the amplitude of EPSCs of responding cells was significantly increased in BDNF2L/2LCk-Cre mice compared to wild-types. Because recordings were conducted in the presence of bicuculline to study excitatory events in isolation, the observed increase in EPSCs cannot be attributed to the observed disinhibition of mutant projection neurons and likely arises from an independent mechanism. Interestingly, findings from a previous study suggested that serotonin might act presynaptically to regulate glutamate release in the BLA (Rainnie 1999), although this aspect of serotonin function remains under studied. This paradoxical role of serotonin relative to its facilitative effect on GABAergic inhibitory drive onto BLA pyramidal neurons might represent a feedback mechanism that prevents excessive inhibition during prolonged serotonin release (Rainnie 1999). In agreement with this model, whereas short-term exposure of wild-type rat slices to serotonin inhibited pyramidal cells, longer treatment resulted in excitatory responses (Rainnie, 1999). Based on those findings, it was postulated that serotonin receptors in glutamatergic presynaptic terminals might have lower affinity to serotonin compared to receptors located in the postsynaptic membrane of GABAergic interneurons. In this context, it is worth noting that elevated extracellular levels of serotonin in the BLA due to deficits in serotonin transporter function were associated with increased amygdala activity following exposure to fearful stimuli in humans and increased anxiety-like behaviors in mice (Hariri et al. 2002; Wellman et al. 2007). Our results suggest that BDNF might act to facilitate the effects of serotonin on excitatory/inhibitory balance in the BLA (Fig. 7). A role of BDNF in maintaining the balance of inhibition and excitation was reported previously in the cortex and hippocampus (Rutherford et al., 1998; Singh et al., 2006).
Figure 7. Modulation of glutamatergic and GABAergic transmission by 5-HT and BDNF in the BLA.

We propose that BDNF influences the balance of excitatory and inhibitory transmission in the BLA thereby affecting overall BLA output. Our data suggest that BDNF positively regulates expression of pre-synaptic Gq-coupled 5-HT2 receptors on GABA interneurons, thereby mediating increases in GABAergic inhibitory drive onto glutamatergic pyramidal neurons induced by 5-HT released by dorsal raphe nucleus (DRN) fibers. As proposed previously (Rainnie 1999), serotonin might also act on 5-HT2 receptors on glutamatergic inputs onto pyramidal neurons to positively regulate glutamate release and prevent prolonged inhibition. Our findings suggest that BDNF might also influence this balancing effect of 5-HT on glutamatergic transmission in the BLA.
After considering the cumulative data, it is plausible that the number of serotonin receptors in glutamatergic terminals in the BLA or their affinity for serotonin is increased by perturbed BDNF signaling with concomitant increases in glutamate release onto pyramidal neurons. This is in contrast to the proposed effect of BDNF deficiency in BLA GABAergic neurons, namely reduced expression of 5-HT2 receptors. This is a plausible scenario because we demonstrated previously that the effect of BDNF on serotonin receptors appears to be context- dependent. Whereas diminished BDNF results in reduced expression of 5-HT2A in the medial PFC (Rios et al. 2006) and as shown here in the BLA, it also results in increased levels of 5-HT2A receptor binding in the hippocampus (Klein et al. 2010). Because we measured transcript levels of 5-HT2 receptors in the BLA, potential expression increases in presynaptic glutamatergic terminals arising from elsewhere would be undetected. Consistent with our model, we show that the observed facilitation in glutamate transmission in pyramidal neurons in BDNF2L/2LCk-Cre mutants can be abrogated by the 5-HT2 receptor antagonist cinanserine.
We reported previously that BDNF2L/2LCk-Cre mice exhibit increased anxiety-like behavior as measured by the light-dark exploration test (Rios et al, 2001). Our present observations of heightened BLA activity due to altered serotonin transmission in BDNF2L/2LCk-Cre mice are interesting in light of findings in patients with generalized anxiety, social phobia, panic disorder, and post traumatic stress disorder (PTSD) (Bryant et al. 2005; Semple et al. 2000; for review see Shin and Liberzon 2010). For example, individuals with PTSD showed elevated amygdala activity in response to salient, nonthreatening stimuli (Bryant et al. 2008) and anticipatory activation of the amygdala has been observed in generalized anxiety disorder (Nitschke et al. 2009). Increased amygdala activation was also reported in anxious individuals in response to fear conditioning (Bremner et al. 2005; Rauch et al. 2000; Williams et al. 2006). Importantly, a role for BDNF in exaggerated amygdala activity was indicated by neuroimaging/fMRI studies in individuals with a functional polymorphism in the bdnf gene. In this elegant study, Solimon et al, (2010) showed that during fear extinction, individuals with the BDNFMet allele displayed enhanced recruitment of the amygdala, a region that normally exhibits diminished activity during extinction trials, relative to non-Met allele carriers. This effect was recapitulated in knock-in mice with humanized BDNFMet alleles.
Conclusions
In summary, we demonstrate that BDNF plays a critical role in serotonin-mediated facilitation of GABAergic and glutamatergic transmission in the BLA. We propose that aberrant responses to serotonin and concomitant increases in excitatory responses of BLA pyramidal neurons are putative mechanisms underlying the emergence of anxiety in individuals with perturbed BDNF function. Gaining a greater understanding of how BDNF may regulate these systems may direct future suitable approaches for the treatment of anxiety disorders.
We examined the role of BDNF in serotonin transmission in the basolateral amygdala
Inhibitory responses of BLA pyramidal neurons to serotonin are impaired in BDNF mutants
BDNF mutant pyramidal neurons exhibit exaggerated excitatory responses to serotonin
The BLA of BDNF mutant mice exhibits reduced content of 5-HT2 receptors
A BLA hyperexcitable circuit in BDNF mutants might underlie their increased anxiety
Acknowledgments
This work was supported by a NIH DK073311 grant to MR. The authors would like to thank the Genomics core at Tufts University, which is supported by the Center for Neuroscience Research (P30 NS047243), for facilitating these studies and Dr. Kathleen Dunlap for helpful discussions pertaining to the electrophysiology experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant Brain Derived Neurotrophic Factor (Valine 66Methionine) Polymorphism Contributes to Developmental and Estrous Stage-Specific Expression of Anxiety-Like Behavior in Female Mice. Biological Psychiatry. 2012 May; doi: 10.1016/j.biopsych.2012.03.032. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med. 2008;38:555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, Gordon E, Williams LM. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Burke TF, Advani T, Adachi M, Monteggia LM, Hensler JG. Sensitivity of hippocampal 5-HT1A receptors to mild stress in BDNF-deficient mice. Int J Neuropsychopharmacol. 2012;10:1–15. doi: 10.1017/S1461145712000466. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;1:2295–22313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Esler M, Nutt DJ. Anxiety--bridging the heart/mind divide. J Psychopharmacol. 2010;24:633–638. doi: 10.1177/0269881109103800. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008;64(10):884–90. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/-) mixe. J Neurochem. 2003;85:1139–47. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology. 2009;34:410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Klein AB, Santini MA, Aznar S, Knudsen GM, Rios M. Changes in 5-HT2A-mediated behavior and 5-HT2A- and 5-HT1A receptor binding and expression in conditional brain-derived neurotrophic factor knock-out mice. Neuroscience. 2010;169:1007–1016. doi: 10.1016/j.neuroscience.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen E, Vöikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castrén E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26(1):166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, Kunz D, Gallinat J. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhav TR, Pei Q, Zetterstrom TS. Serotonergic cells of the rat raphe nuclei express mRNA of tyrosine kinase B (trkB), the high-affinity receptor for brain derived neurotrophic factor (BDNF) Brain Res Mol Brain Res. 2001;93(1):56–63. doi: 10.1016/s0169-328x(01)00183-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid- containing neurons in the rat basolateral amygdala. Neurosci Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007;146:306–320. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Beitz AJ, Larson AA, Kuriyama R, Sellitto C, Madl JE. Co-localization of glutamate and tubulin in putative excitatory neurons of the hippocampus and amygdala: an immunohistochemical study using monoclonal antibodies. Neuroscience. 1989;30:405–421. doi: 10.1016/0306-4522(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1362. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rios M, Lambe EK, Liu R, Teillon S, Liu J, Akbarian S, Roffler-Tarlov S, Jaenisch R, Aghajanian GK. Severe deficits in 5-HT2A -mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–30. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52(4):701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: An immunohistochemical study. Neuroscience. 1990;36:431–447. doi: 10.1016/0306-4522(90)90439-b. [DOI] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Henneberger C, Bteances D, Arevalo MA, Rodriguez-Tebar A, Grantyn R. Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons. J Neurosci. 2006;26:7189–200. doi: 10.1523/JNEUROSCI.5474-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Clark MS, Rind HB, Whittemore SR, Russo AF. BDNF induction of tryptophan hydroxylase mRNA levels in the rat brain. J Neurosci Res. 1998;52(2):149–158. doi: 10.1002/(SICI)1097-4547(19980415)52:2<149::AID-JNR3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19(11):RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15(1):80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal cortocolimbic morphology in serotonin transpoter knock-out mice. Journal of Neuroscience. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]