Abstract
Background
Compared to whites, African Americans have a greater incidence of diabetes, decreased control, and higher rates of micro-vascular complications. A peer mentorship model could be a scalable approach to improving control in this population and reducing disparities in diabetic outcomes.
Objective
To determine whether peer mentors or financial incentives are superior to usual care in helping African American Veterans improve their glycosylated hemoglobin (HbA1c) levels.
Design
A six month randomized controlled trial. (ClinicalTrials.gov registration number: NCT01125956)
Setting
The Philadelphia VA Medical Center.
Patients
African American veterans, age 50-70 years old, with persistently poor diabetes control.
Measurements
Change in HbA1c at 6 months
Intervention
118 participants were randomized to one of the three arms. Usual care participants were notified of their starting HbA1c and recommended goals for HbA1c. Those in the peer mentor arm were assigned a peer mentor who formerly had poor glycemic control but now had good control (HbA1c < 7.5%) who was asked to talk with the participant at least once a week. Peer mentors were matched on race, sex, and age. Those in the financial incentive arm could earn $100 by dropping their HbA1c by one point and $200 by dropping it by two points or to a HbA1c of 6.5%.
Results
Mentors and mentees talked the most in the first month (mean calls 4: range 0-30) and dropped to a mean of 2 calls (range 0-10) by the sixth month. HbA1c dropped from 9.9% to 9.8% in the control arm, 9.8% to 8.7% in the peer mentor arm and from 9.5% to 9.1% in the financial incentive arm. Mean change in HbA1c from baseline to 6 months relative to control was −1.07 (95% CI −1.84 to −0.31) in the peer mentor arm and −0.45 (95% CI −1.23 to 0.32) in the financial incentive arm.
Limitations
The study included only veterans and lasted only 6 months.
Conclusions
Peer mentorship improved glucose control in a cohort of African American Veterans with diabetes.
Management of Diabetes Mellitus has proven difficult because many of the most critical elements of disease management occur outside of clinical encounters. Intensive clinic based programs have proven effective in improving diabetes management, but such programs are resource intensive with declining effectiveness over time. Support from families and friends is often not a viable alternative because many patients are socially isolated, others may not want to engage relatives or friends in discussions about their medical problems, and family and friends may be unable to assume a caretaker role (1).
Disease-specific social support has been shown to improve diabetes self-management behaviors and may be particularly beneficial when the support comes from a peer with the same chronic condition (2-6). In interventions with diabetics, peer support has been shown to be effective in improving medication adherence, diet, exercise, blood glucose monitoring, and most recently glucose control (7-11). Prior interventions have introduced peer support through group visits or nurse phone calls or home visits from community health workers; however, these require expensive professional or semi-professional support staff (12-18). A more informal, flexible means of providing one-on-one peer support through volunteer peer coaches or mentors could potentially provide similar benefits at lower cost.
Financial incentives could enhance diabetes self-care. Financial incentives show promise in domains of behavior such as medication adherence (19), diet and exercise (20), and smoking (21), where people’s short time horizons lead them to favor immediate benefits at the expense of delayed costs (22-24). As far as we know, financial incentives have not been tested as a means to improving diabetes control.
To test the efficacy of these emerging means to promote health behaviors, we performed a randomized controlled trial of peer mentoring and financial incentives aimed at improving glucose control in African American veterans with persistently poor diabetes control.
METHODS
Design Overview
Participants were randomized to one of three arms in parallel: usual care, peer mentoring or financial incentives. Study investigators were blinded to the allocation and results until study completion. We used the Philadelphia VA Medical Center (PVAMC) lab for all study based blood draws. Phlebotomists were unaware of the study. The study was completed with one un-blinded research assistant. Informed consent was obtained from all participants and mentors with a different consent process for participants and mentors. The PVAMC institutional review board approved all aspects of the study. Enrollment occurred between October 2009 and April 2010 with follow-up completed by October 2010.
Setting and Participants
We identified patients seen at the PVAMC with at least two International Classification of Diseases −9 250 codes and with at least two HbA1cs drawn in the last three years. Inclusion criteria for participants were: age 50-70, self-identified race of black or African American, and persistently poor diabetes control. We chose to perform a single race study to specifically determine if this intervention would be effective in an African American population as a potential approach to reduce disparities given that African Americans are disproportionately adversely affected by diabetes, poor control and complications from poor control (25-29). Persistently poor diabetes control was defined as having the last two glycosylated hemoglobin levels (HbA1c) in the electronic medical record > 8%, with the last measure being within three months of enrollment. All participants also had an HbA1c drawn on the day of enrollment and at the end of six months. One person whose baseline HbA1c was < 7% was excluded from the study due to concerns that the intervention might lead to dangerous hypoglycemia. Potential participants were identified from the electronic medical record on an ongoing basis.
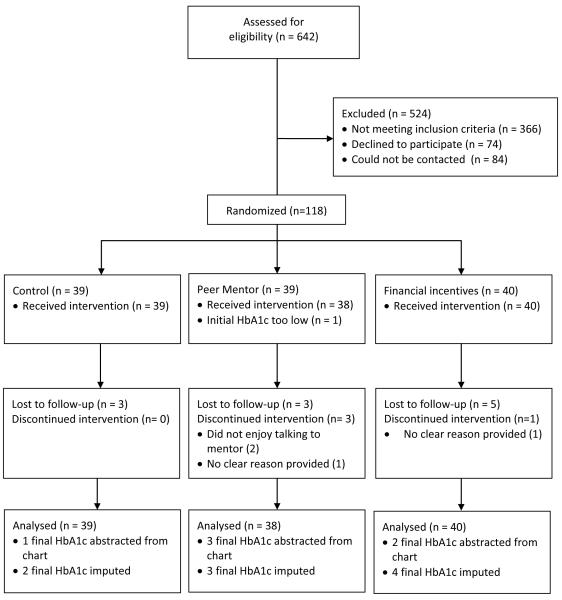
Of 642 charts of patients with diabetes that were reviewed, 366 did not meet eligibility (mostly because the patient was not African American or had not had a recent HbA1c). We were able to contact 192 (70%) of the 276 potential eligible participants, of whom 74 (27%) declined participation, leaving us with 118 participants of whom 39 patients were assigned to the control arm, 39 to peer mentoring, and 40 to financial incentives (Figure 1).
Figure 1. Study Flow Diagram.
Randomization and Intervention
We created a file in Excel (Microsoft Office 2007, Redmond, WA) with 40 allocations per arm. Using the random number generator function we gave each arm assignment a random number and put the ordered numbers in envelopes. Sealed envelopes were shuffled and stacked and the research assistant took the top envelope after consent was obtained to determine arm assignment. Neither blocking nor stratification was used in the process.
A short baseline survey was administered at the initial visit for which participants were paid $25. All participants were called the day following enrollment and notified of their starting HbA1c and informed of the American Diabetes Association and VA recommendations regarding HbA1c targets. All participants were paid $25 for returning 6 months later for the repeat HbA1c test. No additional intervention was provided to the control arm. We did not influence provider clinical care. Reimbursements were provided in the form of a VA voucher which the veterans could redeem for cash.
Those in the peer mentoring arm were matched to a peer mentor within 1-3 weeks. Guided by our own qualitative research (30), peer mentors were all African American patients who previously had been in poor control but were currently in good control (defined as an HbA1c of > than 8% in the past 3 years and ≤ 7.5% within 3 months of enrollment). Peer mentors were matched by gender and age (+/− 10 years). Active recruitment of mentors only occurred after a participant had been randomized into the peer mentor arm. Potential mentors were identified and recruited in a similar manner as participants. Of 72 eligible mentors contacted, 27 refused to participate and 7 did not show up for training. One potential mentor was excluded at the baseline visit because he was incoherent. The overall participation rate of those contacted was 51%. Nobody reported they had been assigned to someone they knew.
Peer mentors participated in an hour long one-on-one training informed by motivational interviewing techniques (31). The training guide started with asking the mentor, by talking with the mentee, to learn the mentee’s story, understand the mentee’s motivations, help the mentee identify the differences between their behaviors and goals, and help the mentee identify a realistic plan for goal achievement. Open ended questions were encouraged and modeled. Mentors were also taught how to follow-up and assess progress. Sample questions were provided. However, mentors were also encouraged to draw on their own experiences. Calls were not monitored. No face-to-face meetings between mentors and mentees were required. Peer mentors were given the phone number of their mentee and informed they would receive $20 per month if the mentee confirmed they talked at least once a week. Once a month, peer mentors were contacted to provide training reinforcement and asked about their interactions with their mentee. Mentors were given $25 at the end of the training and for completing the short exit interview.
Study participants randomized to the financial incentive arm were told they could earn $100 at six months if their HbA1c dropped by one point and $200 if it dropped by two points or to 6.5% -- a level chosen instead of more aggressive targets. A day after enrollment, participants were notified by phone of their starting HbA1c and personal goals. For example a participant with a starting HbA1c of 11% was told they could earn $100 if their final HbA1c was between 9.1-10% and $200 if it was 9% or lower. A participant with a starting HbA1c of 8% could earn $100 if their final HbA1c was between 6.6-7% and $200 if it was 6.5% or below.
Outcomes and Follow-Up
Our primary pre-specified outcome was change in assessed HbA1c with treatment assignments as randomized and incorporating all available data. When available, HbA1c values drawn during routine clinical practice and within 30 days of the intended study end date were used for patients who missed their study follow-up appointment: otherwise we imputed follow-up values.
All participants were called monthly to assess for hypoglycemic symptoms to determine the safety of the interventions (a pre-specified secondary aim). Participants were asked how many times in the last month they had low blood sugar symptoms and how many times in the last month they had severe low blood sugar symptoms such as passing out or needing help to treat the reaction. If the latter question was greater than zero, follow-up questions asked about what kind of help they needed and if they went to the hospital. Events in which participants passed out or required assistance, went to the emergency room, or were hospitalized were considered potential study related serious adverse events (SAE). Patients also frequently reported hospitalization unrelated to hypoglycemic symptoms. These cases were reviewed, blinded to arm by one of the authors (JAL). All hypoglycemic events were considered potentially study related. Finally, short qualitative exit interviews were conducted with 28 participants and 24 mentors in the peer mentoring arm. Participants and mentors were asked if they liked the program, what were the best and worst aspects of the program and how it could be improved.
Statistical Analysis
We based our sample size estimate on a clinically relevant difference of 1.5 (32) for the change in HbA1c in the intervention groups, relative to control. To account for the two comparisons of interest, peer mentoring versus control and financial incentives versus control, we used a two-sided significance of 0.025 for each comparison. Assuming a standard deviation between 1.5 and 2.0 (17) and equal variances across time and groups, and within-subjects correlation of 0.5-0.6, with power at 0.80 we estimated that 21-38 patients per treatment group would be required.
We evaluated change in HbA1c as the dependent variable. We included baseline HbA1c as an adjustment variable since the maximum possible change in HbA1c is limited by the biological lower bound. We included as additional adjustment variables patient characteristics that were not balanced between intervention groups and control. To assess balance, we calculated standardized differences between each intervention group and control for each characteristic and included variables whose standardized difference was greater than 10% as main effects in a linear additive model. We selected change in HbA1c instead of final HbA1c as the dependent variable because its distribution was consistent with the assumed normality for linear regression. Our primary analyses are based on everyone enrolled, as randomized, except for the one person excluded at baseline due to a low HbA1c We used multiple imputation to generate values for each subject with missing follow-up data (33). Our multiple imputation procedure simulated a multivariate normal distribution for all variables in the primary analysis model. Each of ten imputed complete data sets were analyzed and the results combined for inference (33). All analyses were completed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
The multiple imputation method assumes data are missing at random (34, 35) or that missingness depends on observed variables only. We performed additional analyses to assess the robustness of our results and appropriateness of this assumption. First, we confirmed that all patient characteristics associated with missing final HbA1c were included in the imputation model. We then repeated the primary analysis, including only participants who had complete data. We also repeated the primary analysis including time in the study as a covariate, to account for the longer times between baseline and follow-up HbA1c tests in the peer mentor arm (which occurred due to the matching process). The results of the sensitivity analyses were similar to the original model, and we report only the results from the original model.
Not all participants had complete responses for the six monthly follow-up calls assessing hypoglycemic symptoms. We checked the amount of missing information by comparing the proportion of patients who completed 0-3 calls with those who completed 4-6 calls by arm. Minor hypoglycemic events were summarized by treatment arm, based on the proportion of completed monthly follow-up calls in which 0, 1-3, or >3 minor hypoglycemic events were reported. We compared the occurrence of hypoglycemic events between groups by modeling the ordinal event outcome in a multinomial generalized linear mixed model, after checking the proportional odds assumption. We included a fixed effect for study arm and accounted for clustering of repeated measures within subjects with random subject intercepts. In order to assess the representativeness of our results we compared the rates of minor hypoglycemic events among participants who completed 0-3 versus 4-6 monthly calls overall, and by study arm. To do this, we modeled the ordinal event outcome as above, and included follow up call completion status, study arm, and an interaction term between study arm and call completion status as independent variables.
Role of the Funding Source
The work was funded by a National Institute of Aging Roybal Center pilot grant. Funders were not involved in the design, conduct or reporting of the study.
RESULTS
The enrollment rate of those contacted and eligible was 61% for patients with poor control and 52% for mentors. The only statistically significant difference between the groups at baseline was the number of people with complications from diabetes (Table 1). The mean baseline HbA1c was 9.9% (SD 1.6) in the control arm, 9.8% (SD 1.8) in the peer mentor arm, and 9.5% (SD 1.2) in the financial incentive arm. The mean baseline HbA1c for peer mentors (based on chart review that made them eligible for the study) was 6.7% (SD 0.6).
Table 1.
Participant Characteristics
| Arm 1 Control (n = 39) |
Arm 2 Mentors (n = 38) |
Arm 3 Incentives (n = 40) |
|
|---|---|---|---|
| Mean Age, (SD) | 60 (4) | 60 (5) | 59 (5) |
| Male, % | 92 | 100 | 90 |
| Education ≤12 years, % | 64 | 68 | 50 |
| Married, % | 46 | 58 | 38 |
| On Insulin, % | 72 | 71 | 63 |
| Diabetes >10 years, % | 67 | 55 | 52 |
| Any Complication from Diabetes, % | 92 | 82 | 98 |
| Smoker Current, % | 33 | 47 | 28 |
| All Healthcare at VA, % | 74 | 74 | 73 |
| Good Self-Reported Adherence, % | 67 | 79 | 80 |
| Mean Baseline HbA1c, (SD) | 9.9 (1.6) | 9.8 (1.8) | 9.5 (1.2) |
| Mean Days Between Tests, (SD) | 185 (11) | 195 (15) | 185 (13) |
SD = standard deviation, VA = Veterans Administration
On average, HbA1c dropped from: 9.9% to 9.8% in the control arm, 9.8% to 8.7% in the peer mentor arm, and from 9.5% to 9.1% in the financial incentive arm (Figure 2). After adjusting for covariates the mean change relative to control (Table 2) was −1.07 points (95% CI-1.84 to −0.31) in the peer mentor arm and −0.45 points (95% CI −1.23 to 0.32) in the financial incentive arm.
Figure 2. Change in HbA1c for Each Participant by Arm.
Table 2.
Mean Change in HbA1c (%)
| Usual Care Arm |
Peer Mentor Arm |
P Value | Financial Incentive Arm |
P Value | |
|---|---|---|---|---|---|
| Mean Change from Baseline (95% CI) |
−0.01 (−0.52:0.51) | −1.08 (−1.62:-0.54) | −0.46 (−1.02:0.10) | ||
| Mean Change Relative to Control (95% CI) *† |
−1.07 (−1.84: −0.31) | 0.006 | −0.45 (−1.23: 0.32) | 0.25 |
Model covariates: baseline HbA1c, marital status, insulin use, diabetes co-morbidities, duration of diabetes, and self-reported adherence
Missing final HbA1c for 9 patients were handled by using multiple imputation multivariate normal models, including change in HbA1c(final-initial), treatment arm, baseline HbA1c, marital status, insulin use, diabetes co-morbidities, duration of diabetes, and selfreported adherence
CI = confidence interval
There were only two SAEs due to hypoglycemia (Table 3). No enrollee was removed from the study for a SAE. Taking into account repeated measures within participants, we found no evidence of significant differences between treatment groups in the occurrence of minor hypoglycemic events.
Table 3.
Harms
| Arm 1 Control (n = 39) |
Arm 2 Mentors (n = 38) |
Arm 3 Incentives (n = 40) |
|
|---|---|---|---|
| Deaths | 0 | 0 | 0 |
| Any Emergency Room Visit or Hospitalization |
7 | 8 | 10 |
| Passing Out, Emergency Room Visit or Hospitalization Secondary to Hypoglycemia |
1 | 0 | 1 |
| Minor Hypoglycemic Symptoms | # calls = 201 | # calls = 174 | # calls = 188 |
| 0 | 142 (71%) | 107 (61%)) | 121 (64%) |
| 1-3 | 38 (19%) | 52 (30%) | 51 (27%) |
| >3 | 21 (10%) | 15 (9%) | 16 (9%) |
Mentors and mentees talked the most in the first month, with a mean of 4 (range 0-30) calls per month. 14 mentors (37%) received payment for talking at least four times during the first month. By the sixth month the mean number of calls was 2 (range 0-10), and only 6 mentors (16%) received payment. We do not know if declining calls reflected decreased motivation or perceived reduction in need; we did not observe a dose response relationship between the number of calls made and change in HbA1c.
Twenty eight of 38 (74%) participants and 24 of 37 (65%) mentors completed the exit survey. Compared to participants who did not complete the exit survey, participants who completed the exit survey were similar. Mentors who completed the exit interview compared to those who missed it, were less likely to be married and more likely to have had diabetes for more than 10 years but these differences were not statistically different due to small sample size. In participant exit interviews 14/28 felt the mentoring experience was educational and 5/28 mentioned they appreciated the common understanding and life experiences. While 6/28 participants complained of too little contact. 20/28 liked that the mentor had diabetes and felt it was an important part of the program. Participants liked best the support provided (14/28), the education provided (9/28) and the ability to commiserate with mentors (6/28). Complaints about the program included difficulty getting in touch (4/28) and lack of compatibility with the mentor (3/28). 11/28 participants felt no changes were necessary, while 6/28 requested more calls, and 8/28 requested face-to-face meetings. Mentors appreciated helping others (12/24), communicating with their mentee (7/24) and the teaching process (7/24). 15/24 mentors thought it was important that they at one time did not have good control. Mentors complained about scheduling calls (5/24), disinterested mentees (5/24) and talking about non-diabetes related issues (4/24). 15/24 recommended face-to-face meetings, and 5/24 felt we should ensure greater willingness in the mentee.
DISCUSSION
In this well tolerated randomized controlled trial, a six month intervention of peer mentors had a statistically significant impact in improving glucose control. On average, participants in the peer mentor arm compared to the control arm dropped their HbA1c by close to 1 point. Although we also saw a drop of 0.5 in HbA1c compared to control in the financial incentive arm, it was not statistically significant; however, because of wide confidence intervals we cannot conclusively state the intervention was ineffective.
Matching peers by race, sex, and age made the peer mentor intervention innately culturally competent in that peers and mentees came from the same cultural background (1, 36). Because the intervention relied heavily on mentors’ personal experience, training was minimal and easy to implement. The effect size observed was large compared to many other behavior interventions (37-39).
Several factors may have contributed to the success of the peer mentor intervention. First, the peer intervention may have benefited from a culture of camaraderie among the veteran participants. Second, a long history of mistreatment and distrust in the health care system (40, 41) may make peer support particularly effective for minorities. Third, both intervention groups in our study may have benefited from our stringent inclusion criteria. In a prior study that successfully used reciprocal peer support for veterans with diabetes, the intervention was especially successful relative to nurse care management for those with a baseline HbA1c of >8% (11). We chose a group with persistently poor control since patients with chronically poor glucose control are at greatest risk from complications. Fourth, we provided mentors with $20 to talk at least 4 times per month to their mentee. This is itself a financial incentive, albeit a small one, and may have motivated mentors to call more frequently which must be considered when contemplating both the efficacy and the cost of the program.
Peer mentoring was done completely by phone, increasing its broad applicability and scalability. Although both participants and peer mentors indicated that they would have appreciated face-to-face introductions, the peer mentor arm was remarkably effective without such an introduction. An intervention of this sort could be especially effective in rural or suburban settings where contact by telephone is relatively easy, while frequent visits to the health care provider for provider care or group support might be relatively difficult. Finally, perhaps the most obvious attraction of this type of peer mentoring is that it is virtually free, almost certainly enhancing its cost-effectiveness relative to more costly interventions such as nurse care management, telemedicine and group medical appointments (17, 18, 42).
In this study no violations of privacy or safety concerns were raised by participants. While additional privacy safeguards could be implemented, for these programs to work, the mentee needs to be willing to divulge some personal information about their difficulty controlling their disease. Making this clear up front to participants is essential.
We may not have observed as large an improvement in HbA1c in the financial incentive arm as we did in the peer mentor arm because of the lack of feedback on glycemic control between the first visit and the follow-up six months later. There is growing evidence that financial incentives are more effective when there is frequent feedback (19-21, 23, 43). Financial incentives are controversial. Opponents of financial incentives worry about undue influence in low-income populations and blaming of individuals for their health status (44, 45). Others feel that financial incentives encourage patients taking a more active role in promoting their own health (23, 46). Financial incentives that reward healthy behaviors can be seen as non-punitive; however, programs where people are penalized for behaviors such as smoking are seen by some as unfair (44, 47, 48). The American College of Physicians (ACP) has come out in favor of incentive programs with the caveat that they should be evidence-based, respect autonomy, and be culturally sensitive (49). While this study does nothing to resolve this debate, it does add to the existing evidence.
We chose to limit inclusion in this study to patients who had both of their last two HbA1cs above 8%. People with poor control may be asked to return to clinic more frequently. Given recent evidence that intensive glucose lowering may not be optimal (50, 51) we targeted patients who would clearly benefit from improved control and were successful in finding a group with very poor baseline control who did not respond to usual care as evidenced by an improvement in the HbA1c of only 0.01 for those in the control arm. However, this design does limit the generalizability of the study and we do not know if the intervention would have been just as effective in a population with only one HbA1c reading above 8%.
Patients in the study were all African Americans veterans at one institution. Further research should examine the efficacy of a similar intervention on a broader population.
Future studies are necessary to determine the sustainability of these effects. Behavioral interventions effects frequently wane after the completion of the intervention (20, 21, 52, 53). One possible approach to maintaining sustainability would be to transition those who achieve control from mentee to mentor roles. Prior research has found that peer support is not only beneficial to those receiving it, but also to those giving it (1), because mentors may be highly motivated to maintain control to set a good example. In conclusion, this peer mentor intervention shows promise as a scalable approach to creating a mechanism to help patients at higher risk of diabetic complications improve HbA1c levels.
Acknowledgments
Funding for this project was supported by the National Institute of Aging Roybal Center 1P30AG034546 Volpp principal investigator
References
- 1.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003 Apr;30(2):170–195. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd CE, Wing RR, Orchard TJ, Becker DJ. Psychosocial correlates of glycemic control: the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. Diabetes Res Clin Pract. 1993 Aug-Sep;21(2-3):187–195. doi: 10.1016/0168-8227(93)90068-g. [DOI] [PubMed] [Google Scholar]
- 3.Glasgow RE, Toobert DJ. Social environment and regimen adherence among type II diabetic patients. Diabetes Care. 1988 May;11(5):377–386. doi: 10.2337/diacare.11.5.377. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero L, Spirito A, Bond A, Coustan D, McGarvey S. Impact of social support and stress on compliance in women with gestational diabetes. Diabetes Care. 1990 Apr;13(4):441–443. doi: 10.2337/diacare.13.4.441. [DOI] [PubMed] [Google Scholar]
- 5.Tillotson LM, Smith MS. Locus of control, social support, and adherence to the diabetes regimen. Diabetes Educ. 1996 Mar-Apr;22(2):133–139. doi: 10.1177/014572179602200206. [DOI] [PubMed] [Google Scholar]
- 6.Kulik JA, Mahler HI. Emotional support as a moderator of adjustment and compliance after coronary artery bypass surgery: a longitudinal study. J Behav Med. 1993 Feb;16(1):45–63. doi: 10.1007/BF00844754. [DOI] [PubMed] [Google Scholar]
- 7.Joseph DH, Griffin M, Hall RF, Sullivan ED. Peer coaching: an intervention for individuals struggling with diabetes. Diabetes Educ. 2001 Sep-Oct;27(5):703–710. doi: 10.1177/014572170102700511. [DOI] [PubMed] [Google Scholar]
- 8.Wilson W, Pratt C. The impact of diabetes education and peer support upon weight and glycemic control of elderly persons with noninsulin dependent diabetes mellitus (NIDDM) Am J Public Health. 1987 May;77(5):634–635. doi: 10.2105/ajph.77.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyserling TC, Ammerman AS, Samuel-Hodge CD, et al. A diabetes management program for African American women with type 2 diabetes. Diabetes Educ. 2000 Sep-Oct;26(5):796–805. doi: 10.1177/014572170002600508. [DOI] [PubMed] [Google Scholar]
- 10.Keyserling TC, Samuel-Hodge CD, Ammerman AS, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care. 2002 Sep;25(9):1576–1583. doi: 10.2337/diacare.25.9.1576. [DOI] [PubMed] [Google Scholar]
- 11.Heisler M, Vijan S, Makki F, Piette JD. Diabetes Control With Reciprocal Peer Support Versus Nurse Care Management. Ann Intern Med. 2010 Oct 19;153(8):507–515. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heisler M. Different models to mobilize peer support to improve diabetes self-management and clinical outcomes: evidence, logistics, evaluation considerations and needs for future research. Fam Pract. 2009;27(suppl 1):i23–i32. doi: 10.1093/fampra/cmp003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Matthews BA, Baker F, Hann DM, Denniston M, Smith TG. Health status and life satisfaction among breast cancer survivor peer support volunteers. Psychooncology. 2002 May-Jun;11(3):199–211. doi: 10.1002/pon.550. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz CE, Sendor M. Helping others helps oneself: response shift effects in peer support. Soc Sci Med. 1999 Jun;48(11):1563–1575. doi: 10.1016/s0277-9536(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 15.Jaber R, Braksmajer A, Trilling JS. Group visits: a qualitative review of current research. J Am Board Fam Med. 2006 May-Jun;19(3):276–290. doi: 10.3122/jabfm.19.3.276. [DOI] [PubMed] [Google Scholar]
- 16.Clancy DE, Cope DW, Magruder KM, Huang P, Wolfman TE. Evaluating concordance to American Diabetes Association standards of care for type 2 diabetes through group visits in an uninsured or inadequately insured patient population. Diabetes Care. 2003 Jul;26(7):2032–2036. doi: 10.2337/diacare.26.7.2032. [DOI] [PubMed] [Google Scholar]
- 17.Kirsh S, Watts S, Pascuzzi K, O’Day ME, Davidson D, Strauss S, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007 Oct;16(5):349–353. doi: 10.1136/qshc.2006.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trento M, Passera P, Bajardi M, Tomalino M, Grassi G, Borgo E, et al. Lifestyle intervention by group care prevents deterioration of Type II diabetes: a 4-year randomized controlled clinical trial. Diabetologia. 2002 Sep;45(9):1231–1239. doi: 10.1007/s00125-002-0904-8. [DOI] [PubMed] [Google Scholar]
- 19.Volpp KG, Loewenstein G, Troxel AB, Doshi J, Price M, Laskin M, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpp KG, John L, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial Incentive-based Approaches for Weight Loss: A Randomized Trial. JAMA. 2008;300:2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. New Engl J Med. 2009 Feb 12;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 22.O’Donoghue T, Rabin M. Doing it now or later. Am Econ Rev. 1999;89:103–124. [Google Scholar]
- 23.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007 Nov 28;298(20):2415–2417. doi: 10.1001/jama.298.20.2415. [DOI] [PubMed] [Google Scholar]
- 24.Volpp KG, Pauly MV, Loewenstein G, Bangsberg D. P4P4P: An Agenda for Research on Pay for Performance for Patients. Health Aff. 2009 Jan;28(1):206–214. doi: 10.1377/hlthaff.28.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention, National Institutes of Health, American Diabetes Association . National Diabetes Fact Sheet. U.S. Department of Health and Human Services; Atlanta, GA: 2007. [Google Scholar]
- 26.Pleis JL JW, Ward BW, Summary health statistics for U.S. adults: National Health Interview Survey, 2008 National Center for Health Statistics. Vital Health Stat. 2009;10(242):1–167. [PubMed] [Google Scholar]
- 27.Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the veterans affairs diabetes trial. Diabetes Care. 2005 Aug;28(8):1954–1958. doi: 10.2337/diacare.28.8.1954. [DOI] [PubMed] [Google Scholar]
- 28.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003 Aug;26(8):2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi AN, Grebla RC, Wright SM, Washington DL. Despite Improved Quality Of Care In The Veterans Affairs Health System, Racial Disparity Persists For Important Clinical Outcomes. Health Affairs. 2011 Apr 1;30(4):707–715. doi: 10.1377/hlthaff.2011.0074. [DOI] [PubMed] [Google Scholar]
- 30.Shacter HE, Shea JA, Akhabue E, Sablani N, Long JA. A qualitative evaluation of racial disparities in glucose control. Ethn Dis. 2009;19(2):121–127. [PubMed] [Google Scholar]
- 31.Rollnick S, Miller WR. What is motivational interviewing? Behavioural and Cognitive Psychotherapy. 1995;23:325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 32.America Diabetes Association Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2002;25:S28–S32. doi: 10.2337/diacare.26.2007.s28. [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB. Multiple Imputation for Nonresponse in Surveys. J Wiley & Sons; New York: 1987. [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd Edition Wiley-Interscience; New Jersey: 2002. [Google Scholar]
- 35.Donders A, van der Heijden GJMG, Stingen T, Moons KGM. A gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Webel AR, Okonsky J, Trompeta J, Holzemer WL. A Systematic Review of the Effectiveness of Peer-Based Interventions on Health-Related Behaviors in Adults. Am J Public Health. 2010 Feb 1;100(2):247–253. doi: 10.2105/AJPH.2008.149419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peek ME, Cargill A, Huang ES. Diabetes Health Disparities. Med Care Res Rev. 2007 Oct 1;2007(5 suppl):101S–156S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gary TL, Genkinger JM, Guallar E, Peyrot M, Brancati FL. Meta-Analysis of Randomized Educational and Behavioral Interventions in Type 2 Diabetes. Diabetes Educ. 2003 May-Jun;29(3):488–501. doi: 10.1177/014572170302900313. 2003. [DOI] [PubMed] [Google Scholar]
- 39.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-Management Education for Adults With Type 2 Diabetes. Diabetes Care. 2002 Jul 1;25(7):1159–1171. doi: 10.2337/diacare.25.7.1159. 2002. [DOI] [PubMed] [Google Scholar]
- 40.Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health. 1997 Nov;87(11):1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008 Jun;23(6):827–833. doi: 10.1007/s11606-008-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone RA, Rao RH, Sevick MA, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care. Mar;33(3):478–484. doi: 10.2337/dc09-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haisley E, Volpp KG, Pellathy T, Loewenstein G. Promoting Completion of Health Risk Assessments with Lottery Incentives. Am J Health Promot. 2011 doi: 10.4278/ajhp.100729-ARB-257. In Press. [DOI] [PubMed] [Google Scholar]
- 44.Bishop G, Brodkey AC. Personal responsibility and physician responsibility--West Virginia’s Medicaid plan. N Engl J Med. 2006 Aug 24;355(8):756–8. doi: 10.1056/NEJMp068170. [DOI] [PubMed] [Google Scholar]
- 45.Shaw J. Is it acceptable for people to be paid to adhere to medication? No. Bmj. 2007 Aug 4;335(7613):233. doi: 10.1136/bmj.39286.422639.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunstein CR, Thaler RH. Libertarian paternalism is not an oxymoron. The University of Chicago Law Review. 2003;70:1159–1202. [Google Scholar]
- 47.Halpern SD, Madison KM, Volpp KG. Patients as mercenaries? The ethics of using financial incentives in the war on unhealthy behaviors. Circ Cardiovasc Qual Outcomes. 2009;2:514–516. doi: 10.1161/CIRCOUTCOMES.109.871855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson SD, Lieber SR. Financial Penalties for the Unhealthy? Ethical Guidelines for Holding Employees Responsible for Their Health. Health Affairs. 2009;28:845–52. doi: 10.1377/hlthaff.28.3.845. [DOI] [PubMed] [Google Scholar]
- 49.Mitnick S, Snyder L, Hood VL. Ethical considerations for the use of patient incentives to promote personal responsibility for health: West Virginia medicaid and beyond. Position paper of the American College of Physicians. 2010 [Google Scholar]
- 50.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 52.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A Self-Regulation Program for Maintenance of Weight Loss. New Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 53.Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with High or Low Protein Content and Glycemic Index for Weight-Loss Maintenance. New Engl J Med. 363(22):2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]