Abstract
Numerous reporting guidelines are available to help authors write higher quality manuscripts more efficiently. Almost 200 are listed on the EQUATOR (Enhancing the Quality and Transparency of Health Research) Network’s website and they vary in authority, usability, and breadth, making it difficult to decide which one(s) to use. This paper provides consistent information about guidelines for preventive medicine and public health and a framework and sequential approach for selecting them.
EQUATOR guidelines were reviewed for relevance to target audiences; selected guidelines were classified as “core” (frequently recommended) or specialized, and the latter were grouped by their focus. Core and specialized guidelines were coded for indicators of authority (simultaneous publication in multiple journals, rationale, scientific background supporting each element, expertise of designers, permanent website/named group), usability (presence of checklists and examples of good reporting), and breadth (manuscript sections covered). Discrepancies were resolved by consensus. Selected guidelines are presented in four tables arranged to facilitate selection: core guidelines, all of which pertain to major research designs; guidelines for additional study designs, topical guidelines, and guidelines for particular manuscript sections. A flow diagram provides an overview. The framework and sequential approach will enable authors as well as editors, peer reviewers, researchers, and systematic reviewers to make optimal use of available guidelines to improve the transparency, clarity, and rigor of manuscripts and research protocols and the efficiency of conducing systematic reviews and meta-analyses.
Background
CONSORT, the first reporting guideline to gain traction among journal editors, merged two initiatives in the mid-1990’s, spurred by systematic review practitioners and methodologists.1 The very name of the first (1996) CONSORT Statement, the Consolidated Standards of Reporting Trials,1 acknowledged these earlier initiatives. CONSORT and subsequent reporting guidelines were developed to improve the transparency and rigor of journal articles reporting biomedical research, and to promote consistency in both what is reported and how it is reported.2
Such guidelines have now been expanded to cover many types of health research,3 and the majority of high impact medical journals4 (e.g., New England Journal of Medicine, Lancet, JAMA) now require coverage of elements specified in reporting guidelines.5 Discriminating use of reporting guidelines can have enormous value, alerting researchers, authors, peer reviewers, journal editors, and systematic reviewers to common errors in both reporting and the conduct of empiric studies and, thus, helping to avoid these errors.6,7
Finding reporting guidelines has been made easier by the creation of the EQUATOR (Enhancing the QUality And Transparency Of health Research) network and its Library for Health Research Reporting at www.equator-network.org. In fact, several sources, including Uniform Requirements,8 no longer specify which reporting guideline(s) to use, but simply refer the reader to EQUATOR. Since being published in January, 2010,9,10 the EQUATOR catalogue of reporting guidelines has been expanded through systematic searches11 five times,12 most recently (October 2011) by 35 new guidelines, bringing the total to 191.13 A novice user, however accomplished an author, may find the sheer number of possibilities overwhelming and be unclear how to evaluate a guideline. Some guidelines are explicitly designed to be used with other listed guidelines (e.g., TREND with CONSORT). Most guidelines vary in their authority, usability, and breadth. Navigating this thicket requires more than a simple listing of what is available. Thus, the purpose of this paper is to provide information on the authority, usability, and breadth of guidelines included in the EQUATOR catalogue that are relevant to preventive medicine and public health. We present this information together with a framework and sequential approach for selecting and using relevant guidelines.
Methods
Selection of guidelines from EQUATOR’s Catalogue of Reporting Guidelines
EQUATOR’s Catalogue is comprehensive for published guidelines available in English and served as the sole source of possible guidelines. Because some of the guidelines are what EQUATOR calls “highly specialized”, with a focus on specific medical conditions or procedures (e.g., intra-arterial cerebral thrombolysis for acute ischemic stroke), those with the greatest relevance to preventive medicine and public health were selected from the guidelines mentioned in author instructions for journals with the highest ISI impact factors in their respective categories: (a) the highest-ranked 40 “public, environmental, and occupational health” and (b) the 10 highest-ranked “general and internal medicine” journals.4 Next, the authors, representing behavioral sciences, epidemiology, and public policy, reviewed the remaining guidelines in November 2011.
Designating the selected guidelines as “core” or “specialized”
Selected guidelines were categorized as “core” or specialized; specialized guidelines were subdivided by topic. Core designation was based on having been mentioned by name six or more times in 1) the author instructions for the 50 journals described above, 2) the list of guidelines in EQUATOR’s right-hand navigation panel, which highlights basic guidelines, 3) the list of guidelines previously specified in Uniform Requirements for the Submission of Manuscripts to Biomedical Journals, and 4) the National Library of Medicine list of “research reporting guidelines and initiatives”.14
All selected guidelines, both core and specialized, were coded by two authors of this paper for characteristics contributing to authority, usability, and breadth. Indicators of authority included stating a rationale, having been developed by a named group e.g., CONSORT Group, maintaining a website, and explicitly describing, in the text or on the website, the expertise of those involved in guideline design. A rationale was defined as being based on a survey of the literature or other evidence of omissions or errors in reporting and/or conducting studies. Aims, goals, or justifications lacking these elements were not considered rationales. Further indicators of authority included simultaneous publication of the guideline, supportive editorials, and explanation of the scientific background of each reporting element, with supporting citations for 75 percent or more of the explanations. Early guidelines presented examples in separate “explanation and elaboration” (E&E) documents; later guidelines often incorporate this information into the initial publication.
Second, indicators of usability included presenting a checklist with definitions of the included elements and examples of good reporting from published sources. Third, guidelines were coded for breadth, i.e., the parts of the manuscript covered. Because guidelines differ in breadth, several guidelines may be needed to cover all sections of a manuscript. The protocol from Moher and associates’ 2011 review of guidelines contains several of these coding elements.15
Based on experience, it was anticipated that there might be a need to use multiple guidelines to write a specific paper; thus, specialized guidelines were grouped in a logical sequence for ease of use. Discrepancies in data extraction and grouping were resolved by consensus.
Results
Fifty-one guidelines from the EQUATOR catalogue were chosen as most relevant to preventive medicine and public health. Excluded, for example, were guidelines pertaining to dentistry and music therapy. Five guidelines were designated as “core” guidelines, representing a range of study design: randomized controlled trials (CONSORT); non-randomized trials (TREND); cohort, case-control, and cross-sectional studies (STROBE); systematic reviews and meta-analyses (PRISMA); and studies of diagnostic accuracy (STARD) (Table 1). Most authors will find one of these guidelines a key resource in preparing their papers.
Table 1.
Core Guidelines
| RCTs CONSORT 2010 rev | Non-RCTs TREND 2004 | Observational Studies in Epidemiology: STROBE 2007 | Systematic Reviews and Meta-Analyses PRISMA 2009 | Diagnostic Accuracy Studies STARD 2003 | |
|---|---|---|---|---|---|
| Group, Web site | CONSORT Group www.consort-statement.org/ | HIV/AIDS Prevention Research Systhesis Team www.cdc.gov/trendstatement/ | STROBE Initiative www.strobe-statement.org/ | PRISMA Group www.prisma-statement.org/ | STARD Group www.stard-statement.org/ |
| Expertise | Clinical trials, statistics, biomedical editors | HIV/AIDS prevention, research, public health practice, journal editors | Epidemiology, methods, statistics, research, journal editors | Systematic review, methods, clinical med, medical editors, a consumer | Research, methods, editors, reps of prof orgs |
| Explanations | E&E doc + website | [CONSORT E&E doc] | E&E doc | E&E doc | E&E doc |
| Examples | E&E doc | [CONSORT E&E doc] | E&E doc | E&E doc | E&E doc |
| Flow diagram | ✓ | x | x | ✓ | ✓ |
| Additional features | Definitions, contents of an abstract; baseline demographics example. | Distinguishes new items from CONSORT items. | Draft checklist for conference abstracts on website. | Evolution of revision; comparison of two. | |
| Notes; Open access citation + no. simultaneous publications | Replaced CONSORT 2001; 2010 BMC Med2 +10 E&E: 2010 BMJ29 +1 |
Focus=behavioral & public health interventions; Am J Public Health,30 + editorial | Cohort, case-control, cross-sectional studies; 2007 PLoS Med31 +7 E&E: 2007 PLoS Med32 +2 |
Replaced QUOROM; 2009 PLoS Med33 +5 E&E: 2009 PLoS Med34 +2 |
2003 BMJ35 + 6 others; +8 later E&E: 2003 Ann Intern Med36 +1; +1 later |
✓included
x not included
E&E doc: Explanation and elaboration document
All have rationales and checklists.
The core guidelines (Table 1) all present rationales, have permanent websites, were written by named groups (except TREND), have simultaneous publications or (for TREND) a supporting editorial, and, most importantly, give the scientific background for every specified element; in each case these explanations met the stated criteria. The explanations and examples may be found in separate publications (CONSORT, STROBE, and PRISMA), as a “background document” (STARD’s website), or within the guideline document itself TREND. A signal feature of CONSORT is the flow diagram, with numbers rather than percentages; PRISMA and STARD have a similar feature. All five have checklists; four offer additional features in their texts or on their websites (e.g., STROBE’s definitions of study designs). CONSORT and its E&E document have been updated in 2001 and 2010 since their original publication in 1996; PRISMA is an update of what was previously called QUOROM (QUality Of Reporting Of Meta-analysis).
The next group of guidelines (n=31) comprises additional study designs organized under the broad headings used on EQUATOR, with further subdivision to differentiate subgroups that assist readers in identifying guidelines of interest: Experimental studies (n=4), observational studies (n=8), reliability studies (n=1), meta-analyses (n=1), qualitative research (n=3), economic evaluations (n=6), health administration (n=1), statistics (n=4), quality improvement studies (n=2), and participatory action research (n=1) (Table 2). Authors who do not find a fit in Table 1 (e.g., for a qualitative study) should check Table 2, where they will find, for example, COREQ for qualitative interviews and focus groups. Authors who do find a good fit with a guideline from Table 1 also should check Table 2, for additional, related guidelines. For example, after choosing STROBE from Table 1 for a cross-sectional study using an Internet survey, adding CHERRIES (Internet surveys) from Table 2 will help in reporting the appropriate information about sample selection. Another (2003)16 guideline for surveys in Table 2 has few indications of authority compared to STROBE, but offers a perspective on non-epidemiologic surveys.
Table 2.
Guidelines for Other Designs and Analyses
| Designs | Citations | Characteristics | Breadth | |||||
|---|---|---|---|---|---|---|---|---|
| Ti | Ab | Int | Re | D | ||||
| EXPERIMENTAL STUDIES | ||||||||
| TRIALS | Non-inferiority and equivalence | CONSORT ext; 2006 JAMA37 | xRationale xExplanations ✓Examples xExpertise ✓Group: www.consort-statement.org/extensions/ |
✓ | ✓ | ✓ | ✓ | |
| Comparative effectiveness | 2009 Value Health38 | xRationale ✓Explanations xExamples xExpertise ✓Group: ISPOR Good Research Practices for Retrospective Database Analysis Task Force + guidelines for appropriate research questions |
✓ | ✓ | ✓ | ✓ | ||
| Pragmatic trials | CONSORT ext; 2008 BMJ39 | xRationale ✓Explanations xExamples xExpertise ✓Group: Pragmatic Trials in Healthcare www.consort-statement.org/extensions/ |
✓ | ✓ | ✓ | |||
| Cluster RCTs | CONSORT ext; 2004 BMJ,21 BMJ [ed] 200440 | ✓Rationale ✓Explanations ✓Examples xExpertise ✓Group: http://www.consort-statement.org/extensions/ |
✓ | ✓ | ✓ | ✓ | ✓ | |
| OBSERVATIONAL STUDIES | ||||||||
| BIOMARKERS | Molecular epidemiology (biomarker) studies |
STROBE ext; STROBE-ME; 2011 PloS Med41 +6 |
xRationale ✓Explanations ✓Examples ✓Expertise: epidemiology, biostatistics, lab science ✓Group: Initiated by Environmental Cancer Risk, Nutrition & Individual Susceptibilty European Network of Excellence |
✓ | ✓ | ✓ | ✓ | ✓ |
| Tumour marker prognostic studies | REMARK; 2005 J Natl Cancer Inst42 +5 | ✓Rationale ✓Explanations xExamples ✓Expertise ✓Group: Nat’l Cancer Inst & Eur Org for Res & Treatment of Cancer http://cdp.cancer.gov/scientificPrograms/pacct/remark.htm |
✓ | ✓ | ✓ | ✓ | ||
| GENETIC STUDIES | Genetic association studies |
STROBE ext; STREGA; 2009 PLoS Med43 +6 +3 eds E&E: website |
✓Rationale ✓Explanations xExamples ✓Expertise: epidmiology, genetics, statistics, journal editors ✓Group: www.strega-statement.org |
✓ | ✓ | ✓ | ✓ | ✓ |
| Immunogenomic studies |
STREGA ext; STREIS; 2011 Tissue Antigens44 |
xRationale ✓Explanations ✓Examples xExpertise ✓Group: Immunogenomics Data Analysis Working Group http://igdawg.org/streis.html |
✓ | ✓ | ||||
| Genetic risk prediction studies |
GRIPS; 2011 PLoS Med45 +9 E&E: 2011 J Clin Epidemiol46 +3 |
✓Rationale ✓Explanations ✓Examples ✓Expertise: risk predicttion, epidemiology, genetics, methods, statistics, journal editors | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SURVEYS | Internet e-surveys | CHERRIES; 2004 J Med Internet Res47 [ed] | ✓Rationale xExplanations xExamples ✓Expertise: author is journal’s editor | |||||
| Momentary self-report | 2002 Ann Behav Med48 | ✓Rationale xExplanations xExamples xExpertise | ||||||
| Survey research | 2003 Int J Qual Health Care16 | xRationale xExplanations xExamples xExpertise | ✓ | ✓ | ✓ | ✓ | ||
| RELIABILITY AND AGREEMENT STUDIES | ||||||||
| Reliability & agreement studies | GRRAS; 2011 Clin Epidemiol49 +1 [ed] | xRationale ✓Explanations xExamples ✓Expertise: instrument development, evaluation, reliability & agreement estimation, sys tematic review | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SYSTEMATIC REVIEWS AND META-ANALYSES | ||||||||
| Meta-analyses: epidemiology | MOOSE; 2000 JAMA50 | ✓Rationale ✓Explanations xExamples ✓Expertise: clinical med, trials, statistics, epidemiology, social science, journal editors ✓Group: MOOSE group (no website) |
✓ | ✓ | ✓ | ✓ | ||
| QUALITATIVE RESEARCH | ||||||||
| Interviews, focus groups | COREQ; 2007 Int J Qual Health care51 | ✓Rationale ✓Explanations ✓Examples xExpertise | ✓ | |||||
| Qualitative research in psychology | 1999 Br J Clin Psychol52 | ✓Rationale xExplanations ✓Examples ✓Expertise: Soc for Psychotherapy workshop (1993), Am Psychological Assoc Symposium (1994) | ✓ | ✓ | ||||
| Qualitative research | RATS; 2003 book53 | xRationale xExplanations xExamples xExpertise Modified for Biomed Central http://www.biomedcentral.com/info/ifora/rats |
✓ | ✓ | ||||
| ECONOMIC EVALUATIONS | ||||||||
| Cost-effectiveness analyses | 1996 JAMA54 | xRationale xExplanations ✓Examples ✓Expertise: CEA, clinicalmed, ethics, health outcomes measurement ✓Group: Panel on Cost-Effectiveness in Health & Medicine |
✓ | ✓ | ||||
| Cost-effectiveness analyses with clin trials | 2005 Value Health55 | xRationale ✓Explanations ✓Examples xExpertise ✓Group: Internatl Soc for Pharmacoeconomics & Outcomes Res RCT-Cost Effectiveness Analysis Task Force http://www.ispor.org/workpaper/clinical_trial.asp |
✓ | ✓ | ||||
| Economic evaluations for trial-based studies & decision analytic models | 2005 Int J Technol Assess Health Care56 | xRationale xExplanations ✓Examples xExpertise | ✓ | ✓ | ||||
| Economic evaluations (modeling studies) | 1998 Pharmacoeconomics57 | xRationale xExplanations ✓Examples xExpertise | ✓ | ✓ | ✓ | ✓ | ||
| Economic evaluation w/RCTs | 2011 BMJ58 | xRationale xChecklist xExplanations xExamples xExpertise | ✓ | ✓ | ||||
| Economic evaluation (modeling) | 2011 BMJ59 | xRationale xChecklist xExplanations xExamples xExpertise | ✓ | ✓ | ||||
| HEALTH ADMINISTRATION | ||||||||
| Validation studies of health admin data | STARD modification; 2011 J Clin Epidemiol60 | ✓Rationale xExplanations xExamples ✓Expertise: research using health administrative data | ✓ | |||||
| STATISTICAL METHODS & ANALYSES | ||||||||
| Bayesian: health-care evaluations | BayesWatch; 2000 Health Technol Assess61 | ✓Rationale ✓Explanations ✓Examples xExpertise | ✓ | ✓ | ✓ | ✓ | ||
| Bayesian: clinical studies | ROBUST; 2005 J Clin Epidemiol62 | ✓Rationale xExplanations xExamples ✓Expertise: Bayesian analysis of clinical studies | ✓ | ✓ | ||||
| Heterogeneity in effects | 2010 Trials63 | xRationale ✓Explanations xExamples xExpertise | ✓ | ✓ | ||||
| Subgroup analysis in clinical trials | 2007 N Engl J Med64 | xRationale xExplanations xExamples xExpertise | ✓ | ✓ | ✓ | ✓ | ||
| QUALITY IMPROVEMENT STUDIES | ||||||||
| Quality improvement studies | SQUIRE; 2008 Qual Saf Health Care65 | xRationale ✓Explanation xExamples ✓Expertise: authors, publication guideline developers, peer reviewers, journal editors | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Quality improvement studies | 1999 Qual Health Care66 | xRationale ✓Explanation xExamples ✓Expertise: journal editors | ✓ | ✓ | ✓ | |||
| OTHER REPORTING GUIDELINES | ||||||||
| Participatory action research | 2010 Couns Psychol67 | xRationale ✓Explanations ✓Examples xExpertise | ✓ | ✓ | ✓ | ✓ | ||
Notes: All listed address the methods section of papers; all have checklists unless otherwise noted.
Six Table 2 guidelines are “extensions” of either CONSORT or STROBE. All of the official CONSORT extensions are being revised in keeping with the 2010 revision of the parent guideline. In addition to the CONSORT and STROBE groups, seven more guidelines are group efforts (e.g., REMARK, for tumor marker prognostic studies). The remainder are the work of one or more individual authors rather than named groups (e.g., CHERRIES for Internet surveys). A majority (n=17) of the Table 2 guidelines do not provide a rationale, but almost all (n=29) include a checklist with definitions, and the majority offer explanations of checklist elements (n=17). Fewer include examples (n=13). All cover the methods section; more than half cover at least three of the five other sections of a paper (n=17).
The next group of guidelines (n=9) addresses research topics rather than designs (Table 3). Topical guidelines cover a wide range of subjects, conditions, treatments, and outcomes; examples include health informatics, HIV interventions, and quality of life. All those in Table 3 have checklists and two-thirds (n=6) have explanations of included items. Seven discuss all sections of a paper and one covers only the methods and results sections. Four offer examples of good reporting. In addition to these 9, there are numerous highly specific guidelines that may be helpful with particular study types and topics, such as economic evaluations of fall prevention research. To access such fine-grained guidelines, there is a search engine at the EQUATOR website.
Table 3.
Guidelines for Specialized Topics
| Topic | Citation(s) | Characteristics | Ti | Ab | I | R | D |
|---|---|---|---|---|---|---|---|
| Behavioural medicine | Unofficial CONSORT ext; 2003 Ann Behav Med68 | xRationale ✓Explanations ✓Examples xExpertise | ✓ | ✓ | ✓ | ✓ | ✓ |
| eHealth interventions | 2010 Patient Educ Couns69 | xRationale ✓Explanations ✓Examples xExpertise | ✓ | ✓ | ✓ | ✓ | ✓ |
| Biospecimen reporting | BRISQ; 2011 J Proteome Res70 +1 | xRationale ✓Explanations ✓Examples ✓Expertise: lab science, clinical med, pathology, statistics, patient advocacy, biobanking, professional societies | |||||
| Evaluation studies in health informatics | STARE-HI; 2009 Int J Med Inform71 | ✓Rationale xExplanations xExamples ✓Expertise: journal’s eds, reviewers, authors, readers ✓Group: Med Informatics & Am Med Informatics Assoc work groups on evaluation http://iig.umit.at/efmi/starehi.htm |
✓ | ✓ | ✓ | ✓ | ✓ |
| Harms | CONSORT ext; 2004 Ann Intern Med72 | ✓Rationale ✓Explanations xExamples ✓Expertise: journal editors, experts in related fields ✓Group: http://www.consort-statement.org/extensions/data/harms/ |
✓ | ✓ | ✓ | ✓ | ✓ |
| HIV intervention research | 2004 AIDS Educ Prev73 | ✓Rationale xExplanations xExamples xExpertise ✓Group: Behavioral Intervention Research Branch, Divisions of HIV/AIDS Prevention, Centers for Disease Control & Prevention |
✓ | ||||
| Non- pharmacologic treatments |
CONSORT ext; 2008 Ann Intern Med,74 E&E: 2008 Ann Intern Med75 |
✓Rationale ✓Explanations ✓Examples ✓Expertise: “content”, methods, editors ✓Group: http://www.consortstatement.org/extensions/ |
✓ | ✓ | ✓ | ✓ | ✓ |
| Outbreak reports of nosocomial infections | ORION; 2007 Lancet Infect Dis76 +2 | xRationale ✓Explanations xExamples ✓Expertise: Authors, editors, content, learned soc ✓Group: Infectious Disease Research Network www.idrn.org/orion.php |
✓ | ✓ | ✓ | ✓ | ✓ |
| Quality of life in clinical trials | 1996 Qual Life Res77 | ✓Rationale xExplanations xExamples xExpertise | ✓ | ✓ | ✓ | ✓ | ✓ |
Note: All guidelines listed address the methods section of papers. All guidelines have checklists unless otherwise noted.
The fourth group of guidelines (n=6) focuses on sections of a manuscript, e.g., the abstract or the discussion section (Table 4). The most authoritative and broad source is Uniform Requirements for Submission of Manuscripts to Biomedical Journals (URM). If the selected guidelines do not cover a particular section or if the instructions are very general (e.g., “include the study type in the title” or “use a structured abstract”), URM is the default (available under “Guidance developed by editorial groups” at EQUATOR or directly at http://www.icmje.org/). An extension of CONSORT addresses abstracts and two guidelines address descriptions of literature searches (e.g., STARLITE, supplementing PRISMA and MOOSE). Specific perspectives on aspects of discussion sections are also available.
Table 4.
Parts of a Manuscript
| Guideline | Citation(s) | Characteristics | Breadth | |||||
|---|---|---|---|---|---|---|---|---|
| Ti | Ab | I | M | R | D | |||
| CONSORT for Abstracts (RCTs)a | 2008 Lancet78 E&E: 2008 PLoS Med79 |
✓Rationale ✓Explanations ✓Examples (website) xExpertise ✓Group: CONSORT group www.consort-statement.org/extensions/data/abstracts |
✓ | |||||
| Literature searches | STARLITE; 2006 J Med Libr Assoc80 | ✓Rationale xExplanations xExamples xExpertise | ✓ | |||||
| 2010 Int J Technol Assess Health Care81 | ✓Rationale xExplanations xExamples xExpertise | ✓ | ||||||
| Narrative sections | 2005 Ann Emerg Med82 | xRationale xChecklist ✓Explanations xExamples xExpertise | ✓ | ✓ | ✓ | ✓ | ||
| Structured discussions | 1999 BMJ83 | xRationale xExplanations xExamples xExpertise | ✓ | |||||
| Research recommendations | 2006 BMJ84,85 | xRationale xExplanations ✓Examples ✓Expertise: BMJ Pub Grp, Centre for Reviews & Dissem, Nat’l Coord Centre for Health Tech Assess, Nat’l Instit for Health & Clin Excellence, Scottish Intercollegiate Guidelines Network, UK Cochrane Center | ✓ | |||||
note: All items have checklists unless otherwise noted.
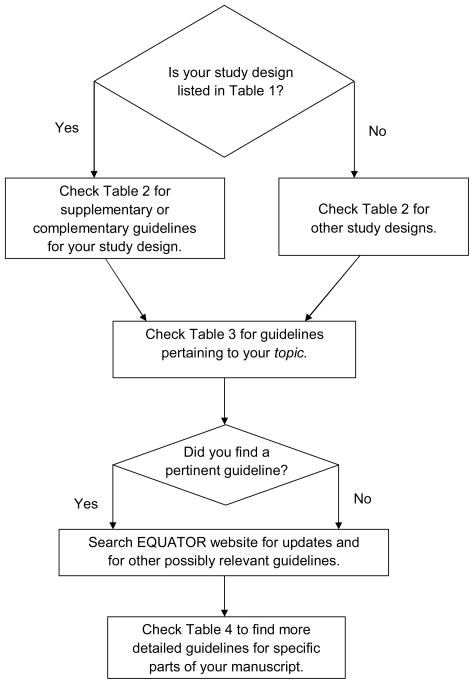
Thus, it is suggested that Tables 1–4, representing a division of guidelines into logical groups, be used in sequence, as illustrated (Figure 1). These groups may be expanded in the future through new guidelines and extensions of existing guidelines; see EQUATOR’s section on “reporting guidelines under development”.17 Consolidation and evaluation of guidelines also is occurring. For example, Moher’s group has done a systematic review to identify guidelines for reporting survey research and to compare and critique those available; they concluded that there was no consensus on items to be included and that a new, validated guideline should be developed, possibly building on STROBE.18
Figure 1.
Discussion
Impact of reporting guidelines
Guidelines have gained momentum in the number of journals endorsing particular guidelines or at least referring authors to the EQUATOR Network. Two of the “core” guidelines in Table 1 (CONSORT and STROBE) now have growing “families” of related guidelines, sanctioned by the group that keeps the core guideline updated. These “families” are positive developments, in that each new guideline is specifically designed as a supplement to the original, re-using those aspects which are common to both, and making it easy for users to follow them. CONSORT, the most extensively studied of reporting guidelines, has had a modest positive influence on the quality of reporting;19,20 the CONSORT extension for cluster randomized trials21 has been reported to have improved identification of trials as cluster designs, but there has been little improvement in the frequency of inappropriate statistical analyses.22
It is not unreasonable for journal editors to make a distinction between guidelines or standards and requirements. As discussed by the American Psychological Association’s Working Group on Journal Article Reporting Standards (the JARS group) in offering their recommendations to the APA Publications and Communication Board, “By not calling them ‘requirements,’ … [we] felt the standards should be given the weight of authority while retaining for authors and editors the flexibility to use the standards in the most efficacious fashion.”3, p. 847
Benefits of reporting guidelines to several user groups
Systematic reviewers provided the impetus for the creation of reporting guidelines, out of their frustration with missing, unclear, and erroneous information that made it perilous to describe study samples, interventions, outcomes, and the risk of bias and, therefore, to draw appropriate conclusions.23 Moreover, reporting guidelines provide guidance about study characteristics to code and definitions for the codebook as well as more informative titles and abstracts that make it faster to select citations for inclusion.
The utility of reporting guidelines for authors seems self-evident: They prescribe necessary information, in a sequence and form which is standardized within a specific field. Indeed, many journals require the submission of a completed checklist. Indicating the use of reporting guidelines with a statement such as “Items are reported in accordance with ----” allows searchers to find such articles to monitor adoption of guidelines in addition to indicating the authority for items included in the manuscript. The concern of the JARS group and others regarding space limitations and complete reporting3 is more easily resolved in this age of electronic publishing, with easy access to supplemental material stored online.
Some guidelines stress that journal peer reviewers and editors should not use a guideline’s checklist as a first screening tool for publication. For example, CONSORT 2010 states, “The items should elicit clear pronouncements of how and what the authors did, but do not contain any judgments on how and what the authors should have done. Nor is it appropriate to use the checklist to construct a ‘quality score.’”24 Nevertheless, clearer reporting of guideline-specified information makes it easier to evaluate a study’s strengths and weaknesses.
CONSORT (among others) also disclaims its value in designing a research protocol. “Note that the Statement does not include recommendations for designing, conducting, and analyzing trials. It solely addresses the reporting of what was done and what was found.”24 First, reporting elements that have not been anticipated may leave the investigator at a loss when it comes time to write the manuscript. Second, having a clear understanding of the definitions and acceptable operations of certain reporting elements, e.g., “intent to treat analysis”, should result in clearer and higher quality protocols. Third, for types of studies prone to inappropriate research questions or analyses, guidelines such as CONSORT for Reporting of Noninferiority and Equivalence Randomized Trials provide advice on appropriate questions and analyses.
The EQUATOR website is likely to continue to be the long-term go-to place to discover the existence of specific reporting guidelines, as well as those under development.25 Moher’s recent review and evaluation of guideline creation processes15 is designed to be part of a possible future rating system for reporting guidelines,15,26 but such a rating system is not yet in place.9,10
Limitations
Several potential limitations in this paper must be noted. Reliance on the EQUATOR Network as the source of guidelines is viewed by the authors of this paper as a relatively minor flaw because of the pre-eminent position of the Network, the quality of its periodic searches, and the ease of going to a single source for virtually all reporting guidelines relevant to the public health and preventive medicine literature. It should be noted, however, that the influential RE-AIM framework (reach, effectiveness, adoption, implementation and maintenance)27 is not included in the EQUATOR database, despite proposing an expansion to CONSORT to cover external validity.28 EQUATOR also does not necessarily list papers which critique current survey guidelines; the one mentioned above18 was not found on EQUATOR, despite authors who are deeply involved in the EQUATOR network; it might be found by searching PubMed.
Further limitations are that in the tables, the tradeoff of space versus nuanced description means that some characteristics receiving an “x” were partially present. The relatively simple coding scheme used here to indicate “authority” does not purport to address validity, a complex task underway by the EQUATOR network team. When faced with a guideline with few indicators of authority, EQUATOR’s section on reporting guidelines under development is again a valuable point of contact. Ultimately, users must make their own judgments as to which guideline(s) are potentially of greatest value to them, whether they are writing a paper or systematic review, preparing a research proposal, or providing peer review. By using the sequential approach outlined here and the indicators of authority, usability, and breadth in picking 1) a core guideline, 2) a supplementary or specialty secondary guideline, 3) a topic-based guideline if available, and 4) appropriate resources for specific parts of the manuscript, a user can make optimal use of guidelines and provide much-needed transparency and rigor.
Acknowledgments
The authors wish to acknowledge with thanks the many thoughtful comments we received from the journal’s reviewers.
NEC, SAK, KAS, and LRT are supported by pre-doctoral Fellowships from, WAC and Y-CL by post-doctoral Fellowships from, and KP and PDM are partially supported by the University of Texas School of Public Health Cancer Education and Career Development Program - National Cancer Institute/NIH Grant #2 R25 CA57712. MYC is supported by NCI grant #5 K07 CA140159-03.
Footnotes
The authors declare they have no financial disclosures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
Note: In cases of multiple concurrent publication, we have when possible chosen a version appearing in an open-access journal.
- 1.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 2.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.APA [American Psychological Association], Publications and Communications Board Working Group on Journal Article Reporting Standards. Reporting standards for research in psychology: Why do we need them? What might they be? Am Psychol. 2008;63(9):839–851. doi: 10.1037/0003-066X.63.9.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuters Thomson. ISI Web of Knowledge: Journal Citation Reports. 2010. Science Edition. Last updated: 2011 Sep 28 [cited 2012 Apr 19] [Google Scholar]
- 5.CONSORT Endorsers - Journals. Last updated: 2011 Aug 4 [cited 2012 Jan 23]. Available: < http://www.consort-statement.org/about-consort/consort-endorsement/consort-endorsers---journals/>.
- 6.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF QUOROM Group. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Br J Surg. 2000;87(11):1448–1454. doi: 10.1046/j.1365-2168.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1(1):2. doi: 10.1186/1471-2288-1-2. Available: http://www.biomedcentral.com/1471-2288/1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Committee of Medical Journal Editors. Uniform Requirements for Submission of Manuscripts to Biomedical Journals. 2007 [Google Scholar]
- 9.Simera I, Altman DG, Moher D, Schulz KF, Hoey J. Guidelines for reporting health research: the EQUATOR network’s survey of guideline authors. PLoS Med. 2008;5(6):e139. doi: 10.1371/journal.pmed.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 11.EQUATOR Network. Identification of reporting guidelines (Search strategy for MEDLINE database) Last updated: 2009 Apr. Available: < http://www.equator-network.org/resource-centre/; To see how we identified reporting guidelines click here.>.
- 12.EQUATOR Network. EQUATOR Network publications. Last updated: 2011 Oct 20 [cited 2012 Apr 19]. Available: < http://www.equator-network.org/about-equator/equator-publications0/equator-network-publications-2010/>.
- 13.EQUATOR Network. New reporting guidelines added to our Library. EQUATOR Newsletter. Last updated: 2011 Nov [cited 2012 Apr 19]. Available: < http://www.equator-network.org/mod_cms/news/newsletter_view.aspx?id=1076>.
- 14.National Library of Medicine. Research Reporting Guidelines and Initiatives: By Organization. Available: < http://www.nlm.nih.gov/services/research_report_guide.html>.
- 15.Moher D, Weeks L, Ocampo M, Seely D, Sampson M, Altman DG, Schulz KF, Miller D, Simera I, Grimshaw J, Hoey J. Describing reporting guidelines for health research: A systematic review. J Clin Epidemiol. 2011;64(7):718–742. doi: 10.1016/j.jclinepi.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15(3):261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 17.EQUATOR Network. Reporting Guidelines under Development. Last updated: 2012 Apr 2 [cited 2012 Apr 19]. Available: < http://www.equator-network.org/resource-centre/library-of-health-research-reporting/reporting-guidelines-under-development/>.
- 18.Bennett C, Khangura S, Brehaut JC, Graham ID, Moher D, Potter BK, Grimshaw JM. Reporting guidelines for survey research: An analysis of published guidance and reporting practices. PLoS Med. 2010;8(8):e1001069. doi: 10.1371/journal.pmed.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Jones A, Lepage L. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285(15):1992–1995. doi: 10.1001/jama.285.15.1992. [DOI] [PubMed] [Google Scholar]
- 20.Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, Gaboury I. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185(5):263–267. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 21.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328(7441):702–708. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivers NM, Taljaard M, Dixon S, Bennett C, McRae A, Taleban J, Skea Z, Brehaut JC, Boruch RF, Eccles MP, Grimshaw JM, Weijer C, Zwarenstein M, Donner A. Impact of CONSORT extension for cluster randomised trials on quality of reporting and study methodology: review of random sample of 300 trials, 2000–8. BMJ. 2011;343:d5886. doi: 10.1136/bmj.d5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullen PD, Ramírez G. The promise and pitfalls of systematic reviews. Annu Rev Public Health. 2006;27:81–102. doi: 10.1146/annurev.publhealth.27.021405.102239. [DOI] [PubMed] [Google Scholar]
- 24.Antes G. The new CONSORT statement. BMJ. 2010;340:c1432. doi: 10.1136/bmj.c1432. [DOI] [PubMed] [Google Scholar]
- 25.EQUATOR Network. Reporting Guidelines Under Development. Last updated: 2011 Dec 7 [cited 2012 Jan 24]. Available: < http://www.equator-network.org/resource-centre/library-of-health-research-reporting/reporting-guidelines-under-development/>.
- 26.Simera I, Altman DG, Moher D, Schulz KF, Hoey J. Guidelines for reporting health research: The EQUATOR network’s survey of guideline authors. PLoS Med. 2008;5(6):e139. doi: 10.1371/journal.pmed.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzewaltowski DA, Glasgow RE, Klesges LM, Estabrooks PA, Brock E. RE-AIM: evidence-based standards and a Web resource to improve translation of research into practice. Ann Behav Med. 2004;28(2):75–80. doi: 10.1207/s15324796abm2802_1. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361–366. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenbroucke JP, von EE, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326(7379):41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 37.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 38.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report--Part I. Value Health. 2009;12(8):1044–1052. doi: 10.1111/j.1524-4733.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 39.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell MJ. Extending CONSORT to include cluster trials. BMJ. 2004;328(7441):654–655. doi: 10.1136/bmj.328.7441.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JP, Kirsch-Volders M, Matullo G, Phillips DH, Schoket B, Stromberg U, Vermeulen R, Wild C, Porta M, Vineis P. STrengthening the Reporting of OBservational studies in Epidemiology - Molecular Epidemiology (STROBE-ME): an extension of the STROBE Statement. PLoS Med. 2011;8(10):e1001117. doi: 10.1371/journal.pmed.1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97(16):1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 43.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von EE, Khoury MJ, Cohen B, vey-Smith G, Grimshaw J, Scheet P, Gwinn M, Williamson RE, Zou GY, Hutchings K, Johnson CY, Tait V, Wiens M, Golding J, van DC, McLaughlin J, Paterson A, Wells G, Fortier I, Freedman M, Zecevic M, King R, Infante-Rivard C, Stewart A, Birkett N. STrengthening the REporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009;6(2):e22. doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollenbach JA, Mack SJ, Gourraud PA, Single RM, Maiers M, Middleton D, Thomson G, Marsh SG, Varney MD. A community standard for immunogenomic data reporting and analysis: proposal for a STrengthening the REporting of Immunogenomic Studies statement. Tissue Antigens. 2011;78(5):333–344. doi: 10.1111/j.1399-0039.2011.01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssens AC, Ioannidis JP, van Duijn CM, Little J, Khoury MJ. Strengthening the reporting of Genetic RIsk Prediction Studies: the GRIPS Statement. PLoS Med. 2011;8(3):e1000420. doi: 10.1371/journal.pmed.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssens AC, Ioannidis JP, Bedrosian S, Boffetta P, Dolan SM, Dowling N, Fortier I, Freedman AN, Grimshaw JM, Gulcher J, Gwinn M, Hlatky MA, Janes H, Kraft P, Melillo S, O’Donnell CJ, Pencina MJ, Ransohoff D, Schully SD, Seminara D, Winn DM, Wright CF, van Duijn CM, Little J, Khoury MJ. Strengthening the reporting of Genetic RIsk Prediction Studies (GRIPS): explanation and elaboration. J Clin Epidemiol. 2011;64(8):e1–e22. doi: 10.1016/j.jclinepi.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6(3):e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone AA, Shiffman S. Capturing momentary, self-report data: a proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- 49.Kottner J, Audige L, Brorson S, Donner A, Gajewski BJ, Hrobjartsson A, Roberts C, Shoukri M, Streiner DL. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64(1):96–106. doi: 10.1016/j.jclinepi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 51.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 52.Elliott R, Fischer CT, Rennie DL. Evolving guidelines for publication of qualitative research studies in psychology and related fields. Br J Clin Psychol. 1999;38(Pt 3):215–229. doi: 10.1348/014466599162782. [DOI] [PubMed] [Google Scholar]
- 53.Clark JP. How to peer review a qualitative manuscript. In: Godlee F, Jefferson T, editors. Peer Review in Health Sciences. 2. London: BMJ Books; 2003. pp. 219–235. [Google Scholar]
- 54.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(16):1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 55.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, Reed S. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8(5):521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 56.Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care. 2005;21(2):165–171. [PubMed] [Google Scholar]
- 57.Nuijten MJ, Pronk MH, Brorens MJ, Hekster YA, Lockefeer JH, De Smet PA, Bonsel G, Van der KA. Reporting format for economic evaluation. Part II: Focus on modelling studies. Pharmacoeconomics. 1998;14(3):259–268. doi: 10.2165/00019053-199814030-00003. [DOI] [PubMed] [Google Scholar]
- 58.Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ. 2011;342:d1548. doi: 10.1136/bmj.d1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766. doi: 10.1136/bmj.d1766. [DOI] [PubMed] [Google Scholar]
- 60.Benchimol EI, Manuel DG, To T, Griffiths AM, Rabeneck L, Guttmann A. Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol. 2011;64(8):821–829. doi: 10.1016/j.jclinepi.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Bayesian methods in health technology assessment: a review. Health Technol Assess. 2000;4(38):1–130. [PubMed] [Google Scholar]
- 62.Sung L, Hayden J, Greenberg ML, Koren G, Feldman BM, Tomlinson GA. Seven items were identified for inclusion when reporting a Bayesian analysis of a clinical study. J Clin Epidemiol. 2005;58(3):261–268. doi: 10.1016/j.jclinepi.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: A proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 65.Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney S. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17(Suppl 1):i3–i9. doi: 10.1136/qshc.2008.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moss F, Thompson R. A new structure for quality improvement reports. Qual Health Care. 1999;8(2):76. doi: 10.1136/qshc.8.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith L, Rosenzweig L, Schmidt M. Best practices in the reporting of participatory action research: Embracing both the forest and the trees. Counselling Psychologist. 2010;38(8):1115–1138. [Google Scholar]
- 68.Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, Spring B, Trudeau KJ, Whitlock EP. Evidence-based behavioral medicine: what is it and how do we achieve it? Ann Behav Med. 2003;26(3):161–171. doi: 10.1207/S15324796ABM2603_01. [DOI] [PubMed] [Google Scholar]
- 69.Baker TB, Gustafson DH, Shaw B, Hawkins R, Pingree S, Roberts L, Strecher V. Relevance of CONSORT reporting criteria for research on eHealth interventions. Patient Educ Couns. 2010;81(Suppl):s77–s86. doi: 10.1016/j.pec.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, Hayes DF, Hainaut P, Kim P, Mansfield E, Potapova O, Riegman P, Rubinstein Y, Seijo E, Somiari S, Watson P, Weier HU, Zhu C, Vaught J. Biospecimen reporting for improved study quality (BRISQ) J Proteome Res. 2011;10(8):3429–3438. doi: 10.1021/pr200021n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Talmon J, Ammenwerth E, Brender J, de Keizer N, Nykanen P, Rigby M. STARE-HI: Statement on Reporting of Evaluation studies in Health Informatics. Int J Med Inform. 2009;78(1):1–9. doi: 10.1016/j.ijmedinf.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, Moher D. Better reporting of harms in randomized trials: An extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 73.Flores SA, Crepaz N. Quality of study methods in individual- and group-level HIV intervention research: critical reporting elements. AIDS Educ Prev. 2004;16(4):341–352. doi: 10.1521/aeap.16.4.341.40396. [DOI] [PubMed] [Google Scholar]
- 74.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Methods and processes of the CONSORT Group: Example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med. 2008;148(4):W60–W66. doi: 10.7326/0003-4819-148-4-200802190-00008-w1. [DOI] [PubMed] [Google Scholar]
- 75.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 76.Stone SP, Cooper BS, Kibbler CC, Cookson BD, Roberts JA, Medley GF, Duckworth G, Lai R, Ebrahim S, Brown EM, Wiffen PJ, Davey PG. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect Dis. 2007;7(4):282–288. doi: 10.1016/S1473-3099(07)70082-8. [DOI] [PubMed] [Google Scholar]
- 77.Staquet M, Berzon R, Osoba D, Machin D. Guidelines for reporting results of quality of life assessments in clinical trials. Qual Life Res. 1996;5(5):496–502. doi: 10.1007/BF00540022. [DOI] [PubMed] [Google Scholar]
- 78.Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008;371(9609):281–283. doi: 10.1016/S0140-6736(07)61835-2. [DOI] [PubMed] [Google Scholar]
- 79.Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5(1):e20. doi: 10.1371/journal.pmed.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Booth A. “Brimful of STARLITE”: Toward standards for reporting literature searches. J Med Libr Assoc. 2006;94(4):421–9. e205. [PMC free article] [PubMed] [Google Scholar]
- 81.Niederstadt C, Droste S. Reporting and presenting information retrieval processes: The need for optimizing common practice in health technology assessment. Int J Technol Assess Health Care. 2010;26(4):450–457. doi: 10.1017/S0266462310001066. [DOI] [PubMed] [Google Scholar]
- 82.Schriger DL. Suggestions for improving the reporting of clinical research: the role of narrative. Ann Emerg Med. 2005;45(4):437–443. doi: 10.1016/j.annemergmed.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Docherty M, Smith R. The case for structuring the discussion of scientific papers [editorial] BMJ. 1999;318(7193):1224–1225. doi: 10.1136/bmj.318.7193.1224. Available: http://www.bmj.com/cgi/reprint/318/7193/1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown MA. Social support during pregnancy: A unidimensional or multidimensional construct? Nurs Res. 1986;35:4–9. [PubMed] [Google Scholar]
- 85.Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, Forbes C, Glanville J, Hicks NJ, Moody J, Twaddle S, Timimi H, Young P. How to formulate research recommendations. BMJ. 2006;333(7572):804–806. doi: 10.1136/bmj.38987.492014.94. Available: http://www.bmj.com/cgi/reprint/333/7572/804. [DOI] [PMC free article] [PubMed] [Google Scholar]