Abstract
TERE1/UBIAD1 is involved in SCCD (Schnyder crystalline corneal dystrophy) and multiple human cancers. So far, the molecular mechanism of TERE1/UBIAD1 in tumourigenesis is unclear. Here, the expression levels of hTERT and TERE1/UBIAD1 in pathologically proven Chinese TCC (transitional cell carcinoma) samples were measured. It was found that decreased TERE1/UBIAD1 expression is closely related to both an increased hTERT expression and activation of Ras–MAPK signalling. Chemically modified TERE1 siRNA oligos were used to knock down TERE1 expression in human L02 cells. Cells transfected with TERE1 siRNA oligos underwent significant cell proliferation. When the levels of hTERT expression and ERK phosphorylation were measured, it was found that both of them increased in the above transfected cells, suggesting the activation of Ras–MAPK signalling. Addition of the MEK inhibitor U0126 into the transfected L02 cells described above inhibited ERK phosphorylation and hTERT expression. Our result is the initial demonstration that down-regulation of TERE1 activates Ras–MAPK signalling and induces subsequent cell proliferation. TERE1 might be a new negative regulator of Ras–MAPK signalling, which plays a pivotal role in the cell proliferation of multiple human cancers.
Keywords: cell proliferation, hTERT, MAPK, TERE1/UBIAD1
Abbreviations: GAPDH, human glyceraldehyde 3-phosphate dehydrogenase; IHC, immunohistochemistry; MAPK, mitogen-activated protein kinase; MC, mock control; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NC, negative group; SCCD, Schnyder crystalline corneal dystrophy; SREBP, sterol response element binding protein; siRNA, small interfering RNA; TCC, transitional cell carcinoma
1. Introduction
Bladder transitional cell carcinoma is the fourth most common cancer in the United States and accounts for 52810 new cases and 10180 cancer-related deaths per year (Jemal et al., 2009; Kaufman et al., 2009). TERE1, a potential TCC (transitional cell carcinoma) tumour suppressor, is widely expressed in various human tissues (McGravey et al., 2001). The human TERE1 gene encodes a protein of 338 amino acids mapped to chromosome 1p36.11–36.33. Analysis on loss of heterozygosity indicates that this region may be responsible for multiple tumour types. It was reported that TERE1 is down-regulated in prostate carcinoma as well (McGravey et al., 2003). Overexpression of TERE1 in prostate cancer cell lines inhibited the proliferation of tumour cells (McGravey et al., 2003).
TERE1 was also cloned as UBIAD1, encoding a potential prenyltransferase responsible for SCCD (Schnyder crystalline corneal dystrophy) (Orr et al., 2007; Yellore et al., 2007; Weiss et al., 2007), a rare autosomal dominant eye disease characterized by an abnormal increase in cholesterol and phospholipid deposition in the cornea. It was reported that overexpression of TERE1 can reduce cholesterol levels in bladder and prostate cancer cells by 20–50% (Fredericks et al., 2009). On the other hand, higher cholesterol levels in tumour cells lower TERE1 expression through SREBP (sterol response element binding protein) (Fredericks et al., 2009). Whether or not down-regulation of TERE1 directly causes tumourigenesis remains to be determined.
Telomerase plays a vital role in most human cancers. It is composed of the catalytic protein subunit hTERT and the RNA subunit hTR (human telomerase RNA). The induction of hTERT expression results in increased telomerase activity and contributes, as part of a multistep process, to human carcinogenesis (Nakamura et al., 1997; Müller, 2002).
The precise spatiotemporal regulation of hTERT and intracellular Ras–MAPK (mitogen-activated protein kinase) signalling is critical for cells to maintain homoeostasis. Aberrant activation of Ras–MAPK signalling has been shown to be a key contributing factor in many types of human cancers. The promoter region of hTERT possesses the binding site for multiple Ets transcription factors. When MAPK signalling is activated by extracellular stimuli, these Ets transcription factors, phosphorylated by activated ERK (extracellular-signal-regulated kinase), initiate the transcription of hTERT (Kyo et al., 2008; Maida et al., 2002). The mechanism for hTERT transcriptional regulation during bladder carcinogenesis remains unclear.
Discovery of novel regulators, especially inhibitors of Ras–MAPK signalling and hTERT, represents a promising strategy for cancer treatment (Cech et al., 2004; Roberts and Der, 2007).
In this paper, chemically modified siRNA (small interfering RNA) oligos were exploited to knock down the expression of TERE1. It was found that down-regulation of TERE1 activates Ras–MAPK signalling, inducing hTERT transcription and subsequent cell proliferation.
2. Materials and methods
2.1. Collection of tissue specimen
Eighty-three pathologically proven TCC samples were obtained at surgery from patients (with consent) at Tongji hospital of Tongji Medical College. The procedure of sample collection was approved by the Medical Faculty Ethics Committee of Tongji Medical College (Table 1). Each tumour sample was staged and graded according to the TNM (tumour, node, metastasis) staging system (UICC, International Union Against Cancer). Homologous normal human bladder epithelium tissues (18 samples) served as control.
Table 1. Expression levels of TERE1 and hTERT in 101 bladder tissue samples from Chinese population.
A total of 83 pathologically proven TCC bladder samples plus 18 healthy bladder samples were obtained at surgery. The expression level of TERE1 and hTERT were measured by IHC. The TNM classification of urinary bladder carcinoma was according to the standard of the UICC.
| Total | TERE1 | hTERT | |
|---|---|---|---|
| Characteristic | n = 83 | n [Positive (%)] | n [Positive (%)] |
| Age (years) | |||
| 34–76 | |||
| Stage | |||
| Ta | 17 | 16 (94.12) | 11 (64.71) |
| T1 | 19 | 13 (68.42) | 15 (78.95) |
| T2 | 22 | 6 (27.27) | 20 (90.91) |
| T3–4 | 25 | 4 (16.00) | 24 (96.00) |
| Histological grade | |||
| G1 | 25 | 19 (76.00) | 16 (64.00) |
| G2 | 27 | 14 (51.85) | 23 (85.19) |
| G3–4 | 31 | 5 (16.13) | 29 (93.55) |
| Healthy bladder tissues | 18 | 16 (88.89) | 1 (5.56) |
2.2. IHC (immunohistochemistry)
Tissue samples were fixed, embedded, cut to desired thickness (5 μm), mounted onto slides, deparaffinized and then rehydrated. The slides were blocked and incubated with either affinity purified anti-TERE1 (Abcam) or anti-hTERT primary antibody (Santa Cruz) overnight at 4°C. Following serveral washes, the slides were incubated with biotin-conjugated rabbit IgG for 60 min followed by streptavidin-conjugated peroxidase, 3′3-diaminobenzidine and counterstained with Haematoxylin.
2.3. RNA isolation and RT-PCR analysis
Total RNA was extracted from bladder tissues using TRIzol reagent (Invitrogen). For RT-PCR analysis (Toyobo), the primers for TERE1 were sense: 5′-CTC TGAAACTGGAGCACTTGG-3′ and antisense: 5′-GCCAGAATGATGCCAAAGAC-3′ for 30 cycles (94°C for 45 s, 58°C for 45 s, 72°C for 90 s). The primers for hTERT were sense: 5′-CGGAAGAGTGTCTGGAGCAA-3′ and antisense: 5′-GGATGAAGCGGAGTCTGGA-3′ for 30 cycles (94°C for 45 s, 60°C for 45 s, 72°C for 90 s). GAPDH (human glyceraldehyde 3-phosphate dehydrogenase) (sense: 5′-CTCAGACACCATGGGGAAGGTGA-3′ and antisense: 5′-ATGATCTTGAGGCTGTTGTCATA-3′) was used as internal control.
2.4. Western blot analysis
Total protein from cells or tissues was extracted with the RIPA lysis buffers according to manufacturer’s instructions (Abcam) and quantified with the BCA (bicinchoninic acid) Protein Assay Kit (Bio-Rad). Equal amounts of protein samples were separated by SDS/PAGE and were transferred on to PVDF membranes (Millipore). The membrane was blocked in TBST (Tris buffered saline with Tween 20) containing 5% non-fat milk and 0.1% Tween 20 and probed overnight at 4°C with primary antibodies (Rabbit anti-GAPDH antibody, Cell Signaling Technology; TERE1 specific affinity purified polyclonal antibody, Abcam; hTERT antibody, Santa Cruz Biotechnology; anti-ERK1/2 antibody and anti-phospho-ERK1/2 monoclonal antibody, Promega). Following the wash, the membrane was incubated with a peroxidase-conjugated secondary antibody for rabbit IgG raised in Goat (Cell Signaling Technology) for 1 h, and the protein was detected using ECL detection system (Amersham Pharmacia Biotech). Image analysis and quantification was performed using a scanning densitometer.
2.5. Cell culture and siRNA techniques
Human cell line T24 and L02 were obtained from A.T.C.C. and cultured according to supplier’s instructions. SiRNA oligos were synthesized by Invitrogen as follows:
Negative control siRNA,
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense)
5′-ACGUGACACGUUCGGAGAATT-3′(antisense);
TERE1-291 siRNA,
5′-GUAAUUUGGUCAACACUUATT-3′(sense)
5′-UAAGUGUUGACCAAAUUACCG3-′(antisense);
TERE1-22 siRNA,
5′-CAAGGGCAUUGACCACAAATT-3′ (sense)
5′-UUUGUGGUCAAUGCCCUUGGA-3′ (antisense)
For each transfection, oligomer–Lipofectamine™ 2000 complexes were prepared by mixing siRNA oligos with Lipofectamine™ 2000 and added to each well containing cells and medium. For mock control, only Lipofectamine™ 2000 was added. For negative control, random siRNA oligos were used. As for Ras–MAPK signalling analysis, before harvesting, cells were pretreated with 25 μM MEK [MAPK/ERK (extracellular signal regulated kinase) kinase]-specific inhibitor U0126 for 30 min (Promega). Extractes or lysates from cells were then harvested for either RT-PCR or Western blot assay.
2.6. Cell viability and cell growth rate assay
Cell viability was determined using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell proliferation kit (Roche Diagnostics). Cells were seeded in a 96-well plate (2×103/well) and treated with TERE1 siRNA oligos for 48 h. Cells were then incubated with MTT and quantified with an ELISA plate reader (Yim et al., 2009).
To measure cell proliferation (Karreth et al., 2009), 1.5×105 cells were plated in each well of a six-well plate. siRNA with a final concentration of 100 nM were used 4 h following the transfection. Viable cells were counted everyday using a haematocytometer with Trypan Blue (Sigma) staining.
2.7. Statistical analyses
Data were analysed using SPSS 11.0 software. Results were expressed as the mean±S.D., and the difference was determined by the t test. P-value <0.05 was considered significant.
3. Results
3.1. The association of TERE1 down-regulation with hTERT up-regulation in bladder carcinoma tissues
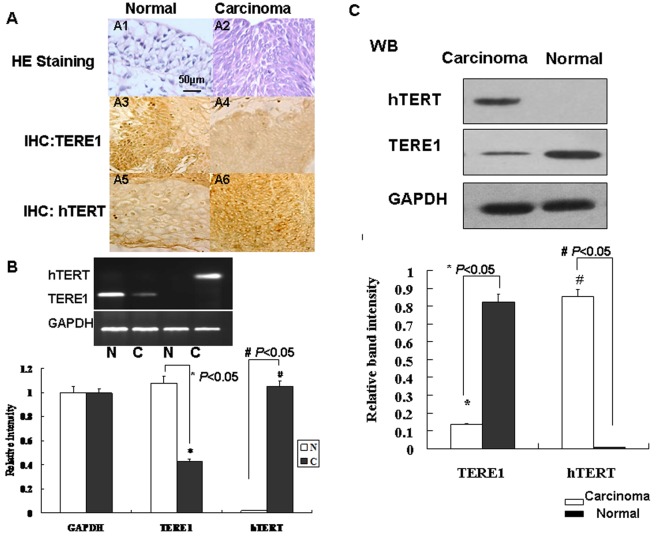
A total of 83 pathologically proven TCC samples were obtained at surgery from Chinese patients (Table 1). Immunohistochemical staining with TERE1 and hTERT antibodies revealed that, in normal bladder tissues, TERE1 is abundant, and hTERT is barely detectable. In bladder carcinoma tissues, TERE1 is down-regulated and hTERT is up-regulated (Figure 1A).
Figure 1. Expression level of human TERE1 and hTERT in bladder carcinoma tissues.
(A) Immunohistochemical staining of normal bladder and bladder carcinoma tissues. (A1–A2) Haematoxylin and eosin staining; (A3–A4): TERE1 staining; (A5–A6): hTERT staining. IHC: immunohistochemistry. Scale bar, 50 μm. (B) mRNA expression level of human TERE1 and hTERT in normal bladder tissue (N) and bladder carcinoma tissue (C). Relative intensities of bands are graphed. Values are means±S.D. of three separate experiments. (C) Analysis of TERE1 and hTERT level in normal and carcinoma bladder tissues with Western blot.
Statistically (Table 1), the severer tumour phenotype is usually accompanied by a smaller percentage of samples that express TERE1 and a higher percentage of samples that express hTERT, e.g. 16.13% of G3–4 samples compared with 76% of G1 samples showed TERE1 expression, while 93.55% of G3–4 samples compared with 64.00% of G1 samples showed hTERT expression. Similar results were obtained in clinical bladder cancer staging samples, e.g. 16.00% of T3–4 samples compared with 94.12% of Ta samples showed TERE1 expression, while 96% of T3–4 samples compared with 64.71% of Ta samples showed hTERT expression.
The expression levels of TERE1 and hTERT in both bladder carcinoma and normal bladder tissues were analysed in both mRNA and protein levels. In bladder carcinoma tissues, the mRNA level of TERE1 decreased, while the mRNA level of hTERT increased (Figure 1B). hTERT was almost undetectable in normal bladder tissue. Compared with normal bladder tissues, bladder carcinoma tissues showed a decrease in TERE1 and an increase in hTERT expression (Figure 1C).
3.2. Down-regulation of TERE1 expression causing the up-regulation of hTERT and cell proliferation
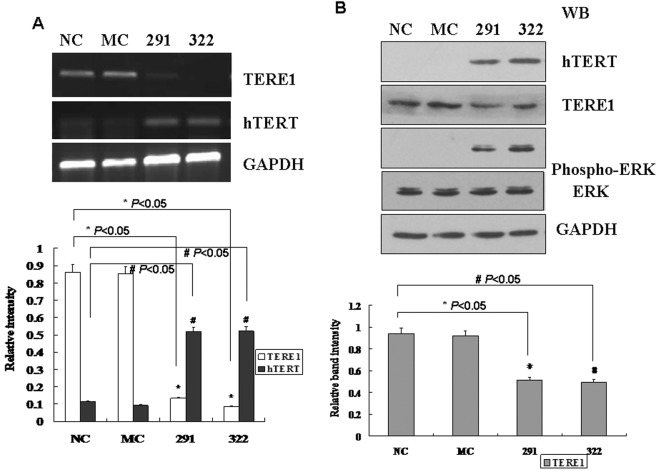
Since TERE1 is widely expressed in various human cell types, human L02 cells were utilized to study the relationship between TERE1 and tumourigenesis. To test if down-regulation of TERE1 is the direct cause for tumourigenesis, chemically modified siRNA oligos were used to knock down the expression level of TERE1 in human L02 cells. TERE1-291, 322-siRNA oligos down-regulated the expression of TERE1 in both mRNA and protein levels (Figure 2). Where TERE1 expression was knocked down by siRNA oligos, hTERT expression significantly increased in both mRNA and protein levels.
Figure 2. Knocking down TERE1 expression with siRNA oligos up-regulates hTERT expression.
(A) mRNA expression level of TERE1 and hTERT in human L02 cells transfected with TERE1 siRNA oligos. NC, negative control (random siRNA oligos); MC, mock control (no siRNA oligos); 291, TERE1-291-siRNA oligos; 322, TERE1-322-siRNA oligos. (B) Protein level of TERE1 and hTERT in the above experiment. TERE1 expression was silenced by TERE1 siRNA oligos. The relative intensities of TERE1 bands are shown in the graph. The protein level of hTERT increased (P<0.05) in TERE1 siRNA-transfected cells. ERK phosphorylation level increased as well.
The effect of TERE1 down-regulation on cell proliferation was further measured in TERE1-291, 322-siRNA transfected cells using MTT assay (Figure 3). Addition of TERE1-291, 322-siRNA into L02 cells caused a dramatic increase in the number of viable cells (Figure 3A). Trypan Blue exclusion tests were performed to count the number of cells following the transfection over a 3-day period (Figure 3B). Cells transfected with TERE1 siRNA 291 and 322 experienced dramatic cell proliferation. Knocking down the TERE1 expression resulted in cell proliferation.
Figure 3. Induction of cell proliferation by TERE1 siRNA transfection.
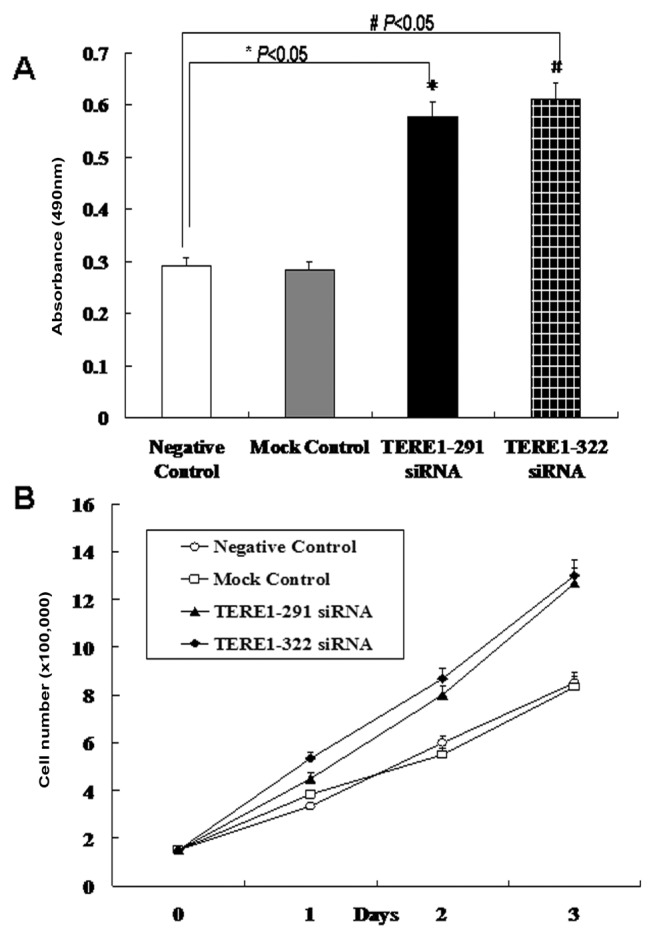
(A) Human L02 cells transfected with TERE1 siRNA were cultured and quantified by MTT assay. *P<0.05 compared with NC, #P<0.05 compared with NC. Values are means±SD of three separate experiments. (B) Count of cell numbers over 3 days following the transfection with two different TERE1-siRNA oligos (291, 322). Cell numbers are means±SD of three separate experiments.
3.3. Down-regulation of TERE1 activating the Ras–MAPK signalling pathway
Ras–MAPK signalling might be the mechanism for the activation of hTERT expression and the formation of bladder carcinoma in TERE1 down-regulated tissues. To test this hypothesis, phosphorylation levels of ERK in L02 transfected with TERE1 siRNA oligos were measured by Western blot analysis (Figure 2B). Compared with the NC (negative group, random siRNA oligos) and the MC (mock control, no siRNA oligos), phosphorylated protein levels of p42ERK and p44ERK increased significantly. Down-regulation of TERE1 increased the level of ERK phosphorylation and possibly activated Ras–MAPK signalling.
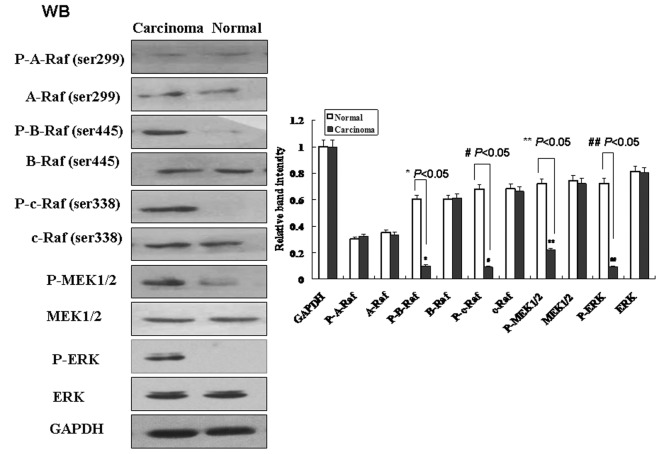
To verify the above results in bladder carcinoma tissues, phosphorylation levels of key molecules within the Ras–MAPK signalling pathway such as Raf, MEK and ERK were further measured using Western blot analysis (Figure 4). Compared with normal tissue, phosphorylated protein levels of B-Raf (Ser455), C-Raf (Ser338), MEK (1/2) and ERK increased significantly in bladder carcinoma (P<0.05). However, total protein levels of A-Raf, B-Raf (Ser455), C-Raf (Ser338), MEK (1/2) and ERK as well as the phosphorylation levels of A-Raf (Ser299) in bladder carcinoma tissues bear no significant differences compared with normal bladder tissues. Therefore, down-regulation of TERE1 directly activates Ras–MAPK signalling pathway in bladder carcinoma tissues. The finding is consistent with the results from cell culture experiments. Based upon our results, it is possible that TERE1/UBIAD1 affects the formation of bladder carcinoma through Ras–MAPK signal transduction pathway.
Figure 4. Activation of Ras–MAPK signalling in bladder carcinoma tissues.
Compared with normal bladder tissues, phosphorylation levels of B-Raf (Ser455), C-Raf (Ser338), MEK (1/2) and ERK significantly increased in bladder carcinoma tissues. The relative intensities of bands are shown in diagram. Values are means±SD of three separate experiments.
3.4. Inhibition of MAPK phosphorylation reducing the level of hTERT expression in TERE1 down-regulated cells
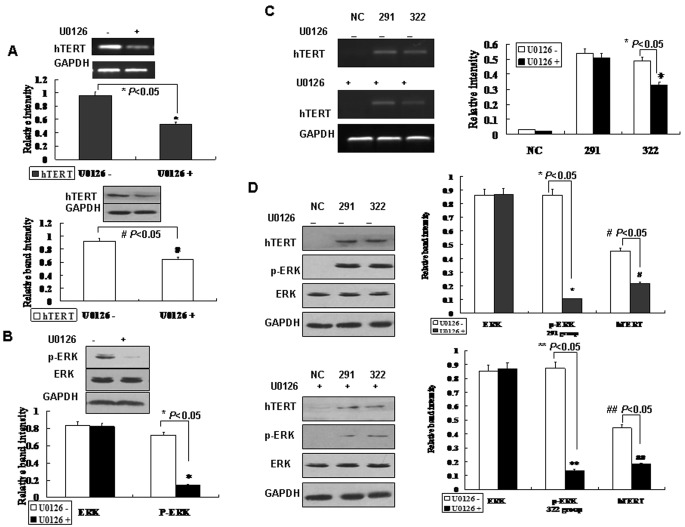
Since Ras–MAPK signalling is involved in the activation of hTERT expression and cell proliferation in TERE1 down-regulated cells, tests were performed to determine if inhibition of MAPK phosphorylation would decrease hTERT expression. T24 cell lines pretreated with U0126 (an MEK1/2-specific inhibitor) showed decreased hTERT expression in both mRNA and protein levels (Figure 5A). In addition, the level of ERK phosphorylation in T24 cells pretreated with U0126 decreased as expected (Figure 5B). This result indicates that the inhibition of ERK phosphorylation will partially decrease the expression of hTERT.
Figure 5. U0126 partially blocking the activation of Ras–MAPK signalling induced by TERE1 down-regulation.
(A) T24 cells treated with U0126 showed decreased hTERT expression in both mRNA and protein levels. (B) ERK phosphorylation is inhibited by U0126 in T24 cells. (C) Human L02 cells transfected with TERE1 291- and 322-siRNA oligos and pretreated with U0126 (middle panel) showed decreased mRNA level of hTERT compared with samples without U0126 (upper panel). (D) Human L02 cells transfected with TERE1 291- and 322-siRNA oligos were treated with U0126, which decreased hTERT expression and ERK phosphorylation. The intensities of the bands were compared in the diagram.
The effect of U0126 in L02 cells transfected with TERE1 siRNA oligos was also studied. hTERT expression was detected at both transcriptional and translational level in TERE1-291, 322-siRNA transfected cells. If cells transfected with TERE1 siRNA oligos were pretreated with U0126, the mRNA level (Figure 5C) and the protein level (Figure 5D) of hTERT decreased. ERK phosphorylation also decreased in cells transfected with TERE1 siRNA oligos (Figure 5D). The fact that U0126 can block hTERT expression induced by TERE1 down-regulation suggests that Ras–MAPK signalling might play a critical role in regulating hTERT transcription and subsequent bladder cell proliferation in TERE1 down-regulated tissues.
4. Discussion
Blocking TERE1 expression with siRNA directly activates the Ras–MAPK signal transduction pathway, resulting in up-regulation of hTERT transcription and cell proliferation.
There seems to be two roles for the TERE1 protein. First, it is possibly a putative tumour suppressor with the ability to suppress carcinogenesis in TCC tumours. Decreased expression of TERE1 was associated with bladder and prostate carcinoma (McGravey et al., 2001, 2003). Overexpression of TERE1 in TCC cell line J82 alleviated the tumour phenotype, inhibiting the progression of cell cycle in cancer cells. Later, it was discovered that single copy loss of the TERE1 gene caused the dominant phenotype of SCCD (Orr et al., 2007).
It was proposed by Fredericks et al. (2009) that the main function of TERE1 is to lower cholesterol inside the cell. Higher cholesterol levels might provide an apoptotic escape mechanism for tumour cells and affect the integrity of lipid raft growth signalling complexes such as TGFβ, Akt, etc. On the other hand, tumour cells with high levels of cholesterol may have low levels of TERE1, since high-level cholesterol would preclude the activation of TERE1 transcription by SREBP (Fredericks et al., 2009). In this scenario, down-regulation of TERE1 may be the result of tumourigenesis.
Based upon our results, it was shown that in cells transfected with TERE1 siRNA, there is a conspicuous cell proliferation compared with cells transfected with random oligos (Figure 3). The expression level of hTERT was dramatically increased, implying tumourigenesis (Figure 2). In our previous study of the Drosophila heix gene (Hong and Rubin, 1998), which encodes an orthologue of the human TERE1 protein, the malignant blood tumour phenotype was observed with increased number of Drosophila blood cells in the loss of function heix mutant (Hong and Rubin, 1998; McGravey et al., 2001), which is consistent with our result.
Until now, no growth signalling pathway has been identified to account for cell proliferation in TERE1 down-regulated cells. We showed for the first time that Ras–MAPK signalling contributes to TERE1 down-regulation induced bladder cell proliferation. Owing to the fact that down-regulation of TERE1 activates Ras–MAPK signalling, TERE1 might be a potential new negative regulator of MAPK signalling pathway.
In both TCC cell line T24 and the transfected human cell line L02, U0126 (Ma et al., 2005) blocked the ERK phosphorylation and the hTERT expression. Since U0126 can block Ras–MAPK activation in TERE1 down-regulated cells, TERE1 might function upstream of MEK in Ras–MAPK cascade. It was reported by Fredericks et al. (2009) that overexpression of TERE1 inhibited growth of bladder cancer cell line J82 and prostate cancer cell lines LNCaP and PC-3. TERE1 overexpression also inhibited tumourigenesis of J82 bladder cancer cells in nude mice. Since PTEN was lost in J82, LNCaP and PC-3 cell lines (Abounader et al., 2009; Puzio-Kuter et al., 2009), it seems that overexpression of TERE1 can suppress the PTEN loss of function phenotype, implying that TERE1 might function between RTK and MEK possibly through the Ras–MAPK pathway. In humans, bacterial two-hybrid screen has identified a physical interaction between the TERE1 protein and the C-terminal of apoE protein, suggesting that TERE1 might be involved in apoE signalling (McGravey et al., 2005). The addition of apoE into human 293 cells decreased the MAPK phosophorylation, suggesting that protein complex of TERE1 and apoE might suppress Ras–MAPK signalling.
5. Conclusion
In summary, we have shown that down-regulation of TERE1 directly causes the initiation of hTERT expression and subsequent cell proliferation. Ras–MAPK signalling pathway is the first identified activated growth signalling pathway in TERE1 down-regulated cells. Inhibition of Ras–MAPK signalling with MEK-specific inhibitor U0126 might partially suppress hTERT expression in TERE1 down-regulated cells. TERE1 might be a novel negative regulator of Ras–MAPK signalling and serve as a potential therapeutic target for bladder carcinoma.
Acknowledgements
We thank Dr Nanqin Gan for critical comments and scientific help.
Footnotes
This project was supported in part by grants from NSFC (People’s Republic of China, no. 30971608), 863 Grant of Ministry of Science and Technology (People’s Republic of China, no. 2007AA09Z449), NSF of the Hubei Province (People’s Republic of China, 2009CDB074), The Specific Key Project of Novel Medicine Discovery (People’s Republic of China, 2009ZX09301-014) and International Collaboration Programs of Wuhan Science and Technology Bureau (People’s Republic of China, no. 201070934334).
Author contribution
Yanzhi Xia took part in the whole study, wrote the manuscript and was responsible for the study. Xiong Wei performed the RNA isolation, RT-PCR analysis, Western blot analysis, siRNA analysis, cell viability and growth rate assay. Shimin Wu took part in collection of tissue specimen and IHC. Bo Wang took part in cell culture collection and statistical analysis. Ximing Wang and Ling Hong edited the manuscript and supervised the entire experiments.
References
- Abounader R. Interactions between PTEN and receptor tyrosine kinase pathways and their implications for glioma therapy. Expert Rev Anticancer Ther. 2009;9:235–45. doi: 10.1586/14737140.9.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–9. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Fredericks WJ, Wang H, Sepulveda J, McGarvey T, Malkowicz SB. TERE1/UBAID1 and TBL2 proteins Regulate cholesterol levels in bladder cancer cells. Abstract from American Urological Association, Chicago, IL, USA. 2009 [Google Scholar]
- Hong L, Rubin GM. “L(2)35Fc, a novel transmembrane protein required for suppression of melanotic tumors”. 39th Annual Drosophila Research Conference, March 25–29, Washington, DC; 1998. p. 128. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Karreth FA, DeNicola GM, Winter SP, Tuveson DA. C-Raf inhibits MAPK activation and transformation by B-RafV600E. Mol Cell. 2009;36:477–86. doi: 10.1016/j.molcel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–38. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Maida Y, Kyo S, Kanaya T, Wang Z, Yatabe N, Tanaka M, Nakamura M, Ohmichi M, Gotoh N, Murakami S, Inoue M. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene. 2002;21:4071–9. doi: 10.1038/sj.onc.1205509. [DOI] [PubMed] [Google Scholar]
- McGravey TW, Nguyaen T, Malkowicz SB. An interaction between apolipoprotein E and TERE1 with a possible association with bladder tumor formation. J Cell Biochem. 2005;95:419–28. doi: 10.1002/jcb.20432. [DOI] [PubMed] [Google Scholar]
- McGravey TW, Nguyaen T, Puthiyaveettil R, Tomaszewski JE, Malkowicz SB. TERE1, a novel gene affecting growth regulation in prostate carcinoma. Prostate. 2003;54:144–55. doi: 10.1002/pros.10174. [DOI] [PubMed] [Google Scholar]
- McGravey TW, Nguyaen T, Tomaszewski JE, Monson FC, Malkowicz SB. Isolation and characterization of the TERE1 gene, a gene downregulated in transitional cell carcinoma of the bladder. Oncogene. 2001;20:1042–51. doi: 10.1038/sj.onc.1204143. [DOI] [PubMed] [Google Scholar]
- Müller MH. Telomerase: its clinical relevance in the diagnosis of bladder cancer. Oncogene. 2002;21:650–5. doi: 10.1038/sj.onc.1205071. [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–9. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Orr A, Dubé MP, Marcadier J, Jiang H, Federico A, George S, Seamone C, Andrews D, Dubord P, Holland S. Mutations in the UBIAD1 gene, encoding a potential prenyltransferase, are causal for Schnyder crystalline corneal dystrophy. PlosOne. 2007;2:e685. doi: 10.1371/journal.pone.0000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C, Abate-Shen C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–80. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf–MEK–ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Weiss JS, Kruth HS, Kuivaniemi H, Tromp G, White PS, Winters RS, Lisch W, Henn W, Denninger E, Krause M. Mutations in the UBIAD1 gene on chromosome short arm 1, region 36, cause Schnyder crystalline corneal dystrophy. H Invest Ophthalmol Vis Sci. 2007;48:5007–12. doi: 10.1167/iovs.07-0845. [DOI] [PubMed] [Google Scholar]
- Yellore VS, Khan MA, Bourla N, Rayner SA, Chen MC, Sonmez B, Momi RS, Sampat KM, Gorin MB, Aldave AJ. Identification of mutations in UBIAD1 following exclusion of coding mutations in the chromosome 1p36 locus for Schnyder crystalline corneal dystrophy. Mol Vis. 2007;13:1777–82. [PubMed] [Google Scholar]
- Yim EK, Peng G, Dai H, Hu R, Li K, Lu Y, Mills GB, Meric-Bernstam F, Hennessy BT, Craven RJ, Lin SY. Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell. 2009;15:304–14. doi: 10.1016/j.ccr.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]