Abstract
Genetically manipulated transparent animals were already explored in many species for in vivo study of gene expression, transplantation analysis and cancer biology. However, there are no reports about transparent animals as in vitro genetic resources. In the present study, fin-derived cells from glass catfish (Krytopterus bicirrhis), naturally transparent fish with a visible skeleton and internal organs, were isolated after culturing fin explants and characterized using cryopreservation and cell cycle analysis. The cells grew well in DMEM (Dulbecco's modified Eagle's medium) containing 1% (v/v) P/S (penicillin–streptomycin) and 10% (v/v) fetal bovine serum at 26°C and showed increased cryopreservation efficiency with the slow-freezing method in the presence of 15% dimethyl sulfoxide. In addition, cell cycle analysis was evaluated based on flow cytometric analysis, and culturing to confluence (>85%) was more effective for synchronizing cells at the G0/G1 stages than roscovitine treatment (<75%). This is the first report about cell isolation from transparent animals. The results from testing the cell's viability following cryopreservation and subjecting the cells to cycle analysis can be useful tools for genetic resource management.
Keywords: cell cryopreservation, cell cycle analysis, cell isolation, fin cells, glass catfish
Abbreviations: DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; P/S, penicillin–streptomycin; SCNT, somatic cell nuclear transfer
1. Introduction
Development of fish cell lines has become an important tool for biomedical research. Several cell lines from a variety of fish species have been published in previous studies due to their potential use in virus isolation and toxicological and cytogenetic studies (Wolf and Mann, 1980). However, few studies have tested viability of fish cells following cryopreservation or subjected the cells to cycle analysis to determine if the cells could be used as nucleus carriers.
Using somatic cells as nucleus carriers must be considered for fish bearing a valuable phenotype or genotype, and the importance of somatic cells as nucleus carriers can be enhanced when their gametes and embryos are not available (Mauger et al., 2006; Moritz and Labbe, 2008). Cryopreservation technology of somatic cells is one possible way to use cells as nucleus carriers. Cryopreservation of fish-cultured cells for fish genome banking has been described in many papers (Chou et al., 1989; Zhang et al., 1998; Wang et al., 2003). Also, generating fish with cloning technology [SCNT (somatic cell nuclear transfer)], representative method using nucleus carriers, can be accomplished by transferring a nucleus from a somatic cell into an oocyte that has had its nucleus removed. In SCNT technology, cell cycle stage is one of the most important factors that determine the success of the development of cloned embryos (Campbell et al., 1996; Gibbons et al., 2002; Lee et al., 2005). It was reported that cloned embryos using donor nuclei from the G1- or G0-phase would be better for reprogramming than those from cells in other cycle stages (Prather et al., 1992; Campbell et al., 1994).
Transparent animal models, which result from genetic manipulation, have been reported in other studies, including frog (Fisher et al., 2007), zebrafish (White et al., 2008) and medaka (Wakamatsu et al., 2001) for in vivo study of gene expression, transplantation analysis and cancer biology. However, using transparent species as an in vitro genetic resource has yet to be explored. The glass catfish (or ghost catfish) Krytopterus bicirrhis, which is native to Southeastern Asian countries, has a naturally transparent body with a visible skeleton and internal organs. In the case of glass catfish, the mature state of the fish is still uncertain, and its availability is almost limited to the wild (Lim, 1999), so the value of somatic cells as nucleus carriers could be empathized in this species. We aimed to establish and characterize a cell line from the glass catfish by testing the cell's viability following cryopreservation and subjecting the cells to cycle analysis.
2. Materials and methods
2.1. Fin cell culturing
All experiments using catfish were carried out according to institutional guidelines.
Ten glass catfish (weighing about 1.0 g and 6.0 cm in length) were imported from Indonesia and reared in a 70 litre glass aquarium equipped with aeration at a temperature of 26°C. Before excising tissue, the fish were anaesthetized with 0.6 mg/ml ethyl 3-aminobenzoate methanesulfonate salt (Sigma–Aldrich) for 5 min.
Under sterile conditions, parts of the caudal, pectoral and abdominal fins were cut from the fish and washed three times in PBS (Gibco) with 1% (v/v) P/S (penicillin–streptomycin; Gibco). After the final wash, the fin tissue was chopped into small explants and cultured in 35-mm culture dishes (Nunc) with the culture medium: DMEM (Dulbecco's modified Eagle's medium; Gibco) with 1% (v/v) P/S and 10% (v/v) FBS (fetal bovine serum; Gibco). A 10% collagenase type I (Sigma–Aldrich) solution was added to the culture medium for enzymatic digestion and removed after 3 days. The culture dishes were placed in an incubator at 26°C with a humidified atmosphere of 5% CO2, and the culture medium was changed every 3 days until the cells reached confluency.
When a complete monolayer had formed in primary culture, the medium was decanted off, and 0.25% trypsin–EDTA (Sigma–Aldrich) was added for 1 to 2 min to detach the cells from the dish. After washing with PBS, the cells were divided into two culture dishes with culture medium for subculturing. Three replicates of cells from passage seven were used to examine the cells' growth rate and cryopreservation and perform cell cycle analysis.
To test the growth rate among cells from different fin types (caudal, pectoral and abdominal), viable cells from each fin were counted with a haemocytometer using a Trypan Blue assay and individually cultured under the previously mentioned conditions. After 5 days, viable cells were re-counted, and the growth rates were compared (growth rate = the number of viable cells counted on day 5/the number of viable cells counted on day 0).
2.2. Cell cryopreservation
The cell cryopreservation methods of Mauger et al. (2006) were used with some modifications. To compare two freezing methods (slow and fast), viable cells (106 cells/ml) were suspended in freezing medium: culture medium supplemented with an additional 10% FBS and 5% DMSO (Sigma–Aldrich). For the slow-freezing method, cells in cryotubes (Nunc) were placed in a Cryo 1°C Freezing Container (Nalgene) and stored in a −80°C freezer overnight then transferred to a liquid nitrogen tank (−196°C) for storage. After 5 days, the cells were thawed in a 37°C water bath and cultured with new culture medium. For the fast-freezing method, on the other hand, cells in cryotubes were directly put in a liquid nitrogen tank (−196°C) and thawed after 5 days.
To find the optimal DMSO concentration, viable cells (106 cells/ml) were suspended in three kinds of freezing medium containing different DMSO concentrations. The composition of the freezing medium was as follows: freezing medium 1 was culture medium supplemented with a 10% FBS and 5% DMSO, freezing medium 2 was culture medium supplemented with a 10% FBS and 10% DMSO and freezing medium 3 was culture medium supplemented with a 10% FBS and 15% DMSO. Cells were cryopreserved using the slow-freezing method and thawed as described above.
Cell viability was determined with a haemocytometer using a Trypan Blue assay before and after cryopreservation to calculate survival rates (survival rate = the number of viable cells after cryopreservation×100/the number of viable cells before cryopreservation).
2.3. Cell cycle analysis
The effects of culturing to confluence and roscovitine treatment on inducing cell cycle arrest at the G0-/G1-phase were determined. To examine the effect of confluence, the cells were fixed and analysed after they had reached 100% confluency. To examine the effect of roscovitine (Sigma–Aldrich) treatment, the cells were grown to 50% confluency then subjected to 10 μM roscovitine treatment for 24 h before being harvested and fixed for analysis.
The cell cycle analysis methods of Choresca et al. (2009) were used with some modifications. Briefly, the harvested cells (105 cells/ml) were resuspended in 0.3 ml PBS and fixed by adding 0.7 ml cold ethanol (70%) then incubating the cells at 4°C for 48 h. The fixed cells were resuspended in 0.25 ml PBS containing 5 μl of 10 mg/ml RNase A (Sigma–Aldrich) and incubated at 37°C for 1 h, then stained by adding 10 μl of 1 mg/ml propidium iodide (Sigma–Aldrich). The fixed and stained cells were analysed with a BD FACS Calibur (Becton Dickinson) flow cytometer at 488 nm. Histogram plots were created using the Cell Quest program, and the percentages of cells within various phases of the cell cycle were calculated using the WinMDI program under the methods of Fried et al. (1976).
2.4. Statistical analysis
The data were analysed by a Student's t test or an ANOVA (analysis of variance) using the statistical program for social sciences (SPSS version 17.0). Data were expressed as the means±S.E.M., and differences were considered significant when P<0.05.
3. Results and discussion
3.1. Fin cell culturing
The fin cell culture technique used in this study offers an elegant way to develop primary cultures without sacrificing the fish (Prasanna et al., 2000). Therefore, this technique could be a practical way of obtaining cell cultures from valuable fish such as the glass catfish, the mature state of which is still uncertain.
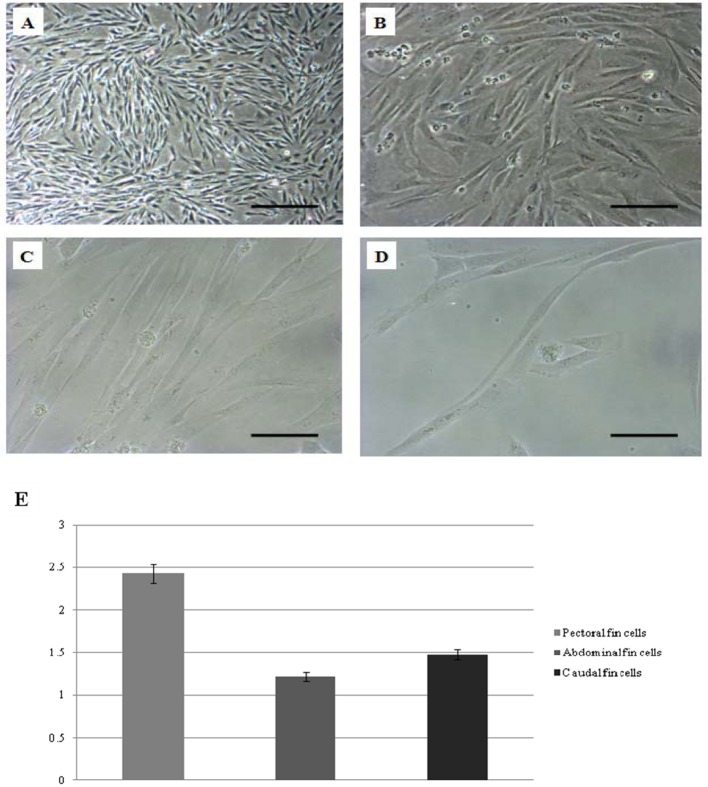
Cells derived from the caudal, abdominal and pectoral fins have reached confluency within 2 to 3 weeks (data not shown). DMEM supplemented with 10% FBS (v/v) was determined to be the optimal growth medium, and 26°C was the best temperature for maintaining the primary cell cultures. Additionally, the cell cultures could be maintained for 13 to 15 days without medium change. Photomicrographs of cultured cells at passage seven are shown (Figures 1A to 1D).
Figure 1. Photomicrograph of cultured cells from glass catfish and the growth rate comparison of three fintypes (caudal, pectoral and abdominal).
(A) Monolayer formation from pectoral fin at passage seven, scale bar = 0.2 mm. (B) Scale bar = 80 μm. (C) Scale bar = 40 μm. (D) Single cell from pectoral fin at passage seven, scale bar = 40 μm. (E) Differences in growth rate among three fin types (means±S.E.M.).
Among the different fin types (caudal, pectoral and abdominal), the growth rate of pectoral fin cells was significantly higher (2.43±0.11, P<0.05) than the growth rate of abdominal and caudal fins cells (1.22±0.05 and 1.48±0.06, respectively) (Figure 1E). Based on this data, it can be hypothesized that cells in pectoral fins have a higher proliferation ability than cells from the other fins.
3.2. Cell cryopreservation
In the present study, we developed an optimized protocol to cryopreserve fin cells from glass catfish. DMSO is the most commonly used cryoprotectant for cultured cells, including fish cells, due to its low molecular weight and penetration capacity (Freshney, 2000). The toxicity of DMSO was demonstrated by the decreased percentage of recovery and adhesion rate compared with fresh fin cells (Mauger et al., 2006), but the DMSO in the freezing medium could be removed by successive washes, leaving only trace amounts (Chen et al., 2007).
The cryopreservation efficiency for two freezing methods is shown in (Table 1). After thawing, the cells that were processed with the slow-freezing method showed significantly higher cell recovery than cells preserved with the fast-freezing method (P<0.05). Based on this result, it can be speculated that DMSO cannot protect cells sufficiently in the fast-freezing method because of the slow penetration of the cryoprotectant into the fin cells.
Table 1. Effect of different freezing conditions (freezing methods comparison and freezing medium comparison) on recovery rate of glass catfish fin cells.
Percentage of recovery rate (means±S.E.M.).
| Freezing methods comparison | Freezing medium comparison (three different DMSO concentrations) | |||
|---|---|---|---|---|
| Fast freezing | Slow freezing | Freezing medium 1 (5% DMSO) | Freezing medium 2 (10% DMSO) | Freezing medium 3 (15% DMSO) |
| 64.4467±3.5317 | 7.3233±1.4953 | 67.6733±2.5318 | 85.0000±4.3899 | 95.8833±0.3383 |
Practically, the concentrations of DMSO used for frozen cell suspensions are 10% to 20% (Donahoe et al., 1977; Jezek et al., 2002; Shinohara et al., 2002). For cryopreservation of established cells, the optimal concentration of DMSO was 15%, which was comparable with other data (Donahoe et al., 1977; Jezek et al., 2002; Shinohara et al., 2002), but there was no significant difference between 10% and 15% DMSO concentrations in terms of cell recovery (Table 1). Even though the 5% DMSO concentration has been successfully used for cell cryopreservation in the previous paper (Keros et al., 2005), and the concentration could be lowered to 5% for cryopreservation in this study, this concentration had significantly lower efficiency compared with the 10% or 15% concentrations (P<0.05).
3.3. Cell cycle analysis
Several methods have been employed in mammals for obtaining somatic cells in the G0/G1 stage of the cell cycle for SCNT application (Prather et al., 1992, Campbell et al., 1994; Gibbons et al., 2002), but few studies were reported in fish. We used two treatments (roscovitine and culturing to confluence) to determine the relative proportions of glass catfish fin cells in different phases of the cell cycle, particularly the G0/G1 phase. The chemicals for treatment were chosen by the result of Choresca et al. (2009).
The relative percentage of the proportions of cells in the G0/G1, S and G2/M stages were calculated (Table 2), and representative histograms for the cell cycle stage distributions of fin cells of glass catfish treated with different culture conditions are shown (Figure 2). In our study, culturing to confluence yielded over 85% of cells arrested at the G0/G1 phase (85.6833±0.9988). Culture to confluence has been known to cause cells to arrest at the G0/G1 phase by contact inhibition and appears to be one of the most widely used methods prior to SCNT (Campbell et al., 2007).
Table 2. Effect of different cell culture conditions on the synchronization of cell cycles of glass catfish fin cells.
Percentage of different cell cycle stages (means±S.E.M.).
| Cell culture conditions | G0/G1 | S | G2/M |
|---|---|---|---|
| Culturing to confluence | 85.6833±0.9988 | 3.5867±0.3534 | 6.6667±0.2472 |
| 10 μM Roscovitine | 74.4433±1.9999 | 7.3833±0.2185 | 17.6933±2.0720 |
Figure 2. Typical histograms of DNA content obtained using flow cytometry of glass catfish fin cells at (A) culturing to confluence, (B) 10 μM roscovitine treatment.
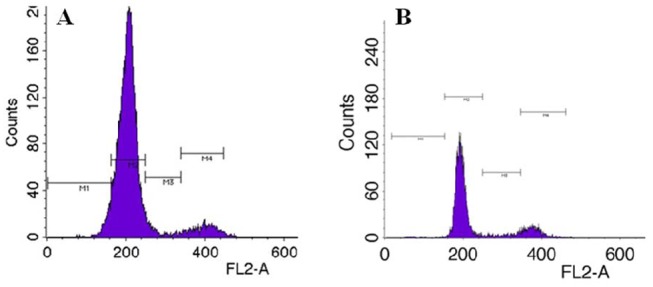
Recently, roscovitine, a specific CDK (cyclin-dependent kinase) 2 inhibitor was used to arrest cells in the G0-/G1-phase. This chemical was recommended due to the slightly higher proportion of cells in G0/G1 and the faster synchronizing response after the onset of treatment compared with the other protocols (Choresca et al., 2009; Koo et al., 2009). In our study, roscovitine treatment was also effective for synchronizing cells at the G0/G1 stages (74.4433±1.9999) but showed significantly lower efficiency than culturing to confluence (P<0.05). Additionally, the use of a chemical agent such as roscovitine for cell cycle synchronization prior to SCNT can impose potentially stressful or toxic conditions on the cells (Gerger et al., 2010).
4. Conclusions
In our study, fin-derived cells from glass catfish were successfully isolated after culturing fin explants, and the cells were viable after cryopreservation. Cryopreservation with 15% DMSO using the slow-freezing method allowed for a 95% recovery rate of cells; therefore, the whole procedure can be considered for cryopreservation of glass catfish genome. In addition, methods for producing G0 and G1 cell populations were evaluated for further SCNT application, and culturing to confluence alone was able to induce glass catfish fin cells to arrest at the G0 and G1 stages (>85%) compared with roscovitine treatment (<75%). Therefore, we provide sufficient data that it is best not to use chemicals on donor nuclei cells prior to SCNT to avoid the impairment of cell viability associated with cell death or apoptosis. These tested procedures on characterization of glass catfish fin cells, including testing cryopreservation and cycle analysis, demonstrate that these somatic cells could be a reliable depository, and these conclusions are highly interesting when cells are used for genetic resource management.
Footnotes
This study was supported through MKE [grant numbers 2009-67-10033839, 2009-67-10033805], and NRF [grant number M10625030005-508-10N250300510].
Author contribution
Jee Eun Han took a part in the experimental development of the work as well as in the discussion of results and writing of the manuscript. Byeong Chun Lee and Se Chang Park directed the whole work and discussions. Casiano Choresca, Jr. participated in the cell culture and cryopreservation, and Ok Jae Koo, Hyun Ju Oh and So Gun Hong participated in cell cycle analysis. Ji Hyung Kim, Sang Phil Shin and Jin Woo Jun helped with cell cultures and cryopreservation.
References
- Campbell KH, Loi P, Cappai P, Wilmut I. Improved development to blastocyst of bovine nuclear transfer embryos reconstructed during the presumptive S-phase of the enucleated activated oocyte. Biol Reprod. 1994;50:1385–93. doi: 10.1095/biolreprod50.6.1385. [DOI] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Titchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–6. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Campbell KH, Fisher P, Chen WC, Choi I, Kelly RD, Lee JH. Somatic cell nuclear transfer: past, present and future perspectives. Theriogenology. 2007;68S:S214–31. doi: 10.1016/j.theriogenology.2007.05.059. [DOI] [PubMed] [Google Scholar]
- Chen GR, Ge RS, Lin H, Dong L, Sottas CM, Hardy MP. Development of a cryopreservation protocol for Leydig cells. Hum Reprod. 2007;22:2160–8. doi: 10.1093/humrep/dem169. [DOI] [PubMed] [Google Scholar]
- Choresca CH, Koo OJ, Oh HJ, Hong SG, Gomez DK, Kim JH. Different culture conditions used for arresting the G0/G1 phase of the cell cycle in goldfish (Carassius auratus) caudal fin-derived fibroblasts. Cell Biol Int. 2009;33:65–70. doi: 10.1016/j.cellbi.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Chou SC, Taylor JD, Tchen TT. Isolation of melanized cell lines with stable phenotypes from a goldfish erythrophoroma cell line and cryopreservation of these cells by the use of autologous serum. In Vitro Cell Dev Biol. 1989;25:813–20. doi: 10.1007/BF02623665. [DOI] [PubMed] [Google Scholar]
- Donahoe PK, Ito Y, Hendren WH. The preservation of Mullerian inhibiting substance during long-term freezing of testicular fragments. Cryobiology. 1977;14:534–42. doi: 10.1016/0011-2240(77)90163-8. [DOI] [PubMed] [Google Scholar]
- Fisher SC, Riva ID, Vila C. See-through frog offers inside information. Nature. 2007;449:521. [Google Scholar]
- Freshney RI. Culture of animal cells: a manual of basic techniques. New York: A.R. Liss; 2000. [Google Scholar]
- Fried J, Perez A, Clarkson B. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J Cell Biol. 1976;71:172–81. doi: 10.1083/jcb.71.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerger RP, Ribeiro ES, Forell F, Bertolini LR, Rodrigues JL, Ambrósio CE. In vitro development of cloned bovine embryos produced by handmade cloning using somatic cells from distinct levels of cell culture confluence. Genet Mol Res. 2010;9:295–302. doi: 10.4238/vol9-1gmr690. [DOI] [PubMed] [Google Scholar]
- Gibbons J, Arat S, Rzucidlo J, Miyoshi K, Waltenburg R, Respess D. Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cells. Biol Reprod. 2002;66:895–900. doi: 10.1095/biolreprod66.4.895. [DOI] [PubMed] [Google Scholar]
- Jezek D, Schulze W, Kalanj-Bognar S, Vukelic Z, Milavec-Puretic V, Krhen I. Effects of various cryopreservation media and freezing–thawing on the morphology of rat testicular biopsies. Andrologia. 2002;33:368–78. doi: 10.1046/j.1439-0272.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20:1676–87. doi: 10.1093/humrep/deh797. [DOI] [PubMed] [Google Scholar]
- Koo OJ, Hossein MS, Hong SG, Martinez-Conejero JA, Lee BC. Cell cycle synchronization of canine ear fibroblasts for somatic cell nuclear transfer. Zygote. 2009;17:37–43. doi: 10.1017/S096719940800498X. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ. Dogs cloned from adult somatic cells. Nature. 2005;436:641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Encyclopedia of tropical fishes, 1st edn. Korea: Maya; 1999. [Google Scholar]
- Mauger PE, Le Bail PY, Labbe C. Cryobanking of fish somatic cells: optimizations of fin explant culture and fin cell cryopreservation. Comp Biochem Physiol B. 2006;144:29–37. doi: 10.1016/j.cbpb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Moritz C, Labbe C. Cryopreservation of goldfish fins and optimization for field scale cryobanking. Cryobiology. 2008;56:181–8. doi: 10.1016/j.cryobiol.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Prasanna I, Lakra WS, Ogale SN, Bhonde RR. Cell culture from fin explant of endangered golden masheer, Tor putitora (Hamilton). Curr Sci. 2000;79:93–5. [Google Scholar]
- Prather RS, Stumpf TT, Rickords LF. Nuclear transplantation as a method of producing genetically identical livestock. Anim Biotechnol. 1992;3:67–79. [Google Scholar]
- Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in vitro microinsemination. Hum Reprod. 2002;17:3039–45. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Pristyazhnyuk S, Kinoshita M, Tanaka M, Ozato K. The see-through medaka: a fish model that is transparent throughout life. Proc Natl Acad Sci USA. 2001;98:10046–50. doi: 10.1073/pnas.181204298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, LaPatra S, Zeng L, Zhao Z, Lu Y. Establishment, growth, cryopreservation and species of origin identification of three cell lines from white sturgeon, Acipenser transmontanus. Methods Cell Sci. 2003;25:211–20. doi: 10.1007/s11022-004-9120-x. [DOI] [PubMed] [Google Scholar]
- Wolf K, Mann JA. Poikilotherm vertebrate cell lines and viruses: a current listing for fishes. In Vitro Cell Dev Biol Plant. 1980;16:168–79. doi: 10.1007/BF02831507. [DOI] [PubMed] [Google Scholar]
- White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–9. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cooper RK, Wolters WR, Tiersch TR. Isolation, culture and characterization of a primary fibroblast cell line from channel catfish. Cytotechnology. 1998;26:83–90. doi: 10.1023/A:1007911619537. [DOI] [PMC free article] [PubMed] [Google Scholar]